Abstract
Aldehyde dehydrogenase 2 (aldh2) serves an important role in the development of organ injury. Therefore, the present study investigated the effects of aldh2 on the oxidative stress response in a mouse model of ketamine-induced cystitis (KIC). A total of 60 8-week-old male Institute of Cancer Research wild-type (WT) mice and 45 aldh2 knock-out (KO) mice were randomized to receive low-dose ketamine (30 mg/kg), high-dose ketamine (60 mg/kg) or normal saline (controls). At 4, 8 and 12 weeks post-injection, bladder tissues were harvested and used to investigate the protective mechanisms of aldh2 on bladder function. The results demonstrated that aldh2 KO mice exhibited significant weight loss following chronic ketamine injection compared with that in WT mice. Furthermore, ketamine treatment increased the urination rate (P<0.05), pathological score (P<0.05), levels of the oxidative stress product malondialdehyde (P<0.05) in addition to reducing the expression of the anti-oxidative stress enzyme superoxide dismutase (P<0.05) and glutathione-SH (P<0.05). Oxidative stress in aldh2 KO mice was also found to significantly enhance the expression of proteins associated with the NF-κB signaling pathway, which promoted the expression of inducible nitric oxide synthase (P<0.05) and cyclooxygenase-2 (P<0.05) further. Finally, aldh2 KO mice demonstrated higher severity of fibrosis in the submucosal and muscular layers of the bladder. In conclusion, the present study suggests that aldh2 serves a protective role in preventing inflammation and fibrosis in KIC.
Keywords: ketamine-induced cystitis, aldehyde dehydrogenase 2, oxidative stress, cyclooxygenase-2, inducible nitric oxide synthase
Introduction
Ketamine, also known as K powder, is a substance that has been widely abused in recent years (1). Chronic ketamine abusers frequently suffer from severe lower urinary tract syndrome (LUTS), which is characterized by the excessive frequency of urination, nocturia, suprapubic discomfort and occasional hematuria (2). Pathologically, it is similar to interstitial cystitis, also known as ketamine-induced cystitis (KIC) (3). However, the pathophysiology of bladder dysfunction in patients with KIC remains to be elucidated.
Accumulating evidence has indicated that oxidative stress-mediated injury serves an important role in KIC (4,5). During oxidative stress, ATP production from the mitochondrial respiratory chain triggers bladder hyperactivity. Ketamine injections increase the production of intracellular reactive oxygen species (ROS), which stimulates electron leakage from the mitochondrial respiratory chain complexes (4). ROS can be removed from the body by enzymes (for example, SOD and GSH-px) and non-enzymatic (for example GSH, Vit C and Vit E) antioxidants, which serves as the main defense system (6). During oxidative stress, high ROS levels induce lipid peroxidation and the formation of malondialdehyde (MDA), which is an end-product of oxidation that influences the mitochondrial respiratory chain complex and the activities of key enzymes (For example, SOD and GSH) (7). Superoxide dismutase (SOD) and glutathione-sulfhydryl (GSH) are the main antioxidant enzymes that degrade and scavenge free radicals in vivo (8). A previous study revealed that chronic ketamine treatment increased MDA levels in the rat bladder and reduced the expression of antioxidant enzymes SOD and GSH (Mi et al, 2016, unpublished data). This indicated that ketamine can target the bladder urothelium to release ROS and induce inflammation in the bladder. The release of ROS in bladder urothelium is mediated by the cytochrome C oxidase pathway, which induces detrusor hyperactivity. Furthermore, oxidative stress triggers the production of large quantities of proinflammatory mediators nitric oxide (NO) and prostaglandin E2 from macrophages. These products are generated by inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), respectively (9). These inflammatory mediators increase vascular permeability in the urothelium, resulting in ulceration, erythrocyte accumulation (hemorrhage), monocyte infiltration and increased interstitial fibrosis between the detrusor smooth muscle tracts in the rat bladder injured by ketamine treatment (10).
Aldehyde dehydrogenase 2 (aldh2) is a mitochondrial enzyme that regulates aldehyde metabolism by eliminating cytotoxic aldehydes, thereby reducing oxidative stress and inhibiting the production of ROS-related toxic products (11). In particular, ~40% of the population in Asia has a defective aldh2 gene compared with that in Europe and Africa, where the prevalence is <5% (12). Individuals with the aldh2 allele deficiency are highly susceptible to adverse reactions to ethanol and other stimulatory factors (For example, 4-hydroxy-2-nonenal and hypoxia) due to the accumulation of aldehydes caused by the lack of aldh2 enzymes. Aldh2 has been previously identified as a potential prognostic marker for bladder urothelial carcinoma (13). In a follow-up study performed in the USA, aldh2 variants were found to be associated with a shorter time to first recurrence of bladder cancer (14). This finding was supported by another previous study by Ferreira-Teixeira et al (15), who revealed that aldh2 has the potential to predict the progression and metastasis of invasive bladder cancer. Although a number of studies have demonstrated the anti-oxidant effect of aldh2 in bladder tumors, its role in cystitis remains poorly understood.
It has been previously reported that ketamine treatment induces the translocation of the NF-κB subunit p65 into the nucleus, activates COX-2 expression and production of prostaglandin E2 in bladder tissues (16). As an important anti-oxidative stress protein in the body, aldh2 has been found to suppress the production of ROS and the related toxic aldehyde product MDA, in turn inhibiting apoptosis by suppressing the NF-κB signaling pathway in human pulmonary artery smooth muscle cells (5). Based on these aforementioned findings, the present study hypothesized that aldh2 may serve a role in the development of KIC.
Materials and methods
Animal models
A total of 45 aldh2 knock-out (aldh2KO) and 60 wild-type (WT) male Institute of Cancer Research (ICR) mice (weight, 25±5 g; age, 8 weeks) were used for the present study. The aldh2KO mice were obtained from the Genomics Center Laboratory of Guangxi Medical University (Nanning, China; Fig. S1). The genotype of the mice was verified by PCR using tail tissues cut from the aldh2 KO mice (17). The aldh2 specific primer (DNA) was designed on the BLAST website (https://blast.ncbi.nlm.nih.gov/Blast.cgi): Forward, 5'-GCTGGGCTGACAAGTACCAT-3' and reverse, 5'-TTGATCAAGTTGGCCACGTA-3' (Takara Bio Technology Co., Ltd.). The aldh2 genotype was verified by Takara TaqTM Version 2.0 (Code No. R004Q; Takara Biotechnology Co., Ltd.) according to the manufacturer's instructions. The thermocycling conditions were as follows: Initial denaturation at 98˚C for 10 sec, followed by 30 cycles of 55˚C for 30 sec and 72˚C for 1 min. Agarose gel (1.5%) and ethidium bromide (0.5 µg/ml) were used in the experiment. WT mice were purchased from Hunan Slack Jingda Experimental Animal Co., Ltd. All mice were kept at 23±1˚C and 50±5% humidity, with 12 h light/dark cycles and had free access to food and water. The present study was approved by the Ethics Committee of Guangxi Medical University (approval no. 20180129).
Firstly, 15 WT mice were randomly divided into the ketamine group, which received 30 mg/kg for 4 and 8 weeks to produce the ICR mouse model or the saline group, which received normal saline (saline group) for 4 and 8 weeks, according to the ICR mouse model previously established by Yeung et al (18). Four groups were used with 3-4 WT mice within each group. Ketamine hydrochloride injections (100 mg/2 ml) were purchased from China Fujian Gutian Pharmaceutical Co., Ltd. Injections were administered by an intraperitoneal injection every day at 9 am, where an injection volume of 1 ml liquid volume (saline or ketamine solution) was used to mimic chronic ketamine abuse. All mice were weighed weekly to adjust the doses of ketamine given (30 mg/kg). Mice were sacrificed by cervical dislocation following the 4- and 8-week treatments after an anesthetic (40 mg/kg intravenous pentobarbital; Sigma-Aldrich; Merck KGaA) was administered. Bladder tissues were subsequently obtained and the expression levels of aldh2 was quantified using quantitative PCR (qPCR), as aformentioned.
