Abstract
BACKGROUND
Cabozantinib inhibits tyrosine kinases, including vascular endothelial growth factor receptors 1, 2, and 3, MET, and AXL, which are implicated in the progression of hepatocellular carcinoma and the development of resistance to sorafenib, the standard initial treatment for advanced disease. This randomized, double-blind, phase 3 trial evaluated cabozantinib as compared with placebo in previously treated patients with advanced hepatocellular carcinoma.
METHODS
A total of 707 patients were randomly assigned in a 2:1 ratio to receive cabozantinib (60 mg once daily) or matching placebo. Eligible patients had received previous treatment with sorafenib, had disease progression after at least one systemic treatment for hepatocellular carcinoma, and may have received up to two previous systemic regimens for advanced hepatocellular carcinoma. The primary end point was overall survival. Secondary end points were progression-free survival and the objective response rate.
RESULTS
At the second planned interim analysis, the trial showed significantly longer overall survival with cabozantinib than with placebo. Median overall survival was 10.2 months with cabozantinib and 8.0 months with placebo (hazard ratio for death, 0.76; 95% confidence interval [CI], 0.63 to 0.92; P = 0.005). Median progression-free survival was 5.2 months with cabozantinib and 1.9 months with placebo (hazard ratio for disease progression or death, 0.44; 95% CI, 0.36 to 0.52; P<0.001), and the objective response rates were 4% and less than 1%, respectively (P = 0.009). Grade 3 or 4 adverse events occurred in 68% of patients in the cabozantinib group and in 36% in the placebo group. The most common high-grade events were palmar–plantar erythrodysesthesia (17% with cabozantinib vs. 0% with placebo), hypertension (16% vs. 2%), increased aspartate aminotransferase level (12% vs. 7%), fatigue (10% vs. 4%), and diarrhea (10% vs. 2%).
CONCLUSIONS
Among patients with previously treated advanced hepatocellular carcinoma, treatment with cabozantinib resulted in longer overall survival and progression-free survival than placebo. The rate of high-grade adverse events in the cabozantinib group was approximately twice that observed in the placebo group. (Funded by Exelixis; CELESTIAL ClinicalTrials.gov number, NCT01908426.)
THE RATE OF DEATH FROM LIVER CANCER is rising faster than the rate of death from any other cancer in the United States.1,2 The systemic treatment options available for most cases are limited.3–5 Despite several advances,6–10 outcomes in the majority of patients remain poor, and additional treatment options are needed.
The vascular endothelial growth factor (VEGF) pathway is an established therapeutic target in hepatocellular carcinoma, but the clinical benefit from targeting this pathway has been modest, which suggests that inhibition of additional signaling pathways may improve efficacy.11 Like VEGF, the receptor tyrosine kinases MET and AXL are induced by tumor hypoxia.12,13 MET and AXL play diverse roles in tumor biology, including promotion of the epithelial-to-mesenchymal transition, invasion, and metastasis,14,15 and both kinases are implicated in resistance to antiangiogenic therapy.16–18 High expression of MET or AXL may be associated with poor prognosis in patients with hepatocellular carcinoma,19,20 and increased MET expression or activation has been associated with previous sorafenib treatment in patients with hepatocellular carcinoma and with sorafenib resistance in preclinical models.21–24
Cabozantinib, an inhibitor of tyrosine kinases including VEGF receptors 1, 2, and 3, MET, and AXL, inhibits tumor growth in murine models of hepatocellular carcinoma.23,25 In a phase 2, randomized discontinuation trial, cabozantinib showed clinical activity in patients with advanced hepatocellular carcinoma, regardless of whether they had received previous treatment with sorafenib26; median overall survival was 11.5 months and median progression-free survival was 5.2 months. On the basis of these results, we conducted a randomized, double-blind, placebo-controlled, phase 3 trial to evaluate cabozantinib (Cabometyx, Exelixis) in previously treated patients with advanced hepatocellular carcinoma.
METHODS
PATIENTS
Eligible patients were 18 years of age or older, had received a pathological diagnosis of hepatocellular carcinoma that was not amenable to curative treatment, and had Child–Pugh class A liver function (a score of 5 to 6 points out of a possible 15, with higher scores indicating more advanced liver disease; the score is the total of five clinical measures of liver function: total bilirubin, serum albumin, prothrombin time, ascites, and hepatic encephalopathy). Eligible patients had received previous treatment with sorafenib and had had disease progression after at least one systemic treatment for hepatocellular carcinoma, but they could have received up to two previous systemic treatments. Additional inclusion criteria were an Eastern Cooperative Oncology Group (ECOG) performance-status score of 0 or 1 (on a 5-point scale, with higher scores indicating greater disability), adequate hematologic measures, and adequate renal function. Patients could not have had previous treatment with cabozantinib and could not have uncontrolled clinically significant illness. Additional eligibility criteria are listed in the Supplementary Appendix, available with the full text of this article at NEJM.org.
TRIAL DESIGN
In this double-blind, phase 3 trial, patients were randomly assigned, in a 2:1 ratio, to receive cabozantinib or placebo. Randomization was performed at a central location through an interactive response system with the use of permuted blocks, stratified according to etiologic factor (hepatitis B virus [HBV], with or without hepatitis C virus [HCV]; HCV without HBV; or other), geographic region (Asia or other), and evidence of extrahepatic spread of disease, macrovascular invasion, or both (yes or no).
