Abstract
Objective:
To examine predictors of pathological parametrial invasion in clinical stage IIB cervical cancer, and to examine prognostic factors in pathological stage IIB disease.
Methods:
This study is an ancillary analysis of a nation-wide retrospective cohort examining 6,003 clinical stage IB-IIB cervical cancers. Women with clinical stage IIB disease who underwent primary radical hysterectomy with lymphadenectomy were examined (n = 714). Multivariate analysis was performed to identify independent clinico-pathological factors for pathological parametrial invasion and to identify independent prognostic factors in pathological stage IIB disease.
Results:
Parametrial invasion was identified on the surgical specimen in 400 cases (56.0%, 95% confidence interval 52.4–59.7). On multivariate analysis, deep stromal invasion (DSI, adjusted-OR 3.922), multiple pelvic nodal metastases (adjusted-OR 3.266), lympho-vascular space invasion (adjusted-OR 2.333), and uterine corpus invasion (adjusted-OR 1.656) remained independent tumor factors for pathological parametrial invasion. In classification-tree models, tumors with DSI and multiple pelvic nodal metastases had the highest incidence of pathological parametrial invasion (75.0–87.7%); contrary, tumors without DSI had the lowest incidence (21.9%). Among patients with pathological stage IIB disease, the absolute difference in 5-year disease-free survival rates was 57.2%, ranging between 80.9% in those with squamous histology with none/single pelvic nodal metastasis and 23.7% in those with non-squamous histology with multiple pelvic nodal metastases.
Conclusion:
In clinical stage IIB cervical cancer, accuracy for pathological parametrial invasion is low-modest. With absence of DSI, only one in five clinical stage IIB diseases has pathological stage IIB disease. Survival of pathological stage IIB varies widely and is largely dependent on nodal factors.
Keywords: Cervical cancer, Stage IIB, Radical hysterectomy, Parametrial invasion, Surgical-pathological factor
Introduction
In Japan, nearly 25% of women with cervical cancer are diagnosed with stage II disease [1], and approximately 30–50% of women with stage II cervical cancer undergo primary surgical treatment with radical hysterectomy and pelvic lymphadenectomy, as shown in the recent national statistics [1,2]. This practice pattern is unique, as a non-surgical approach with definitive radiotherapy is the standard therapy for stage IIB cervical cancer in the United States [3].
One advantage of primary surgical treatment for clinical stage IIB disease is the opportunity to assess surgical-pathological factors in hysterectomy specimens. This is particularly important because of the relatively poor accuracy of clinical staging via physician’s examination for true pathological parametrial invasion in clinical early-stage disease and the potential for over-diagnosis and overtreatment [4]. There is the possibility that a considerable percentage of women thought to have stage IIB disease but who indeed have no high/intermediate risk factors in the surgical specimen are unnecessarily exposed to radiation-related toxicities. Therefore, identifying the predictors of true parametrial tumor invasion in clinical stage IIB cervical cancer is of utmost importance to avoid overtreatment with definitive radiotherapy.
Moreover, survival specific to pathological stage IIB cervical cancer has not been completely elucidated, and identifying prognosticators will be useful to characterize survival in this disease spectrum. The objectives of the study were (i) to examine predictors of pathological parametrial invasion in clinical stage IIB cervical cancer, and (ii) to examine prognostic factors in pathological stage IIB disease.
Materials and methods
Data source and eligibility
This is a retrospective ancillary analysis of the Japanese Gynecologic Oncology Group (JGOG) study [5–10]. In the original study, consecutive cases of clinical stage IB-IIB cervical cancer treated with radical hysterectomy and pelvic lymphadenectomy between 2004 and 2008 were collected from 116 JGOG-designated institutions (N = 6,003). The study period for the data acquisition was between 2012 and 2013. Women with clinical stage IIB disease who underwent primary surgery were eligible for the study, and those who received neoadjuvant chemotherapy were excluded from the study. Institutional Review Board approval was properly obtained for the study.
Clinical information
Variables ascertained from the database for this analysis were patient age, clinical stage, surgical-pathological factors (histology subtypes, tumor size, parametrial involvement, deep stromal invasion (DSI), uterine corpus invasion, ovarian metastasis, lympho-vascular space invasion [LVSI], nodal status [pelvic and paraaortic], and peritoneal cytology), adjuvant therapy type (whole pelvic radiotherapy alone, concurrent chemo-radiotherapy [CCRT], and systemic chemotherapy), and survival outcomes (disease-free survival [DFS] and cause-specific survival [CSS]). Anatomical recurrent sites were designated as local-recurrence (vaginal cuff and pelvis) or distant-recurrence (other than local-recurrence). Surgical-pathological factors were abstracted from archived medical records by investigators at each participating site.
Study definition
Age cutoff was based on our prior study [6]. Histology subtypes were grouped as squamous, adenocarcinoma, adenosquamous, and others. DSI was defined as tumor invasion into the outer half of the uterine cervix [8,11]. Large tumor size was defined as tumor diameter of >4 cm. Pathological stage IIB disease was defined by the presence of tumor in the parametrial tissue of surgical specimen. Nodal metastasis was grouped as none, single, or multiple, based on the impact on survival demonstrated by prior studies [7,12]. Patients who did not undergo para-aortic lymphadenectomy were considered clinically negative for metastasis in this study. DFS was defined as the time interval between surgery and the first recurrence or cervical cancer death, and CSS was defined as the time interval between surgery and death from cervical cancer.
Statistical consideration
The primary objective of this study was to examine the predictors of pathological parametrial tumor invasion in clinical stage IIB cervical cancer. The secondary objective of the study was to examine prognostic factors in women with pathological stage IIB cervical cancer.
