Abstract
Objective
The aim of this study was to determine the association between weight change patterns and survival outcomes of women with endometrial cancer.
Methods
This retrospective study examined surgically-staged endometrial cancer cases with available weight information between 1999 and 2013 (n = 665). Proportional body mass index (delta-BMI) change at 6 months, 1 and 2 years after hysterectomy was compared with baseline BMI and correlated to patient demographics, tumor characteristics, treatment type, and disease-free survival (DFS) and overall survival (OS).
Results
Mean BMI was 35.6, and 69 % of cases were obese. At 6 months, 1 and 2 years after surgery, 39.1, 51.6, and 57.0 % of the study population, respectively, gained weight compared with pre-treatment baseline. In univariate analysis, 6-month delta-BMI change was significantly associated with DFS and OS, demonstrating bidirectional effects (both p < 0.001): 5-year rates, ≥15.0 % delta-BMI loss (33.5 and 59.1 %), 7.5–14.9 % loss (67.3 and 70.0 %), <7.5 % loss (87.8 and 95.7 %), <7.5 % gain (87.2 and 90.3 %), 7.5–14.9 % gain (64.6 and 67.6 %), and ≥15.0 % gain (32.5 and 66.7 %). In multivariable analysis controlling for age, ethnicity, baseline BMI, histology, grade, stage, chemotherapy, and radiotherapy, 6-month delta-BMI change remained an independent prognostic factor for DFS and OS (all p < 0.05): adjusted hazard ratios, ≥15 % delta-BMI loss (3.35 and 5.39), 7.5–14.9 % loss (2.35 and 4.19), 7.5–14.9 % gain (2.58 and 3.33), and ≥15.0 % gain (2.50 and 3.45) compared with <7.5 % loss. Similar findings were observed at a 1-year time point (p < 0.05). Baseline BMI was not associated with survival outcome (p > 0.05).
Conclusion
Our results demonstrated that endometrial cancer patients continued to gain weight after hysterectomy, and post-treatment weight change had bidirectional effects on survival outcome.
Endometrial cancer continues to be the most common gynecologic malignancy in the US.1 While obesity is a well-recognized risk factor for developing endometrial cancer,2 prognostic implications of body habitus in endometrial cancer seems contradictory. Some studies have shown that increased body habitus is associated with increased endometrial cancer mortality; however, these studies were in a predominantly non-obese population (proportion of obesity, 32–40 %) and may therefore not represent a typical endometrial cancer population.3–5 Other studies in predominantly obese populations (proportion of obesity, 54–68 %) have concluded that obesity is not associated with survival outcome of endometrial cancer.6,7 Their findings are based on the rationale that the majority of endometrial cancer is estrogen-dependent disease related to excess adiposity that is associated with low-grade and early-stage disease and a better prognosis.8 Taken together, identifying how body habitus affects disease prognosis is an important consideration in the management of women with endometrial cancer.
Recently, post-treatment weight change patterns have been reported to have a prognostic effect on survival in certain types of cancer. For instance, post-treatment weight gain is associated with an increased risk of recurrence in breast cancer, while weight change patterns were not associated with survival outcomes in patients with colon cancer.9,10 Additionally, considerable weight loss is regarded as a poor survival indicator of cancer patients in general.11 To date, little is known about the association of post-treatment weight change patterns and endometrial cancer prognosis. The objective of this study was to examine the association between weight change patterns and survival outcomes of women with endometrial cancer.
PATIENTS AND METHODS
After Institutional Review Board approval was obtained, cases were identified by utilizing the divisional database for endometrial cancer. Eligibility criteria were consecutive cases of surgically-staged endometrial cancer diagnosed and managed at the Los Angeles County Medical Center between 1999 and 2013, with available weight information during follow-up care. Sarcoma, endometrial hyperplasia, metastatic cancer to the endometrium, and non-hysterectomy cases were excluded from the study. Among the eligible cases, patient demographics, tumor characteristics, postoperative treatment patterns, weight information, and survival outcomes were obtained from medical records. The STROBE guideline was consulted for observational study. Parts of the study population were within the context of our prior studies6,12–14 Collected patient demographics included patient age, ethnicity, and medical comorbidities, while tumor characteristics included histologic subtype, grade, and stage. Tumor grade was defined as per the International Federation of Gynecology and Obstetrics (FIGO) criteria, and cancer stage was re-classified based on the 2009 FIGO system.15 Postoperative treatments included systemic chemotherapy and radiotherapy.
Weight information included baseline body mass index [BMI; calculated as weight (kg)/(height (m)2)] at the time of surgical staging, and BMI at the additional three time points after surgical staging (6 months, 1 and 2 years). This time period was chosen because the majority of endometrial cancer recurrences occur within the first 2 years after treatment.16 Then, a half faction (1 year) and a quarter fraction (6 months) of the 2-year time window were set for interval weight change assessment. The usual practice during this time included routine post-treatment surveillance visits scheduled every 3–6 months in the first 2 years, followed by every 6 months up to 5 years after surgery, and then followed by annual visits until 10 years after surgery.17 BMI was classified as <30, 30–39.9, or ≥40 kg/m2 per the World Health Organization (WHO) definition.18 Survival outcomes included disease-free survival (DFS) and overall survival (OS). DFS was defined as the time interval between the date of endometrial cancer surgery and the date of the first recurrence or the last follow-up date if there was no recurrence, and OS was defined as the time interval between the date of endometrial cancer surgery and the date of death due to endometrial cancer or the last follow-up date if the patient is alive.
