Abstract
Background
Brain metastases (BM) cause symptoms that supportive medications can alleviate. We assessed whether racial disparities exist in supportive medication utilization after BM diagnosis.
Methods
Medicare-enrolled patients linked with the Surveillance, Epidemiology, and End Results program (SEER) who had diagnoses of BM between 2007 and 2016 were identified. Fourteen supportive medication classes were studied: non-opioid analgesics, opioids, anti-emetics, anti-epileptics, headache-targeting medications, steroids, cognitive aids, antidepressants, anxiolytics, antidelirium/antipsychotic agents, muscle relaxants, psychostimulants, sleep aids, and appetite stimulants. Drug administration ≤30 days following BM diagnosis was compared by race using multivariable logistic regression.
Results
Among 17,957 patients, headache aids, antidepressants, and anxiolytics were prescribed less frequently to African Americans (odds ratio [95% CI] = 0.81 [0.73–0.90], P < 0.001; OR = 0.68 [0.57–0.80], P < 0.001; and OR = 0.68 [0.56–0.82], P < 0.001, respectively), Hispanics (OR = 0.83 [0.73–0.94], P = 0.004 OR = 0.78 [0.64–0.97], P = 0.02; and OR = 0.63 [0.49–0.81], P < 0.001, respectively), and Asians (OR = 0.81 [0.72–0.92], P = 0.001, OR = 0.67 [0.53–0.85], P = 0.001, and OR = 0.62 [0.48–0.80], P < 0.001, respectively) compared with non-Hispanic Whites. African Americans also received fewer anti-emetics (OR = 0.75 [0.68–0.83], P < 0.001), steroids (OR = 0.84 [0.76–0.93], P < 0.001), psychostimulants (OR = 0.14 [0.03–0.59], P = 0.007), sleep aids (OR = 0.71 [0.61–0.83], P < 0.001), and appetite stimulants (OR = 0.85 [0.77–0.94], P = 0.002) than Whites. Hispanic patients less frequently received antidelirium/antipsychotic drugs (OR = 0.57 [0.38–0.86], P = 0.008), sleep aids (OR = 0.78 [0.64–0.94, P = 0.01), and appetite stimulants (OR = 0.87 [0.76–0.99], P = 0.04). Asian patients received fewer opioids (OR = 0.86 [0.75–0.99], P = 0.04), anti-emetics (OR = 0.83 [0.73–0.94], P = 0.004), anti-epileptics (OR = 0.83 [0.71–0.97], P = 0.02), steroids (OR = 0.81 [0.72–0.92], P = 0.001), muscle relaxants (OR = 0.60 [0.41–0.89], P = 0.01), and appetite stimulants (OR = 0.87 [0.76–0.99], P = 0.03). No medication class was prescribed significantly less frequently to Whites.
Conclusions
Disparities in supportive medication prescription for non-White/Hispanic groups with BM exist; improved provider communication and engagement with at-risk patients is needed.
Key Points
1. Patients with BM commonly experience neurologic symptoms.
2. Supportive medications improve quality of life among patients with BM.
3. Non-White patients with BM receive fewer supportive medications than White patients.
Keywords: supportive care, brain metastases, pain, race, disparities, medications, palliative care
Importance of the Study.
BM are associated with significant neurologic symptomatology with potential for deleterious impact on patient quality of life. Supportive medications have a significant role in the alleviation of such symptoms, and an increasing body of literature has linked effective palliative care/symptom management to improved oncologic outcomes. To our knowledge, there have been no previous studies that have specifically addressed pharmacologic symptom management among patients with BM on a population-based level. In this SEER-Medicare study of 17,957 patients with BM, we found that African American, Hispanic, and Asian patients were generally less likely to receive opioids, anti-emetics, anti-epileptics, steroids, antidepressants, anxiolytics, antidelirium/antipsychotic agents, muscle relaxants, psychostimulants, sleep aids, and appetite stimulants compared with non-Hispanic white patients. Given the link between effective palliative care and improved oncologic outcomes, our work highlights the need for practice-based changes to identify and mitigate disparities in supportive medication prescription.
Brain metastases (BM) occur in approximately 10–40% of patients with cancer and represent a significant source of mortality and morbidity.1,2 Common symptoms in patients with BM include headaches, nausea, fatigue, anorexia, anxiety, depression, mental status changes, cognitive decline, insomnia, seizures, and focal neurologic deficits.3,4 Moreover, local therapies for BM, including surgery and radiation, which are commonly utilized given the guarded efficacy of most systemic agents, can be associated with significant complications and symptomatology.5,6 Prompt recognition and effective management of symptoms is essential for optimizing patient quality of life and potentially more significant oncologic outcomes; several randomized studies have demonstrated that modalities promoting palliation of symptoms in patients with advanced cancer yield improvements in survival.7–9 While it is known that racial disparities exist with respect to cancer-related outcomes,10,11 recent studies have suggested that such disparities may also extend to management of symptoms, alleviation of pain, and access to psychiatric care.12,13
Older patients with BM represent a population for whom supportive care is especially important. Elderly patients may be particularly vulnerable to the sequelae of disease complications and treatment-related side effects given their baseline neurologic and functional status and reduced ability to tolerate therapy.14 To our knowledge, no prior population-based studies assessing utilization of supportive medications among patients with BM have been published. We therefore sought to evaluate the prevalence of supportive medication utilization among Medicare patients with newly diagnosed BM and assess whether racial disparities exist with regard to prescription of such medications.