In addition, 45 WT and 45 KO mice were randomly divided into the following groups: i) WT normal saline control (WNS); ii) KO normal saline control (KNS); iii) WT low-dose ketamine (WLK; 30 mg/kg); iv) KO low-dose ketamine (KLK; 30 mg/kg); v) WT high-dose ketamine (WHK; 6 mg/kg); and vi) KO high-dose ketamine (KHK; 60 mg/kg). Each group was then further divided into 3 subgroups (4, 8 and 12 weeks, 5 mice in each). Following ketamine administration, mice bladder tissues were obtained from each mouse following euthanasia at 4, 8 and 12 weeks as aforementioned.
Micturition behavior
Micturition frequency was monitored as previously described by Gu et al (19). Briefly, the short-term micturition frequency of freely moving mice was observed at the end of weeks 4, 8 and 12 following ketamine treatment. At these time-points, mice were placed in a metabolic cage containing a mesh filter pad, where the short-term urination frequency was recorded. A filter paper was soaked with a saturated copper sulfate solution (CuSO4.5H2O) which was dehydrated at 200˚C for 1 h prior to use. When the urine come to contact with the filter paper, the anhydrous CuSO4 was rehydrated and turned blue. After allowing the mice to move freely for 2 h, the filter paper was removed from the cage and the number of urination events was determined by counting the number of blue dots on the filter paper. Overlapping urination points with distinctly different edges were considered separate urination events and urine points >0.2 cm in diameter were counted (Fig. S2).
Determination of ketamine metabolites in serum and urine
A total of 1 ml of blood was obtained from the mouse's tail. Then the blood was separated by centrifugation at 2,000 x g for 10 min at 4°C. The concentration of ketamine and norketamine in the serum and urine (1 ml) was determined using high-performance liquid chromatography. Briefly, samples were collected on the day before the mice were euthanized. The final mobile phase was prepared via mixing ammonium bicarbonate solution (5 mM) adjusted with concentrated ammonia to a pH of 11.3 and acetonitrile in a ratio of 70:30. A Phenomenex LUX® AMP (Phenomenex) 3 µm, 150x4.6 mm column served as the stationary phase. All chemicals were of analytical grade. Chiral separation experiments were carried out with an Agilent 1260 Series Liquid Chromatograph (Agilent Technologies Inc.), equipped with an autosampler and a diode array detector. Each analysis was performed at ambient column temperature or a column temperature of 40°C. The measurements were performed under isocratic conditions with a flow rate of 0.5 ml/min and an injection volume of 1 µl. Ultraviolet detection was performed at 200 nm. Data evaluation was performed via a ChemStation for LC 3D Systems Rev. C. 01.07SR2 software (Agilent Technologies GmbH).
Measurement of oxidative stress parameters
Bladder tissues were quickly excised from mice and washed thoroughly with ice-cold normal PBS (pH 7.2). Tissues were sectioned into small pieces by the shear with liquid nitrogen and then homogenized using a glass homogenizer in ice-cold PBS. Next, the solution was centrifuged at 20,000 rpm (41,800 x g) for 10 min at 4°C. The supernatant was used to estimate the levels of oxidative stress indicators SOD, GSH and MDA using their respective ELISA kits (SOD, cat. no. 706002; GSH, cat. no. 703002; MDA, grant no. 700870; Cayman Chemical Company), according to the manufacturer's protocols.
Histopathology and immunohistochemical examinations
Bladder tissues were fixed in 4% phosphate-buffered paraformaldehyde for one day at room temperature, dehydrated in an ascending ethanol gradient, cleared in xylene and embedded in paraffin. The paraffinized tissues were then cut into 5-µm sections and stained with hematoxylin and eosin (H&E) and Masson's trichrome for 5 min at room temperature. Sections were then examined under a light microscope (magnification, x100, x200, x400; Olympus Corporation).
Immunohistochemical examination was performed using the Zhongshan Jinqiao Detection kit (OriGene Technologies, Inc.). Briefly, the tissue sections were deparaffinized and immersed in 3% H2O2 for 30 min to quench endogenous peroxidase activity. After being blocked with 2% BSA at room temperature for 30 min, the sections were incubated with iNOS antibody (1:400; cat. no. AF0199; Affinity Biosciences), COX-2 antibody (1:500; cat. no. 12282; Cell Signaling Technology, Inc.) overnight at 4°C. Following incubation with the primary antibodies, the tissue sections were incubated with the appropriate biotinylated secondary antibody (1:5,00; cat. no. TA130016; OriGene Technologies, Inc.) for 30 min at room temperature; after which, they were incubated with 3,3'-diaminobenzidine for 2 min and lightly counterstained with hematoxylin for 1 min at room temperature. Lastly, sections were then examined under a light microscope (magnification, x200; Olympus Corporation).
Western blotting
Harvested bladder tissues (10 mg) were homogenized in liquid nitrogen and re-suspended. The lysates were centrifuged at 14,500 x g for 15 min at 4˚C. Protein concentrations were quantified using a bicinchoninic acid Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.) and 5X protein loading buffer (Beyotime Institute of Biotechnology) was add to the supernatant (specifically 1:4). The samples (25 µg total protein/lane) were separated using 10% SDS-PAGE gel electrophoresis and then transferred onto PVDF membranes. After that, membranes were blocked with 5% BSA at room temperature for 30 min. The membranes were incubated with primary antibodies at 4˚C overnight and then washed with TBS-supplemented with 0.1% Tween-20. The primary antibodies used were as follows: iNOS (1:1,000; cat. no. 131205; Cell Signaling technology, Inc.), Aldh2 (1:1,000; cat. no. 108306; Abcam), COX-2 (1:1,000; cat. no. 12282; Cell Signaling technology, Inc.), NF-κB (1:1,500; cat. no. 8242; Cell Signaling technology, Inc.), α-smooth muscle actin (α-SMA; 1:1,000; cat. no. 68463; Cell Signaling technology, Inc.), transforming growth factor-β (TGF-β; 1:1,000; cat. no. 3711; Cell Signaling technology, Inc.), fibronectin (1:1,000; cat. no. AF5335; Affinity Biosciences) and β-actin (1:5,000; cat. no. 12262; Cell Signaling technology, Inc.). This was followed by incubation with the horseradish peroxidase (HRP)-conjugated secondary antibodies (1:1,000; cat. no. A0208; Beyotime Institute of Biotechnology) at 37˚C for 1 h. The membranes were next incubated with Novex ECL HRP chemiluminescent substrate reagent kits (Invitrogen; Thermo Fisher Scientific, Inc.) for 30 sec at room temperature and exposed to X-ray film. Protein signals were quantified by scanning densitometry using a FluorChem Q system (Alpha Innotech Corporation). The results of western blotting were quantified using Quantity One Version 4.4.0 software (Bio-Rad Laboratories, Inc.).
