Abstract
Background
Toxoplasma gondii is an obligate parasite of all warm-blooded animals around the globe. Once infecting a cell, it manipulates the host’s DNA damage response that is yet to be elucidated. The objectives of the present study were three-fold: (i) to assess DNA damages in T. gondii-infected cells in vitro; (ii) to ascertain causes of DNA damage in T. gondii-infected cells; and (iii) to investigate activation of DNA damage responses during T. gondii infection.
Methods
HeLa, Vero and HEK293 cells were infected with T. gondii at a multiplicity of infection (MOI) of 10:1. Infected cells were analyzed for a biomarker of DNA double-strand breaks (DSBs) γH2AX at 10 h, 20 h or 30 h post-infection using both western blot and immunofluorescence assay. Reactive oxygen species (ROS) levels were measured using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA), and ROS-induced DNA damage was inhibited by a ROS inhibitor N-acetylcysteine (NAC). Lastly, DNA damage responses were evaluated by detecting the active form of ataxia telangiectasia mutated/checkpoint kinase 2 (ATM/CHK2) by western blot.
Results
γH2AX levels in the infected HeLa cells were significantly increased over time during T. gondii infection compared to uninfected cells. NAC treatment greatly reduced ROS and concomitantly diminished γH2AX in host cells. The phosphorylated ATM/CHK2 were elevated in T. gondii-infected cells.
Conclusions
Toxoplasma gondii infection triggered DNA DSBs with ROS as a major player in host cells in vitro. It also activated DNA damage response pathway ATM/CHK2. Toxoplasma gondii manages to keep a balance between survival and apoptosis of its host cells for the benefit of its own survival. 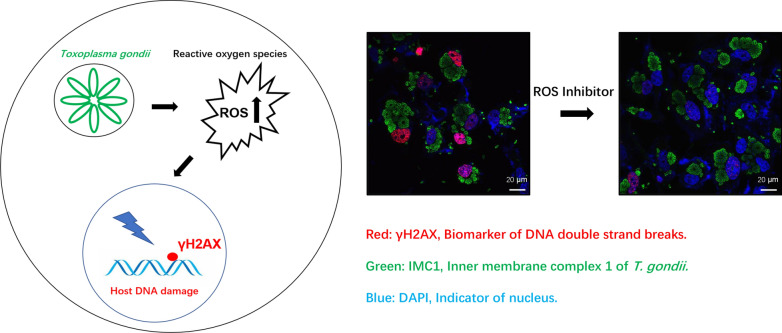
Keywords: Toxoplasma gondii, DNA damage, Reactive oxygen species, DNA damage response
Background
The protozoan parasite Toxoplasma gondii infects almost all warm-blooded animals including humans worldwide [1]. It modulates some biological processes of the infected cell, such as autophagy and apoptosis, to facilitate its survival and proliferation [2, 3]. DNA damage was also reported in the retina and the peripheral blood cells of T. gondii-infected mice [4, 5]. However, it remains to be elucidated how T. gondii causes host DNA damage and what the DNA damage responses are. DNA damage appears in different forms such as single-strand breaks (SSBs), double-strand breaks (DSBs), missing bases and chemical modification of bases, etc. [6]. DNA damage may be caused by various endogenous or exogenous factors. Examples are reactive oxygen species (ROS) and ultraviolet light and chemical reagents, respectively [7].
ROS are naturally generated from metabolic and biochemical reactions and are a major source of the endogenous stress [8]. Superoxide, hydrogen peroxide, hydroxyl radicals and singlet oxygen are all ROS. High levels of ROS can oxidize DNA molecules leading to base oxidization, SSBs and DSBs [9]. Increasing lines of evidence clearly show that ROS are triggered by bacterial, viral and parasitic pathogens in host cells during infection [10]. Further, ROS affect DNA integrity of host cells although it is debatable whether ROS are always detrimental to infecting pathogens [11].
According to its virulence in the mouse model, the protozoan T. gondii is divided into three vastly different virulent groups, i.e. Type I, II and III. Interestingly, infections by both avirulent Type III (CTG strain) and virulent Type I (GT1 strain) trigger ROS production in the infected macrophages. CTG-strain parasites are mainly cleared by the naive macrophages in a manner depending on NADPH oxidase-generated ROS without activation of interferon gamma [12]. Due to ROS’ potential detrimental impact on DNA, we explored the association between elevated ROS levels and host DNA damage during T. gondii infection. We used γH2AX, a well-characterized marker for DNA DSBs, to demonstrate DNA damage in T. gondii-infected HeLa cells. γH2AX levels increased over time during T. gondii infection and ROS was the major player in the DNA DSBs. Furthermore, the DNA damage response pathway ataxia telangiectasia mutated/checkpoint kinase 2 (ATM/CHK2) was activated, suggesting roles of DNA damage in regulation of other biological processes as well in these T. gondii-infected host cells. Together, our data reveal the major role of ROS in host DNA damage during T. gondii infection, and lay a foundation for better understanding how T. gondii interacts with its host from the view of DNA damage.
Methods
Cells, parasites and mice
Vero, HEK293T and HeLa cells were all obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in DMEM (Biological Industries, Kibbutz, Israel) supplemented with a final concentration of 10% fetal bovine serum (FBS) (Biological Industries) and 1% penicillin-streptomycin-glutamine (Gibco, Carlsbad, USA). All cells were treated with MycAwayTM elimination reagent (Yeason, Shanghai, China), followed by testing using One-Step Mycoplasma Detection Kit (Yeason) to make sure they were Mycoplasma free. Both the T. gondii RHΔku80 strain and EGFP-RHΔku80 strain were serially passaged as tachyzoites in Vero cells in our own laboratory as previously described [13]. Briefly, tachyzoites were collected in the supernatant of centrifugation of spontaneously ruptured T. gondii-infected Vero cells at 300g for 5 min at room temperature. They were further cleaned by passing through a syringe filter of 5-μm pore size (Millipore, Darmstadt, Germany). Parasites were used to infect fresh Vero cells at a multiplicity of infection (MOI) of 10:1 after quantification using a hemocytometer. Six-week-old BALB/c mice were obtained from Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China), and housed in a ventilated cage kept under a conditioned temperature of 25 °C with a light/dark cycle of 14 h/10 h.
