Abstract
Background
Inhibiting Notch is a promising anti-cancer strategy as it plays a critical role in cancer stem cells maintenance and tumour angiogenesis. BMS-986115is anorally active, selective inhibitor of gamma-secretase mediated Notch signalling.
Method
Two dose escalation schedules (Arm-A continuous daily schedule and Arm-B intermittent 2 times weekly schedule) of BMS-986115 were evaluated in advanced solid tumour patients. The primary objective was to establish the safety, tolerability and Maximum Tolerated Dose (MTD) of BMS-986115.
Results
Thirty six patients (24 in Arm A and 12 in Arm B) were treated. The most frequent treatment related adverse advents were diarrhoea (72%), hypophosphataemia (64%), and nausea (61%). The MTD was 1.5 mg daily in Arm A but not established in Arm B. Four patients in Arm A and 2 in Arm B experienced dose limiting toxicities (grade 3 nausea, diarrhoea, pruritus/urticaria and ileus). BMS-986115 showed dose related increase in exposure within the dose range tested. Target inhibition of Notch pathway related genes was observed. Three patients in Arm A and 2 in Arm B achieved stable disease for more than 6 months.
Conclusion
The daily oral dosing of BMS-986115 is safe and tolerable with biological activity demonstrated by continuous target engagement and Notch signalling inhibition.
Keywords: BMS-986115, Oral NOTCH inhibitor, Gamma-secretase inhibitor, Phase I trial
Introduction
Notch receptors are highly conserved type I transmembrane glycoproteins that regulate critical cellular functions including differentiation, cell fate determination, proliferation, self-renewal, and survival [1]. Oncogenic activation of the Notch pathway, either through mutation, gene rearrangement or over-expression, is implicated in both hematologic malignancies, solid tumours including breast cancer, melanoma, non-small cell lung cancer (NSCLC), colorectal carcinoma and in desmoid fibromatosis [2–10]. Furthermore, Notch plays an important role in maintenance and survival of cancer stem cells (CSCs) [11–14] and tumour angiogenesis [15, 16]. Thus, there is strong rationale for development of Notch inhibitors as anti-cancer therapy.
BMS-986115 is a potent and selective inhibitor of gamma-secretase mediated Notch signalling and inhibits all 4 mammalian Notch receptors (Notch1 to 4) with low nanomolar median inhibitory concentrations (IC50s). Nonclinical experiments showed that BMS-986115 was effective as a single agent in the treatment of human T-ALL xenograft models, and demonstrated anti-tumour activity against 5 out of 7 solid tumour xenografts evaluated, including 3 breast, 1 NSCLC, and 1 pancreatic carcinoma. In animal studies, the main pharmacological toxicities included dose-dependent gastrointestinal (GI) toxicity, lymphoid depletion, and interruption of ovarian follicle maturation. Notch inhibitor mediated GI toxicity, which is characterized by goblet cell metaplasia throughout the intestines, is the result of on-target drug-induced differentiation of small intestinal and colonic progenitors to the secretory (goblet) cell fate. As prolonged inhibition of the Notch pathway is associated with increased GI toxicity, an oral Notch inhibitor with a short half-life that allows transient rather than continuous target engagement is desirable. BMS-986115 has a projected human half-life of approximately 15 h (BMS-986115: Investigator brochure (version 1). Bristol-Myers Squibb Company; 2013) providing shorter duration of target inhibition than other intravenous Notch inhibitors with longer half-lives [17].
In this open label phase I dose escalation study, BMS-986115 was administered as an oral single agent on once daily continuous (QD) and two times weekly intermittent (BIW) schedules. The primary objective of the study was to assess safety and tolerability and to establish the maximum tolerated dose (MTD) of oral doses of BMS-986115 in subjects with advanced solid tumours. The secondary objectives were to assess pharmacokinetics, pharmacodynamics and preliminary anti-tumour activity of BMS-986115.
Patients and methods
The study was an ascending multiple-dose Phase I study and conducted in accordance with the Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice Guidelines. The study was approved by Research Ethics Board at each participating sites and registered at www.clinicaltrials.gov with registration number NCT01986218. The first patient was enrolled on 11th December 2013 and the study was completed on 29th March 2016. There were no subjects on study at the time of study completion.
Patient selection
Male and female subjects who were 18 years or older and had a histological or cytological diagnosis of advanced solid tumours refractory to or relapsed from standard therapies were eligible. The inclusion criteria included life expectancy of ≥3 months, ECOG performance status 0–1, and adequate organ function including ECG parameters. Subjects were required to have an adequate lipid profile as increases in triglycerides were seen in the multiple dose rat study with BMS-986115. Lactating or pregnant women were not eligible and all subjects were needed to comply with effective contraception methods as per protocol. The main exclusion criteria were: symptomatic or unstable brain metastases, any serious medical disorders including active infection and significant cardiovascular disease, major thromboembolic events or bleeding diathesis within 6 months of study entry, current or recent GI disease that increases the risk of diarrhoea, any major surgery or GI disorder that would interfere with administration of oral medications, conditions requiring chronic systemic glucocorticoid use, therapeutic anticoagulation, and concomitant second malignancies (except adequately treated in situ cancers and non-melanomatous skin cancers). Patients with hepatitis C, hepatitis B or HIV were excluded with an exception of those who have achieved viral cure for prior hepatitis C infection. Prior exposure to BMS-986115 or other Notch inhibitors was not allowed and those with a history of allergy to Notch inhibitors including BMS-986115related compounds were excluded. Patients required a washout period of at least 6 months for prior therapy with nucleoside analogues, 6 weeks for prior therapy with nitrosoureas, mitomycin C and liposomal doxorubicin and 4 weeks for other anticancer therapies. The prohibited concomitant medications included herbal supplements, medications causing Torsades de Pointes, strong inhibitors or inducers of CYP3A4 or P-gp, and sensitive substrates of CYP3A4 with a narrow therapeutic window.
Study design
The dose escalation phase was initially conducted using an accelerated titration design [18] in which a single subject was enrolled in each cohort, and was switched to a 3 + 3 design with modified Fibonacci dose escalation if any of the following occurred in a single-subject cohort: grade 2 treatment-related diarrhoea lasting >36 h despite appropriate medical management; or any other grade 2 treatment-related adverse advent (AE), excluding asymptomatic electrolyte abnormalities that were manageable with appropriate supplements, alopecia and fatigue lasting ≤5 days. An additional subject (i.e., a second subject in the accelerated titration phase, and a fourth subject in the 3 + 3 phase) may be enrolled in a dose escalation cohort to allow for subjects that become not DLT-evaluable, following agreement between the Investigators and the study sponsor.