Materials and Methods
Patient Population and Study Design
The Surveillance, Epidemiology, and End Results (SEER) program captures information from 34.6% of the population with cancer in the United States15 and publishes information on demographics, clinical parameters, treatment, and survival. The SEER-Medicare database links Medicare claims files to SEER data for approximately 93% of Medicare enrollees in SEER cancer registries.16
We used the SEER-Medicare database to identify patients with a diagnosis of BM between 2007 and 2016, the most recent year that SEER-Medicare data have been released. Data relating to prescription of medications were derived from Part D claims, which are available from 2007–2016. We included patients with 3 or more claims associated with a diagnosis code from the International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification, for secondary malignant neoplasm of the brain, cerebral meninges, and spinal cord (ICD-9-CM 198.3; ICD-10-CM C79.31, ICD-10-CM 79.32), an approach associated with a 97% sensitivity and 99% specificity when using claims data to identify patients with BM.17 We further limited our cohort to patients with Part D coverage in the year of BM diagnosis (N = 20,395). We subsequently excluded patients with diagnoses at autopsy or death certificate or for whom the date of death varied by greater than 3 months when comparing SEER and Medicare files (N = 2,356). Patients for whom race was unknown were also excluded (N = 17), leaving 18,022 patients in the cohort. Race was subdivided into non-Hispanic White (which will be referred to simply as “White” in succeeding sections), African American, Hispanic, Asian/Pacific Islander, and other. We removed the “other” race category (N = 65) given that many cells in our tables had ≤10 patients and therefore could not be presented per SEER-Medicare policy. The final cohort therefore consisted of 17,957 patients.
To determine the date of BM diagnosis, we used the date of the first ICD-9-CM/ICD-10-CM code for secondary malignant neoplasm of the brain, cerebral meninges, or spinal cord, a previously validated approach associated with a 92% sensitivity for predicting the actual date of BM diagnosis to within 30 days.18
Statistical Methodology
We assessed 14 separate classes of supportive medications, including non-opioid analgesics, opioids, anti-emetics, anti-epileptics, headache-targeting medications, steroids, cognitive aids, antidepressants, anxiolytics, antidelirium/antipsychotic agents, muscle relaxants, psychostimulants, sleep aids, and appetite stimulants. A complete list of the 14 classes of medications we evaluated, as well as the specific medications used to subpopulate each category, is provided in Supplementary Table 1. We favored an inclusive approach when delineating medications that could potentially be used for palliation of a specific symptom. The list was generated through an extensive review of the literature and carefully modified by a palliative care physician (K.A.L.). The presence of any Part D claim in the first month following BM diagnosis for a medication in Supplementary Table 1 was deemed to be an indication that the medication was prescribed shortly after a diagnosis of BM.
Our objective was to characterize the relationship between race and receipt of supportive medications in the first month following the diagnosis of BM using multivariable logistic regression. We used multivariable models adjusted for age, sex, marital status, ZIP code–level high school completion rate and median household income, year of BM diagnosis, Charlson comorbidity index (CCI) as assessed by the Deyo et al method,19 and primary tumor site to estimate the adjusted association between race and receipt of a particular class of supportive medication. In order to control for patients who were on a particular medication prior to their diagnosis of BM, we also included a covariate depicting whether the patient received the class of medication in question in the 2 months prior to diagnosis.
Baseline categorical covariates were compared across racial subgroups with the chi-square test. Normally distributed and nonnormally distributed continuous covariates were compared by race using ANOVA and the Kruskal–Wallis tests, respectively. Individual logistic models were created for each class of supportive medications mentioned above. Interaction models with a term linking racial subgroup and year of BM diagnosis were also generated for each class of medication to evaluate changes in medication utilization over time by race. Analyses were performed using SAS v9.4. A two-sided P < 0.05 was considered statistically significant. This study was approved by our institutional review board.
