Abstract
Background and Objectives:
To examine characteristics and survival outcome of women with endometrial cancer who declined postoperative radiotherapy.
Methods:
A retrospective study was conducted to examine surgically-treated grade 1-2 stage IB and grade 3 stage IA-IB endometrioid endometrial cancer in the Surveillance, Epidemiology, and End Results Program between 1983 and 2013 (n = 10 613). Associations of patient declination for guideline-based postoperative radiotherapy and clinico-pathological demographics or survival outcome were examined on multivariable analysis.
Results:
There were 323 (3.0%) women who declined adjuvant radiotherapy. Women who declined postoperative radiotherapy were more likely to be older, White, Western U.S. residents, and register in recent years (all, adjusted-P < 0.05). On multivariable analysis, patient declination for guideline-based postoperative radiotherapy remained an independent prognostic factor for decreased endometrial cancer-specific survival in unstaged grade 1-2 stage IB or staged/unstated grade 3 stage IA-IB diseases (adjusted-hazard ratio 1.84, 95% confidence interval 1.34-2.51, P = 0.001). Association of patient declination for guideline-based postoperative radiotherapy and decreased overall survival remained independent in the entire cohort on multivariable analysis (adjuvant-hazard ratio 1.71, 95% confidence interval 1.44-2.02, P < 0.001).
Conclusions:
Our study suggested that patient compliance to guideline-based postoperative radiotherapy is a prognostic factor for women with stage I endometrioid endometrial cancer.
Keywords: adjuvant radiotherapy, compliance, endometrial cancer, survival outcome
1 ∣. INTRODUCTION
In 2017, endometrial cancer remains the most common gynecologic malignancy in the United States, and over 61 000 cases are estimated to be diagnosed with this disease.1 The majority of endometrial cancer patients undergo primary surgical treatment with total hysterectomy, salpingo-oophorectomy, and possible lymphadenectomy in the presence of risk factors.2 Surgical specimens are valuable to determine cancer stage, patient prognosis, and additional treatment to decrease recurrence risk among patients whom the tumors express certain risk factors.3
Radiotherapy is considered among the effective treatment modalities as adjuvant therapy for endometrial cancer. The American Society of Radiation Oncology (ASTRO) recently released evidence-based clinical practice guidelines on postoperative radiotherapy for women with endometrial cancer,4 which has been endorsed by the American Society of Clinical Oncology (ASCO).5 Unlike surgical treatment where the patient generally requires only one time of treatment session, radiotherapy generally requires multiple treatment sessions over weeks of time duration to complete the treatment course. For this reason, patient compliance to adherent the treatment schedule has been an important factor for treatment response in radiotherapy.
Association between patient non-compliance for radiotherapy and decreased treatment outcome has been reported in various types of cancer including breast and head-neck cancers.6-8 However, evidence examining the effects of patient compliance on survival outcome has been lacking in endometrial cancer. The aim of the study was to examine characteristics of women who declined postoperative radiotherapy and to assess survival outcomes of women who declined postoperative radiotherapy for endometrial cancer.
2 ∣. MATERIALS AND METHODS
This is a retrospective observational study utilizing the Surveillance, Epidemiology, and End Results Program that is a population-based tumor registry in the United States covering approximately 28% of the population.9 This publicly available and deidentified database is supported and managed by the National Cancer Institute since 1973. The data entry is performed by the certified cancer registrars, and survival data are linked with state mortality records and National Death Index for verification. The Institutional Review Board in University of Southern California exempted this study because of the use of publicly available deidentified data. To outline the observational study results, the STROBE guidelines were consulted for this study.10
Eligible cases for this study were consecutive stage I endometrioid endometrial cancer cases that underwent primary hysterectomy between 1983 and 2013. SEER*Stat 8.2.1 was used to extract the dataset for the 1973-2013 case records, and the cases between 1973 and 1982 were excluded from this analysis due to lack of information on the surgical procedures. Cases with uterine sarcomas and metastatic tumors to the uterus were excluded. Women who received radiotherapy prior to hysterectomy and unknown postoperative radiotherapy type were also excluded from the analysis.
Based on the ASTRO guidelines, we identified the cases that met the recommendation criteria for postoperative radiotherapy for endometrial cancer.4 These cases included grade 1-2 stage IB disease, and grade 3 stage IA diseases, and grade 3 stage IB disease. Then, patients who received the adjuvant radiotherapy modality per the ASTRO guidelines were identified: vaginal brachytherapy or whole pelvic radiotherapy for grade 1-2 stage IB disease; vaginal brachytherapy or whole pelvic radiotherapy for grade 3 stage IA disease. For women with grade 3 stage IB, while whole pelvic radiotherapy is recommended per the guidelines, a recent study examined the practice pattern for adjuvant radiotherapy for this particular patient population demonstrated a significant increase in vaginal brachytherapy use and therefore we included both vaginal brachytherapy and whole pelvic radiotherapy as the choices of adjuvant radiotherapy.11 Among the cases that met with the recommendation criteria for postoperative radiotherapy as above, the code described as “refused” in the radiotherapy modality section was considered the surrogate marker for patient non-compliance in a way of declination of postoperative radiotherapy in our study.
Clinical information abstracted from the database included patient demographics, tumor characteristics, treatment patterns, and survival outcome. Patient demographics at cancer diagnosis included chronological age (<60 vs ≥60 years), calendar year (1983-1999, 2000-2009, and 2010-2013), race (White, Black, Hispanic, Asian, and others), marital status (single, married, and others), and registry area (West, Central, and East). Tumor characteristics included cancer stage (IA, IB, and INOS), grade (1, 2, and 3), histology subtypes (endometrioid), and tumor size (<2 vs ≥2 cm). Treatment patterns included hysterectomy types (simple vs extended), performance of pelvic lymphadenectomy (performed vs not performed), and postoperative radiotherapy (vaginal brachytherapy, whole pelvic radiotherapy with or without vaginal brachytherapy). Survival outcome included cause-specific survival and overall survival.