Delta-BMI change was defined as the interval BMI change between the two time points (expressed as a percentage of the starting BMI). For example, if the patient’s BMI at the surgical staging of endometrial cancer and 6 months after surgery was 40 and 42, the 6-month delta-BMI change was calculated as 100 × (42–40)/40 = 5 %. In this study, delta-BMI change was defined as minimum-mild (<7.5 % loss or gain), moderate (7.5–14.9 % loss or gain), and excess (≥15.0 % loss or gain) (see electronic supplementary Method S1).
The primary analysis was to examine the pattern of weight change after endometrial cancer surgery (6 months, 1 and 2 years), and the secondary analysis was to examine the association between the interval weight change pattern and survival outcome (DFS and OS). Continuous variables were assessed for normality using the Kolmogorov–Smirnov test, expressed as mean (standard deviation) or median (range). Statistical significance of continuous variables in multiple groups was examined with a one-way analysis of variance (ANOVA) test. Categorical or ordinal variables were assessed using the χ2 test. Survival analysis was performed using the log-rank test for univariate analysis and a Cox’s proportional hazard regression model for multivariate analysis, expressed with hazard ratio (HR) and 95 % confidence interval (CI). Covariates entered in the final model were the variables with a cutoff value being p < 0.10 in univariate analysis. The Kaplan–Meier method was used to construct survival curves. A p value < 0.05 was considered statistically significant (two-tailed), and the Statistical Package of the Social Science (SPSS) version 12.0 (SPSS Inc., Chicago, IL, USA) was used for the analysis.
RESULTS
Overall, 841 women were diagnosed with endometrial cancer during the study period. Of these, 70 (8.3 %) women did not undergo hysterectomy-based surgical staging. Among 771 women who underwent hysterectomy-based surgical staging, 7 (0.9 %) women were excluded because of a lack of baseline BMI data. Of the remaining 764 women, records were examined for the availability of postoperative BMI results at 6-month, 1-year, and 2-year time points; 99 (13.0 %) were excluded due to the lack of the information for any of the three postoperative time points. Collectively, 665 women with endometrial cancer who underwent hysterectomy-based surgical staging with available postoperative BMI information in at least one of three time points represented the study population (n = 585 for the 6-month time point, n = 594 for the 1-year time point, and n = 375 for the 2-year time point).
Patient demographics are shown in Table 1. The mean age was 52.5 years, and the majority of the study population was Hispanic or Latina (70.8 %). Mean baseline BMI was 35.6 kg/m2, and the majority of patients were obese (69.0 %). Medical comorbidities were prevalent, with hypertension being the most common (55.3 %). The majority of endometrial cancers were endometrioid histology (82.9 %), grade 1 tumor (53.1 %), and stage I disease (70.2 %). Approximately one-quarter of patients received postoperative chemotherapy (26.5 %). Postoperative radiotherapy was administered to nearly one-third of the patients (34.5 %), with whole pelvic radiotherapy (WPRT) being the most common modality (22.1 %). The median follow-up time was 36.4 months (range 6.0–163.3) for the entire cohort; there were 94 (14.1 %) recurrences, with the median time-to-recurrence being 14.2 months; and there were 59 (8.9 %) deaths due to endometrial cancer, with the median time to death being 28.4 months.
TABLE 1.
Patient demographics
| N = 665 | |
|---|---|
| Age (years) | 52.5 (±10.1) |
| <50 | 237 (35.6) |
| ≥50 | 428 (64.4) |
| Ethnicity | |
| Caucasian | 68 (10.2) |
| African | 28 (4.2) |
| Hispanic | 471 (70.8) |
| Asian | 98 (14.7) |
| BMI (kg/m2) | 35.6 (±9.6) |
| <30 | 206 (31.0) |
| 30–39.9 | 273 (41.1) |
| ≥40 | 186 (28.0) |
| Hypertension | |
| No | 297 (44.7) |
| Yes | 368 (55.3) |
| Diabetes mellitus | |
| No | 453 (68.1) |
| Yes | 212 (31.9) |
| Hypercholesterolemia | |
| No | 500 (75.2) |
| Yes | 165 (24.8) |
| Histologic subtype | |
| Endometrioid | 551 (82.9) |
| Serous | 33 (5.0) |
| Clear cell | 13 (2.0) |
| Mixed | 63 (9.5) |
| Others | 5 (0.8) |
| Grade | |
| 1 | 353 (53.1) |
| 2 | 164 (24.7) |
| 3 | 148 (22.3) |
| Stage | |
| I | 467 (70.2) |
| II | 54 (8.1) |
| III | 99 (14.9) |
| IV | 45 (6.8) |
| Postoperative chemotherapy | |
| None | 489 (73.5) |
| Carboplatin + paclitaxel | 161 (24.2) |
| Other | 15 (2.3) |
| Postoperative radiotherapy | |
| None | 435 (65.4) |
| ICBT alone | 83 (12.5) |
| WPRT ± ICBT | 147 (22.1) |
Data are expressed as mean (±SD) or n (%)
111 (16.7 %) cases received both chemotherapy and radiotherapy
BMI body mass index, ICBT intracavitary brachytherapy, WPRT whole pelvic radiotherapy, SD standard deviation
Weight change patterns during the postoperative course were examined. During the postoperative follow-up, the proportions of any class of obesity were 69.0, 67.1, 71.6, and 72.3 % at baseline, 6-month, 1-year, and 2-year time points, respectively. At the 6-month time point, 39.1 % of patients gained weight after surgery, with the median delta-BMI change being 2.4 %, and the remaining 60.1 % of patients lost weight, with the median delta-BMI change being −3.4 %. At the 1-year time point, 51.6 % of patients gained weight compared with pre-treatment weight (median delta-BMI change 4.3 %), and the remaining 48.4 % of patients lost weight (−3.0 %). At the 2-year time point, the proportion of patients who gained weight compared with pre-hysterectomy weight was increased to 57.1 % (median delta-BMI changes, 4.6 %), and the remaining 42.9 % of patients lost weight (−3.5 %). Across the three observed time points, there were 330 patients who were available for weight information at all time points (electronic supplementary Fig. S1). The most common weight change pattern was sustained BMI above the baseline level throughout the 2-year follow-up after surgery (30.6 %), and this group had a significantly increasing proportion of obesity over time (baseline, 6-month, 1-year, and 2-year time points: 55.4, 68.3, 73.3, and 74.3 %; p = 0.016). There were groups of patients with rebound weight gain after a period of postoperative weight loss (26.7 %).