A binary logistic regression model was used to identify the independent predictors for pathological stage IIB disease. In this multivariate analysis, all the covariates with P < 0.05 on univariate analysis were entered in the initial model, and the conditional backward method was used to retain only the covariates with P < 0.05 in the final model. Magnitude of statistical significance was expressed with adjusted-odds ratios (OR) and 95% confidence intervals (CI). The Hosmer-Lemeshow test was used to assess goodness-of-fit in the final model, and a P > 0.05 or greater was considered to be a good-fit model [13].
In an attempt to identify the specific patterns of predictors for pathological stage IIB disease, a recursive partitioning analysis was performed to construct a classification-tree model for risk patterns [14]. All independent risk factors of pathological stage IIB disease were entered in the final analytic model, and the chi-square automatic interaction detector method was used for the model. Among the determined nodes in this analysis, incidences of pathological stage IIB disease were estimated. Similarly, a recursive partitioning analysis was used to identify the specific patterns for survival among women with pathological stage IIB disease.
The Kaplan-Meier method was used to construct the survival curves, and the log-rank test was used to examine statistical difference between the curves. On multivariate analysis, Cox proportional hazard regression models with the conditional backward method were used to identify independent predictors for survival outcome. Magnitude of statistical significance was expressed with adjusted-hazard ratio (HR) and 95%CI.
Various sensitivity analyses were conducted to examine the robustness of the study. First, anatomical sites of tumor recurrence were examined based on pathological parametrial status. In addition, the association between adjuvant therapy and anatomical site of tumor recurrent was examined. This is based on the rationale that surgically-treated women with pathological stage IIB cervical cancer are considered to be a high-risk group for which adjuvant therapy is recommended [3]. A recent study showed that systemic chemotherapy and radiotherapy had comparable effects on survival but exhibited variable recurrence patterns [6].
The inverse probability of treatment weighting (IPTW) based on propensity score was used to adjust for the background differences between the two groups (pathological parametrial involvement: yes versus no) [15]. All the patient demographics, surgical-pathological factors, and adjuvant treatment types were fitted in the binary logistic regression model to estimate the predicted probability with propensity score in each patient (ranging between 0 and 1). Patients in the pathological IIB group were weighted as 1/propensity score whereas those in the no pathological IIB group were weighted as 1/(1-propensity score). Distributions after weighting were assessed with standardized difference, and a value of ≤0.10 indicated a good balance between the two groups.
The variance inflation factor (VIF) was determined among covariates in multivariate analysis, and VIF of ≥2 was defined as multicollinearity in this study. On multivariate analyses, overadjustment was assessed with the ratio of events-of-interest per the entered covariates, and a ratio of <10 was interpreted as overadjustment in this study. A P < 0.05 was considered statistically significant (two-tailed hypothesis). Statistical Package for Social Science software (IBM SPSS, version 24.0, Armonk, NY, USA) was used for all the analyses. The STROBE guidelines were utilized to outline the results as recommended for retrospective cohort studies [16].
Results
The study selection schema is shown in Fig. S1. 714 women with clinical stage IIB cervical cancer who underwent primary radical hysterectomy and pelvic lymphadenectomy with known parametrial status on surgical specimens comprised the study population. Surgical-pathological characteristics of patients with clinical stage IIB disease are shown in Table 1. The mean age at surgery was 52.2. The most common histology type was squamous cell carcinoma (68.2%). Pathological stage IIB disease was confirmed in 400 (56.0%, 95%CI 52.4–59.7) cases in the study population.
Table 1.
Patient demographics (N = 714).
| Age (years) | 52.2 (±12.0) |
| <50 | 278 (38.9%) |
| ≥50 | 436 (61.1%) |
| Year | |
| 2004 | 143 (20.0%) |
| 2005 | 155 (21.7%) |
| 2006 | 153 (21.4%) |
| 2007 | 143 (20.0%) |
| 2008 | 120 (16.8%) |
| Histology | |
| SCC | 487 (68.2%) |
| Adeno | 149 (20.9%) |
| AS | 63 (8.8%) |
| Others | 15 (2.1%) |
| Tumor size | |
| ≤2 cm | 31 (4.3%) |
| 2.1 –4.0 cm | 321 (45.0%) |
| 4.1 –6.0 cm | 275 (38.5%) |
| >6.0 cm | 61 (8.5%) |
| Missing | 26 (3.6%) |
| Parametrial involvement | |
| No | 314 (44.0%) |
| Yes | 400 (56.0%) |
| DSI | |
| Not involved | 116 (16.2%) |
| Involved | 540 (75.6%) |
| Missing | 58 (8.1%) |
| LVSI | |
| No | 138 (19.3%) |
| Yes | 533 (74.6%) |
| Missing | 43 (6.0%) |
| Uterine corpus | |
| Not involved | 510 (71.4%) |
| Involved | 191 (26.8%) |
| Missing | 13 (1.8%) |
| Ovarian metastasis | |
| No | 681 (95.4%) |
| Yes | 21 (2.9%) |
| Missing | 12 (1.7%) |
| Pelvic lymph node | |
| Not involved | 359 (50.3%) |
| Single metastasis | 120 (16.8%) |
| Multiple metastasis | 224 (31.4%) |
| Missing | 11 (1.5%) |
| Para-aortic lymph node | |
| Not involved | 141 (19.7%) |
| Single metastasis | 15 (2.1%) |
| Multiple metastasis | 27 (3.8%) |
| Clinically not involved | 531 (74.4%) |
| Peritoneal cytology | |
| No malignancy | 327 (45.8%) |
| Malignant cells | 39 (5.5%) |
| Not performed | 346 (48.5%) |
| Missing | 2 (0.3%) |
| Adjuvant therapy | |
| CCRT | 256 (35.9%) |
| RT alone | 198 (27.7%) |
| Chemotherapy alone | 132 (18.5%) |
| RT/chemotherapy | 21 (2.9%) |
| None | 78 (10.9%) |
| Missing | 29 (4.1%) |
Mean (±SD) or number (%) per column are shown. Abbreviations: SCC, squamous cell carcinoma; Adeno, adenocarcinoma; AS, adenosquamous; DSI, deep stromal invasion; LVSI, lympho-vascular space invasion; CCRT, concurrent chemoradiotherapy; and RT, radiotherapy.