Reverse transcription (RT)-qPCR (RT-qPCR) Total RNA extraction and RT
Minced bladder tissues were treated with TRIzol® reagent (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol, which were fully lysed and mixed. A total of 50% by volume of chloroform was added and the upper aqueous phase was collected following centrifugation (4°C; 15,000 x g). Equal volumes of isopropanol were added and RNA was precipitated by centrifugation (4°C; 15,000 x g). RNA was washed with 70% alcohol, dried and dissolved in RNA-free enzyme water. Subsequently, the purity and concentration of the isolated RNA were detected using an ultra-micronucleic acid detector AIGS (SuiZhen Biotechnology Co., Ltd.). RNA was reverse-transcribed to cDNA using a reverse transcription kit MonAmp™ Mix (cat. no. MR05201; Monad Biotech Co., Ltd.), according to the manufacturer's protocol. Obtained cDNA was stored at -20˚C until use.
qPCR and data analysis
The qPCR reaction system consisted of 5 µl SYBR Green Premix Taq (cat. no. RN04006M; Monad Biotech Co., Ltd.), 1 µl cDNA, 0.3 µl forward primer (10 µM), 0.3 µl reverse primer (10 µM) and 3.4 µl H2O. The thermocycling conditions were as follows: Initial denaturation at 95˚C for 30 sec, followed by 40 cycles of 95˚C for 5 sec, 60˚C for 30 sec and 72˚C for 15 sec. Dissolution curve was produced using the standard dissolution curve program (Agilent Aria Software; version 1.5; Agilent Technologies, Inc.). The PCR reaction was carried out on an ABI 7500 quantitative PCR instrument (Thermo Fisher Scientific, Inc.). The relative mRNA expression data were calculated using the 2-ΔΔCq method (20). The relative expression value of a gene was normalized against the expression of Actb from control group (WNS) mRNA. The Data was analyzed using the SPSS 22.0 software (IBM Corp). Primer sequences are presented in Table I.
Table I.
Oligonucleotide sequences of the primer pairs used for reverse transcription-quantitative PCR.
| Primer | Forward sequence (5'-3') | Reverse sequence (5'-3') |
|---|---|---|
| β-actin | CAGCCTTCCTTCTTGGGTAT | TGGCATAGAGGTCTTTACGG |
| COX-2 | CAGATGACTGCCCAACTCCC | TGAACCCAGGTCCTCGCTTA |
| iNOS | TGGAGCGAGTTGTGGATTGT | GTGAGGGCTTGGCTGAGTGA |
| NF-κB | ACACGAGGCTACAACTCTGC | GGTACCCCCAGAGACCTCAT |
| α-SMA | GTACCCAGGCATTGCTGACA | GCTGGAAGGTAGACAGCGAA |
| TGF-β | AGGGCTACCATGCCAACTTC | CCACGTAGTAGACGATGGGC |
| Fibronectin | ATGAGAAGCCTGGATCCCCT | GGAAGGGTAACCAGTTGGGG |
| Aldh2 | TTCGGGGACGTAAAAGACGG | GGTGTCCTTCTCCGGCATAG |
COX-2, cyclooxygenase 2; iNOS, inducible nitric oxide synthase; α-SMA, α-smooth muscle actin; TGF-β, transforming growth factor β; aldh2, aldehyde dehydrogenase 2.
Statistical analysis
In the aldh2 gene expression experiment, 4 mice were used. While in the rest of the experiments, 5 mice were analysed. All data are presented as mean ± standard error of the mean. Statistical analyses were performed using the Graph Pad Prism software (version 7.0; GraphPad Software, Inc.). Mean differences were compared with one-way ANOVA followed by multiple comparison by Tukey's test. P<0.05 was considered to indicate a statistically significant difference.
Results
Ketamine increases aldh2 mRNA expression in WT mice
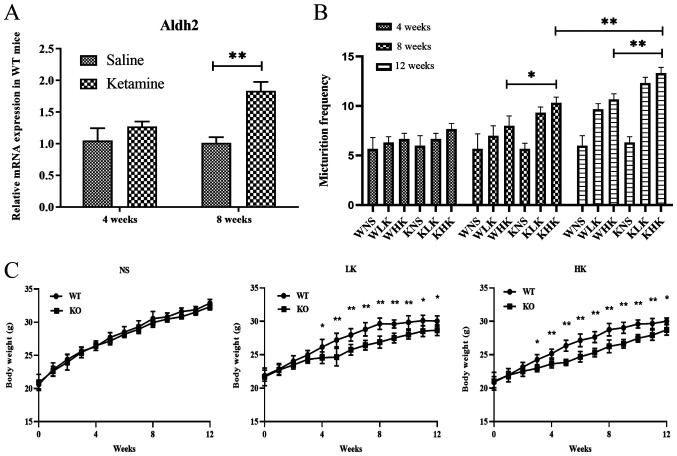
The mRNA expression levels of aldh2 in bladder tissues from WT mice were measured after ketamine treatment. At 4 weeks, the relative aldh2 mRNA levels were found to be 1.06±0.37 in the saline group and 1.38±0.15 in the ketamine group (Fig. 1A). There was no significant difference between these two groups (P=0.078). At 8 weeks, the relative mRNA levels of aldh2 in the WT mice in the ketamine treatment group was 1.84±0.24, which was significantly higher compared with 1.02±0.15 in the saline group (P<0.01; Fig. 1A). These results suggest that long-term treatment with ketamine increased the expression of aldh2 mRNA in the bladder tissues of WT mice.
Figure 1.
Ketamine treatment increases aldh2 mRNA expression in WT mice and increases urination frequency and reduces body weight in KO mice. (A) Reverse transcription-quantitative PCR was performed to measure the mRNA expression of aldh2 in WT mice at 4 and 8 weeks following saline and ketamine treatment. (B) Changes in urination frequency of KO and WT mice during 4-, 8- and 12-week ketamine treatments. (C) Weekly weight changes of KO and WT mice (each week, group WT vs. group KO). Data were presented as the mean ± standard error of the mean from ≥3 experimental repeats. *P<0.05 and **P<0.01. Aldh2, aldehyde dehydrogenase 2; WT, wild-type; KO, knock-out; WNS, wild-type normal saline control group; WLK, wild-type low-dose ketamine group; WHK, wild-type high-dose ketamine group; KNS, knock-out normal saline control group; KLK, knock-out low-dose ketamine group; KHK, knock-out high-dose ketamine group; NS, normal saline; LK, low-dose ketamine; HK, high-dose ketamine; w, week.
Ketamine treatment increases the frequency of urination and suppresses weight gain in aldh2 KO mice
Results of Aldh2 KO and WT mice were compared, which revealed that prolonged ketamine treatment caused urinary dysfunction (Fig. 1B) and suppressed weight gain (Fig. 1C) in KO mice. At week 8, the frequency of urination in the KHK group was 10.33±0.47, which was significantly higher compared with 8.00±0.82 in the WHK group (P<0.05; Fig. 1B). Similar trends were recorded at week 12 (KHK vs. WHK; 13.33±0.47 vs. 10.67±0.47; P<0.01; Fig. 1B). In terms of body weight, the weight of mice in the HK group at week 12 was 28.76±0.93 g in WT mice and 27.67±1.12 g in KO mice (P=0.032), where the average weight gain from the beginning of the experiment to week 12 was 9.12±1.03 g in WT mice and 7.68±0.69 g in KO mice (P=0.042) in the HK group (Fig. 1C). Similar results were observed in the LK group (Fig. 1C). There were no significant differences in urination frequency or body weight changes between WT and KO mice in the NS group. These results suggest that ketamine treatment significantly hindered weight gain whilst increasing urination frequency in KO mice compared with that in WT mice.
Ketamine metabolism is unaffected by aldh2 KO
The potential effects of aldh2 on ketamine metabolism in WT and aldh2 KO mice was next investigated. According to the serum and urine measurements of ketamine and norketamine, the levels in the Aldh2 KO group did not differ significantly compared with those in the WT group (Table II). These observations suggest that ketamine and its metabolites induced similar toxic effects in bladder tissues in WT and KO mice.
Table II.
