Abstract
Objective.
Uterine adenosarcoma (UAS) is a rare gynecologic malignancy and the significance of lymph node metastasis on survival has not been well studied.
Methods.
A retrospective study was performed utilizing the Surveillance, Epidemiology, End Results Program to examine UAS (n = 994), endometrial stromal sarcoma (ESS, n = 2910), and uterine leiomyosarcoma (LMS, n = 5506) diagnosed between 1973 and 2013. The impact of lymph node metastasis on cause-specific survival (CSS) was cross-compared by multivariable analysis. Systematic literature review was conducted to examine the impact of nodal metastasis on progression-free survival (PFS) in UAS.
Results.
UAS had the lowest incidence of lymph node metastasis among the sarcoma subtypes examined (UAS 2.9%, LMS 3.4%, and ESS 6.6%, P < 0.001). Lymph node metastasis was independently associated with decreased CSS in all three tumor types (all, P < 0.01); however, magnitudes of statistical significance of lymph node metastasis for CSS were similar across the three tumor types: adjusted-hazard ratio (aHR) for UAS 2.34, ESS 2.43, and LMS 2.10. Systematic literature review identified 230 unique cases of surgically treated UAS. On multivariable analysis, lymph node metastasis (aHR 4.72) had the greatest degree of significance for PFS compared to other tumor factors including sarcomatous overgrowth (aHR 2.88), heterologous elements (aHR 2.08), and deep myometrial invasion (aHR 1.51). Large tumor, deep myometrial invasion, and sarcomatous overgrowth were associated with increased risk of lymph node metastasis (all, P < 0.05).
Conclusion.
While uterine adenosarcoma had a low incidence of lymph node metastasis, the impact of lymph node metastasis on survival was comparable to ESS or LMS.
Keywords: Uterine adenosarcoma, Lymph node metastasis, Endometrial stromal sarcoma, Leiomyosarcoma
1. Introduction
Uterine sarcoma is a rare gynecologic malignancy, comprising approximately 3% of all uterine tumors. In 2016, an estimated 1800 new cases of uterine sarcoma are anticipated in the United States [1,2]. From 1988 to 2001, the rate of death from uterine sarcoma has increased from 7.6 to 9.1% of all uterine malignancies [3]. The most common histologic subtypes in uterine sarcoma are leiomyosarcoma (LMS, 63%) followed by endometrial stromal sarcoma (ESS, 21%) [4]. Uterine adenosarcoma (UAS), first described approximately four decades ago [5], is a rare histology type accounting for 2–5% of all uterine sarcomas [1].
The International Federation of Gynecology and Obstetrics (FIGO) revised a new classification and staging system for uterine sarcomas in 2009 designed to reflect their unique biological characteristics across each sarcoma [6], and specific criteria are currently used to stage UAS. In contrast to LMS and ESS, UAS commonly arises from the endometrium and is histologically characterized by an admixture of benign glandular epithelial and a malignant stromal sarcomatous components [5, 7]. The majority of UAS are low-grade and have a low malignant potential, with 5-year cause-specific survival (CSS) approaching 48–79% [8–11]. Due to the rarity of UAS, epidemiology, clinical manifestations, and the impact of lymph node metastasis in patients with this tumor remained understudied. Available evidence examining lymph node metastasis in UAS has primarily been derived from case reports of which making their findings difficult to adopt in general population [9,10,12]. The aim of this study was to examine the significance of lymph node metastasis on survival outcome of women with UAS.
2. Materials and Thethods
2.1. Study design and eligibility
University of Southern California Institutional Review Board (IRB) exempted the use of publicly available deidentified data, The Surveillance, Epidemiology, and End Results (SEER) Program database for this study. SEER is a population-based database launched in 1973 that is supported and managed by the National Cancer Institute in the United States. The SEER database covers approximately 27.8% of the US population from 11 states and 7 areas. SEER*Stat 8.3.2 was used to sort the dataset (1973–2013), accessed on May 18, 2016.
Cases were examined for eligibility in this study by a diagnosis within the category “Corpus uteri/Uterus NOS,” which was then limited to those subcategorized by malignancy. Within the extracted dataset, uterine sarcoma cases were identified and grouped into UAS, ESS and LMS by histology codes. Patients with endometrial cancer, carcinosarcoma, tumors metastatic to the uterus, and other rare sarcoma subtypes (liposarcoma, rhabdomyosarcoma, or other histologic types of sarcoma) were excluded. Variables obtained from the database included patient demographics, tumor characteristics, treatment patterns, and survival outcome. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were consulted for this observational study [13].
2.2. Clinical information
Patient demographics abstracted included age and year at diagnosis, ethnicity, marital status and registration area. Tumor characteristics included histologic subtype, stage, grade, tumor size, depth of myometrial tumor invasion, and lymph node status. In this study, the ICD-O-3 SEER Site/Histology Validation List and the World Health Organization histological classification were used for grouping histologic subtypes as shown in Table S1 [14]. Stage was reclassified according to the American Joint Committee on Cancer 7th Edition staging criteria [15].
Treatment patterns included type of hysterectomy-based surgery and postoperative radiotherapy. Lymph node metastasis was evaluated by the results from Regional Lymph Node section. If not specified in this section, lymph node metastasis was considered no lymph node assessment. For survival outcome, both cause-specific survival (CSS) and all-cause mortality (overall survival [OS]) were collected. CSS were defined as the time interval between the initial tumor diagnosis and the date of death from uterine sarcoma. OS were defined as the time interval between the initial tumor diagnosis and the date of death from any causes. Patients were censored if alive at the last follow-up.
2.3. Systematic review
To evaluate progression-free survival (PFS) in women with UAS, we conducted a comprehensive systematic literature search per the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines for systematic review [16]. A public search engine PubMed/MEDLINE was utilized with the entry keyword “uterine adenosarcoma” limited to English literature (searched on September 20, 2016). Eligible publications included case reports, case series, and cohort study, of UAS received hysterectomy. The references listed in each identified article were also reviewed and eligible studies were enrolled in the analysis. Among these, publications lacking sufficient patient demographics, hysterectomy status, histology results, treatment details, survival outcome and follow-up were excluded from the analysis. Age, area and year of publication, surgical pathology results (stage, depth of myometrial tumor invasion, sarcomatous overgrowth, sarcoma element [homologous versus heterologous], and lymph node status), treatment pattern (hysterectomy type, adjuvant radiotherapy, and adjuvant chemotherapy), and survival outcome for PFS were abstracted from the publication. Sarcomatous overgrowth was defined by >25% of the tumor consisting of a sarcomatous component based on prior study [10,17,18].
2.4. Statistical analysis
The primary interest of analysis was to examine characteristics and CSS of women with node-positive UAS. The secondary interest of analysis was to compare the impact of nodal metastasis on PFS to other tumor factors in women with surgically-treated UAS.