Correlations between 6-month weight change patterns and clinicopathological factors were examined (Table 2). Delta-BMI change was associated with age, ethnicity, baseline BMI, hypertension, histologic subtype, grade, stage, and adjuvant chemotherapy and radiotherapy (all p < 0.05). The majority of significant variables showed a bidirectional association to delta-BMI change. That is, excess (≥15 % loss or gain) and moderate (7.5–14.9 % loss or gain) delta-BMI changes were associated with older age (p = 0.034), and non-endometrioid histology (p = 0.035), higher grade (p = 0.001), higher stage (p <0.001), and greater prevalence of postoperative radiotherapy (p = 0.038) and chemotherapy (p = 0.001) compared with minimum–mild delta-BMI change (<7.5 % loss or gain). An inverse correlation was seen between delta-BMI change and baseline BMI (p < 0.001). Similar results were seen in the correlation between 1-year delta-BMI change and age, baseline BMI, stage, and postoperative chemotherapy (all p < 0.05) (electronic supplementary Table S1). Antiglycemic agent was not associated with delta-BMI change in diabetic patients (electronic supplementary Table S2).
TABLE 2.
Correlations between 6-month delta-BMI change and clinicopathological factors
| ≥15 % loss [n = 19 (2.9 %)] |
7.5–14.9 % loss [n = 61 (9.2 %)] |
<7.5 % lossa [n = 272 (40.9 %)] |
<7.5 % gain [n = 201 (30.2 %)] |
7.5–14.9 % gain [n = 20 (3.0 %)] |
≥15 % gain [n = 12 (1.8 %)] |
p value | |
|---|---|---|---|---|---|---|---|
| Age (years) | 56.5 (±8.7) | 50.8 (±9.9) | 53.2 (±10.1) | 51.0 (±10.3) | 54.3 (±10.3) | 55.9 (±11.1) | 0.034 |
| <50 | 3 (15.8) | 27 (44.3) | 89 (32.7) | 82 (40.8) | 5 (25) | 4 (33.3) | |
| ≥50 | 16 (84.2) | 34 (55.7) | 183 (67.3) | 119 (59.2) | 15 (75) | 8 (66.7) | |
| Ethnicity | 0.008 | ||||||
| Caucasian | 3 (15.8) | 12 (19.7) | 28 (10.3) | 16 (8.0) | 2 (10) | 1 (8.3) | |
| African | 2 (10.5) | 3 (4.9) | 14 (5.1) | 4 (2.0) | 0 | 3 (25.0) | |
| Hispanic | 11 (57.9) | 37 (60.7) | 192 (70.6) | 152 (75.6) | 16 (80) | 4 (33.3) | |
| Asian | 3 (15.8) | 9 (14.8) | 38 (14.0) | 29 (14.4) | 2 (10) | 4 (33.3) | |
| Pre-treatment BMI | 36.6 (±10.3) | 40.1 (±12.8) | 36.1 (±9.1) | 34.7 (±9.1) | 30.9 (±5.9) | 26.0 (±5.3) | <0.001 |
| <30 | 5 (26.3) | 14 (23.0) | 74 (27.2) | 70 (34.8) | 10 (50) | 11 (91.7) | |
| 30–39.9 | 9 (47.4) | 19 (31.1) | 116 (42.6) | 80 (39.8) | 9 (45) | 1 (8.3) | |
| ≥40 | 5 (26.3) | 28 (45.9) | 82 (30.1) | 51 (25.4) | 1 (5) | 0 | |
| Hypertension | 0.026 | ||||||
| No | 6 (31.6) | 32 (52.5) | 112 (41.2) | 99 (49.3) | 9 (45) | 10 (83.3) | |
| Yes | 13 (68.4) | 29 (47.5) | 160 (58.8) | 102 (50.7) | 11 (55) | 2 (16.7) | |
| Diabetes mellitus | 0.11 | ||||||
| No | 12 (63.2) | 42 (68.9) | 170 (62.5) | 146 (72.6) | 13 (65) | 11 (91.7) | |
| Yes | 7 (36.8) | 19 (31.1) | 102 (37.5) | 55 (27.4) | 7 (35) | 1 (8.3) | |
| Hypercholesterolemia | 0.53 | ||||||
| No | 15 (78.9) | 50 (82.0) | 204 (75) | 150 (74.6) | 17 (85) | 11 (91.7) | |
| Yes | 4 (21.1) | 11 (18.0) | 68 (25) | 51 (25.4) | 3 (15) | 1 (8.3) | |
| Histologic subtype | 0.035 | ||||||
| Endometrioid | 13 (68.4) | 49 (80.3) | 223 (82.0) | 176 (87.6) | 16 (80) | 6 (50) | |
| Serous | 2 (10.5) | 4 (6.6) | 12 (4.4) | 9 (4.5) | 2 (10) | 2 (16.7) | |