Among those who did not have pathological stage IIB disease (n = 314, 44.0%), stage IIA disease (29.6%) was the most common pathological stage followed by stage IB2 disease (28.0%) and stage IB1 disease (25.8%). The extent of pelvic nodal metastasis was significantly associated with the presence of para-aortic nodal metastasis: 0.6% for no pelvic nodal metastasis, 1.7% for single-metastasis, and 17.0% for multiple-metastasis (P < 0.001).
Predictors of pathological stage IIB disease among clinical stage IIB disease were examined (Table 2). Tumors in pathological stage IIB disease were characterized by significantly higher incidences of DSI (84.0% versus 65.0%), LVSI (84.5% versus 62.1%), pelvic nodal metastasis (any 60.8% versus 32.2%; and multiple 43.0% versus 16.6%), large tumor size (52.3% versus 40.5%), para-aortic lymph node metastasis (any 8.8% versus 2.2%; multiple 5.5% versus 1.6%), uterine corpus invasion (35.8% versus 19.4%), and ovarian metastasis (4.3% versus 1.3%) compared to cases without pathological stage IIB disease (all, P < 0.05). Women with pathological stage IIB disease were also more likely to receive CCRT after surgery (41.3% versus 29.0%, P < 0.001). On multivariate analysis, age ≥50 (adjusted-OR 1.427), DSI (adjusted-OR 3.920), LVSI (adjusted-OR 2.333), uterine corpus invasion (adjusted-OR 1.656), and multiple pelvic lymph node metastases (adjusted-OR 3.266) remained independent predictors for pathological stage IIB disease (all, P < 0.05; Table 2).
Table 2.
Clinico-pathological factors for pathological stage IIB disease.
| Characteristics | Pathological stage IIB |
P-valuea | aOR (95%CI) | P-value | |
|---|---|---|---|---|---|
| No | Yes | ||||
| Number | n = 314 | n = 400 | |||
| Age (years) | 50.7 (±11.6) | 53.4 (±12.2) | 0.003 | ||
| <50 | 136 (43.3%) | 142 (35.5%) | 1 | ||
| ≥50 | 178 (56.7%) | 258 (64.5%) | 1.427 (1.013–2.009) | 0.042 | |
| Year | 0.109 | ||||
| 2004 | 62 (19.7%) | 81 (20.3%) | |||
| 2005 | 82 (26.1%) | 73 (18.3%) | |||
| 2006 | 67 (21.3%) | 86 (21.5%) | |||
| 2007 | 57 (18.2%) | 86 (21.5%) | |||
| 2008 | 46 (14.6%) | 74 (18.5%) | |||
| Histology | 0.682 | ||||
| SCC | 207 (65.9%) | 280 (70.0%) | |||
| Adeno | 69 (22.0%) | 80 (20.0%) | |||
| AS | 31 (9.9%) | 32 (8.0%) | |||
| Others | 7 (2.2%) | 8 (2.0%) | |||
| Tumor size | 0.001 | ||||
| ≤2 cm | 21 (6.7%) | 10 (2.5%) | |||
| 2.1–4.0 cm | 150 (47.8%) | 171 (42.8%) | |||
| 4.1–6.0 cm | 108 (34.4%) | 167 (41.8%) | |||
| >6.0 cm | 19 (6.1%) | 42 (10.5%) | |||
| Missing | 16 (5.1%) | 10 (2.5%) | |||
| DSI | <0.001 | <0.001b | |||
| Not involved | 91 (29.0%) | 25 (6.3%) | 1 | ||
| Involved | 204 (65.0%) | 336 (84.0%) | 3.920 (2.341–6.564) | <0.001 | |
| Missing | 19 (6.1%) | 39 (9.8%) | 5.631 (2.316–13.69) | <0.001 | |
| LVSI | <0.001 | 0.001b | |||
| No | 100 (31.8%) | 38 (9.5%) | 1 | ||
| Yes | 195 (62.1%) | 338 (84.5%) | 2.333 (1.470–3.703) | <0.001 | |
| Missing | 19 (6.1%) | 24 (6.0%) | 1.612 (0.679–3.827) | 0.279 | |
| Uterine corpus | <0.001 | 0.039b | |||
| Not involved | 253 (80.6%) | 257 (64.3%) | 1 | ||
| Involved | 56 (17.8%) | 135 (33.8%) | 1.656(1.118–2.452) | 0.012 | |
| Missing | 5 (1.6%) | 8 (2.0%) | 0.902 (0.222–3.827) | 0.886 | |
| Ovarian metastasis | 0.047 | ||||
| No | 306 (97.5%) | 375 (93.8%) | |||
| Yes | 4 (1.3%) | 17 (4.3%) | |||
| Missing | 4 (1.3%) | 8 (2.0%) | |||
| Pelvic lymph node | <0.001 | <0.001b | |||
| Not involved | 206 (65.6%) | 153 (38.3%) | 1 | ||
| Single metastasis | 49 (15.6%) | 71 (17.8%) | 1.504 (0.961–2.353) | 0.074 | |
| Multiple metastasis | 52 (16.6%) | 172 (43.0%) | 3.266 (2.177–4.899) | <0.001 | |
| Missing | 7 (2.2%) | 4 (1.0%) | 0.566 (0.149–2.149 | 0.403 | |
| Para-aortic lymph node | 0.003 | ||||
| Not involved | 66 (21.0%) | 75 (18.8%) | |||
| Single metastasis | 2 (0.6%) | 13 (3.3%) | |||
| Multiple metastasis | 5 (1.6%) | 22 (5.5%) | |||
| Clinically not involved | 241 (76.8%) | 290 (72.5%) | |||
| Peritoneal cytology | 0.001 | ||||
| No malignancy | 135 (43.0%) | 192 (48.0%) | |||
| Malignant cells | 7 (2.2%) | 32 (8.0%) | |||
| Not performed | 171 (54.5%) | 175 (43.8%) | |||
| Missing | 1 (0.3%) | 1 (0.3%) | |||
| Adjuvant therapy | <0.001 | ||||
| CCRT | 91 (29.0%) | 165 (41.3%) | |||
| RT alone | 81 (25.8%) | 117 (29.3%) | |||
| Chemotherapy alone | 55 (17.5%) | 77 (19.3%) | |||
| RT/chemotherapy | 11 (3.5%) | 10 (2.5%) | |||
| None | 60 (19.1%) | 18 (4.5%) | |||
| Unknown | 16 (5.1%) | 13 (3.3%) | |||
Univariate analysis.