Antibodies and mouse sera
The antibodies to the following molecules were purchased from various sources as indicated. γH2AX (20E3, Cell Signaling Technology, Danvers, USA) was used to detect DNA DSBs. Actin and the cleaved caspase 3 (Abcam, Cambridge, UK) were used as a loading control and apoptotic marker, respectively. ATM, ATM-S1981, CHK2 and CHK2-T68 (Cell Signaling) were used to document DNA damage responses. HRP-conjugated goat anti-mouse IgG and anti-rabbit IgG (Fude, Hangzhou, China) were used in the western blot. Donkey anti-mouse Alexa Fluor 488 and goat anti-rabbit Alexa Fluor 594 (Invitrogen, Carlsbad, USA) were used in the immunofluorescence assay (IFA). Anti-T. gondii inner membrane complex 1 (IMC1) and surface protein 1 (SAG1) mouse sera, kind gifts from colleagues Miss Mi Lin and Miss Mingxiu Zhao (College of Animal Sciences, Zhejiang University), were used to detect T. gondii in IFA and western blot, respectively. Toxoplasma gondii positive serum was harvested from BALB/c mice 6 days post-infection (pi) and used to block T. gondii invasion into HeLa cells. Negative serum was collected from the same mice prior to infection and used as controls.
Western blot
Western blot was carried out as previously described [13]. Briefly, cells were lysed by incubation for 30 min in ice-cold radioimmunoprecipitation assay lysis buffer supplemented with a protease inhibitor cocktail (Bimake, Shanghai, China). The soluble proteins derived from the supernatants of the centrifugation of the cellular lysates at 12,000g, 4 °C for 10 min were measured using a BCA assay kit (Fude, Hangzhou, China) and were then subjected to SDS-PAGE. The 0.22 μm PVDF membrane (Millipore, Darmstadt, Germany) blotted with proteins was blocked with 5% skimmed milk (Sangon, Shanghai, China) in Tris buffered saline containing 0.5% Tween-20 (TBST) followed by incubation in suitable primary antibodies. The membranes were then probed with appropriate HRP-conjugated secondary antibodies (Fude). Membranes were rinsed thrice in TBS (10 min each) at each interval. Signals were immediately documented using the ChemiDoc™ chemiluminescence system (Bio-Rad, Hercules, USA) after membranes were exposed to ECL substrates (Fude).
IFA
IFA was carried out as previously described [12]. Briefly, cells grown on coverslips in a 24-well plate were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min and permeabilized with 0.25% triton-PBS for 10 min. They were incubated with the primary antibodies for 1 h after being blocked in 1% bovine serum albumin for 1 h followed by submerging in suitable Alexa-fluor-conjugated secondary antibodies for 1 h in the dark. Afterwards they were counterstained with DAPI (Sigma-Aldrich, Saint Louis, USA) for 1 min. At each interval, the cells were rinsed thrice with PBS (10 min each). The coverslips were then mounted to a glass slide and cell images were obtained using an Olympus IX81 FV1000 confocal microscope (Olympus, Monolith, Japan).
ROS measurement
ROS measurement and inhibition was carried out as previously described [14, 15]. Briefly, HeLa cells were seeded on 24-well plates and infected with T. gondii RHΔku80 strain parasites at a multiplicity of infection (MOI) of 10:1. Twenty-four hours post-infection (hpi), infected cells were treated with the ROS inhibitor N-acetylcysteine (NAC) at 50 μm for 1 h, 2 h or 4 h. NAC, a precursor of cysteine and glutathione, cellularly functions as an antioxidant via the redox potential of thiols or via increasing glutathione levels [16]. Following NAC treatment and after being rinsed 3 times in PBS, infected cells were incubated in 10 μm 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; MCE, Monmouth, USA) in the dark at 37 °C and 5% CO2 for 30 min. H2DCFDA is a non-fluorescent precursor that is intracellularly oxidized by ROS to form a highly fluorescent product DCF. Finally, fluorescence signals of cells were measured using a Synergy 2 plate reader (Biotek, Winooski, USA) at 485 nm exiting wavelength.
Quantification and statistical analysis
Quantification of the western-blot protein bands was performed by Image J. Western blot data from Image J and fluorescence data from a plate reader representing triplicate samples were analyzed by GraphPad Prism 7.0 (GraphPad, La Jolla, USA). Statistical analysis was performed by one-way ANOVA and Student’s t-test with P ≤ 0.05 being considered significant.
