Abstract
Pain is more prevalent in women for reasons that remain unclear. We have identified a mechanism of injury-free nociceptor sensitization and opioid-induced hyperalgesia (OIH) promoted by prolactin (PRL) in females. PRL signals through mutually inhibitory long (PRLR-L) and short (PRLR-S) receptor isoforms, and PRLR-S activation induces neuronal excitability. PRL and PRLR expression were higher in females. CRISPR-mediated editing of PRLR-L promoted nociceptor sensitization and allodynia in naïve, uninjured female mice that depended on circulating PRL. Opioids, but not trauma-induced nerve injury, decreased PRLR-L promoting OIH through activation of PRLR-S in female mice. Deletion of both PRLR-L and PRLR-S (total PRLR) prevented, whereas PRLR-L overexpression rescued established OIH selectively in females. Inhibition of circulating PRL with cabergoline, a dopamine D2 agonist, up-regulated PRLR-L and prevented OIH only in females. The PRLR-L isoform therefore confers protection against PRL-promoted pain in females. Limiting PRL/PRLR-S signaling pharmacologically or with gene therapies targeting the PRLR may be effective for reducing pain in a female-selective manner.
INTRODUCTION
Prolactin (PRL) is an endocrine polypeptide hormone that is released from the pituitary and extrapituitary sources and is now recognized to have many biological functions beyond its role in lactation and maternal behavior (1). The major physiological inhibitor of circulating PRL is dopamine, released from tuberoinfundibular dopamine (TIDA) neurons in the arcuate nucleus of the hypothalamus (2, 3). Dopamine is transported to the anterior pituitary where it inhibits PRL secretion from lactotrophs via dopaminergic D2 receptors (4). Patients with prolactinoma associated with hyperprolactinemia suffer from increased incidence of headache and are treated by lowering circulating PRL with dopamine agonists (5, 6). In rodents and humans, alternative splicing generates PRLR-L (long receptor) and PRLR-S (short receptor) isoforms of PRLR that have identical extracellular domains but differ in the length and sequence of their cytoplasmic domains (7). PRLR-S is thought to regulate excitability of female sensory neurons by modulation of colocalized ion channels, including the transient receptor potential vanilloid 1 (TRPV1) (8, 9). Studies in heterologous expression systems have suggested a mutual functional inhibition between PRLR-L and PRLR-S through intracellular mechanisms (8, 10–12), although this concept has never been tested in vivo. Women have higher circulating PRL than men and greater sensitivity to experimental pain (13). Nevertheless, women do not normally live in pain. We speculated that a possible shift in the balance of PRLR isoform expression during pathological pain conditions may affect nociceptor sensitization and pain in females.
Although opioids are used to treat pain, paradoxically, these drugs have also been associated with nociceptor sensitization and allodynia, commonly referred to as opioid-induced hyperalgesia (OIH) (14, 15). OIH has been reported after the use of opioids in both drug abusers and in patients with pain (16). An additional, well-known side effect of opioids is the disruption of hormonal function that includes transient hyperprolactinemia and galactorrhea, most often observed in females (17, 18). We therefore determined whether the PRL/PRLR system might be disrupted by opioid administration in the absence of injury. We used a model of trauma-induced neuropathic pain as a comparator. We found that expression of PRLR isoforms is sexually dimorphic. Expression of PRLR-L and PRLR-S were much greater in female than in male dorsal root ganglia (DRGs). In females, decreasing PRLR-L sensitized nociceptors and elicited allodynia. In addition, treatment with opioids, but not nerve injury, promoted nociceptor sensitization and allodynia through down-regulation of PRLR-L. Conversely, decreasing circulating PRL increased the expression of PRLR-L and provided protective effects against nociceptor sensitization and allodynia. Overexpression of PRLR-L reversed OIH selectively in females. Thus, our studies reveal a mechanism of nociceptor sensitization, pain, and OIH in females and suggest approaches for sex-based pain therapies.
RESULTS
Circulating prolactin concentrations and PRLR isoform expression are sexually dimorphic in mice
Consistent with previous reports (19, 20), we detected higher serum PRL in female mice compared with males (Fig. 1A). Using PRLR-LCre/− crossed with GFPflx/− mice to visualize PRLR-L–positive neurons, we observed that the average number of these cells was higher in female than in male mice (Fig. 1B). Using Western blot analysis, we found that both PRLR-S and PRLR-L isoforms were observed in DRGs from naïve female mice. However, very low expression of PRLR-S and PRLR-L were detected in DRGs from males. Neither PRLR isoform was observed in the dorsal lumbar spinal cord in either females or males (Fig. 1C). The results show sexually dimorphic expression of PRL, as well as of PRLR isoforms, and provide a basis for investigating the role of the PRL/PRLR pathway in nociceptor sensitization and pain in females.
Fig. 1. PRLR-L expression in DRGs protects female mice from nociceptor sensitization and tactile allodynia.
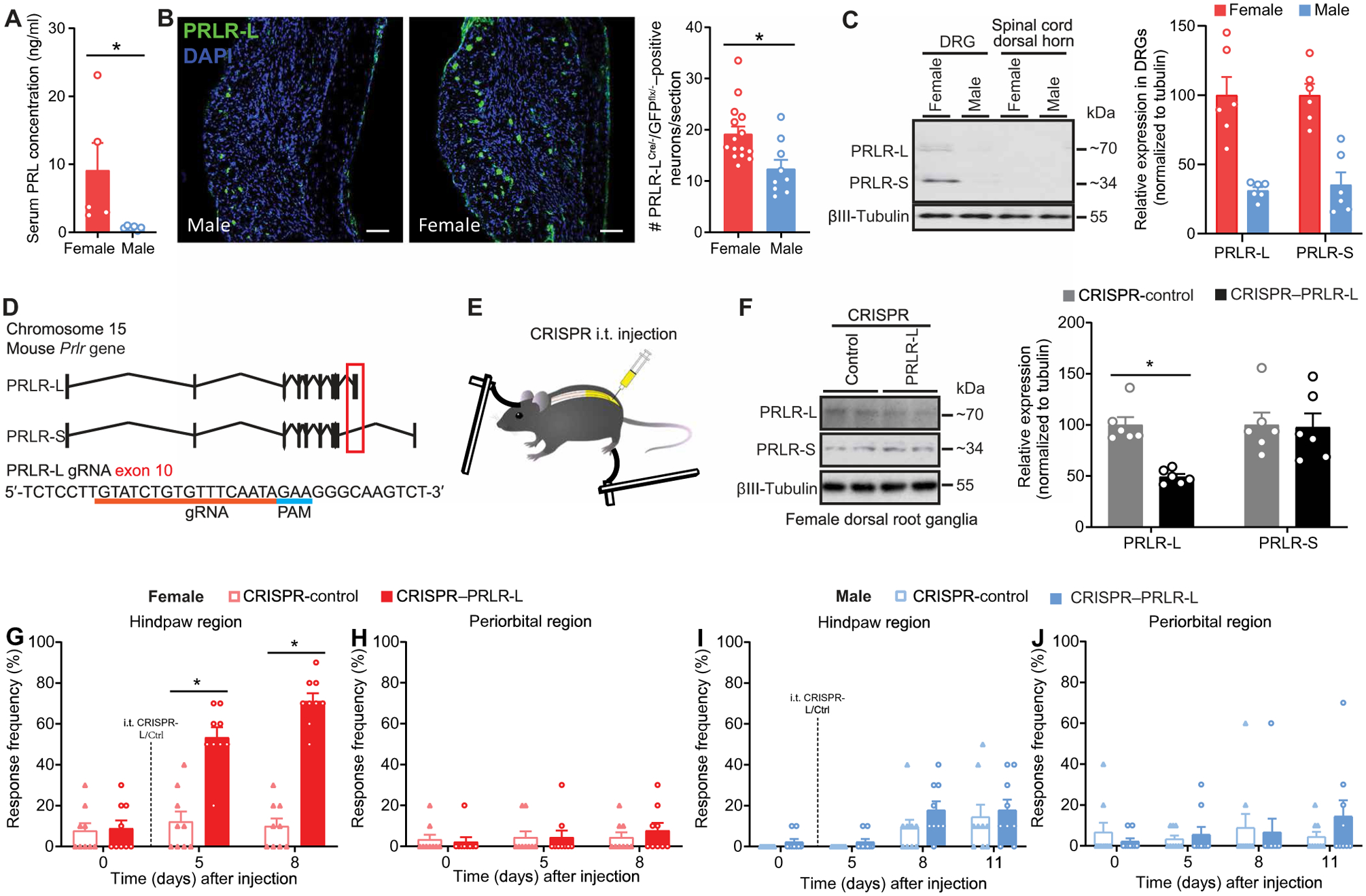
(A) Serum concentration of PRL in naïve female and male mice (female, n = 5; male, n = 6; P = 0.0043). (B) Representative images (left) and quantification (right) of PRLR-LCre/−/GFPflx/− in L3–5 DRGs from female and male mice (female, n = 5; male, n = 3). Scale bars, 100 μm (left panels). DAPI, 4′,6-diamidino-2-phenylindole. (C) Representative Western blot images of PRLR isoform protein expression in L3–5 DRGs and spinal cord dorsal horn tissues from naïve female and male mice (n = 6 per group per sex). (D) Schematic of Cas9/gRNA-targeting at exon 10 of mouse Prlr gene coding for PRLR-L. Protospacer adjacent motif (PAM) sequence is highlighted by a blue line. Exon 10 is indicated by a red box. The gRNA sequence is underlined in red pairs with its DNA target followed by an NGG sequence. (E) Schematic of the intrathecal (i.t.) administration of CRISPR vectors followed by periorbital and hindpaw tactile allodynia testing with von Frey filaments in mice. (F) Verification of intrathecal CRISPR–PRLR-L editing efficiency in DRGs of naïve female mice from (G) and (H) (left: representative Western blot images; right: quantification) (n = 6 per group; PRLR-L, P = 0.0043; PRLR-S, P = 0.5887). (G to J) Effect of intrathecal CRISPR–PRLR-L on hindpaw and periorbital allodynia in naïve female (G and H) and male (I and J) mice (n = 9 per group per sex; female: hindpaw P < 0.0001, periorbital P = 0.2822; male: hindpaw P = 0.7116, periorbital P = 0.1099). Two-tailed Mann-Whitney test (A, B, and F). Two-way repeated-measures ANOVA with Sidak’s multiple comparisons test (G to J). *P < 0.05. Group data are expressed as means ± SEM. Each data point represents an individual animal.
PRLR-L expression in DRGs protects against nociceptor sensitization and tactile allodynia promoted by high concentrations of circulating PRL in females
Studies using overexpression strategies in isolated trigeminal ganglion (TG) cells suggested mutually inhibitory actions of PRLR-L and PRLR-S isoforms (8). We therefore investigated whether the PRLR-L could have a physiological protective role in the presence of high concentrations of circulating PRL in females. We used a CRISPR-Cas9 approach with a guide RNA (gRNA) that targets the PRLR-L isoform by inducing indels on the exon 10 of the Prlr gene, specific to PRLR-L (Fig. 1D). We delivered a plasmid allowing for simultaneous expression of the PRLR-L gRNA and the nuclease Cas9 intrathecally (Fig. 1E) to efficiently decrease protein expression of PRLR-L in L3–5 DRGs from naïve female mice (Fig. 1F). After intrathecal CRISPR–PRLR-L editing, we observed a time-dependent development of hindpaw allodynia in female mice (Fig. 1G). The lack of effect on von Frey responses in the periorbital region of the same female mice suggests that the behavioral consequences of intrathecal CRISPR–PRLR-L intervention were localized to the segmental level, providing an important internal control (Fig. 1H). In contrast, intrathecal CRISPR–PRLR-L administration did not result in changes in tactile responses in naïve male mice (Fig. 1, I and J), confirming a lack of effect of low expression of PRLR-L in males. These observations show that decreased expression of PRLR-L in DRG neurons is sufficient for the induction of hindpaw allodynia only in female mice.