Continuous variables were assessed by one-way ANOVA or Kruskal-Wallis H test, and expressed as mean (± standard deviation) or median (range) as appropriate. Ordinal and categorical variables were analyzed using the chi-square test, and magnitude of statistical significance was expressed with odds ratio (OR) and 95% confidence interval (CI). Univariable and multivariable analyses for survival outcome were performed by log-rank test and a Cox proportional hazard regression test, respectively. Covariates included in the final multivariable model consisted of patient demographics, tumor factors and treatment patterns. Endpoint probability for survival was expressed as a hazard ratio (HR) with 95%CI.
The Joinpoint Regression Program (version 4.3.1.0) provided by the National Cancer Institute was utilized for evaluation of temporal (calendar year) trends in UAS [19]. Time point data were examined every calendar year to identify temporal change. The presence of annual trend was examined with a linear segmented regression test, and log-transformation was performed to determine annual percent change of the slope.
The variance inflation factor (VIF) was determined among the covarates in the multivariable analysis, and VIF ≥2.0 was interpreted as multicollinearity (myometrial invasion, tumor size and nodal metastasis for cancer stage). Survival curves were constructed with Kaplan-Meier method. All statistical tests were two-tailed, and P < 0.05 was considered to be statistically significant. Statistical Package for the Social Sciences (SPSS, version 24.0, Chicago, IL) was used for the analysis.
3. Results
Selection criteria are shown in Fig. 1. There were 10,577 cases of uterine sarcoma identified during the study period and there were 1167 cases excluded due to a diagnosis of rhabdomyosarcoma, liposarcoma, and other sarcoma histologic types. The remaining 9410 cases were eligible for the analysis, divided into three subgroups: UAS (n = 994), ESS (n = 2910), and LMS (n = 5506).
Fig. 1.
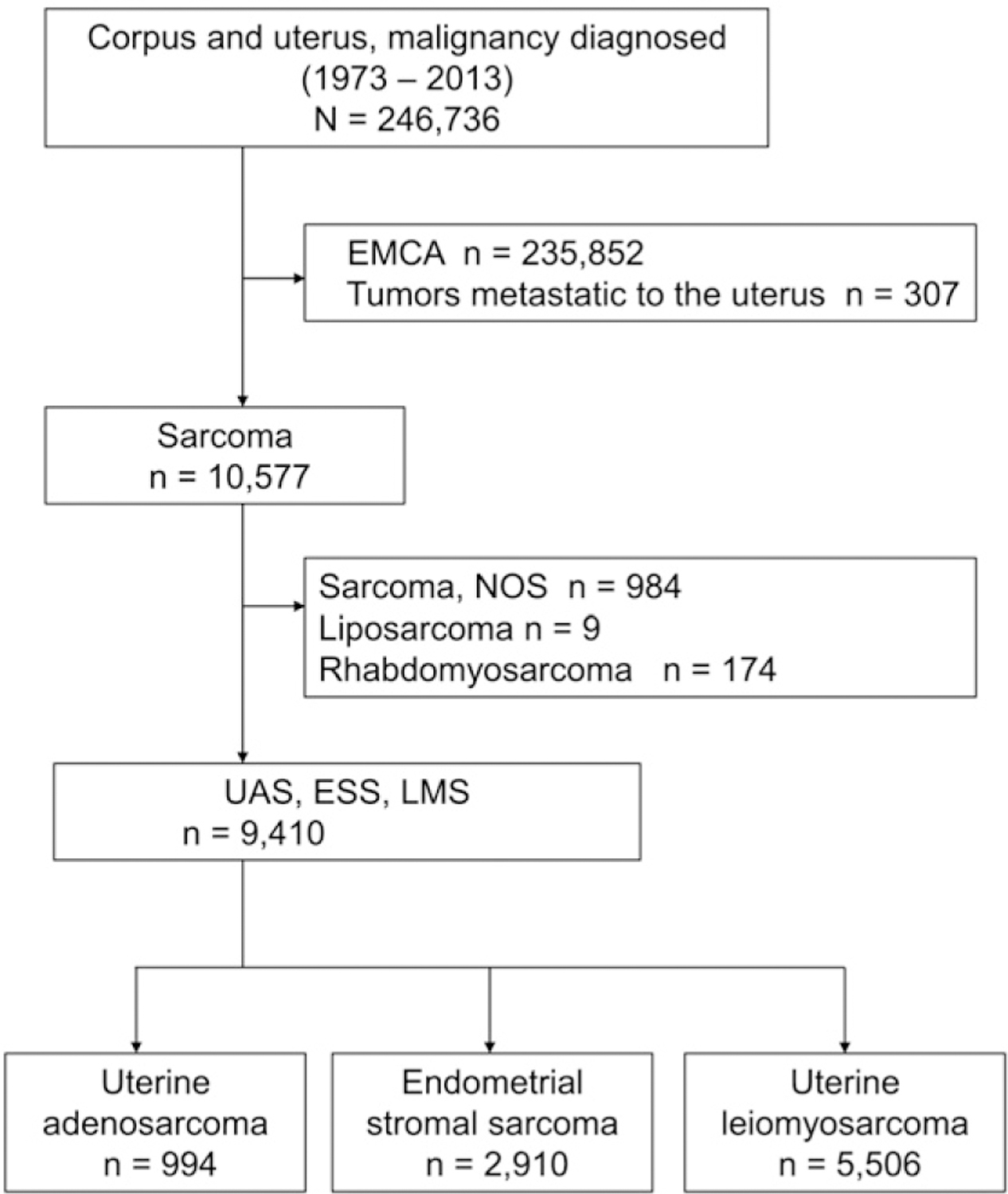
Selection criteria for SEER cohort. Abbreviations; EMCA, endometrial cancer; NOS, not otherwise specified; UAS, uterine adenosarcoma; ESS, endometrial stromal sarcoma; and LMS, uterine leiomyosarcoma.
Demographics and clinical characteristics of patients with UAS, ESS, and LMS are listed in Table 1. Compared to ESS and LMS, patients with UAS were more likely to be over age 60 years, Caucasian, single, and to have undergone simple hysterectomy (all, P < 0.001). Tumor characteristics associated with UAS included early-stage disease (56.3%), <50% myometrial invasion (39.5%), and small tumor size (all, P < 0.001). UAS had the lowest incidence of lymph node metastasis among the three tumor types (UAS 2.9%, LMS 3.4%, and ESS 6.6%, P < 0.001).
Table 1.