Results
Toxoplasma gondii infection triggered DNA DSBs in host cells in vitro
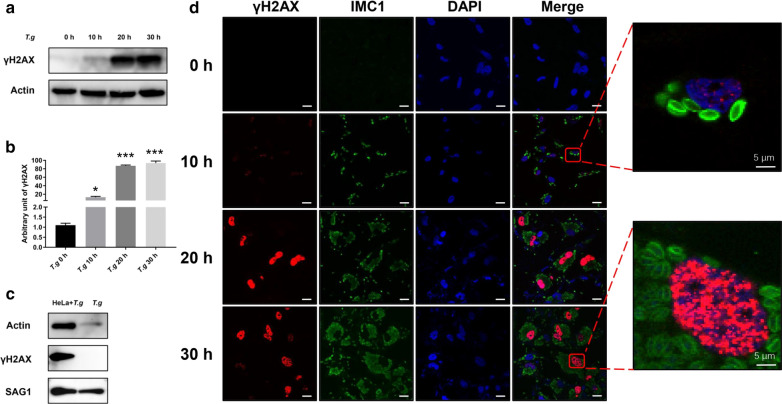
DSBs are one of the most common forms of DNA damage [17]. Phosphorylation at the ser139 position of H2AX named γH2AX is a well-characterized DSBs marker of the mammalian cells [18]. To study host cell DNA damage by T. gondii infection in vitro, we infected Vero, HEK293T or HeLa cells with T. gondii RHΔku80 strain parasites at a MOI of 10:1. A similar effect was observed among all the three types of cells upon T. gondii infections. Hela cells were used throughout the entire study description here unless another cell type was specifically identified. The cells were harvested at 0 h (uninfected control) 10, 20 or 30 hpi and analyzed by western blot to detect γH2AX. Compared to 0 h, γH2AX levels in the infected HeLa cells at 10 h, 20 h, and 30 h had increased approximately 10-, 85- and 90-fold (10 h: ANOVA, F(3, 8) = 13.87, P = 0.0323; 20 h: ANOVA, F(3, 8) = 13.87, P = 0.0002; 30 h: ANOVA, F(3, 8) = 13.87, P = 0.0005) (Fig. 1a, b), respectively. A similar trend was observed in the infected Vero and HEK293T cells as well (data not shown). We next determined the source of γH2AX since Fig. 1a, b did not show whether it originated from the infected host cells, parasites themselves or both. Tachyzoites were harvested by passing the infected Hela cells at 24 hpi through a 27-gauge syringe needle numerous times. They were then cleaned up with a 5-μm filter followed by cellular lysis by sonication. Their γH2AX levels were contrasted to those of the infected cells by western blot. γH2AX was abundantly detected in the infected HeLa cells. In contrast, γH2AX levels in parasites themselves were below the detectable level (Fig. 1c). These data clearly show that γH2AX predominately originated from the host cells rather than the parasites. To confirm γH2AX location in T. gondii infected cells, we carried out IFA using anti-IMC1 mouse serum and antibody to γH2AX to pinpoint T. gondii and DNA damage, respectively. Relative to uninfected cells, highly elevated γH2AX signals were detected in the nuclei of T. gondii-infected HeLa cells 20 or 30 hpi (Fig. 1d). Taken together, our data unequivocally demonstrated that T. gondii infection induced DSBs in host cells in vitro.
Fig. 1.
DNA damage of the T. gondii-infected host cells in vitro. Antibodies to γH2AX and actin, SAG1 serum and IMC serum were all used at a 1:1000 dilution for the western blot and at a 1:200 dilution for the immunofluorescence assay (IFA). a HeLa cells were infected with T. gondii RHΔku80 at a MOI of 10:1. γH2AX levels were detected by western blot at 0 h (uninfected control), 10 h, 20 h or 30 h post-infection. Actin was used as a loading control. b Quantification of γH2AX shown in a. Mean ± SD (n = 3) of γH2AX levels are shown with that of uninfected cells set as one arbitrary unit. c γH2AX levels were measured by western blot in T. gondii-infected HeLa cells and purified T. gondii parasites that were freshly isolated from infected Hela cells. Actin and SAG1 were used as a loading control for host cells and T. gondii, respectively. d γH2AX of uninfected (0 h) and infected HeLa cells at 10 h, 20 h or 30 h post-infection by IFA. IMC1 mouse serum and DAPI were used to indicate T. gondii and nuclei, respectively. Key: red, γH2AX; green, IMC1; blue, DAPI. All experiments were performed in triplicate. *P ≤ 0.05, ***P ≤ 0.001. Scale-bars: 30 μm
Toxoplasma gondii induced DSBs were irrelevant to apoptosis and depended on its invasion
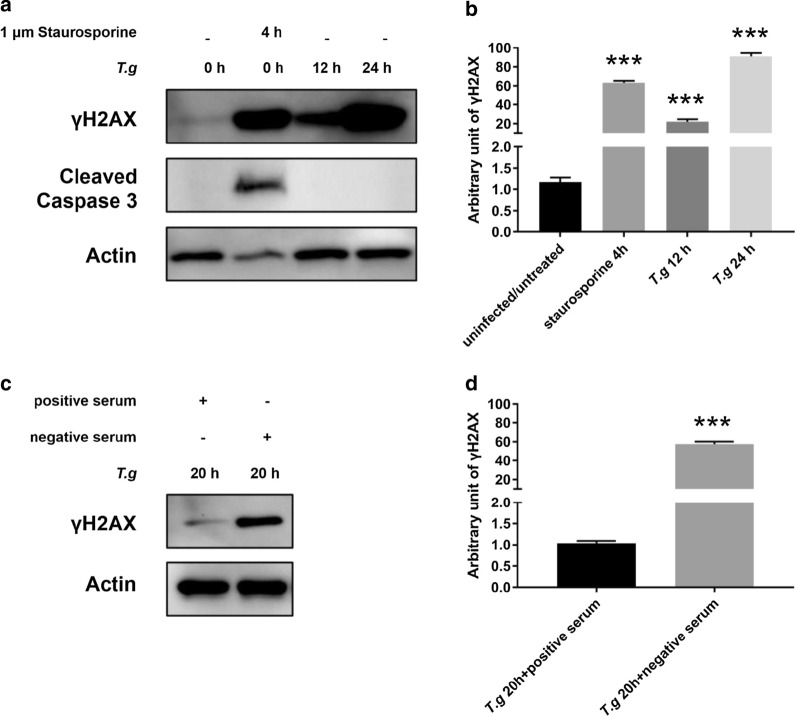
It has been reported that γH2AX occurs in early apoptosis when DNA fragmentation just starts [19]. Toxoplasma gondii initiates apoptosis in some type of cells whereas it inhibits the process in others [20]. Our next experiments tested whether apoptosis influenced γH2AX levels during T. gondii infection. To this end, HeLa cells were treated with staurosporine, an apoptosis inducer [21], at 1 μm for 4 h and apoptosis was monitored by an early apoptotic signal, the cleaved caspase 3 in the western blot [22]. While γH2AX were detected in both staurosporine-treated HeLa cells and T. gondii-infected (12 h and 24 h) HeLa cells, the cleaved caspase 3 was only detected in staurosporine-treated cells with no trace of it being detected in T. gondii-infected cells (treated: ANOVA: F(3, 8) = 80.51, P = 0.0003; 12 h: ANOVA: F(3, 8) = 80.51, P = 0.0002; 24 h: ANOVA: F(3, 8) = 80.51, P = 0.0003) (Fig. 2a, b). These data clearly showed that T. gondii infection did not trigger apoptosis in HeLa cells, which unequivocally ruled out that apoptosis was involved in their DNA damage.
