Abstract
Purpose
While adenomyosis is one of the most common benign histologic findings in hysterectomy specimens of endometrial cancer, demographics of endometrial cancer arising in adenomyosis (EC-AIA) has not been well elucidated. The aim of this study is to evaluate histopathological findings and disease-free survival (DFS) of EC-AIA in comparison to endometrial cancer coexisting with adenomyosis (EC-A).
Methods
EC-AIA cases were identified via a systematic literature search (n = 46). EC-A cases were identified from a historical cohort that underwent hysterectomy-based surgical staging in two institutions (n = 350). Statistical comparisons of the two groups were based on univariate and multivariate analyses.
Results
The EC-AIA group was significantly older than the EC-A group (58.9 versus 53.8, p = 0.002). As to tumor characteristics, 63.6% of EC-AIA cases reported tumor within the myometrium without endometrial extension. The EC-AIA group was significantly associated with more non-endometrioid histology (23.9 versus 14.8%; p = 0.002) and deep myometrial tumor invasion (51.6 versus 19.4%; p < 0.001) than EC-A. Tumor grade, stage, and nodal metastasis risk were similar (all, p > 0.05). In a univariate analysis, the EC-AIA group had a significantly decreased DFS compared to EC-A (5-year rates, 72.2 versus 85.5%, p = 0.001). After controlling for age, histology, tumor grade, and stage, EC-AIA remained an independent prognostic factor associated with decreased DFS compared to EC-A (adjusted-hazard ratio 2.87, 95% confidence interval 1.44–5.70, p = 0.031).
Conclusion
Our study demonstrated that EC-AIA has distinct tumor characteristics and a poorer survival outcome compared to EC-A. This suggests a benefit of recognition of this unique entity as an aggressive variant of endometrial cancer.
Keywords: Endometrial cancer, Adenomyosis, Endometriosis-associated cancer, Survival, Prognosis
Introduction
Endometrial cancer is the most prevalent gynecologic malignancy in the United States, with an estimated 61,380 new cases and 10,920 deaths projected for 2017 [1]. Approximately 2.8% of women are diagnosed with endometrial cancer at some point during their lifetime [2]. Two-thirds (68%) of patients with endometrial cancer are initially diagnosed with the disease confined to the uterus by hysterectomy-based surgical staging [2]. In contrast, adenomyosis is one of the most common pathologic findings of benign gynecological disease in hysterectomy specimens. Adenomyosis is defined as ectopic endometriosis, which invades the endometrium into the myometrium, into the uterus and endometrial glands [3]. The prevalence of adenomyosis is reported to range widely from 18 to 66% [4, 5].
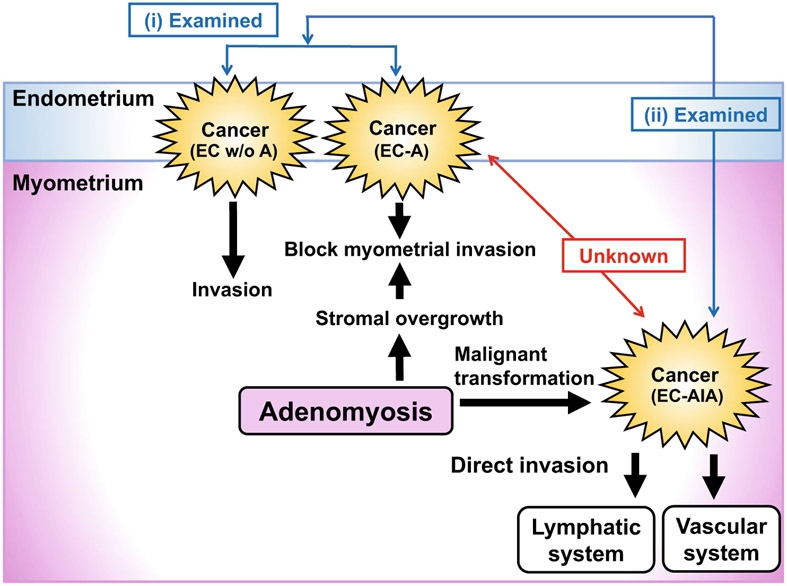
Currently, a population-based study including 9842 people with endometriosis showed that women diagnosed with endometriosis and/or adenomyosis had increased risk of endometrial cancer [6]. Malignant transformation in endometriosis is a well described occurrence in ovarian cancer. Sampson suggested that the ectopic location of endometriosis contributes to the potential for malignant change. Indeed, endometrial cancer arising in adenomyosis (EC-AIA) is reported in approximately 1% of women with endometrial cancer [7]. EC-AIA is characterized as follows: the absence of carcinoma in the normally situated endometrium or elsewhere in the pelvis, the demonstration of cancer arising in the epithelium of the adenomyosis and not invading it from another sources, and the presence of tissue resembling endometrial stromal cells surrounding epithelial glands. However, the clinical features and prognosis of EC-AIA remain controversial. Some studies have shown that EC-AIA was associated with aggressive tumor behavior, which resulted in poor patient prognosis [8-11]. However, the majority of these studies were based on case series. Our previous studies showed (1) that presence of adenomyosis in endometrial cancer is associated with improved survival in endometrial cancer and (2) that EC-AIA was associated with decreased survival compared to endometrial cancer irrespective to adenomyosis status (Fig. 1) [12] [13]. Because these studies did not clearly compare the characteristics and outcomes between EC-AIA and endometrial cancer coexisting with adenomyosis (EC-A), whether or not EC-AIA had distinct differences to EC-A remains unknown. The aim of this study was to conduct a sub-analysis to evaluate the histopathologic findings and prognosis of EC-AIA compared to EC-A within our previous study cohorts, and to investigate the role of adenomyosis in endometrial cancer progression.