Next, we evaluated whether the hindpaw allodynia induced by CRISPR–PRLR-L editing in female mice relies on PRL signaling at PRLR-S in DRGs. We could not design a specific gRNA to target the sequences of all variants of PRLR-S found in mice (21) without affecting PRLR-L. We therefore used a gRNA targeting exon 4, common to all isoforms of the Prlr gene (Fig. 2A), to delete all the PRLR isoforms (Fig. 2B), including PRLR-S, referred to here as CRISPR-Total-PRLR, and evaluated behavioral responses to von Frey filaments (Fig. 2C). This approach resulted in a reduction in protein expression of PRLR-S isoforms in DRGs from female mice that had previously received intrathecal CRISPR–PRLR-L vector (Fig. 2D). CRISPR-Total-PRLR reversed the hindpaw tactile allodynia induced by intrathecal CRISPR–PRLR-L in naïve female mice (Fig. 2, E and F). These results suggest that allodynia induced by the absence of PRLR-L was due to activation of the PRLR-S isoform by PRL. To test whether circulating PRL promoted allodynia induced by intrathecal CRISPR–PRLR-L, we treated mice with cabergoline, a U.S. Food and Drug Administration (FDA)–approved long-lasting dopamine D2 receptor agonist for hyperprolactinemia-related diseases (22). Systemic cabergoline treatment reduced the concentration of circulating PRL. Whereas cabergoline treatment had no effect for the first 24 hours, a reduction in allodynic responses induced by intrathecal CRISPR–PRLR-L was observed starting at 48 hours after injection and was sustained for 5 days after the second injection of cabergoline (Fig. 2G). Periorbital responses were unaffected by the cabergoline treatment (Fig. 2H). Together, these results reveal that the expression of PRLR-L protects against allodynia promoted by circulating PRL to PRLR-S signaling in female mice.
Fig. 2. Allodynia induced by deletion of PRLR-L in female DRGs is mediated by circulating PRL to PRLR-S signaling.
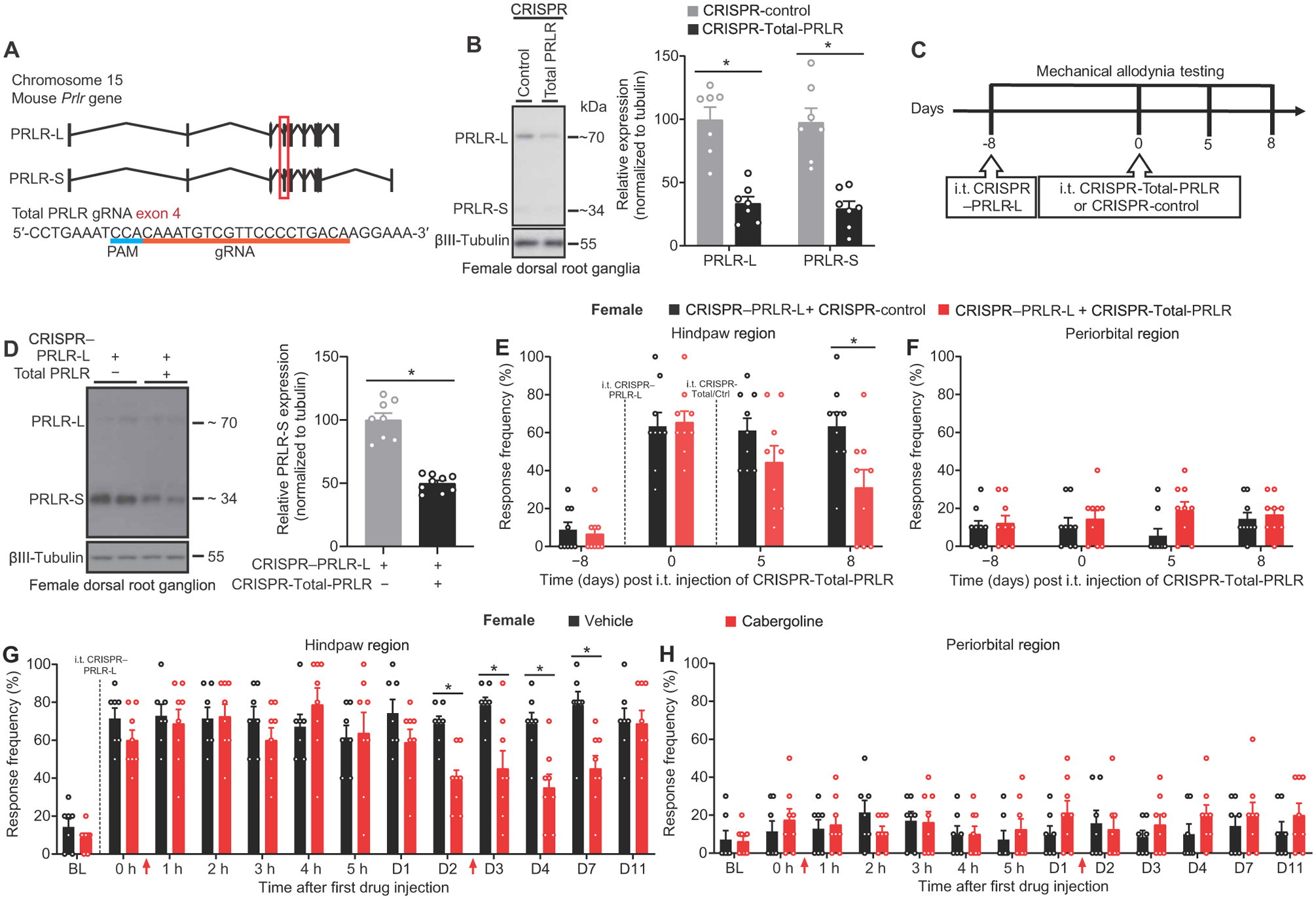
(A) Schematic of the Cas9/gRNA targeting at the exon 4 of mouse Prlr gene coding for both PRLR-L and PRLR-S. Protospacer adjacent motif (PAM) sequence is highlighted by a blue line. Exon 4 is indicated by a red box. The gRNA sequence is underlined in red pairs with its DNA target followed by an NGG sequence. (B) Verification of intrathecal CRISPR-Total-PRLR editing efficiency in L3–−5 DRGs from naïve female mice (left: representative Western blot images; right: quantification) (n = 7 per group; PRLR-L, P = 0.0012; PRLR-S, P = 0.0006). (C) Experiment design of (D) to (F). (D) Verification of intrathecal CRISPR–PRLR-L editing followed by CRISPR-Total-PRLR editing in DRGs of naïve female mice from (E) and (F) (CRISPR-control, n = 8; CRISPR-Total-PRLR, n = 9; P < 0.0001). (E and F) Hindpaw and periorbital allodynia in naïve female mice with intrathecal CRISPR–PRLR-L followed by CRISPR-Total-PRLR (n = 9 per group; hindpaw, P = 0.0058; periorbital, P = 0.2765). (G and H) Effect of cabergoline on hindpaw and periorbital allodynia in naïve female mice that had undergone intrathecal CRISPR–PRLR-L. Drug administrations are indicated by red arrows. (vehicle, n = 7; cabergoline, n = 8; hindpaw, P < 0.0001; periorbital, P = 0.4072). Two-tailed Mann-Whitney test (B and D). Two-way repeated-measures ANOVA with Sidak’s multiple comparisons test (E) to (H). *P < 0.05. Group data are expressed as means ± SEM. Each data point represents an individual animal.
Allodynia after CRISPR-Cas9–induced loss of PRLR-L in naïve female mice is mediated by spinal glutamatergic signaling
To elucidate the mechanisms mediating tactile allodynia after intrathecal CRISPR–PRLR-L in naïve female mice, we assessed the contribution of the nociceptive neuropeptide calcitonin gene-related peptide (CGRP) and excitatory neurotransmitter glutamate (Fig. 3A). Previous studies had demonstrated that capsaicin-evoked CGRP release was enhanced from female, but not male, TG neurons in the presence of PRL (8, 23). Consistent with this observation, we found enhanced capsaicin-evoked CGRP release in the spinal cord tissues from naïve female mice with intrathecal PRLR-L editing, suggesting that PRLR-L disruption promoted sensitization of nociceptors detected at the central terminals in the spinal cord (Fig. 3B). However, in naïve female mice, a single acute intrathecal administration of BIBN 4096, a CGRP receptor antagonist, at doses sufficient to block the allodynia produced by intrathecal CGRP (fig. S1, A and B), did not block hindpaw allodynia caused by PRLR-L editing (Fig. 3C) and did not affect periorbital responses (Fig. 3D). Glutamate is an important transmitter mediating nociceptive transmission from primary afferents to neurons in the spinal cord (24). Consequently, we tested whether glutamate/N-methyl-d-aspartate (NMDA) receptor signaling participates in tactile allodynia induced by PRLR-L editing in naïve female mice. We found that intrathecal administration of the NMDA receptor antagonist MK-801 reversed the hindpaw allodynia (Fig. 3E) without affecting periorbital response (Fig. 3F). Our results demonstrate that decreased expression of PRLR-L in the DRG can induce allodynia in naïve females through glutamate/NMDA receptor signaling.
Fig. 3. Allodynia induced by decreased expression of PRLR-L in female DRGs is mediated by spinal glutamatergic signaling.
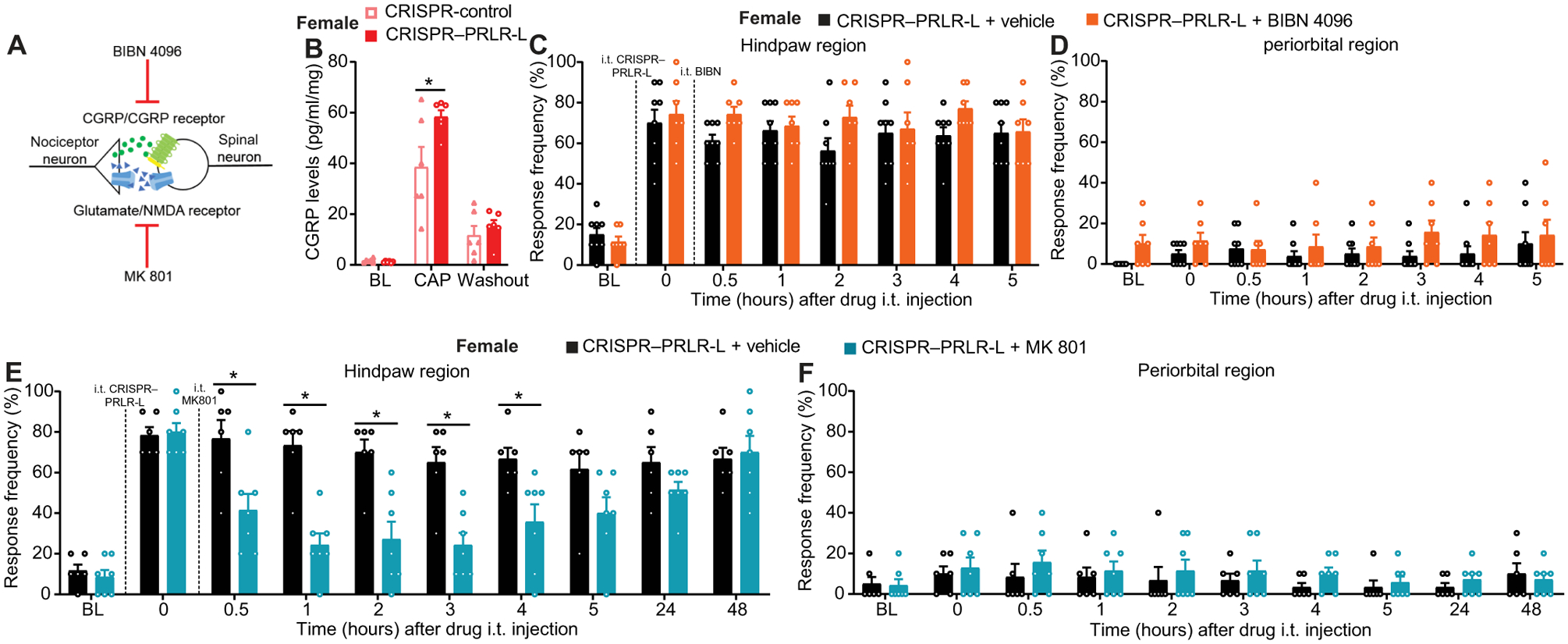
(A) Schematic of experimental investigation of spinal mechanisms underlying allodynia induced by CRISPR-mediated disruption of PRLR-L expression in female DRGs. (B) Concentration of capsaicin (CAP)–evoked CGRP release from the lumbar region of spinal cords isolated from naïve female mice after intrathecal PRLR-L gene editing (n = 6 per group; P = 0.0059). (C to F) Effect of intrathecal administration of BIBN 4096 [(C) and (D), vehicle, n = 8; BIBN 4096, n = 7; hindpaw, P = 0.2272; periorbital, P = 0.6830] or MK-801 [(E) and (F), n = 7 per group; hindpaw, P < 0.0001; periorbital, P = 0.9503] on hindpaw and periorbital allodynia induced by intrathecal CRISPR–PRLR-L in female mice. Two-way repeated-measures ANOVA with Sidak’s multiple comparisons test (B to F). *P < 0.05. Group data are expressed as means ± SEM. Each data point represents an individual animal.