Results
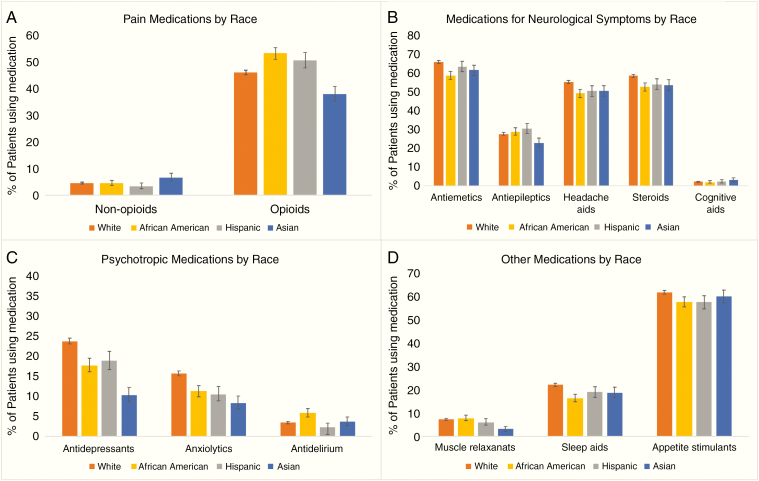
Baseline characteristics for patients stratified by race are depicted in Table 1. There were significant differences among racial subgroups in age, sex, marital status, education, income, year of diagnosis, CCI, and primary tumor site (Table 1). Prevalence of supportive medication use in the first 30 days after diagnosis of BM, stratified by race and medication class, is presented in Figure 1 and Table 2. There were significant differences in receipt of non-opioid analgesics, opioids, anti-emetics, anti-epileptics, headache aids, steroids, antidepressants, anxiolytics, antidelirium/antipsychotic drugs, muscle relaxants, sleep aids, psychostimulants, and appetite stimulants by race (all P < 0.05). There were no significant differences in use of headache aids or cognitive aids by race.
Table 1.
Clinical and demographic characteristics of patients with BM by race
| White (n = 13 511) | African American (n = 2046) | Hispanic (n = 1183) | Asian (n = 1217) | P | |
|---|---|---|---|---|---|
| Age at BM diagnosis, median (interquartile range [IQR]) | 72 (67–77) | 68 (63–74) | 71 (66–77) | 73 (68–78) | <0.001 |
| Sex, no. (%) | <0.001 | ||||
| Female | 7898 (58) | 1326 (65) | 729 (62) | 636 (52) | |
| Male | 5613 (42) | 720 (35) | 454 (38) | 581 (48) | |
| Marital status, no. (%) | <0.001 | ||||
| Married/partnered | 7099 (53) | 556 (27) | 537 (45) | 768 (63) | |
| Unmarried/single | 5715 (42) | 1377 (67) | 583 (49) | 394 (32) | |
| Unknown | 697 (5) | 113 (6) | 63 (5) | 55 (5) | |
| Graduated from high school, a median % (IQR) | 87 (79–93) | 79 (71–85) | 78 (64–87) | 85 (74–91) | <0.001 |
| Household income (per 10K USD), a median (IQR) | 5.2 (3.8–7.0) | 3.6 (2.6–4.8) | 4.6 (3.6–6.1) | 5.6 (4.5–7.7) | <0.001 |
| Residence, no. (%) | <0.001 | ||||
| Urban | 11,819 (87) | 1875 (92) | 1146 (97) | 1213 (100) | |
| Non-urban/unknown | 1692 (13) | 212 (12)b | |||
| Year of BM diagnosis, median (IQR) | 2012 (2010–2014) | 2012 (2009–2014) | 2012 (2010–2014) | 2012 (2010–2014) | 0.02 |
| Charlson comorbidity index, c no. (%) | <0.001 | ||||
| 0–2 | 10,086 (75) | 1367 (67) | 826 (70) | 897 (74) | |
| >2 | 2235 (17) | 520 (25) | 236 (20) | 167 (14) | |
| Unknown | 1190 (9) | 159 (8) | 121 (10) | 153 (13) | |
| Primary tumor site, no. (%) | <0.001 | ||||
| Lung | 7026 (52) | 1099 (54) | 534 (45) | 789 (65) | |
| Breast | 1341 (10) | 369 (18) | 211 (18) | 98 (8) | |
| Melanoma | 588 (4) | 54 (4)b | |||
| Otherd | 1096 (8) | 143 (7) | 167 (14) | 97 (8) | |
| Multiple primaries | 3460 (26) | 428 (21) | 237 (20) | 220 (18) |
aZIP code-level.
bNon-White races were grouped together so as to comply with NCI data policy of not displaying any cells with values ≤10.
cExcluded diagnosis of metastatic cancer so as not to inflate all scores by 6 points.
dIncludes esophageal, testicular, ovarian, kidney, and colon as primary site.
Fig. 1.
Receipt of supportive medications in the first month following diagnosis of BM by class of medication and race: (A) pain medications, (B) medications for neurological symptoms, (C) psychotropic medications, and (D) other medications. Due to NCI data policies that mandate figures do not display results relevant to ≤10 patients, we could not show data on psychostimulants in the figure. Comparisons by racial subgroup via the chi-square test resulted in a P-value less than 0.05 for every class of medications except for cognitive aids.
Table 2.