Biochemical indicators of ketamine in blood and urine of mice in the different experimental groups.
| A, 4 weeks | ||||||
|---|---|---|---|---|---|---|
| C (NS) | LK (30 mg/kg) | HK (60 mg/kg) | ||||
| Indicator | Aldh2-(n=15) | Aldh2+(n=15) | Aldh2-(n=15) | Aldh2+(n=15) | Aldh2-(n=15) | Aldh2+(n=15) |
| Serum Ket (ng/ml) | ND | ND | ND | ND | ND | ND |
| Serum Nket (ng/ml) | ND | ND | ND | ND | ND | ND |
| Urine Ket (ng/ml) | ND | ND | 868±95.9 | 896±94.3 | 1068±98.6 | 1,123±95.3 |
| Urine Nket (ng/ml) | ND | ND | 2,2681±734.2 | 2,0248±870.4 | 23,425±726.9 | 24,786±102.3 |
| B, 8 weeks | ||||||
| C (NS) | LK (30 mg/kg) | HK (60 mg/kg) | ||||
| Indicator | Aldh2-(n=15) | Aldh2+(n=15) | Aldh2-(n=15) | Aldh2+(n=15) | Aldh2-(n=15) | Aldh2+(n=15) |
| Serum Nket (ng/ml) | ND | ND | ND | ND | ND | ND |
| Urine Ket (ng/ml) | ND | ND | 1,076±103.2 | 998±87.3 | 1,324±112.3 | 1,238±109.3 |
| Urine Nket (ng/ml) | ND | ND | 24,564±924.3 | 23,652±898.3 | 27,347±923.4 | 25,863±954.2 |
| C, 12 weeks | ||||||
| C (NS) | LK (30 mg/kg) | HK (60 mg/kg) | ||||
| Indicator | Aldh2-(n=15) | Aldh2+(n=15) | Aldh2-(n=15) | Aldh2+(n=15) | Aldh2-(n=15) | Aldh2+(n=15) |
| Serum Nket (ng/ml) | ND | ND | 1.2±0.32 | 2.3±0.26 | 4.6±0.67 | 3.2±0.34 |
| Urine Ket (ng/ml) | ND | ND | 1,545±162.7 | 1,432±93.3 | 1,636±156.8 | 1,523±196.2 |
| Urine Nket (ng/ml) | ND | ND | 23,691±1,423.3 | 26,538±1,397.2 | 28,877±1,452.3 | 29,376±1,254.8 |
Data are presented as the mean ± standard error of the mean. Ket, ketamine; nket, norketamine; KO, knock-out; WT, wild-type; -, KO; +, WT; C, control; NS, normal saline; LK, low-dose ketamine; HK, high-dose ketamine; Aldh2, aldehyde dehydrogenase 2; Ket, ketamine; Nket, norketamine; ND, not detected.
Ketamine increases oxidative stress parameters in aldh2 KO mice
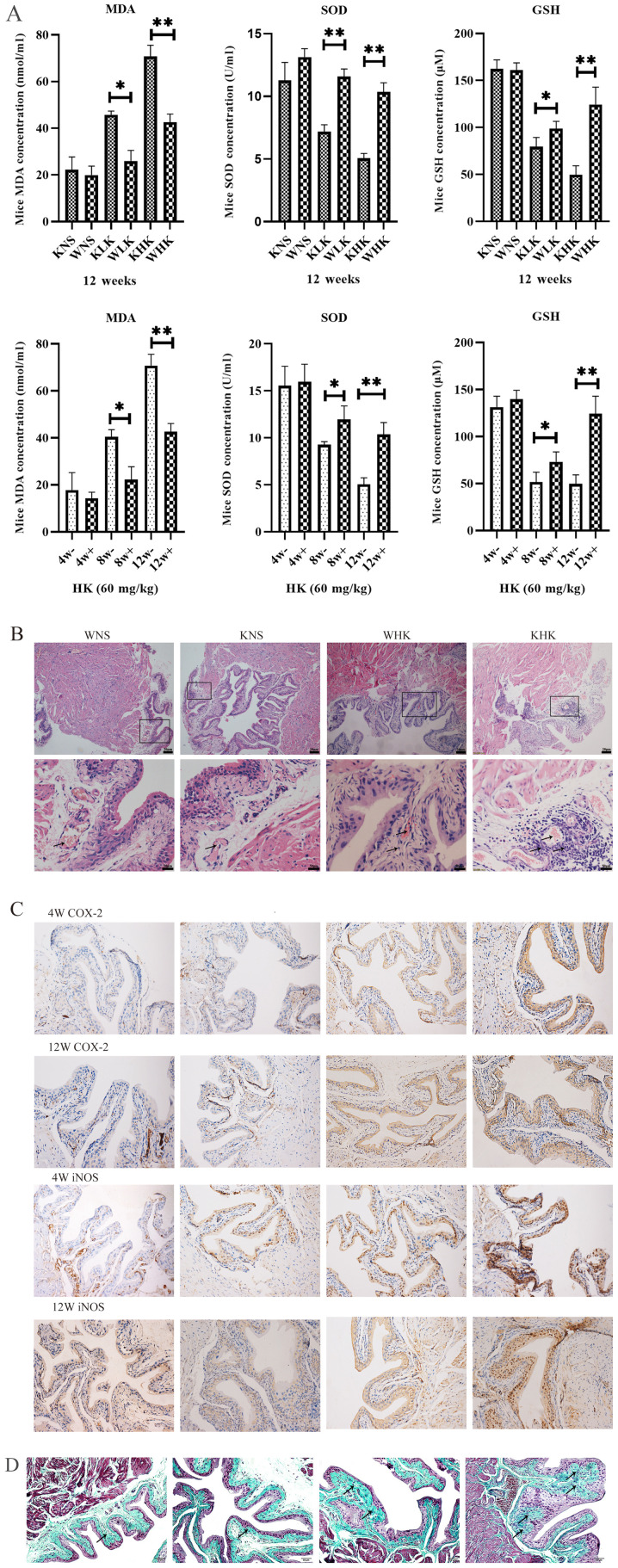
ELISA were performed to quantify the levels of oxidative stress factors in bladder tissues. Results in Fig. 2A demonstrated the effects of different treatment periods and doses of ketamine on oxidative stress in the mouse bladder tissues at 12 weeks in all groups and at 4, 8 and 12 weeks in the HK group. These assays reported that levels of anti-oxidation factors SOD (P<0.01) and GSH (P<0.01) were significantly decreased, whilst levels of the lipid peroxidation indicator MDA (P<0.01) was significantly increased in aldh2 KO mice compared with those in the WT mice at week 12 in the HK group. At week 12, the KHK group exhibited a significant decline in the level of SOD (5.05±0.56 U/ml) and GSH (49.75±13.45 µM) and a significant increase in MDA (70.79±8.28 nmol/ml) in bladder tissues compared with WHK (SOD, 10.35±1.04 U/ml; P<0.01; GSH, 124.49±26.02 µM; P<0.05; MDA, 41.97±5.01 nmol/ml; P<0.01).
Figure 2.