| Clear cell | 1 (5.3) | 1 (1.6) | 4 (1.5) | 4 (2.0) | 2(10) | 0 | |
| Mixed | 3 (15.8) | 7(11.5) | 30 (11.0) | 10 (5.0) | 0 | 4 (33.3) | |
| Others | 0 | 0 | 3 (1.1) | 2 (1.0) | 0 | 0 | |
| Grade | 0.001 | ||||||
| 1 | 4 (21.1) | 29 (47.5) | 148 (54.4) | 125 (62.2) | 9 (45) | 2 (16.7) | |
| 2 | 5 (26.3) | 12 (19.7) | 68 (25) | 45 (22.4) | 5 (25) | 5 (41.7) | |
| 3 | 10 (52.6) | 20 (32.8) | 56 (20.6) | 31 (15.4) | 6 (30) | 5 (41.7) | |
| Stage | <0.001 | ||||||
| I | 7 (36.8) | 36 (59.0) | 204 (75.0) | 158 (78.6) | 10 (50) | 2 (16.7) | |
| II | 0 | 7 (11.5) | 19 (7.0) | 15 (7.5) | 2 (10) | 1 (8.3) | |
| III | 7 (36.8) | 13 (21.3) | 34 (12.5) | 19 (9.5) | 3 (15) | 4 (33.3) | |
| IV | 5 (26.3) | 5 (8.2) | 15 (5.5) | 9 (4.5) | 5 (20) | 5 (41.7) | |
| Postoperative radiotherapy | 0.038 | ||||||
| None | 11 (57.9) | 35 (57.4) | 175 (64.3) | 146 (72.6) | 13 (65) | 5 (41.7) | |
| ICBT alone | 1 (5.3) | 6 (9.8) | 45 (16.5) | 23 (11.4) | 2 (10) | 3 (25) | |
| WPRT ± ICBT | 7 (36.8) | 20 (32.8) | 52 (19.1) | 32 (15.9) | 5 (25) | 4 (33.3) | |
| Postoperative chemotherapy | <0.001 | ||||||
| None | 5 (26.3) | 37 (60.7) | 211 (77.6) | 163 (81.1) | 10 (50) | 5 (41.7) | |
| Carboplatin + paclitaxel | 12 (63.2) | 22 (36.1) | 57 (21.0) | 32 (15.9) | 10 (50) | 7 (58.3) | |
| Other | 2 (10.5) | 2 (3.3) | 4 (1.5) | 6 (3.0) | 0 | 0 |
Data are expressed as mean (±SD) or n (%)
One-way ANOVA or χ2 test for p-values. Significant p values are shown in bold
Including cases with no interval BMI change
BMI body mass index, ICBT intracavitary brachytherapy, WPRT whole pelvic radiotherapy, ANOVA analysis of variance, SD standard deviation
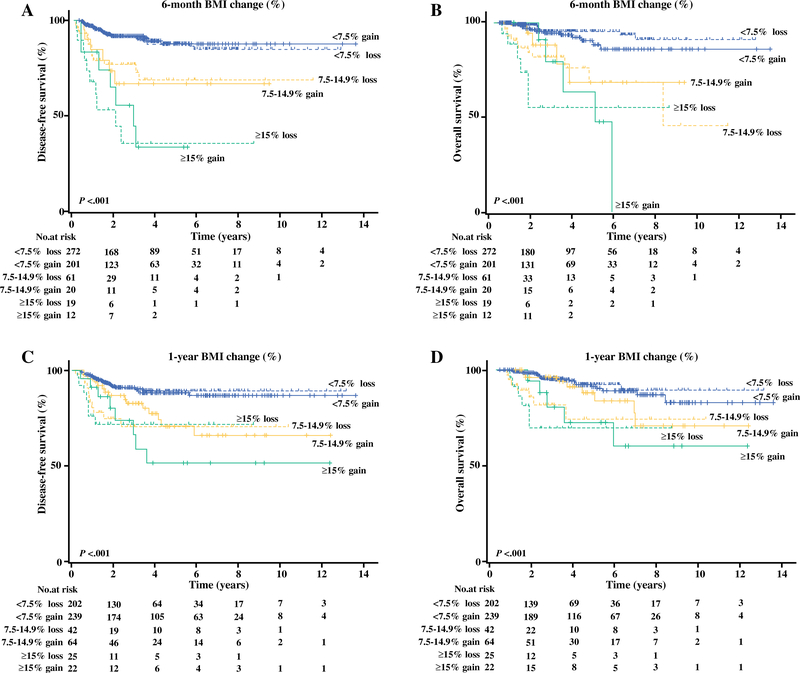
Survival analysis for 6-month delta-BMI change was performed. In univariate analysis, 6-month delta-BMI change was significantly associated with 5-year DFS rates, demonstrating a bidirectional association (33.5 % for excess delta-BMI loss, 67.3 % for 7.5–14.9 loss, 87.8 % for <7.5 % loss, 87.2 % for <7.5 % gain, 64.6 % for 7.5–14.9 % gain, and 32.5 % for ≥15 % gain; p < 0.001) (Fig. 1a). After controlling for age, ethnicity, baseline BMI, histologic subtype, grade, stage, postoperative radiotherapy and chemotherapy, excess and moderate 6-month delta-BMI changes remained independent prognostic factors for decreased DFS compared with <7.5 % loss (adjusted HRs, 3.35 for ≥15 % delta-BMI loss, 2.35 for 7.5–14.9 % loss, 2.58 for 7.5–14.9 % gain, and 2.50 for ≥ C15 % gain; all p < 0.05) (Table 3). Other independent prognostic factors for decreased DFS included age ≥50 years (adjusted HR 1.86; p = 0.037), grade 3 tumor (adjusted HR 2.44; p = 0.006), and stage III–IV disease (adjusted HR 5.51; p < 0.001). Additionally, 6-month delta-BMI change was significantly associated with 5-year OS rates (59.1, 70.0, 95.7, 90.3, 67.6, and 66.7 %; p < 0.001) (Fig. 1b). On multivariate analysis, excess and moderate 6-month delta-BMI changes remained an independent prognostic factor for decreased OS compared with <7.5 % loss (adjusted HRs, 5.39 for ≥15 % delta-BMI loss, 4.19 for 7.5–14.9 % loss, 3.33 for 7.5–14.9 % gain, and 3.45 for 7.5–14.9 % gain; all p < 0.05) (Table 4). Baseline BMI was not associated with DFS and OS (both p > 0.05). A bidirectional association was re-demonstrated between