P-value for interaction. A binary logistic regression model for pathological stage IIB disease. Covariates with P < 0.05 in univariate analysis for patient and tumor characteristics were entered in the initial model (age, tumor size, DSI, LVSI, uterine corpus invasion, ovarian metastasis, pelvic lymph node metastasis, para-aortic lymph node metastasis, and peritoneal cytology results), and conditional backward method was used to retain only covariates with P < 0.05 in the final model (age, DSI, LVSI, uterine corpus, and pelvic lymph node metastasis). Hosmer-Lemeshow test shows P = 0.378. Abbreviations: aOR, adjusted-odds ratio; CI, confidence interval; SCC, squamous cell carcinoma; Adeno, adenocarcinoma; AS, adenosquamous; DSI, deep stromal invasion; LVSI, lympho-vascular space invasion; RT, whole pelvic radiotherapy; CCRT, concurrent chemo-radiotherapy.
Among the 630 cases with data available for these five independent predictors of pathological stage IIB disease, classification-tree analysis was performed to examine the clinico-pathological patterns for pathological stage IIB disease (Fig. 1). The patient group exhibiting the highest incidence of pathological stage IIB disease were those with tumors that had DSI and multiple pelvic lymph node metastases (75.0–87.7%), which represented nearly a quarter of study population (28.7%). Contrary, when there was no DSI, as seen in 18.1% of study population, the incidence of pathological stage IIB was only 21.9%. Even when there was DSI, absence of LVSI and multiple pelvic lymph node metastases (10.6% of study population) was associated with lower incidence of pathological stage IIB disease than average (34.3%).
Fig. 1. Classification-tree model for pathological stage IIB.
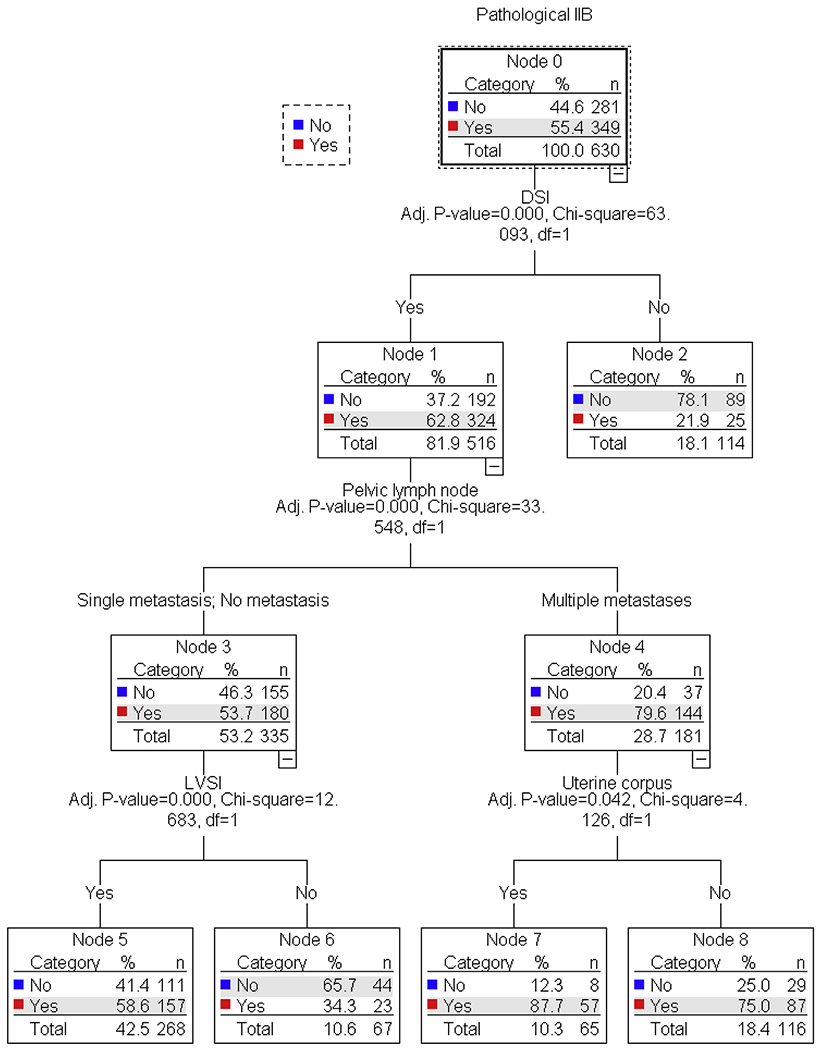
630 women with available results for independent predictors for pathological parametrial invasion was examined for analysis (age, DSI, LVSI, uterine corpus invasion, and pelvic lymph node).
