Abstract
Objective
Synchronous endometrial and ovarian cancer with endometrioid histology at two cancer sites typically presents with early-stage disease and is thought to have a good prognosis. We examined the survival of women with early-stage endometrioid endometrial cancer who had synchronous early-stage endometrioid ovarian cancer.
Methods
This is a retrospective case-control study examining the Surveillance, Epidemiology, and End Result Program between 1973 and 2013. Survival of women with stage I endometrioid endometrial cancer with stage I endometrioid ovarian cancer (n = 839) were compared to women with stage I endometrioid endometrial cancer without synchronous ovarian cancer (n = 123,692) after propensity score matching.
Results
Women with synchronous stage I endometrioid ovarian cancer were more likely to be diagnosed recently, be younger, have stage IA disease, grade 1 tumors, to have undergone lymphadenectomy, and were less likely to receive radiotherapy compared to those without synchronous ovarian cancer (all, P < 0.001). In a propensity score matched model, the presence of synchronous ovarian cancer was not associated with endometrial cancer-specific survival (10-year rates 96.0% versus 95.3%, P = 0.97) or overall survival (85.6% versus 87.2%, P = 0.10). Among tumors with concordant grades at the two cancer sites, survival was similar regardless of presence of synchronous ovarian tumors (grade 1 tumors, 10-year rate for overall survival, 88.2% versus 89.1%, P = 0.40; and grade 2 tumors, 84.0% versus 85.8%, P = 0.78).
Conclusion
Women with stage I endometrioid endometrial cancer with synchronous stage I endometrioid ovarian cancer have a survival outcome similar to those with stage I endometrioid endometrial cancer without synchronous ovarian cancer.
Keywords: Endometrial cancer, Ovarian cancer, Synchronous, Prognosis
1. Introduction
In 2017, endometrial cancer remains the most common gynecologic malignancy in the United States with an estimated 61,000 new cases. The concurrent presence of ovarian cancer with endometrial cancer, so called synchronous endometrial and ovarian cancer (SEOC), is not a rare clinical entity, and it is reported in ~3% of endometrial cancers [1–4]. Historically, SEOC has presented as early-stage disease resulting in a good prognosis [2,5]. A recent study has concluded that SEOC has a survival outcome similar to endometrial cancer without SEOC [6].
One of the obstacles in making a diagnosis of SEOC is that the histologic types are often the same at the two cancer sites with the most common pattern being concordant endometrioid histology [2,5]. In such cases of isolated endometrioid tumors seen in the endometrium and the ovary, diagnostic possibilities include (i) SEOC with stage I endometrioid endometrial cancer and stage I endometrioid ovarian cancer, (ii) metastatic endometrioid endometrial cancer to the ovary (stage III disease), or (iii) metastatic endometrioid ovarian cancer to the endometrium (stage II disease).
Traditionally, the Ulbright and Roth criteria followed by the Scully criteria have been utilized to distinguish SEOC from metastatic endometrial or ovarian cancer [7,8]. However, to date, population-based statistics to determine a survival difference in women with concordant endometrioid SEOC from those with endometrioid endometrial cancer without synchronous ovarian cancer have been lacking. The objective of our study was to examine survival of women with stage I endometrioid endometrial cancer who had synchronous stage I endometrioid ovarian cancer compared to those who had stage I endometrioid endometrial cancer without synchronous ovarian cancer.
2. Materials and methods
2.1. Data source and eligibility criteria
This is a retrospective case-control study utilizing the Surveillance, Epidemiology, and End Results (SEER) Program [9]. SEER is the largest population-based tumor registry in the United States, provided and maintained by the National Cancer Institute since 1973. SEER covers ~28% of the US population, and is publicly available and de-identified. The data entry to this database is performed by certified and trained staff personnel with rigorous quality assurance [10]. The Institutional Review Board at the University of Southern California exempted this study protocol. The STROBE guidelines were utilized to describe the results of the observational study [11].
The SEER18 cases covering 1973–2013 were extracted by using SEER*Stat 8.3.2 by searching cases for “Corpus Uteri/Uterus NOS” limited to malignancy and female sex. The primary endometrial cancer cases were eligible for the study, and sarcoma or metastatic tumors to the uterus from another origin were excluded from analysis. Among the eligible cases, stage I endometrioid type endometrial cancer were examined for the presence of synchronous ovarian cancer.
To identify synchronous ovarian cancer cases, SEER*Stat 8.3.2 was used to generate primary ovarian cancer cases for the same study period. The ovarian cancer dataset and the aforementioned endometrial cancer dataset were then linked. The synchronous ovarian cancer cases were then identified via searching for the same study identification number between the two datasets [12–14]. Women whom the time intervals between the endometrial cancer diagnosis and the ovarian cancer diagnosis were less than 4 months were defined as SEOC.
We used the cutoff value of a 4-month time interval between the two cancer diagnoses based on the logic that endometrial cancer is commonly diagnosed via endometrial biopsy prior to hysterectomy and ovarian cancer is generally diagnosed at the time of subsequent hysterectomy. Indeed, the vast majority of women with endometrial cancer (nearly 90%) undergo hysterectomy within 4 months of the cancer diagnosis [15–17].
2.2. Clinical information
Among the eligible cases the following variables were ascertained from the SEER database: patient demographics, tumor information, treatment types, and survival outcome. Age, year and month at diagnosis, ethnicity, marital status, and registration area were examined for patient demographics. Cancer stage, histologic subtype, tumor grade, and tumor size were collected for tumor characteristics. Use of regional lymphadenectomy and radiotherapy represents treatment type. Survival outcome included cause-specific survival (CSS) and overall survival (OS).
Cancer stage was based on the AJCC 7th surgical-pathological staging classification schema [18]. The ICD-0–3 site/histology validation list and the World Health Organization histological classification were used for identifying endometrioid histology (endometrioid endometrial cancer, 8140–3, 8262–3, 8380–3, 8382–3, and 8383–3; and endometrioid ovarian cancer, 8380–3, 8381–3, 8382–3, and 8383–3) as previously described [19]. CSS was defined as the time interval between the date of endometrial cancer diagnosis and the date of death from endometrial cancer. OS was defined as the time interval between the data of endometrial cancer diagnosis and the data of death for any reason (all-cause). In the SEER database, cause of death is linked with the National Death Index and the state mortality records for validation [20].