Recorded cancer stage was reclassified with the American Joint Committee on Cancer 7th edition surgical-pathological staging classification schema. The World Health Organization histological classifications combined with ICD-0-3 site/histology validation list were used for histologic subtypes.12 Cutoff values for patient age at diagnosis and tumor size were based on prior studies.12,13 Cause-specific survival was defined as the time interval between the date of endometrial cancer diagnosis and the date of death from endometrial cancer with censoring of patients who were alive at the last follow-up and who died of other causes. Overall survival was defined as the time interval between the date of endometrial cancer diagnosis and the date of death from any reason (all-cause), with censoring of patients who were alive at the last follow-up.
For the internal validation of the study, women who met the criteria for high-intermediate risk group in the PORTEC-1 trial were examined.14 PORTEC-1 was a multicenter randomized controlled trial examining the effectiveness of post-hysterectomy pelvic irradiation for women with stage I endometrial cancer, enrolling women with grade 1 with ≥50% tumor invasion, grade 2 with any invasion, and grade 3 with <50% invasion. The trial showed that pelvic irradiation significantly reduced loco-regional recurrence but not all-cause mortality. Based on this trial, women who had at least two out of three risk factors (age ≥60 years, grade 3 tumors, and stage IB) were defined as the high-intermediate risk group. In our study, we examined cases that met these criteria for survival outcome comparing whole pelvic radiotherapy versus patient declination. All factors were available for the analysis in this database.
The primary objective of analysis was to examine contributing factors for patient declination related to postoperative recommended radiotherapy. The secondary objective of analysis was to examine survival outcome of women with stage I endometrioid endometrial cancer who declined postoperative radiotherapy. Among women who met the guideline recommendations for postoperative radiotherapy, we compared women who declined the radiotherapy to those who received adjuvant radiotherapy.
Binary logistic regression models (patient declination vs adjuvant radiotherapy) were used to identify the independent contributing factors for patient declination for postoperative radiotherapy. Covariates entered in the final model of the multivariable analysis were patient demographics, tumor characteristics, and treatment patterns. Magnitudes of statistical significance were expressed with adjusted-odds ratio (OR) and 95% confidence interval (CI). Hosmer-Lemeshow goodness-of-fit test was used for evaluating the final model of multivariable analysis.
The Kaplan-Meier method was used to construct the survival curves between the declination group and the adjuvant radiotherapy group,15 and statistical significance between the curves were assess with log-rank test in univariable analysis. Cox proportional hazard regression models were used to determine the independent prognostic factors for cause-specific survival and overall survival (all-cause) in multivariable analysis,16 and covariates entered in the final model were patient demographics, tumor characteristics, and treatment patterns. Magnitudes of statistical significance were expressed with adjusted-hazard ratio (HR) and 95%CI. The variance inflation factor was determined among covariates in multivariable analysis, and a value of 2 or greater was defined as multicollinearity in this study.17 Over-adjustment in the multivariable model was assessed by the ratio between events of interest and the entered variables in the model, and ratio <10 was defined as model over-adjustment.18,19 All statistical tests were two-tailed, and P-values of less than 0.05 were considered statistical significance. Statistical Package for Social Sciences (SPSS, IBM Corp., version 24.0, Armonk, NY) was used for all the analyses.
3 ∣. RESULTS
Patient selection schema is shown in Fig. 1. Among 235 849 cases of primary endometrial cancer in the database, 219 543 women with non-endometrioid histology, stage II-IV disease, or unknown grade/stage/hysterectomy status, and who received neoadjuvant radiotherapy were excluded from the study. Then, 16 306 women with stage IA-IB endometrioid endometrial cancer who underwent primary hysterectomy were examined for postoperative radiotherapy indication, and 5693 women with grade 1-2 stage IA disease were excluded. The study population therefore composed of 5783 women with grade 1-2 stage IB disease and 4830 women with grade 3 stage IA-IB disease.
FIGURE 1.
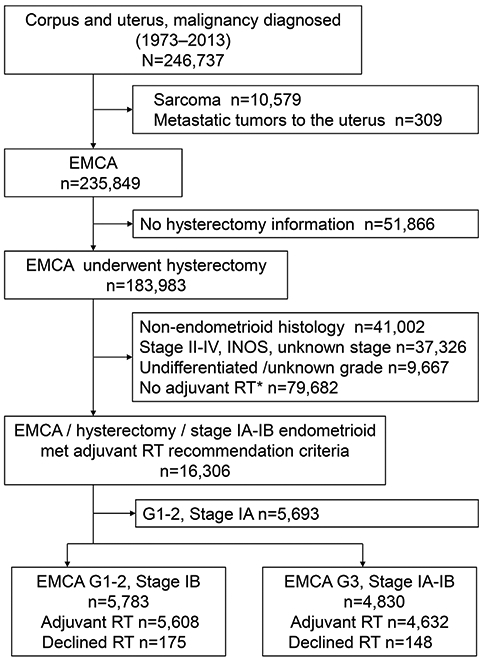
Selection criteria. EMCA, endometrial cancer; G, grade; and RT, radiotherapy. *Including unknown radiation modality type
Patient demographics are shown in Table 1. There were 323 (3.0%, 95%CI 2.7-3.4) women who declined postoperative radiotherapy in our study population. When compared to women who received the postoperative radiotherapy on multivariable analysis, those women who declined the postoperative radiotherapy were more likely to be old (60 years or older vs younger than 60 years, 3.4% vs 2.1%, adjusted-OR 1.52, 95%CI 1.13-2.04, P = 0.005), of White ethnicity (White vs non-White, 3.2% vs 2.6%, adjusted-OR 1.53, 95%CI 1.12-2.10, P = 0.01), and Western United State residents (West vs Central, 4.6% vs 2.4%, adjusted-OR 2.13, 95%CI 1.59-2.86, P < 0.001). Women who were registered in recent years were more likely to decline postoperative radiotherapy (year 2000 and later vs before 2000, 3.2% vs 2.7%, adjusted-OR 1.40, 95%CI 1.04-1.88, P = 0.03). Pelvic lymphadenectomy status was not associated with patient declination for postoperative radiotherapy (adjusted-P = 0.69).