Fig. 1.
Schema for study design. Prior studies examined (i) endometrial cancer coexisting with adenomyosis (EC-A) compared to endometrial cancer without adenomyosis (EC w/o A), (ii) endometrial cancer arising in adenomyosis (EC-AIA) compared to endometrial cancer regardless of adenomyosis status. However, the relation between EC-AIA and EC-A is still unknown. EC-AIA defined as follows: (1) the carcinoma must not be situated in the endometrium or elsewhere in the pelvis, (2) the carcinoma must be seen to arise from the epithelium of adenomyosis and not to have invaded from another source, (3) endometrial stromal cells should be surrounding the aberrant glands to support the diagnosis of adenomyosis
Materials and methods
Study design and eligibility
Approval for Institutional Review Board (IRB) was obtained at the University of Southern California, and an exploratory investigation was performed. This study defined the case group as EC-AIA, and the control group as historically confirmed endometrial cancer co-existing with adenomyosis. For the case group, a systematic literature search was conducted by using public search engines PubMED and MEDLINE with entry keywords “endometrial cancer” AND “adenomyosis” in English literature on August 7, 2015. Eligible cases were EC-AIA with adequate description for clinical information and pathological results. These cases of EC-AIA were reported within the context of our prior study [12].
For the control group, patients with a diagnosis of endometrial cancer co-existing with adenomyosis (EC-A) underwent surgical staging including hysterectomy, and specimens were examined at Los Angeles County Medical Center and Keck Medical Center of University of Southern California between January 1, 2000 and August 31, 2015. All the pathology reports were examined for the presence of EC-AIA, and no cases of EC-AIA were found in the control group. Patients were excluded from this study if diagnosed with endometrial cancer without adenomyosis, or with other histologic diagnoses including uterine sarcoma, carcinosarcoma, and endometrial hyperplasia. The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guideline was consulted when reporting a case-control study [14]. Some of the patients in this study were within the context of our previous studies [12, 13, 15, 16].
Clinical information
For eligible cases, the following information was abstracted: (1) patient demographics (2) tumor characteristics, and (3) survival outcomes. Patient demographics included age and ethnicity. In addition, the year and country of publication, menopausal status, and presenting symptoms were collected for the case group. Tumor characteristics included histologic subtype, grade, stage, depth of myometrial tumor invasion, lymph node metastasis, and estrogen receptor (ER) expression. Tumor expansion to the endometrial layer was also abstracted. The survival outcomes included disease-free survival (DFS) and overall survival (OS).
Definition
EC-AIA was defined by the previously described diagnostic criteria; (1) cancer must not be located in the endometrium or other place in pelvis, (2) cancer arises from the epithelium of adenomyosis and not to be invading from another source, and (3) endometrial stromal cells surround the aberrant glands to support the diagnosis of adenomyosis [17]. EC-AIA involved endometrium defined as a continuous transition from the adenomyotic epithelium in the myometrium to adenocarcinoma, which extended to the endometrium. It were contradictory to the first criteria, however, we accepted the modified criteria supported by the immunostaining results base on prior reports. [10, 18]. EC-A was defined as adenomyosis accompanied with endometrial cancer concurrently, presenting with endometrial glands and stroma in the myometrium, away from the endometrial junction. Cancer stage was evaluated based on the 2009 FIGO system [19]. Histologic subtypes were grouped as endometrioid, serous, clear cell, or other adenocarcinoma. Tumor grade was grouped into low-grade versus high-grade. Grade 1 and 2 endometrioid tumors were categorized as low-grade. Grade 3 endometrioid, serous, and clear cell tumors were categorized as high-grade. Deep myometrial tumor invasion was defined as the presence of tumor in the outer half of the myometrial layer (≥50%). The extent of myometrial invasion in EC-AIA was based on descriptions. If their was deep invasion without detail, it was allocated to the deep invasion group. Nodal metastasis was evaluated for pelvic and/or para-aortic lymph nodes. DFS was defined as the time interval between the date of hysterectomy and the date of the first recurrence of endometrial cancer, or the last follow-up date. OS was defined as the time interval between the date of hysterectomy and the date of death due to endometrial cancer, or the last follow-up date if the patient was alive. The co-investigators entered the data into the de-identified database, and the principal investigator of the study examined the database for accuracy, consistency, and quality.
Statistical analysis
The primary interest of analysis was to examine DFS and OS between the case and control groups. The secondary aim of the analysis was to identify the characteristics of EC-AIA. The continuous variable for age was examined for normality by the Kolmogorov-Smirnov test, and was expressed with mean (±standard deviation) as appropriate. Statistical significance of continuous variables was assessed with student t test or Mann-Whitney U test as appropriate. Categorical or ordinal variables were expressed with number (%), and statistical significance was examined by Chi-square test or the Fisher’s exact test as appropriate. For survival analysis, Kaplan–Meier method and a log-rank test was used for univariate analysis. A Cox proportional hazard regression model was used for multivariate analysis. Covariates with p < 0.10 in the univariate analysis were initially entered into the multivariate model. The final model in the multivariate analysis were EC-AIA (no or yes), age (<50, 50–59, and ≥60 years), ethnicity (Caucasian, African, Hispanic, or Asia), histology (endometrioid, serous, clear cell, and other), grade (low versus high-grade), and stage (I, II, III, and IV). Subsequently, it was expressed with hazard ratio (HR) and 95% CI. The Kaplan–Meier method was used to construct survival curves. All statistical tests were two-tailed, and p value less than 0.05 was considered to be statistically significant. Statistical Package for the Social Sciences (SPSS, version 22.0, Chicago, IL, USA) was used for the analysis.