2.3. Statistical considerations
The primary interest of analysis was to examine the survival outcome of women with stage I endometrioid SEOC (case group) compared to those with stage I endometrioid endometrial cancer without synchronous ovarian cancer (control group). The secondary objective of the analysis was to examine survival outcome of women with stage I concordant grade 1 endometrioid SEOC compared to those with stage I grade 1 endometrioid endometrial cancer. Similar analysis was performed for concordant grade 2 cases.
We used propensity score matching to adjust the background differences between the two groups (SEOC and non-SEOC). A multivariable logistic regression analysis was used to compute the propensity score for SEOC for each case; patient demographics, tumor characteristics, and treatment pattern were entered in the model. An automated algorithm was utilized for one-to-one propensity score matching between the SEOC group and the non-SEOC group, and the cutoff of the propensity score difference was defined as 1%.
Survival curves were constructed with the Kaplan-Meier method [21], and a log-rank test was used to assess statistical significance between the curves via univariable analysis. A Cox proportional hazard regression model was used to assess the association of SEOC and survival outcome on univariable analysis [22], and the magnitudes of statistical significance were expressed with hazard ratio (HR) and 95% confidence interval (CI). All hypotheses were two-sided, and a P-value of less than 0.05 was considered statistically significant. Statistical Package for Social Sciences (IBM SPSS, version 24.0, Armonk, NY) was used for the analysis.
3. Results
3.1. Patient selection
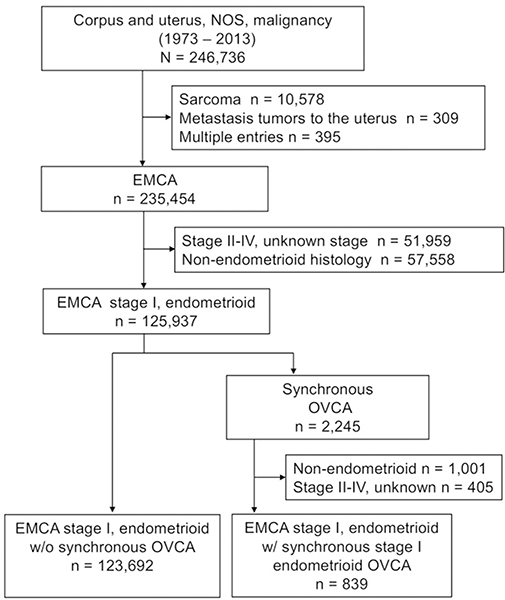
The patient selection schema is shown in Fig. 1. Among 246,736 cases of uterine malignancy between 1973 and 2013, there were 235,454 cases of primary endometrial cancer. Of those, there were 125,937 cases with stage I endometrioid endometrial cancer. Among those, there were 2,245 (1.8%, 95% CI 1.7 to 1.9) cases of synchronous ovarian cancer with any histology/stage of disease. After excluding cases with non-endometrioid/stage II–IV disease, there were 839 cases of stage I endometrioid endometrial cancer with synchronous stage I endometrioid ovarian cancer, representing the case group. The remaining 123,692 cases of stage I endometrioid endometrial cancer without synchronous ovarian cancer comprised the control group.
Fig. 1. Study schema.
Abbreviations: EMCA, endometrial cancer; and OVCA, ovarian cancer.
3.2. Clinical characteristics
Patient demographics are shown in Table 1. Women with stage I endometrioid endometrial cancer and synchronous stage I endometrioid ovarian cancer were more likely to be younger and single but less likely to be of Black race compared to those with stage I endometrioid endometrial cancer without synchronous ovarian cancer (all, P < 0.001). The diagnosis of stage I endometrioid endometrial cancer with synchronous stage I endometrioid ovarian cancer was more likely to be made in recent years (P < 0.001). The endometrial component of stage I endometrioid SEOC was more likely to be stage IA and less likely to be grade 3 compared to non-synchronous stage I endometrioid endometrial cancer (both, P < 0.001). Women with stage I endometrioid endometrial cancer and synchronous stage I endometrioid ovarian cancer were more likely to undergo regional lymphadenectomy (71.2% versus 47.3%, P < 0.001) and less likely to receive radiotherapy (10.8% versus 15.7%, P < 0.001) compared to those with stage I endometrioid endometrial cancer without synchronous ovarian cancer.
Table 1.
Patient demographics (N = 124,531).