The peripheral PRL/PRLR system is not critical for trauma-induced neuropathic pain in female mice
Because decreased expression of PRLR-L was sufficient to induce hindpaw tactile allodynia in naïve female mice, we asked whether PRLR-L expression might be disrupted in female mice with trauma-induced chronic pain. Spared nerve injury (SNI) is a commonly used model of neuropathic pain that produces unilateral hindpaw allodynia in mice (25). We found that SNI did not alter protein expression of PRLR-L and PRLR-S (fig. S2A). In addition, intrathecal CRISPR-Total-PRLR editing failed to prevent or reverse hindpaw tactile allodynia in SNI animals (fig. S2, B and C). These results suggest that allodynia observed in this trauma-induced neuropathic pain model in female mice is not dependent on the PRL/PRLR system.
Opioids disrupt PRL/PRLR in DRGs, thereby promoting OIH in female mice
Opioids are known to transiently increase serum PRL concentrations (26–28). In addition, opioids elicit OIH (16), but whether OIH is related to PRL is not known. To determine whether disruption of PRL secretion or PRLR isoform expression may contribute to OIH, we treated adult female and male mice subcutaneously with a single control or morphine pellet; we confirmed results in some studies by repeated twice daily subcutaneous morphine injections. After implantation of the subcutaneous morphine pellet (fig. S3) or with repeated morphine injections (fig. S4, A and B), allodynia was observed in both male and female mice. Serum PRL concentration was increased at day 1 after morphine pellet implantation in female mice (control versus morphine: 6.16 ± 2.22 versus 19.50 ± 4.02 ng/ml). In males, PRL concentration was also higher in morphine-treated mice (control versus morphine: 1.06 ± 0.30 versus 3.06 ± 0.72 ng/ml) (Fig. 4A). Consistent with previous reports (26–28), increased PRL concentration after the morphine pellet treatment or repeated morphine injection was not maintained (Fig. 4A and fig. S4C), suggesting that sustained PRL increase is not required for OIH. We then tested whether protein expression of PRLR-L and PRLR-S isoforms in DRGs of female mice could be altered by sustained morphine exposure. We found that either morphine pellet treatment or daily injection of morphine suppressed the expression of PRLR-L protein but had no effect on PRLR-S expression in female DRGs. We found that the expression ratio of PRLR-L to PRLR-S in DRGs was reduced by 67.6 ± 14.8% in morphine-pelleted female mice compared with control-pelleted mice (Fig. 4B), and by 66.1 ± 12.1% in morphine-injected mice compared with saline-injected mice (fig. S4D). Because we used two antibodies with different affinities for the PRLR-L or PRLR-S isoforms (see Materials and Methods), we were unable to determine the absolute expression ratio of PRLR-L/PRLR-S.
Fig. 4. Down-regulation of PRLR-L in DRGs is sufficient for the development of female OIH.
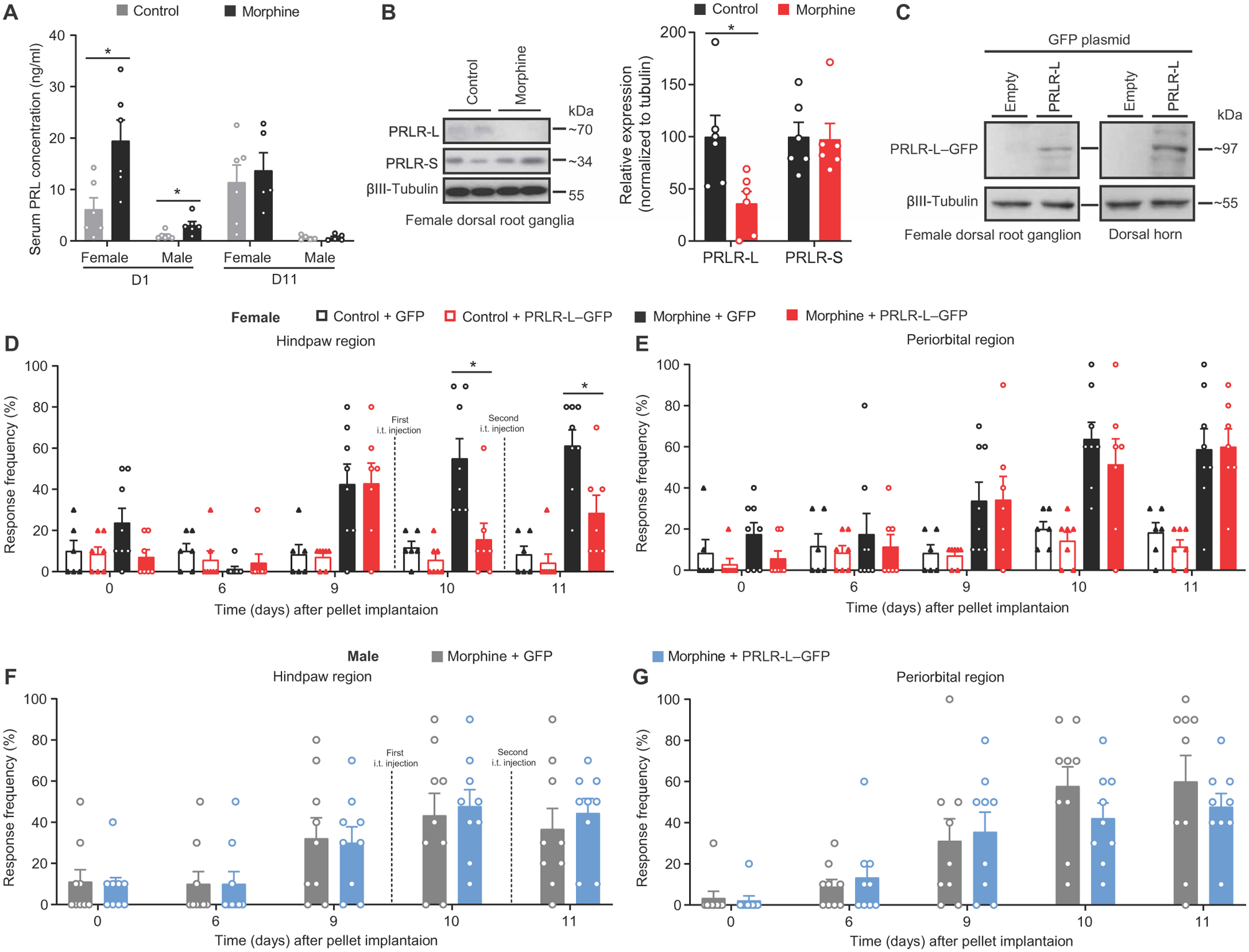
(A) Serum concentration of PRL at days 1 and 11 after morphine or control pellet implantation in mice (day 1: n = 6 per group per sex; female, P = 0.0260; male, P = 0.0260; day 11: female: control n = 6, morphine n = 5, P = 0.6623; male: n = 5 per group, P = 0.8413). (B) Representative images (left) and quantification (right) of PRLR isoform expression in DRGs from control or morphine pelleted female mice (n = 6 per group; PRLR-L, P = 0.0411; PRLR-S, P = 0.8182). (C) Representative Western blot images of PRLR-L–GFP expression in DRGs and spinal cord dorsal horns from naïve female mice with intrathecal delivery of PRLR-L–GFP plasmid (n = 3 per group). (D to G) Effect of intrathecal delivery of PRLR-L–GFP plasmid on hindpaw and periorbital allodynia in control or morphine pelleted female (D and E) and morphine pelleted male (F and G) mice (female: control + GFP, n = 6; control + PRLR-L–GFP, n = 7; morphine + GFP, n = 8; morphine + PRLR-L–GFP, n = 7; hindpaw P < 0.0001, periorbital P = 0.0003; male: n = 9 per group; hindpaw P = 0.8989, periorbital P = 0.3352). Two-tailed Mann-Whitney test (A and B). Two-way repeated-measures ANOVA with Sidak’s multiple comparisons test (D to G). *P < 0.05. All data are expressed as means ± SEM. Each data point represents an individual animal.
To test whether morphine-induced PRLR-L down-regulation might be responsible for allodynia in females, we attempted to increase PRLR-L expression in DRGs by intrathecal delivery of a plasmid coding for PRLR-L fused to GFP (Fig. 4C) in female mice with confirmed OIH. One day after the plasmid injection, we observed reversal of hindpaw allodynia (Fig. 4D), demonstrating the restoration of the protective function of the PRLR-L in females. Periorbital allodynia induced by morphine, measured in the same mice, was still present (Fig. 4E), again providing an internal control confirming the contribution of PRL/PRLR signaling to hindpaw OIH from systemic morphine treatment. In contrast, overexpression of PRLR-L did not affect allodynic responses in male mice (Fig. 4, F and G). These data suggest a PRLR-dependent mechanism mediating OIH selectively in females.
Suppression of PRL/PRLR-S inhibits nociceptor activity and OIH in a female-specific manner
We next investigated whether down-regulation of PRLR-L induced by opioids would result in amplified consequences of PRL at PRLR-S. Because PRLR-S activation in sensory neurons has been linked to increased neuronal activity (8), we used a microelectrode array (MEA) to test whether exogenous PRL could increase the spontaneous firing of cultured DRG sensory neurons from opioid-treated female mice. Firing of DRG neurons from control pellet implanted mice was not observed. In contrast, DRG neurons from female mice with confirmed OIH fired spontaneously at a rate that approximately doubled after PRL application (Fig. 5, A and B). To test whether amplified signaling at PRLR-S might promote OIH in females, we edited the Prlr gene with a gRNA directed against exon 4 (CRISPR-Total-PRLR) before morphine treatment. Ablation of Prlr by intrathecal CRISPR-Total-PRLR (Fig. 5C) prevented the development of hindpaw, but not periorbital, allodynia in female mice (Fig. 5, D and E). The same treatment had no effect on either hindpaw or periorbital allodynia in males (Fig. 5, F and G).
Fig. 5. Opioid-induced nociceptor activity and OIH in females is mediated by PRLR-S.