Patient demographics and clinical characteristics of uterine adenosarcoma, endometrial stromal sarcoma, and leiomyosarcoma (N = 9410).
| Characteristic | UAS |
ESS |
LMS |
P-value |
|---|---|---|---|---|
| n = 994 | n = 2910 | n = 5506 | ||
| Age (y) | 58.4 (±14.9) | 54.2 (±14.0) | 54.6 (±12.4) | <0.001 |
| ≥60 | 480 (48.3%) | 923 (31.7%) | 167 (30.3%) | |
| <60 | 514 (51.7%) | 1987 (68.3%) | 3835 (69.7%) | |
| Ethnicity | <0.001 | |||
| Caucasian | 652 (65.6%) | 1891 (65.0%) | 3410 (62.1%) | |
| African American | 131 (13.2%) | 394 (13.5%) | 950 (17.3%) | |
| Hispanic | 113 (11.4%) | 357 (12.3%) | 674 (12.2%) | |
| Asian | 70 (7.0%) | 214 (7.4%) | 362 (6.6%) | |
| Others | 28 (2.8%) | 54 (1.9%) | 100 (1.8%) | |
| Marital status | <0.001 | |||
| Single | 194 (19.5%) | 489 (16.8%) | 1020 (18.5%) | |
| Married | 488 (49.1%) | 1647 (56.6%) | 3080 (55.9%) | |
| Others | 312 (31.4%) | 774 (26.6%) | 1406 (25.5%) | |
| Registry Area | 0.014 | |||
| West | 525 (52.8%) | 1498 (51.5%) | 2849 (51.7%) | |
| Central | 193 (19.4%) | 708 (24.3%) | 1239 (22.5%) | |
| East | 276 (27.8%) | 704 (24.2%) | 1418 (25.8%) | |
| Year at diagnosis | <0.001 | |||
| 1973–1999 | 236 (23.7%) | 946 (32.5%) | 1979 (35.9%) | |
| 2000–2009 | 523 (52.6%) | 1383 (47.5%) | 2406 (43.7%) | |
| 2010–2013 | 235 (23.6%) | 581 (20.0%) | 1121 (20.4%) | |
| Stage | <0.001 | |||
| I | 560 (56.3%) | 1138 (39.1%) | 2024 (36.8%) | |
| II | 13 (1.3%) | 79 (2.7%) | 140 (2.5%) | |
| III | 33 (3.3%) | 226 (7.8%) | 241 (4.4%) | |
| IV | 51 (5.1%) | 620 (21.3%) | 1480 (26.9%) | |
| Unknown | 337 (33.9%) | 847 (29.1%) | 1621 (29.4%) | |
| Myometrial invasion | <0.001 | |||
| ≤50% | 393 (39.5%) | 663 (22.8%) | 883 (16.0%) | |
| >50% | 43 (4.3%) | 87 (3.0%) | 64 (1.2%) | |
| Unknown | 558 (56.1%) | 2160 (74.2%) | 4559 (82.8%) | |
| Lymph nodes metastasis | <0.001 | |||
| Negative | 848 (85.3%) | 2006 (68.9%) | 3561 (64.7%) | |
| Positive | 29 (2.9%) | 192 (6.6%) | 188 (3.4%) | |
| Unknown | 117 (11.8%) | 712 (24.5%) | 1757 (31.9%) | |
| Grade | <0.001 | |||
| 1 | 109 (11.0%) | 465 (16.0%) | 314 (5.7%) | |
| 2 | 158 (15.9%) | 926 (31.8%) | 573 (10.4%) | |
| 3a | 173 (17.4%) | 914 (31.4%) | 2003 (36.4%) | |
| Unknown | 554 (55.7%) | 605 (20.8%) | 2616 (47.5%) | |
| Tumor size | <0.001 | |||
| ≤2.0 cm | 82 (8.2%) | 183 (6.3%) | 145 (2.6%) | |
| 2.1–5.0 cm | 186 (19.7%) | 453 (15.6%) | 463 (8.4%) | |
| >5.0 cm | 318 (33.6%) | 1084 (37.2%) | 3096 (56.3%) | |
| Unknown | 408 (41.0%) | 1190 (40.9%) | 1802 (32.7%) | |
| Surgery type | <0.001 | |||
| No surgery | 46 (4.6%) | 293 (10.1%) | 466 (8.5%) | |
| Total/pan/simple hyst | 720 (72.4%) | 1848 (63.5%) | 3383 (61.4%) | |
| Others | 228 (22.9%) | 769 (26.4%) | 1657 (30.1%) | |
| Radiotherapy | <0.001 | |||
| No adjuvant radiation | 746 (75.1%) | 1850 (63.6%) | 3335 (60.6%) | |
| Adjuvant radiation | 171 (17.2%) | 493 (16.9%) | 871 (15.8%) | |
| Others | 77 (7.7%) | 567 (19.5%) | 1300 (23.6%) |
Number (%) or mean (±SD) is shown. One-way ANOVA or chi-square test for P-values. Significant P-values are emboldened.
Abbreviations: UAS, uterine adenosarcoma; ESS, endometrial stromal sarcoma; LMS, leiomyosarcoma; and hyst, hysterectomy.
Included undifferentiated type.
Temporal trend analysis (Fig. S1) revealed an increase in number of uterine sarcomas reported in the database from 1973 to 2013. The proportion of UAS among three sarcoma types combined also significantly increased from 1978 to 1997 (annual percent change 10.0, 95%CI 7.0–13.2, P < 0.001) and reached a plateau after 1997. Over a 35-year time interval, the proportion of UAS increased from 1.0% (95%CI 0.4–1.6) in 1978 to 12.7% (95%CI 10.5–14.9) in 2013 among the three subtypes of sarcoma evaluated (risk difference 11.7%, 95%CI 9.7–13.7).
Survival analysis was performed among the patients with UAS, ESS, and LMS. Among 9410 patients with these three uterine sarcoma subtypes, the median follow-up time was 6.5 years for all cases and 5.1 years for UAS. In total, there were 3899 (41.4%) deaths due to uterine sarcoma in this cohort; 200 (20.1%) from UAS, 866 (29.8%) from ESS, and 2833 (51.5%) from LMS, respectively.
CSS was examined in each sarcoma type (Table 2). On multivariable analysis, lymph node metastasis remained an independent prognostic factor for decreased CSS in all three sarcoma types: 5-year rates for node-positive versus node-negative cases, 43.0% versus 81.8% for UAS (adjusted-P = 0.01), 30.4% versus 76.8% for ESS (adjusted-P < 0.001), and 20.9% versus 49.0% for LMS (adjusted-P < 0.001), respectively. Notably, magnitudes of statistically significant of lymph node metastasis on CSS were similar across the three sarcoma types: adjusted-HR for UAS 2.34 (95%CI 1.29–4.25); adjusted-HR for ESS 2.43 (95%CI 1.99–2.98); and adjusted-HR for LMS 2.10 (95%CI 1.75–2.52). Lymph node metastasis remained an independent prognostic factor for decreased OS in the three sarcoma types (Table S2). Similar to CSS, magnitude of statistical significance of lymph node metastasis on OS were similar across the three tumor subtypes: adjusted-HR for UAS 2.04 (95%CI 1.20–3.46); adjusted-HR for ESS 2.15 (95%CI 1.77–2.61); and adjusted-HR for LMS 2.12 (95%CI 1.79–2.51).