Survival analysis was performed among women with pathological stage IIB disease (n = 400). The median follow-up time of the cases without a survival event was 5.4 years (interquartile range, 4.5–6.8). There were 164 women who developed disease recurrence, and there were 110 women who died of cervical cancer. Amongst the whole cohort, the 5-year DFS and CSS rates were 58.5% and 71.3%, respectively.
Prognostic factors for pathological stage IIB disease were examined (Table 3). On univariate analysis, all examined surgical-pathological factors except for DSI and uterine corpus invasion were significantly associated with DFS (all, P < 0.05). Adjuvant therapy type was not associated with DFS in this study cohort (CCRT versus radiotherapy alone, HR 1.253, 95%CI 0.495–3.172, P = 0.633; systemic chemotherapy versus CCRT, HR 1.236, 95%CI 0.811–1.883, P = 0.325). On multivariate analysis, age <50 (adjusted-HR 1.567), non-SCC histology (adjusted-HR 2.205), multiple pelvic lymph node metastases (adjusted-HR 2.535), and multiple pelvic lymph node metastases (adjusted-HR 2.428) remained independent prognostic factors for decreased DFS (all, P < 0.05). Similarly, age, histology, and multiple pelvic and para-aortic lymph node metastases remained independent prognostic factors for CSS (all, P< 0.05).
Table 3.
Independent prognostic factors in pathological stage IIB cervical cancer.
| Characteristics | Disease-free survival |
Cause-specific survival |
||
|---|---|---|---|---|
| Adjusted-HR (95%CI) | P-value | Adjusted-HR (95%CI) | P-value | |
| Age (years) | ||||
| ≥50 | 1 | 1 | ||
| <50 | 1.567 (1.125–2.181) | 0.008 | 2.166 (1.454–3.224) | <0.001 |
| Histology | ||||
| SCC | 1 | 1 | ||
| Non-SCC | 2.205 (1.587–3.064) | <0.001 | 2.139 (1.446–3.163) | <0.001 |
| Pelvic lymph node | <0.001a | <0.001a | ||
| Not involved | 1 | 1 | ||
| Single metastasis | 1.234 (0.720–2.113) | 0.445 | 1.013 (0.482–2.128) | 0.974 |
| Multiple metastasis | 2.535 (1.726–3.725) | <0.001 | 2.725 (1.686–4.404) | <0.001 |
| Missing | - | - | - | - |
| Para-aortic lymph node | 0.004a | 0.012a | ||
| Not involved | 1 | 1 | ||
| Single metastasis | 1.422 (0.654–3.090) | 0.375 | 1.647 (0.690–3.932) | 0.261 |
| Multiple metastasis | 2.428 (1.286–4.584) | 0.006 | 2.466 (1.163–5.228) | 0.019 |
| Clinically not involved | 0.909 (0.594–1.391) | 0.660 | 0.913 (0.535–1.556) | 0.737 |
Cox proportional hazard regression models for multivariate analysis (conditional backward). Only significant covariates (P < 0.05) in the final models were shown in the table.
P-value for interaction. – suppressed due to number <5. Abbreviations: HR, hazard ratio; CI, confidence interval; SCC, squamous cell carcinoma; Adeno, adenocarcinoma; AS, adenosquamous; DSI, deep stromal invasion; LVSI, lympho-vascular space invasion; RT, whole pelvic radiotherapy; CCRT, concurrent chemo-radiotherapy.
Survival was then examined based on patterns of clinico-pathological factors (Fig. S2). Those with non-SCC tumors with multiple pelvic lymph node metastases (representing 13.5% of population) had the worst 5-year DFS (23.7%), while those with SCC tumors with no or single pelvic nodal metastasis (representing 40.7% of population) had the highest 5-year DFS (80.9%) (Fig. 2). Absolute difference in 5-year DFS rates between the two groups was 57.2%. Similarly, absolute difference in 5-year CSS rates between the most favorable (SCC tumors with non/single pelvic nodal metastasis, 91.0%) and the least favorable (women aged <50 with multiple pelvic nodal metastases, 40.0%) groups was 51.0% (Supplemental Figs. S3–4).
Fig. 2. Disease-free survival of pathological stage IIB cervical cancer based on prognostic factors.