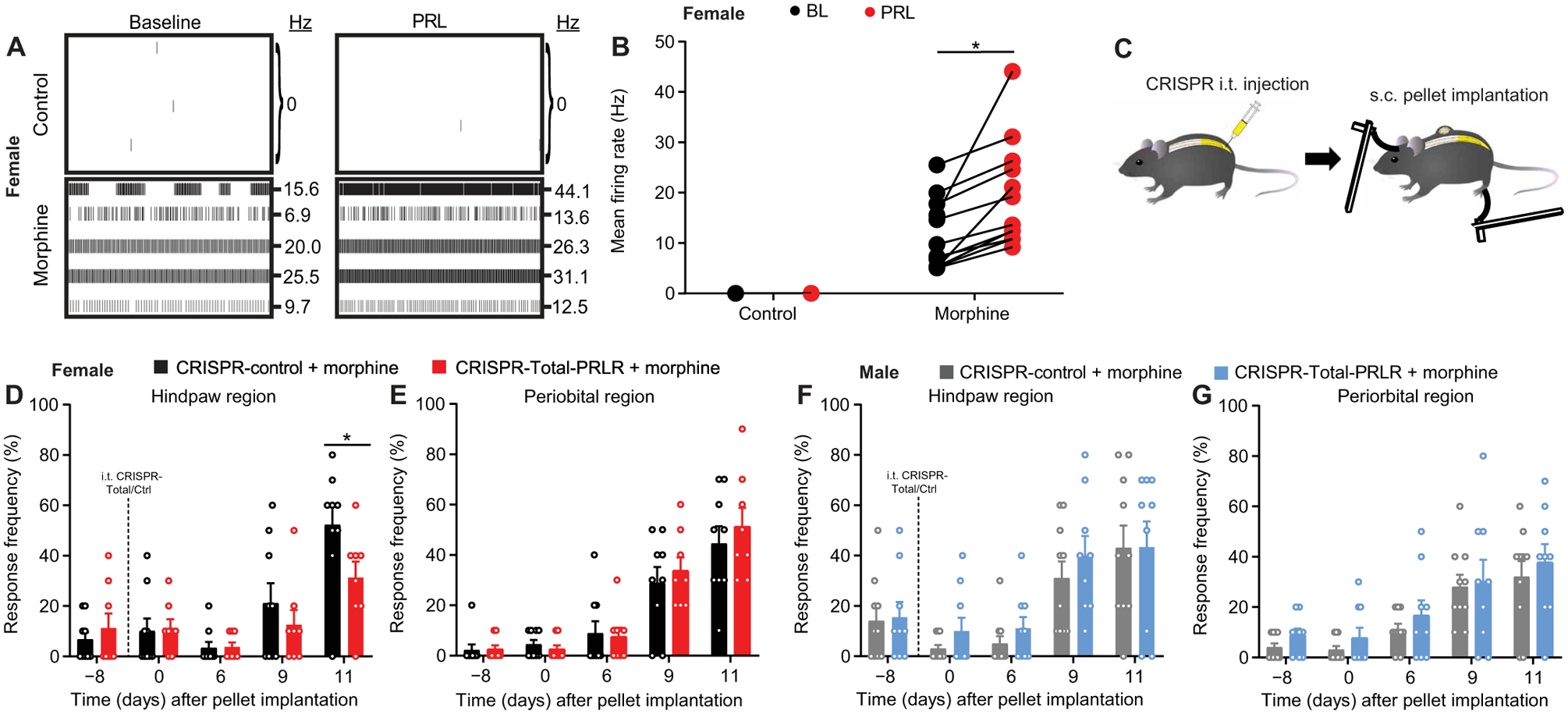
(A and B) Representative MEA recording images (A) and mean action potential firing rates (B) of cultured DRG sensory neurons from control or morphine pelleted female mice before and after exogenous PRL application (n = 12 electrodes per group; P = 0.0005). (C) Schematic of experiment design for (D) to (G). s.c., subcutaneous. Naïve mice were intrathecally injected with CRISPR vectors, followed by subcutaneous pellet implantation and tactile allodynia testing. (D to G) Hindpaw and periorbital allodynia in female (D and E) and male (F and G) mice with intrathecal CRISPR-Total-PRLR treatment before morphine pellet implantation. (n = 9 per group per sex; female: hindpaw P < 0.0001, periorbital P = 0.2822; male: hindpaw P = 0.7116, periorbital P = 0.1099). Two-tailed Wilcoxon test (B). Two-way repeated-measures ANOVA with Sidak’s multiple comparisons test (D to G). *P < 0.05. All data are expressed as means ± SEM. Each data point represents an individual electrode or animal.
To further interrogate the role of circulating PRL signaling at PRLR-S in female mice with OIH and evaluate the possible translational value of pharmacological suppression of circulating PRL, we assessed the efficacy of cabergoline in OIH. Cabergoline treatment markedly lowered circulating PRL in female and male mice implanted with either control or morphine pellets (Fig. 6A). Repeated cabergoline treatment up-regulated PRLR-L expression in DRGs of female mice with control as well as morphine pellets (Fig. 6B). This suggests that nociceptor PRLR-L expression is negatively regulated by circulating PRL in female mice and that treatment with cabergoline can prevent the down-regulation of PRLR-L that may occur in pathological states. Using MEA, we evaluated the spontaneous firing of DRG sensory neurons from morphine-pelleted female mice with repeated injection of cabergoline or vehicle. Increased firing of DRG neurons from female or male mice was observed after morphine pellet and vehicle. Addition of PRL resulted in a further increase in firing of DRG neurons from female mice; no effect of PRL was observed in DRG neurons from male mice. This further supports a lack of sensitivity of male mice to PRL. In contrast, we saw reduced firing of DRG neurons in response to PRL in female mice receiving morphine pellet and cabergoline (Fig. 6C). Next, we assessed whether suppressing circulating PRL/PRLR-S signaling with cabergoline could impair the development of OIH. We observed an attenuation of both hindpaw and periorbital allodynia in female mice receiving morphine and cabergoline, but not vehicle. Note that unlike intrathecal manipulation of PRLR isoform expression, decreasing circulating concentration of PRL reversed OIH in both the hindpaw and periorbital regions, reflecting the consequences of systemic treatment (Fig. 6, D and E). In contrast, cabergoline had no effect on the development of OIH in male mice (Fig. 6, F and G). These data suggest that treatment with cabergoline can prevent the pathological opioid-induced down-regulation of PRLR-L, opioid-induced nociceptor sensitization, and OIH. These findings identify the opioid-induced down-regulation of PRLR-L and subsequent shift toward circulating PRL/PRLR-S signaling as an additional mechanism mediating OIH in females.
Fig. 6. Repeated administration of cabergoline reduces nociceptor excitability and OIH selectively in female mice.
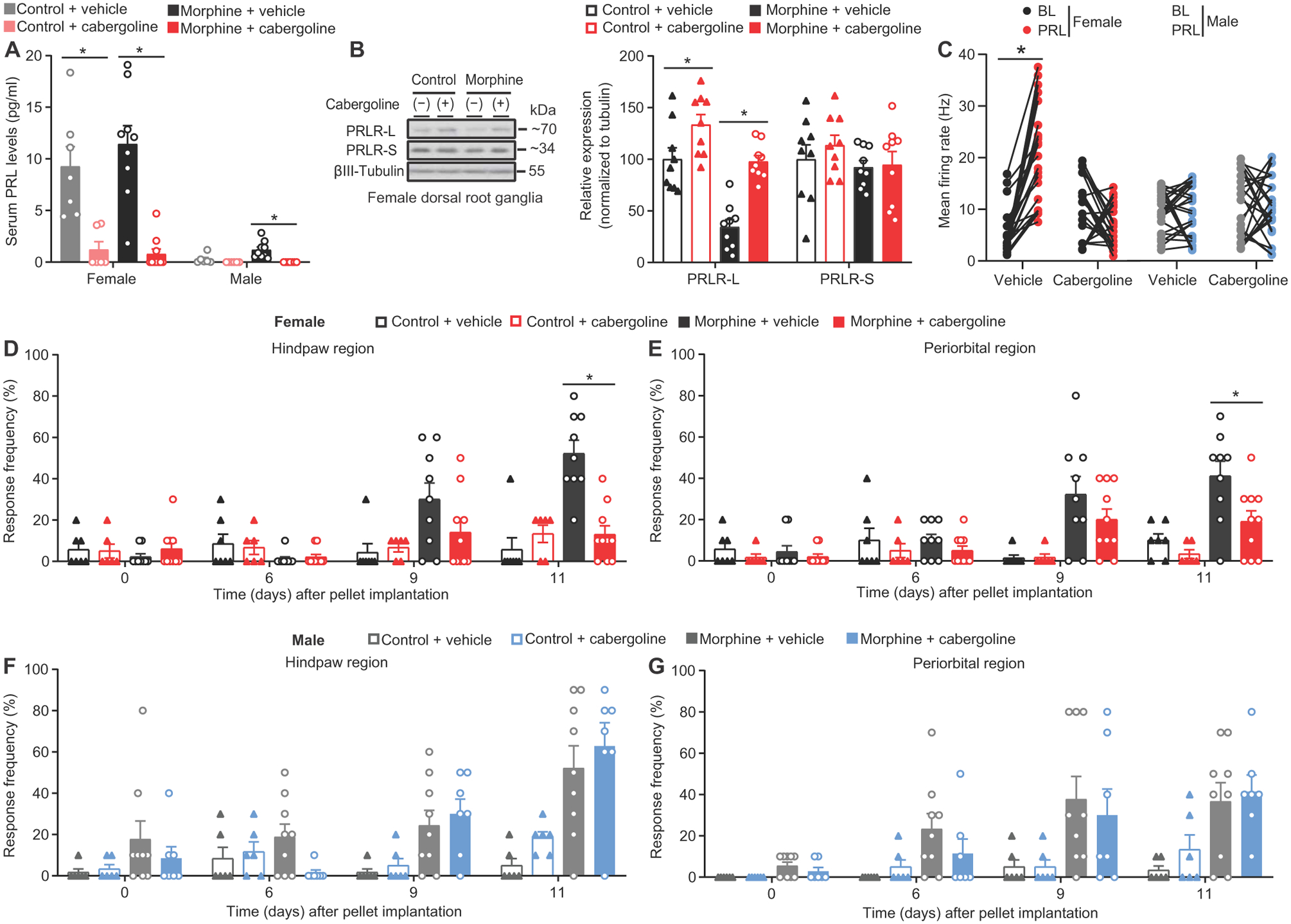
(A) Serum PRL concentration in mice receiving control/morphine pellet and vehicle/cabergoline from (K) to (L) (female: n = 7, 6, 9, and 10, respectively; P < 0.0001; male: n = 6, 6, 9, and 7, respectively; P = 0.0002). (B) Representative Western blot images (left) and quantification (right) of PRLR isoform expression in DRGs from female mice receiving control/morphine pellet and vehicle/cabergoline (n = 9/group; PRLR-L P < 0.000, PRLR-S P = 0.5345). (C) Mean action potential firing rates before and after PRL application in cultured DRG sensory neurons from mice receiving control/morphine pellet and vehicle/cabergoline (female: n = 24 electrodes in the vehicle group, P < 0.0001; n = 22 electrodes in the cabergoline group, P = 0.0645; male: n = 26 electrodes in the vehicle group, P = 0.8380; n = 23 electrodes in the cabergoline group, P = 0.9471). (D to G) Effect of repeated administration of cabergoline on development of opioid-induced hindpaw and periorbital allodynia in female (D and E) and male (F and G) mice from (A) (female: hindpaw P < 0.0001, periorbital P < 0.0001; male: hindpaw P = 0.1684, periorbital P = 0.6690). Two-tailed paired t test (C). One-way ANOVA with Tukey’s multiple comparison test (A and B). Two-way repeated-measures ANOVA with Sidak’s multiple comparisons test (D to G). *P < 0.05. All data are expressed as means ± SEM. Each data point represents an individual electrode or animal.
Mu-opioid receptor is required for opioid-induced down-regulation of PRLR-L in DRGs from female mice
We asked whether the morphine-induced down-regulation of PRLR-L in females with OIH relies on activation of the Mu-opioid receptor (MOR). To determine whether the PRLR-L is colocalized with the MOR in the DRG, we used PRLR-LCre/−/GFPflx/−/MOR-mCherry+/− mice to label PRLR-LCre–positive and MOR-positive cells in same animals. We determined that ~80% of PRLR-LCre/−/GFPflx/−–positive neurons also expressed MOR in DRGs from both males and females (Fig. 7, A and B). This suggests potential interactions between MOR and PRLR-L in female DRG neurons that could elicit OIH and PRLR-L down-regulation. (−)-Oxymorphone is an agonist that binds to the MOR with nanomolar affinity, whereas (+)-oxymorphone exhibits negligible MOR binding at a concentration of 10 μM (table S1). Sustained administration of (−)-oxymorphone resulted in hindpaw and periorbital tactile allodynia, whereas (+)-oxymorphone had no effect (Fig. 7, C and D). Consistent with OIH, we found that (−)-oxymorphone, but not (+)-oxymorphone, induced a decrease of PRLR-L expression in female mice (Fig. 7E).