Table 2.
Multivariable analysis of cause-specific survival for women with uterine adenosarcoma, endometrial stromal sarcoma, and leiomyosarcoma (n = 9410).
| Type of sarcoma | Uterine adenosarcoma (n = 994) |
Endometrial stromal sarcoma (n = 2910) |
Uterine leiomyosarcoma (n = 5506) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Survival rate |
Multivariable |
Survival rate |
Multivariable |
Survival rate |
Multivariable |
||||||
| Characteristic | No. | 5-yr (%) | HR (95%CI) | P-value | No. | 5-yr (%) | HR (95%CI) | P-value | No. | 5-yr (%) | HR (95%CI) | P-value |
| Age (y) | ||||||||||||
| ≥60 | 480 | 76.0 | 1.29 (0.96–1.74) | 0.09 | 923 | 51.3 | 1.38 (1.19–1.61) | <0.001 | 1671 | 35.1 | 1.53 (1.41–1.66) | <0.001 |
| <60 | 514 | 83.8 | 1 | 1987 | 79.6 | 1 | 3835 | 54.3 | 1 | |||
| Ethnicity | ||||||||||||
| Caucasian | 652 | 81.2 | 1 | 1891 | 71.0 | 1 | 3420 | 51.1 | 1 | |||
| African American | 131 | 73.1 | 1.18 (0.79–1.77) | 0.42 | 394 | 59.2 | 1.26 (1.05–1.52) | 0.01 | 950 | 41.7 | 1.37 (1.24–1.51) | <0.001 |
| Hispanic | 113 | 80.8 | 1.32 (0.82–2.14) | 0.26 | 357 | 77.8 | 1.05 (0.82–1.35) | 0.68 | 674 | 47.5 | 1.05 (0.93–1.19) | 0.47 |
| Others | 98 | 81.9 | 0.91 (0.54–1.52) | 0.71 | 268 | 78.4 | 0.99 (0.75–1.31) | 0.97 | 462 | 46.6 | 1.12 (0.98–1.30) | 0.11 |
| Marital status | ||||||||||||
| Single | 194 | 80.9 | 1 | 489 | 66.6 | 1 | 1020 | 45.6 | 1 | |||
| Married | 488 | 85.2 | 0.98 (0.64–1.49) | 0.91 | 1647 | 75.9 | 0.75 (0.62–0.90) | 0.003 | 3080 | 52.7 | 0.83 (0.75–0.92) | <0.001 |
| Others | 312 | 71.4 | 1.43 (0.93–2.19) | 0.11 | 774 | 62.4 | 0.92 (0.74–1.14) | 0.43 | 1406 | 42.2 | 0.99 (0.89–1.12) | 0.97 |
| Registry Area | ||||||||||||
| West | 525 | 81.3 | 1 | 1498 | 73.5 | 1 | 2849 | 46.2 | 1 | |||
| Central | 193 | 75.7 | 1.24 (0.85–1.81) | 0.27 | 708 | 67.6 | 1.09 (0.92–1.29) | 0.32 | 1239 | 51.5 | 0.93 (0.84–1.03) | 0.14 |
| East | 276 | 80.9 | 1.17 (0.81–1.67) | 0.40 | 704 | 68.6 | 0.98 (0.82–1.17) | 0.81 | 1418 | 51.4 | 0.82 (0.75–0.90) | <0.001 |
| Year at diagnosis | ||||||||||||
| 1973–1999 | 236 | 83.3 | 1 | 946 | 73.9 | 1 | 1979 | 54.7 | 1 | |||
| 2000–2009 | 523 | 79.2 | 1.30 (0.91–1.88) | 0.16 | 1383 | 71.4 | 1.05 (0.87–1.26) | 0.68 | 2406 | 44.7 | 1.07 (0.97–1.18) | 0.17 |
| 2010–2012 | 235 | n.r | 1.07 (0.64–1.80) | 0.78 | 581 | n.r. | 1.23 (0.97–1.54) | 0.08 | 1121 | n.r. | 0.98 (0.86–1.13) | 0.82 |
| Grade | ||||||||||||
| 1 | 109 | 93.4 | 1 | 465 | 96.1 | 1 | 314 | 81.8 | 1 | |||
| 2 | 158 | 84.2 | 2.59 (1.14–5.87) | 0.02 | 926 | 94.3 | 1.34 (0.87–2.07) | 0.18 | 573 | 66.7 | 1.82 (1.40–2.36) | <0.001 |
| 3a | 173 | 54.8 | 7.37 (3.41–15.9) | <0.001 | 914 | 32.6 | 14.0 (9.49–20.7) | <0.001 | 2003 | 32.7 | 4.64 (3.68–5.86) | <0.001 |
| Unknown | 554 | 83.9 | 2.43 (1.17–5.05) | 0.02 | 605 | 69.1 | 4.52 (3.02–6.75) | <0.001 | 2616 | 52.3 | 2.72 (2.17–3.42) | <0.001 |
| Tumor size | ||||||||||||
| ≤5.0 cm | 268 | 87.7 | 1 | 636 | 87.3 | 1 | 608 | 69.8 | 1 | |||
| >5.0 cm | 318 | 64.7 | 1.89 (1.28–2.80) | 0.001 | 1084 | 61.2 | 1.91 (1.50–2.43) | <0.001 | 3096 | 42.2 | 2.03 (1.74–2.35) | <0.001 |
| Unknown | 408 | 86.2 | 0.79 (0.51–1.24) | 0.31 | 1190 | 70.5 | 1.36 (1.06–1.76) | 0.016 | 1802 | 52.0 | 1.62 (1.37–1.91) | <0.001 |
| Lymph node status | ||||||||||||
| Negative | 848 | 81.8 | 1 | 2006 | 76.8 | 1 | 3561 | 49.0 | 1 | |||
| Positive | 29 | 43.0 | 2.34 (1.29–4.25) | 0.01 | 192 | 30.4 | 2.43 (1.99–2.98) | <0.001 | 188 | 20.9 | 2.10 (1.75–2.52) | <0.001 |
| Unknown | 117 | 75.8 | 1.71 (1.06–2.74) | 0.03 | 712 | 65.4 | 1.67 (1.37–2.03) | <0.001 | 1757 | 50.4 | 1.21 (1.09–1.35) | <0.001 |
| Surgery type | ||||||||||||
| No surgery | 46 | 59.1 | 4.70 (1.85–11.9) | 0.001 | 293 | 34.5 | 2.82 (2.08–3.82) | <0.001 | 466 | 18.9 | 4.09 (3.42–4.88) | <0.001 |
| Total/pan/simple hyst | 720 | 82.2 | 1 | 1848 | 76.8 | 1 | 3383 | 50.6 | 1 | |||
| Others | 228 | 78.0 | 1.28 (0.90–1.80) | 0.17 | 769 | 69.6 | 1.17 (0.98–1.40) | 0.08 | 1657 | 52.1 | 1.20 (1.09–1.33) | <0.001 |
| Radiation | ||||||||||||
| No adjuvant radiation | 746 | 76.7 | 1 | 1850 | 77.9 | 1 | 3335 | 49.9 | 1 | |||
| Adjuvant radiation | 117 | 74.6 | 1.06 (0.74–1.52) | 0.75 | 493 | 65.8 | 0.91 (0.76–1.09) | 0.30 | 871 | 48.4 | 0.97 (0.88–1.08) | 0.58 |
| Others | 77 | 65.7 | 0.82 (0.37–1.83) | 0.64 | 567 | 52.3 | 0.99 (0.76–1.29) | 0.94 | 1300 | 45.9 | 0.86 (0.74–0.99) | 0.04 |
A Cox proportional hazard regression model for multivariable analysis adjusted for collected covariates (myometrial invasion was not included due to over 50% of lack of data and stage was excluded for the multicollinearity of other factors). Significant P-values are emboldened.