TABLE 1.
Contributing factors for patient declination of postoperative radiotherapy in stage I endometrioid endometrial cancer (N = 10 613)
| Characteristic | Declined RT | Received RT | Adjusted-ORa (95%CI) | P-value |
|---|---|---|---|---|
| Number (%) | 323 (3.0%) | 10 290 (97.0%) | ||
| Age (y) | 70.8 (±11.5) | 65.8 (±10.5) | ||
| <60 | 61 (2.1%) | 2798 (97.9%) | 1 | |
| ≥60 | 262 (3.4%) | 7492 (96.6%) | 1.52 (1.13-2.04) | 0.005 |
| Ethnicity | ||||
| White | 273 (3.2%) | 8383 (96.8%) | 1.53 (1.12-2.10) | 0.01 |
| Non-White | 50 (2.6%) | 1907 (97.4%) | 1 | |
| Marital status | ||||
| Single | 42 (3.1%) | 1331 (96.9%) | 1.37 (0.95-1.96) | 0.09 |
| Married | 132 (2.4%) | 5397 (97.6%) | 1 | |
| Others | 149 (4.0%) | 3562 (96.0%) | 1.65 (1.30-2.11) | <0.001 |
| Registry area | ||||
| West | 231 (4.6%) | 4744 (95.4%) | 2.13 (1.59-2.86) | <0.001 |
| Central | 60 (2.4%) | 2453 (97.6%) | 1 | |
| East | 32 (1.0%) | 3093 (99.0%) | 0.40 (0.26-0.63) | <0.001 |
| Year at diagnosis | ||||
| Before 2000 | 79 (2.7%) | 2794 (97.3%) | 1 | |
| 2000 and after | 277 (3.2%) | 7496 (96.8%) | 1.40 (1.04-1.88) | 0.03 |
| Grade | ||||
| 1-2 | 175 (3.0%) | 5068 (97.0%) | 1 | |
| 3 | 148 (3.1%) | 4682 (96.9%) | 1.12 (0.84-1.49) | 0.43 |
| Stage | ||||
| IA | 76 (2.7%) | 2727 (97.3%) | 1 | |
| IB | 247 (3.2%) | 7563 (96.8%) | 1.10 (0.78-1.53) | 0.59 |
| Tumor size (cm) | ||||
| <2.0 | 19 (2.6%) | 713 (97.4%) | 1 | |
| ≥2.0 | 182 (3.1%) | 5604 (96.9%) | 1.13 (0.69-1.83) | 0.63 |
| Unknown | 122 (3.0%) | 3973 (97.0%) | 1.20 (0.73-1.96) | 0.48 |
| Surgery type | ||||
| Simple hyst | 273 (3.1%) | 8610 (96.9%) | 1 | |
| Others | 50 (2.9%) | 1680 (97.1%) | 0.98 (0.70-1.37) | 0.91 |
| Pelvic lymphadenectomy | ||||
| Performed | 224 (3.0%) | 7134 (97.0%) | 1 | |
| Not performed | 96 (3.2%) | 2895 (96.8%) | 0.95 (0.73-1.24) | 0.69 |
| Unknown | 3 (1.1%) | 261 (98.9%) | 0.34 (0.10-1.20) | 0.08 |
hyst, hysterectomy; OR, odds ratio; CI, confidence interval; and RT, radiotherapy. Number (%) per row or mean (±SD) is shown. A binary logistic regression model for multivariable analysis. All listed covariates were entered in the final model. Significant P-values are emboldened. Grade 3 cases included undifferentiated endometrioid type.
OR for patient declination for postoperative radiotherapy compared to postoperative radiotherapy.
Median follow-up was 71 months for the entire cohort: the patient declination group 55 months and the postoperative radiotherapy group 71.5 months. There were 1166 (11.0%) deaths from endometrial cancer and 3493 (32.9%) deaths from any causes in the study cohort.