Fig. 7. MOR is required for opioid-induced PRLR-L down-regulation and OIH development in female mice.
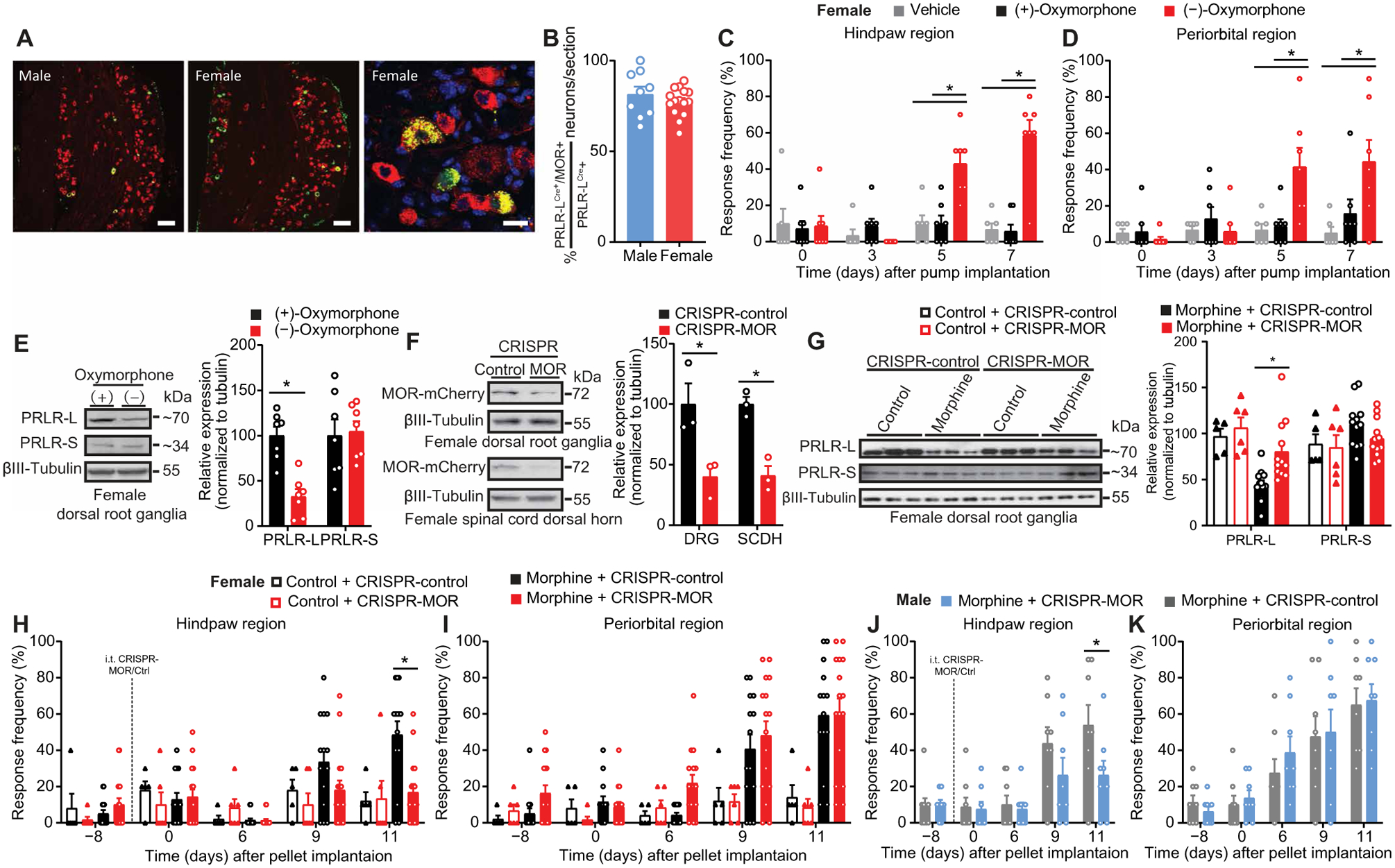
(A) Representative images showing colocalization of MOR-mCherry+/− (red) and PRLR-LCre/− (green) in DRGs from PRLR-LCre/−/GFPflx/−/MOR-mCherry+/− male and female mice. Scale bars, 100 μm (left two panels) and 20 μm (right panel). (B) Quantification of the percentage of PRLR-LCre/MOR-mCherry coexpressing neurons among total PRLR-LCre–expressing neurons in L3–5 DRGs from PRLR-LCre/GFPflx/−/MOR-mCherry+/− mice (male, n = 3; female, n = 5). (C and D) Effect of sustained administration of (+)- or (−)-oxymorphone on hindpaw and periorbital allodynia in female mice [vehicle, n = 6; (+)/(−)-oxymorphone, n = 7; hindpaw P < 0.0001, periorbital P = 0.0001]. (E) Representative Western blot images (left) and quantification (right) of PRLR isoform expression in DRGs of female mice with (+)/(−)-oxymorphone treatment from (C) and (D) (PRLR-L, P = 0.0012; PRLR-S, P = 0.7104). (F) Verification of intrathecal CRISPR-MOR editing efficiency in DRGs and spinal cord dorsal horn tissues from naive MOR-mCherry+/+ female mice [n = 3 per group; DRG, P = 0.0363; SCDH (spinal cord dorsal horn), P = 0.0041]. (G) Representative Western blot images (left) and quantification (right) of PRLR isoform expression in DRGs from control or morphine pelleted female mice from (H) and (I) with intrathecal CRISPR-MOR or CRISPR-control (control + CRISPR-control, n = 5; placebo + CRISPR-MOR, n = 6; n = 12 per morphine group; PRLR-L, P < 0.0001; PRLR-S, P = 0.1628). (H to K) Effect of intrathecal CRISPR-MOR before pellet implantation on hindpaw and periorbital allodynia in female (H and I) and male (J and K) mice (female: control + CRISPR-control, n = 5; control + CRISPR-MOR, n = 6; morphine + CRISPR-control, n = 14; morphine + CRISPR-MOR; n = 16; hindpaw P < 0.0001, periorbital P < 0.0001; male: n = 9 per group; hindpaw P = 0.0324, periorbital P = 0.7675). Two-tailed Mann-Whitney test (B and E). Two-tailed t test (F). One-way ANOVA with Tukey’s multiple comparison test (G). Two-way repeated-measures ANOVA with Sidak’s multiple comparisons test (C, D, and H to K). *P < 0.05. All data are expressed as means ± SEM. Each data point represents an individual animal.
To further test whether MOR activation is required for a decrease in PRLR-L expression and OIH, we designed a gRNA to edit the gene coding MOR, Oprm1. We used MOR-mCherry+/+ mice where mCherry is fused with MOR (29) to determine the efficiency of CRISPR-MOR (Fig. 7F). In female mice with OIH, intrathecal MOR editing prevented the decrease in PRLR-L expression (Fig. 7G) as well as hindpaw allodynia (Fig. 7H). Intrathecal MOR gene editing did not reduce the periorbital allodynia resulting from morphine pellet treatment (Fig. 7I), providing an internal control for the effects of intrathecal editing of the MOR. Similarly, intrathecal MOR editing in male mice also prevented the development of hindpaw, but not periorbital, allodynia (Fig. 7, J and K). Our data suggest that MOR activation drives OIH development in both male and female mice but that, in addition, a specific peripheral PRL/PRLR-S signaling mechanism is unlocked in females to promote OIH.
The peripheral PRL/PRLR system promotes migraine-like pain in female mice
Our data showed that the PRL/PRLR system is important for nociceptor sensitization that occurs in the absence of injury. For this reason, we evaluated the possible contribution of the PRL/PRLR mechanism in a second injury-free pain condition. High concentrations of PRL are associated with increased migraine (30). Female mice that had been implanted with subcutaneous control pellets received a direct injection of PRL on the dura mater via the lambdoid suture, resulting in migraine-like pain (Fig. 8, A and B). Opioids are commonly used as acute headache treatments despite its association with increased risk for development of chronic migraine (31). Female mice were implanted with a morphine pellet and, after resolution of allodynia, demonstrated an increase in sensitivity to dural PRL (Fig. 8, A to D). After morphine pretreatment, lower and previously ineffective doses of PRL were able to produce allodynia (Fig. 8, E and F). These results suggest that the PRL/PRLR system may also play a role in other injury-free pain conditions with female prevalence, including migraine.
Fig. 8. Activation of PRLR in the dural matter induces migraine-like pain responses in female mice.
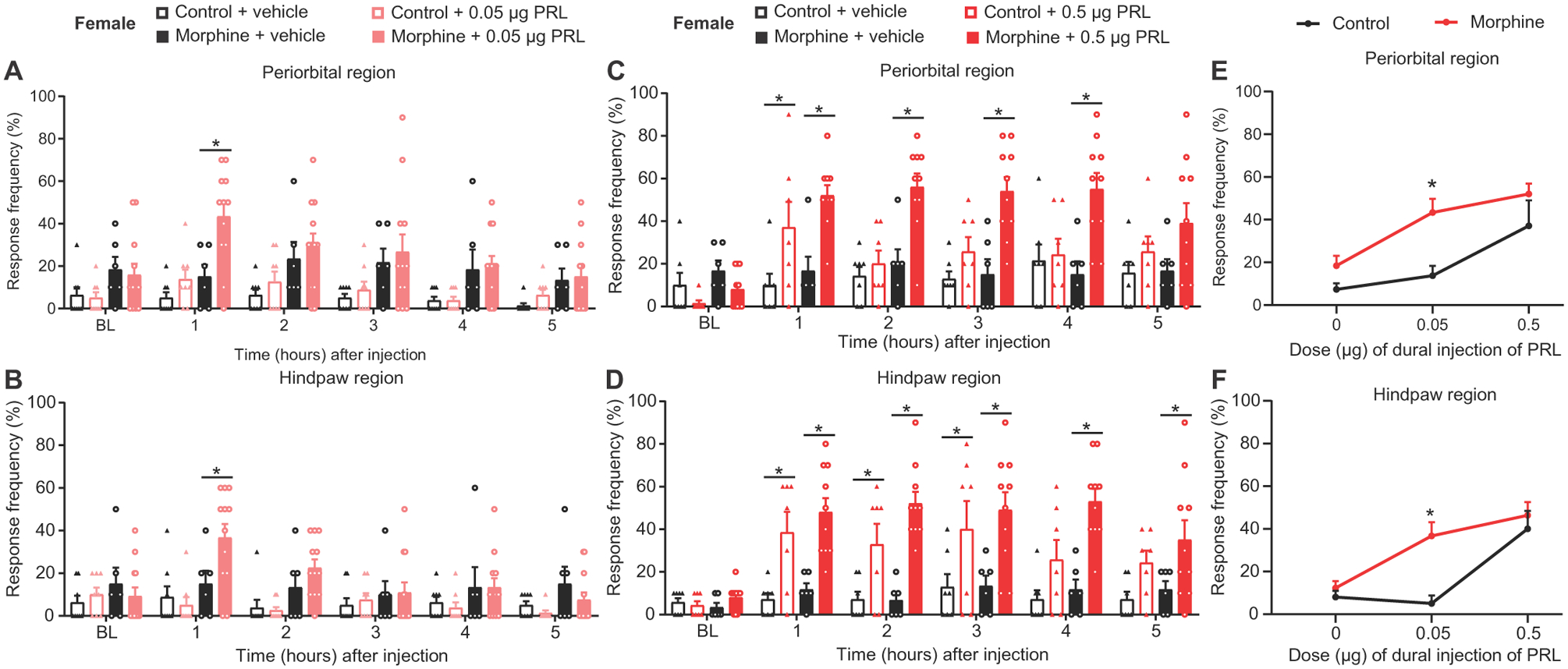
(A and B) Effect of dural injection of 0.05 μg of PRL on periorbital and hindpaw allodynia in control- or morphine-pelleted female mice (control + vehicle, n = 8; control + 0.05 μg PRL, n = 8; morphine + vehicle, n = 6; morphine + 0.05 μg PRL, n = 12; periorbital, P = 0.0002; hindpaw, P < 0.0001). (C and D) Effect of dural injection of 0.5 μg of PRL on periorbital and hindpaw allodynia in control- or morphine-pelleted female mice (control + vehicle, n = 7; morphine + 0.5 μg PRL, n = 7; morphine + vehicle, n = 6; morphine + 0.5 μg PRL, n = 10; periorbital, P < 0.0001; hindpaw, P = 0.0003). (E and F) Dose-response curve for periorbital (E) and hindpaw (F) allodynia produced by dural injection of PRL in female mice from (A) to (D) (periorbital, P = 0.00024; hindpaw, P = 0.0005). Two-way repeated-measures ANOVA with Sidak’s multiple comparisons test (A to F). *P < 0.05. All data are expressed as means ± SEM. Each data point represents an individual animal.