Patients received either a 60-mg tablet of cabozantinib or a matched placebo tablet to be taken orally once per day. Treatment interruptions and dose reductions (to 40 mg and then to 20 mg) were used to manage adverse events. Patients continued the assigned trial regimen as long as they had clinical benefit, as judged by the investigator, or until they had unacceptable toxic effects. Patients were allowed to receive cabozantinib or placebo beyond radiographic progression as long as they continued to have clinical benefit.
END POINTS AND ASSESSMENTS
The primary end point was overall survival, defined as the time from randomization to death from any cause. Secondary efficacy end points were progression-free survival (defined as the time from randomization to radiographic progression or death from any cause, whichever occurred first) and objective response rate (the percentage of patients with a confirmed complete or partial response). Tumors were assessed by computed tomography or magnetic resonance imaging at baseline and every 8 weeks after randomization; assessments were performed until 8 weeks after radiographic progression or discontinuation of cabozantinib or placebo, whichever occurred later. Tumor response and progression were assessed by the investigator according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.27 Safety was evaluated continuously, and the severity of adverse events was assessed by the investigator according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Results of analyses of pharmacokinetics, health-related quality of life, and biomarkers are not reported here.
TRIAL OVERSIGHT
The protocol (available at NEJM.org) was approved by the ethics committee or institutional review board at each center, and the trial was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. Written informed consent was obtained from every patient. An independent data and safety monitoring committee reviewed safety and efficacy during the trial. The trial was designed by the first and last authors in collaboration with the sponsor, and the authors and the sponsor were responsible for data collection and analysis. The authors vouch for the fidelity of the trial to the protocol and for the accuracy and completeness of the data. The first and last authors wrote the first draft of the manuscript in collaboration with the sponsor. Medical writing support was provided by the sponsor.
STATISTICAL ANALYSIS
Up to three analyses of the primary end point of overall survival were planned, when approximately 50%, 75%, and 100% of the expected deaths had occurred. We estimated that a sample size of 760 patients, with a total of 621 deaths, would provide the trial with 90% power to detect a hazard ratio of 0.76 favoring cabozantinib over placebo, with a two-sided log-rank test at a 5% level of significance. Assuming a median overall survival of 8.2 months in the placebo group (as shown in the Brivanib Study in HCC Patients at Risk Post Sorafenib [BRISK-PS]28) and exponential distribution, this would correspond to 32% longer median overall survival (10.8 months) in the cabozantinib group. Inflation of the type 1 error associated with interim analyses was controlled with the use of the Lan–DeMets O’Brien–Fleming alpha spending function.29 If the null hypothesis of no difference in overall survival was rejected at either the first or second interim analysis, testing of secondary end points would proceed, and subsequent analyses of overall survival would not be performed.
Efficacy was assessed in all randomly assigned patients according to the intention-to-treat principle. Safety was assessed in all patients who received at least one dose of the trial regimen. For time-to-event end points, hypothesis testing was performed with the stratified log-rank test with adjustment for the stratification factors used at randomization; median durations and associated 95% confidence intervals were estimated with the Kaplan–Meier method. Hazard ratios were estimated with univariate Cox regression models, with the randomized group as the only predictor. Hazard ratios for overall analyses were calculated from models adjusted for the randomization stratification factors. Hypothesis testing of objective response was performed with the Cochran–Mantel–Haenszel method. All subgroup analyses of overall survival and progression-free survival were prespecified except those based on extrahepatic spread of disease or macrovascular invasion as separate factors and on sorafenib as the only previous therapy. For subgroup analyses, no adjustments were made for multiplicity, and confidence intervals are considered to be descriptive. Hazard ratios for subgroup analyses were calculated from unstratified models except those calculated for the subgroup of patients whose only previous therapy was sorafenib. All analyses were performed with SAS software, version 9.1 or higher (SAS Institute).