DLT period was 35 days (from C1, duration of which was 7 days, to end of cycle 2, duration of which was 28 days). A DLT was defined as any of the following events unless a clear alternative cause was identified: grade (Gr) 4 neutropenia (absolute neutrophil count [ANC] < 500/mm3) lasting ≥5 days; Gr 3 febrile neutropenia lasting >24 h (single temperature of >38.3C or a sustained temperature of ≥38C for >1 h (nonaxillary) with ANC <1000/mm3); Gr 4 febrile neutropenia of any duration; Gr 4 thrombocytopenia (platelets <25,000/mm3) or Gr 3 thrombocytopenia with significant bleeding; Gr ≥3 diarrhoea lasting >24 h despite appropriate medical management; any other drug-related Gr ≥3 non-hematologic AE, except hyperlipidemia in subjects not receiving maximum medical management or electrolyte abnormalities that may be managed with supplements.
For single-subject cohorts, dose escalation occurred in increments of 100% above the previous dose. For the 3 + 3 design (including enrolment of additional subjects in the single-subject cohort with the first occurrence of a Gr ≥ 2 treatment-related AE), the first 3 dose escalations occurred in increments of up to 67%, 50%, and 40%, respectively, and all subsequent dose escalations occurred in increments of 33%. Dose escalation continued until the MTD was established or until the sponsor and investigators concurred that further dose escalation was not warranted. Once MTD was established in a given study arm, approximately 6 additional subjects were to be treated in a dose expansion cohort, at a dose equal to or below the MTD.
Study arms and dosing
BMS-986115 was administered orally once daily (QD) at doses of 0.3, 0.6, 1.2, 1.5, and 2 mg (Arm A) or twice weekly (BIW) on consecutive days at doses of 2, 4, 8 mg (Arm B). Arm A and Arm B were conducted in a sequential manner. The study consisted of a screening period (within 28 days prior to first dose), a treatment period, and a follow-up period approximately 30 days after the last dose of study medication. In the treatment period, the first cycle was 7 days in duration, and all subsequent cycles were 28 days in duration. Subjects received a single dose of BMS-986115 on Cycle 1 Day 1. In Cycle 2 and all subsequent cycles, subjects in Arm A received QD doses of BMS-986115 (i.e., Day 1 to Day 28 inclusive) and subjects in Arm B received BIW doses of BMS-986115 (i.e., Days 1, 2, 8, 9, 15, 16, 22, and 23 of each cycle). Dose interruption (up to 28 days) and/or dose modification (up to 2 dose reductions) were permitted in the event of study drug toxicity. Subjects could continue to receive treatment in the study until they experienced disease progression, unacceptable AEs, or withdrew consent. BMS-986115 was supplied as grey hard gelatin capsules, including a size 1 capsule containing 0.3 mg BMS-986115 and a size 0 capsule containing 2 mg BMS-986115.
Safety and efficacy assessment
Safety assessments consisted of physical examination, vital signs, weight, performance status assessment using ECOG (Eastern Cooperative Oncology Group) criteria and documentation of AEs and serious AEs (SAEs) for all treated subjects using the National Cancer Institute’s Common Toxicity Criteria for Adverse Events v4.03. Laboratory tests including hematology, coagulation, biochemistry, urinalysis, pregnancy test, if applicable, and an ECG were performed during screening, treatment and end of treatment at protocol specified time points and at any time when clinically indicated. Tumour assessments by CT or MRI were performed at baseline and approximately every 8 weeks during treatment, and at the end of treatment or 30-day follow-up if not done within the preceding 8 weeks. The radiological response to therapy was assessed using Response Evaluation Criteria in Solid Tumor (RECIST) 1.1. All subjects who received at least one dose of BMS-986115 were evaluated for safety parameters. Additionally, any occurrence of an SAE from the time of consent until 30 days post discontinuation of study drug dosing was documented. Any occurrence of non-serious AEs was collected from first dose of study drug until 30 days post discontinuation of dosing.
PK assessments
Pre and post treatment EDTA peripheral whole blood samples for the PK determination of BMS-986115 and its equally active metabolite BMT-100948 were collected on days 1 (pre-dose and 0.5, 1, 1.5, 2, 4, and 8 h post-dose), 2 (24 h post first dose), 3 (48 h post first dose), 4 (72 h post first dose) and 5 (120 h post first dose) of cycle 1 for both Arm A and Arm B and, subsequently, days 1 (pre-dose), 15 (pre-dose and 0.5, 1, 2, 4, and 8 h post-dose), and 16 (pre-dose) of cycle 2 for Arm A and days 1 (pre-dose and 1 h post-dose), 2 (pre-dose and 1 h post-dose), 8 (pre-dose), 9 (pre-dose), 15 (pre-dose and 0.5, 1, 3, and 6 h post-dose), 16 (pre-dose and 0.5, 1, 2, 4, and 8 h post dose), 17 (24 h post day 16 dose), 18 (48 h post day 16 dose), day 19 (96 h post day 16 dose), and 22 (pre-dose) of C2 for Arm B. Immediately post collection, blood samples were centrifuged to separate plasma and cells. Plasma concentrations of BMS-986115 and BMT-100948 were determined using a validated liquid chromatography-tandem mass spectrometry assay. Plasma concentration-time data for BMS-986115 and an equally active metabolite BMT-100948 were reported for subjects who received at least one dose of study medication. Non-compartmental analysis (NCA) was performed to characterize the PK parameters for the parent compound (BMS-986115) using nominal time.
PD assessments
Expression of Notch pathway-related genes in peripheral blood was measured as surrogate PD biomarkers for BMS-986115. Blood samples were collected pre-treatment and 2, 4, 8, 24, and 48 h post-treatment on C1D1 (single-dose) on both arms; on Arm A at steady-state pre-treatment and 2, 4, 8 and 24 h post-treatment on C2D15; and in Arm B at steady-state pre-treatment (C2D15 and C2D16) and 2, 4, 8, 24, and 48 h post-treatment on C2D16, and analysed for Hes1 and Deltex1 expression in mRNA extracted from whole blood using RT-qPCR. This qPCR assay was validated to measure the relative expression levels of Hes1 and Deltex1 with respect to the endogenous control gene PPIA. Total RNA from whole blood samples was isolated using QIAGEN PAXgene Blood RNA Extraction Kit (QIAGEN, Cat.# 762,174) according to the manufacturer’s protocol. RNA samples were reverse transcribed to cDNA at input of 1 μg using the SuperScript II Reverse Transcriptase Kit (Invitrogen, Cat.# 18,064–014). Real-time PCR to measure Hes1 and Deltex1 expression was performed following Applied Biosystems Assays-on-Demand protocol using 8 μL of the cDNA sample. All real-time PCR reactions were analyzed in triplicate in a 384-well optical reaction plate at 20 μL per well. Amplification was detected using the 7900HT Fast system under standard thermal cycling conditions. The level of inhibition of Hes1 and Deltex1 expression by oral doses of BMS-986115 in peripheral blood was measured as surrogate PD biomarkers.