DISCUSSION
Sex-dependent pain mechanisms have been linked to gonadal hormones including estrogen and testosterone (13, 32). More recently, it has become clear that PRL, an estrogen-regulated neurohormone, may also be associated with pain in women (8, 23, 33). PRL has been shown to produce sensitization of TRPV1-positive sensory neurons from females through a hypothesized PRL/PRLR-S signaling mechanism; sensitization of sensory neurons from males can be achieved by PRL at much higher doses than required in females (8, 23). Estrogen promotes, whereas testosterone suppresses, the synthesis and secretion of circulating PRL (34) as well as expression of the PRLR (35–37). Considering the mutual inhibition of nociceptor excitability of the PRLR isoforms observed in cultured neurons (8), we hypothesized that endogenous pain-protective mechanisms may result from the expression of PRLR-L that might be disrupted in pathological states. We determined whether PRLR isoform expression is modulated in a sex-dependent manner using experimental mouse models of OIH and whether circulating PRL/PRLR-S signaling can promote nociceptor sensitization and pain in females. We also assessed the role of PRL/PRLR-S signaling in models of neuropathic pain and migraine.
We found that the number of PRLR-LCre–positive neurons and expression of PRLR-L protein were greater in DRGs of female, compared with male, mice. Although PRLR-LCre–positive neurons were observed in male DRGs, Western blot analysis showed only low expression of protein. In contrast, both PRLR-L and PRLR-S protein expression was observed in females. The predominant presence of PRLR-L protein in females raised the possibility that this isoform is a key factor in preventing nociceptor sensitization that could result from relatively high concentrations of circulating PRL. Consistent with previous reports, PRL concentrations were higher in females than in males. When we treated male or female mice with cabergoline, a dopamine D2 agonist, concentrations of PRL were decreased. However, we found that cabergoline increased the expression of PRLR-L but did not alter PRLR-S expression in female mice. This finding suggests that circulating PRL tonically suppresses PRLR-L expression in DRG cells of female mice. We determined the consequences of PRLR-L expression on pain behaviors using CRISPR-Cas9 technology and found that disruption of PRLR-L expression in DRGs was sufficient to produce allodynic responses in naïve female, but not male, mice without injury. Furthermore, after reduction in PRLR-L, the allodynia in female mice was blocked by cabergoline, confirming the essential role of circulating PRL and suggesting that pain was promoted by PRL signaling through PRLR-S. To further test this possibility, we first established allodynia in naïve female mice with intrathecal administration of CRISPR–PRLR-L and then administered a second intrathecal CRISPR vector to edit total PRLR, an indirect strategy to delete PRLR-S. Using this approach, we demonstrated reversal of allodynia induced by intrathecal CRISPR–PRLR-L. These studies reveal that PRLR-L expression protects against pain promoted by circulating PRL signaling at PRLR-S in females. Note that in rodents, cabergoline has been reported to effectively lower serum PRL concentration within 2 hours (38), and this effect persists for about 72 hours (39). Our studies similarly showed that cabergoline lowered serum PRL, but reversal of allodynia was not observed until 2 days after treatment. This observation suggests that after cabergoline, PRL concentration was not sufficiently low to prevent signaling at PRLR-S in females. Rather, reversal of allodynia may depend on subsequent time-related up-regulation of PRLR-L promoted by decreased circulating PRL.
We then explored possible mechanisms by which CRISPR–PRLR-L gene editing might promote pain in the absence of injury in naïve female mice. Because the PRLR has been reported to be expressed primarily in TRPV1- (9, 33) and CGRP-positive neurons (40, 41), we used capsaicin to demonstrate enhanced evoked release of CGRP in spinal cord tissues from female mice that had undergone CRISPR-Cas9–mediated editing of the PRLR-L gene. Although increased evoked release of CGRP was observed, the allodynia induced by editing of the PRLR-L gene was not blocked by a single, intrathecal administration of a CGRP receptor antagonist, BIBN 4096; this dose of BIBN 4096 was effective, however, in blocking intrathecal CGRP-induced allodynia in naïve mice. In contrast, intrathecal administration of MK-801, an NMDA antagonist, effectively blocked the allodynia induced by PRLR-L gene editing. These findings suggest that normally innocuous stimuli applied to the hindpaw of CRISPR–PRLR-L–treated female mice result in an increase in glutamatergic transmission that may contribute to subsequent adaptations that promote spinal sensitization and allodynia (42–44).
Our previous work has shown that pain and stress can engage overlapping circuits in the brain (45, 46). Stress can increase concentration of circulating PRL (1), and the local release of PRL has been shown to contribute to hypersensitivity after incisional injury (47). We therefore determined whether PRLR isoform expression was disrupted in a mouse model of trauma-induced neuropathic pain elicited by injury to peripheral nerves. SNI did not result in changes in PRLR isoform expression in the DRG of female mice. In addition, we found no effect of total PRLR gene editing either before or after induction of chronic neuropathic pain in female mice. Opioids are known to produce hyperalgesia and nociceptor sensitization (14, 15) to transiently increase PRL blood concentration (26–28), and patients on methadone maintenance have been reported to experience hyperprolactinemic galactorrhea (17, 18). We therefore investigated whether PRL/PRLR signaling may influence the injury-free hypersensitivity produced by opioids. Our studies showed that about 80% of DRG cells expressing PRLR-LCre/−/GFPflx/− were also MOR positive, suggesting a potential cellular mechanism by which opioids might modulate PRLR-L function. After administration of morphine from a subcutaneously implanted pellet or with repeated intraperitoneal injections, increased sensitivity to probing with von Frey filaments was observed in the periorbital region and in the hindpaws of both female and male mice, demonstrating the presence of OIH. The morphine pellet also increased the concentration of circulating PRL in both female and male mice, but this effect was not sustained. However, expression of PRLR-L in DRGs from females was decreased after the morphine pellet treatment or with repeated intraperitoneal morphine injections. To determine whether OIH in females was the result of disruption of PRLR-L expression by opioids, we overexpressed PRLR-L through an intrathecal vector injection after the development of OIH and demonstrated rescue of hindpaw, but not periorbital, allodynia in females with OIH. In addition, we delivered a gRNA to disrupt the total PRLR expression at the lumbar spinal level and demonstrated that hindpaw, but not periorbital, OIH was blocked.
To evaluate the translational value of this approach, we repeatedly treated mice with cabergoline and found decreased concentration of circulating PRL in both female and male mice, but periorbital and hindpaw OIH were only blocked in females. Note that unlike selective modulation of PRLR isoforms in the DRG with intrathecal vector manipulations, systemic administration of cabergoline resulted in global blockade of allodynic responses. To confirm that the behavioral effects were due to increased sensitivity to PRL after opioid treatment, we measured excitability of DRG cells taken from female animals treated with control or morphine pellets. We found spontaneous firing only in cells from morphine-treated female mice. Addition of PRL further increased spontaneous firing of cells from morphine-but not control-treated females. The potentiating effect of PRL was not observed in cells from morphine-pelleted females receiving cabergoline. PRL had no effect in cells from morphine-treated male mice regardless of vehicle or cabergoline cotreatment.
Consistent with previous studies (14, 48), morphine produced OIH in both female and male mice. However, we found that mechanisms of OIH are sex dependent. We discovered down-regulation of PRLR-L, PRL-dependent nociceptor sensitization, and OIH selectively in female mice. OIH has been suggested to result from MOR-mediated sensitization of primary afferent nociceptors in female and male mice (14), but nonopioid receptor mechanisms have also been reported (49). We therefore determined whether MOR was necessary for PRLR-L down-regulation in females. We found that only the active enantiomer of oxymorphone, an agonist that shows affinity to the MOR, produced down-regulation of PRLR-L and OIH. In addition, intrathecal CRISPR-Cas9–mediated editing of the MOR gene prevented morphine-induced down-regulation of PRLR-L in lumbar DRGs and blocked hindpaw, but not periorbital, OIH. Because opioids produce only a transient increase in circulating PRL, OIH likely results from the already high concentration of PRL in females after down-regulation of PRLR-L by MOR agonists. PRL signaling at PRLR-S likely leads to the subsequent postsynaptic adaptations contributing to OIH that have previously been reported after opioid administration (42–44). Our results are therefore in agreement with previous conclusions of requirement of peripheral MORs for the establishment of OIH in male mice (14), but now demonstrate an additional female-selective mechanism.
There is a lack of information on whether women are more prone to OIH than men. This is a complex clinical question that likely depends on many factors including menopausal status, pain conditions, as well as opioid use and/or abuse. Note that women are more likely to have chronic pain, which could contribute to the high rates of opioid prescriptions among women of reproductive age (50). In addition, women may be more likely to misuse prescription opioids to cope with pain and other problems such as anxiety or tension (51), even when men and women report similar degrees of pain (52). Thus, women might more likely develop opioid-induced side effects. However, to date, most OIH research has not emphasized possible sex differences. An additional limitation is that although the transcript for PRLR has been identified in human DRG, it remains unclear whether there is sexually dimorphic expression of the PRLR and whether the expression of PRLR is linked to circulating PRL. Future studies are also needed to determine how opioids may regulate the expression of the PRLR-L in DRG neurons. In this regard, we note that PRLR-L is highly colocalized with MOR in DRGs of female mice, and MOR gene editing prevented the opioid-induced decrease in PRLR-L, suggesting that an interaction between MOR and PRLR-L could occur in the same DRG neuron. We cannot exclude, however, extracellular mechanisms involving opioid-induced release of nonopioid mediators from other neurons, satellite glia, or immune cells that may result in PRLR-L degradation and/or decreased translation of PRLR-L mRNA. In vitro cancer research shows that PRLR-L down-regulation requires activation of Janus kinase 2 (JAK2) (53). After chronic morphine treatment, JAK2 expression in DRGs is increased (54), possibly reflecting a mechanism that could lead to the reduction in PRLR-L expression by opioids. We found that inhibition of circulating PRL with cabergoline increased the expression of PRLR-L, suggesting that high concentrations of circulating PRL as found in PRL-secreting tumors might decrease the expression of PRLR-L and promote pain. Stress is also known to increase circulating PRL (1) and may decrease PRLR-L expression, providing a mechanism by which many injury-free, “functional,” female-prevalent pain disorders such as migraine could be triggered (55, 56). Consistent with this possibility, administration of PRL to the dura mater of female mice promoted migraine-like pain that was amplified by pretreatment with morphine. This observation raises the possibility that the PRL/PRLR mechanism may have more general relevance to injury-free pain conditions with female prevalence. In contrast, we found that PRLR-L down-regulation and PRL/PRLR signaling were not involved in trauma-related neuropathic pain. We conclude that in females, decreased expression of PRLR-L is sufficient, but not necessary, to elicit nociceptor sensitization and to promote enhanced evoked excitatory glutamatergic, but not CGRP-mediated, inputs to the spinal cord. We did not evaluate the estrous stage of the mice in these experiments. Nevertheless, our results were highly reproducible, suggesting a robust impact of this mechanism regardless of estrous stage.