Fig. 2.
DNA damage of T. gondii-host cells upon its invasion. Antibodies to γH2AX, actin, and the cleaved caspase 3 were all used at a 1:1000 dilution. a Uninfected HeLa cells were treated with 1 μm staurosporine for 4 h to induce apoptosis. Treated, uninfected and infected HeLa cells were harvested 12 h or 24 h later, γH2AX and the cleaved caspase 3 were detected by western blot. b Quantification of γH2AX shown in a. Mean ± SD (n = 3) of γH2AX levels are shown with that of uninfected cells set as one arbitrary unit. c γH2AX was detected by western blot on HeLa cells 20 h post-infection with positive or negative serum-pretreated EGFP-RHΔku80. d Quantification of γH2AX shown in c. Mean ± SD (n = 3) of γH2AX levels are shown with that of uninfected cells set as one arbitrary unit. All experiments were performed in triplicate. ***P ≤ 0.001
During early infection, T. gondii invades host cells and leads to the formation of a parasitophorous vacuole to facilitate its proliferation [23]. We next examined whether T. gondii triggered host DNA damage before invasion. To this end, EGFP-RHΔku80 T. gondii tachyzoites were incubated with either positive serum from T. gondii-infected BALB/c mice or negative control serum for 1 h at 37 °C prior to infection as previously reported [24]. The positive serum was used to block the invasion of T. gondii. HeLa cells were then infected with serum-treated T. gondii at a MOI of 10:1, infected cells were imaged by fluorescence microscopy to detect EGFP 24 hpi or harvested for western blot analysis of γH2AX at 20 hpi. Compared with negative serum treatment, positive serum treatment dramatically blocked invasion of T. gondii, and decreased γH2AX levels by approximately 98%. (Student’s t-test: t(2) = 17.91, P = 0.0002) (Fig. 2c, d). These results strongly indicate that induction of host DNA damage depends on the invasion of T. gondii.
ROS contributed to host DNA damage
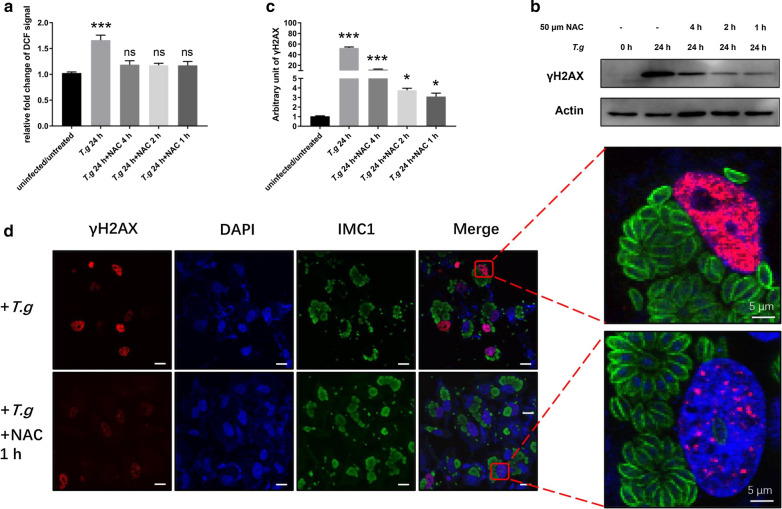
ROS induce oxidative stress, which turns out to cause damages to macromolecules such as DNA, proteins and lipids [25]. Multiple studies have suggested pathogen-induced ROS role in host-cell DNA damage [26–30]. Elevated ROS levels have been reported in T. gondii-infected host cells [12, 31]. We hypothesized that ROS cause host DNA damage during T. gondii infection. To test this hypothesis, we treated T. gondii infected HeLa cells with N-acetylcysteine (NAC), a ROS inhibitor. NAC was applied to the infected cells 24 hpi for up to 4 h. Two methods were used to gauge the impact of ROS on host DNA damage. First, ROS levels of uninfected cells, host cells and NAC-treated host cells were analyzed using H2DCHFA, a non-fluorescent precursor that is converted to fluorescent DCF by ROS. H2DCHFC has been widely used as a probe to detect ROS for 50 years. It detects hydrogen peroxide, hydroxyl radicals and singlet oxygen but not superoxide anion [32]. The higher the ROS level is, the stronger florescence signal it produces. The second method was to monitor generation of γH2AX by western blot and IFA. The results show that ROS levels in the infected Hela cells increased by approximately 70% compared to those of uninfected cells 24 hpi by fluorescence quantification. NAC treatment for as little as 1 h completely offset the influence of T. gondii infection by bringing the ROS levels down almost to the basic level of the uninfected cells (infected: ANOVA: F(4, 10) = 38.85, P = 0.0002; NAC 4 h: ANOVA: F(4, 10) = 38.85, P = 0.8755; NAC 2 h: ANOVA: F(4, 10) = 38.85, P = 0.9321; NAC 1 h: ANOVA: F(4, 10) = 38.85, P = 0.8652) (Fig. 3a). Western blot showed that γH2AX levels in the NAC-treated infected host cells reduced by approximately 85%, 85% and 70% at 1, 2 and 4 h of NAC treatment, respectively, compared to those of the untreated infected host cells (infected: ANOVA: F(4, 10) = 47.55, P = 0.0002; NAC 4 h: ANOVA: F(4, 10) = 47.55, P = 0.0005; NAC 2 h: ANOVA: F(4, 10) = 47.55, P = 0.0341; NAC 1 h: ANOVA: F(4, 10) = 47.55, P = 0.0325) (Fig. 3b, c); quantification of protein bands were performed by Image J. IFA confirmed this dramatic reduction in γH2AX levels in NAC-treated host cells compared to untreated host cells. Further, γH2AX, although weak, remained detectable in these NAC-treated cells (Fig. 3d). These two lines of evidence suggested that diminishing ROS by NAC greatly reduced γH2AX levels in infected cells, indicating that ROS leads to DNA damage during T. gondii infection.