Prevalence of supportive medication prescription following diagnosis of BM
| White (n = 13 511) | African American (n = 2046) | Hispanic (n = 1183) | Asian (n = 1217) | P | |
|---|---|---|---|---|---|
| Pain medications, no. (%) | |||||
| Non-opioid analgesics | 615 (5) | 93 (5) | 40 (3) | 81 (7) | 0.001 |
| Opioids | 6237 (46) | 1091 (53) | 601 (51) | 464 (38) | <0.001 |
| Medications for neurological symptoms, no. (%) | |||||
| Anti-emetics | 8935 (66) | 1206 (59) | 754 (64) | 750 (62) | <0.001 |
| Anti-epileptics | 3753 (28) | 593 (29) | 361 (31) | 280 (23) | <0.001 |
| Headache aids | 7488 (55) | 1008 (49) | 598 (51) | 616 (51) | <0.001 |
| Steroids | 7924 (59) | 1079 (53) | 642 (54) | 655 (54) | <0.001 |
| Cognitive aids | 295 (2) | 42 (2) | 27 (2) | 38 (3) | 0.18 |
| Psychotropic medications, no. (%) | |||||
| Antidepressants | 3177 (24) | 357 (17) | 220 (19) | 124 (10) | <0.001 |
| Anxiolyticsa | 2096 (16) | 226 (11) | 123 (10) | 100 (8) | <0.001 |
| Antidelirium/antipsychotic agents | 454 (3) | 117 (6) | 26 (2) | 43 (4) | <0.001 |
| Other, no. (%) | |||||
| Muscle relaxants | 987 (7) | 161 (8) | 72 (6) | 37 (3) | <0.001 |
| Sleep aids | 2988 (22) | 337 (16) | 225 (19) | 230 (19) | <0.001 |
| Appetite stimulants | 8403 (62) | 1188 (58) | 685 (58) | 735 (60) | <0.001 |
aBenzodiazepines were not covered by Part D until 2013.
Note: Due to NCI data policies that mandate tables do not contain cells with ≤10 patients, we could not show data on psychostimulants in the table above.
In the adjusted primary models examining receipt of each class of supportive medication, several significant racial discrepancies were noted (Table 3). Compared with White patients, African Americans were less likely to receive anti-emetics (odds ratio [95% CI] 0.75 [0.68–0.83], P < 0.001), headache aids (OR = 0.81 [0.73–0.90], P < 0.001), steroids (OR 0.84 [0.76–0.93], P < 0.001), antidepressants (OR 0.68 [0.57–0.80], P < 0.001), anxiolytics (OR 0.68 [0.56–0.82], P < 0.001), psychostimulants (OR 0.14 [0.03–0.59], P = 0.007), sleep aids (OR 0.71 [0.61–0.83], P < 0.001), and appetite stimulants (OR 0.85 [0.77–0.94], P = 0.002). Compared with White patients, Hispanic patients were less likely to receive antidepressants (OR 0.78 [0.64–0.97], P = 0.02), anxiolytics (OR 0.63 [0.49–0.81], P < 0.001), headache aids (OR = 0.83 [0.73–0.94], P = 0.004), antidelirium/antipsychotic drugs (OR 0.57 [0.38–0.86], P = 0.008), sleep aids (OR 0.78 [0.64–0.94, P = 0.01), and appetite stimulants (OR 0.87 [0.76–0.99, P = 0.04), and Asian patients were less likely to receive opioids (OR 0.86 [0.75–0.99], P = 0.04), anti-emetics (OR 0.83 [0.73–0.94], P = 0.004), anti-epileptics (OR 0.83 [0.71–0.97], P = 0.02), headache aids (OR = 0.81 [0.72–0.92], P = 0.001), steroids (OR 0.81 [0.72–0.92], P = 0.001), antidepressants (OR 0.67 [0.53–0.85], P = 0.001), anxiolytics (OR 0.62 [0.48–0.80], P < 0.001), muscle relaxants (OR 0.60 [0.41–0.89], P = 0.01), and appetite stimulants (OR 0.87 [0.76–0.99], P = 0.03). We could not identify a class of medication that was prescribed less commonly to white patients compared with any nonwhite race.
Table 3.