Statistical considerations
Approximately 20 subjects were planned per study arm, including dose escalation and expansion, with 1 to 6 subjects treated per dose level and at least 12 subjects treated at the MTD. The estimation of the MTD was based on the probability of DLT in cycle 1 and 2 for patients in dose escalation. Demographics, baseline characteristics, safety and efficacy measurements were described using descriptive statistics. For PD analysis, geometric mean relative gene expression data for the Notch pathway genes Deltex1 and Hes1 were plotted.
Results
Patient characteristics
A total of 36 patients were treated in the study (24 in Arm A and 12 in Arm B). Patient characteristics are summarized in Table 1. Sixteen (67%) of the 24 subjects treated in Arm A and 6 (50%) of the 12 subjects treated in Arm B continued to receive treatment until disease progression. A total of 9 subjects discontinued treatment after experiencing one or more AEs that were either related to study drug (N = 5, 3 subjects in Arm A and 2 in Arm B) or not related to the study drug (N = 4, 2 subjects in each arm).
Table 1.
Patient characteristics (N = 36)
| Characteristics | N (%) |
|---|---|
| Sex | |
| Male | 17 (47) |
| Female | 19 (53) |
| Age | |
| Mean (SD), years | 58 (14) |
| Range | 23–84 |
| Race | |
| White | 29 (81) |
| Asian | 7 (19) |
| Baseline WHO PS | |
| 0 | 10 (28) |
| 1 | 25 (69) |
| 2 | 1 (3) |
| Cancer types | |
| Colon | 5 (14) |
| Adenoid cystic carcinoma | 5 (14) |
| Cholangiocarcinoma | 3 (8) |
| NSCLC | 2 (6) |
| Renal | 2 (6) |
| Thyroid | 2 (6) |
| Melanoma | 1 (3) |
| Breast | 1 (3) |
| Ovary | 1 (3) |
| Pancreas | 1 (3) |
| Prostate | 1 (3) |
| Rectal | 1 (3) |
| Gastric | 1 (3) |
| Desmoid fibromatosis | 1 (3) |
| Unknown | 1 (3) |
| Other | 8 (22) |
| Prior anti-cancer therapy | |
| Surgery | 36 (100) |
| Radiotherapy | 22 (61) |
| Neo-adjuvant therapy | 6 (17) |
| Adjuvant therapy | 11 (31) |
| Systemic therapy for metastatic disease | 29 (81) |
| Number of prior systemic treatment | |
| 0 | 2 (6) |
| 1 | 12 (33) |
| 2 | 7 (19) |
| 3 | 6 (17) |
| ≥ 4 | 9 (25) |
Dose escalation and MTD
Of 24 patients treated in Arm A, 20 patients were DLT evaluable for 5 QD dose levels evaluated (0.3 mg [N = 2], 0.6 mg [N = 2], 1.2 mg [N = 5], 1.5 mg [N = 6], and 2 mg [N = 5]) (Table 2). Based on the incidence of DLT, the MTD for QD dosing of BMS-986115 was 1.5 mg QD. At this dose level, 1 DLT (Gr 3 nausea) was observed in one of 6 patients treated. No DLT was observed in 5 evaluable patients treated at 1.2 mg QD. The 2 mg QD dose was not tolerated with DLTs in 3 of the 5 DLT-evaluable patients (Gr 3 ileus; Gr 3 nausea; Gr 3 pruritus/urticaria). Of 12 patients treated within Arm B, 10 were DLT evaluable for 3 BIW dose levels (2 mg [N = 2], 4 mg [N = 2], and 8 mg [N = 6]). The MTD was not established for BIW dosing schedule, as the study was terminated prior to this being formally established. None of the two patients treated at 4 mg BIW had DLT. But, a higher dose of 8 mg BIW was not tolerated with DLTs in 2 of the 6 DLT-evaluable subjects (Gr 3 diarrhoea; Gr 3 nausea/dehydration/anorexia with Gr 2 fatigue). Dose limiting toxicities and reasons for treatment discontinuation are summarized in Table 2.
Table 2.
Dose-limiting toxicities and reasons for treatment discontinuation
| Dose level | Total no. of patients | No. of DLT evaluable patients | No. of patients with DLTs | Description of DLT | Description of study drug toxicity that led to treatment discontinuation (N = 5) | Reasons for treatment discontinuation (N) |
|---|---|---|---|---|---|---|
| Arm A- Once daily (QD) schedule | ||||||
| 0.3 mg | 2 | 2 | 0 | NA | NA | Disease progression (N = 2) |
| 0.6 mg | 2 | 2 | 0 | NA | NA | Disease progression (N = 2) |
| 1.2 mg | 6 | 5 | 0 | NA | NA | Disease progression (N = 4) |
| AE unrelated to study drug (N = 1) | ||||||
| Subject request to discontinue treatment (N = 1) | ||||||
| 1.5 mg | 7 | 6 | 1 | G3 nausea | G3 Diarrhoea (N = 1) | Disease progression (N = 2) |
| G3 Nausea concurrent with G2 diarrhoea (N = 1) | Study drug toxicity (N = 3) AE unrelated to study drug (N = 1) | |||||
| G2 Dermatitis acneiform (N = 1) | Subject request to discontinue treatment (N = 1) | |||||
| 2.0 mg | 7 | 5 | 3 | G3 ileus | NA | Disease progression (N = 6) |
| G3 nausea | Other (N = 1) | |||||
| G3 pruritus/urticaria | ||||||
| Arm B- Twice weekly (BIW) schedule | ||||||
| 2 mg | 2 | 2 | 0 | NA | NA | Disease progression (N = 1) |
| Subject request to discontinue treatment (N = 1) | ||||||
| 4 mg | 2 | 2 | 0 | NA | NA | Disease progression (N = 2) |
| 8 mg | 8 | 6 | 2 | G3 diarrhoea | G3 Diarrhoea (N = 1) | Disease progression (N = 3) |
| G3 nausea/dehydration/anorexia | G3 Dehydration and anorexia (N = 1) | Study drug toxicity (N = 2) AE unrelated to study drug (N = 2) | ||||
| Subject request to discontinue treatment (N = 1) | ||||||
Safety
There were no treatment-related deaths. Adverse events (AE) were reported in all 36 (100%) subjects. The most frequently reported AEs (>50% of all treated subjects) regardless of causality were diarrhoea (72%), nausea (69%), hypophosphataemia (67%), fatigue (64%), decreased appetite (58%), and vomiting (53%). Treatment related AEs were reported for 35 (97%) subjects and summarized in Table 3. The most frequent treatment related AEs included diarrhoea (72%), hypophosphataemia (64%),nausea (61%), vomiting(44%), fatigue(44%),decreased appetite (36%), rash (31%), hypokalaemia (28%), and pruritus (25%). In the QD arm, a dose related trend was apparent in the frequency of GI events but this trend was not observed in the BIW arm (Table 3). The most common treatment-related Gr 3 or 4 AEs were hypophosphataemia (39%), diarrhoea (19%), nausea (11%), hypokalaemia (11%) and hyponatraemia (6%) (Table 4). Overall, SAEs were reported for 18 (50%) subjects. Eight (6 in Arm A and 2 in Arm B) (22%) experienced SAEs that were treatment-related: Gr 3 diarrhoea (Arm B), Grade 3 diarrhoea with Gr 2 abdominal pain (Arm A), Gr 2 diarrhoea with Gr 2 dehydration (Arm A), Gr 3 nausea, dehydration and decreased appetite (Arm B), Gr 3 nausea with Gr 3 fatigue and Gr 4 hypophosphataemia (Arm A), Gr 3 colitis (Arm A), Gr 2 anaemia and Gr 3 pulmonary embolism (Arm A), and Gr 3 ileus (Arm A).