Ketamine increases the level of oxidative stress in aldh2 KO mice and aggravates pathological damage. (A) Effects of ketamine on parameters of oxidative stress in WT and KO mice at 4, 8 and 12 weeks as detected by ELISA. Negative control mice were treated with NS. *P<0.05 and **P<0.01. KO, knock-out; NS, normal saline; KHK, knock-out high-dose ketamine group; SOD, superoxide dismutase; GSH, glutathione-sulfhydryl; MDA, malondialdehyde; WHK, wild-type high-dose ketamine group; COX-2, cyclooxygenase 2; iNOS, inducible nitric oxide synthase; WT, wild-type; KNS, knock-out normal saline control group; WNS, wild-type normal saline control group; KLK, knock-out low-dose ketamine group; WLK, wild-type low-dose ketamine group; HK, high-dose ketamine; W, week; -, knock-out; +, wild-type. Ketamine increases the level of oxidative stress in aldh2 KO mice and aggravates pathological damage. (B) Representative hematoxylin and eosin staining images of bladder tissues from KO and WT mice in week 12. Magnification, x100 for the upper images; x400 for the lower images. (C) Representative immunohistochemical staining images of COX-2 and iNOS proteins in the bladder tissues of KO and WT mice in weeks 4 and 12. The cytoplasm and cell membranes exhibiting brown-yellow colors were considered as positive expression of the target protein, which were mainly confined to the bladder epithelium Magnification, x200. (D) Representative Masson trichrome staining images of bladder tissues of KO and WT mice in week 12. Collagen fibers stained green, muscle fibers stained red, and nucleus stained blue-brown. Magnification, x200. Data are presented as the mean ± standard error of the mean from ≥3 experimental repeats. *P<0.05 and **P<0.01. KO, knock-out; NS, normal saline; KHK, knock-out high-dose ketamine group; SOD, superoxide dismutase; GSH, glutathione-sulfhydryl; MDA, malondialdehyde; WHK, wild-type high-dose ketamine group; COX-2, cyclooxygenase 2; iNOS, inducible nitric oxide synthase; WT, wild-type; KNS, knock-out normal saline control group; WNS, wild-type normal saline control group; KLK, knock-out low-dose ketamine group; WLK, wild-type low-dose ketamine group; HK, high-dose ketamine; W, week; -, knock-out; +, wild-type.
Ketamine induces more severe pathological damage in KO mice
At week 12, modest inflammatory cell infiltration and massive intravascular congestion were observed via H&E staining in the submucosal layer of the bladder tissues of mice in the WHK group (Fig. 2B). In tissues from mice in the KHK group, this appeared to be more severe, where the mucosal barrier had disintegrated with numerous inflammatory cells accumulated in the submucosa and extensive edema in the bladder mucosa (Fig. 2B). No edema could be observed in the bladder walls of tissues from the WNS and KNS control groups, where the bladder mucosal barrier was intact (Fig. 2B). All these qualitative descriptions were pointed out by using arrows. Immunohistochemical analysis revealed that COX-2 and iNOS protein expression in KO mice was enhanced beneath the bladder mucosa compared with WT mice (Fig. 2C). In week 4, COX-2 protein expression in KHK group increased more than WHK and iNOS protein expression in KHK and WHK group showed little difference. When in week 12, the staining densities of COX-2 and iNOS in the KHK group were markedly higher compared with those in the WHK group (Fig. 2C). Masson trichome staining of the bladder tissues revealed that the bladder submucosa and muscular layers were not notably affected in the WNS and KNS groups at week 12 (Fig. 2D). By contrast, the lamina propria and submucosa structure had disintegrated in the WHK and KHK groups, where the tissues exhibited diffuse interstitial fibrosis. Furthermore, the area infiltrated by collagen fibers was larger and the thickening of the knot tissues was clearer in the KHK group compared with that in the WHK group. All these qualitative descriptions are presented by arrows.
Inflammation of ketamine-induced cystitis is more severe in KO mice
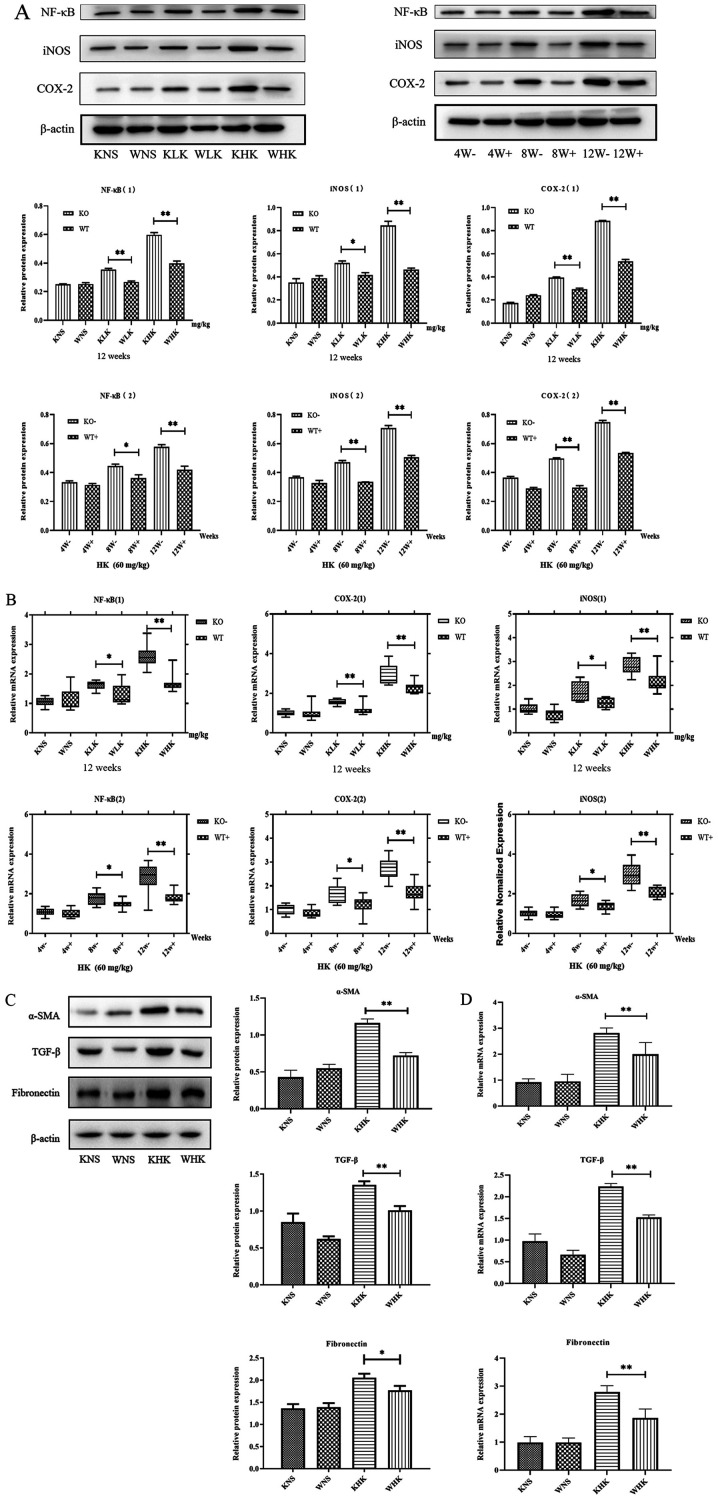
Furthermore, the expression of NF-κB, iNOS and COX-2, indicators associated with inflammation, was verified by measuring their protein and mRNA levels. The results demonstrated that in the KHK group, the protein expression levels of COX-2, iNOS and NF-κB were increased by 1.63 (P<0.01), 1.41 (P<0.01) and 1.51 (P<0.01) fold, respectively, compared with those in the WHK group in week 12, in a concentration-dependent manner (Fig. 3A). In addition, when comparing the LK groups (week 12) or week 8 (HK groups), the results also demonstrated that NF-κB (P<0.05), iNOS (P<0.05) and COX-2 (P<0.01) protein expression was enhanced in KO mice compared with those in WT mice. At 12 weeks in the HK group, the mRNA levels of NF-κB, COX-2 and iNOS were significantly higher (all P<0.01) in the KO group (2.620±0.350, 2.903±0.188 and 3.307±0.242, respectively) compared with those in the WT group (1.884±0.311, 1.787±0.116 and 2.083±0.286, respectively; Fig. 3B). Furthermore, when comparing LK groups (week 12) or week 8 (HK groups), the results also demonstrated that NF-κB (P<0.05), iNOS (P<0.05) and COX-2 (P<0.05) protein expressions were enhanced in KO mice compared with those in WT mice.