Fig. 3.
Reactive oxygen species contributed to DNA damage. HeLa cells were seeded in a 24-well plate and infected with T. gondii RHΔku80 at a MOI of 10:1 for 24 h. N-acetylcysteine (NAC) at 50 μm was then applied to the infected cells for 1, 2 or 4 h. Antibodies to γH2AX, actin, and IMC serum were all used at a 1:1000 dilution for the western blot and at a 1:200 dilution for the immunofluorescence assay (IFA). a NAC treated T. gondii-infected cells were stained with 10 μm H2DCFDA and fluorescence signals were recorded. Data are presented as the mean ± SD (n = 3). b γH2AX was detected by western blot. c Quantification of γH2AX shown in b. Mean ± SD (n = 3) of γH2AX levels are shown with that of uninfected cells set as one arbitrary unit. d γH2AX assayed by IFA. Key: red, γH2AX; green, IMC1; blue, DAPI. All experiments were performed in triplicate. *P ≤ 0.05, ***P ≤ 0.001; ns: not significant. Scale-bars: 30 μm
Host DNA damage response pathway ATM/CHK2 was activated by T. gondii infection
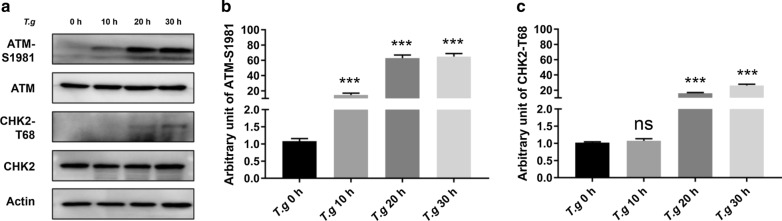
DNA damage response is triggered upon DNA damage in the eukaryotic cells. A network of kinase pathways is involved in this biological process, among which ATM/CHK2 and ATM RAD3-related/checkpoint kinase 1 (ATR/CHK1) are two main pathways responding to DSBs and SSBs, respectively [17]. Since we had already detected DSBs in host cells, we then examined whether ATM/CHK2 pathway was activated in the T. gondii-infected cells. ATM-S1981 and CHK2-T68 are the functionally activated form of ATM and CHK2, respectively [17]. The levels of ATM-S1981 and CHK2-T68 quantified by western blot were approximately 10-, 65-, 70-fold and 1-, 15-, 25-fold, respectively at 10, 20 and 30 hpi compared to the uninfected controls of 0 h (ATM-S1981 10 h: ANOVA: F(3, 8) = 34.28, P = 0.0002; 20 h: ANOVA: F(3, 8) = 34.28, P = 0.0003; 30 h: ANOVA: F(3, 8) = 34.28, P = 0.0005; CHK2-T68 10 h: ANOVA: F(3, 8) = 54.92, P = 0.9981; 20 h: ANOVA: F(3, 8) = 54.92, P = 0.0003; 30 h: ANOVA: F(3, 8) = 54.92, P = 0.0006) (Fig. 4a–c). This result indicated that the ATM/CHK2 pathway was activated during T. gondii infection.
Fig. 4.
ATM/CHK2 activation upon T. gondii infection. a HeLa cells were infected with T. gondii RHΔku80 at a MOI of 10:1. Expression of ATM, ATM-S1981, CHK2 and CHK2-T68 were detected by western blot at 0 h (uninfected control), 10 h, 20 h or 30 h post-infection. All antibodies were used at a 1:1000 dilution. b Quantitation of ATM-S1981 shown in a with ATM-S1981 levels of uninfected cells set as one arbitrary unit. c Quantitative data of CHK2-T68 shown in a with CHK2-T68 levels of uninfected cells set as one arbitrary unit. Data are presented as the mean ± SD (n = 3). All experiments were performed in triplicate. ***P ≤ 0.001; ns: not significant
Discussion
DNA damage is classified as SSBs, DSBs, and chemical modification of bases. These different types of DNA damage can be detected by various markers. We used γH2AX to detect DNA DSBs in the T. gondii infected cells. Other types of DNA damage in the eukaryotic cells can be identified by corresponding suitable markers. For example, anti-8oxoG antibody can be used to detect chemical modification of DNA bases under oxidative stress [33, 34]. Here, DSBs were clearly demonstrated in the T. gondii-infected host cells. Evaluation of other types of DNA damage in these cells is beyond the scope of the present paper and waits to be further performed. Whether T. gondii cells themselves show DSBs is worthy of a brief discussion. We did not detect them in both western blot and IFA using the antibody to γH2AX. Nevertheless, a basal level of DSBs in proliferating T. gondii has been previously reported [35]. One plausible reason for this discrepancy is that the different monoclonal antibodies (mAb) to γH2AX are used in the two studies. Our mAb (20E3), was purchased from Cell Signaling Technology, whereas the mAb (JBW301) in the previous study was purchased from Merck Argentina. mAbs to the same protein may recognize different epitopes. Alternatively, this discrepancy may be due to the different binding capacity of the two mAbs.
It has been reported that T. gondii infection inhibits apoptosis of host cells [36]. According to our data, T. gondii infections do not cause apoptosis in its host cells which aligns well with these earlier reports even though they do not exactly confirm inhibition of apoptosis in the infected cells. In our experiment of blocking invasion of T. gondii, one shortcoming of using mouse serum to inhibit the invasion of host cells by T. gondii is that there might exist antibodies to the molecules that play important roles other than its invasion. Reagents that have been proven to block this parasite invasion are alternatives. This can be achieved by the reagent’s action on either the T. gondii tachyzoites or host cells. One example of the former is protease inhibitors such as BAY11-7082 [37]. The latter includes dynamin inhibitor such as dynasore, a small chemical compound that inhibits dynamin GTPase activity [38]. Due to the technical challenges these compounds were not used in the present study. Nevertheless, the mouse serum containing polyclonal antibodies used in the current study indeed block the parasites’ invasion of HeLa cells (see Additional file 1: Figure S1).
