Abstract
Background
The aim of this study is to evaluate results in terms of local control (LC), overall survival (OS), and toxicity profile and to better identify factors influencing clinical outcome of skull base chordoma treated with proton therapy (PT) and carbon ion radiotherapy (CIRT).
Methods
We prospectively collected and analyzed data of 135 patients treated between November 2011 and December 2018. Total prescription dose in the PT group (70 patients) and CIRT group (65 patients) was 74 Gy relative biological effectiveness (RBE) delivered in 37 fractions and 70.4 Gy(RBE) delivered in 16 fractions, respectively (CIRT in unfavorable patients). LC and OS were evaluated using the Kaplan–Meier method. Univariate and multivariate analyses were performed, to identify prognostic factors on clinical outcomes.
Results
After a median follow-up of 49 (range, 6–87) months, 14 (21%) and 8 (11%) local failures were observed in CIRT and PT group, respectively. Five-year LC rate was 71% in CIRT cohort and 84% in PT cohort. The estimated 5-year OS rate in the CIRT and PT group was 82% and 83%, respectively. On multivariate analysis, gross tumor volume (GTV), optic pathways, and/or brainstem compression and dose coverage are independent prognostic factors of local failure risk. High rate toxicity grade ≥3 was reported in 11% of patients.
Conclusions
Particle radiotherapy is an effective treatment for skull base chordoma with acceptable late toxicity. GTV, optic pathways, and/or brainstem compression and target coverage were independent prognostic factors for LC.
Key Points
• Proton and carbon ion therapy are effective and safe in skull base chordoma.
• Prognostic factors are GTV, organs at risk compression, and dose coverage.
• Dual particle therapy and customized strategy was adopted.
Keywords: carbon ions therapy, chordoma, proton therapy, skull base
Importance of the Study.
Particle radiotherapy plays a key role in the treatment of skull base chordoma, a rare, locally aggressive, radioresistant tumor requiring rather challenging management due to its location. While most of the published series are retrospective, this prospective study on a large number of patients for such a rare tumor evaluates particle radiotherapy outcomes in the view of the personalized medicine era. For the first time in the same series, a dual particle (protons and carbon ions) integrated approach was presented and the choice of particle was based on multiple features regarding tumor characteristics in a customized way, toxicity risks, and post-surgery outcomes. Moreover, prognostic factors for tumor local control have been identified in order to identify more precise criteria to plan further tailored radiation treatment and optimal combination with previous surgery.
Chordoma is a rare primary bone tumor arising from notochord remnants with an incidence of 0.8–1 per million, with approximately one-third arising from the midline clivus and skull base.1,2 Although chordoma is generally considered a slow growing tumor, it is characterized by locally aggressive growth and a propensity for local recurrence (LR), and because of a low potential for metastasis, local control (LC) is the outcome affecting significantly survival.3 The importance of surgery has been well established in the treatment of skull base chordoma (SBC). Complete resection is aimed as the optimal goal of surgical procedure; however, it is often precluded by tumor location and the surrounding critical structure such as brainstem or optic pathways.4,5 Optimal strategy of combination between maximum safe resection and radiotherapy should be pursued in order to obtain the best outcomes, driven by the prognostic factors and personalized on the specific case. For inoperable patients, radiotherapy after biopsy is the treatment of choice.6,7 Postoperative photon radiotherapy has been widely administered, but using moderate radiation doses (<55 Gy) to accommodate the tolerance of dose-limiting structures such as the brainstem, optic nerves and chiasm, and temporal lobes. A dose-response relationship has been demonstrated for SBC where an improved tumor control rate can be achieved with dose higher than 70 Gy.4,8–11 In order to overcome chordoma radioresistance and to obtain satisfactory LC, a dose escalation given with advanced forms of treatment modalities (stereotactic radiotherapy or heavy particles) has become more important in the management of this tumor. Particle beam therapy (protons and carbon ions) appeared to be the most effective radiation modality in the management of SBC, allowing the delivery of high radiation dose levels, maximizing the positive balance between gross tumor volume (GTV) optimal dose coverage and sparing of organs at risk (OARs).12 Furthermore, carbon ions deliver a higher relative biological effectiveness (RBE) in a selective way to the target region and a dose dependency for the RBE chordoma cell lines and dose correlation with tumor control probability addressing to higher dose levels.13–15
The purpose of this study was to evaluate results and safety of proton therapy (PT) or carbon ion radiotherapy (CIRT) of patients with SBC at the National Center for Oncological Hadrontherapy (CNAO), Pavia, Italy and to identify factors influencing LC and overall survival (OS).