moderate–excess 1-year delta-BMI changes and decreased DFS (p < 0.001) (Fig. 1c). On multivariate analysis, excess 1-year delta-BMI change remained an independent prognostic factor associated with decreased DFS (adjusted HR, 3.47 for ≥15 % loss, and 3.05 for ≥15 % gain; both p < 0.05) (electronic supplementary Table S4). Moderate-excess 1-year delta-BMI loss remained as an independent prognostic factor for decreased OS on multivariate analysis (adjusted HR 3.03 for ≥15 % loss, and 4.67 for 7.5–14.9 % loss; both p < 0.05) (Fig. 1d and electronic supplementary Table S4).
FIG. 1.
Survival curves based on 6-month delta-BMI change for a disease-free survival and b overall survival; and 1-year delta-BMI change for c disease-free survival and d overall survival. Log-rank test for p values. BMI body mass index
TABLE 3.
Disease-free survival based on 6-month delta-BMI change
| N | 5-years (%) | Univariate |
Multivariate |
|||
|---|---|---|---|---|---|---|
| HR (95 % CI) | p value | HR (95 % CI) | p value | |||
| Age (years) | 0.001 | |||||
| <50 | 237 | 89.1 | 1 | 1 | ||
| ≥50 | 428 | 77.8 | 2.24 (1.3–3.67) | 1.86 (1.04–3.35) | 0.037 | |
| Ethnicity | <0.001 | |||||
| Non-hispanic | 194 | 71.5 | 1 | 1 | ||
| Hispanic | 471 | 85.8 | 0.50 (0.33–0.75) | 0.65 (0.41–1.04) | 0.073 | |
| Pre-treatment BMI | 0.034 | |||||
| <30 | 206 | 77.3 | 1 | 1 | ||
| ≥30 | 459 | 84.0 | 0.64 (0.43–0.97) | 1.44 (0.86–2.42) | 0.17 | |
| Hypertension | 0.74 | |||||
| No | 297 | 82.6 | 1 | |||
| Yes | 368 | 81.4 | 1.07 (0.71–1.61) | |||
| Diabetes mellitus | 0.57 | |||||
| No | 453 | 81.1 | 1 | |||
| Yes | 212 | 83.5 | 0.88 (0.57–1.37) | |||
| Hypercholesterolemia | 0.87 | |||||
| No | 500 | 81.3 | 1 | |||
| Yes | 165 | 83.1 | 1.04 (0.66–1.64) | |||
| Histologic subtype | <0.001 | |||||
| Endometrioid | 551 | 88.4 | 1 | 1 | ||
| Non-endometrioid | 114 | 50.6 | 6.46 (4.30–9.68) | 1.49 (0.84–2.66) | 0.18 | |
| Grade | <0.001 | |||||
| 1–2 | 517 | 89.6 | 1 | 1 | ||
| 3 | 148 | 54.5 | 7.12 (4.70–10.8) | 2.44 (1.30–4.59) | 0.006 | |
| Stage | <0.001 | |||||
| I–II | 521 | 92.9 | 1 | 1 | ||
| III–IV | 144 | 46.0 | 11.1 (7.07–17.3) | 5.51 (2.72–11.2) | <0.001 | |
| Postoperative chemotherapy | <0.001 | |||||
| None | 489 | 93.1 | 1 | 1 | ||
| Yes | 176 | 52.3 | 9.32 (5.90–14.7) | 1.54 (0.76–3.12) | 0.23 | |
| Postoperative radiotherapy | <0.001 | |||||
| None or ICBT alone | 518 | 86.7 | 1 | 1 | ||
| WPRT ± ICBT | 147 | 67.1 | 2.80 (1.86–4.20) | 1.04 (0.64–1.69) | 0.89 | |
| Delta-BMI change | <0.001 | |||||
| <7.5 % lossa | 272 | 87.8 | 1 | 1 | ||
| <7.5 % gain | 201 | 87.3 | 0.92 (0.50–1.68) | 1.22 (0.65–2.27) | 0.53 | |
| 7.5–14.9 % loss | 61 | 67.0 | 3.08 (1.63–5.82) | 2.35 (1.21–4.56) | 0.011 | |
| 7.5–14.9 % gain | 20 | 64.6 | 3.35 (1.38–8.14) | 2.58 (1.03–6.45) | 0.043 | |
| ≥15 % loss | 19 | 33.5 | 8.96 (4.30–18.7) | 3.35 (1.55–7.25) | 0.002 | |
| ≥15 % gain | 12 | 32.5 | 7.09 (3.07–16.4) | 2.50 (1.01–6.19) | 0.048 | |
Log-rank test for univariate analysis, and a Cox proportional hazard regression model for multivariate analysis. Covariates entered in the final model were the variables with p < 0.10 in univariate analysis. Significant p values are shown in bold
Including cases with no interval BMI change