ROS are inhibited by activating antioxidant enzymes, such as catalase, superoxide dismutase and glutathione peroxidase, or by non-enzymatic antioxidants, such as glutathione and thioredoxin. ROS inhibitor NAC reacts with hypochlorous acid, hydroxyl radical and hydrogen peroxide but not superoxide anion [39]. Our data clearly demonstrated that ROS inhibition by NAC decreased γH2AX levels by a minimum of 70%, leaving a maximum of 30% γH2AX intact. This may be due to the action of superoxide anion, which can be addressed later in a follow-up study by its specific inhibitors such as eugenol, chalcone and derivative, 3’-isopropoxychalcone (H2O7D) [40, 41]. Our result showed that T. gondii infection causes a significant increase in the level of ROS in HeLa cells. Although consistent with some earlier reports [12, 31], these are clearly contradicted to at least one other study. Decreased levels of ROS were observed in T. gondii-infected ARPE-19 cells, a human RPE cell line, that had also been exposed to H2O2 treatment, and T. gondii-infected ARPE-19 cells at a MOI of 5:1 without H2O2 treatment showed ROS levels very similar to the uninfected controls [36]. These contradicting results might be due to different type of cells, i.e. HeLa versus ARPE-19, and different MOI of 10:1 and 5:1. Furthermore, DSBs was repaired in 1 h of ROS removal by NAC, the shortest time point used in the present study, as shown by a dramatical decrease of γH2AX levels. DSBs repair is very likely to occur and be observed in minutes if shorter time points had been included in our experiment.
Activation of the ATM/CHK2 pathway is involved in many biological processes such as DNA damage repair, cell cycle control and apoptosis etc. For example, the active ATM/CHK2 pathway leads to phosphorylation of P53, which is a well-known transcription factor. On one hand, phosphorylation of P53 at Ser25, Ser20 and Ser33 upregulates the expression of DNA binding protein 2, E3 ubiquitin-protein ligase MDM2 and CDC25a. The latter three are the key players in DNA damage repair and cell cycle control, which contribute to survival of wounded cells [42]. On the other hand, phosphorylation of P53 at Ser46 by ATM/CHK2 pathway activates pro-apoptotic genes, leading to apoptosis of wounded cells [43]. We propose that activation of the ATM/CHK2 pathway plays a pivotal role in the host cells to keep a calculated balance between survival and apoptosis during T. gondii infection, probably for the benefit of parasite survival.
Identification of parasite molecules causing host cell DNA damage and their molecular interactions with host cells are beyond the scope of the current study. However, it has been reported that T. gondii ROP18 kinase interacts with host proteins involving DNA repair and other functions [44]. Ocular and brain lesions caused by T. gondii infections are confirmed in human individuals with significant polymorphism of DNA repair genes [45]. Therefore, T. gondii virulence factors associated with DNA damage and host susceptibility to this damage are worth further investigation.
Conclusions
Our studies clearly showed that T. gondii infection induced DNA double-strand breaks in host cells. This damage was irrelative to apoptosis and occurred only after T. gondii invasion. Reactive oxygen species were determined as the major player for DNA double-strand breaks in host cells. The host DNA damage response pathway ATM/CHK2 was activated during T. gondii infection, suggesting the DNA damage process may trigger other biological processes as well. More studies are needed to explore the molecular network of DNA double-strand breaks as well as other types of DNA damage in host cells in the future.
Supplementary information
Additional file 1: Figure S1. Serum of T. gondii-infected BALB/c mice blocked T. gondii invasion to HeLa cells. EGFP-RHΔku80 tachyzoites were pre-incubated with either positive mouse serum collected at 6 days post-infection or negative serum harvested at 0 day prior to infection at a 1:10 dilution for 1 h. Afterwards, these parasites were added to HeLa cells at a multiplicity of infection (MOI) of 10:1 at 0 h when images were taken immediately (left panels). EGFP of the infected HeLa cells was again imaged at 24 hpi by fluorescence microscopy (right panels). Experiments were performed in triplicate. Scale-bars: 100 μm.
Acknowledgements
We are very grateful to the colleagues Miss Mi Lin and Miss Mingxiu Zhao for their sharing anti-T. gondii IMC1 and SAG1 mouse sera, respectively. We also thank Drs Weiren Dong and Ying Shan (College of Animal Sciences, Zhejiang University) for their technical help on operating plate reader and confocal microscope, respectively.
Abbreviations
- MOI
Multiplicity of infection
- ROS
Reactive oxygen species
- NAC
N-acetylcysteine
- IFA
Immunofluorescence assay
- mAb
Monoclonal antibody
- ATM/CHK2
Ataxia telangiectasia mutated/checkpoint kinase 2
- SSBs
Single-strand breaks
- DSBs
Double-strand breaks
- FBS
Fetal bovine serum
- DMEM
Dulbecco’s modified Eagleʼs medium
- IMC1
Inner membrane complex 1
- SAG1
Surface protein 1
- TBS
Tris buffered saline
- PBS
Phosphate-buffered saline
- H2DCFDA
2′,7′-dichlorodihydrofluorescein diacetate
Authors’ contributions
YY, AFD, YMY, XFZ and SYH conceived and supported the study. HHZ designed and performed the laboratory work. LTP and CQY carried out data analysis. XQC purchased experimental materials and performed a part of laboratory work. HHZ and CQY wrote the manuscript. All authors read and approved the final manuscript.
Funding
This project was supported by the National Natural Science Foundation of China (Grant No. 31672543), Zhejiang Province “Sannongliufang” Science and Technology Cooperation Project (Grant No. 2020SNLF007), the Science and Technology Department of Zhejiang (Grant No. 2012C12009-2) and the National Natural Science Foundation of China (Grant No. 31472184).
Availability of data and materials
Data supporting the conclusions of this article are included within the article and its additional file. Data and materials can be available upon reasonable request to the corresponding author.