Adjusteda use of supportive care medications following diagnosis of BM by race (OR = odds ratio)
| White | African American OR [95% CI] | P | Hispanic OR [95% CI] | P | Asian OR [95% CI] | P | |
|---|---|---|---|---|---|---|---|
| Pain medications | |||||||
| Non-opioid analgesics | Ref | 0.89 [0.66–1.20] | 0.44 | 0.69 [0.46–1.05] | 0.08 | 0.97 [0.70–1.34] | 0.86 |
| Opioids | Ref | 0.96 [0.85–1.08] | 0.48 | 1.09 [0.94–1.26] | 0.24 | 0.86 [0.75–0.99] | 0.04 |
| Medications for neurological symptoms | |||||||
| Anti-emetics | Ref | 0.75 [0.68–0.83] | <0.001 | 0.91 [0.80–1.04] | 0.16 | 0.83 [0.73–0.94] | 0.004 |
| Anti-epileptics | Ref | 1.04 [0.92–1.18] | 0.49 | 1.11 [0.95–1.29] | 0.19 | 0.83 [0.71–0.97] | 0.02 |
| Headache aids | Ref | 0.81 [0.73–0.90] | <0.001 | 0.83 [0.73–0.94] | 0.004 | 0.81 [0.72–0.92] | 0.001 |
| Steroids | Ref | 0.84 [0.76–0.93] | <0.001 | 0.90 [0.80–1.03] | 0.12 | 0.81 [0.72–0.92] | 0.001 |
| Cognitive aids | Ref | 0.78 [0.49–1.23] | 0.28 | 1.17 [0.69–1.99] | 0.56 | 1.04 [0.64–1.70] | 0.88 |
| Psychotropic medications | |||||||
| Antidepressants | Ref | 0.68 [0.57–0.80] | <0.001 | 0.78 [0.64–0.97] | 0.02 | 0.67 [0.53–0.85] | 0.001 |
| Anxiolytics | Ref | 0.68 [0.56–0.82] | <0.001 | 0.63 [0.49–0.81] | <0.001 | 0.62 [0.48–0.80] | <0.001 |
| Antidelirium/antipsychotic agentsb | Ref | 1.21 [0.97–1.52] | 0.09 | 0.57 [0.38–0.86] | 0.008 | 1.23 [0.88–1.70] | 0.22 |
| Other | |||||||
| Muscle relaxants | Ref | 0.92 [0.72–1.16] | 0.48 | 0.85 [0.62–1.16] | 0.30 | 0.60 [0.41–0.89] | 0.01 |
| Psychostimulantsb,c | Ref | 0.14 [0.03–0.59] | 0.007 | 0.15 [0.02–1.07] | 0.06 | 0.93 [0.40–2.17] | 0.87 |
| Sleep aids | Ref | 0.71 [0.61–0.83] | <0.001 | 0.78 [0.64–0.94] | 0.01 | 1.00 [0.83–1.20] | 0.98 |
| Appetite stimulants | Ref | 0.85 [0.77–0.94] | 0.002 | 0.87 [0.76–0.99] | 0.04 | 0.87 [0.76–0.99] | 0.03 |
aCovariates were adjusted for age, sex, marital status, ZIP code level high school completion rate and median household income, year of BM diagnosis, Charlson comorbidity index, and primary tumor site.
b,cCovariates for use of prior medication (b) and melanoma as primary cancer site (c) were removed from select models due to nonconvergence.
When assessing trends over time, the only class of medication for which racial disparities appeared to be decreasing was steroid utilization for African Americans relative to White patients (P-interaction = 0.02). While there were no differences across racial subgroups in receipt of cognitive aids found above, African Americans tended to be less likely to receive cognitive aids compared with White patients over time (P-interaction = 0.05).
Discussion
Using prescription claims data across a large, national sample of patients with cancer, we identified significant racial disparities in the use of supportive medications for patients with newly diagnosed BM. African American, Hispanic, or Asian patients were generally less likely to receive opioids, anti-emetics, anti-epileptics, steroids, antidepressants, anxiolytics, antidelirium/antipsychotic agents, muscle relaxants, psychostimulants, sleep aids, and appetite stimulants compared with White patients. We were not able to find a category of medication that was significantly more likely to be prescribed to non-White patients relative to White patients.
Prior studies have demonstrated substantial disparities with respect to oncologic outcomes among non-White patients relative to White patients,10 which have, in part, been explained by socioeconomic factors, disease stage at time of presentation, as well as differences in treatment strategies.11,20,21 Suboptimal management of cancer-related pain among non-White groups has also been consistently demonstrated in prior work,22 although the body of literature evaluating whether such disparities extend to the management of symptoms beyond pain is much more limited. A recent investigation found no differences in opioid prescribing but demonstrated significant racial disparity in use of non-opioid psychotropic medications to treat depression, anxiety, and insomnia among women with breast cancer, with African American women less likely than White women to receive such medications.12
To the authors’ knowledge, ours is the first study to assess disparities in the use of supportive medications among older patients with BM, a population for which supportive management is especially vital. The prognosis for such patients is generally guarded, and systemic therapy typically has limited efficacy, resulting in utilization of local therapies such as radiation and neurosurgical resection, which can both yield additional symptomatology.5,6 Oncologic management of such patients commonly focused on quality of life and supportive medications plays an important role in this capacity.
One possible explanation for the disparities noted in this study relates to cultural stigmas. Perceptions regarding mental illnesses among patients of certain cultural backgrounds may discourage successful engagement with psychiatric services.23 Epidemiological studies regarding individuals of non-Western backgrounds have shown associations between psychiatric diagnoses and feelings of shame24 or social rejection.24 Such perceptions are especially common among Asian cultures, which may, in part, account for the lower rate of antidepressant and anxiolytic medication utilization observed among Asian patients in our study. Moreover, it is thought that Asian patients are more likely to “somaticize” psychiatric symptoms and present with physical symptoms, such as bodily pains, indigestion, and insomnia, while White patients are more likely to present with affective symptoms.25 Their differential presentation highlights the need for providers to recognize such “atypical” presentations and tailor their screening evaluations for depression accordingly. Psychiatric comorbidities not only affect quality of life but have also been associated with worse oncologic outcomes,26 with randomized trials demonstrating an improvement in survival when depression is treated among cancer patients.27 Therefore, it is imperative for providers to actively screen all patients for depression and anxiety and offer treatments in line with their cultural values.