| Characteristics | Before matching |
After matching |
||||
|---|---|---|---|---|---|---|
| |
OVCA (+) |
OVCA (−) |
P-value | OVCA (+) |
OVCA (−) |
P-value |
| Number | n = 839 | n = 123,692 | n = 839 | n = 839 | ||
| Age | 50.7 (± 10.5) | 62.0 (± 12.0) | <0.001 | 50.7 (± 10.5) | 51.7 (± 11.5) | 0.42 |
| <40 | 104 (12.4%) | 4,322 (3.5%) | 104 (12.4%) | 96 (11.4%) | ||
| 40–49 | 308 (36.7%) | 12,641 (10.2%) | 308 (36.7%) | 317 (37.8%) | ||
| 50–59 | 290 (34.6%) | 35,632 (28.8%) | 290 (34.6%) | 268 (31.9%) | ||
| ≥60 | 137 (16.3%) | 71,097 (57.5%) | 137 (16.3%) | 158 (18.8%) | ||
| Race/ethnicity | <0.001 | 0.55 | ||||
| White | 659 (78.5%) | 98,666 (79.8%) | 659 (78.5%) | 656 (78.2%) | ||
| Black | 17 (2.0%) | 6,409 (5.2%) | 17 (2.0%) | 25 (3.0%) | ||
| Hispanic | 80 (9.5%) | 9,598 (7.8%) | 80 (9.5%) | 84 (10.0%) | ||
| Asian | 69 (8.2%) | 6,626 (5.4%) | 69 (8.2%) | 57 (6.8%) | ||
| Others | 14 (1.7%) | 2,393 (1.9%) | 14 (1.7%) | 17 (2.0%) | ||
| Year at diagnosis | <0.001 | 0.10 | ||||
| 1973–1979 | 11 (1.3%) | 5,920 (4.8%) | 11 (1.3%) | 22 (2.6%) | ||
| 1980–1989 | 40 (4.8%) | 11,139 (9.0%) | 40 (4.8%) | 58 (6.9%) | ||
| 1990–1999 | 154 (18.4%) | 23,314 (18.8%) | 154 (18.4%) | 158 (18.8%) | ||
| 2000–2009 | 435 (51.8%) | 54,864 (44.4%) | 435 (51.8%) | 418 (49.8%) | ||
| 2010–2013 | 199 (23.7%) | 28,455 (23.0%) | 199 (23.7%) | 183 (21.8%) | ||
| Marital status | <0.001 | 0.18 | ||||
| Single | 252 (30.0%) | 17,604 (14.2%) | 252 (30.0%) | 249 (29.7%) | ||
| Married | 443 (52.8%) | 68,412 (55.3%) | 443 (52.8%) | 417 (49.7%) | ||
| Others | 144 (17.2%) | 37,676 (30.5%) | 144 (17.2%) | 173 (20.6%) | ||
| Registry area | 0.13 | 0.46 | ||||
| West | 469 (55.9%) | 65,053 (52.6%) | 469 (55.9%) | 452 (53.9%) | ||
| Central | 170 (20.3%) | 27,922 (22.6%) | 170 (20.3%) | 191 (22.8%) | ||
| East | 200 (23.8%) | 30,717 (24.8%) | 200 (23.8%) | 196 (23.4%) | ||
| Stage | <0.001 | 0.16 | ||||
| IA | 704 (83.9%) | 93,447 (75.5%) | 704 (83.9%) | 351 (85.2%) | ||
| IB | 67 (8.0%) | 16,721 (13.5%) | 67 (8.0%) | 18 (4.4%) | ||
| I NOS | 68 (8.1%) | 13,524 (10.9%) | 68 (8.1%) | 43 (10.4%) | ||
| Grade | <0.001 | 0.65 | ||||
| 1 | 435 (51.8%) | 61,258 (49.5%) | 435 (51.8%) | 423 (50.4%) | ||
| 2 | 248 (29.6%) | 36,572 (29.6%) | 248 (29.6%) | 239 (28.5%) | ||
| 3 | 48 (5.7%) | 12,990 (10.5%) | 48 (5.7%) | 55 (6.6%) | ||
| Unknown | 108 (12.9%) | 12,872 (10.4%) | 108 (12.9%) | 122 (14.5%) | ||
| Size | 0.55 | 0.46 | ||||
| ≤2.0 cm | 104 (12.4%) | 16,274 (13.2%) | 104 (12.4%) | 100 (11.9%) | ||
| >2. cm | 291 (34.7%) | 40,843 (33.0%) | 291 (34.7%) | 270 (32.2%) | ||
| Unknown | 444 (52.9%) | 66,575 (53.8%) | 444 (52.9%) | 469 (55.9%) | ||
| Lymphadenectomy | <0.001 | 0.31 | ||||
| Not performed | 212 (25.3%) | 57,019 (46.1%) | 212 (25.3%) | 231 (27.5%) | ||
| Performed | 597 (71.2%) | 58,531 (47.3%) | 597 (71.2%) | 570 (67.9%) | ||
| Unknown | 30 (3.6%) | 8,142 (6.6%) | 30 (3.6%) | 38 (4.5%) | ||
| Radiotherapy | <0.001 | 0.20 | ||||
| No | 743 (88.6%) | 100,951 (81.6%) | 743 (88.6%) | 728 (86.8%) | ||
| Yes | 91 (10.8%) | 19,456 (15.7%) | 91 (10.8%) | 99 (11.8%) | ||
| Unknown | 5 (0.6%) | 3,258 (2.7%) | 5 (0.6%) | 12 (1.4%) | ||
Number (%) per column or mean (±standard deviation) is shown. P-values are results of univariable analysis, and significant P-values are emboldened. Abbreviations: OVCA, synchronous stage I endometrioid ovarian cancer; and NOS, not otherwise specified.
3.3. Survival analysis after propensity score matching
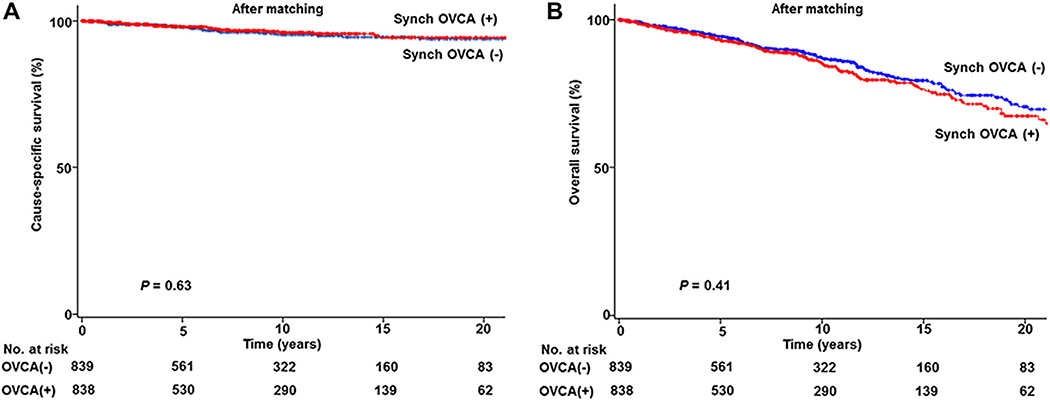
Given the large difference in patient background characteristics between the two groups, a propensity score match was performed (Table 1). After matching, demographics were similar between the two groups (all, P > 0.05). Median follow-up time was 7.3 years for the SEOC group and 7.6 years for the non-SEOC group, respectively. There were 30 and 25 deaths from endometrial cancer in the SEOC group and the non-SEOC group, respectively. There were 131 and 130 deaths from any causes in the SEOC group and the non-SEOC group, respectively. In a post-matching model, univariable analysis showed that women with stage I endometrioid endometrial cancer and synchronous of stage I endometrioid ovarian cancer had a CSS similar to those with stage I endometrioid endometrial cancer without synchronous ovarian cancer (10-year rate, 96.0% versus 95.3%, HR 0.88, 95% CI 0.52 to 1.49, P = 0.63; Fig. 2A). Moreover, OS was similar between the two groups (10-year rate, 85.6% versus 87.2%, HR 1.11, 95% CI 0.87 to 1.41, P = 0.41; Fig. 2B).