BMI body mass index, 5-years (%) 5-years rate, HR hazard ratio, CI confidence interval, ICBT intracavitary brachytherapy, WPRT whole pelvic radiotherapy
TABLE 4.
Overall survival based on 6-month delta-BMI change
| N | 5-years (%) | Univariate |
Multivariate |
|||
|---|---|---|---|---|---|---|
| HR (95 % CI) | p value | HR (95 % CI) | p value | |||
| Age (years) | 0.042 | |||||
| <50 | 237 | 92.7 | 1 | 1 | ||
| ≥50 | 428 | 85.9 | 1.82 (1.01–3.27) | 1.15 (0.56–2.37) | 0.71 | |
| Ethnicity | 0.021 | |||||
| Non-hispanic | 194 | 82.2 | 1 | 1 | ||
| Hispanic | 471 | 90.8 | 0.55 (0.32–0.92) | 0.65 (0.35–1.20) | 0.17 | |
| Pre-treatment BMI | 0.06 | |||||
| <30 | 206 | 84.5 | 1 | 1 | ||
| ≥30 | 459 | 90.3 | 0.62 (0.37–1.03) | 1.19 (0.62–2.28) | 0.60 | |
| Hypertension | 0.92 | |||||
| No | 297 | 88.4 | 1 | |||
| Yes | 368 | 88.4 | 0.98 (0.58–1.63) | |||
| Diabetes mellitus | 0.38 | |||||
| No | 453 | 88.0 | 1 | |||
| Yes | 212 | 89.3 | 0.78 (0.44–1.37) | |||
| Hypercholesterolemia | 0.61 | |||||
| No | 500 | 88.4 | 1 | |||
| Yes | 165 | 88.4 | 0.86 (0.47–1.56) | |||
| Histologic subtype | <0.001 | |||||
| Endometrioid | 551 | 94.1 | 1 | 1 | ||
| Non-endometrioid | 114 | 62.0 | 8.86 (5.25–15.0) | 2.06 (1.01–4.20) | 0.048 | |
| Grade | <0.001 | |||||
| 1–2 | 517 | 94.8 | 1 | 1 | ||
| 3 | 148 | 65.6 | 7.88 (4.62–13.4) | 2.20 (0.97–5.01) | 0.06 | |
| Stage | <0.001 | |||||
| I–II | 521 | 95.8 | 1 | 1 | ||
| III–IV | 144 | 65.8 | 14.3 (7.56–26.8) | 5.56 (2.04–15.1) | 0.001 | |
| Postoperative chemotherapy | <0.001 | |||||
| None | 489 | 95.7 | 1 | 1 | ||
| Yes | 176 | 69.0 | 10.6 (5.74–19.7) | 1.61 (0.66–3.96) | 0.30 | |
| Postoperative radiotherapy | <0.001 | |||||
| None or ICBT alone | 518 | 91.6 | 1 | 1 | ||
| WPRT ± ICBT | 147 | 79.4 | 3.01 (1.80–5.02) | 1.13 (0.60–2.14) | 0.70 | |
| Delta-BMI change | <0.001 | |||||
| <7.5 % lossa | 272 | 95.7 | 1 | 1 | ||
| <7.5 % gain | 201 | 90.3 | 1.78 (0.78–4.06) | 2.02 (0.87–4.70) | 0.10 | |
| 7.5–14.9 % loss | 61 | 70.0 | 6.72 (2.90–15.6) | 4.19 (1.73–10.2) | 0.001 | |
| 7.5–14.9 % gain | 20 | 67.6 | 5.03 (1.58–16.0) | 3.33 (1.01–11.1) | 0.049 | |
| ≥15 % loss | 19 | 59.1 | 14.4 (5.22–39.9) | 5.39 (1.83–15.9) | 0.002 | |
| ≥15 % gain | 12 | 66.7 | 10.3 (3.50–30.3) | 3.50 (1.08–11.3) | 0.036 | |
Log-rank test for univariate analysis, and a Cox proportional hazard regression model for multivariate analysis. Covariates entered in the final model were the variables with p < 0.10 in univariate analysis. Significant p values are shown in bold
Including cases with no interval BMI change
BMI body mass index, 5-years (%) 5-year rate, HR hazard ratio, CI confidence interval, ICBT intracavitary brachytherapy, WPRT whole pelvic radiotherapy
DISCUSSION
The key findings of this study are that endometrial cancer patients continued to gain weight after surgical staging and that both weight loss and gain patterns were significantly associated with survival outcome of endometrial cancer. A parabolic relationship was demonstrated between the interval weight change and the risk of cancer recurrence/mortality. Interestingly, the magnitudes of significance for survival outcome were similar between weight loss and gain, endorsing the importance of monitoring weight changes during postoperative surveillance follow-up.