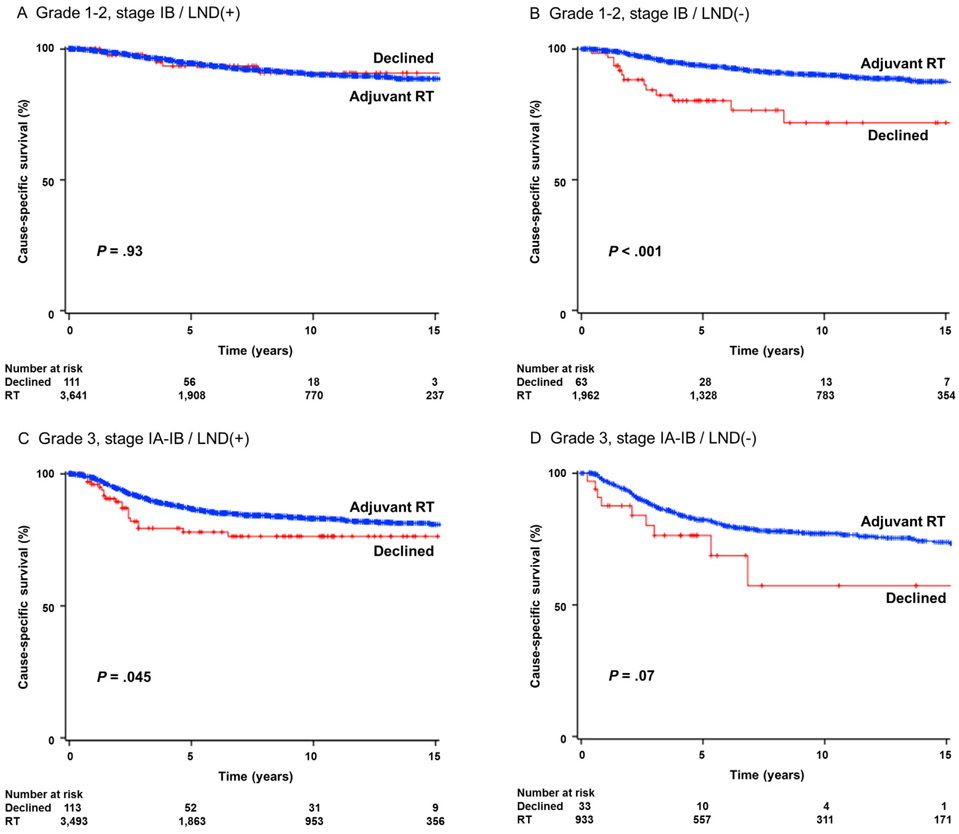
Cause-specific survival was examined. On univariable analysis, women who declined postoperative radiotherapy had a significantly lower 5-year cause-specific survival rate compared to those who received the postoperative radiotherapy in grade 1-2 stage IB disease if pelvic lymphadenectomy was not performed (declination vs radiotherapy, 80.1% vs 93.8%, P < 0.001); conversely, cause-specific rates were similar between women who declined postoperative radiotherapy and those who received when pelvic lymphadenectomy was performed (94.6% vs 93.6%, P = 0.93; Fig. 2A,B). Among 3606 women with grade 3 stage IA-IB disease who underwent pelvic lymphadenectomy, patient declination for postoperative radiotherapy was significantly associated with decreased cause-specific survival compared to those who received postoperative radiotherapy (5-year rates 78.1% vs 86.9%, P = 0.045); similarly, among 966 women who did not undergo pelvic lymphadenectomy for grade 3 stage IA-IB disease, the 5-year cause-specific survival rate was lower in the patient declination group than the radiotherapy group but it did not reach statistical significance (76.4% vs 82.3%, P = 0.07; Fig. 2C,D).
FIGURE 2.
Survival curves for cause-specific survival. Log-rank test for P-value. Survival curves were constructed per the Kaplan-Meier method for cause-specific survival for grade 1-2 stage IB disease with lymphadenectomy (panel A), grade 1-2 stage IB disease without lymphadenectomy (panel B), grade 3 stage IA-IB disease with lymphadenectomy (panel C), and grade 3 stage IA-IB without lymphadenectomy (panel D). LND, pelvic lymphadenectomy
A multivariable analysis was performed to examine the association of patient declination for postoperative radiotherapy and cause-specific survival among the subgroups of women with grade 1-2 stage IB disease who did not undergo pelvic lymphadenectomy and grade 3 stage IA-IB disease with or without pelvic lymphadenectomy (Table 2). After controlling for patient demographics, tumor characteristics, and treatment patterns, patient declination of postoperative radiotherapy remained an independent prognostic factor for decreased cause-specific survival compared to the guideline-preferred radiotherapy (adjusted-HR 1.84, 95%CI 1.34-2.51, P < 0.001). Older age, Non-White ethnicity, Central U.S. residence, higher grade and stage, large tumor, and no lymphadenectomy were independently associated with decreased cause-specific survival (all, adjusted-P < 0.01; Table 2).
TABLE 2.
Multivariable analysis of cause-specific survival with endometrial cancer (n = 6597)
| Survival (%) |
Multivariable |
||||
|---|---|---|---|---|---|
| Characteristics | No. | 5-yr | 10-yr | HR (95%CI) | P-value |
| Age (y) | |||||
| <60 | 1890 | 92.4 | 89.7 | 1 | |
| ≥60 | 4707 | 86.3 | 81.6 | 1.94 (1.63-2.32) | <0.001 |
| Ethnicity | |||||
| White | 5280 | 88.3 | 84.6 | 1 | |
| Non-White | 1317 | 86.9 | 81.3 | 1.26 (1.06-1.49) | 0.01 |
| Marital status | |||||
| Single | 837 | 90.0 | 85.4 | 1.01 (0.81-1.27) | 0.92 |
| Married | 3452 | 88.8 | 84.8 | 1 | |
| Others | 2308 | 86.3 | 82.3 | 1.07 (0.93-1.24) | 0.35 |
| Registry area | |||||
| West | 3221 | 88.2 | 83.9 | 0.87 (0.74-1.03) | 0.10 |
| Central | 1576 | 86.9 | 83.2 | 1 | |
| East | 1800 | 88.9 | 84.9 | 0.91 (0.75-1.10) | 0.31 |
| Year at diagnosis | |||||
| Before 2000 | 2050 | 87.8 | 83.9 | 1 | |
| 2000 and after | 4547 | 88.3 | 83.9 | 0.96 (0.82-1.12) | 0.57 |
| Grade | |||||
| 1-2 | 2025 | 93.4 | 89.6 | 1 | |
| 3 | 4572 | 85.6 | 81.4 | 3.15 (2.56-3.89) | <0.001 |
| Stage | |||||
| IA | 2548 | 89.7 | 86.1 | 1 | |
| IB | 4049 | 87.1 | 82.7 | 1.78 (1.52-2.07) | <0.001 |
| Size (cm) | |||||
| <2.0 | 488 | 93.6 | 91.6 | 1 | |
| ≥2.0 | 3440 | 87.1 | 82.2 | 1.89 (1.34-2.66) | <0.001 |
| Unknown | 2669 | 88.3 | 84.5 | 1.63 (1.16-2.30) | <0.001 |
| Surgery type | |||||
| Simple hyst | 5363 | 88.2 | 84.2 | 1 | |
| Others | 961 | 87.2 | 83.1 | 1.04 (0.86-1.27) | 0.68 |
| Pelvic lymphadenectomy | |||||
| Performed | 3606 | 86.6 | 82.8 | 1 | |
| Not performed | 2991 | 89.7 | 85.3 | 1.13 (1.10-1.56) | 0.003 |
| Adjuvant radiotherapy | |||||
| Received | 6388 | 88.4 | 84.3 | 1 | |
| Declined | 209 | 78.4 | 73.1 | 1.84 (1.34-2.51) | <0.001 |
hyst, hysterectomy; HR, hazard ratio; CI, confidence interval. Women with grade 1-2 stage IB disease who did not undergo pelvic lymphadenectomy and women with grade 3 stage IA-IB disease with or without pelvic lymphadenectomy were examined. Results of a Cox proportional hazard regression model for multivariable analysis are shown. All the listed covariates were entered in the final model. Significant P-values are emboldened.