Fig. 2. Survival curves based on synchronous ovarian cancer status.
Log-rank test for P-values. (A) CSS and (B) OS are shown for stage I endometrioid endometrial cancer with synchronous stage I endometrioid ovarian cancer (red lines) compared to stage I endometrioid endometrial cancer without synchronous ovarian cancer (blue lines) based on propensity score matching status. Abbreviations: Synch OVCA, synchronous stage I endometrioid ovarian cancer; CSS, cause-specific survival from endometrial cancer; and OS, overall survival from all-cause.
3.4. Concordant tumor grade at two cancer sites
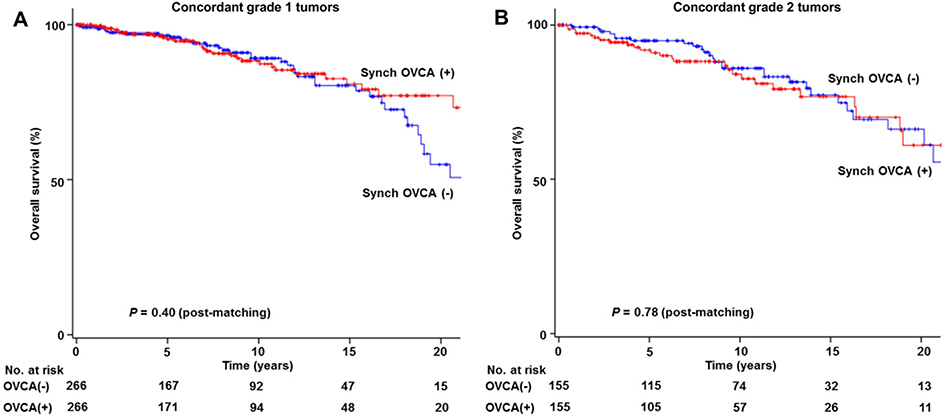
In a sensitivity analysis, survival outcome was examined in women with concordant tumor grade at the two cancer sites in a post-matching model. First, concordant grade 1 endometrioid tumors at the two cancer sites (n = 266) were compared to grade 1 endometrioid endometrial cancer without synchronous ovarian cancer (n = 61,258; Table 2). After propensity score matching, OS was similar between the two groups (10-year rate, 88.2% versus 89.1%, HR 0.81, 95% CI 0.50 to 1.32, P = 0.40; Fig. 3A). Similarly, in the concordant grade 2 tumor cases (Table 3), presence of synchronous ovarian cancer was not associated with OS (10-year rate, 84.0% versus 85.8%, HR 1.07, 95% CI 0.64 to 1.82, P = 0.78; Fig. 3B).
Table 2.
Patient demographics (concordant grade 1 tumor cohort).
| Characteristics | Before matching |
After matching |
||||
|---|---|---|---|---|---|---|
| |
OVCA (+) |
OVCA (−) |
P-value | OVCA (+) |
OVCA (−) |
P-value |
| Number | n = 266 | n = 61,258 | n = 266 | n = 266 | ||
| Age | 49.7 (± 10.2) | 60.4 (± 11.9) | <0.001 | 49.7 (± 10.2) | 50.5 (± 12.3) | 0.10 |
| <40 | 39 (14.7%) | 2,742 (4.5%) | 39 (14.7%) | 44 (16.5%) | ||
| 40–49 | 98 (36.8%) | 7,526 (12.3%) | 98 (36.8%) | 101 (38.0%) | ||
| 50–59 | 95 (35.7%) | 19,063 (31.1%) | 95 (35.7%) | 72 (27.1%) | ||
| ≥60 | 34 (12.8%) | 31,927 (52.1%) | 34 (12.8%) | 49 (18.4%) | ||
| Race/ethnicity | 0.003 | 0.66 | ||||
| White | 199 (74.8%) | 48,692 (79.5%) | 199 (74.8%) | 186 (69.9%) | ||
| Black | 5 (1.9%) | 2,610 (4.3%) | 5 (1.9%) | 9 (3.4%) | ||
| Hispanic | 33 (12.4%) | 5,167 (8.4%) | 33 (12.4%) | 38 (14.3%) | ||
| Asian | 25 (9.4%) | 3,453 (5.6%) | 25 (9.4%) | 27 (10.2%) | ||
| Others | 4 (1.5%) | 1,336 (2.2%) | 4 (1.5%) | 6 (2.3%) | ||
| Year at diagnosis | 0.002 | 0.67 | ||||
| 1973–1979 | 1 (0.4%) | 2,553 (4.2%) | 1 (0.4%) | 1 (0.4%) | ||
| 1980–1989 | 12 (4.5%) | 5,255 (8.6%) | 12 (4.5%) | 14 (5.3%) | ||
| 1990–1999 | 49 (18.4%) | 11,321 (18.5%) | 49 (18.4%) | 52 (19.5%) | ||
| 2000–2009 | 141 (53.0%) | 28,498 (46.5%) | 141 (53.0%) | 124 (46.6%) | ||
| 2010–2013 | 63 (23.7%) | 13,631 (22.3%) | 63 (23.7%) | 75 (28.2%) | ||
| Marital status | <0.001 | 0.73 | ||||
| Single | 77 (28.9%) | 9,052 (14.8%) | 77 (28.9%) | 84 (31.6%) | ||
| Married | 149 (56.0%) | 35,009 (57.2%) | 149 (56.0%) | 140 (52.6%) | ||
| Others | 40 (15.0%) | 17,197 (28.1%) | 40 (15.0%) | 42 (15.8%) | ||
| Registry area | 0.11 | 0.64 | ||||
| West | 159 (59.8%) | 32,724 (53.4%) | 159 (59.8%) | 150 (56.4%) | ||
| Central | 48 (18.0%) | 13,459 (22.0%) | 48 (18.0%) | 48 (18.0%) | ||
| East | 59 (22.2%) | 15,075 (24.6%) | 59 (22.2%) | 68 (25.6%) | ||
| Stage | 0.038 | 0.97 | ||||
| IA | 230 (86.5%) | 49,133 (80.2%) | 230 (86.5%) | 230 (86.5%) | ||
| IB | 16 (6.0%) | 5,440 (8.9%) | 16 (6.0%) | 15 (5.6%) | ||
| I NOS | 20 (7.5%) | 6,685 (10.9%) | 20 (7.5%) | 21 (7.9%) | ||
| Size | 0.37 | 0.98 | ||||
| ≤2.0 cm | 35 (13.2%) | 9,644 (15.7%) | 35 (13.2%) | 36 (13.5%) | ||
| >2.0 cm | 82 (30.8%) | 17,032 (27.8%) | 82 (30.8%) | 83 (31.2%) | ||
| Unknown | 149 (56.0%) | 34,582 (56.5%) | 149 (56.0%) | 147 (53.5%) | ||
| Lymphadenectomy | <0.001 | 0.07 | ||||
| Not performed | 71 (26.7%) | 34,019 (55.5%) | 71 (26.7%) | 96 (36.1%) | ||
| Performed | 186 (69.9%) | 23,458 (38.3%) | 186 (69.9%) | 161 (60.5%) | ||
| Unknown | 9 (3.4%) | 3,781 (6.2%) | 9 (3.4%) | 9 (3.4%) | ||
| Radiotherapy | 0.35 | 0.57 | ||||
| No | 245 (92.1%) | 55,534 (90.7%) | 245 (92.1%) | 251 (94.4%) | ||