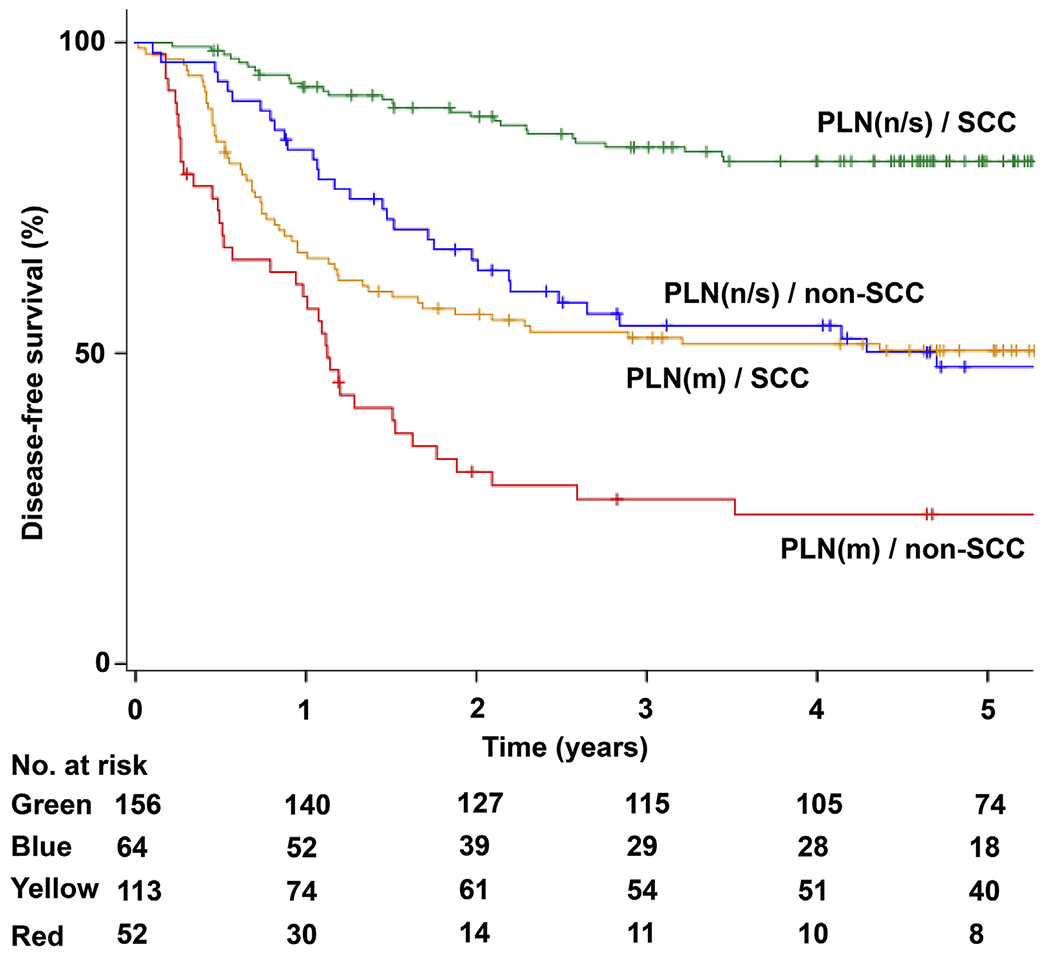
Y-axis is truncated at 5-year time point. Abbreviations: PLN, pelvic lymph node metastasis; n, negative metastasis; s, single metastasis; m, multiple metastasis; and SCC, squamous cell carcinoma.
Anatomical recurrent sites were examined. Patient demographics were well matched between the pathological IIB group and the non-pathological IIB group after IPTW analysis (all, standardized difference ≥0.10; Supplemental Table S1). There was no difference in the local-recurrence rates between the two groups (20.4% versus 18.6%, P = 0.385; Supplemental Table S2). However, women in the pathological IIB group had a significantly higher risk of distant-recurrence compared to those in the non-pathological IIB group (23.5% versus 14.6%, P < 0.001). When the risk of distant-recurrence was examined based on adjuvant therapy types (Supplemental Table S3), radiotherapy-based treatment was associated with an increased risk of distant-recurrence, whereas systemic chemotherapy was associated with a decreased risk of distant-recurrence in the pathological IIB group compared to the non-pathological IIB group (all, P < 0.05).
Discussion
In our study, only nearly half of women with clinical stage IIB cervical cancer had pathological stage IIB disease. In a review of the literature, the diagnostic accuracy of clinical examination for stage IIB cervical cancer is as low as ~20% (range, 21 –55%) [4]. Based on these statistics, our study was amongst the highest in accuracy for diagnosing clinical stage IIB disease. However, at the same time, more than 40% of our study patients were still over-diagnosed with clinical stage IIB disease, similarly to what has been reported in the literature (range, 50–75%) [4]. Due to the fact that absence of parametrial tumor involvement makes patients more suitable candidates for surgical treatment rather than definitive radiotherapy, improving the accuracy of clinical diagnosis of stage IIB disease is necessary.
In this setting, our study identified five predictors of pathological parametrial invasion, which will be useful to help surgeons guide management of women with clinical stage IIB disease. These factors include old age, DSI, LVSI, uterine corpus invasion, and multiple pelvic nodal metastases. When both DSI and multiple pelvic nodal metastases are present, accuracy for diagnosis of pathological stage IIB disease is significantly higher, nearing 80%. This association supports the theory that cervical tumors first invade deeply into the cervical stroma, followed by invasion of parametrial tissue and then spread to pelvic nodes [7,12].
In contrast, when tumors did not have DSI, accounting for 18.2% of clinical stage IIB disease, the incidence of parametrial invasion was considerably low (~20%). Thus, evaluation of DSI could be a key step in the preoperative management of women with clinical stage IIB disease. In the United States, the National Comprehensive Cancer Network (NCCN) guidelines state that pelvic magnetic resonance imaging (MRI) is a “considered” imaging modality to assess local tumor extension [3]. In Europe, the European Society of Urogenital Radiology (ESUR) states that MRI is the imaging modality of choice in cervical cancer [17]. Given the wide range in accuracy for diagnosis of true parametrial invasion in clinical stage IIB disease is largely depending on presence of DSI in our study, we respectfully recommend evaluation of DSI with MRI in clinical stage IIB disease. In addition, certain studies suggest the utility of MRI to predict parametrial invasion in early-stage cervical cancer [18–20], given a recent systematic review that reported considerably higher accuracy with MRI compared to clinical examination (84% versus 40%) [21]. This additionally supports routine use of MRI in clinical stage IIB cervical cancer.