Ethics approval and consent to participate
Animal experiments were carried out under strict adherence to the guidelines on use of experimental animals of the People’s Republic of China. Animal use for this study was approved by the Experimental Animal Ethics Committee of Zhejiang University (permit number: ZJU20160240).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haohan Zhuang, Chaoqun Yao, equal contributors
Contributor Information
Haohan Zhuang, Email: 11617028@zju.edu.cn.
Chaoqun Yao, Email: chyao@rossvet.edu.kn.
Xianfeng Zhao, Email: zxf0903@126.com.
Xueqiu Chen, Email: chenxueqiu@zju.edu.cn.
Yimin Yang, Email: 353287374@qq.com.
Siyang Huang, Email: siyang.huang@hotmail.com.
Lingtao Pan, Email: 1472380978@qq.com.
Aifang Du, Email: afdu@zju.edu.cn.
Yi Yang, Email: yangyi0607@zju.edu.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13071-020-04324-7.
References
- 1.Molan A, Nosaka K, Hunter M, Wang W. Global status of Toxoplasma gondii infection: systematic review and prevalence snapshots. Trop Biomed. 2019;36:898–925. [PubMed] [Google Scholar]
- 2.Mammari N, Halabi MA, Yaacoub S, Chlala H, Darde ML, Courtioux B. Toxoplasma gondii modulates the host cell responses: an overview of apoptosis pathways. Biomed Res Int. 2019;2019:6152489. doi: 10.1155/2019/6152489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Weiss LM, Orlofsky A. Host cell autophagy is induced by Toxoplasma gondii and contributes to parasite growth. J Biol Chem. 2009;284:1694–1701. doi: 10.1074/jbc.M807890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribeiro DA, Pereira PCM, Machado JM, Silva SB, Pessoa AWP, Salvadori DMF. Does toxoplasmosis cause DNA damage? An evaluation in isogenic mice under normal diet or dietary restriction. Mutat Res Genet Toxicol Environ Mutagen. 2004;559:169–176. doi: 10.1016/j.mrgentox.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 5.El-Sayed NM, Aly EM. Toxoplasma gondii infection can induce retinal DNA damage: an experimental study. Int J Ophthalmol. 2014;7:431–436. doi: 10.3980/j.issn.2222-3959.2014.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakem R. DNA-damage repair; the good, the bad, and the ugly. EMBO J. 2008;27:589–605. doi: 10.1038/emboj.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedberg EC, McDaniel LD, Schultz RA. The role of endogenous and exogenous DNA damage and mutagenesis. Curr Opin Genet Dev. 2004;14:5–10. doi: 10.1016/j.gde.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Srinivas US, Tan BWQ, Vellayappan BA, Jeyasekharan AD. ROS and the DNA damage response in cancer. Redox Biol. 2019;25:101084. doi: 10.1016/j.redox.2018.101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang FC. Antimicrobial actions of reactive oxygen species. mBio. 2011;2:e00141. doi: 10.1128/mBio.00141-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paiva CN, Bozza MT. Are reactive oxygen species always detrimental to pathogens? Antioxid Redox Signal. 2014;20:1000–1037. doi: 10.1089/ars.2013.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matta SK, Patten K, Wang Q, Kim B-H, MacMicking JD, Sibley LD. NADPH oxidase and guanylate binding protein 5 restrict survival of avirulent Type III strains of Toxoplasma gondii in naive macrophages. mBio. 2018;9:e01393. doi: 10.1128/mBio.01393-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun H, Wang S, Zhao X, Yao C, Zhuang H, Huang Y, et al. Targeted overexpression of cyclic AMP-dependent protein kinase subunit in Toxoplasma gondii promotes replication and virulence in host cells. Vet Parasitol. 2017;243:248–255. doi: 10.1016/j.vetpar.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Raihan O, Brishti A, Li Q, Zhang Q, Li D, Li X, et al. SFRS11 loss leads to aging-associated cognitive decline by modulating LRP8 and ApoE. Cell Rep. 2019;28:78–90. doi: 10.1016/j.celrep.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Sun PR, Gao FF, Choi HG, Zhou W, Yuk J-M, Kwon J, et al. Dipenyleneiodonium induces growth inhibition of Toxoplasma gondii through ROS induction in ARPE-19 cells. Korean J Parasitol. 2019;57:83–92. doi: 10.3347/kjp.2019.57.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halasi M, Wang M, Chavan TS, Gaponenko V, Hay N, Gartel AL. ROS inhibitor N-acetyl-L-cysteine antagonizes the activity of proteasome inhibitors. Biochem J. 2013;454:201–208. doi: 10.1042/BJ20130282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanz MC, Dibitetto D, Smolka MB. DNA damage kinase signaling: checkpoint and repair at 30 years. EMBO J. 2019;38:e101801. doi: 10.15252/embj.2019101801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma A, Singh K, Almasan A. Histone H2AX phosphorylation: a marker for DNA damage. Methods Mol Biol. 2012;920:613–626. doi: 10.1007/978-1-61779-998-3_40. [DOI] [PubMed] [Google Scholar]
- 19.Rogakou EP, Nieves-Neira W, Boon C, Pommier Y, Bonner WM. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem. 2000;275:9390–9395. doi: 10.1074/jbc.275.13.9390. [DOI] [PubMed] [Google Scholar]
- 20.Luder CGK, Gross U, Lopes MF. Intracellular protozoan parasites and apoptosis: diverse strategies to modulate parasite-host interactions. Trends Parasitol. 2001;17:480–486. doi: 10.1016/S1471-4922(01)02016-5. [DOI] [PubMed] [Google Scholar]
- 21.Belmokhtar CA, Hillion J, Segal-Bendirdjian E. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene. 2001;20:3354–3362. doi: 10.1038/sj.onc.1204436. [DOI] [PubMed] [Google Scholar]
- 22.Walters J, Pop C, Scott FL, Drag M, Swartz P, Mattos C, et al. A constitutively active and uninhibitable caspase-3 zymogen efficiently induces apoptosis. Biochem J. 2009;424:335–345. doi: 10.1042/BJ20090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rastogi S, Cygan AM, Boothroyd JC. Translocation of effector proteins into host cells by Toxoplasma gondii. Curr Opin Microbiol. 2019;52:130–138. doi: 10.1016/j.mib.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirahata T, Shimizu K, Suzuki N. Effects of immune lymphocyte products and serum antibody on multiplication of Toxoplasma in murine peritoneal macrophages. Parasitol Res. 1976;49:11–23. doi: 10.1007/BF00445014. [DOI] [PubMed] [Google Scholar]