Abbreviations: n.r., cases did not reach 5-year follow-up; 5-yr, 5-year proportion; and hyst, hysterectomy.
Included undifferentiated type.
Use of hysterectomy was associated with improved CSS for UAS: 5-year rates for non-surgical management versus hysterectomy, 59.1% versus 82.2% on multivariable analysis (P = 0.001). There were 276 (27.8%) patients aged <50 years with UAS among the 977 cases, and ovarian conservation was performed in 38 (13.8%) cases in this population. Ovarian conservation was not associated with CSS in women <50 years of age with UAS (5-year rates; ovarian conservation 91.4% versus oophorectomy 85.0%, P = 0.33) on univariable analysis.
Survival analysis was performed across the three sarcoma types (n = 9410). On univariable analysis, UAS was significantly associated higher CSS rate compared to ESS and LMS (5-year rates; 80.1% for UAS, 70.9% for ESS, and 48.8% for LMS, P < 0.001; Fig. S2). We then examined CSS stratified by nodal status (Table S3). Among the 6415 node-negative cases, UAS was significantly associated with higher CSS rate compared to ESS and LMS (5-year rates; 81.8% for UAS, 76.8% for ESS, and 49.0% for LMS, P < 0.001). On multivariable analysis, UAS remained the most favorable tumor type for both CSS and OS among the three sarcoma subtypes evaluated in node-negative cases (P < 0.001). In contrast, among the 409 node-positive cases, although UAS had the highest CSS rate, tumor histology did not reach statistical significance for CSS (2-year survival rates; 49.2% for UAS, 37.6% for ESS, and 33.6% for LMS, P = 0.15) for both univariable and multivariable analysis.
3.1. Systematic review
Selection schema is shown in Fig. S3. Our search identified 125 articles, of which titles and abstracts were screened. Of these, 50 articles meeting our criteria were reviewed and 230 cases were examined. There was no cohort study examining the impact of lymph node metastasis on survival of women with UAS identified in this search.
Demographic results of a systematic review for lymph node status in UAS are summarized in Table 3. The rate of lymph node metastasis for UAS was 4.0% among 230 cases. Sarcomatous overgrowth, heterologous element, deep myometrial tumor invasion were reported in 39.6%, 15.2%, and 9.2% of the cases, respectively. Median follow-up time was 33.0 (range, 1.0–238) months. There were 115 (50.0%) cases of disease recurrence and 84 (36.5%) cases of death due to UAS. On univariable analysis, lymph node metastasis was significantly associated with decreased PFS (2-year rates for node-positive versus node-negative cases, 0% versus 79.6%, P < 0.001; Fig. 2A). Multivariable analysis revealed that lymph node metastasis (aHR 4.72) had the largest magnitude of significance for PFS followed by sarcomatous overgrowth (aHR 2.88; Fig. 2B), heterologous components (aHR 2.08; Fig. 2C), and deep myometrial invasion (aHR 1.51; Fig. 2D) (Table 4). Similar findings were seen for CSS: lymph node metastasis (aHR 6.24), heterologous components (aHR 3.63) sarcomatous overgrowth (aHR 3.02), and deep myometrial invasion (aHR 1.62).
Table 3.
Uterine adenosarcoma systematic review patient demographics (n = 230).
| Characteristic | No. (%) |
|---|---|
| Age (y) | 57.3 (±16.1) |
| ≥60 | 109 (47.4%) |
| <60 | 108 (47.0%) |
| Unknown | 13 (5.6%) |
| Area of publication | |
| North America | 188 (81.8%) |
| Asia | 28 (12.2%) |
| Europe | 10 (4.3%) |
| Others | 4 (1.7%) |
| Year of publication | |
| 1974–1999 | 106 (46.1%) |
| 2000–2009 | 20 (8.7%) |
| 2010–2015 | 104 (45.2%) |
| Stage | |
| I | 179 (77.8%) |
| II | 21 (9.1%) |
| III | 15 (6.5%) |
| IV | 8 (3.5%) |
| Unknown | 7 (3.1%) |
| Myometrial invasion | |
| ≤50% | 139 (60.4%) |
| >50% | 21 (9.2%) |
| Unknown | 70 (30.4%) |
| Sarcomatous overgrowth | |
| No | 68 (29.6%) |
| Yes | 91 (39.6%) |
| Unknown | 71 (30.9%) |
| Heterologous components | |
| No | 119 (51.7%) |
| Yes | 35 (15.2%) |
| Unknown | 76 (33.3%) |
| Lymph node metastasis | |
| No | 122 (53.0%) |
| Yes | 9 (4.0%) |
| Unknown | 99 (43.0%) |
| Surgery type | |
| Total/pan/simple hyst | 217 (94.3%) |
| Other hysterectomy | 13 (5.7%) |
| Adjuvant radiation | |
| No | 188 (81.7%) |
| Yes | 35 (15.3%) |
| Unknown | 7 (3.0%) |
| Adjuvant chemotherapy | |
| No | 197 (85.7%) |
| Yes | 26 (11.3%) |
| Unknown | 7 (3.0%) |
Number (%) or mean (±SD) is shown. Abbreviations: hyst, hysterectomy.
Fig. 2.