Results
The selection of the EC-AIA group was performed using internet search engines with entry keywords. 588 articles initially matched in this study. Of those, 544 articles were not relevant to study, and were excluded. The remaining 34 articles were reviewed entirely. Six articles were further excluded due to separate foci of adenomyosis and cancer. Twenty-four articles which described a total of 46 cases of EC-AIA represent the study population for statistical analysis [8, 10, 11, 18, 20-42] (Supplemental Figure S1). In the control group, 350 cases of endometrial cancer that had surgical staging during the study period were examined for statistical analysis (Los Angeles County Medical Center n = 296 and Keck Medical Center of University of Southern California n = 54). These did not comprise any EC-AIA cases.
Clinical and pathological variables were compared between the EC-AIA groups and EC-A groups in Table 1. Patients in the EC-AIA group were significantly older (58.9 versus 53.8 years; p = 0.002) and more often Asian (56.8 versus 14.0%: p < 0.001) compared to patients in the EC-A group. More than three quarter of the patients in the EC-AIA group were postmenopausal (81.4%). Tumor characteristics of the EC-AIA group were significantly associated with more non-endometrioid histology (23.9 versus 14.8%; p = 0.002) and deep myometrial tumor invasion (51.6 versus 19.4%; p < 0.001) than EC-A. The tumors in the EC-AIA group were more often high-grade (31.7 versus 19.3%), stage III-IV in stage (24.4 versus 17.7%), and positive of nodal metastasis (27.3 versus 17.9%) than EC-A, although this did not reach statistical significance (all; p > 0.05). Tumors with EC-AIA were also less likely to be positive for ER expression than tumors with EC-A (14.3 versus 93.4%; p < 0.001). Endometrial biopsy was performed preoperatively in 25 cases, and 9 out of the 25 cases were diagnosed with endometrial cancer. 30 (65.2%) cases of EC-AIA were incidentally discovered in the hysterectomy specimens.
Table 1.
Patient demographics
| EC-AIA | EC-A | p value | |
|---|---|---|---|
| No. | n = 46 | n = 350 | |
| Age | 58.9 (±9.9) | 53.8 (±0.55) | 0.002 |
| <50 | 7 (15.6%) | 109 (31.1%) | |
| 50–59 | 16 (35.5%) | 137 (39.2%) | |
| ≥60 | 22 (48.9%) | 104 (29.7%) | |
| Ethnicity | <0.001 | ||
| Caucasian | 14 (31.8%) | 72 (20.6%) | |
| African American | 3 (6.8%) | 13 (3.7%) | |
| Hispanic | 2 (4.6%) | 216 (61.7%) | |
| Asian | 25 (56.8%) | 49 (14.0%) | |
| Histology | 0.002 | ||
| Endometrioid | 35 (76.1%) | 298 (85.2%) | |
| Serous | 7 (15.2%) | 19 (5.4%) | |
| Clear cell | 3 (6.5%) | 4 (1.1%) | |
| Other | 1 (2.2%) | 29 (8.3%) | |
| Grade | 0.06 | ||
| low-grade | 28 (68.3%) | 281 (80.7%) | |
| high-grade | 13 (31.7%) | 67 (19.3%) | |
| Deep myometrial invasion | <0.001 | ||
| No | 15 (48.4%) | 272 (77.7%) | |
| Yes | 16 (51.6%) | 68 (19.4%) | |
| Stage | 0.43 | ||
| I | 30 (73.2%) | 268 (76.6%) | |
| II | 1 (2.4%) | 20 (5.7%) | |
| III | 6 (14.6%) | 46 (13.1%) | |
| IV | 4 (9.8%) | 16 (4.6%) | |
| Nodal metastasis* | 0.22 | ||
| Negative | 24 (72.7%) | 128 (82.1%) | |
| Positive | 9 (27.3%) | 28 (17.9%) | |
| Not performed | 13 | 194 | |
| ER expression | <0.001 | ||
| Negative | 12 (85.7%) | 7 (6.5%) | |
| Positive | 2 (14.3%) | 100 (93.4%) | |
| Not evaluated | 33 | 243 | |
Student t test or Chi square for p values. Mean (±SD) or number (%) is shown
1 missing data for age, 2 missing data for ethnicity, 7 missing data for grade, 25 missing data for deep myometrial invasion, and 5 missing data for stage. EC-AIA endometrial cancer arising in adenomyosis, ER estrogen receptor
Nodal metastasis for pelvic and/or para-aortic lymph nodes
Significant P-values are shown in bold
In addition, demographics and tumor characteristics were compared between EC-AIA tumors confined to the myometrium and EC-AIA with tumors extending into the endometrium (Table 2). Twenty-eight patients in the EC-AIA group were found to have tumor confined within the myometrial layer of the uterus without invasion to the endometrium. Age, symptoms, grade, histology, and stage were similar between the two groups (all, p > 0.05). There was no difference for 5-year DFS rates between the two groups (72.3 versus 70.8%; p = 0.84).