RESULTS
PATIENTS
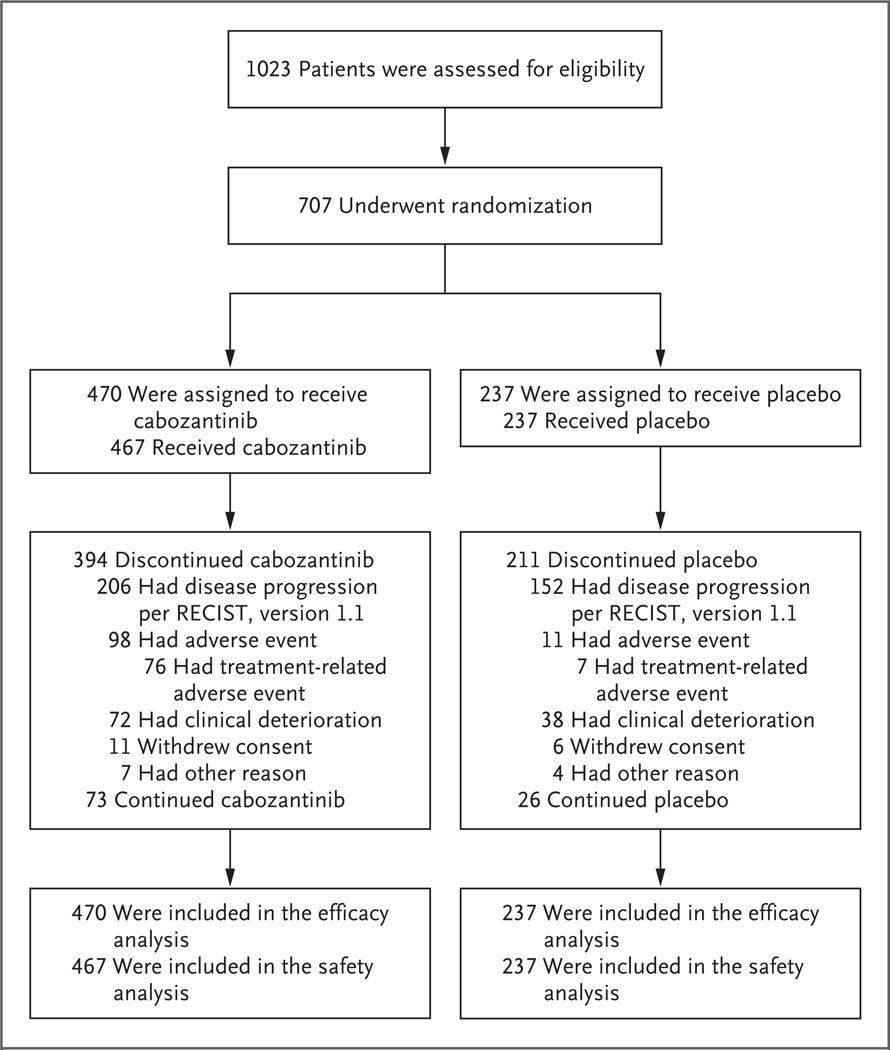
From September 2013 through September 2017, a total of 773 patients underwent randomization at 95 centers in 19 countries. As of the data cutoff date of June 1, 2017, for the second interim analysis, 707 patients had undergone randomization: 470 patients had been assigned to receive cabozantinib, and 237 to receive placebo; these patients made up the intention-to-treat population for efficacy analyses (Fig. 1). The safety population comprised 704 patients: 467 patients who received cabozantinib and 237 who received placebo. As of the data cutoff date, 73 patients (16%) in the cabozantinib group and 26 (11%) in the placebo group were still following the assigned trial regimen. The most common reason for discontinuation of cabozantinib or placebo was radiographic disease progression. Baseline demographics and clinical characteristics were balanced between the groups (Table 1, and Table S1 in the Supplementary Appendix). All the patients had previously received sorafenib, and 27% had received two previous systemic anticancer regimens for advanced hepatocellular carcinoma.
Figure 1. Eligibility, Randomization, and Follow-up.
RECIST denotes Response Evaluation Criteria In Solid Tumors.
Table 1.
Basic Baseline Characteristics.*
| Characteristic | Cabozantinib (N = 470) | Placebo (N = 237) |
|---|---|---|
| Median age (range) — yr | 64 (22–86) | 64 (24–86) |
| Sex — no. (%) | ||
| Male | 379 (81) | 202 (85) |
| Female | 91 (19) | 35 (15) |
| Geographic region — no. (%) | ||
| Asia† | 116 (25) | 59 (25) |
| Europe | 231 (49) | 108 (46) |
| Canada and United States | 108 (23) | 59 (25) |
| Australia and New Zealand | 15 (3) | 11 (5) |
| ECOG performance-status score — no. (%)‡ | ||
| 0 | 245 (52) | 131 (55) |
| 1 | 224 (48) | 106 (45) |
| 2 | 1 (<1) | 0 |
| Etiologic factor — no. (%)§ | ||
| HBV | 178 (38) | 89 (38) |
| HCV | 113 (24) | 55 (23) |
| Dual HBV and HCV infection | 8 (2) | 4 (2) |
| Alcohol use | 112 (24) | 39 (16) |
| Nonalcoholic steatohepatitis | 43 (9) | 23 (10) |
| Other | 24 (5) | 16 (7) |
| Unknown | 75 (16) | 47 (20) |
| Extrahepatic spread of disease — no. (%) | 369 (79) | 182 (77) |
| Macrovascular invasion — no. (%) | 129 (27) | 81 (34) |
| Extrahepatic spread of disease, macrovascular invasion, or both — no. (%) | 398 (85) | 200 (84) |
There were no significant differences (P<0.05) between the groups at baseline. Percentages may not total 100 because of rounding. More details are provided in Table S1 in the Supplementary Appendix. HBV denotes hepatitis B virus, and HCV hepatitis C virus.
Asia included Hong Kong, South Korea, Singapore, and Taiwan.
Eastern Cooperative Oncology Group (ECOG) performance-status scores are assessed on a 5-point scale, with higher scores indicating greater disability. Although patients were required to have a score of 0 or 1, a few patients had a score of 2.
Etiologic factors were assessed according to case-report forms. Some patients had more than one factor.