Table 3.
All grades study drug related AEs occurred in at least 5% of all patients (safety cohort)
| Drug related adverse events | Arm A- once daily (QD) schedule |
Arm B- twice weekly (BIW) schedule |
Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.3 mg (N = 2) n (%) | 0.6 mg (N = 2) n (%) | 1.2 mg (N = 6) n (%) | 1.5 mg (N = 7)n (%) | 2.0 mg (N = 7) n (%) | Total (N = 24) n (%) | 2 mg (N = 2) n (%) | 4 mg (N = 2) n (%) | 8 mg (N = 8) n (%) | Total (N = 12) n (%) | All patients (N = 36) n (%) | |
| Gastrointestinal | |||||||||||
| Diarrhoea | 0 | 1 (50) | 6 (100) | 6 (86) | 5 (71) | 18 (75) | 1 (50) | 0 | 7 (88) | 8 (67) | 26 (72) |
| Nausea | 0 | 1 (50) | 4 (67) | 5 (71) | 5 (71) | 15 (63) | 1 (50) | 0 | 6 (75) | 7 (58) | 22 (61) |
| Vomiting | 0 | 1 (50) | 2 (33) | 4 (57) | 5 (71) | 12 (50) | 0 | 0 | 4 (50) | 4 (33) | 16 (44) |
| Abdominal pain | 0 | 0 | 0 | 1 (14) | 1 (14) | 2 (8) | 1 (50) | 0 | 0 | 1 (8) | 3 (8) |
| Dry mouth | 0 | 0 | 1 (17) | 0 | 1 (14) | 2 (8) | 0 | 0 | 1 (13) | 1 (8) | 3 (8) |
| Dyspepsia | 0 | 0 | 1 (17) | 0 | 1 (14) | 2 (8) | 1 (50) | 0 | 0 | 1 (8) | 3 (8) |
| Flatulence | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (50) | 2 (25) | 3 (25) | 3 (8) |
| Abdominal distension | 0 | 0 | 0 | 1 (14) | 0 | 1 (4) | 0 | 1 (50) | 0 | 1 (8) | 2 (6) |
| Mouth ulceration | 0 | 0 | 1 (17) | 0 | 1 (14) | 2 (8) | 0 | 0 | 1 (13) | 1 (8) | 3 (8) |
| Metabolism and nutrition | |||||||||||
| Hypophosphataemia | 2 (100) | 1 (50) | 6 (100) | 4 (57) | 3 (43) | 16 (67) | 1 (50) | 1 (50) | 5 (63) | 7 (58) | 23 (64) |
| Decreased Appetite | 0 | 0 | 2 (33) | 1 (14) | 5 (71) | 8 (33) | 0 | 0 | 5 (63) | 5 (42) | 13 (36) |
| Hypokalaemia | 0 | 0 | 3 (50) | 2 (29) | 2 (29) | 7 (29) | 0 | 1 (50) | 2 (25) | 3 (25) | 10 (28) |
| Dehydration | 0 | 0 | 2 (33) | 0 | 1 (14) | 3 (13) | 0 | 0 | 2 (25) | 2 (17) | 5 (14) |
| Hypocalcaemia | 0 | 0 | 0 | 1 (14) | 0 | 1 (4) | 0 | 0 | 1 (13) | 1 (8) | 2 (6) |
| Hyponatraemia | 0 | 0 | 0 | 1 (14) | 0 | 1 (4) | 0 | 0 | 1 (13) | 1 (8) | 2 (6) |
| Skin and subcutaneous tissue | |||||||||||
| Rash | 1 (50) | 1 (50) | 3 (50) | 2 (29) | 1 (14) | 8 (33) | 0 | 0 | 3 (38) | 3 (25) | 11 (31) |
| Pruritus | 1 (50) | 0 | 0 | 2 (29) | 3 (43) | 6 (25) | 0 | 1 (50) | 2 (25) | 3 (25) | 9 (25) |
| Dry skin | 0 | 0 | 1 (17) | 1 (14) | 1 (14) | 3 (13) | 0 | 0 | 2 (25) | 2 (17) | 5 (14) |
| Rash maculopapular | 0 | 0 | 0 | 0 | 2 (29) | 2 (8) | 0 | 1 (50) | 2 (25) | 3 (25) | 5 (14) |
| Dermatitis acneiform | 0 | 0 | 0 | 4 (57) | 0 | 4 (17) | 0 | 0 | 0 | 0 | 4 (11) |
| Constitutional | |||||||||||
| Fatigue | 0 | 0 | 2 (33) | 4 (57) | 4 (57) | 10 (42) | 1 (50) | 1 (50) | 4 (50) | 6 (50) | 16 (44) |
| Respiratory | |||||||||||
| Dysphonia | 0 | 0 | 0 | 1 (14) | 3 (43) | 4 (17) | 0 | 0 | 1 (13) | 1 (8) | 5 (14) |
| Cough | 0 | 0 | 0 | 2 (29) | 1 (14) | 3 (13) | 0 | 0 | 1 (13) | 1 (8) | 4 (11) |
| Increased upper airway secretion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (50) | 2 (25) | 3 (25) | 3 (8) |
| Productive cough | 0 | 0 | 0 | 1 (14) | 0 | 1 (4) | 0 | 0 | 1 (13) | 1 (8) | 2 (6) |
| Laryngeal pain | 0 | 0 | 0 | 2 (29) | 0 | 2 (8) | 0 | 0 | 0 | 0 | 2 (6) |
| Investigations | |||||||||||
| Blood creatinine increased | 0 | 0 | 0 | 0 | 1 (14) | 1 (4) | 0 | 0 | 1 (13) | 1 (8) | 2 (6) |
| Weight decreased | 0 | 0 | 1 (17) | 1 (14) | 0 | 2 (8) | 0 | 0 | 0 | 0 | 2 (6) |
| Nervous system | |||||||||||
| Dysgeusia | 0 | 0 | 2 (33) | 0 | 1 (14) | 3 (13) | 0 | 1 (50) | 1 (13) | 2 (17) | 5 (14) |
| Infection | |||||||||||
| Sinusitis | 1 (50) | 0 | 1 (17) | 0 | 0 | 2 (8) | 0 | 0 | 0 | 0 | 2 (6) |
| Eye disorder | |||||||||||
| Vision blurred | 0 | 0 | 0 | 1 (14) | 0 | 1 (4) | 0 | 0 | 1 (13) | 1 (8) | 2 (6) |
Table 4.