Table 2.
Significance of EC-AIA expanding into the endometrium
| Endometrium extension |
p value | ||
|---|---|---|---|
| No | Yes | ||
| No. | n = 28 | n = 16 | |
| Age | 59.3 ± 10.2 | 58.2 ± 10.0 | 0.91 |
| <50 | 4 (14.8%) | 3 (18.8%) | |
| 50–59 | 10 (37.0%) | 5 (31.2%) | |
| ≥60 | 13 (48.2%) | 8 (50.0%) | |
| Symptom | 0.24 | ||
| Abnormal uterine bleeding | 9 (32.1%) | 9 (56.3%) | |
| Abdominal-pelvic pain | 8 (28.6%) | 5 (31.3%) | |
| Other symptoms | 3 (10.7%) | 1 (6.2%) | |
| Asymptomatic | 8 (28.6%) | 1 (6.2%) | |
| Grade | 0.99 | ||
| low-grade | 18 (69.2%) | 9 (64.3%) | |
| high-grade | 8 (30.8%) | 5 (35.7%) | |
| Histology | 0.40 | ||
| Endometrioid | 21 (75.0%) | 12 (80.0%) | |
| Serous | 4 (14.3%) | 3 (20.0%) | |
| Clear cell | 3 (10.7%) | 0 | |
| Stage | 0.16 | ||
| I | 20 (80.0%) | 8 (57.2%) | |
| II | 0 | 1 (7.1%) | |
| III | 2 (8.0%) | 4 (28.6%) | |
| IV | 3 (12.0%) | 1 (7.1%) | |
Student t test, Chi-square, and Fisher’s exact test for p value
Mean (±SD) or number (%) is shown
1 missing data for age and histology, 4 missing data for grade, and 5 missing data for stage
EC-AIA and endometrial cancer arising in adenomyosis
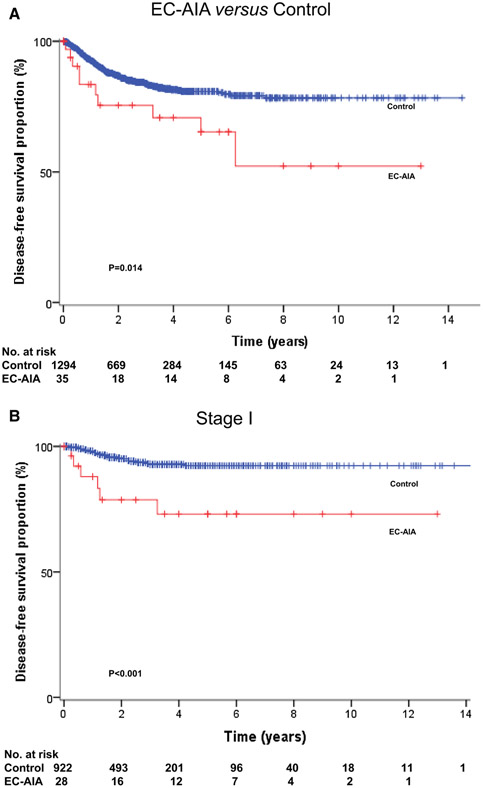
The survival analysis was performed and the independent risk factors for disease-free survival in all cases were evaluated (Table 3). The median follow-up time between the date of EC-AIA diagnosis and the last follow-up was 28.6 months. There were 10 cases (21.7%) of recurrence of disease and 6 deaths (13%) due to cancer progression reported in the study. A significantly decreased DFS was observed in the EC-AIA group in the univariate analysis when compared with patients of the EC-A group (5-year rates 72.2 versus 85.5%, p = 0.001, Fig. 2a). Furthermore, older age, Asian ethnicity, non-endometrioid histology, high-grade tumor, and advanced stage were also associated with decreased DFS in all cases (all, p < 0.05). Even in cases with earlier disease stage, the decreased DFS was significantly associated with stage I EC-AIA compared with stage I EC-A (5-year rates 77.0 versus 92.5%, p < 0.001, Fig. 2b). In the multivariate analysis, EC-AIA remained a statistically independent risk factor for DFS in all cases (HR 2.87; 95% CI 1.44–5.70; p = 0.031). Other independent risk factors associated with decreased DFS included age 50–59 (HR 4.57; 95% CI 1.49–14.0; p = 0.008), age ≥60 (HR 5.11; 95% CI 1.52–17.1; p = 0.008), stage III (HR 3.68; 95% CI 1.59–8.49; p = 0.002), and stage IV disease (HR 18.4; 95% CI 6.45–52.7; p < 0.001). Survival analysis was performed for all cases (Supplement Table 1). Decreased OS was significantly associated with EC-AIA (5-year rates: 86.0 versus 90.8%, p = 0.031) compared to the EC-A group in the univariate analysis. However, EC-AIA did not remain as a statistically independent risk factor for OS in multivariate analysis (p = 0.13).
Table 3.