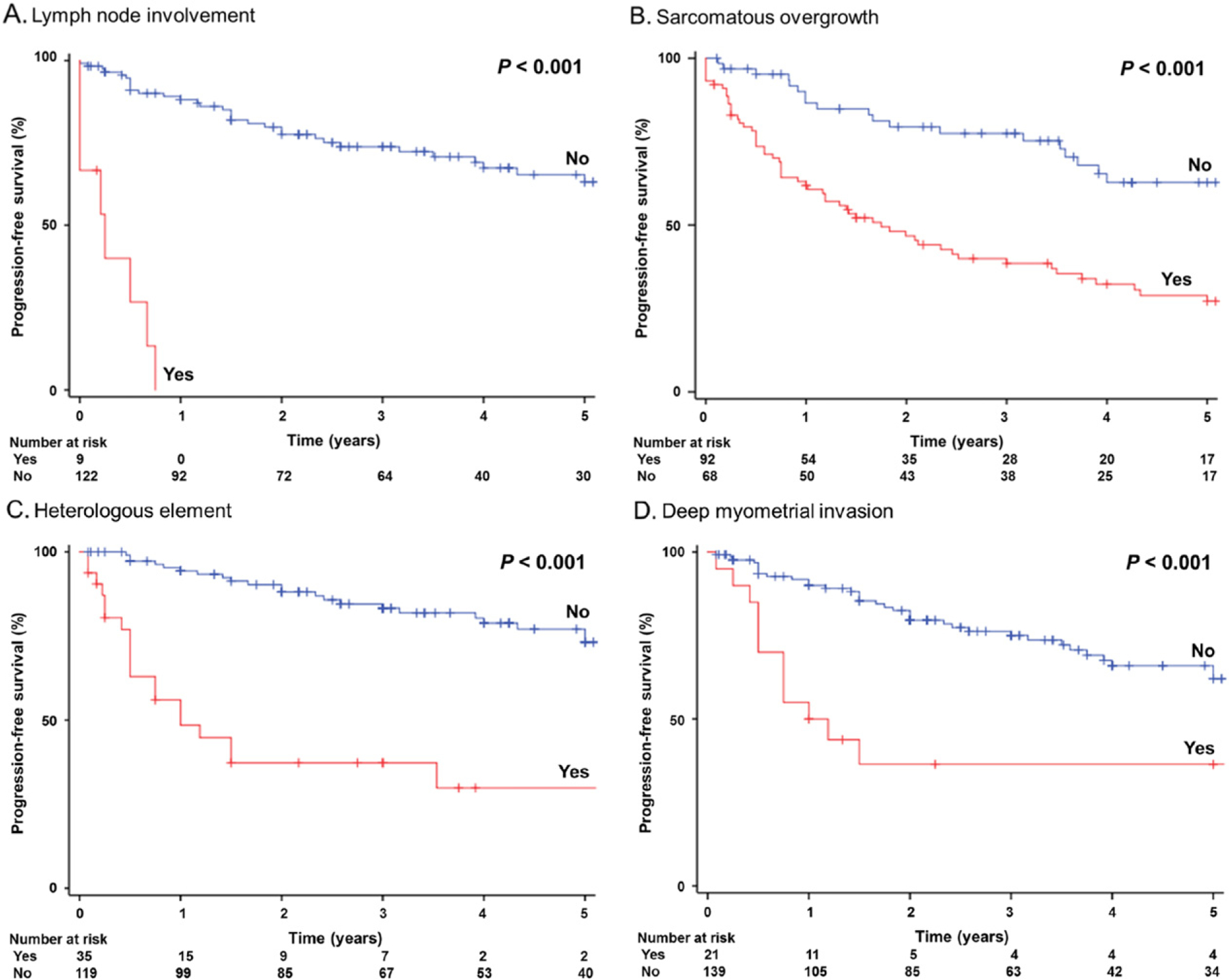
Survival curves for women with uterine adenosarcoma by systematic review. Log-rank test for P-values. Progression-free survival curves were constructed for: lymph node involvement (panel A), sarcomatous overgrowth (panel B), heterologous element (panel C) and deep myometrial invasion (panel D).
Table 4.
Multivariable analysis of survival in women with uterine adenosarcoma by systematic review (n = 230).
| Progression-free survival |
Cause-specific survival |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Survival |
Univariable |
Multivariable |
Survival |
Univariable |
Multivariable |
||||||
| Characteristic | No | 2-yr (%) | HR (95%CI) | P-value | HR (95%CI) | P-value | 2-yr (%) | HR (95%CI) | P-value | HR (95%CI) | P-value |
| Lymph node metastasis | <0.001 | <0.001 | |||||||||
| No | 122 | 79.6 | 1 | 1 | 86.2 | 1 | 1 | ||||
| Yes | 9 | 0 | 20.0 (7.83–51.1) | 4.72 (0.92–24.2) | 0.06 | 0 | 27.0 (10.0–72.7) | 6.24 (1.17–33.4) | 0.03 | ||
| Sarcomatous overgrowth | <0.001 | <0.001 | |||||||||
| No | 68 | 79.4 | 1 | 1 | 92.9 | 1 | 1 | ||||
| Yes | 92 | 46.8 | 3.39 (2.07–5.55) | 2.88 (0.99–8.40) | 0.053 | 63.2 | 5.95 (2.93–12.1) | 3.02 (0.70–13.1) | 0.14 | ||
| Heterologous elements | <0.001 | <0.001 | |||||||||
| No | 119 | 90.2 | 1 | 1 | 96.1 | 1 | 1 | ||||
| Yes | 35 | 36.8 | 4.95 (2.74–8.92) | 2.08 (0.70–6.12) | 0.19 | 42.0 | 7.88 (3.97–15.6) | 3.63 (0.90–14.6) | 0.07 | ||
| Myometrial invasion | <0.001 | <0.001 | |||||||||
| ≤50% | 139 | 82.4 | 1 | 1 | 87.2 | 1 | 1 | ||||
| >50% | 21 | 36.1 | 3.07 (1.60–5.88) | 1.51 (0.48–4.72) | 0.48 | 50.5 | 4.27 (2.08–8.76) | 1.62 (0.47–5.58) | 0.44 | ||
A Cox proportional hazard regression model for multivariable analysis adjusted for collected covariates. Significant P-values are emboldened. Abbreviations: 2-yr, 2-year proportion.
Risk factor for nodal metastasis was examined in UAS (Table S4). Both SEER and systematic literature review cohorts showed that >50% myometrial tumor invasion was significantly associated with increased the risk of nodal metastasis (ORs 7.74 and 8.73; both cohorts P < 0.05) on univariable analysis. Large tumor size (OR 3.55 in the SEER cohort) and sarcomatous overgrowth (OR 8.75 in the systematic review) were also associated with increased the risk of nodal metastasis (both, P < 0.05). Extents of risk factors were correlated to lymph node metastasis risk (Fig. S4A–B), and presence of multiple risk factors was significantly associated with increased risk of nodal metastasis (range, 12.5–18.2%). Contrary, absence of these risk factors was associated with low risk of lymph node metastasis (1.3–1.6%).