Grade 3 and 4 adverse advents suspected to be study drug related (safety cohort)
| Drug related adverse events | Arm A- once daily (QD) schedule |
Arm B- twice weekly (BIW) schedule |
Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.3 mg (N = 2) n (%) | 0.6 mg (N = 2) n (%) | 1.2 mg (N = 6) n (%) | 1.5 mg (N = 7)n (%) | 2.0 mg (N = 7) n (%) | Total (N = 24) n (%) | 2 mg (N = 2) n (%) | 4 mg (N = 2) n (%) | 8 mg (N = 8) n (%) | Total (N = 12) n (%) | All patients (N = 36) n (%) | |
| Gastrointestinal | |||||||||||
| Diarrhoea | 0 | 0 | 1 (17) | 2 (29) | 1 (14) | 4 (17) | 1 (50) | 0 | 2 (25) | 3 (25) | 7 (19) |
| Nausea | 0 | 0 | 0 | 2 (29) | 1 (14) | 3 (13) | 0 | 0 | 1 (13) | 1 (8) | 4 (11) |
| Vomiting* | 0 | 0 | 0 | 0 | 1 (14) | 1 (4) | 0 | 0 | 0 | 0 | 1 (3) |
| Abdominal pain* | 0 | 0 | 0 | 1 (14) | 0 | 1 (4) | 0 | 0 | 0 | 0 | 1 (3) |
| Colitis* | 0 | 0 | 1 (17) | 0 | 0 | 1 (4) | 0 | 0 | 0 | 0 | 1 (3) |
| Ileus* | 0 | 0 | 0 | 0 | 1 (14) | 1 (4) | 0 | 0 | 0 | 0 | 1 (3) |
| Metabolism and nutrition | |||||||||||
| 1 (50) | 0 | 2 (33) | 2 (29) | 3 (43) | 8 (33) | 1 (50) | 1 (50) | 4 (50) | 6 (50) | 14 (39) | |
| Hypophosphataemia | |||||||||||
| Hypokalaemia* | 0 | 0 | 1 (17) | 1 (14) | 2 (29) | 4 (17) | 0 | 0 | 0 | 0 | 4 (11) |
| Hyponatraemia | 0 | 0 | 0 | 1 (14) | 0 | 1 (4) | 0 | 0 | 1 (13) | 1 (8) | 2 (6) |
| Decreased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (13) | 1 (8) | 1 (3) |
| Appetite† | |||||||||||
| Dehydration† | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (13) | 1 (8) | 1 (3) |
| Hypocalcaemia† | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (13) | 1 (8) | 1 (3) |
| Skin and subcutaneous tissue | 0 | ||||||||||
| Pruritus* | 0 | 0 | 0 | 0 | 1 (14) | 1 (4) | 0 | 0 | 0 | 0 | 1 (3) |
| Urticaria* | 0 | 0 | 0 | 0 | 1 (14) | 1 (4) | 0 | 0 | 0 | 0 | 1 (3) |
| Constitutional | |||||||||||
| Fatigue* | 0 | 0 | 0 | 1 (14) | 0 | 1 (4) | 0 | 0 | 0 | 0 | 1 (3) |
| Respiratory | |||||||||||
| Cough* | 0 | 0 | 0 | 0 | 1 (14) | 1 (4) | 0 | 0 | 0 | 0 | 1 (3) |
| Pulmonary embolism* | 0 | 0 | 0 | 1 (14) | 0 | 1 (4) | 0 | 0 | 0 | 0 | 1 (3) |
| Blood and lymphatic | |||||||||||
| Leucocytosis† | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (13) | 1 (8) | 1 (3) |
Only observed in Arm A.
Only observed in Arm B
Treatment exposure
Nine subjects (25%) discontinued treatment due to AEs (Table 2), including 5 subjects who discontinued due to treatment-related diarrhoea with or without nausea (N = 3), dermatitis acneiform (N = 1), or dehydration and anorexia (N = 1). Four other subjects discontinued treatment due to non-treatment related AEs. The median duration of time on treatment was 5.6 weeks (range 0.9–57.9 weeks) for the overall study population, and was similar for subjects in Arm A (median 5.8 weeks, range 1.7–57.9 weeks) and those in Arm B (median 5.5 weeks, range 0.9–48.4 weeks).
Pharmacokinetic (PK) analysis
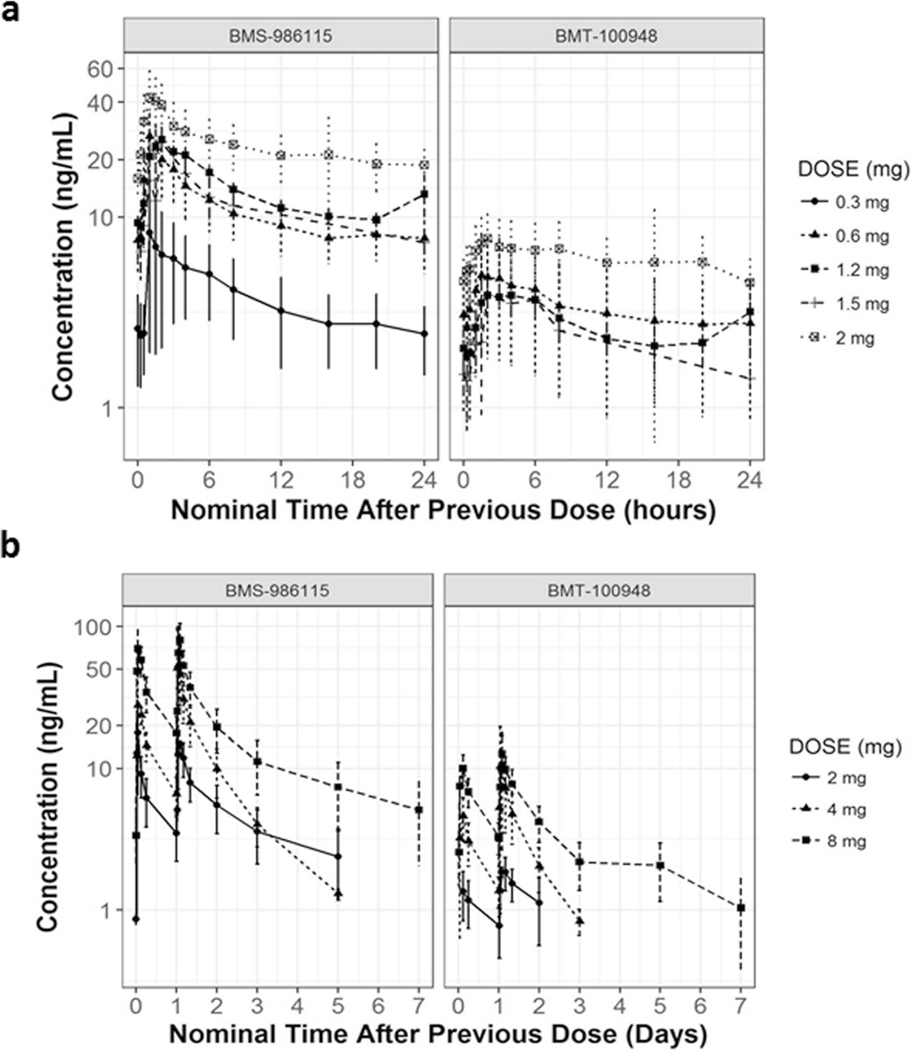
Plasma concentration levels of BMS-986115 (parent) and BMT-100948 (active metabolite) during cycle 2 in patients who received at least one dose of BMS-986115 in the once daily regimen (Arm A) and in the twice weekly regimen (Arm B) were summarized in Fig. 1a and b respectively. Results from NCA using nominal time performed to characterize the PK parameters for the parent compound BMS-986115 in patients who received once daily regimen are presented in Supplementary Table 1. BMS-986115 was rapidly absorbed following single/multiple-dose administration over the 0.3-mg to 8-mg dose range, and the median Tmax was within a range of 1 h to 3 h. There was a dose-related increase for both Cmax and AUCtau after BMS-986115 administration following single/multiple-dose