Risk factors for disease-free survival in all cases
| No. | 5-year (%) | Univariate |
Multivariate |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |||
| Age | 0.035 | |||||
| <50 | 116 | 93.6 | 1 | 1 | ||
| 50–59 | 150 | 81.2 | 4.24 (1.47–12.4) | 4.57 (1.49–14.0) | 0.008 | |
| ≥60 | 122 | 78.9 | 5.47 (1.85–16.2) | 5.11 (1.52–17.1) | 0.008 | |
| Ethnicity | 0.002 | |||||
| Caucasian | 85 | 76.4 | 1 | |||
| African American | 16 | 85.2 | 1.32 (0.37–4.68) | |||
| Hispanic | 217 | 89.8 | 0.38 (0.17–0.82) | |||
| Asian | 69 | 72.6 | 1.63 (0.76–3.48) | |||
| Histology | <0.001 | |||||
| Endometrioid | 326 | 88.7 | 1 | 1 | ||
| Serous | 26 | 58.6 | 4.85 (2.19–10.8) | 1.20 (0.39–3.72) | 0.75 | |
| Clear cell | 7 | 33.3 | 21.3 (7.25–62.7) | 3.90 (0.46–32.9) | 0.21 | |
| Other | 28 | 64.2 | 3.30 (1.36–8.02) | 1.91 (0.72–5.08) | 0.20 | |
| Grade | <0.001 | |||||
| low-grade | 305 | 89.7 | 1 | 1 | ||
| high-grade | 73 | 68.8 | 4.45 (2.35–8.43) | 1.72 (0.71–4.18) | 0.23 | |
| Stage | <0.001 | |||||
| I | 295 | 90.3 | 1 | 1 | ||
| II | 21 | 88.2 | 1.13 (0.26–4.88) | 1.78 (0.39–7.99) | 0.46 | |
| III | 51 | 70.0 | 3.81 (1.88–7.73) | 3.68 (1.59–8.49) | 0.002 | |
| IV | 18 | 29.1 | 14.4 (6.64–31.2) | 18.4 (6.45–52.7) | <0.001 | |
| EC-AIA | 0.001 | |||||
| No | 350 | 85.5 | 1 | 1 | ||
| Yes | 35 | 72.2 | 3.18 (1.57–6.45) | 2.87 (1.44–5.70) | 0.031 | |
Log-rank test for univariate analysis, and Cox proportional hazard regression test for multivariate analysis. Significant p values are emboldened
EC-AIA endometrial cancer arising in adenomyosis, HR hazard ratio, CI confidence interval, 5-yr 5-year survival proportion
Significant P-values are shown in bold
Fig. 2.
Disease-free survival in case-control cases. Log-rank test for p values. Kaplan-Meier method for survival curves for a DFS for all cases, b OS for all cases, and c DFS for stage I
Discussion
The two key findings of our study are: (1) endometrial cancer arising in adenomyosis was associated with poor survival outcome compared to endometrial cancer co-existing with adenomyosis; (2) cancer arising from adenomyosis has occurred, and this endometrial cancer is likely to have aggressive tumor features.
The relation between endometrial cancer and adenomyosis has been documented; however, the role of adenomyosis in endometrial cancer progression remains unclear [8-13]. Our data suggests that adenomyosis may have a protective effect against cancer progression. The overall survival in endometrial cancer coexisting with adenomyosis was relatively higher compared to general reports of survival in endometrial cancer [2]. This positive prognostic result may be explained mechanically with the contribution of adenomyosis blocking cancer invasion with its surrounding hypertrophic and hyperplastic myometrial stroma (Fig. 1). Meanwhile, adenomyosis has a unique molecular cytokine environment [43]. Increased secretion of cytokines, including interferon (INF)-α,-γ, tumor necrosis factor (TNF)-α, and interleukin (IL)-10, is observed. IFNα/γ regulates and activates immune responses, and leads to increase in tumor immunity [44]. In addition, both TNFα and IL-6 stimulates the growth of endometrial and endometriosis stroma [45]. IL10 acts as an anti-inflammatory cytokine and controls tumor growth [46]. TNF-α induces apoptotic cell death, resulting in anti-tumor effect [47]. Consequently, increased INF- α,-γ, IL-10, and TNF-α leads to anti-tumor effect, and may result in protective effects against cancer progression.
Conversely, our study also shows that endometrial cancer arising in adenomyosis is also associated with an extremely poor prognosis and is more likely to have characteristics of deep myometrial tumor invasion as well as high-grade, advanced stage, and to have positive nodal metastasis. This finding may be explained by mechanisms behind cancer progression in EC-AIA (Fig. 1). Cancer initially occurs within the myometrial layer and easily reaches the myometrial stroma due to the lack of an anatomical barrier in the basal layer of endometrium [48]. The direct invasion of cancer in the myometrial stroma easily spreads to the lymphatic and vascular systems. Consequently, once cancer was arising in the myometrium in the adenomyosis, there was no significance in the prognosis of EC-AIA expanding into the endometrium. This mechanism can partially explain the poor prognosis of EC-AIA. However, malignant transformation of adenomyosis is still unclear in many molecular aspects. Few studies have described the association of adenomyosis and the loss of heterozygosity in the DNA mismatch repair gene (hMSH2, hMLH1, p16 and GALT) [49, 50]. Both genetic and epigenetic alterations in this multistep processes need to be considered in order to further evaluate whether these genes are related to malignant transformation of endometriosis.