EFFICACY
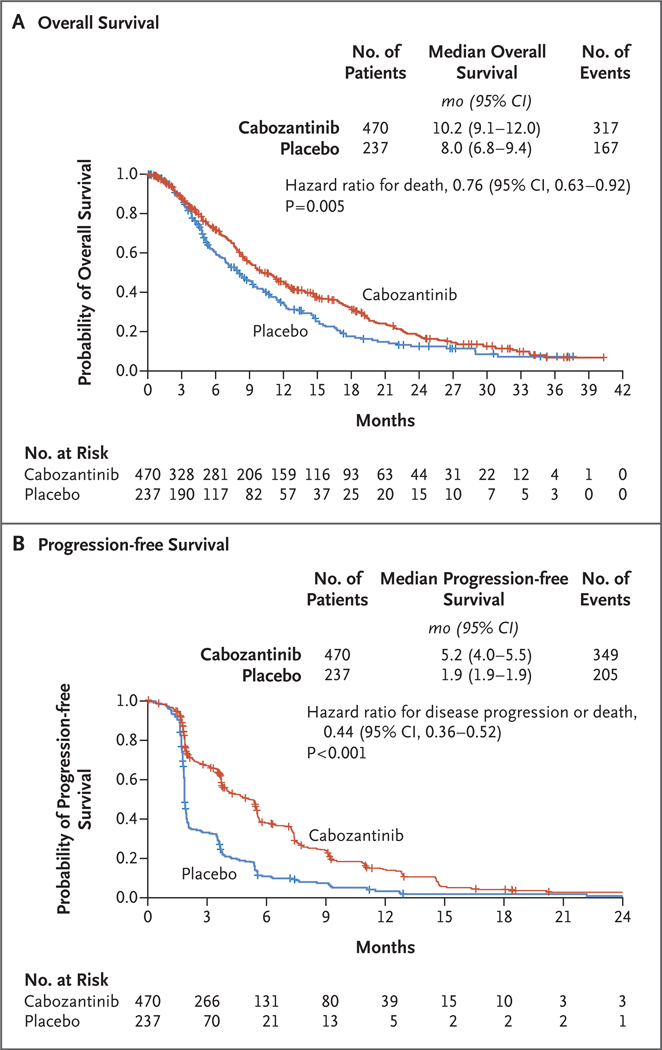
The median overall survival was 10.2 months (95% confidence interval [CI], 9.1 to 12.0) in the cabozantinib group and 8.0 months (95% CI, 6.8 to 9.4) in the placebo group (Fig. 2A). The stratified hazard ratio for death was 0.76 (95% CI, 0.63 to 0.92), and the stratified log-rank P value was 0.005, which met the criterion for statistical significance. Overall survival was significantly longer with cabozantinib than with placebo at the second planned interim analysis, which had a data cutoff date of June 1, 2017, and included 484 deaths, representing 78% of the 621 deaths planned for the prespecified final analysis. The stopping boundary according to the prespecified alpha-spending function was a P value of 0.02. Landmark estimates of overall survival according to the Kaplan–Meier method at 6, 12, 18, and 24 months showed a higher percentage of patients alive in the cabozantinib group than in the placebo group at each time point (Table S2 in the Supplementary Appendix). As of June 2017, a total of 123 patients (26%) in the cabozantinib group and 78 (33%) in the placebo group had received subsequent systemic or local liver-directed anticancer therapy that did not include radiation (Table S3 in the Supplementary Appendix). These overall survival results are consistent with the findings of the first interim analysis, which had a data cutoff date of June 2016 and included 321 patient deaths, representing 52% of the 621 deaths planned for the prespecified final analysis. At that time point, the observed hazard ratio for death was 0.71 and the P value was 0.0041, which did not cross the stopping boundary for the first interim analysis (P = 0.0037).
Figure 2. Kaplan–Meier Analysis of Overall Survival and Progression-free Survival.
Overall survival was defined as the time from randomization to death from any cause, and progression-free survival as the time from randomization to radiographic progression or death from any cause. Tick marks indicate censored data.
The median progression-free survival according to RECIST, version 1.1, as assessed by the investigator, was 5.2 months (95% CI, 4.0 to 5.5) in the cabozantinib group and 1.9 months (95% CI, 1.9 to 1.9) in the placebo group. The stratified hazard ratio for disease progression or death was 0.44 (95% CI, 0.36 to 0.52; P<0.001 by stratified log-rank test) (Fig. 2B). The objective response rate according to RECIST, version 1.1, was 4% (18 partial responses among 470 patients) in the cabozantinib group and less than 1% (1 partial response among 237 patients) in the placebo group (P = 0.009) (Table S4 in the Supplementary Appendix). Disease control (defined as a partial response or stable disease) was achieved in 64% of the patients (300 patients) in the cabozantinib group, as compared with 33% (79 patients) in the placebo group.