Stage-specific survival analysis for PFS was performed based on adjuvant treatment pattern (Fig. S5A–B). Chemotherapy had a 5-year higher PFS rate compared to radiotherapy in stage I disease (72.0% versus 31.8%, P = 0.063) whereas radiotherapy had a higher 5-year PFS rate compared to chemotherapy in stage II–IV disease (8.6% versus 31.7%, P = 0.28) but it did not reach statistical difference. The commonly used chemotherapy agents were ifosfamide (39.4%), cisplatin (36.4%) and doxorubicin (24.2%). Whole pelvic radiotherapy (48.7%) was the most common radiotherapy choice followed by vaginal brachytherapy alone (23.1%).
4. Discussion
Past decades have witnessed UAS being understudied due to its rarity. Therefore, we aimed to determine clinical risk factors associated with decreased survival, which may guide optimal management of this rare tumor. In this population-based study, the proportion of lymph node metastasis in UAS was lower compared to other uterine sarcoma subtypes. However, lymph node metastasis in UAS still portended a worse prognosis. Our study showed that UAS with lymph node metastasis is associated with both decreased CSS and OS. Moreover, the impact of lymph node metastasis on CSS in UAS was similar to that of LMS, a tumor typically associated with a high risk of recurrence and death [20].
Our study also shows that hysterectomy improves CSS compared to non-surgical management. Therefore, initial treatment via hysterectomy is recommended but the benefit of routine lymphadenectomy in patients with UAS remains uncertain. Lymph node evaluation at the initial surgery may help to determine prognosis and the potential need for adjuvant therapy. However, no optimal adjuvant or systemic treatment strategy has been identified for patients with UAS in the past. In our systematic literature review, although statistical significance was not met, a trend toward improved PFS is suggested for patients with early-stage disease who receive adjuvant chemotherapy (Fig. S5A). Prior case series have reported that adjuvant chemotherapy with sarcoma-based regimens, such as anthracyclines doxorubicin, cisplatin, ifosfamide, and paclitaxel may benefit patients with UAS at high-risk of recurrence or death [21,22]. Because the limited sample size makes interpretation of the result difficult to adopt, additional data are needed to determine the best adjuvant therapy regimen for patients with high-risk UAS.
Previously, sarcomatous overgrowth [10,17,18], myometrial tumor invasion [12,17,23], heterologous components and lymphovascular space invasion (LVSI) [9,10,24,25] have been described as prognostic histologic features for UAS. Sarcomatous overgrowth has been demonstrated in 8–65% of UAS, carrying a higher risk of recurrence (2-year recurrence rate, 70–82%) and a poor prognosis (5-year OS rate, 22–31%) [7,9,10,18]. Additionally, the prevalence of deep myometrial tumor invasion is estimated in 3–11% of UAS. While some studies report a worsened prognosis in this setting of deep myometrial tumor invasion (5-year OS rate, 0–50%), others do not [10,12,23]. Similarly, heterologous components are seen in 10–15% of UAS and are associated with loco-regional recurrence and poor prognosis (5-year OS rate, 25–30%) [8]. Some studies have previously reported LVSI, a surrogate marker for lymph node metastasis, in 9–16% of UAS specimens, and an association with decreased survival (5-year OS rate, 4–40%) [8–10]. However, these studies were limited by small patient sample size.
Due to the low incidence of lymph node metastasis in UAS, data have been missing for survival impact of lymph node metastasis in UAS in the past. Generally, the rarity of UAS has made it difficult to perform large-scale studies to identify risk factors for survival. For this reason, we conducted a systematic literature review and evaluated demographics associated with recurrence in UAS. Notably, our analysis demonstrated that lymph node metastasis had the greatest impact on PFS among other tumor factors including sarcomatous overgrowth, the presence of heterologous elements, and deep myometrial tumor invasion. Thus, our results will be useful to identify women at high risk of recurrence.
The strengths of our study are its population-based design with accommodation for large-scale sample size and long-term follow-up. The SEER database was particularly useful for analyzing cases of this rare malignancy which require a large sample size for adequate evaluation. Additionally, systematic literature review empowered and validated the quality of the study. For instance, lymph node metastasis for UAS rate was similar between the SEER data and systematic literature review (2.3% versus 4.0%) with the consistent findings of decreased survival outcome with lymph node metastasis. In addition, recurrence information is not captioned in the SEER database and therefore the systematic literature review is filling this missing gap.
Limitations of our study include its retrospective nature, and possible confounding variables such as a history of tamoxifen use, prior pelvic radiation, and hereditary conditions are missing for the analysis [8–10]. Additionally, this database is unable to generate solid information about the initial chemotherapy. Selection bias may exist because only published cases were used for the systematic literature review. In addition, lack of central pathology review is a weakness of the study as the diagnosis of such rare disease may differ across pathologists. Lastly, it was unknown if lymphadenectomy was performed for grossly abnormal appearing nodes in the pre-/intra-operative assessment, or if microscopic metastasis to otherwise normal appearing lymph nodes were identified in routine lymphadenectomy in the SEER database. Without this information, solid recommendation for routine lymphadenectomy is difficult to draw.
Clinical implications of our study may be in the area of selective lymphadenectomy for UAS. That is, our study found a low incidence of nodal metastasis rate in UAS that may not be feasible to do apply the concept for universal lymphadenectomy as suggested previously [8]. In this setting, our results of risk factors for nodal metastasis by utilizing uterine factors will be useful to identify a subgroup of women at high-risk of lymph node metastasis in whom benefits from additional lymphadenectomy. That is, presence of any risk factors among sarcomatous overgrowth, large tumor size >5 cm, and >50% myometrial tumor invasion were associated with significantly increased risk of lymph node metastasis (5.3–15.4%) with possible additive effects in multiple risk factors (12.5–18.2%).
Because diagnostic accuracy for these risk factors during hysterectomy via frozen section is likely suboptimal and so is for diagnosing UAS, it would be important for surgeons to counsel the patient that the secondary surgery for lymphadenectomy may be needed if the tumor expresses the risk factors for nodal metastasis. Given the era of minimally-invasive surgery, laparoscopic approach will be reasonable for lymphadenectomy. If UAS is preoperative diagnosed via endometrial sampling, evaluating a presence of sarcomatous overgrowth and obtaining a systematic imaging to assess suspicious nodes and large tumor size in the uterus prior to hysterectomy-based surgical treatment will guide surgeons to assess the necessity of lymphadenectomy.
Supplementary Material
HIGHLIGHTS.
Uterine adenosarcoma (UAS) has a low incidence rate (<3%) of lymph node metastasis.