administration over the 0.3-mg to 8-mg dose range. Following multiple once daily dose administration of BMS-986115, the median half-life ranged from 17.24 h to 28.01 h. BMS-986115 accumulated in plasma following once daily administration in Arms A and B. The median accumulation ratios (AUCtau ratio of Day 15/Day 1 values) of BMS-986115 after once daily dosing were 2.64, 3.9, 3.66, 2.33, and 2.19 for 0.3-, 0.6-, 1.2-, 1.5-, and 2-mg doses, respectively. Accumulation ratios (AUCtau ratio of Day 16/Day 1) of BMS-986115 after two consecutive days dosing in a week (Arm B) were 2.47, 3.01, and 2.77 for 2-, 4- and 8-mg doses, respectively. Plasma concentrations of BMT-100948 were low relative to plasma concentrations of the parent drug (BMS-986115). Median Cmax% ratios for BMT-100948 to that of BMS-986115 after a single dose on Day 1 ranged from 5 to 13%, and after multiple doses on Day 15 ranged from 12 to 16%, respectively. Similarly, median AUCtau ratio for BMT-100948 to that of BMS-986115 after a single dose on Day 1 ranged from 14% - 22%, and after multiple doses on Day 15 ranged from 17% - 27%, respectively.
Fig. 1.
Pharmacokinetics of BMS-986115 (parent) and BMT-100948 (metabolite) at steady state (week 3) in patients who received: a once daily regimen (Arm A), b twice weekly regimen (Arm B)
PD analysis
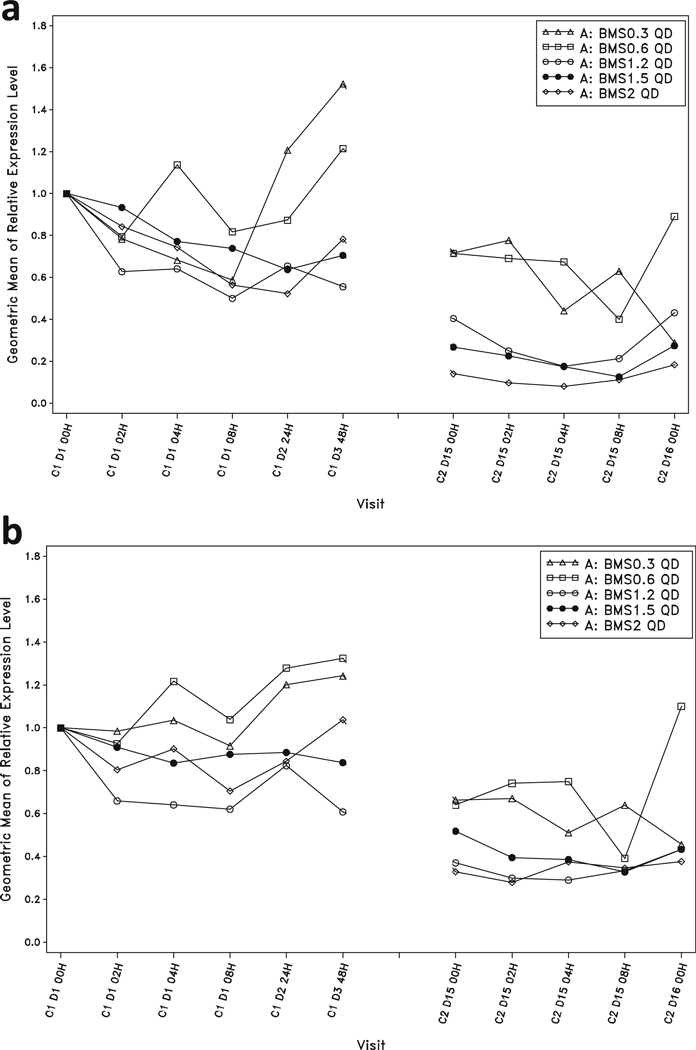
Relative expression levels of the Notch pathway genes Hes1 and Deltex1 in peripheral blood were examined as surrogate markers of the PD activity of BMS-986115. After 2 weeks of dosing at the highest tolerated doses of 1.2 mg QD and 1.5 mg QD, geometric mean relative expression of Hes1 was continuously inhibited by >50% below baseline, with peak inhibition of >80% (Fig. 2). Similarly, geometric mean relative expression of Deltex1 was continuously inhibited by greater than 45% for the entire dosing interval, with peak inhibition of >60% (Fig. 2). At the highest tolerated dose level of 4 mg BIW, geometric mean relative expression of Hes1 was inhibited by >75% for at least 48 h of the weekly dosing cycle and Deltex1 was inhibited by greater than 50%.
Fig. 2.
Geometric means of relative expression levels in peripheral blood following a single dose and once daily dosing with BMS-986115: a Hes1, and b Deltex1
Efficacy
Of the 36 treated subjects, 21 (58%) subjects had progressive disease and 11 (31%) had stable disease (SD) as a best tumour response and 4 were not evaluable. Of the 11 subjects with SD, 5 (1 clear cell renal cell carcinoma, 1 fibromatosis, 2 adenoid cystic carcinoma, and 1 retroperitoneal liposarcoma) had SD for more than 6 months (Supplementary Figure 1). The patient with clear cell renal cell carcinoma, who had documented disease progression prior to study entry, achieved SD with maximum tumor shrinkage of 23.9% after ~5.5 months of treatment. In addition, the patient with fibromatosis, who did not have documented disease progression before study entry, achieved SD with maximum disease shrinkage of 16.5% after ~9 months of treatment. Two patients with adenoid cystic carcinoma and one patient with retroperitoneal liposarcoma were documented t ohave slowly progressive-disease before entering the study. The patient with retroperitoneal liposarcoma actually achieved SD with maximum tumor shrinkage of 13.8% after ~7.5 months of treatment while two patients with adenoid cystic carcinoma experienced continued slow progression of disease during treatment. All 3 patients who had prolonged SD with disease shrinkage were treated with the pharmacodynamically active doses of BMS-986115 (1.2 mg QD [renal cell carcinoma case], 1.5 mg QD [fibromatosis case], and 2 mg QD [retroperitoneal liposarcoma case]).