| Yes | 20 (7.5%) | 4,862 (7.9%) | 20 (7.5%) | 14 (5.3%) | ||
| Unknown | 1 (0.4%) | 862 (1.4%) | 1 (0.4%) | 1 (0.4%) | ||
Number (%) per column or mean (±standard deviation) is shown. P-values are results of univariable analysis, and significant P-values are emboldened. Abbreviations: OVCA, synchronous stage I endometrioid ovarian cancer; and NOS, not otherwise specified.
Fig. 3. Survival curves for sensitivity analysis.
Log-rank test for P-values. Overall survival is shown for stage I endometrioid endometrial cancer with synchronous stage I endometrioid ovarian cancer (red lines) compared to stage I endometrioid endometrial cancer without synchronous ovarian cancer (blue lines) based on propensity score matching status: panel A, concordant grade 1 tumors at the two cancer sites: and panel B, concordant grade 2 tumors at the two cancer sites. Abbreviations: Synch OVCA, synchronous stage I endometrioid ovarian cancer.
Table 3.
Patient demographics (concordant grade 2 tumor cohort).
| Characteristics | Before matching |
After matching |
||||
|---|---|---|---|---|---|---|
| |
OVCA (+) |
OVCA (−) |
P-value | OVCA (+) |
OVCA (−) |
P-value |
| Number | n = 155 | n = 36,572 | n = 155 | n = 155 | ||
| Age | 50.9 (± 10.1) | 63.5 (± 11.8) | <0.001 | 50.9 (± 10.1) | 52.8 (± 12.2) | 0.59 |
| <40 | 16 (10.3%) | 923 (2.5%) | 16 (10.3%) | 19 (12.3%) | ||
| 40–49 | 62 (40.0%) | 3,069 (8.4%) | 62 (40.0%) | 52 (33.5%) | ||
| 50–59 | 50 (32.3%) | 9,489 (25.9%) | 50 (32.3%) | 50 (32.3%) | ||
| ≥60 | 27 (17.4%) | 23,091 (63.1%) | 27 (17.4%) | 34 (21.9%) | ||
| Race/ethnicity | 0.15 | 0.96 | ||||
| White | 130 (83.9%) | 29,427 (80.5%) | 130 (83.9%) | 125 (80.6%) | ||
| Black | 2 (1.3%) | 1,997 (5.5%) | 2 (1.3%) | 3 (1.9%) | ||
| Hispanic | 14 (9.0%) | 2,655 (7.3%) | 14 (9.0%) | 16 (10.3%) | ||
| Asian | 8 (5.2%) | 1,903 (5.2%) | 8 (5.2%) | 10 (6.5%) | ||
| Others | 1 (0.6%) | 590 (1.6%) | 1 (0.6%) | 1 (0.6%) | ||
| Year at diagnosis | 0.09 | 0.59 | ||||
| 1973–1979 | 2 (1.3%) | 948 (2.6%) | 2 (1.3%) | 4 (2.6%) | ||
| 1980–1989 | 7 (4.5%) | 3,854 (10.5%) | 7 (4.5%) | 11 (7.1%) | ||
| 1990–1999 | 35 (22.6%) | 8,294 (22.7%) | 35 (22.6%) | 34 (21.9%) | ||
| 2000–2009 | 80 (51.6%) | 16,377 (44.8%) | 80 (51.6%) | 83 (53.5%) | ||
| 2010–2013 | 31 (20.0%) | 7,099 (19.4%) | 31 (20.0%) | 23 (14.8%) | ||
| Marital status | <0.001 | 0.68 | ||||
| Single | 50 (32.3%) | 5,032 (13.8%) | 50 (32.3%) | 47 (30.3%) | ||
| Married | 79 (51.0%) | 19,724 (53.9%) | 79 (51.0%) | 76 (49.0%) | ||
| Others | 26 (16.8%) | 11,816 (32.3%) | 26 (16.8%) | 32 (20.6%) | ||
| Registry area | 0.11 | 0.76 | ||||
| West | 76 (49.0%) | 18,553 (50.7%) | 76 (49.0%) | 81 (52.3%) | ||
| Central | 29 (18.7%) | 8,660 (23.7%) | 29 (18.7%) | 30 (19.4%) | ||
| East | 50 (32.3%) | 9,359 (25.6%) | 50 (32.3%) | 44 (28.4%) | ||
| Stage | 0.018 | 0.99 | ||||
| IA | 126 (91.3%) | 26,739 (73.1%) | 126 (91.3%) | 126 (91.3%) | ||
| IB | 14 (9.0%) | 6,464 (17.7%) | 14 (9.0%) | 14 (9.0%) | ||
| I NOS | 15 (9.7%) | 3,372 (9.2%) | 15 (9.7%) | 15 (9.7%) | ||
| Size | 0.65 | 0.49 | ||||
| ≤2.0 cm | 16 (10.3%) | 3,881 (10.6%) | 16 (10.3%) | 23 (14.8%) | ||
| >2.0 cm | 64 (41.3%) | 13,782 (37.7%) | 64 (41.3%) | 60 (38.7%) | ||
| Unknown | 75 (48.4%) | 18,909 (51.7%) | 75 (48.4%) | 72 (46.5%) | ||
| Lymphadenectomy | <0.001 | 0.37 | ||||
| Not performed | 43 (27.7%) | 14,290 (39.1%) | 43 (27.7%) | 36 (23.2%) | ||
| Performed | 108 (69.7%) | 19,465 (53.2%) | 108 (69.7%) | 111 (71.6%) | ||
| Unknown | 4 (2.6%) | 2,817 (7.7%) | 4 (2.6%) | 8 (5.2%) | ||
| Radiotherapy | 0.02 | 0.78 | ||||
| No | 133 (85.8%) | 27,904 (76.3%) | 133 (85.8%) | 137 (884%) | ||
| Yes | 20 (12.9%) | 7,671 (21.0%) | 20 (12.9%) | 16 (10.3%) | ||
| Unknown | 2 (1.3%) | 997 (2.7%) | 2 (1.3%) | 2 (1.3%) | ||
Number (%) per column or mean (±standard deviation) is shown. P-values are results of univariable analysis, and significant P-values are emboldened. Abbreviations: OVCA synchronous stage I endometrioid ovarian cancer; and NOS, not otherwise specified.
4. Discussion