Several hypotheses can be proposed for the causality between weight gain and an increased risk of cancer recurrence and mortality. Of these, the most commonly described etiology may be the unopposed estrogen theory from excess adipose tissue. Enhanced conversion of androgen to estradiol in adipose tissue has been described to result in a constant mitogenic stimulation that can potentially trigger tumor progression.2 Hyperinsulinemia related to obesity may be another etiology as it promotes insulin-like growth factor secretion (IGF-1) which can activate mitogenic and pro-angiogenic pathways while inhibiting apoptosis.19 A role of inflammation is also an important consideration. Obesity is a state of chronic inflammation that can trigger mitogenic effects, including tumor promoter release from inflammatory cells. These cells can also generate reactive oxygen species.20 Furthermore, pro-inflammatory cytokines secreted by adipocytes have been implicated in tumor cell growth and progression.21
The role of adipocytes in the tumor microenvironment deserves special attention. It has been suggested that adipocytes can be reprogrammed to cancer-associated adipocytes, which promote adhesion, migration, and invasion of tumor cells, as well as releasing fatty acids that are used by cancer cells as a source of energy.20,21 In addition, nutritional excess is a main inducer of cellular stress that can lead to the activation of endoplasmic reticulum stress in visceral adipocytes. This has been significantly related to aggressive tumor behavior resulting in poor survival outcome of endometrial cancer.22
An important factor for weight gain and decreased survival outcome in cancer patients is possible suboptimal chemotherapy or radiotherapy in obese patients. A recent meta-analysis reported that up to 40 % of obese patients receive limited and reduced doses of chemotherapy when not based on actual body weight, and this has been suggested to be the main contributing factor related to poor survival outcome of obese patients in gynecologic cancer.23 A similar concept applies to the suboptimal efficacy of radiotherapy in the obese population, and obesity is known to be a prognostic factor for increased risk of recurrence after WPRT for pelvic cancer.24,25 Therefore, it is paramount that chemotherapy doses are calculated by actual body weight.23
Other possible associations linking weight gain and poor outcome of endometrial cancer include low levels of physical activity and low socioeconomic status. Reduced physical activity and sedentary lifestyle are related to obesity, and it is suggested that even light exercise and moderate physical activity were associated with improved OS in endometrial cancer patients.2,26 Low socioeconomic status is reported as an independent predictor for poor cancer treatment adherence, resulting in decreased survival outcome.27 Because low socioeconomic status is associated with an increased risk of obesity,28 this association may be an explanation for weight gain and decreased survival in endometrial cancer.
In the present study, moderate/excess weight loss was associated with poor survival in endometrial cancer patients. Similar to this project’s findings, several other studies have suggested that any weight loss after cancer diagnosis was associated with lower response to chemotherapy, increased toxicity, and reduced survival rates.11,29 There are several possible explanations for this link. First, weight loss may lead to a significant impairment of the immune system, particularly deficits in cell-mediated immunity, which can result in tumor progression.30 Second, reduction in body mass may increase toxicity to adjuvant therapy and result in decreased survival outcome.31 Finally, cachexia syndrome is characterized by a systemic inflammatory response, anorexia, and weight loss.32 This may result in impaired immune function, propensity for infections, tolerance to anticancer treatments, and psychosocial distress.32
CONCLUSIONS
Awareness of the potential significance of post-treatment weight change patterns on survival outcome of women with endometrial cancer needs to be considered in practice. Routine and accurate monitoring of weight change, patient education and counseling based on weight change patterns, as well as proper referrals for diet and exercise programs, are suggested as an integral part of a multidisciplinary approach in the management of endometrial cancer patients. Further investigation into postoperative weight changes in the endometrial cancer patient population and patient outcomes is warranted.
Supplementary Material
Acknowledgments
FUNDING Ensign Endowment for Gynecologic Cancer Research (to Koji Matsuo).
Footnotes
DISCLOSURE The authors did not report any potential conflicts of interest in the study.