We reveal a sex-dependent mechanism promoting pain at the level of the nociceptor, the fundamental building block of pain. These observations provide avenues for therapy in women, including the use of dopamine agonists such as cabergoline or bromocriptine, drugs that have shown effectiveness in multiple medical conditions caused by hyperprolactinemia (57). Clinical trials of prolactinoma-related headache have shown therapeutic benefit of dopamine agonists (5). We note that dopaminergic agonists could have off-target actions at other receptors (58) and that when used for Parkinson’s disease, have been implicated in heart complications (59); it has consistently been shown that long-term use of cabergoline for hyperprolactinemia does not increase the risk of cardiovascular reactivity (60, 61), likely due to the differences in doses used in the treatments of these conditions. Note that cabergoline, like all drugs, may have off-target effects at other receptors. Thus, cabergoline-like dopamine agonists could be useful for long-term treatment of OIH and other PRL-promoted pain conditions in women (fig. S5). Treatment with dopamine agonists may target the PRLR system and nociceptor sensitization in two ways: by lowering circulating PRL and also up-regulating PRLR-L. These actions would provide benefit in preventing the development of OIH in women without impairing the therapeutic actions of opioids and likely also in treatment of other sensitized states including, for example, stress-related pain conditions. A second therapeutic approach might include antibodies targeting PRL or the PRLR such as BAY 1158061, which do not require central nervous system penetration (62). In vivo gene therapies are increasingly common in medicine and could be used to edit the total PRLR or to increase the expression of PRLR-L (63–65). Our studies reveal actionable mechanisms for therapies, allowing improved treatment of pain in women. The prevention of OIH would also be valuable in diminishing the negative outcomes of opioids that have resulted in the current opioid epidemic in the United States.
MATERIALS AND METHODS
Study design
Our study goal was to identify the mechanisms mediating sexual dimorphism in pain transmission and processing at the level of nociceptors and to provide potential therapeutic strategies for pain control in women. We hypothesized that PRLR-L in DRGs protects against pain promoted by PRLR-S signaling in women, whereas down-regulation of PRLR-L mediates OIH and other injury-free pain conditions including migraine but not trauma-induced neuropathic pain in a female-selective manner. Therefore, overexpression of PRLR-L, deletion of total PRLR in DRGs, or lowering of circulating PRL concentrations by FDA-approved dopamine D2 agonist might provide benefit in preventing OIH, migraine, and maybe other PRLR-S–mediated pain conditions in women. The research objects were C57BL/6 and transgenic mice as well as cultured DRG cells from mice. Calculation of sample size was determined on the basis of previous experiments using g-power statistics, indicating that 6 to 10 animals per group are required for α < 0.05. The exact n numbers used in each study are indicated in the respective figure legends. For behavioral experiments, data from animals that died or had severe health problems in the middle of the experiments were excluded (<5%). Experiments were completed in multiple time periods in both male and female mice, ensuring that replication was observed. Western blotting, MEA, and immunohistochemical imaging data were reproduced in multiple mice. Animals were assigned randomly to experimental and control groups. The experimenters were blinded for animal allocation and behavioral testing until all data collection was complete. In the case of experiments with repeated drug administration, tissue collection, performance of Western blotting and MEA experiments, and data analysis, the experimenter was not blinded.
Statistical analysis
Data are expressed as means ± SEM. Each data point represents an individual score. Statistical analyses were performed using GraphPad Prism 8 (GraphPad, La Jolla, CA). Differences between mean values of two groups were evaluated by two-tailed Mann-Whitney test, two-tailed Student’s t test, two-tailed Wilcoxon test, and two-tailed paired t test. Mean differences of more than two groups were analyzed using one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons post hoc test. Differences in time-course experiments were assessed by two-way ANOVA with Sidak’s multiple comparison post hoc test. Differences were considered to be statistically significant when P < 0.05. Exact P values and sample sizes are indicated in the respective figure legends.
Supplementary Material
Acknowledgments:
We thank B. Roth at UNC for binding assay support through the National Institute of Mental Health’s Psychoactive Drug Screening Program, contract no. HHSN-271-2013-00017-C (NIMH PDSP). Funding: This work was supported by the National Institutes of Health (NIH) awards 1R01 NS098772 (R.K.), 1R01 DA042852 (R.K.), K08NS104272 (A.P.), 1R01 NS102161 (A.A.), R01 NS106902 (F.P. and V.N.), and P01DA041307 (F.P.). A portion of this work was supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD 20892 (K.R.).
Footnotes
SUPPLEMENTARY MATERIALS
stm.sciencemag.org/cgi/content/full/12/529/eaay7550/DC1
Materials and Methods
Fig. S1. Intrathecal pretreatment of BIBN 4096 prevents hindpaw allodynia induced by intrathecal injection of CGRP in naïve female mice.
Fig. S2. The PRL/PRLR system is not critical for the development of trauma-induced neuropathic pain in female mice.
Fig. S3. Subcutaneous implantation of a single morphine pellet induces hindpaw and periorbital allodynia in female and male mice.
Fig. S4. Alteration in the PRL/PRLR system in female and male mice with daily injections of morphine.
Fig. S5. Schematic of circulating PRL/PRLR system mediating nociceptor sensitization and OIH in females.
Table S1. Binding affinity of (−)- and (+)-oxymorphone.
Data file S1. Raw data.
Competing interests: F.P. has served as a consultant or received research funding from Voyager, SiteOne Therapeutics, Nektar, Amgen, Acadia, Blackthorn, Teva, Eli Lilly, Hoba, Allergan, Ipsen, and Proximagen and has served as a founder of Catalina Pharma, Scientific Advisory Board Regulonix, and Condor Pharma. R.K. is a stakeholder in Regulonix Holding Inc. D.D. has served as a consultant for Amgen, University Health Network, Daniel Edelman Inc., Autonomic Technologies, Axsome, Allergan, Alder, Biohaven, Charleston Laboratories, Promius, Eli Lilly, eNeura, Neurolief, Novartis, Ipsen, Impel, Satsuma, Supernus, Theranica, Teva, WL Gore, Nocira, XoC, Zosano, Upjohn (Division of Pfizer), Pieris, Revance, Equinox, Salvia, and Amzak Health; received honoraria from Foresite Capital, ZP Opco, Oppenheimer, Association of Translational Medicine, HealthLogiX, MediCom Worldwide, MedLogix Communications, Mednet, Electrocore, Miller Medical, PeerView, WebMD Health/Medscape, Chameleon, Academy for Continued Healthcare Learning, Sun Pharma (India), Universal Meeting Management, Haymarket, Global Scientific Communications, Global Life Sciences, Global Access Meetings, UpToDate (Elsevier), Oxford University Press, Cambridge University Press, and Wolters Kluwer Health; has received research support from the Department of Defense, National Institutes of Health, Henry Jackson Foundation, Sperling Foundation, American Migraine Foundation, and Patient Centered Outcomes Research Institute (PCORI); and holds stock options/patents or is a shareholder/board director for Aural Analytics, Healint, Theranica, Second Opinion/Mobile Health, EpiPen (Options/Board), Nocira, Matterhorn/Ontologics (Options/Board), King-Devick Technologies (Options/Board), Precon Health (Options/Board), and patent 17189376.1–1466:vTitle: Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis. A.P. is a founder of Catalina Pharma and has served as a member of Scientific Advisory Board of Regulonix, and consultant for Condor Pharma. Other authors declare that they have no competing interests.
Data and materials availability: All data associated with this study are present in the paper or the Supplementary Materials.
REFERENCES AND NOTES
- 1.Freeman ME, Kanyicska B, Lerant A, Nagy G, Prolactin: Structure, function, and regulation of secretion. Physiol. Rev 80, 1523–1631 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Leong DA, Frawley LS, Neill JD, Neuroendocrine control of prolactin secretion. Annu. Rev. Physiol 45, 109–127 (1983). [DOI] [PubMed] [Google Scholar]
- 3.Ben-Jonathan N, Hnasko R, Dopamine as a prolactin (PRL) inhibitor. Endocr. Rev 22, 724–763 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald P, Dinan TG, Prolactin and dopamine: What is the connection? A review article. J. Psychopharmacol 22, 12–19 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Kallestrup M-M, Kasch H, Østerby T, Nielsen E, Jensen TS, Jørgensen JO, Prolactinoma-associated headache and dopamine agonist treatment. Cephalalgia 34, 493–502 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Bosco D, Belfiore A, Fava A, De Rose M, Plastino M, Ceccotti C, Mungari P, Iannacchero R, Lavano A, Relationship between high prolactin levels and migraine attacks in patients with microprolactinoma. J. Headache Pain 9, 103–107 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Jonathan N, LaPensee CR, LaPensee EW, What can we learn from rodents about prolactin in humans? Endocr. Rev 29, 1–41 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belugin S, Diogenes AR, Patil MJ, Ginsburg E, Henry MA, Akopian AN, Mechanisms of transient signaling via short and long prolactin receptor isoforms in female and male sensory neurons. J. Biol. Chem 288, 34943–34955 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patil MJ, Henry MA, Akopian AN, Prolactin receptor in regulation of neuronal excitability and channels. Channels 8, 193–202 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gadd SL, Clevenger CV, Ligand-independent dimerization of the human prolactin receptor isoforms: Functional implications. Mol. Endocrinol 20, 2734–2746 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Qazi AM, Tsai-Morris C-H, Dufau ML, Ligand-independent homo- and heterodimerization of human prolactin receptor variants: Inhibitory action of the short forms by heterodimerization. Mol. Endocrinol 20, 1912–1923 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Xie Y-L, Hassan SA, Qazi AM, Tsai-Morris CH, Dufau ML, Intramolecular disulfide bonds of the prolactin receptor short form are required for its inhibitory action on the function of the long form of the receptor. Mol. Cell. Biol 29, 2546–2555 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartley EJ, Fillingim RB, Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth 111, 52–58 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, Sotoudeh C, Clark JD, Barres BA, Bohlen CJ, Scherrer G, Loss of μ opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat. Med 23, 164–173 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Araldi D, Khomula EV, Ferrari LF, Levine JD, Fentanyl induces rapid onset hyperalgesic priming: Type I at peripheral and type II at central nociceptor terminals. J. Neurosci 38, 2226–2245 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L, A comprehensive review of opioid-induced hyperalgesia. Pain Physician 14, 145–161 (2011). [PubMed] [Google Scholar]
- 17.Katz N, Mazer NA, The impact of opioids on the endocrine system. Clin. J. Pain 25, 170–175 (2009). [DOI] [PubMed] [Google Scholar]
- 18.La Torre D, Falorni A, Pharmacological causes of hyperprolactinemia. Ther. Clin. Risk Manag 3, 929–951 (2007). [PMC free article] [PubMed] [Google Scholar]
- 19.Charlton HM, Speight A, Halpin DMG, Bramwell A, Sheward WJ, Fink G, Prolactin measurements in normal and hypogonadal (hpg) mice: Developmental and experimental studies. Endocrinology 113, 545–548 (1983). [DOI] [PubMed] [Google Scholar]
- 20.Sinha YN, Salocks CB, Vanderlaan WP, Prolactin and growth hormone levels in different inbred strains of mice: Patterns in association with estrous cycle, time of day, and perphenazine stimulation. Endocrinology 97, 1112–1122 (1975). [DOI] [PubMed] [Google Scholar]
- 21.Ormandy CJ, Binart N, Helloco C, Kelly PA, Mouse prolactin receptor gene: Genomic organization reveals alternative promoter usage and generation of isoforms via alternative 3’-exon splicing. DNA Cell Biol 17, 761–770 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Verhelst J, Abs R, Maiter D, van den Bruel A, Vandeweghe M, Velkeniers B, Mockel J, Lamberigts G, Petrossians P, Coremans P, Mahler C, Stevenaert A, Verlooy J, Raftopoulos C, Beckers A, Cabergoline in the treatment of hyperprolactinemia: A study in 455 patients. J. Clin. Endocrinol. Metab 84, 2518–2522 (1999). [DOI] [PubMed] [Google Scholar]