Discussion
The clinical development of Notch inhibitors has been hampered by on-target GI toxicity and most drugs have not been tolerable at daily continuous schedule [19–21]. Our study, however, demonstrates that an oral gamma secretase inhibitor BMS-986115 could be tolerable with continuous QD dosing with acceptable PK profile and pharmacodynamic target engagement evidenced by continuous inhibition of Notch pathway genes Hes1 and Deltex1 at 1.2 mg QD and 1.5 mg QD, the MTD. This demonstration, in our opinion, is an important milestone in the clinical development of Notch inhibitors.
Notch plays both tumour suppressive and oncogenic role in a context dependent manner and loss of function as well as gain of function mutations were found across multiple solid tumour types [5, 22]. Despite the hope that gain of function mutations could potentially be used as a biomarker for response, clinical activity of Notch inhibitors in T cell acute lymphoblastic leukaemia, where Notch1 mutations are seen in >50% of cases [2], was modest [23] highlighting the biological complexity of Notch pathway. Nonetheless, Notch signalling inhibition using gamma-secretase inhibitors was shown to produce anti-tumour activity in multiple solid tumours in the published literature including high-grade gliomas, thyroid cancer, melanoma, colorectal carcinoma with neuroendocrine features and also in desmoid tumours [10, 19–21, 24] indicating that there is a subset of patients who could indeed derive clinical benefit from Notch inhibitors.
Potential predictive biomarkers of response to Notch inhibitors are also emerging. In ~10% of triple negative breast cancer (TNBC), NOTCH1 and NOTCH2 re-arrangements that cause constitutive receptor activation were found by next generation sequencing [25]. TNBC cells with NOTCH1 re-arrangements were sensitive to MRK-003, a gamma secretase inhibitor, in vitro and in vivo. In contrast those with NOTCH2 rearrangement were resistant to MRK-003 [25]. NOTCH1 re-arrangements were associated with high levels of activated NOTCH1 intracellular domain (N1-ICD) and there was a correlation between N1-ICD expression measured by immunohistochemistry and responsiveness to MRK-003 [25]. Multiple solid tumours, in fact, have Notch NRR domain mutations with variable frequencies [25] and patients with tumours harbouring these mutations could be selected for future Notch inhibitor trials.
Notch signalling is diverse and cross talks with several key oncogenic pathways including RAS, PI3K/AKT, VEGF and oestrogen receptor pathways [23]. Safety and tolerability of oral notch inhibitors combined with insulin like growth factor 1 inhibitor [26] as well as m-TOR inhibitor [27, 28] has been previously reported. Identification of rational combination strategies that can be employed within the appropriate biological and clinical contexts will be critical in further clinical development of Notch inhibitors in patients with solid tumours.
Despite the demonstration of safety and tolerability, our study was terminated early for business reasons making it difficult to ultimately draw any firm conclusion. Our results, however, show that continuous target inhibition of the Notch pathway can be achieved using tolerable QD doses of BMS-986115. At the MTD 1.5 mg QD, the only DLT observed was Gr 3 nausea (N = 1). Although Gr 3/4 diarrhoea related to the study treatment was observed in 2 of 7 patients (29%) treated at this dose level, these events resolved to Gr 1 within 24 h with appropriate medical management. No DLT was observed at 1.2 mg QD dosing. Encouragingly, we also observed prolonged SD of >6 months with disease shrinkage in 3 patients treated at dose levels that are pharmacodynamically active. It is regrettable, however, that we did not manage to perform correlative analyses on tumors to identify potential biomarkers of response in these patients because of early termination of the study.
In summary, our findings indicate that oral continuous QD doses of an oral gamma-secretase inhibitor BMS-986115 could be tolerable and may be clinically active in selected tumor types. However, although manageable, the risk of gastrointestinal toxicities remains relatively high in patients treated with continuous schedule and intermittent schedule may be better tolerated and preferable. In our study, although 8 mg BIW schedule was not found to be tolerated, 2 patients tolerated 4 mg BIW dosing, and we could have tested an intermediate dose on the same schedule if the study was not terminated early. The findings from our study reinforce the fact that careful dose selection and scheduling may improve tolerability of oral Notch inhibitors while maintaining biological activity and therapeutic efficacy.
Supplementary Material
Acknowledgements
Authors would like to thank the patients and study teams at each participating site; Dr. Lillian Siu from the Princess Margaret Cancer Centre, Toronto, Canada; Lisu Wang and John Cogswell from Bristol-Myers Squibb, Hopewell, New Jersey, USA; Sumitha Kurian, Shweta Ramayya, Ankit Jinager, and Deepa Joshi from Biocon BMS R&D Center, Syngene International Ltd., Bangalore, India.
Funding This study was sponsored and funded by Bristol-Myers Squibb.
Footnotes
Compliance with ethical standards
Conflict of interest Gaurav Bajaj, Bing He, Tian Chen, Lili Zhu, Zhenhao Qi, and Bruce S. Fischer are employees and stockholders of Bristol-Myers Squibb. Sharath Poojary and Shashwati Basak are employees of Syngene International Ltd., which has business relationship with Bristol-Myers Squibb. Other authors declare no conflict of interest.
Ethical approval This article does not contain any studies with animals performed by any of the authors. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10637-018-0597-6) contains supplementary material, which is available to authorized users.