We noted that the simultaneous presence of stage I endometrioid ovarian cancer and stage I endometrioid endometrial cancer does not impact survival. In a review of the literature, the findings regarding this association have been inconsistent. Historically, women with SEOC have been thought to have a better prognosis as shown in studies of women in the mid-1990s to early-2000s [2,5,23]. Constructive comments from others included that these studies had relatively small sample sizes and lacked a control arms [6].
Because women with SEOC seem to have distinguishing characteristics compared to the non-SEOC counterpart, Heitz et al. recently conducted a matched analysis by utilizing data from five institutions in 2013 [6]. Their study findings were remarkable in that prognosis of women with SEOC were comparable to those with endometrial or ovarian cancer alone. However, the study size was limited (n = 77 for SEOC group), making an interpretation of their results difficult to adopt. Our study is considerably larger (n = 839 for SEOC cases) and included an adequate control group. Collectively, our results suggest that survival of women with stage I endometrioid endometrial cancer with synchronous stage I endometrial ovarian cancer were not inferior to those with stage I endometrioid endometrial cancer without synchronous ovarian cancer. The lack of impact on mortality may be due in part to the favorable prognosis associated with these low grade tumors.
The diagnosis of SEOC is made generally and largely based upon the histologic findings of tumor in the endometrium and the ovary [7,8]. When histologic types are discordant at the two cancer sites, making the diagnosis of SEOC is clear. On the contrary, making a diagnosis of SEOC is challenging when the histologic types are concordant as tumor metastasis needs to be ruled out. Commonly, SEOC is more likely to be stage I disease with endometrioid histology [2,5]. For this reason, we limited the study population only to the stage I disease with concordant endometrioid histology in our analysis to make the interpretation more meaningful.
Utilizing this analytic approach, we found that survival of women with stage I endometrioid endometrial cancer with synchronous stage I endometrioid ovarian cancer is comparable to those with stage I endometrioid endometrial cancer without synchronous ovarian cancer. This result endorses and reassures the current diagnostic criteria for SEOC proposed by Ulbright and Roth in the mid-1980s and affirmed by Scully et al. in the late-1990s [7,8]. Therefore, adherence to these diagnostic criteria for SEOC would be paramount to identify this disease particularly when the histology types are concordant at the two cancer sites.
Despite the strict diagnostic criteria for SEOC, misclassification may still occur. Recent robust and comprehensive molecular studies have shown that a large fraction of SEOC cases in which the diagnosis was made by clinico-pathological criteria were found to be metastatic endometrial or ovarian cancer [24,25]: 10 out of 11 cases [24], and 23 out of 38 cases [25]. In addition, the concordant clonality between the endometrial and ovarian tumors in stage I low-grade SEOC suggests that tumor dissemination from one organ to the other is only restricted between the endometrium and the ovary (termed as “restricted microenvironment”) whereas the high-grade counterpart spreads beyond these two organs [24,25].
Not only do these studies highlight the limitations of the current histology-based diagnostic criteria for SEOC, but also they suggest a new concept as “a disease of isolated metastatic phenomenon” in the pathophysiology and diagnosis of SEOC that would need further validation and clarification [24–26]. Because women with metastatic endometrial or ovarian cancer benefits from receiving adjuvant chemotherapy after surgery, an accurate diagnosis of SEOC is an important part in the management of women with concurrent presence of tumors in the endometrium and the ovary. In our study, molecular clonality results were not available to confirm the diagnosis of SEOC. Integrating molecular testing would be highly useful in the diagnostic process of SEOC.