- 23.Patil MJ, Ruparel SB, Henry MA, Akopian AN, Prolactin regulates TRPV1, TRPA1, and TRPM8 in sensory neurons in a sex-dependent manner: Contribution of prolactin receptor to inflammatory pain. Am. J. Physiol. Endocrinol. Metab 305, E1154–E1164 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Mello R, Dickenson AH, Spinal cord mechanisms of pain. Br. J. Anaesth 101, 8–16 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Decosterd I, Woolf CJ, Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain 87, 149–158 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Pintér-Kübler B, Ferenczi S, Núnez C, Zelei E, Polyák A, Milanés MV, Kovács KJ, Differential changes in expression of stress- and metabolic-related neuropeptides in the rat hypothalamus during morphine dependence and withdrawal. PLOS ONE 8, e67027 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deyo SN, Swift RM, Miller RJ, Fang VS, Development of tolerance to the prolactin-releasing action of morphine and its modulation by hypothalamic dopamine. Endocrinology 106, 1469–1474 (1980). [DOI] [PubMed] [Google Scholar]
- 28.Mioduszewski R, Zimmermann E, Critchlow V, Effects of morphine dependence, withdrawal and tolerance on prolactin and growth hormone secretion in the rat. Life Sci 30, 1343–1348 (1982). [DOI] [PubMed] [Google Scholar]
- 29.Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch J-L, Koch M, Kessler P, Hentsch D, Birling M-C, Koutsourakis M, Vasseur L, Veinante P, Kieffer BL, Massotte D, A mu–delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct. Funct 220, 677–702 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavestro C, Rosatello A, Marino MP, Micca G, Asteggiano G, High prolactin levels as a worsening factor for migraine. J. Headache Pain 7, 83–89 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB, Acute migraine medications and evolution from episodic to chronic migraine: A longitudinal population-based study. Headache 48, 1157–1168 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley III JL, Sex, gender, and pain: A review of recent clinical and experimental findings. J. Pain 10, 447–485 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diogenes A, Patwardhan AM, Jeske NA, Ruparel NB, Goffin V, Akopian AN, Hargreaves KM, Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J. Neurosci 26, 8126–8136 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gill-Sharma MK, Prolactin and male fertility: The long and short feedback regulation. Int. J. Endocrinol 2009, 687259 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leondires MP, Hu Z-Z, Dong J, Tsai-Morris C-H, Dufau ML, Estradiol stimulates expression of two human prolactin receptor isoforms with alternative exons-1 in T47D breast cancer cells. J. Steroid Biochem. Mol. Biol 82, 263–268 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Lerant A, Freeman ME, Ovarian steroids differentially regulate the expression of PRL-R in neuroendocrine dopaminergic neuron populations: A double label confocal microscopic study. Brain Res 802, 141–154 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Smirnova OV, Petraschuk OM, Kelly PA, Immunocytochemical localization of prolactin receptors in rat liver cells: I. Dependence on sex and sex steroids. Mol. Cell. Endocrinol 105, 77–81 (1994). [DOI] [PubMed] [Google Scholar]
- 38.Jefferson F, Ehlen JC, Williams NS, Montemarano JJ, Paul KN, A dopamine receptor d2-type agonist attenuates the ability of stress to alter sleep in mice. Endocrinology 155, 4411–4421 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eguchi K, Kawamoto K, Uozumi T, Ito A, Arita K, Kurisu K, In vivo effect of cabergoline, a dopamine agonist, on estrogen-induced rat pituitary tumors. Endocr. J 42, 153–161 (1995). [DOI] [PubMed] [Google Scholar]
- 40.Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P, Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci 18, 145–153 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Patil M, Hovhannisyan AH, Wangzhou A, Mecklenburg J, Koek W, Goffin V, Grattan D, Boehm U, Dussor G, Price TJ, Akopian AN, Prolactin receptor expression in mouse dorsal root ganglia neuronal subtypes is sex-dependent. J. Neuroendocrinol 31, e12759 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrini F, Trang T, Mattioli T-AM, Laffray S, Del’Guidice T, Lorenzo L-E, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu J-M, Cahill CM, Salter MW, De Koninck Y, Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl− homeostasis. Nat. Neurosci 16, 183–192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou H-Y, Chen S-R, Chen H, Pan H-L, Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J. Neurosci 30, 4460–4466 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drdla R, Gassner M, Gingl E, Sandkühler J, Induction of synaptic long-term potentiation after opioid withdrawal. Science 325, 207–210 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Xie JY, De Felice M, Kopruszinski CM, Eyde N, LaVigne J, Remeniuk B, Hernandez P, Yue X, Goshima N, Ossipov M, King T, Streicher JM, Navratilova E, Dodick D, Rosen H, Roberts E, Porreca F, Kappa opioid receptor antagonists: A possible new class of therapeutics for migraine prevention. Cephalalgia 37, 780–794 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nation KM, De Felice M, Hernandez PI, Dodick DW, Neugebauer V, Navratilova E, Porreca F, Lateralized kappa opioid receptor signaling from the amygdala central nucleus promotes stress-induced functional pain. Pain 159, 919–928 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patil MJ, Green DP, Henry MA, Akopian AN, Sex-dependent roles of prolactinand prolactin receptor in postoperative pain and hyperalgesia in mice. Neuroscience 253, 132–141 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roeckel L-A, Utard V, Reiss D, Mouheiche J, Maurin H, Robé A, Audouard E, Wood JN, Goumon Y, Simonin F, Gaveriaux-Ruff C, Morphine-induced hyperalgesia involves mu opioid receptors and the metabolite morphine-3-glucuronide. Sci. Rep 7, 10406 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah M, Choi S, Toll-like receptor-dependent negative effects of opioids: A battle between analgesia and hyperalgesia. Front. Immunol 8, 642 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ailes EC, Dawson AL, Lind JN, Gilboa SM, Frey MT, Broussard CS, Honein MA; Centers for Disease Control and Prevention (CDC), Opioid prescription claims among women of reproductive age—United States, 2008–2012. MMWR Morb. Mortal. Wkly Rep 64, 37–41 (2015). [PMC free article] [PubMed] [Google Scholar]
- 51.Substance Use in Women (2018); https://www.drugabuse.gov/. [Google Scholar]
- 52.McHugh RK, DeVito EE, Dodd D, Carroll KM, Potter JS, Greenfield SF, Connery HS, Weiss RD, Gender differences in a clinical trial for prescription opioid dependence. J. Subst. Abuse Treat 45, 38–43 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swaminathan G, Varghese B, Thangavel C, Carbone CJ, Plotnikov A, Kumar KGS, Jablonski EM, Clevenger CV, Goffin V, Deng L, Frank SJ, Fuchs SY, Prolactin stimulates ubiquitination, initial internalization, and degradation of its receptor via catalytic activation of Janus kinase 2. J. Endocrinol 196, R1–R7 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Li H, Tao R, Wang J, Xia L, Upregulation of miR-375 level ameliorates morphine analgesic tolerance in mouse dorsal root ganglia by inhibiting the JAK2/STAT3 pathway. J. Pain Res 10, 1279–1287 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sauro KM, Becker WJ, The Stress and Migraine Interaction (2009; http://www.ncbi.nlm.nih.gov/pubmed/19619238), pp. 1378–1386. [DOI] [PubMed]
- 56.Martinez-Lavin M, Biology and therapy of fibromyalgia. Stress, the stress response system, and fibromyalgia. Arthritis Res. Ther 9, 216 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Webster J, Piscitelli G, Polli A, D’Alberton A, Falsetti L, Ferrari C, Fioretti P, Giordano G, L’Hermite M, Ciccarelli E, Crosignani PG, De Cecco L, Fadini R, Faglia G, Flamigni C, Tamburrano G, Ismail I, Scanlon MF; (European multicentre cabergoline study group), The efficacy and tolerability of long-term cabergoline therapy in hyperprolactinaemic disorders: An open, uncontrolled, multicentre study. Clin. Endocrinol. (Oxf) 39, 323–329 (1993). [DOI] [PubMed] [Google Scholar]
- 58.Sharif NA, McLaughlin MA, Kelly CR, Katoli P, Drace C, Husain S, Crosson C, Toris C, Zhan G-L, Camras C, Cabergoline: Pharmacology, ocular hypotensive studies in multiple species, and aqueous humor dynamic modulation in the Cynomolgus monkey eyes. Exp. Eye Res 88, 386–397 (2009). [DOI] [PubMed] [Google Scholar]
- 59.Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E, Dopamine agonists and the risk of cardiac-valve regurgitation. N. Engl. J. Med 356, 29–38 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Auriemma RS, Pivonello R, Perone Y, Grasso LFS, Ferreri L, Simeoli C, Iacuaniello D, Gasperi M, Colao A, Safety of long-term treatment with cabergoline on cardiac valve disease in patients with prolactinomas. Eur. J. Endocrinol 169, 359–366 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Khare S, Lila AR, Patil R, Phadke M, Kerkar P, Bandgar T, Shah NS, Long-term cardiac (valvulopathy) safety of cabergoline in prolactinoma. Indian J. Endocrinol. Metab 21, 154–159 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nave R, Jodl S, Hoffmann A, Gashaw I, Zollmann F, Berse M, Höchel J, Krätzschmar J, Rohde B, Monoclonal antibody against prolactin receptor: A randomized placebo-controlled study evaluating safety, tolerability, and pharmacokinetics of repeated subcutaneous administrations in postmenopausal women. Reprod. Sci 26, 523–531 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Mout R, Ray M, Lee Y-W, Scaletti F, Rotello VM, In Vivo delivery of CRISPR/Cas9 for therapeutic gene editing: Progress and challenges. Bioconjug. Chem 28, 880–884 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsu PD, Lander ES, Zhang F, Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F, Genome engineering using the CRISPR-Cas9 system. Nat. Protoc 8, 2281–2308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heckl D, Kowalczyk MS, Yudovich D, Belizaire R, Puram RV, McConkey ME, Thielke A, Aster JC, Regev A, Ebert BL, Generation of mouse models of myeloid malignancy with combinatorial genetic lesions using CRISPR-Cas9 genome editing. Nat. Biotechnol 32, 941–946 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moutal A, Sun L, Yang X, Li W, Cai S, Luo S, Khanna R, CRMP2–Neurofibromin interface drives NF1-related pain. Neuroscience 381, 79–90 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brittain JM, Duarte DB, Wilson SM, Zhu W, Ballard C, Johnson PL, Liu N, Xiong W, Ripsch MS, Wang Y, Fehrenbacher JC, Fitz SD, Khanna M, Park C-K, Schmutzler BS, Cheon BM, Due MR, Brustovetsky T, Ashpole NM, Hudmon A, Meroueh SO, Hingtgen CM, Brustovetsky N, Ji R-R, Hurley JH, Jin X, Shekhar A, Xu X-M, Oxford GS, Vasko MR, White FA, Khanna R, Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca2+ channel complex. Nat. Med 17, 822–829 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bogulavsky JJ, Gregus AM, Kim PT-H, Costa ACS, Rajadhyaksha AM, Inturrisi CE, Deletion of the glutamate receptor 5 subunit of kainate receptors affects the development of morphine tolerance. J. Pharmacol. Exp. Ther 328, 579–587 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bryant HU, Yoburn BC, Inturrisi CE, Bernton EW, Holaday JW, Morphine-induced immunomodulation is not related to serum morphine concentrations. Eur. J. Pharmacol 149, 165–169 (1988). [DOI] [PubMed] [Google Scholar]
- 71.Das S, Kelschenbach J, Charboneau R, Barke RA, Roy S, Morphine withdrawal stress modulates lipopolysaccharide-induced interleukin 12 p40 (IL-12p40) expression by activating extracellular signal-regulated kinase 1/2, which is further potentiated by glucocorticoids. J. Biol. Chem 286, 29806–29817 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Juni A, Klein G, Pintar JE, Kest B, Nociception increases during opioid infusion in opioid receptor triple knock-out mice. Neuroscience 147, 439–444 (2007). [DOI] [PubMed] [Google Scholar]
- 73.Luiz AP, Schroeder SD, Rae GA, Calixto JB, Chichorro JG, Contribution and interaction of kinin receptors and dynorphin A in a model of trigeminal neuropathic pain in mice. Neuroscience 300, 189–200 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL, Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 (1994). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