Provider-driven factors, including communication barriers between providers and non-White patients, may also explain disparities in supportive medication prescribing. Prior work on patients with limited English proficiency (LEP) has demonstrated that such patients report poorer comprehension of their conditions28 and worse symptom control.29 Even if supportive medications are being offered to LEP patients at the same rate as non-LEP patients, it is possible that LEP patients have concerns about these medications, related to side effects or risk for long-term dependence, and that providers are inadequately addressing patients’ questions due to language barriers and/or use of medical jargon.30 Communication barriers could also result in providers failing to recognize patients’ true burden of symptoms, leading to fewer prescriptions and inadequate symptom management. Despite federal regulations mandating utilization of medical interpreters, interpreters are not consistently utilized across clinical practices.31 When professional interpreters are not used, LEP patients are less likely to receive appropriate medical services and more likely to report worse symptom management and experience poorer clinical outcomes.32,33
Our data highlight the need for oncologists to screen all patients with BM for cancer- and treatment-related symptoms and carefully discuss use of supportive medications. Unlike diagnoses that can be made through laboratory data or imaging, assessment of symptom burden relies on effective communication between patients and providers. Oncologists should consider structural changes to overcome communication barriers, such as systemic integration of professional interpreters into oncology clinics, translation of common symptom-related questionnaires into different languages, and increased allotment of time for visits with LEP patients.
Discrepancies across multiple classes of supportive medications have profound implications for patients with BM. Randomized studies on patients with metastatic disease have previously shown that early symptom management not only improves patient quality of life but also results in longer survival.7,8 Undertreatment of pain and nausea are common causes for both local and systemic treatment interruptions among patients with cancer,34,35 and such interruptions have been linked to worse oncologic outcomes.36 Prompt recognition of symptoms allows for intervention before adverse consequences, such as treatment interruptions, can take place. In addition to affecting patient quality of life, poor supportive care and symptom management among non-White groups likely contribute to the gap in progression and survival-based oncologic outcomes among White and non-White cancer patients. Whether differences in prescription of supportive drugs represent unique symptom profiles among patients of different races or true disparities in management needs to be further assessed in future studies.
Prior literature suggests that racial and socioeconomic differences may influence cancer stage at initial presentation, with non-White patients being more likely than White patients to present with more advanced disease.37,38 It is conceivable that patients who present at more advanced stages may be less likely to undergo aggressive treatments due to poor prognosis, an unfavorable risk-benefit ratio, and/or poor functional status, among other factors. Such patients may instead opt for inpatient hospice, in which case medications they receive may not be captured by Medicare Part D claims. Moreover, given that several of the medications we examined are routinely administered in the peritreatment period, including dexamethasone and levetiracetam, the lower rates of surgery39 and/or stereotactic radiosurgery40,41 in non-White groups may also account for some of the medication disparities we note. Ultimately, as mentioned above, further studies are needed to better identify the exact source of the medication-related disparities we identified in this study.
In 2017, the American Society of Clinical Oncology (ASCO) passed formal guidelines advocating for the early integration of palliative care into standard oncology practice.42 The guideline was based on multiple randomized controlled trials demonstrating both quality of life and survival benefit when early palliative care was added to usual oncology care. More specifically, it called for palliative care involvement, including symptom, distress, and functional status management, within 8 weeks of diagnosis for patients with advanced cancer. While this particular guideline was released after the period that our analysis pertains to (2007–2016), it was supported by several earlier studies7,43 and represented an update to ASCO recommendations that had already been made in preceding years.44 Whether the gaps in supportive medication utilization across racial subgroups noted in our study will close following more widespread implementation of this ASCO guideline remains to be seen.
Our study should be considered in the context of its limitations. First, this study was performed with the SEER-Medicare database and included only those patients on Medicare Part D. Therefore, the results of our analysis should be extrapolated to the general population of patients with BM with caution. Second, claims data cannot reliably be used for identification of metastatic involvement of many organs, such as lung, liver, and bone. Accordingly, the National Cancer Institute advises caution regarding use of SEER-Medicare data to identify metastases after initial cancer diagnosis, particularly those for which reimbursement is unaffected by diagnostic codes.45 However, because BM are largely treated with local modalities (radiation therapy and/or resection), diagnostic/billing codes for brain-directed treatments are useful indicators of intracranial involvement. Moreover, the use of health insurance claims data for identification of BM has been previously validated with high sensitivity (97%) and specificity (99%) relative to manual chart review.17 SEER-Medicare data have subsequently been successfully used to identify patients with BM by other investigators as well.46 Another limitation of this study is the overlap in medications across categories; supportive care medications often have multiple indications, and particular medications we examined therefore appeared in more than one category. As a result, it should be noted that there is some degree of correlation within our results and the disparities we note. Another limitation of this study is that data on patient-reported symptoms and quality of life were not available; therefore, we were unable to assess whether the disparities we noted in supportive medication prescription translated to worse symptom management as experienced by patients. Future institutional studies should incorporate quality of life–related questionnaires for patients with BM to better understand whether such correlations between supportive medication use and symptom management exist.