There were multiple strengths of this study. First, this is a population-based study with amongst the largest sample size for SEOC in the literature. Second, we used strict selection criteria and limited the cohort to only stage I disease and endometrioid histology making the study population clearer. Lastly, propensity score matching enriched the quality of statistical analysis. However, we acknowledge a number of limitations. First, this is a retrospective study and there may be confounding factors that we could not include. For example, salient tumor characteristics such as absence of lympho-vascular space invasion in the endometrial tumor to endorse the diagnosis of SEOC was not available in the SEER database. Second, central pathology review to confirm SEOC could not be performed in this study, and the inter-observervariability across the pathologists as well as exact use of the criteria-based diagnosis were not assessable. Lastly, the SEER database does not have information for chemotherapy. However, it is unlikely that this missing information alters our study results because stage I endometrioid ovarian cancer or stage I endometrioid endometrial cancer generally does not require adjuvant chemotherapy [27,28].
Another weakness of this study is that we were not able to compare the survival outcome of women with concordant stage I endometrioid SEOC to women with stage III endometrioid endometrial cancer metastatic to the ovary or women with stage II endometrioid ovarian cancer metastatic to the endometrium because the SEER coding does not specify the detail of metastatic patterns (fallopian tube, ovary, or uterine surface involvement for stage III endometrial cancer; and endometrium versus other pelvic organ for stage II ovarian cancer). However, when compared to historical data for survival of women with stage III endometrioid endometrial cancer or stage II endometrioid ovarian cancer, survival of women with concordant stage I endometrioid SEOC in our study was superior [29,30]. Therefore, misclassification of metastatic endometrial (or ovarian) cancer to SEOC is less likely in this study cohort. Finally, the study covers a long time period between 1973 and 2013. While this inclusion was useful to capture a large number of cases of a relatively rare clinical entity, it may impact the outcome in that medical and surgical practices have evolved during the study period.
The clinical utility of this study is in the area of (i) patient counseling regarding information for survival outcome for stage I endometrioid SEOC and (ii) the importance of molecular testing for SEOC. The latter can be especially essential because the results would impact the postoperative management. Without molecular testing, there may be a possibility of under-diagnosis of stage III endometrioid endometrial cancer (ovarian metastasis) and stage II endometrioid ovarian cancer (endometrial metastasis) as stage I endometrioid SEOC [24,25]. However, recent data have suggested that large fraction of SEOC tumors are in fact metastatic cases based on clonality analysis. This implies that metastatic endometrial or ovarian cancer diagnosed as stage I SEOC may have a similar prognosis as genuine stage I SEOC even when chemotherapy is omitted [31]. Therefore, whether the effect of chemotherapy and survival of women with SEOC diagnosed by clinico-pathological characteristics in whom molecular testing indicates metastatic endometrial or ovarian cancer differs from those with genuine SEOC with consistency between clinico-pathological criteria and molecular testing merits further investigation.
HIGHLIGHTS.
Synchronous endometrial and ovarian cancer (SEOC) is not a rare clinical entity.
Historically women with SEOC have known to have a good prognosis.
This study compared survival of stage I endometrioid SEOC to non-SEOC.
Stage I endometrioid SEOC and stage I non-SEOC had similar survival outcomes.
Concordant tumor grade was not associated with survival for stage I SEOC.
Acknowledgments
Financial support
Supported by the Ensign Endowment for Gynecologic Cancer Research (K.M.)
Footnotes
Disclosure
Consultant for Tesaro and Clovis Oncology (J.D.W.). The other authors did not report any potential conflicts of interest.
References
- [1].Chiang YC, Chen CA, Huang CY, Hsieh CY, Cheng WF, Synchronous primary cancers of the endometrium and ovary, Int. J. Gynecol. Cancer 18 (2008) 159–164. [DOI] [PubMed] [Google Scholar]
- [2].Zaino R, Whitney C, Brady MF, DeGeest K, Burger RA, Buller RE, Simultaneously detected endometrial and ovarian carcinomas–a prospective clinicopathologic study of 74 cases: a gynecologic oncology group study, Gynecol. Oncol. 83 (2001) 355–362. [DOI] [PubMed] [Google Scholar]
- [3].Williams MG, Bandera EV, Demissie K, Rodriguez-Rodriguez L, Synchronous primary ovarian and endometrial cancers: a population-based assessment of survival, Obstet. Gynecol. 113 (2009) 783–789. [DOI] [PubMed] [Google Scholar]
- [4].AlHilli MM, Dowdy SC, Weaver AL, St Sauver JL, Keeney GL, Mariani A, Podratz KC, Bakkum-Gamez JN, Incidence and factors associated with synchronous ovarian and endometrial cancer: a population-based case-control study, Gynecol. Oncol. 125 (2012) 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Soliman PT, Slomovitz BM, Broaddus RR, Sun CC, Oh JC, Eifel PJ, Gershenson DM, Lu KH, Synchronous primary cancers of the endometrium and ovary: a single institution review of 84 cases, Gynecol. Oncol. 94 (2004) 456–462. [DOI] [PubMed] [Google Scholar]
- [6].Heitz F, Amant F, Fotopoulou C, Battista MJ, Wimberger P, Traut A, Fisseler-Eckhoff A, Harter P, Vandenput I, Sehouli J, Schmidt M, Kimmig R, du Bois R, du Bois A, Synchronous ovarian and endometrial cancer–an international multicenter case-control study, Int. J. Gynecol. Cancer 24 (2013) 54–60. [DOI] [PubMed] [Google Scholar]
- [7].Scully RE, Young RH, Clement PB, Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament, Atlas of Tumor Pathology, Armed Forces Institute of Pathology, Bethesda, MD, 1998. [Google Scholar]
- [8].Ulbright TM, Roth LM, Metastatic and independent cancers of the endometrium and ovary: a clinicopathologic study of 34 cases, Hum. Pathol 16 (1985) 28–34. [DOI] [PubMed] [Google Scholar]
- [9].The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute, https://seer.cancer.gov/, Accessed date: 1 July 2017.