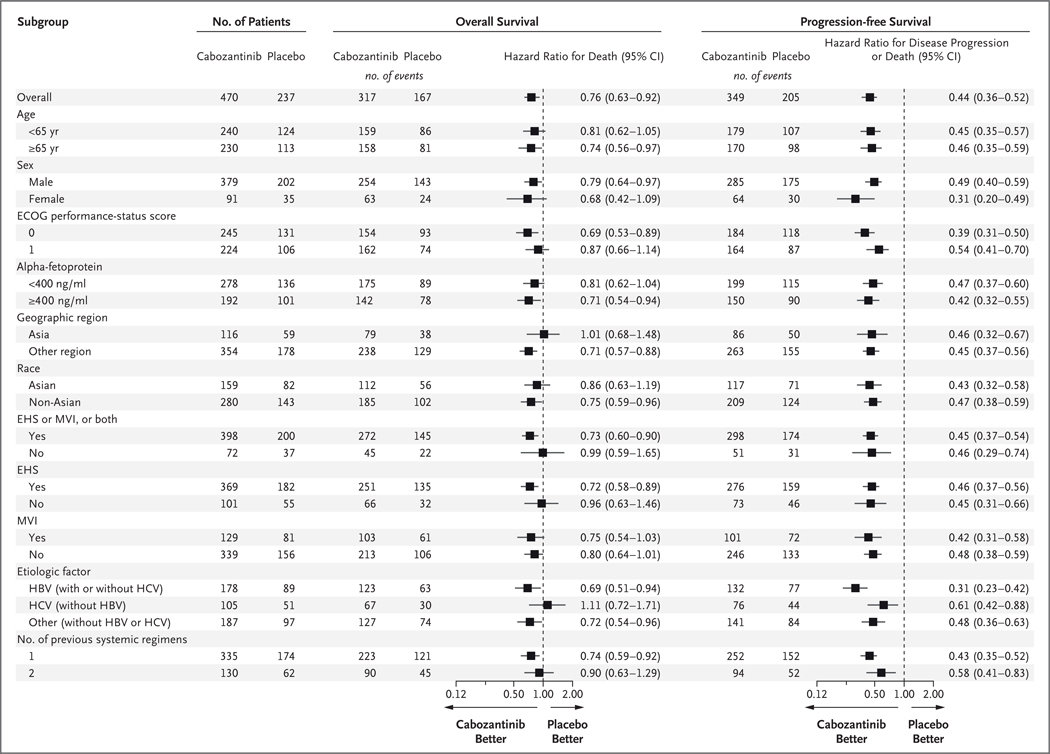
Subgroup analyses of progression-free survival consistently favored cabozantinib, which showed the clinical activity of cabozantinib across subgroups of patients with various etiologic factors and demographic characteristics (Fig. 3, and Table S5 in the Supplementary Appendix). The results for overall survival across subgroups were more variable. In the subgroup of patients whose only previous systemic therapy was sorafenib, the median overall survival was 11.3 months with cabozantinib and 7.2 months with placebo (stratified hazard ratio for death, 0.70; 95% CI, 0.55 to 0.88), and the median progression-free survival was 5.5 months with cabozantinib and 1.9 months with placebo (stratified hazard ratio for disease progression or death, 0.40; 95% CI, 0.32 to 0.50).
Figure 3. Overall Survival and Progression-free Survival in Selected Subgroups.
Eastern Cooperative Oncology Group (ECOG) performance-status scores are assessed on a 5-point scale, with higher scores indicating greater disability. Race was reported by the patient. EHS denotes extrahepatic spread of disease, HBV hepatitis B virus, HCV hepatitis C virus, and MVI macrovascular invasion.
SAFETY
The median duration of receipt of the trial drug or placebo was 3.8 months in the cabozantinib group and 2.0 months in the placebo group. Dose reductions occurred in 291 patients (62%) in the cabozantinib group and in 30 patients (13%) in the placebo group. The median average daily dose was 35.8 mg for cabozantinib and 58.9 mg for placebo, with a median time to first dose reduction of 38 days in the cabozantinib group. The rate of discontinuation of cabozantinib or placebo owing to adverse events that were considered to be related to the trial regimen was 16% (76 patients) in the cabozantinib group and 3% (7 patients) in the placebo group. Adverse events leading to treatment discontinuation in more than 1.0% of patients in the cabozantinib group were palmar–plantar erythrodysesthesia, fatigue, decreased appetite, diarrhea, and nausea.
Adverse events of any grade regardless of causality were reported in 99% of the patients in the cabozantinib group and in 92% in the placebo group, and adverse events of grade 3 or 4 were reported in 68% of the patients in the cabozantinib group and in 36% in the placebo group (Table 2). The most common grade 3 or 4 adverse events in the cabozantinib group were palmar–plantar erythrodysesthesia (17%, vs. 0% with placebo), hypertension (16% vs. 2%), increased aspartate aminotransferase level (12% vs. 7%), fatigue (10% vs. 4%), and diarrhea (10% vs. 2%). The most common adverse events of any grade leading to dose reductions in the cabozantinib group were palmar–plantar erythrodysesthesia (22%), diarrhea (10%), fatigue (7%), hypertension (7%), and increased aspartate aminotransferase level (6%). Serious adverse events were reported in 50% of the patients who received cabozantinib and in 37% of the patients who received placebo. A serious adverse event was defined as an adverse event of any grade that caused death, was life-threatening, resulted in hospitalization or prolongation of hospitalization, was deemed medically important, or resulted in disability or birth defect. Grade 5 adverse events occurring within 30 days after the last dose of cabozantinib or placebo were reported in 55 patients (12%) in the cabozantinib group and in 28 (12%) in the placebo group and were commonly related to disease progression. Grade 5 adverse events that were considered to be related to cabozantinib or placebo were reported in 6 patients in the cabozantinib group (one event each of hepatic failure, bronchoesophageal fistula, portal-vein thrombosis, upper gastrointestinal hemorrhage, pulmonary embolism, and the hepatorenal syndrome) and in 1 patient in the placebo group (hepatic failure).