Nodal metastasis is an independent risk factor for survival in uterine UAS.
Deep invasion, large tumor, and sarcomatous overgrowth increase nodal metastasis.
Acknowledgments
Financial support
Ensign Endowment for Gynecologic Cancer Research (K.M.)
Footnotes
Disclosure statement
There is no conflict of interest in all authors for the study.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2017.01.012.
References
- [1].D’Angelo E, Prat J, Uterine sarcomas: a review, Gynecol. Oncol 116 (2010) 131–139. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2016, CA Cancer J. Clin 66 (2016) 7–30. [DOI] [PubMed] [Google Scholar]
- [3].Ueda SM, Kapp DS, Cheung MK, Shin JY, Osann K, Husain A, Teng NN, Berek JS, Chan JK, Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths, Am. J. Obstet. Gynecol 198 (2008) 218, e1–6. [DOI] [PubMed] [Google Scholar]
- [4].Trope CG, Abeler VM, Kristensen GB, Diagnosis and treatment of sarcoma of the uterus. A review, Acta Oncol 51 (2012) 694–705. [DOI] [PubMed] [Google Scholar]
- [5].Clement PB, Scully RE, Mullerian adenosarcoma of the uterus. A clinicopathologic analysis of ten cases of a distinctive type of mullerian mixed tumor, Cancer 34 (1974) 1138–1149. [DOI] [PubMed] [Google Scholar]
- [6].Prat J, FIGO staging for uterine sarcomas, Int. J. Gynaecol. Obstet 104 (2009) 177–178. [DOI] [PubMed] [Google Scholar]
- [7].D’Angelo E, Spagnoli LG, Prat J, Comparative clinicopathologic and immunohistochemical analysis of uterine sarcomas diagnosed using the World Health Organization classification system, Hum. Pathol 40 (2009) 1571–1585. [DOI] [PubMed] [Google Scholar]
- [8].Clement PB, Scully RE, Mullerian adenosarcoma of the uterus: a clinicopathologic analysis of 100 cases with a review of the literature, Hum. Pathol 21 (1990) 363–381. [DOI] [PubMed] [Google Scholar]
- [9].Kaku T, Silverberg SG, Major FJ, Miller A, Fetter B, Brady MF, Adenosarcoma of the uterus: a gynecologic oncology group clinicopathologic study of 31 cases, Int. J. Gynecol. Pathol 11 (1992) 75–88. [PubMed] [Google Scholar]
- [10].Carroll A, Ramirez PT, Westin SN, Soliman PT, Munsell MF, Nick AM, Schmeler KM, Klopp AH, Fleming ND, Uterine adenosarcoma: an analysis on management, outcomes, and risk factors for recurrence, Gynecol. Oncol 135 (2014) 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Arend R, Bagaria M, Lewin SN, Sun X, Deutsch I, Burke WM, Herzog TJ, Wright JD, Long-term outcome and natural history of uterine adenosarcomas, Gynecol. Oncol 119 (2010) 305–308. [DOI] [PubMed] [Google Scholar]
- [12].Bernard B, Clarke BA, Malowany JI, McAlpine J, Lee CH, Atenafu EG, Ferguson S, Mackay H, Uterine adenosarcomas: a dual-institution update on staging, prognosis and survival, Gynecol. Oncol 131 (2013) 634–639. [DOI] [PubMed] [Google Scholar]
- [13].von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies, BMJ 335 (2007) 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].http://seer.cancer.gov/icd-o-3/ (accessed November 3rd, 2016).
- [15].Edge SB, Compton CC, The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM, Ann. Surg. Oncol 17 (2010) 1471–1474. [DOI] [PubMed] [Google Scholar]
- [16].Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB, Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group, JAMA 283 (2000) 2008–2012. [DOI] [PubMed] [Google Scholar]
- [17].Tanner EJ, Toussaint T, Leitao MM Jr., Hensley ML, Soslow RA, Gardner GJ, Jewell EL, Management of uterine adenosarcomas with and without sarcomatous overgrowth, Gynecol. Oncol 129 (2013) 140–144. [DOI] [PubMed] [Google Scholar]
- [18].Krivak TC, Seidman JD, McBroom JW, MacKoul PJ, Aye LM, Rose GS, Uterine adenosarcoma with sarcomatous overgrowth versus uterine carcinosarcoma: comparison of treatment and survival, Gynecol. Oncol 83 (2001) 89–94. [DOI] [PubMed] [Google Scholar]
- [19].Kim HJ, Fay MP, Feuer EJ, Midthune DN, Permutation tests for joinpoint regression with applications to cancer rates, Stat. Med 19 (2000) 335–351. [DOI] [PubMed] [Google Scholar]
- [20].Tse KY, Crawford R, Ngan HY, Staging of uterine sarcomas, Best Pract. Res. Clin. Obstet. Gynaecol 25 (2011) 733–749. [DOI] [PubMed] [Google Scholar]
- [21].Yamagami W, Susumu N, Ninomiya T, Kuwahata M, Takigawa A, Nomura H, Kataoka F, Tominaga E, Banno K, Tsuda H, Aoki D, A retrospective study on combination therapy with ifosfamide, adriamycin and cisplatin for progressive or recurrent uterine sarcoma, Mol. Clin. Oncol 2 (2014) 591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pautier P, Floquet A, Gladieff L, Bompas E, Ray-Coquard I, Piperno-Neumann S, Selle F, Guillemet C, Weber B, Largillier R, Bertucci F, Opinel P, Duffaud F, Reynaud-Bougnoux A, Delcambre C, Isambert N, Kerbrat P, Netter-Pinon G, Pinto N, Duvillard P, Haie-Meder C, Lhomme C, Rey A, A randomized clinical trial of adjuvant chemotherapy with doxorubicin, ifosfamide, and cisplatin followed by radiotherapy versus radiotherapy alone in patients with localized uterine sarcomas (SARCGYN study). A study of the French Sarcoma Group, Ann. Oncol 24 (2013) 1099–1104. [DOI] [PubMed] [Google Scholar]
- [23].Zaloudek CJ, Norris HJ, Adenofibroma and adenosarcoma of the uterus: a clinicopathologic study of 35 cases, Cancer 48 (1981) 354–366. [DOI] [PubMed] [Google Scholar]
- [24].Clarke BA, Mulligan AM, Irving JA, McCluggage WG, Oliva E, Mullerian adenosarcomas with unusual growth patterns: staging issues, Int. J. Gynecol. Pathol 30 (2011) 340–347. [DOI] [PubMed] [Google Scholar]
- [25].Lazar RI, Straja T, Bratucu B, Uterine adenosarcoma metastasizing to the retroperitoneum. The impact of vascular involvement, J. Med. Life 5 (2012) 145–148. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