The standard treatment for clinical stage IIB cervical cancer is CCRT in the United States [3]. Contrary, a considerable number of women with clinical stage IIB disease undergo primary surgery with radical hysterectomy in Japan [1,2]. The exact reason for this statistic remains unknown, but it is speculated that surgeons may consider clinical examination to have low accuracy in diagnosing pathological stage IIB disease as shown in this study [4]. Another possibility may include availability of radiotherapy at different treatment centers as well as patient preferences and refusal of radiotherapy from a historical standpoint in Japan.
One of the remarkable findings in our study is that nearly 40% of women with pathological stage IIB cervical cancer had multiple pelvic nodal metastases. This is particularly important clinically because the presence of multiple pelvic nodal metastases was significantly associated with increased risk of para-aortic nodal metastasis [7,12]. Therefore, when performing radical hysterectomy for women with clinical stage IIB disease, para-aortic lymphadenectomy is recommended when there is evidence of multiple pelvic nodal metastases. Moreover, in women with clinical stage IIB disease who are planned to undergo definitive CCRT, pretreatment assessment of para-aortic nodes with retroperitoneal lymphadenectomy would be particularly applicable when there is suspicion for multiple pelvic nodal metastases in order to determine the radiation field.
Survival of women with pathological stage IIB cervical cancer ranges widely depending on additional prognostic factors, including multiple nodal metastases, histology, and age. The absolute survival difference between the groups with the most and least favorable survival outcomes exceeded 50%, implying that stage IIB disease is not a single disease entity and tailored assessment of these factors is needed. Without additional risk factors, survival of pathological stage IIB disease can be comparable to what reported in stage IB1 disease with tumor size 2–4 cm: 5-year CSS rate of 91.7% among those with SCC tumors with non/single pelvic lymph node metastasis shown in our study versus 5-year OS rate of 91.9% among those with stage IB1 (2–4 cm) disease shown in the JCOG0806-A study [22].
CSS in our study population of clinical stage IIB cervical cancer (5-year rate, 71.3%) was similar to what has been reported in the literature (55–77%) [4]. Notably, our study showed that survival was similar across the three adjuvant therapy types in women with pathological stage IIB disease. This supports our recent analysis that demonstrated comparable survival of women with node-positive high-risk stage IB-IIB cervical cancer regardless of modality of adjuvant therapy [10]. However, survival outcomes were similar between the CCRT group and the radiotherapy alone group, which is in contrast to a prior clinical trial that demonstrated benefit of additional chemotherapy during whole pelvic radiotherapy for high-risk early-stage cervical cancer [23]. This difference in outcome is likely due to the difference in patient population, since the aforementioned study enrolled clinical stage IA2-IIA disease whereas we only examined clinical stage IIB disease.
Recent studies have shown that the benefit of concurrent chemotherapy during radiotherapy diminishes if the tumor exhibits more high-risk surgical-pathological factors [24,25]. Currently, there is an ongoing clinical trial evaluating additional systemic chemotherapy after standard treatment for high-risk early-stage cervical cancer with surgery followed by CCRT (RTOG-0724) [26], and this trial will ultimately address the utility of additional systemic chemotherapy in early-stage cervical cancer exhibiting multiple high-risk factors similar to our study population. This treatment approach may be particularly applicable when tumors have pathological parametrial invasion. In our study, pathological parametrial involvement was a risk factor for distant-recurrence. Radiotherapy was found to reduce the risk of local-recurrence, and systemic chemotherapy was found to reduce the risk of distant-recurrence. Thus, combining these two modalities by administering both pelvic irradiation and systemic chemotherapy may be the way to improve survival in pathological stage IIB cervical cancer.
A strength of our study is that this is one of the largest sample sizes examining solely stage IIB cervical cancer. Median follow-up time exceeded 5 years, implying the adequacy of survival analysis. A limitation of this study is that certain biases inherent to retrospective study exist. For instance, exact indications or factors involved in the decision regarding type of adjuvant therapy were not able to be assessed in this study. There are also multiple confounders missing in the study. For example, we could not analyze type of pathological parametrial invasion (gross, microscopic, and LVSI) in this study. Moreover, there is no information for nodal assessment (grossly abnormal versus microscopic metastasis).
In conclusion, in clinical stage IIB cervical cancer, the accuracy of clinical staging for predicting pathological parametrial invasion appears low to modest. Because proper patient selection for surgical treatment leads to reduced morbidity and improves both quality of care and survival, further emphasis needs to be placed on the current limitation of clinical assessment for parametrial involvement. Based on the results of this study, assessing DSI with MRI could be the key to improving clinicians’ ability to predict pathological stage IIB disease.
Supplementary Material
Acknowledgement
We thank all the JGOG institutions participated in this study and the JGOG Cervical Cancer Committee members for administrative work for the study. We also thank Dr. Rachel S. Mandelbaum for her scientific input on this study.
Footnotes
Disclosure statement
Honorarium, Chugai (K.M.); Book editorial, Springer, and meeting expense, OVAL (K.M.); none for other authors.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejso.2019.02.019.
References
- [1].Saito T, Katabuchi H. Annual report of the committee on gynecologic Oncology, Japan society of obstetrics and gynecology: patient Annual report for 2013 and treatment annual report for 2008. J Obstet Gynaecol Res 2016;42:1069–79. [DOI] [PubMed] [Google Scholar]
- [2].Mikami M, Aoki Y, Sakamoto M, Shimada M, Takeshima N, Fujiwara H, et al. Surgical principles for managing stage IB2, IIA2, and IIB uterine cervical cancer (Bulky Tumors) in Japan: a survey of the Japanese Gynecologic Oncology Group. Int J Gynecol Cancer 2014;24:1333–40. [DOI] [PubMed] [Google Scholar]
- [3].National Comprehensive Cancer Network Clinical Practice Guideline in Oncology. Cervical Cancer. <https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf> (accessed 1/29/2019).