Table 2.
Adverse Events.*
| Event | Cabozantinib (N = 467) | Placebo (N = 237) | ||||
|---|---|---|---|---|---|---|
| Any Grade | Grade 3 | Grade 4 | Any Grade | Grade 3 | Grade 4 | |
| number of patients (percent) | ||||||
| Any adverse event | 460 (99) | 270 (58) | 46 (10) | 219 (92) | 80 (34) | 6 (3) |
| Diarrhea | 251 (54) | 45 (10) | 1 (<1) | 44 (19) | 4 (2) | 0 |
| Decreased appetite | 225 (48) | 27 (6) | 0 | 43 (18) | 1 (<1) | 0 |
| Palmar–plantar erythrodysesthesia | 217 (46) | 79 (17) | 0 | 12 (5) | 0 | 0 |
| Fatigue | 212 (45) | 49 (10) | 0 | 70 (30) | 10 (4) | 0 |
| Nausea | 147 (31) | 10 (2) | 0 | 42 (18) | 4 (2) | 0 |
| Hypertension | 137 (29) | 73 (16) | 1 (<1) | 14 (6) | 4 (2) | 0 |
| Vomiting | 121 (26) | 2 (<1) | 0 | 28 (12) | 6 (3) | 0 |
| Increase in aspartate aminotransferase level | 105 (22) | 51 (11) | 4 (1) | 27 (11) | 15 (6) | 1 (<1) |
| Asthenia | 102 (22) | 31 (7) | 1 (<1) | 18 (8) | 4 (2) | 0 |
| Dysphonia | 90 (19) | 3 (1) | 0 | 5 (2) | 0 | 0 |
| Constipation | 87 (19) | 2 (<1) | 0 | 45 (19) | 0 | 0 |
| Abdominal pain | 83 (18) | 7 (1) | 1 (<1) | 60 (25) | 10 (4) | 0 |
| Weight loss | 81 (17) | 5 (1) | 0 | 14 (6) | 0 | 0 |
| Increase in alanine aminotransferase level | 80 (17) | 23 (5) | 0 | 13 (5) | 5 (2) | 0 |
| Mucosal inflammation | 65 (14) | 8 (2) | 0 | 5 (2) | 1 (<1) | 0 |
| Pyrexia | 64 (14) | 0 | 0 | 24 (10) | 1 (<1) | 0 |
| Upper abdominal pain | 63 (13) | 3 (1) | 0 | 31 (13) | 0 | 0 |
| Cough | 63 (13) | 1 (<1) | 0 | 26 (11) | 0 | 0 |
| Peripheral edema | 63 (13) | 4 (1) | 0 | 32 (14) | 2 (1) | 0 |
| Stomatitis | 63 (13) | 8 (2) | 0 | 5 (2) | 0 | 0 |
| Dyspnea | 58 (12) | 15 (3) | 0 | 24 (10) | 1 (<1) | 0 |
| Rash | 58 (12) | 2 (<1) | 0 | 14 (6) | 1 (<1) | 0 |
| Ascites | 57 (12) | 17 (4) | 1 (<1) | 30 (13) | 11 (5) | 0 |
| Dysgeusia | 56 (12) | 0 | 0 | 5 (2) | 0 | 0 |
| Hypoalbuminemia | 55 (12) | 2 (<1) | 0 | 12 (5) | 0 | 0 |
| Headache | 52 (11) | 1 (<1) | 0 | 16 (7) | 1 (<1) | 0 |
| Thrombocytopenia | 52 (11) | 16 (3) | 0 | 1 (<1) | 0 | 0 |
| Insomnia | 49 (10) | 1 (<1) | 0 | 17 (7) | 0 | 0 |
| Dizziness | 48 (10) | 2 (<1) | 0 | 15 (6) | 0 | 0 |
| Dyspepsia | 47 (10) | 0 | 0 | 7 (3) | 0 | 0 |
| Anemia | 46 (10) | 18 (4) | 1 (<1) | 19 (8) | 12 (5) | 0 |
| Back pain | 46 (10) | 5 (1) | 0 | 24 (10) | 1 (<1) | 0 |
| Increase in serum bilirubin level | 45 (10) | 10 (2) | 4 (1) | 17 (7) | 2 (1) | 2 (1) |
| Decrease in platelet count | 45 (10) | 13 (3) | 4 (1) | 7 (3) | 2 (1) | 0 |
Listed are adverse events, regardless of causality, that were reported in at least 10% of patients in either group. Severity was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
DISCUSSION
This randomized, phase 3 trial showed that cabozantinib treatment significantly prolonged survival in patients with previously treated advanced hepatocellular carcinoma. The median overall survival was 10.2 months with cabozantinib and 8.0 months with placebo, with a hazard ratio for death of 0.76. Corresponding to this survival benefit, a longer duration of progression-free survival was also observed: the median progression-free survival was 5.2 months with cabozantinib and 1.9 months with placebo, with a hazard ratio for disease progression or death of 0.44. Subgroup analyses of progression-free survival suggested that cabozantinib had clinical activity across subgroups of patients with various etiologic factors and across subgroups with other baseline characteristics. Subgroup analyses of overall survival were more variable, with broader confidence intervals. Hazard ratios in subgroups can be affected by statistical variability from evaluation of smaller populations or imbalances in prognostic factors or subsequent anticancer therapies. It is noteworthy that in an analysis of overall survival, the hazard ratio for death was 0.69 in patients with disease caused by HBV and 1.11 in patients with HCV, and the hazard ratio for death was 0.86 in patients of Asian race but 1.01 in patients enrolled in Asia. Further analyses are necessary to help understand these differences.