- [10].National Cancer Registrars Association, http://www.ncra-usa.org/i4a/pages/index.cfm?pageid=1, Accessed date: 1 July 2017.
- [11].von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies, BMJ 335 (2007) 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rodriguez AM, Kuo YF, Goodwin JS, Risk of colorectal cancer among long-term cervical cancer survivors, Med. Oncol 31 (2014) 943. [DOI] [PubMed] [Google Scholar]
- [13].Matsuo K, Machida H, Stone RL, Soliman PT, Thaker PH, Roman LD, Wright JD, Risk of subsequent ovarian cancer after ovarian conservation in young women with stage I endometrioid endometrial cancer, Obstet. Gynecol 130 (2017) 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Matsuo K, Machida H, Horowitz MP, Shahzad MM, Guntupalli SR, Roman LD, Wright JD, Risk of metachronous ovarian cancer after ovarian conservation in young women with stage I cervical cancer, Am. J. Obstet. Gynecol (2017) (in-press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Elit LM, O’Leary EM, Pond GR, Seow HY, Impact of wait times on survival for women with uterine cancer, J. Clin. Oncol 32 (2013) 27–33. [DOI] [PubMed] [Google Scholar]
- [16].Shalowitz DI, Epstein AJ, Buckingham L, Ko EM, Giuntoli, RL 2nd, Survival implications of time to surgical treatment of endometrial cancers, Am. J. Obstet. Gynecol. 216 (2016) 268.e1–268.e18. [DOI] [PubMed] [Google Scholar]
- [17].Matsuo K, Opper NR, Ciccone MA, Garcia J, Tierney KE, Baba T, Muderspach LI, Roman LD, Time interval between endometrial biopsy and surgical staging for type I endometrial cancer: association between tumor characteristics and survival outcome, Obstet. Gynecol 125 (2015) 424–433. [DOI] [PubMed] [Google Scholar]
- [18].Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A(Eds.), AJCC Cancer Staging Manual, 7th ed.Springer, New York, 2010. [Google Scholar]
- [19].Matsuo K, Machida H, Shoupe D, Melamed A, Muderspach LI, Roman LD, Wright JD, Ovarian conservation and overall survival in young women with early-stage low-grade endometrial cancer, Obstet. Gynecol 128 (2016) 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].National Death Index, https://www.cdc.gov/nchs/ndi/, Accessed date: 1 July 2017.
- [21].Kaplan EL, Meier P, Nonparametric estimation from incomplete observations, J. Am. Stat. Assoc. 53 (1958) 457–481. [Google Scholar]
- [22].Cox DR, Regression models and life-tables, J. R. Stat. Soc. Ser. B Stat Methodol. 34 (1972) 187–220. [Google Scholar]
- [23].Sheu BC, Lin HH, Chen CK, Chao KH, Shun CT, Huang SC, et al. , Int. J. Gynaecol. Obstet. 51 (1995) 141–146. [DOI] [PubMed] [Google Scholar]
- [24].Anglesio MS, Wang YK, Maassen M, Horlings HM, Bashashati A, Senz J, Mackenzie R, Grewal DS, Li-Chang H, Karnezis AN, Sheffield BS, McConechy MK Kommoss F, Taran FA, Staebler A, Shah SP, Wallwiener D, Brucker S, Gilks CB, Kommoss S, Huntsman DG, Synchronous endometrial and ovarian carcinomas: evidence of clonality, J. Natl. Cancer Inst. 108 (2016) (djv428). [DOI] [PubMed] [Google Scholar]
- [25].Brinkmann D, Ryan A, Ayhan A, McCluggage WG, Feakins R, Santibanez-Koref MF, Mein CA, Gayther SA, Jacobs IJ, A molecular genetic and statistical approach for the diagnosis of dual-site cancers, J. Natl. Cancer Inst. 96 (2004) 1441–1446. [DOI] [PubMed] [Google Scholar]
- [26].Chao A, Wu RC, Jung SM, Lee YS, Chen SJ, Lu YL, Tsai CL, Lin CY, Tang YH, Chen MY, Huang HJ, Chou HH, Huang KG, Chang TC, Wang TH, Lai CH, Implication of genomic characterization in synchronous endometrial and ovarian cancers of endometrioid histology, Gynecol. Oncol. 143 (2016) 60–67. [DOI] [PubMed] [Google Scholar]
- [27].Ovarian cancer. NCCN Clinical Practice Guidelines in Oncology (NCCN Guideline) https://www.nccn.org/professionals/physician_gls/f_guidelines.asp (accessed 7/1/2017).
- [28].Uterine neoplasms. NCCN Clinical Practice Guidelines in Oncology (NCCN Guideline) https://www.nccn.org/professionals/physician_gls/f_guidelines.asp (accessed 7/1/2017).
- [29].Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, Ngan HY, Pecorelli S, Beller U, Carcinoma of the ovary. FIGO 26th annual report on the results of treatment in gynecological cancer, Int. J. Gynaecol. Obstet 95 (2006) S161–92. [DOI] [PubMed] [Google Scholar]
- [30].Boll D, Karim-Kos HE, Verhoeven RH, Burger CW, Coebergh JW, van de Poll-Franse LV, van Doorn HC, Increased incidence and improved survival in endometrioid endometrial cancer diagnosed since 1989 in The Netherlands: a population based study, Eur. J. Obstet. Gynecol. Reprod. Biol 166 (2013) 209–214. [DOI] [PubMed] [Google Scholar]
- [31].Dizon DS, Bierre MJ, Making a difference: distinguishing two primaries from metastasis in synchronous tumors of the ovary and uterus, J. Natl. Cancer Inst. 108 (2016) (djv442). [DOI] [PubMed] [Google Scholar]