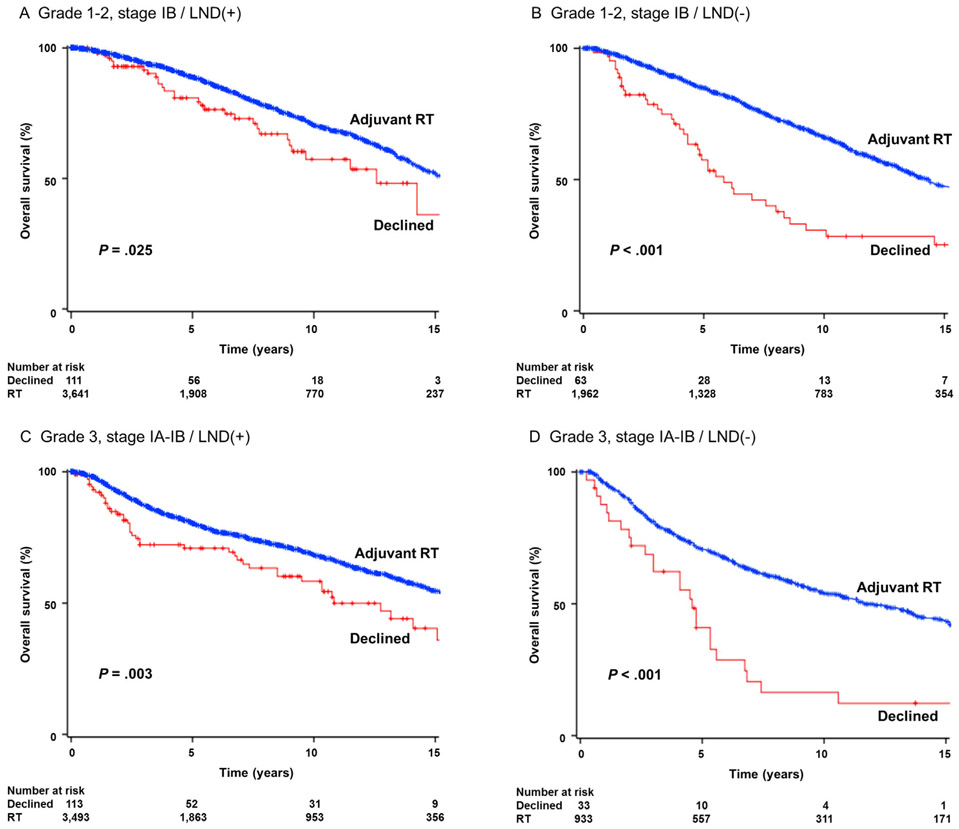
All-cause mortality was examined. On univariable analysis, patient declination of postoperative radiotherapy was significantly associated with decreased overall survival in women with grade 1-2 stage IB disease who underwent lymphadenectomy (5-year rates for patient declination vs postoperative radiotherapy, 80.9% vs 88.9%, P = 0.025; Fig. 3A). In the group of women with grade 1-2 stage IB disease who did not undergo pelvic lymphadenectomy, clinical significance of patient declination for postoperative radiotherapy was more eminent (57.0% vs 85.0%, P < 0.001; Fig. 3B). For grade 3 stage IA-IB disease, women who declined postoperative radiotherapy had a significantly lower 5-year overall survival rate compared to those who received radiotherapy in the lymphadenectomy cases (71.0% vs 80.8%, P = 0.003; Fig. 3C) and the non-lymphadenectomy cases (40.1% vs 70.9%, P < 0.001; Fig. 3D).
FIGURE 3.
Survival curves for overall survival. Log-rank test for P-value. Survival curves were constructed per the Kaplan-Meier method for overall survival for grade 1-2 stage IB disease with lymphadenectomy (panel A), grade 1-2 stage IB disease without lymphadenectomy (panel B), grade 3 stage IA-IB disease with lymphadenectomy (panel C), and grade 3 stage IA-IB without lymphadenectomy (panel D). LND, pelvic lymphadenectomy
On multivariable analysis controlling for patient demographics, tumor characteristics, and treatment patterns in the entire cohort (Table 3), patient declination for postoperative radiotherapy remained an independent prognostic factor for decreased overall survival compared to the postoperative radiotherapy (adjusted-HR 1.71, 95%CI 1.44-2.02, P < 0.001). Additionally, older age, single marital status, higher grade tumor, higher stage disease, large tumor size, and no lymphadenectomy were independently associated with decreased cause-specific survival (all, adjusted-P < 0.05; Table 3).