The safety results for cabozantinib were consistent with results from an earlier phase 2 study involving patients with hepatocellular carcinoma26 and with the known safety profile of cabozantinib. The most common adverse events were similar to those observed with other VEGF-receptor tyrosine kinase inhibitors in patients with hepatocellular carcinoma. Adverse events were managed with dose modifications and supportive care. Dose reductions occurred in the majority of patients, and the rate of discontinuation due to adverse events from cabozantinib or placebo was 16%. The median average daily dose of cabozantinib was 35.8 mg, which was similar to the median dose (43 mg) received in a phase 3 trial involving patients with advanced renal-cell carcinoma, which also showed therapeutic efficacy.30
The patient population included in this trial represents a small percentage of patients with hepatocellular carcinoma. Because the survival of patients who have hepatocellular carcinoma with Child–Pugh liver disease of class B or worse is determined by liver failure, and it may be impossible to discern any effect of treatment on the cancer, it is justified to exclude these patients from pivotal clinical trials. Thus, as with all other agents approved for treatment of hepatocellular carcinoma, additional studies are required to confirm the safety and efficacy of cabozantinib in patients with more compromised liver function or poorer performance status.
MET expression has been shown to increase in tumors after sorafenib exposure in patients with hepatocellular carcinoma, which underscores a possible role for MET in the development of sorafenib resistance.21,22 Tivantinib, an allosteric inhibitor of MET, was evaluated in a phase 3 trial involving patients pretreated with sorafenib who had high tumor MET expression, but it did not result in longer overall survival or progression-free survival than placebo.22 By inhibiting MET and AXL in addition to VEGF receptors, cabozantinib targets multiple oncogenic and angiogenic pathways, which may provide additional efficacy and help overcome resistance to agents that target VEGF receptors.14–18,23,24 Cabozantinib also improved clinical outcomes in patients with advanced renal-cell carcinoma after previous antiangiogenic therapy, which further supports a role for targeting MET and AXL in overcoming resistance to VEGF-pathway inhibition.30,31
In conclusion, treatment with cabozantinib, a tyrosine kinase inhibitor that targets MET, VEGF receptors, and AXL, resulted in longer overall survival and progression-free survival than placebo in patients with previously treated advanced hepatocellular carcinoma. Adverse events were consistent with the known safety profile of cabozantinib, and the rate of high-grade adverse events in the cabozantinib group was approximately twice that observed in the placebo group.
Supplementary Material
Acknowledgments
Supported by Exelixis. Dr. Meyer is funded in part by the University College London Hospitals Biomedical Research Centre.
Dr. Abou-Alfa reports receiving consulting fees and advisory board fees from Bayer and BMS; Dr. Meyer, receiving grant support and consulting fees from Bayer and BTG, and consulting fees from BMS, Merck, and Eisai; Dr. Cheng, receiving consulting fees from BMS, Ono, MSD, and BeiGene, advisory board fees from Novartis, and consulting fees and honoraria from Bayer and Merck; Dr. El-Khoueiry, receiving advisory board fees and consulting fees from Bristol-Myers Squibb and Bayer, advisory board fees from Eisai, Novartis, Roche, Exelixis, Celgene and CytomX, grant support and advisory board fees from AstraZeneca, grant support from Astex, and fees for serving on a speakers’ bureau from Merrimack; Dr. Rimassa, receiving advisory board fees from Lilly, Bayer, Sirtex Medical, and Exelixis, consulting fees and travel support from ArQule and Ipsen, and lecture fees from AstraZeneca and AbbVie; Dr. Park, receiving advisory board fees from BMS, Midatech, and AstraZeneca, advisory board fees and honoraria from Ono and Eisai, and honoraria from Bayer; Dr. Blanc, receiving advisory board fees from Bayer, BMS, Lilly Oncology, Shire, and Onxeo; Dr. Bolondi, receiving advisory board fees and lecture fees from Bayer, BMS, Sirtex, and Guerbet, and lecture fees from Eli Lilly, Meda-Pharm, and Bracco; Dr. Klümpen, serving on an advisory board for Ipsen; Dr. Zagonel, receiving consulting fees, advisory board fees, and fees for serving on a speakers’ bureau from Bristol-Myers Squibb, consulting fees and advisory board fees from Celgene, consulting fees, advisory board fees, and travel support from Merck, fees for serving on a speakers’ bureau and travel support from Bayer and Roche, and fees for serving on a speakers’ bureau from Pfizer and Janssen; Mr. Hessel and Dr. Schwab, being employed by and holding stock in Exelixis; Dr. Borgman-Hagey, being employed by and holding stock in Exelixis; Dr. Kelley, receiving grant support and travel support paid to her institution, provision of trial drugs, and printing and processing costs from AstraZeneca, grant support paid to her institution from Acceleron, grant support paid to her institution and provision of trial drugs from Adaptimmune, Eli Lilly, MedImmune, Celgene, Regeneron, Merck, Tekmira, Novartis, and Taiho, grant support and fees for serving on a steering committee paid to her institution, and provision of trial drugs from Agios, grant support and advisory board fees paid to her institution, and provision of trial drugs from Bayer and Bristol-Myers Squibb, grant support paid to her institution from Sanofi and Debio, and fees for serving on a steering committee paid to her institution from TARGET Pharma Solutions.