Figure 3.
Ketamine treatment induces inflammation and fibrosis in KO mice. (A) Effects of ketamine on inflammation protein COX-2, iNOS and NF-κB levels in mice. *P<0.05 and **P<0.01. Data are presented as the mean ± standard error of the mean from ≥3 experimental repeats. KO, knock-out; KHK, KO high-dose ketamine group; WHK, wild-type high-dose ketamine group; COX-2, cyclooxygenase 2; iNOS, inducible nitric oxide synthase; WT, wild-type; α-SMA, α-smooth muscle actin; TGF-β, transforming growth factor β; KNS, knock-out normal saline control group; WNS, wild-type normal saline control group; KLK, knock-out low-dose ketamine group; WLK, wild-type low-dose ketamine group; W, weeks; -, knock-out; +, wild-type; HK, high-dose ketamine. Ketamine treatment induces inflammation and fibrosis in KO mice. (B) mRNA expression levels of inflammatory proteins COX-2, iNOS and NF-κB in the mouse bladder tissues were detected by reverse transcription-quantitative PCR. (C) Western blotting and (D) reverse transcription-quantitative PCR were performed to measure the expression of α-SMA, TGF-β and fibronectin in mouse bladder tissues at week 12. *P<0.05 and **P<0.01. Data are presented as the mean ± standard error of the mean from ≥3 experimental repeats. KO, knock-out; KHK, KO high-dose ketamine group; WHK, wild-type high-dose ketamine group; COX-2, cyclooxygenase 2; iNOS, inducible nitric oxide synthase; WT, wild-type; α-SMA, α-smooth muscle actin; TGF-β, transforming growth factor β; KNS, knock-out normal saline control group; WNS, wild-type normal saline control group; KLK, knock-out low-dose ketamine group; WLK, wild-type low-dose ketamine group; W, weeks; -, knock-out; +, wild-type; HK, high-dose ketamine.
Fibrosis levels in ketamine-induced cystitis is more severe in KO mice
Additionally, the protein expression levels of fibrotic markers α-SMA (P<0.01), TGF-β (P<0.01) and fibronectin (P<0.05) were found to be significantly higher in mice in the KHK group compared with those in mice in the WHK group (Fig. 3C), consistent with changes in the expression of inflammatory proteins in KO and WT mice. Subsequently, RT-qPCR analysis revealed that the mRNA expression levels of α-SMA, TGF-β and fibronectin in KHK vs. WHK mice were 2.814±0.342 vs. 1.967±0.536, 2.237±0.118 vs. 1.524±0.102 and 2.791±0.395 vs. 1.866±0.551, respectively (all P<0.01) in week 12.
Discussion
To the best of our knowledge, the present study is the first to investigate the impact of the aldh2 gene on ketamine-induced cystitis. Aldh2 is a metabolic enzyme of certain aldehydes, including 4-hydroxynonenal and MDA, which has a number of enzymatic functions, including dehydrogenase and esterase. In addition, aldh2 oxidizes 4-hydroxynonenal, MDA and other oxidation products and converts them into non-toxic acids, thereby reducing the degree of inflammation and inhibiting apoptosis. Using this function, aldh2 has been previously reported to protect the kidneys, liver, heart and other organs from damage (21). Hu et al (22) demonstrated that inhibiting aldh2 expression aggravated the inflammatory reaction in rats with sepsis, increasing kidney damage. In another study, Wimborne et al (23) previously revealed that activation of aldh2 reduced the hepatotoxic effects of ethanol and acetaminophen. Additionally, Xu et al (24) reported that aldh2 prevented myocardial damage associated with pulmonary hypertension. These previous studies demonstrated that that aldh2 served important functions in regulating inflammation leading up to organ damage. The low expression of aldh2 was found to associate closely with renal fibrosis, myocardial fibrosis and urinary tract fibrosis caused by urothelial tumors (25-27). Tang et al (25) revealed that aldh2 can be used as a common potential genetic target for prognosis of various renal fibrosis diseases (renal fibrosis caused by unilateral ureteral obstruction, ischemia-reperfusion injury or cisplatin-induced). Mali et al (26) previously discovered that heart damage and myocardial fibrosis as a result of chronic hyperglycemia are associated with reduced aldh2 activity. A whole genome analysis conducted by Wu et al (27) demonstrated aldh2 is a potential prognostic marker for urothelial carcinoma, where aldh2 expression associated with that of urothelial fibrosis genes. However, the mechanism of aldh2 in the development of KIC remains unclear. Due to its significant anti-inflammatory and antifibrotic effects, the present study hypothesized that aldh2 has a high probability of being a novel KIC prevention target.
The results of the present study demonstrated that following long-term treatment of ketamine, the expression of aldh2 in mice was significantly upregulated. Previous studies have reported that the pathogenesis of KIC is associated with inflammation and fibrosis (28,29). Therefore, the present study hypothesized that following long-term treatment of ketamine, the aldh2 gene may be upregulated to protect against adverse factors, including oxidative stress, inflammation and fibrosis in vivo. To investigate this, the long-term effects of ketamine on aldh2 KO mice were compared with those in WT mice.
KIC is a dynamic and complex process that is closely associated with oxidative stress, which is in turn associated with excessive ROS production (4). The effects of ROS on micturition reflexes have been previously demonstrated in several pathological states of the bladder, including cyclophosphamide-induced hemorrhagic cystitis, ionizing radiation cystitis and partial bladder outlet obstruction (30). High intracellular levels of ROS and the imbalance between the oxidative and antioxidant systems promotes lipid peroxidation and increases the formation of aldehydes, including that of MDA, which is the end product in kidney tissues. Additionally, GSH and SOD inactivate ROS production in the mitochondria by enhancing the activity of catalase in peroxisomes. Therefore, any aberrant changes in GSH and SOD can cause serious damage to cellular DNA, lipids and proteins (5). The present study demonstrated that under the same doses of ketamine, the levels of oxidative stress in aldh2 KO mice were significantly higher compared with that in WT mice. In addition, ketamine treatment significantly reduced the expression of antioxidant enzymes whilst enhancing the production of MDA in aldh2 KO mice.
During KIC, ROS stimulates NF-κB activation, leading to downstream signaling pathways to induce tissue damage (31,32). Generally, NF-κB is inactive under physiological conditions. Once activated by phosphorylation, NF-κB dissociates from its inhibitory unit, nuclear factor of κ light polypeptide gene enhancer in B-cells inhibitor β, freeing the active p65-NF-κB. p65-NF-κB then migrates to the nucleus, where it binds to its promoter sequence to activate the expression of pro-inflammatory cytokines (33). In a previous study, hyaluronan instillation treatment significantly inhibited the activation of the NF-κB signaling pathway by suppressing oxidative stress (34). The results of the present study revealed that the expression of the active NF-κB unit in aldh2 KO mice was higher compared with that in WT, indicating a more stressful condition. Treating aldh2 KO mice with ketamine activated the NF-κB pathway, which may be associated with the absence of aldh2 in its anti-oxidative stress effects. COX-2 and iNOS are sensitive markers of inflammation caused by oxidative stress (35). Previous studies have demonstrated that COX-2 regulated the inflammatory response in rats with KIC via the NF-κB pathway (15,31), such that COX-2 overexpression can serve an important role in altering the urinary pattern during KIC (36). Numerous previous studies on cyclophosphamide-induced cystitis (37,38) revealed the role of COX-2 in bladder overactivity, which is associated with inflammation or hypertrophy. Additionally, treatment with COX-2 inhibitors has been demonstrated to improve ifosfamide-induced bladder injury and alter urinary overactivity (39). iNOS is usually expressed in macrophages. When activated by pathogens or cytokines, such as ROS, it synthesizes nitric oxide (NO) to promote cell apoptosis (40). A previous study reported that iNOS was upregulated in cyclophosphamide-induced cystitis (38). Other studies have previously revealed that ketamine or its urinary metabolites exerted direct toxic effects on bladder epithelial cells due to the activation of iNOS in the mitochondria and the resulting high levels of NO in urine (19,36). In the present study, COX-2 and iNOS were found to be highly expressed in aldh2 KO mice.