Recently, based on molecular genetic studies, ovarian carcinogenesis was explained by categorizing them in two groups: Type I, which is clinically indolent and usually presents with low-grade cancer, showing KRAS, phosphatase and tensin homologue (PTEN), and phosphatidylinositol-4 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3-CA) mutations [51]. These mutations exhibit slow cancer progression from a defined benign precursor lesion: the corresponding endometrioid, clear cell, and low-grade serous cancers. Type II, which is aggressive, high-grade and usually present in advanced-stage disease, shows TP53 and HER2 mutations [52, 53]. Some studies showed that the high frequency of genetic mutations in PTEN, KRAS and AT-rich interactive domain 1A gene (ARID1A) is related with the onset of endometriosis-associated ovarian cancer of endometrioid and clear cell histologies [54]. Indeed, endometrioid and clear cell were the most common histologies of EC-AIA in our study. ARID1A is found in approximately 30–48% of the endometrioid and in 41–57% of the clear cell subtypes of endometriosis-associated ovarian cancer, and in approximately 40% of contiguous endometriosis cases, adjacent to cancerous tissue [54-56]. Genetic mutations in ARID1A have been described as an early event in carcinogenesis of endometrial cancer and may be a particular feature of cancer arising from endometrial glandular epithelium [57]. Unfortunately, the gene mutations have not been explored nor described in our reviewed literature on EC-AIA.
Meta-analyses showed epidemiologic evidence that endometriosis-associated ovarian cancer has favorable characteristics, such as type I ovarian cancer [58]. On the other hand, this study suggested that EC-AIA had quite a different prognosis and resulted in a dismal survival outcome. Additionally, previous studies assessed the prognosis between EC-AIA and high-grade EMCA, with a similar prognosis between the two groups [13]. Our findings proposed that EC-AIA may behave like a type II ovarian cancer, which is aggressive and presents with high-grade, deep myometrial invasion and advanced-stage disease. Decreased ER expression in EC-AIA seems to be less amenable hormone therapy. Further molecular genetics based study of EC-AIA to elucidate this entity is warranted. Currently, a prospective study is underway to identify prognostic biomarkers including ARID1A for endometrial cancer [59]. These results will contribute to explaining the pathogenesis of the EC-AIA endometrial cancer subtype.
The strength of this study was that its case-control design was relatively large in size compared to previous literature describing EC-AIA, and adenomyosis pathology was well described in both the control group and the EC-AIA group. However, a limitation of this study was that the sample size of the EC-AIA group was relatively small and due to its rarity, only 46 cases were detected during the 50 years covered in this study. There may be the possibility that this is a type II error, and that this influenced OS in multivariate analysis. Another limitation is that this is a retrospective study. A case-control study is not a typical approach, which means it may be missing potential confounding factors such as area, time, and treatments that patients received. Selection bias may also exist because only published cases were used for the systematic literature review. Additionally, lack of central pathology review is a weakness of the study as the diagnosis of such rare disease may differ across pathologists and it is not entirely clear how one can be certain that the carcinoma “arises” within one particular area of the endometrial epithelium (eutopic versus ectopic/adenomyosis). Therefore, the optimal study to elucidate EC-AIA entity would be a prospective study with a larger sample size to support our results.
In conclusion, the survival outcome of endometrial cancer coexisting with adenomyosis differs from that of EC-AIA. The mechanism based on its pathogenesis remains unclear in our study and needs further investigation.
Supplementary Material
Acknowledgements
The study was supported by the Ensign Endowment for Gynecologic Cancer Research (K.M.).
Financial support Ensign Endowment for Gynecologic Cancer Research (K.M.).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval All information in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Abstract of the study was presented at 47th Annual Meeting of Society of Gynecologic Oncologists, San Diego, CA, March 19–22, 2016.
Electronic supplementary material The online version of this article (doi:10.1007/s00404-017-4375-z) contains supplementary material, which is available to authorized users.
References
- 1.Siegel RL, Miller KD, Jemal A (2017) Cancer statistics. CA Cancer J Clin 67:7–30 [DOI] [PubMed] [Google Scholar]
- 2. [Accessed 27 Jan 2016]; http://seer.cancer.gov/statfacts/html/corp.html.