TABLE 3.
Multivariable analysis of overall survival with endometrial cancer (N = 10 613)
| Survival (%) |
Multivariable |
||||
|---|---|---|---|---|---|
| Characteristics | No. | 5-yr | 10-yr | HR (95%CI) | P-value |
| Age (y) | |||||
| <60 | 2859 | 91.0 | 84.0 | 1 | |
| ≥60 | 7754 | 79.5 | 60.0 | 2.86 (2.58-3.18) | <0.001 |
| Ethnicity | |||||
| White | 8656 | 82.6 | 65.9 | 1 | |
| Non-White | 1957 | 82.5 | 67.8 | 1.00 (0.91-1.11) | 0.96 |
| Marital status | |||||
| Single | 1373 | 85.6 | 70.9 | 1.20 (1.07-1.36) | 0.02 |
| Married | 5529 | 85.3 | 71.8 | 1 | |
| Others | 3711 | 77.5 | 56.7 | 1.47 (1.37-1.58) | <0.001 |
| Registry area | |||||
| West | 4975 | 82.1 | 65.4 | 0.96 (0.88-1.04) | 0.27 |
| Central | 2513 | 81.5 | 64.7 | 1 | |
| East | 3125 | 84.1 | 69.0 | 0.92 (0.84-1.02) | 0.10 |
| Year at diagnosis | |||||
| Before 2000 | 2873 | 80.6 | 63.2 | 1 | |
| 2000 and after | 7740 | 83.5 | 68.3 | 1.01 (0.92-1.10) | 0.90 |
| Grade | |||||
| 1-2 | 5783 | 86.9 | 68.3 | 1 | |
| 3 | 4830 | 77.5 | 63.6 | 1.73 (1.59-1.88) | <0.001 |
| Stage | |||||
| IA | 2803 | 81.8 | 68.8 | 1 | |
| IB | 7810 | 82.8 | 65.0 | 1.61 (1.45-1.78) | <0.001 |
| Size (cm) | |||||
| <2.0 | 732 | 88.0 | 74.8 | 1 | |
| ≥2.0 | 5786 | 81.9 | 65.4 | 1.25 (1.09-1.44) | 0.002 |
| Unknown | 4095 | 82.4 | 65.6 | 1.17 (1.02-1.35) | 0.03 |
| Surgery type | |||||
| Simple hyst | 8883 | 82.8 | 66.3 | 1 | |
| Others | 1730 | 81.4 | 65.2 | 1.11 (1.02-1.22) | 0.02 |
| Pelvic lymphadenectomy | |||||
| Performed | 2991 | 84.6 | 69.5 | 1 | |
| Not performed | 7358 | 79.5 | 61.2 | 1.37 (1.26-1.48) | <0.001 |
| Unknown | 264 | 69.5 | 53.0 | 1.62 (1.38-1.90) | <0.001 |
| Adjuvant radiotherapy | |||||
| Received | 10 290 | 83.0 | 66.8 | 1 | |
| Declined | 323 | 67.4 | 47.0 | 1.71 (1.44-2.02) | <0.001 |
hyst, hysterectomy; HR, hazard ratio; CI, confidence interval. Results of a Cox proportional hazard regression model for multivariable analysis are shown. All the listed covariates were adjusted for collected covariates. Significant P-values are emboldened.
There were 5715 women who met the inclusion criteria for the PORTEC-1 trial. There were 277 women who declined the adjuvant radiotherapy. Women who declined adjuvant radiotherapy had a significantly decreased cause-specific survival compared to those who received whole pelvic radiotherapy (5-year rates, 82.3% vs 87.7%, P = 0.016). On multivariable analysis, patient declination remained an independent predictor for decreased cause-specific survival compared to whole pelvic radiotherapy (adjusted-HR 1.58, 95%CI 1.16-2.15, P = 0.004; Table S1). Similar results were observed for overall survival (Table S1).
4 ∣. DISCUSSION
Adherence to evidence-based treatment guidelines is a prognostic factor for cancer patients. For instance, in ovarian cancer, adherence to guideline-based treatment for primary surgery and postoperative chemotherapy is associated with improved survival.20 A similar study in cervical cancer also showed decreased survival in patients who were not treated according to guidelines.21 Both the care provider-side and the patient-side can be factored for the guideline adherence, and these possible guideline adherent factors include hospital volume, patient medical comorbidities, and patient non-compliance.20,22 In endometrial cancer, prior studies mainly examined guideline adherence to surgical treatment recommendation or mixed with other malignancies, and no study has solely examined the association of patient non-compliance for postoperative radiotherapy and survival.23-29
Our results of decreased survival in non-compliant women with endometrial cancer for postoperative radiotherapy are consistent with past studies reporting decreased survival related to non-compliance to radiotherapy shown in other malignancies.6,7,29 Therefore, our study not only validates their results but also endorses that patient compliance for radiotherapy is an important factor for prognosis of women with endometrial cancer.
Various studies have tried to identify the predictors for patient non-compliance for radiotherapy. In one large-scale study that examined patients who received external bream radiotherapy, diagnosis of endometrial cancer was reported as a strong predictor for non-compliance.30 Low socioeconomic status and prolonged treatment fractions were also found to be the predictors for radiotherapy non-compliance.29-31 In a tumor registry study for early-stage endometrial cancer, having multiple medical comorbidities significantly reduced the likelihood of receiving postoperative radiotherapy.23 In our study, old age was associated with increased risk of radiotherapy declination. Because older women with endometrial cancer are more likely to have multiple medical comorbidities,32 this can be a possible indirect causality of our findings. However, lack of information for medical comorbidities, detailed socioeconomic status, or other pertinent information such as geography/distance to radiation center, and social deprivation in this database limited the ability to examine the true association of age and patient declination.