- [4].Suprasert P, Srisomboon J, Kasamatsu T. Radical hysterectomy for stage IIB cervical cancer: a review. Int J Gynecol Cancer 2005;15:995–1001. [DOI] [PubMed] [Google Scholar]
- [5].Matsuo K, Shimada M, Yamaguchi S, Kigawa J, Tokunaga H, Tabata T, et al. Neoadjuvant chemotherapy with taxane and platinum followed by radical hysterectomy for stage IB2–IIB cervical cancer: impact of histology type. J Clin Med 2019;8:e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Matsuo K, Shimada M, Yamaguchi S, Kanao H, Nakanishi T, Saito T, et al. Identifying a candidate population for ovarian conservation in young women with clinical stage IB-IIB cervical cancer. Int J Cancer 2018;142:1022–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Matsuo K, Shimada M, Saito T, Takehara K, Tokunaga H, Watanabe Y, et al. Risk stratification models for para-aortic lymph node metastasis and recurrence in stage IB-IIB cervical cancer. J Gynecol Oncol 2018;29:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Matsuo K, Shimada M, Yokota H, Satoh T, Katabuchi H, Kodama S, et al. Effectiveness of adjuvant systemic chemotherapy for intermediate-risk stage IB cervical cancer. Oncotarget 2018;8:106866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Matsuo K, Shimada M, Mikami M. Ovarian conservation for young women with clinical stage IB-IIB cervical cancer in Japan. J Gynecol Oncol 2017;28: e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Matsuo K, Shimada M, Aoki Y, Sakamoto M, Takeshima N, Fujiwara H, et al. Comparison of adjuvant therapy for node-positive clinical stage IB-IIB cervical cancer: systemic chemotherapy versus pelvic irradiation. Int J Cancer 2017;141:1042–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ebina Y, Yaegashi N, Katabuchi H, Nagase S, Udagawa Y, Hachisuga T, et al. Japan Society of Gynecologic Oncology guidelines 2011 for the treatment of uterine cervical cancer. Int J Clin Oncol 2015;20:240–8. [DOI] [PubMed] [Google Scholar]
- [12].Matsuo K, Grubbs BH, Mikami M. Quality and quantity metrics of pelvic lymph node metastasis and risk of para-aortic lymph node metastasis in stage IB-IIB cervical cancer. J Gynecol Oncol 2018;29:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med 1997;16:965–80. [DOI] [PubMed] [Google Scholar]
- [14].Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745–51. [DOI] [PubMed] [Google Scholar]
- [15].Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34: 3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Balleyguier C, Sala E, Da Cunha T, Bergman A, Brkljacic B, Danza F, et al. Staging of uterine cervical cancer with MRI: guidelines of the European society of urogenital Radiology. Eur Radiol 2011;21:1102–10. [DOI] [PubMed] [Google Scholar]
- [18].Bourgioti C, Chatoupis K, Rodolakis A, Antoniou A, Tzavara C, Koutoulidis V, et al. Incremental prognostic value of MRI in the staging of early cervical cancer: a prospective study and review of the literature. Clin Imaging 2016;40:72–8. [DOI] [PubMed] [Google Scholar]
- [19].Lee JY, Youm J, Kim TH, Cho JY, Kim MA, Suh DH, et al. Preoperative MRI criteria for trials on less radical surgery in Stage IB1 cervical cancer. Gynecol Oncol 2014;134:47–51. [DOI] [PubMed] [Google Scholar]
- [20].Lee JY, Youm J, Kim JW, Cho JY, Kim MA, Kim TH, et al. Identifying a low-risk group for parametrial involvement in microscopic Stage IB1 cervical cancer using criteria from ongoing studies and a new MRI criterion. BMC Canc 2015;15:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Thomeer MG, Gerestein C, Spronk S, van Doorn HC, van der Ham E, Hunink MG. Clinical examination versus magnetic resonance imaging in the pretreatment staging of cervical carcinoma: systematic review and metaanalysis. Eur Radiol 2013;23:2005–18. [DOI] [PubMed] [Google Scholar]
- [22].Kato T, Takashima A, Kasamatsu T, Nakamura K, Mizusawa J, Nakanishi T, et al. Clinical tumor diameter and prognosis ofpatients with FIGO stage IB1 cervical cancer (JCOG0806-A). Gynecol Oncol 2015;137:34–9. [DOI] [PubMed] [Google Scholar]
- [23].Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 2000;18:1606–13. [DOI] [PubMed] [Google Scholar]
- [24].Matsuo K, Mabuchi S, Okazawa M, Matsumoto Y, Tsutsui T, Fujita M, et al. Utility of risk-weighted surgical-pathological factors in early-stage cervical cancer. Br J Canc 2013;108:1348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Matsuo K, Mabuchi S, Okazawa M, Kawano M, Kuroda H, Kamiura S, et al. Clinical implication of surgically treated early-stage cervical cancer with multiple high-risk factors. J Gynecol Oncol 2015;26:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chemotherapy and Pelvic Radiation Therapy With or Without Additional Chemotherapy in Treating Patients With High-Risk Early-Stage Cervical Cancer After Radical Hysterectomy. NIH U.S. National Library of Medicine, Clinical-Trials.gov. <https://clinicaltrials.gov/ct2/show/NCT00980954 (accessed 1/29/ 2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