- 3.Ferenczy A (1998) Pathophysiology of adenomyosis. Hum Reprod Update 4:312–322 [DOI] [PubMed] [Google Scholar]
- 4.Vercellini P, Vigano P, Somigliana E et al. (2006) Adenomyosis: epidemiological factors. Best Pract Res Clin Obstet Gynaecol 20:465–477 [DOI] [PubMed] [Google Scholar]
- 5.Kairi-Vassilatou E, Kontogianni K, Salamalekis M et al. (2004) A clinicopathological study of the relationship between adenomyosis and other hormone-dependent uterine lesions. Eur J Gynaecol Oncol 25:222–224 [PubMed] [Google Scholar]
- 6.Kok VC, Tsai HJ, Su CF et al. (2015) The risks for ovarian, endometrial, breast, colorectal, and other cancers in women with newly diagnosed endometriosis or adenomyosis: a population-based study. Int J Gynecol Cancer 25:968–976 [DOI] [PubMed] [Google Scholar]
- 7.Samson John A (1925) Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Arch Surg 10:1–72 [Google Scholar]
- 8.Puppa G, Shozu M, Perin T, Nomura K et al. (2007) Small primary adenocarcinoma in adenomyosis with nodal metastasis: a case report. BMC Cancer 7:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuo K, Gualtieri MR, Cahoon SS et al. (2016) Contributing factors for menopausal symptoms after surgical staging for endometrial cancer. Menopause 23:535–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abushahin N, Zhang T, Chiang S et al. (2011) Serous endometrial intraepithelial carcinoma arising in adenomyosis: a report of 5 cases. Int J Gynecol Pathol 30:271–281 [DOI] [PubMed] [Google Scholar]
- 11.Mori M, Furusawa A, Kino N et al. (2015) Rare case of endometrioid adenocarcinoma arising from cystic adenomyosis. J Obstet Gynaecol Res 41:324–328 [DOI] [PubMed] [Google Scholar]
- 12.Matsuo K, Moeini A, Machida H et al. (2016) Tumor characteristics and survival outcome of endometrial cancer arising in adenomyosis: an exploratory analysis. Ann Surg Oncol 23:959–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuo K, Cahoon SS, Gualtieri M et al. (2014) Significance of adenomyosis on tumor progression and survival outcome of endometrial cancer. Ann Surg Oncol 21:4246–4255 [DOI] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M et al. (2007) Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335:806–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuo K, Garcia-Sayre J, Medeiros F et al. (2015) Impact of depth and extent of lymphovascular space invasion on lymph node metastasis and recurrence patterns in endometrial cancer. J Surg Oncol 112:669–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuo K, Opper NR, Ciccone MA et al. (2015) Time interval between endometrial biopsy and surgical staging for type I endometrial cancer: association between tumor characteristics and survival outcome. Obstet Gynecol 125:424–433 [DOI] [PubMed] [Google Scholar]
- 17.Colman HI, Rosenthal AH (1959) Carcinoma developing in areas of adenomyosis. Obstet Gynecol 14:342–348 [PubMed] [Google Scholar]
- 18.Hernandez E, Woodruff JD (1980) Endometrial adenocarcinoma arising in adenomyosis. Am J Obstet Gynecol 138:827–832 [DOI] [PubMed] [Google Scholar]
- 19.Taga S, Sawada M, Nagai A et al. (2014) A case of endometrioid adenocarcinoma arising from adenomyosis. Case Rep Obstet Gynecol 2014:569295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bae HS, Kim IS, Kang JS et al. (2014) Endometrioid adenocarcinoma arising from adenomyosis after black cohosh with St John’s wort. J Obstet Gynaecol 34:213–214 [DOI] [PubMed] [Google Scholar]
- 21.Heo SH, Lee KH, Kim JW et al. (2011) Unusual manifestation of endometrioid adenocarcinoma arising from subserosal cystic adenomyosis of the uterus: emphasis on MRI and positron emission tomography CT findings. Br J Radiol 84:e210–e212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boes AS, Tousseyn T, Vandenput I et al. (2011) Pitfall in the diagnosis of endometrial cancer: case report of an endometrioid adenocarcinoma arising from uterine adenomyosis. Eur J Gynaecol Oncol 32:431–434 [PubMed] [Google Scholar]
- 23.Kazandi M, Zeybek B, Terek MC et al. (2010) Grade 2 endometrioid adenocarcinoma arising from adenomyosis of the uterus: report of a case. Eur J Gynaecol Oncol 31:719–721 [PubMed] [Google Scholar]
- 24.Hirabayashi K, Yasuda M, Kajiwara H et al. (2009) Clear cell adenocarcinoma arising from adenomyosis. Int J Gynecol Pathol 28:262–266 [DOI] [PubMed] [Google Scholar]
- 25.Ohta Y, Hamatani S, Suzuki T et al. (2008) Clear cell adenocarcinoma arising from a giant cystic adenomyosis: a case report with immunohistochemical analysis of laminin-5 gamma2 chain and p53 overexpression. Pathol Res Pract 204:677–682 [DOI] [PubMed] [Google Scholar]
- 26.Motohara K, Tashiro H, Ohtake H et al. (2008) Endometrioid adenocarcinoma arising in adenomyosis: elucidation by periodic magnetic resonance imaging evaluations. Int J Clin Oncol 13:266–270 [DOI] [PubMed] [Google Scholar]
- 27.Izadi-Mood N, Samadi N, Sarmadi S et al. (2007) Papillary serous carcinoma arising from adenomyosis presenting as intramural leiomyoma. Arch Iran Med 10:258–260 [PubMed] [Google Scholar]
- 28.Hsu MI, Chou SY, Lin SE et al. (2006) Very early stage adenocarcinoma arising from adenomyosis in the uterus. Taiwan J Obstet Gynecol 45:346–349 [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi K, Tateiwa H, Hamana S et al. (2006) Invasive adenocarcinoma arising from adenomyosis in a septate uterus. Acta Obstet Gynecol Scand 85:1146–1147 [DOI] [PubMed] [Google Scholar]