Our results showed that women who declined postoperative radiotherapy were less likely to undergo lymphadenectomy. Per the current guidelines, a proportion of women in our study population would likely have been recommended to undergo comprehensive lymphadenectomy.2 Therefore, there is a possibility that those women who declined postoperative radiotherapy were also more likely to decline other guideline-recommended treatment intervention such as lymphadenectomy or chemotherapy, as demonstrated in other malignancies.7 Chemotherapy might improve survival when there is a conglomerate of high risk factors present such as high-grade disease with deep invasion.3 This database does not have information for chemotherapy but adjuvant chemotherapy is generally not considered as the standard for stage I endometrioid type of endometrial cancer. In this study, we did not include cases with high-grade non-endometrioid tumor and/or stage II-IV disease in order to eliminate the effect of chemotherapy.
Definition of patient non-compliance to radiotherapy varies across the studies from missing multiple fractions to declination.6,30 In our study, it is likely that those women who were coded as “refused” for radiotherapy were most likely recommended postoperative radiotherapy by care providers after hysterectomy-based surgery but did not receive any radiotherapy. However, it was unknown if these patients initially received a certain fraction of radiotherapy then declined in the middle of the radiotherapy course due to an adverse event. Similarly, among women who were coded as undergoing radiotherapy treatment, it was unknown it these patients completed the whole treatment session. Therefore, there may be a possible misclassification in our study.
Historically, postoperative radiotherapy has been shown to reduce the risk of pelvic recurrence but not improve cause-specific survival of women with early-stage endometrial cancer.33 Our results validated this concept of postoperative radiotherapy in early-stage endometrial cancer in that cause-specific survival of women who declined postoperative radiotherapy was similar compared to those who received postoperative radiotherapy in grade 1-2 stage IB disease with use of lymphadenectomy. However, of our interest, patient declination of postoperative radiotherapy was an independent prognostic factor for increased all-cause mortality compared to postoperative radiotherapy in the same group. This may possibly imply that women who declined postoperative radiotherapy were more likely to be non-adherent to treatment recommendation for other medical condition resulting in deaths from non-endometrial cancer reasons. As aforementioned, low socioeconomic status and medical comorbidities are likely the causality of this association and further study will be warranted.
The landmark studies composing the current ASTRO practice guidelines for adjuvant radiotherapy in the treatment of women with stage I endometrial cancer include the PORTEC-1 trial and the GOG-99 trial.14,33 In the current study, we performed a sub-analysis per the PORTEC-1 criteria because the ASTRO guidelines and PORTEC-1 have slightly different recommendation criteria for adjuvant radiotherapy and all the factors were available in the database. For instance, the ASTRO guidelines do not factor patient age while the PORTEC-1 trial does. That is, a patient with age younger than 60 years with grade 1-2 stage IB disease or a patient with younger than 60 years with grade 3 stage IA disease do not meet the PORTEC-1 criteria but meet the ASTRO guideline criteria. In both analytic approaches, we found that patient declination to adjuvant radiotherapy is associated with decreased survival outcome, endorsing an importance of patient compliance to adjuvant radiotherapy in the management of women with stage I endometrioid endometrial cancer.
Another limitation and weakness of our study is that we were not able to assess which type of radiotherapy the patient indeed declined to receive. While vaginal brachytherapy is the preferred radiotherapy modality in grade 1-2 stage IB disease, whole pelvic radiotherapy is also recognized as an alternative treatment.4 This database recodes the modality of radiotherapy that the patient received, however, there is no information for which type of radiation the patient was offered and declined to receive. Therefore, the rationale of patient declination per the radiotherapy type was not able to evaluate in this study.
In addition, this study spans more than few decades, and there is a practice pattern change in both lymphadenectomy and postoperative radiotherapy during time which may impact patient declination.11 Lastly, this database does not have certain tumor information such as lymphovascular space invasion that can impact on radiotherapy recommendation. For instance, the GOG-99 trial demonstrated that lymphovascular space invasion is one of three factors for the high-intermediate risk group where the radiotherapy is recommended.14 Therefore, we were not able to examine the significance of patient declination for adjuvant radiotherapy per their criteria in this study. Strengths of our study include a homogenous study population limited to stage I endometrioid type endometrial cancer chosen per the guideline-based criteria and reproduced findings across sub-groups.
A clinical implication of the study is to reassert the importance of patient education/counseling among patients at risk of non-compliance. Spending adequate time to discuss the rationale of treatment goals, involvement of patient family or other support, removing unrevealed psychosocial barriers between care provider and patient, and arranging communication between patients may be useful strategies to improve patient treatment compliance.
5 ∣. CONCLUSION
Women who are older, non-White, Western U.S. residents, or diagnosed in more recent years were more likely to decline postoperative radiotherapy. Moreover, women with stage I endometrioid endometrial cancer who declined guideline-based postoperative radiotherapy had a decreased survival compared to those who received the adjuvant radiotherapy.