Conclusions
In this population-based study of nearly 18,000 patients with newly diagnosed BM, we found significant racial disparities in the use of supportive medications in the first month following diagnosis. This finding is especially important given increasing awareness of the relationship between symptom management via effective palliative care and improved quality of life and survival. Our findings underscore the need for oncologists to better identify and manage symptom burden among non-White patients with BM. Future studies should focus on how providers can minimize disparities among patients with BM by reducing communication barriers and more effectively screening for symptoms.
Funding
No funding was required for this study.
Conflict of interest statement. Daniel N. Cagney is a recipient of research support from NH Theraguix. Dr Aizer reports research funding from Varian Medical Systems and consulting fees from Novartis. The remaining authors declare no conflicts of interest.
Authorship statement. Study conception/design: NL, KAL, AAA; data collection/analysis/interpretation: all authors; statistical analysis: NL, PC, AAA; drafting of the manuscript: NL, AAA; manuscript editing/critical revision of the manuscript: all authors; supervision: KAL, AAA.
Supplementary Material
References
- 1. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48–54. [DOI] [PubMed] [Google Scholar]
- 2. Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Gonçalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 2012;32(11):4655–4662. [PubMed] [Google Scholar]
- 3. Bezjak A, Adam J, Barton R, et al. . Symptom response after palliative radiotherapy for patients with brain metastases. Eur J Cancer. 2002;38(4):487–496. [DOI] [PubMed] [Google Scholar]
- 4. Chow E, Fan G, Hadi S, Wong J, Kirou-Mauro A, Filipczak L. Symptom clusters in cancer patients with brain metastases. Clin Oncol. 2008;20(1):76–82. [DOI] [PubMed] [Google Scholar]
- 5. Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD. Radiosurgery with or without whole-brain radiotherapy for brain metastases: the patients’ perspective regarding complications. Am J Clin Oncol. 2005;28(2):173–179. [DOI] [PubMed] [Google Scholar]
- 6. Jereczek-Fossa BA, Marsiglia HR, Orecchia R. Radiotherapy-related fatigue. Crit Rev Oncol Hematol. 2002;41(3):317–325. [DOI] [PubMed] [Google Scholar]
- 7. Temel JS, Greer JA, Muzikansky A, et al. . Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. [DOI] [PubMed] [Google Scholar]
- 8. Basch E, Deal AM, Dueck AC, et al. . Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Denis F, Basch E, Septans AL, et al. . Two-year survival comparing web-based symptom monitoring vs routine surveillance following treatment for lung cancer. JAMA. 2019;321(3):306–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aizer AA, Wilhite TJ, Chen MH, et al. . Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120(10):1532–1539. [DOI] [PubMed] [Google Scholar]
- 11. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol. 2018;36(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Check DK, Samuel CA, Rosenstein DL, Dusetzina SB. Investigation of racial disparities in early supportive medication use and end-of-life care among Medicare beneficiaries with stage IV breast cancer. J Clin Oncol. 2016;34(19):2265–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pinheiro LC, Check DK, Rosenstein D, Reeder-Hayes KE, Dusetzina S. Examining potential gaps in supportive medication use for US and foreign-born Hispanic women with breast cancer. Support Care Cancer. 2019;27(5):1639–1646. [DOI] [PubMed] [Google Scholar]
- 14. Chang S, Goldstein NE, Dharmarajan KV. Managing an older adult with cancer: considerations for radiation oncologists. Biomed Res Int. 2017;2017:1695101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Surveillance, Epidemiology, and End Results (SEER) Program; National Cancer Institute. Overview of the SEER Program.https://seer.cancer.gov/about/overview.html. Accessed September 16, 2019.
- 16. National Cancer Institute, Division of Cancer Control and Population Sciences. SEER-Medicare: How the SEER & Medicare Data Are Linked.https://healthcaredelivery.cancer.gov/seermedicare/overview/linked.html. Accessed September 16, 2019.