- 30.Couto D, Mota F, Silva T, de Oliveira C (2004) Adenocarcinoma arising in adenomyosis: report of an unusual case. Acta Obstet Gynecol Scand 83:406–408 [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi K, Yamanaka Y, Hamana S et al. (2004) Invasive adenocarcinoma arising from uterine adenomyosis involving the rectosigmoid colon. Int J Gynecol Cancer 14:1004–1006 [DOI] [PubMed] [Google Scholar]
- 32.Koshiyama M, Suzuki A, Ozawa M et al. (2002) Adenocarcinomas arising from uterine adenomyosis: a report of four cases. Int J Gynecol Pathol 21:239–245 [DOI] [PubMed] [Google Scholar]
- 33.Kawana K, Shirai T, Jimbo H et al. (2002) Endometrial cytology in early diagnosis of adenocarcinoma arising from adenomyosis uteri. Acta Cytol 46:612–614 [PubMed] [Google Scholar]
- 34.Sasaki T, Sugiyama T, Nanjo H et al. (2001) Endometrioid adenocarcinoma arising from adenomyosis: report and immunohistochemical analysis of an unusual case. Pathol Int 51:308–313 [DOI] [PubMed] [Google Scholar]
- 35.Ichikawa Y, Takano K, Higa S et al. (2001) Endometrial carcinoma coexisting with pregnancy, presumed to derive from adenomyosis: a case report. Int J Gynecol Cancer 11:488–490 [DOI] [PubMed] [Google Scholar]
- 36.Takai N, Akizuki S, Nasu K et al. (1999) Endometrioid adenocarcinoma arising from adenomyosis. Gynecol Obstet Invest 48:141–144 [DOI] [PubMed] [Google Scholar]
- 37.Hayata T, Tanaka Y, Miyakawa I (1994) Endometrial cancer associated with adenomyosis. Int J Gynaecol Obstet 44:76–77 [DOI] [PubMed] [Google Scholar]
- 38.Kuwashima Y, Uehara T, Kishi K et al. (1994) Intramural adenocarcinoma of the uterus, arisen from adenomyosis uteri, showing unique histologic appearances. Report of two cases. Eur J Gynaecol Oncol 15:418–423 [PubMed] [Google Scholar]
- 39.Zhang SQ (1992) Adenoacanthoma developing in adenomyosis uteri. Chin Med J 105:343–346 [PubMed] [Google Scholar]
- 40.Woodruff JD, Erozan YS, Genadry R (1986) Adenocarcinoma arising in adenomyosis detected by atypical cytology. Obstet Gynecol 67:145–148 [PubMed] [Google Scholar]
- 41.Winkelman J, Robinson R (1966) Adenocarcinoma of endometrium involving adenomyosis. Report of an unusual case and review of the literature. Cancer 19:901–908 [DOI] [PubMed] [Google Scholar]
- 42.Olsen K (1955) An unusual case of adenocarcinoma-adenomyosis of the uterus with diffuse embolic lung metastases. Acta Obstet Gynecol Scand 34:269–272 [DOI] [PubMed] [Google Scholar]
- 43.Sotnikova N, Antsiferova I, Malyshkina A (2002) Cytokine network of eutopic and ectopic endometrium in women with adenomyosis. Am J Reprod Immunol 47:251–255 [DOI] [PubMed] [Google Scholar]
- 44.Dunn GP, Bruce AT, Sheehan KC et al. (2005) A critical function for type I interferons in cancer immunoediting. Nat Immunol 6:722–729 [DOI] [PubMed] [Google Scholar]
- 45.Khan KN, Masuzaki H, Fujishita A et al. (2005) Interleukin-6-and tumour necrosis factor alpha-mediated expression of hepatocyte growth factor by stromal cells and its involvement in the growth of endometriosis. Hum Reprod 20:2715–2723 [DOI] [PubMed] [Google Scholar]
- 46.Wang F, Li H, Yang Z et al. (2009) Expression of interleukin-10 in patients with adenomyosis. Fertil Steril 91:1681–1685 [DOI] [PubMed] [Google Scholar]
- 47.van Horssen R, Ten Hagen TL, Eggermont AM (2006) TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist 11:397–408 [DOI] [PubMed] [Google Scholar]
- 48.Mehasseb MK, Bell SC, Brown L et al. (2011) Phenotypic characterisation of the inner and outer myometrium in normal and adenomyotic uteri. Gynecol Obstet Invest 71:217–224 [DOI] [PubMed] [Google Scholar]
- 49.Martini M, Ciccarone M, Garganese G et al. (2002) Possible involvement of hMLH1, p16(INK4a) and PTEN in the malignant transformation of endometriosis. Int J Cancer 102:398–406 [DOI] [PubMed] [Google Scholar]
- 50.Koike N, Tsunemi T, Uekuri C et al. (2013) Pathogenesis and malignant transformation of adenomyosis (review). Oncol Rep 29:861–867 [DOI] [PubMed] [Google Scholar]
- 51.Pearce CL, Templeman C, Rossing MA et al. (2012) Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol 13:385–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kurman RJ, Shih Ie M (2011) Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer-shifting the paradigm. Hum Pathol 42:918–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurman RJ, Shih Ie M (2010) The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 34:433–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiegand KC, Shah SP, Al-Agha OM et al. (2010) ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med 14(363):1532–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lowery WJ, Schiidkraut JM, Akushevich L et al. (2012) Loss of ARID1A-associated protein expression is a frequent event in clear cell and endometrioid ovarian cancers. Int J Gynecol Cancer 22:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ayhan A, Mao TL, Seckin T et al. (2012) Loss of ARID1A expression is an early molecular event in tumor progression from ovarian endometriotic cyst to clear cell and endometrioid carcinoma. Int J Gynecol Cancer 22:1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiegand KC, Lee AF, Al-Agha OM et al. (2011) Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. J Pathol 224:328–333 [DOI] [PubMed] [Google Scholar]
- 58.Kim HS, Kim TH, Chung HH et al. (2014) Risk and prognosis of ovarian cancer in women with endometriosis: a meta-analysis. Br J Cancer 110:1878–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Visser NC, Bulten J, van der Wurff AA et al. (2015) PIpelle Prospective ENDOmetrial carcinoma (PIPENDO) study, preoperative recognition of high risk endometrial carcinoma: a multicentre prospective cohort study. BMC Cancer 15:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.