Supplementary Material
Acknowledgments
Funding information
Ensign Endowment for Gynecologic Cancer Research
Footnotes
DISCLOSURE
There is no conflict of interest in all the authors.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Siegel RL, Miller MD, Jemal A. Cancer statistics. CA Cancer J Clin. 2017;2017:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Uterine neoplasms. NCCN Clinical Practice Guidelines in Oncology. Available at: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (accessed on 12/18/2016). [Google Scholar]
- 3.Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet. 2012; 379:1352–1360. [DOI] [PubMed] [Google Scholar]
- 4.Klopp A, Smith BD, Alektiar K, et al. The role of postoperative radiation therapy for endometrial cancer: executive summary of an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2014;4:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer LA, Bohlke K, Powell MA, et al. Postoperative radiation therapy for endometrial cancer: American Society of Clinical Oncology clinical practice guideline endorsement of the american society for radiation oncology evidence-based guideline. J Clin Oncol. 2015;33:2908–2913. [DOI] [PubMed] [Google Scholar]
- 6.Chu QD, Caldito G, Miller JK, Townsend B. Postmastectomy radiation for N2/N3 breast cancer: factors associated with low compliance rate. J Am Coll Surg. 2015;220:659–669. [DOI] [PubMed] [Google Scholar]
- 7.Badakhshi H, Gruen A, Sehouli J, Budach V, Boehmer D. The impact of patient compliance with adjuvant radiotherapy: a comprehensive cohort study. Cancer Med. 2014;2:712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franciosi V, Fumagalli M, Biscari L, et al. Compliance and outcomes in locally advanced head and neck cancer patients treated with alternating chemo-radiotherapy in clinical practice. Tumori. 2003;89:20–25. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer statistics. Available at: https://seer.cancer.gov/registries/ (accessed on 12/19/2016). [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ (Clinical Research ed) 2007; 335:806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuo K, Machida H, Ragab OM, Takiuchi T, Pham HQ, Roman LD. Extent of pelvic lymphadenectomy and use of adjuvant vaginal brachytherapy for early-stage endometrial cancer. Gynecol Oncol. 2017;144:515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuo K, Machida H, Shoupe D, et al. Ovarian conservation and overall survival in young women with early-stage low-grade endometrial cancer. Obstet Gynecol. 2016;128:761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355:1404–1411. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 17.Mansfield ER, Helms BP. Detecting multicollinearity. Am Stat. 1982;36:158–160. [Google Scholar]
- 18.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. [DOI] [PubMed] [Google Scholar]
- 19.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bristow RE, Chang J, Ziogas A, Anton-Culver H. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet Gynecol. 2013;121:1226–1234. [DOI] [PubMed] [Google Scholar]
- 21.Chiew KL, Chong S, Duggan KJ, Kaadan N, Vinod SK. Assessing guideline adherence and patient outcomes in cervical cancer. Asia Pac J Clin Oncol. 2016. in-press. [DOI] [PubMed] [Google Scholar]
- 22.Erickson BK, Martin JY, Shah MM, Straughn JM Jr., Leath CA 3rd. Reasons for failure to deliver National Comprehensive Cancer Network (NCCN)-adherent care in the treatment of epithelial ovarian cancer at an NCCN cancer center. Gynecol Oncol. 2014; 133:142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boll D, Verhoeven RH, van der Aa MA, Lybeert ML, Coebergh JW, Janssen-Heijnen ML. Adherence to national guidelines for treatment and outcome of endometrial cancer stage I in relation to comorbidity in southern Netherlands 1995–2008. Eur J Cancer. 2011; 47:1504–1510. [DOI] [PubMed] [Google Scholar]
- 24.van Lankveld MA, Koot NC, Peeters PH, van Leeuwen JS, Jurgenliemk-Schulz IM, van Eijkeren MA. Compliance to surgical and radiation treatment guidelines in relation to patient outcome in early stage endometrial cancer. J Eval Clin Pract. 2006;12:196–201. [DOI] [PubMed] [Google Scholar]
- 25.Bakkum-Gamez JN, Mariani A, Dowdy SC, et al. The impact of surgical guidelines and periodic quality assessment on the staging of endometrial cancer. Gynecol Oncol. 2011;123:58–64. [DOI] [PubMed] [Google Scholar]
- 26.du Bois A, Strutas D, Buhrmann C, et al. Impact of treatment guidelines and implementation of a quality assurance program on quality of care in endometrial cancer. Onkologie. 2009;32:493–498. [DOI] [PubMed] [Google Scholar]
- 27.Mandato VD, Formisano D, Pirillo D, et al. Province wide clinical governance network for clinical audit for quality improvement in endometrial cancer management. Int J Gynecol Cancer. 2011;22:94–100. [DOI] [PubMed] [Google Scholar]
- 28.Battista MJ, Steiner E, Rieks N, et al. Nationwide analysis on surgical staging procedures and systemic treatment for patients with endometrial cancer in Germany. Int J Gynecol Cancer. 2012;23: 105–112. [DOI] [PubMed] [Google Scholar]
- 29.Ohri N, Rapkin BD, Guha C, Kalnicki S, Garg M. Radiation therapy noncompliance and clinical outcomes in an urban academic cancer center. Int J Radiat Oncol Biol Phys. 2016;95:563–570. [DOI] [PubMed] [Google Scholar]
- 30.Ohri N, Rapkin BD, Guha D, et al. Predictors of radiation therapy noncompliance in an urban academic cancer center. Int J Radiat Oncol Biol Phys. 2015;91:232–238. [DOI] [PubMed] [Google Scholar]
- 31.Arrossi S, Matos E, Zengarini N, Roth B, Sankaranayananan R, Parkin M. The socio-economic impact of cervical cancer on patients and their families in Argentina, and its influence on radiotherapy compliance. Results from a cross-sectional study. Gynecol Oncol. 2007;105: 335–340. [DOI] [PubMed] [Google Scholar]
- 32.Doo DW, Guntupalli SR, Corr BR, et al. Comparative surgical outcomes for endometrial cancer patients 65 years old or older staged with robotics or laparotomy. Ann Surg Oncol. 2015;22:3687–3694. [DOI] [PubMed] [Google Scholar]
- 33.Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.