References
- 1.Borggrefe T, Oswald F (2009) The notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci 66(10):1631–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weng AP, Ferrando AA, Lee W, Morris JP 4th, Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC (2004) Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306(5694):269–271 [DOI] [PubMed] [Google Scholar]
- 3.Jundt F, Schwarzer R, Dorken B (2008) Notch signaling in leukemias and lymphomas. Curr Mol Med 8(1):51–59 [DOI] [PubMed] [Google Scholar]
- 4.Tzoneva G, Ferrando AA (2012) Recent advances on NOTCH signaling in T-ALL. Curr Top Microbiol Immunol 360:163–182 [DOI] [PubMed] [Google Scholar]
- 5.Koch U, Radtke F (2010) Notch signaling in solid tumors. Curr Top Dev Biol 92:411–455 [DOI] [PubMed] [Google Scholar]
- 6.Reedijk M (2012) Notch signaling and breast cancer. Adv Exp Med Biol 727:241–257 [DOI] [PubMed] [Google Scholar]
- 7.Korkaya H, Wicha MS (2009) HER-2, notch, and breast cancer stem cells: targeting an axis of evil. Clin Cancer Res 15(6):1845–1847 [DOI] [PubMed] [Google Scholar]
- 8.Miele L, Golde T, Osborne B (2006) Notch signaling in cancer. Curr Mol Med 6(8):905–918 [DOI] [PubMed] [Google Scholar]
- 9.Rosati E, Sabatini R, Rampino G, Tabilio A, di Ianni M, Fettucciari K, Bartoli A, Coaccioli S, Screpanti I, Marconi P (2009) Constitutively activated Notch signaling is involved in survival and apoptosis resistance of B-CLL cells. Blood 113(4):856–865 [DOI] [PubMed] [Google Scholar]
- 10.Kummar S, O’Sullivan Coyne G, Do KT, Turkbey B, Meltzer PS, Polley E, Choyke PL, Meehan R, Vilimas R, Horneffer Y, Juwara L, Lih A, Choudhary A, Mitchell SA, Helman LJ, Doroshow JH, Chen AP (2017) Clinical activity of the gamma-secretase inhibitor PF-03084014 in adults with desmoid tumors (aggressive fibromatosis). J Clin Oncol 35(14):1561–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolos V et al. (2009) Notch signalling in cancer stem cells. Clin Transl Oncol 11(1):11–19 [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Sullenger BA, Rich JN (2012) Notch signaling in cancer stem cells. Adv Exp Med Biol 727:174–185 [DOI] [PubMed] [Google Scholar]
- 13.Pannuti A, Foreman K, Rizzo P, Osipo C, Golde T, Osborne B, Miele L (2010) Targeting Notch to target cancer stem cells. Clin Cancer Res 16(12):3141–3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reya T, Clevers H (2005) Wnt signalling in stem cells and cancer. Nature 434(7035):843–850 [DOI] [PubMed] [Google Scholar]
- 15.Roca C, Adams RH (2007) Regulation of vascular morphogenesis by Notch signaling. Genes Dev 21(20):2511–2524 [DOI] [PubMed] [Google Scholar]
- 16.Phng LK, Gerhardt H (2009) Angiogenesis: a team effort coordinated by notch. Dev Cell 16(2):196–208 [DOI] [PubMed] [Google Scholar]
- 17.Takebe N, Nguyen D, Yang SX (2014) Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol Ther 141(2):140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon R, Rubinstein L, Arbuck SG, Christian MC, Freidlin B, Collins J (1997) Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst 89(15):1138–1147 [DOI] [PubMed] [Google Scholar]
- 19.Pant S, Jones SF, Kurkjian CD, Infante JR, Moore KN, Burris HA, McMeekin DS, Benhadji KA, Patel BKR, Frenzel MJ, Kursar JD, Zamek-Gliszczynski MJ, Yuen ESM, Chan EM, Bendell JC (2016) A first-in-human phase I study of the oral Notch inhibitor, LY900009, in patients with advanced cancer. Eur J Cancer 56:1–9 [DOI] [PubMed] [Google Scholar]
- 20.Krop I, Demuth T, Guthrie T, Wen PY, Mason WP, Chinnaiyan P, Butowski N, Groves MD, Kesari S, Freedman SJ, Blackman S, Watters J, Loboda A, Podtelezhnikov A, Lunceford J, Chen C, Giannotti M, Hing J, Beckman R, LoRusso P (2012) Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J Clin Oncol 30(19):2307–2313 [DOI] [PubMed] [Google Scholar]
- 21.Tolcher AW, Messersmith WA, Mikulski SM, Papadopoulos KP, Kwak EL, Gibbon DG, Patnaik A, Falchook GS, Dasari A, Shapiro GI, Boylan JF, Xu ZX, Wang K, Koehler A, Song J, Middleton SA, Deutsch J, DeMario M, Kurzrock R, Wheler JJ (2012) Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J Clin Oncol 30(19):2348–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang NJ, Sanborn Z, Arnett KL, Bayston LJ, Liao W, Proby CM, Leigh IM, Collisson EA, Gordon PB, Jakkula L, Pennypacker S, Zou Y, Sharma M, North JP, Vemula SS, Mauro TM, Neuhaus IM, LeBoit PE, Hur JS, Park K, Huh N, Kwok PY, Arron ST, Massion PP, Bale AE, Haussler D, Cleaver JE, Gray JW, Spellman PT, South AP, Aster JC, Blacklow SC, Cho RJ (2011) Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proc Natl Acad Sci U S A 108(43):17761–17766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espinoza I, Miele L (2013) Notch inhibitors for cancer treatment. Pharmacol Ther 139(2):95–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Messersmith WA, Shapiro GI, Cleary JM, Jimeno A, Dasari A, Huang B, Shaik MN, Cesari R, Zheng X, Reynolds JM, English PA, McLachlan KR, Kern KA, LoRusso PM (2015) A phase I, dose-finding study in patients with advanced solid malignancies of the oral gamma-secretase inhibitor PF-03084014. Clin Cancer Res 21(1):60–67 [DOI] [PubMed] [Google Scholar]
- 25.Stoeck A, Lejnine S, Truong A, Pan L, Wang H, Zang C, Yuan J, Ware C, MacLean J, Garrett-Engele PW, Kluk M, Laskey J, Haines BB, Moskaluk C, Zawel L, Fawell S, Gilliland G, Zhang T, Kremer BE, Knoechel B, Bernstein BE, Pear WS, Liu XS, Aster JC, Sathyanarayanan S (2014) Discovery of biomarkers predictive of GSI response in triple-negative breast cancer and adenoid cystic carcinoma. Cancer Discov 4(10):1154–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brana I, Berger R, Golan T, Haluska P, Edenfield J, Fiorica J, Stephenson J, Martin LP, Westin S, Hanjani P, Jones MB, Almhanna K, Wenham RM, Sullivan DM, Dalton WS, Gunchenko A, Cheng JD, Siu LL, Gray JE (2014) A parallel-arm phase I trial of the humanised anti-IGF-1R antibody dalotuzumab in combination with the AKT inhibitor MK-2206, the mTOR inhibitor ridaforolimus, or the NOTCH inhibitor MK-0752, in patients with advanced solid tumours. Br J Cancer 111(10):1932–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz-Padilla I, Hirte H, Oza AM, Clarke BA, Cohen B, Reedjik M, Zhang T, Kamel-Reid S, Ivy SP, Hotte SJ, Razak AAR, Chen EX, Brana I, Wizemann M, Wang L, Siu LL, Bedard PL (2013) A phase Ib combination study of RO4929097, a gamma-secretase inhibitor, and temsirolimus in patients with advanced solid tumors. Investig New Drugs 31(5):1182–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piha-Paul SA, Munster PN, Hollebecque A, Argilés G, Dajani O, Cheng JD, Wang R, Swift A, Tosolini A, Gupta S (2015) Results of a phase 1 trial combining ridaforolimus and MK-0752 in patients with advanced solid tumours. Eur J Cancer 51(14):1865–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.