Numerous animal studies have demontrated that long-term ketamine treatment can lead to bladder fibrosis, which is an important cause of bladder abnormalities, including decreased capacity, lower compliance and impaired detrusor function (41,42). Song et al (41) demonstrated that the inflammatory mediators in KIC increased the expression of collagen type-I (COL-I) and α-SMA, which leads to thickening of the bladder basement membrane. Furthermore, Shen et al (42) conducted whole genome analysis and reported that the expression of fibrotic genes, including fibronectin, TGF-β1 and COL-I, were upregulated in bladder tissues with KIC. This enriched expression was considered to be a sensitive marker of active fibrosis development. The present study revealed that compared with WT mice, the severity of ketamine-induced fibrosis in aldh2 KO mice was considerably higher and the expression of α-SMA, TGF-β1 and fibronectin were significantly higher. These results indicated that the expression of aldh2 alleviates bladder fibrosis induced by KIC. Due to the effects of inflammation and fibrosis in vivo, aldh2 KO mice gained weight slower and urinated more frequently compared with that in WT mice. This may be due to the chronic physiological stress caused by inflammation and fibrosis, which were increased in aldh2 KO mice.
The results of the present study revealed that aldh2 inhibited the NF-κB signaling pathway, thereby preventing fibrosis by suppressing lipid peroxidation in KIC and reducing COX-2 and iNOS expression. Data from the present study advises that Asian people should refrain from ingesting ketamine, since they are at a higher risk of developing mutations in the aldh2 gene, which can result in the development of severe LUTS and bladder contracture. Future studies should include cellular tests and crowd verification experiments to validate the results of the present study. Future studies are also required to clarify whether ketamine-induced cystitis is race-specific.
Supplementary Material
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81860142) and the Natural Science Foundation of Guangxi Zhuang Autonomous Region (grant no. 2017GXNSFAA198279).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author's contributions
XJX and HM designed this study. XJX and SHC performed the experiments. XJX, SHC and HM performed the statistical analysis. XJX and HM drafted and revised the manuscript. HM contributed reagents, materials and experimental platforms. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Guangxi Medical University (approval no. 20180129).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wang JW, Kivovich V, Gordon L. Ketamine abuse syndrome: Hepatobiliary and urinary pathology among adolescents in Flushing, NY. Pediatr Emerg Care. 2017;33:e24–e26. doi: 10.1097/PEC.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 2.Persson J. Wherefore ketamine? Curr Opin Anaesthesiol. 2010;23:455–60. doi: 10.1097/ACO.0b013e32833b49b3. [DOI] [PubMed] [Google Scholar]
- 3.Parkin MC, Turfus SC, Smith NW, Halket JM, Braithwaite RA, Elliott SP, Osselton MD, Cowan DA, Kicman AT. Detection of ketamine and its metabolites in urine by ultra high pressure liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;876:137–142. doi: 10.1016/j.jchromb.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 4.Liu KM, Chuang SM, Long CY, Lee YL, Wang CC, Lu MC, Lin RJ, Lu JH, Jang MY, Wu WJ, et al. Ketamine-induced ulcerative cystitis and bladder apoptosis involve oxidative stress mediated by mitochondria and the endoplasmic reticulum. Am J Physiol Renal Physiol. 2015;309:F318–F331. doi: 10.1152/ajprenal.00607.2014. [DOI] [PubMed] [Google Scholar]
- 5.Xu T, Liu S, Ma T, Jia Z, Zhang Z, Wang A. Aldehyde dehydrogenase 2 protects against oxidative stress associated with pulmonary arterial hypertension. Redox Biol. 2017;11:286–296. doi: 10.1016/j.redox.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bondeva T, Wolf G. Reactive oxygen species in diabetic nephropathy: Friend or foe? Nephrol Dial Transplant. 2014;29:1998–2003. doi: 10.1093/ndt/gfu037. [DOI] [PubMed] [Google Scholar]
- 7.Kim MJ, Lim Y. Protective effect of short-term genistein supplementation on the early stage in diabetes-induced renal damage. Mediators Inflamm. 2013;2013(510212) doi: 10.1155/2013/510212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Choi YJ, Kim HS, Lee J, Chung J, Lee JS, Choi JS, Yoon TR, Kim HK, Chung HY. Down-regulation of oxidative stress and COX-2 and iNOS expressions by dimethyl lithospermate in aged rat kidney. Arch Pharm Res. 2014;37:1032–1038. doi: 10.1007/s12272-014-0332-6. [DOI] [PubMed] [Google Scholar]
- 10.Lin HC, Lee HS, Chiueh TS, Lin YC, Lin HA, Lin YC, Cha TL, Meng E. Histopathological assessment of inflammation and expression of inflammatory markers in patients with ketamine-induced cystitis. Mol Med Rep. 2015;11:2421–2428. doi: 10.3892/mmr.2014.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge W, Guo R, Ren J. AMP-dependent kinase and autophagic flux are involved in aldehyde dehydrogenase-2-induced protection against cardiac toxicity of ethanol. Free Radic Biol Med. 2011;51:1736–48. doi: 10.1016/j.freeradbiomed.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke TK, Adams MJ, Davies G, Howard DM, Hall LS, Padmanabhan S, Murray AD, Smith BH, Campbell A, Hayward C, et al. Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N=112 117) Mol Psychiatry. 2017;22:1376–1384. doi: 10.1038/mp.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu S, Chen J, Dong P, Zhang S, He Y, Sun L, Zhu J, Cheng Y, Li X, Tang A, et al. Global gene expression profiling identifies ALDH2, CCNE1 and SMAD3 as potential prognostic markers in upper tract urothelial carcinoma. BMC Cancer. 2014;14(836) doi: 10.1186/1471-2407-14-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrew AS, Gui J, Hu T, Wyszynski A, Marsit CJ, Kelsey KT, Schned AR, Tanyos SA, Pendleton EM, Ekstrom RM, et al. Genetic polymorphisms modify bladder cancer recurrence and survival in a USA population-based prognostic study. BJU Int. 2015;115:238–247. doi: 10.1111/bju.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira-Teixeira M, Parada B, Rodrigues-Santos P, Alves V, Ramalho JS, Caramelo F, Sousa V, Reis F, Gomes CM. Functional and molecular characterization of cancer stem-like cells in bladder cancer: A potential signature for muscle-invasive tumors. Oncotarget. 2015;6:36185–201. doi: 10.18632/oncotarget.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuang SM, Lu JH, Lin KL, Long CY, Lee YC, Hsiao HP, Tsai CC, Wu WJ, Yang HJ, Juan YS. Epigenetic regulation of COX2 expression by DNA hypomethylation via NFkappaB activation in ketamineinduced ulcerative cystitis. Int J Mol Med. 2019;44:797–812. doi: 10.3892/ijmm.2019.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin S, Chen J, Chen L, Histen G, Lin Z, Gross S, Hixon J, Chen Y, Kung C, Chen Y, et al. ALDH2(E487K) mutation increases protein turnover and promotes murine hepatocarcinogenesis. Proc Natl Acad Sci USA. 2015;112:9088–9093. doi: 10.1073/pnas.1510757112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeung LY, Rudd JA, Lam WP, Mak YT, Yew DT. Mice are prone to kidney pathology after prolonged ketamine addiction. Toxicol Lett. 2009;191:275–278. doi: 10.1016/j.toxlet.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Gu D, Huang J, Yin Y, Shan Z, Zheng S, Wu P. Long-term ketamine abuse induces cystitis in rats by impairing the bladder epithelial barrier. Mol Biol Rep. 2014;41:7313–22. doi: 10.1007/s11033-014-3616-5. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Choi JW, Kim JH, Cho SC, Ha MK, Song KY, Youn HD, Park SC. Malondialdehyde inhibits an AMPK-mediated nuclear translocation and repression activity of ALDH2 in transcription. Biochem Biophys Res Commun. 2011;404:400–406. doi: 10.1016/j.bbrc.2010.11.131. [DOI] [PubMed] [Google Scholar]