We thank the patients, their families, the investigators, the site staff, and the trial teams; David W. Markby (Exelixis) for assistance with medical writing; and Karen O’Leary and Michael Raffin (Fishawack Communications) for editorial assistance, which was funded by Exelixis.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5): E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual report to the nation on the status of cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 2016; 122: 1312–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abou-Alfa GK, Jarnagin W, Lowery M, et al. Liver and bile duct cancer In: Niederhuber JE, Armitage JO, Doroshow JH, Kastan MB, Tepper JE, eds. Abeloff’s clinical oncology. 5th ed Philadelphia: Saunders, 2014:1373–96. [Google Scholar]
- 4.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: hepatobiliary cancers, version 4. 2017. (https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf). [DOI] [PMC free article] [PubMed]
- 5.European Association for the Study of the Liver, European Organisation for Research and Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56: 908–43. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–90. [DOI] [PubMed] [Google Scholar]
- 7.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10: 25–34. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389: 56–66. [DOI] [PubMed] [Google Scholar]
- 9.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389: 2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Opdivo. Princeton, NJ: Bristol-Myers Squibb, July 2017. (package insert).
- 11.Abou-Alfa GK, Venook AP. The antiangiogenic ceiling in hepatocellular carcinoma: does it exist and has it been reached? Lancet Oncol 2013; 14(7): e283e288. [DOI] [PubMed] [Google Scholar]
- 12.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 2003; 3: 347–61. [DOI] [PubMed] [Google Scholar]
- 13.Rankin EB, Giaccia AJ. The receptor tyrosine kinase AXL in cancer progression. Cancers (Basel) 2016; 8(11): E103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012;12: 89–103. [DOI] [PubMed] [Google Scholar]
- 15.Graham DK, DeRyckere D, Davies KD, Earp HS. The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat Rev Cancer 2014; 14: 769–85. [DOI] [PubMed] [Google Scholar]
- 16.Shojaei F, Lee JH, Simmons BH, et al. HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res 2010; 70: 10090–100. [DOI] [PubMed] [Google Scholar]
- 17.Sennino B, Ishiguro-Oonuma T, Wei Y, et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov 2012; 2: 270–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L, Liu XD, Sun M, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene 2016; 35: 2687–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueki T, Fujimoto J, Suzuki T, Yamamoto H, Okamoto E. Expression of hepatocyte growth factor and its receptor, the c-met proto-oncogene, in hepatocellular carcinoma. Hepatology 1997; 25:619–23. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Wang K, Yan Z, et al. Axl expression stratifies patients with poor prognosis after hepatectomy for hepatocellular carcinoma. PLoS One 2016; 11(5): e0154767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rimassa L, Abbadessa G, Personeni N, et al. Tumor and circulating biomarkers in patients with second-line hepatocellular carcinoma from the randomized phase II study with tivantinib. Oncotarget 2016;7: 72622–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rimassa L, Assenat E, Peck-Radosavljevic M, et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol 2018; 19:682–93. [DOI] [PubMed] [Google Scholar]
- 23.Xiang Q, Chen W, Ren M, et al. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin Cancer Res 2014; 20: 2959–70. [DOI] [PubMed] [Google Scholar]
- 24.Firtina Karagonlar Z, Koc D, Iscan E, Erdal E, Atabey N. Elevated hepatocyte growth factor expression as an autocrine c-Met activation mechanism in acquired resistance to sorafenib in hepatocellular carcinoma cells. Cancer Sci 2016; 107: 40716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 2011; 10: 2298–308. [DOI] [PubMed] [Google Scholar]
- 26.Kelley RK, Verslype C, Cohn AL, et al. Cabozantinib in hepatocellular carcinoma: results of a phase 2 placebo-controlled randomized discontinuation study. Ann Oncol 2017; 28: 528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 28.Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol 2013; 31: 3509–16. [DOI] [PubMed] [Google Scholar]
- 29.DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med 1994; 13:1341–52. [DOI] [PubMed] [Google Scholar]
- 30.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016; 17: 917–27. [DOI] [PubMed] [Google Scholar]
- 31.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373: 1814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.