- 25.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu L, Xie C. A lucid review of Helicobacter pylori-induced DNA damage in gastric cancer. Helicobacter. 2019;24:e12631. doi: 10.1111/hel.12631. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro DA, Calvi SA, Picka MM, Persi E, de Carvalho TB, Caetano PK, et al. DNA damage and nitric oxide synthesis in experimentally infected balb/c mice with Trypanosoma cruzi. Exp Parasitol. 2007;116:296–301. doi: 10.1016/j.exppara.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Kocyigit A, Keles H, Selek S, Guzel S, Celik H, Erel O. Increased DNA damage and oxidative stress in patients with cutaneous leishmaniasis. Mutat Res Genet Toxicol Environ Mutagen. 2005;585:71–78. doi: 10.1016/j.mrgentox.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Zgur-Bertok D. DNA damage repair and bacterial pathogens. PLoS Pathog. 2013;9:11. doi: 10.1371/journal.ppat.1003711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Williams V, Filippova M, Filippov V, Duerksen-Hughes P. Viral carcinogenesis: factors inducing DNA damage and virus integration. Cancers. 2014;6:2155–2186. doi: 10.3390/cancers6042155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou W, Quan JH, Lee YH, Shin DW, Cha GH. Toxoplasma gondii proliferation require down-regulation of host Nox4 expression via activation of PI3 Kinase/Akt signaling pathway. PLoS ONE. 2013;8:e66306. doi: 10.1371/journal.pone.0066306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Zhong Z, Xu Z, Chen L, Wang Y. 2′,7 ′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: forty years of application and controversy. Free Radic Res. 2010;44:587–604. doi: 10.3109/10715761003709802. [DOI] [PubMed] [Google Scholar]
- 33.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravanat JL, Cadet J, Douki T. Oxidatively generated DNA lesions as potential biomarkers of in vivo oxidative stress. Curr Mol Med. 2012;12:655–671. doi: 10.2174/156652412800792651. [DOI] [PubMed] [Google Scholar]
- 35.Munera Lopez J, Ganuza A, Bogado SS, Munoz D, Ruiz DM, Sullivan WJ, Jr, et al. Evaluation of ATM kinase inhibitor KU-55933 as potential anti-Toxoplasma gondii agent. Front Cell Infect Microbiol. 2019;13:26. doi: 10.3389/fcimb.2019.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi SH, Park SJ, Cha GH, Quan JH, Chang NS, Ahn MH, et al. Toxoplasma gondii protects against H2O2-induced apoptosis in ARPE-19 cells through the transcriptional regulation of apoptotic elements and downregulation of the p38 MAPK pathway. Acta Ophthalmol. 2011;89:E350–E356. doi: 10.1111/j.1755-3768.2011.02113.x. [DOI] [PubMed] [Google Scholar]
- 37.Han Y, Adeyemi OS, Bin Kabir MH, Kato K. Screening of compound libraries for inhibitors of Toxoplasma growth and invasion. Parasitol Res. 2020;119:1675–1681. doi: 10.1007/s00436-020-06673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caldas LA, Attias M, de Souza W. Dynamin inhibitor impairs Toxoplasma gondii invasion. FEMS Microbiol Lett. 2009;301:103–108. doi: 10.1111/j.1574-6968.2009.01799.x. [DOI] [PubMed] [Google Scholar]
- 39.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine - its reaction with hydrogen-peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-X. [DOI] [PubMed] [Google Scholar]
- 40.Chniguir A, Pintard C, Liu D, Pham My-Chan D, El-Benna J, Bachoual R. Eugenol prevents fMLF-induced superoxide anion production in human neutrophils by inhibiting ERK1/2 signaling pathway and p47phox phosphorylation. Sci Rep. 2019;9:18540. doi: 10.1038/s41598-019-55043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang TL, Yeh SH, Leu YL, Chern CY, Hsu HC. Inhibition of superoxide anion and elastase release in human neutrophils by 3 ‘-isopropoxychalcone via a cAMP-dependent pathway. Br J Pharmacol. 2006;148:78–87. doi: 10.1038/sj.bjp.0706712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roos WP, Thomas AD, Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer. 2016;16:20–33. doi: 10.1038/nrc.2015.2. [DOI] [PubMed] [Google Scholar]
- 43.Pietsch EC, Sykes SM, McMahon SB, Murphy ME. The p53 family and programmed cell death. Oncogene. 2008;27:6507–6521. doi: 10.1038/onc.2008.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng L, Chen Y, Chen L, Shen Y, Shen J, An R, et al. Interactions between the ROP18 kinase and host cell proteins that aid in the parasitism of Toxoplasma gondii. Acta Trop. 2012;122:255–260. doi: 10.1016/j.actatropica.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Smolarz B, Wilczynski J, Nowakowska D. DNA repair mechanisms and Toxoplasma gondii infection. Arch Microbiol. 2014;196:1–8. doi: 10.1007/s00203-013-0944-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Serum of T. gondii-infected BALB/c mice blocked T. gondii invasion to HeLa cells. EGFP-RHΔku80 tachyzoites were pre-incubated with either positive mouse serum collected at 6 days post-infection or negative serum harvested at 0 day prior to infection at a 1:10 dilution for 1 h. Afterwards, these parasites were added to HeLa cells at a multiplicity of infection (MOI) of 10:1 at 0 h when images were taken immediately (left panels). EGFP of the infected HeLa cells was again imaged at 24 hpi by fluorescence microscopy (right panels). Experiments were performed in triplicate. Scale-bars: 100 μm.
Data Availability Statement
Data supporting the conclusions of this article are included within the article and its additional file. Data and materials can be available upon reasonable request to the corresponding author.