- 17. Eichler AF, Lamont EB. Utility of administrative claims data for the study of brain metastases: a validation study. J Neurooncol. 2009;95(3):427–431. [DOI] [PubMed] [Google Scholar]
- 18. Lamba N, Kearney RB, Mehanna E, et al. . Utility of claims data for identification of date of diagnosis of brain metastases [published online ahead of print January 6, 2020]. Neuro Oncol. 2020. doi:10.1093/neuonc/noz245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 20. Clegg LX, Li FP, Hankey BF, Chu K, Edwards BK. Cancer survival among US whites and minorities: a SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch Intern Med. 2002;162(17):1985–1993. [DOI] [PubMed] [Google Scholar]
- 21. Silber JH, Rosenbaum PR, Clark AS, et al. . Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013;310(4):389–397. [DOI] [PubMed] [Google Scholar]
- 22. Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: causes and consequences of unequal care. J Pain. 2009;10(12):1187–1204. [DOI] [PubMed] [Google Scholar]
- 23. Atdjian S, Vega WA. Disparities in mental health treatment in U.S. racial and ethnic minority groups: implications for psychiatrists. Psychiatr Serv. 2005;56(12):1600–1602. [DOI] [PubMed] [Google Scholar]
- 24. Wong E, Collins R, Cerully J, Seelam R, Roth E. Racial and ethnic differences in mental illness stigma and discrimination among Californians experiencing mental health challenges. Rand Health Q. 2017;6(2):6. [PMC free article] [PubMed]
- 25. Kalibatseva Z, Leong FT. Depression among Asian Americans: review and recommendations. Depress Res Treat. 2011;2011:320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40(11):1797–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Giese-Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011;29(4):413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feddock CA, Hoellein AR, Griffith CH 3rd, et al. . Can physicians improve patient satisfaction with long waiting times? Eval Health Prof. 2005;28(1):40–52. [DOI] [PubMed] [Google Scholar]
- 29. Chan A, Woodruff RK. Comparison of palliative care needs of English- and non-English-speaking patients. J Palliat Care. 1999;15(1):26–30. [PubMed] [Google Scholar]
- 30. Scheppers E, van Dongen E, Dekker J, Geertzen J, Dekker J. Potential barriers to the use of health services among ethnic minorities: a review. Fam Pract. 2006;23(3):325–348. [DOI] [PubMed] [Google Scholar]
- 31. Diamond LC, Wilson-Stronks A, Jacobs EA. Do hospitals measure up to the national culturally and linguistically appropriate services standards? Med Care. 2010;48(12):1080–1087. [DOI] [PubMed] [Google Scholar]
- 32. Karliner LS, Jacobs EA, Chen AH, Mutha S. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature. Health Serv Res. 2007;42(2):727–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Silva MD, Genoff M, Zaballa A, et al. . Interpreting at the end of life: a systematic review of the impact of interpreters on the delivery of palliative care services to cancer patients with limited English proficiency. J Pain Symptom Manage. 2016;51(3):569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fesinmeyer MD, Mehta V, Blough D, Tock L, Ramsey SD. Effect of radiotherapy interruptions on survival in Medicare enrollees with local and regional head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2010;78(3):675–681. [DOI] [PubMed] [Google Scholar]
- 35. Wyatt G, Sikorskii A, Tesnjak I, Victorson D, Srkalovic G. Chemotherapy interruptions in relation to symptom severity in advanced breast cancer. Support Care Cancer. 2015;23(11):3183–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ohri N, Rapkin BD, Guha C, Kalnicki S, Garg M. Radiation therapy noncompliance and clinical outcomes in an urban academic cancer center. Int J Radiat Oncol Biol Phys. 2016;95(2):563–570. [DOI] [PubMed] [Google Scholar]
- 37. Virnig BA, Baxter NN, Habermann EB, Feldman RD, Bradley CJ. A matter of race: early-versus late-stage cancer diagnosis. Health Aff (Millwood). 2009;28(1):160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24(11):1666–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haider AH, Scott VK, Rehman KA, et al. . Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482–92.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ascha MS, Funk K, Sloan AE, Kruchko C, Barnholtz-Sloan JS. Disparities in the use of stereotactic radiosurgery for the treatment of lung cancer brain metastases: a SEER-Medicare study. Clin Exp Metastasis. 2020;37(1):85–93. [DOI] [PubMed] [Google Scholar]
- 41. Kann BH, Park HS, Johnson SB, Chiang VL, Yu JB. Radiosurgery for brain metastases: changing practice patterns and disparities in the United States. J Natl Compr Canc Netw. 2017;15(12):1494–1502. [DOI] [PubMed] [Google Scholar]
- 42. Ferrell BR, Temel JS, Temin S, et al. . Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35(1):96–112. [DOI] [PubMed] [Google Scholar]
- 43. Bakitas M, Lyons KD, Hegel MT, et al. . Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith TJ, Temin S, Alesi ER, et al. . American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012;30(8):880–887. [DOI] [PubMed] [Google Scholar]
- 45. National Cancer Institute. SEER-Medicare Linked Database.https://healthcaredelivery.cancer.gov/seermedicare/considerations/measures.html#13. Accessed November 1, 2019.
- 46. Halasz LM, Weeks JC, Neville BA, Taback N, Punglia RS. Use of stereotactic radiosurgery for brain metastases from non-small cell lung cancer in the United States. Int J Radiat Oncol Biol Phys. 2013;85(2):e109–e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.