- 22.Hu JF, Wang HX, Li HH, Hu J, Yu Y, Gao QQ. Inhibition of ALDH2 expression aggravates renal injury in a rat sepsis syndrome model. Exp Ther Med. 2017;14:2249–2254. doi: 10.3892/etm.2017.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wimborne HJ, Hu J, Takemoto K, Nguyen N, Jaeschke H, Lemasters JJ, Zhong Z. Aldehyde dehydrogenase-2 activation decreases acetaminophen hepatotoxicity by prevention of mitochondrial depolarization. Toxicol Appl Pharmacol. 2020;396(114982) doi: 10.1016/j.taap.2020.114982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu T, Liu SY, Ma TT, Jia ZY, Zhang ZF, Wang AM. Aldehyde dehydrogenase 2 protects against oxidative stress associated with pulmonary arterial hypertension. Redox Biol. 2017;11:286–296. doi: 10.1016/j.redox.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang S, Huang T, Jing H, Huang Z, Chen H, Fan Y, Zhong J, Zhou J. Aldehyde dehydrogenase-2 acts as a potential genetic target for renal fibrosis. Life Sci. 2019;239(117015) doi: 10.1016/j.lfs.2019.117015. [DOI] [PubMed] [Google Scholar]
- 26.Mali VR, Pan G, Deshpande M, Thandavarayan RA, Xu J, Yang XP, Palaniyandi SS. Cardiac mitochondrial respiratory dysfunction and tissue damage in chronic hyperglycemia correlate with reduced aldehyde Dehydrogenase-2 activity. PLoS One. 2016;11(e0163158) doi: 10.1371/journal.pone.0163158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu S, Chen JH, Dong P, Zhang SQ, He YY, Sun L, Zhu JL, Cheng YB, Li XX, Tang AF, et al. Global gene expression profiling identifies ALDH2, CCNE1 and SMAD3 as potential prognostic markers in upper tract urothelial carcinoma. BMC Cancer. 2014;14(836) doi: 10.1186/1471-2407-14-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q, Wu Q, Wang J, Chen Y, Zhang G, Chen J, Zhao J, Wu P. Ketamine analog methoxetamine induced inflammation and dysfunction of bladder in rats. Int J Mol Sci. 2017;18(117) doi: 10.3390/ijms18010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim A, Yu HY, Heo J, Song M, Shin JH, Lim J, Yoon SJ, Kim Y, Lee S, Kim SW, et al. Mesenchymal stem cells protect against the tissue fibrosis of ketamine-induced cystitis in rat bladder. Sci Rep. 2016;6(30881) doi: 10.1038/srep30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiba K, Yamaguchi K, Ando M, Miyake H, Fujisawa M. Expression pattern of testicular claudin-11 in infertile men. Urology. 2012;80:1161.e13–e17. doi: 10.1016/j.urology.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 31.Juan YS, Lee YL, Long CY, Wong JH, Jang MY, Lu JH, Wu WJ, Huang YS, Chang WC, Chuang SM. Translocation of NF-κB and expression of cyclooxygenase-2 are enhanced by ketamine-induced ulcerative cystitis in rat bladder. Am J Pathol. 2015;185:2269–2285. doi: 10.1016/j.ajpath.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 32.Xi XJ, Zeng JJ, Lu Y, Chen SH, Jiang ZW, He PJ, Mi H. Extracellular vesicles enhance oxidative stress through P38/NF-kB pathway in ketamine-induced ulcerative cystitis. J Cell Mol Med. 2020;24:7609–7624. doi: 10.1111/jcmm.15397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Q, Lenardo MJ, Baltimore D. 30 years of NF-κB: A blossoming of relevance to human pathobiology. Cell. 2017;168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee YL, Lin KL, Chuang SM, Lee YC, Lu MC, Wu BN, Wu WJ, Yuan SF, Ho WT, Juan YS. Elucidating mechanisms of bladder repair after hyaluronan instillation in ketamine-induced ulcerative cystitis in animal model. Am J Pathol. 2017;187:1945–1959. doi: 10.1016/j.ajpath.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Arena A, Zimmer TS, van Scheppingen J, Korotkov A, Anink JJ, Mühlebner A, Jansen FE, van Hecke W, Spliet WG, van Rijen PC, et al. Oxidative stress and inflammation in a spectrum of epileptogenic cortical malformations: Molecular insights into their interdependence. Brain Pathol. 2019;29:351–365. doi: 10.1111/bpa.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chuang SM, Liu KM, Li YL, Jang MY, Lee HH, Wu WJ, Chang WC, Levin RM, Juan YS. Dual involvements of cyclooxygenase and nitric oxide synthase expressions in ketamine-induced ulcerative cystitis in rat bladder. Neurourol Urodyn. 2013;32:1137–1143. doi: 10.1002/nau.22367. [DOI] [PubMed] [Google Scholar]
- 37.Hu VY, Malley S, Dattilio A, Folsom JB, Zvara P, Vizzard MA. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;284:R574–R585. doi: 10.1152/ajpregu.00465.2002. [DOI] [PubMed] [Google Scholar]
- 38.Oter S, Korkmaz A, Oztas E, Yildirim I, Topal T, Bilgic H. Inducible nitric oxide synthase inhibition in cyclophosphamide induced hemorrhagic cystitis in rats. Urol Res. 2004;32:185–189. doi: 10.1007/s00240-003-0398-y. [DOI] [PubMed] [Google Scholar]
- 39.Macedo FY, Mour ão LT, Palheta RC Jr, Juca DM, Lima RC Jr, Neto Jde S, Magalhaes PJ, Santos AA, Souza MH, Brito GA, Ribeiro RA. Cyclooxygenase-2 contributes to functional changes seen on experimental hemorrhagic cystitis induced by ifosfamide in rat urinary bladder. Cancer Chemother Pharmacol. 2011;67:935–943. doi: 10.1007/s00280-010-1392-z. [DOI] [PubMed] [Google Scholar]
- 40.Kumar A, Singh KP, Bali P, Anwar S, Kaul A, Singh OP, Gupta BK, Kumari N, Noor Alam M, Raziuddin M, et al. iNOS polymorphism modulates iNOS/NO expression via impaired antioxidant and ROS content in P. vivax and P. falciparum infection. Redox Biol. 2018;15:192–206. doi: 10.1016/j.redox.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song M, Yu HY, Chun JY, Shin DM, Song SH, Choo MS, Song YS. The fibrosis of ketamine, a noncompetitive N-methyl-d-aspartic acid receptor antagonist dose-dependent change in a ketamine-induced cystitis rat model. Drug Chem Toxicol. 2016;39:206–212. doi: 10.3109/01480545.2015.1079916. [DOI] [PubMed] [Google Scholar]
- 42.Shen CH, Wang SC, Wang ST, Lin SM, Wu JD, Lin CT, Liu YW. Evaluation of urinary bladder fibrogenesis in a mouse model of long-term ketamine injection. Mol Med Rep. 2016;14:1880–1890. doi: 10.3892/mmr.2016.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.