Electronic supplementary material The online version of this article (doi:10.1245/s10434–016-5237–9) contains supplementary material, which is available to authorized users.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Fader AN, Arriba LN, Frasure HE, von Gruenigen VE. Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol. 2009;114:121–27. [DOI] [PubMed] [Google Scholar]
- 3.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. [DOI] [PubMed] [Google Scholar]
- 5.Arem H, Chlebowski R, Stefanick ML, Anderson G, Wactawski-Wende J, Sims S, et al. Body mass index, physical activity, and survival after endometrial cancer diagnosis: results from the Women’s Health Initiative. Gynecol Oncol. 2012;128:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuo K, Cahoon SS, Gualtieri M, Scannell CA, Jung CE, Takano T, et al. Significance of adenomyosis on tumor progression and survival outcome of endometrial cancer. Ann Surg Oncol. 2014;21:4246–55. [DOI] [PubMed] [Google Scholar]
- 7.Nevadunsky NS, Van Arsdale A, Strickler HD, Moadel A, Kaur G, Levitt J, et al. Obesity and age at diagnosis of endometrial cancer. Obstet Gynecol. 2014;124:300–6. [DOI] [PubMed] [Google Scholar]
- 8.Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet. 2012;379:1352–60. [DOI] [PubMed] [Google Scholar]
- 9.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370–8. [DOI] [PubMed] [Google Scholar]
- 10.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Nelson H, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008;26:4109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glare P, Sinclair C, Downing M, Stone P, Maltoni M, Vigano A. Predicting survival in patients with advanced disease. Eur J Cancer. 2008;44:1146–56. [DOI] [PubMed] [Google Scholar]
- 12.Matsuo K, Opper NR, Ciccone MA, Garcia J, Tierney KE, Baba T, et al. Time interval between endometrial biopsy and surgical staging for type I endometrial cancer: association between tumor characteristics and survival outcome. Obstet Gynecol. 2015;125:424–33. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo K, Hom MS, Moeini A, Machida H, Takeshima N,Roman LD, et al. Significance of monocyte counts on tumor characteristics and survival outcome of women with endometrial cancer. Gynecol Oncol. 2015;138:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuo K, Yessaian AA, Lin YG, Pham HQ, Muderspach LI, Liebman HA, et al. Predictive model of venous thromboembolism in endometrial cancer. Gynecol Oncol. 2013;128:544–51. [DOI] [PubMed] [Google Scholar]
- 15.Pecorelli S Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–4. [DOI] [PubMed] [Google Scholar]
- 16.van Wijk FH, van der Burg ME, Burger CW, Vergote I, van Doorn HC. Management of recurrent endometrioid endometrial carcinoma: an overview. Int J Gynecol Cancer. 2009;19:314–20. [DOI] [PubMed] [Google Scholar]
- 17.Salani R, Backes FJ, Fung MF, Holschneider CH, Parker LP, Bristow RE, et al. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am J Obstet Gynecol. 2011;204:466–78. [DOI] [PubMed] [Google Scholar]
- 18.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000; 894:i–xii, 1–253. Available at: http://www.uptodate.com/contents/obesity-in-adults-prevalence-screening-and-evaluation/abstract/20 Accessed 1 Jul 2015. [PubMed] [Google Scholar]
- 19.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. [DOI] [PubMed] [Google Scholar]
- 20.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831:1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuo K, Gray MJ, Yang DY, Srivastava SA, Tripathi PB, Sonoda LA, et al. The endoplasmic reticulum stress marker, glucose-regulated protein-78 (GRP78) in visceral adipocytes predicts endometrial cancer progression and patient survival. Gynecol Oncol. 2012;128:552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horowitz NS, Wright AA. Impact of obesity on chemotherapy management and outcomes in women with gynecologic malignancies. Gynecol Oncol. 2015;138:201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2012;63:800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frumovitz M, Jhingran A, Soliman PT, Klopp AH, Schmeler KM, Eifel PJ. Morbid obesity as an independent risk factor for disease-specific mortality in women with cervical cancer. Obstet Gynecol. 2014;124:1098–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel AV, Feigelson HS, Talbot JT, McCullough ML, Rodriguez C, Patel RC, et al. The role of body weight in the relationship between physical activity and endometrial cancer: results from a large cohort of US women. Int J Cancer. 2008;123:1877–82. [DOI] [PubMed] [Google Scholar]
- 27.Bristow RE, Chang J, Ziogas A, Campos B, Chavez LR, Anton-Culver H. Sociodemographic disparities in advanced ovarian cancer survival and adherence to treatment guidelines. Obstet Gynecol. 2015;125:833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinsa GD, Goryakin Y, Fumagalli E, Suhrcke M. Obesity and socioeconomic status in developing countries: a systematic review. Obes Rev. 2012;13:1067–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491–7. [DOI] [PubMed] [Google Scholar]
- 30.Chandra RK. Nutrition and the immune system: an introduction. Am J Clin Nutr. 1997;66:460S–3S. [DOI] [PubMed] [Google Scholar]
- 31.Kizer NT, Thaker PH, Gao F, Zighelboim I, Powell MA, Rader JS, et al. The effects of body mass index on complications and survival outcomes in patients with cervical carcinoma undergoing curative chemoradiation therapy. Cancer. 2010;117: 948–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blum D, Omlin A, Fearon K, Baracos V, Radbruch L, Kaasa S, et al. Evolving classification systems for cancer cachexia: ready for clinical practice? Support Care Cancer. 2010;18:273–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.