Materials and Methods
From November 2011 to December 2018, one hundred thirty-five consecutive patients with SBC were treated with PT or CIRT at CNAO. Treatments were delivered in accordance with 2 phase II prospective clinical trials (CNAO 01/2011 and CNAO S12/2012/C for PT and CIRT, respectively) approved by the CNAO Ethical Review Board for obtaining “CE” mark approval for CNAO synchrotrone as a medical device.16 Trials ended in 2013, then patients were prospectively enrolled in a clinical registry study with the same inclusion/exclusion criteria of the phase II clinical trials. All enrolled patients gave their written informed consent for treatment and the use of their anonymized data for research and educational purposes.
Inclusion criteria were: histologically proven SBC; curative treatment (at least 70 Gy[RBE] for PT or 70.4 Gy[RBE] for CIRT); any surgical resection degree (macroscopic complete, not complete, or only biopsy); any clinical timing (new diagnosed or recurrent disease); good performance status (Karnofsky performance status ≥60); age ≥5 years old; follow-up of at least 6 months; written informed consent of the patient (or the legal guardians). Exclusion criteria were: metastatic disease; no histological diagnosis; previous radiotherapy in affected region; concomitant chemotherapy; extensive metal instrumentation/implants; inability to deliver prescribed dose without overdose to normal structures; pregnancy. The outcomes evaluated in the study were: LC, distant progression free survival (DPFS), OS, identification of prognostic factors, toxicity profile. Recurrent cases were all reviewed: the follow-up MRI sequences where the recurrence was detected were fused with rigid registration based on anatomical landmarks with the planning CT scans to allow dosimetric analyses.
Treatment Planning and Delivery
Each patient was treated in supine position. Immobilization was performed using a customized thermoplastic head-mask and mouth-bites. A simulation CT scan (2 mm slice thickness) without contrast enhancement was performed for treatment plan calculation. Image fusion with diagnostic MRI sequences was performed using non-deformable algorithms based on anatomical landmarks.
The GTV was contoured on MR images performed with the patient immobilized in the same setup conditions as for the simulation CT. T2-weighted axial images were routinely employed and contours were checked and modified on post-contrast T1-weighted images. Patients were treated according to a sequential boost protocol. The prescription dose for PT is 74 Gy(RBE) delivered in 37 fractions of 2 Gy(RBE): 54 Gy(RBE) to the low risk (LoR) volume and 20 Gy(RBE) to the high risk (HR) volume. CIRT prescription dose is 70.4 Gy(RBE) delivered in 16 fractions of 4.4 Gy(RBE): 39.6 Gy(RBE) to the LoR and 30.8 Gy(RBE) to the HR volume, respectively.
The HR clinical target volume (CTV-HR) included GTV with 3–5 mm safety margin modified according to both the anatomy and the surgical pathway, to include the HR areas of tumor recurrence. In case of macroscopically radical resection, the CTV-HR is limited to include the resection margins. The CTV-LoR is obtained by adding 5 mm (isotropic expansion) to CTV-HR, then it is modified according to preoperative extension of disease, surgical pathway, and postoperative changes. The HR planning target volume (PTV) and PTV-LoR encompass the CTV-HR and CTV-LoR, respectively with a uniform three-dimensional margin of 2 mm.17 The particle choice (PT or CIRT) was personalized for each patient upon the following criteria: macroscopic disease at pretreatment MRI scan (unfavorable conditions: the presence and larger GTV); histological characteristics (unfavorable conditions: dedifferentiated subtypes, higher proliferative index); pattern of macroscopic disease extension (unfavorable conditions: mucosal or brain infiltration/involvement) and proximity to critical organs (unfavorable conditions: abutment/compression of brainstem, optic pathways); toxicity risks patient-related; post-surgical anatomic changes and complications (unfavorable conditions: dural defect and cerebrospinal fluid fistula). In the presence of unfavorable pretreatment tumor characteristics with no factors that can predispose to high grade toxicity (as extended mucosal or brain infiltration or post-surgical fistula), the choice would be tailored toward CIRT. Furthermore, in the absence of macroscopic disease (GTV not identified), CIRT is usually not indicated in the clinical practice. Dose constraints for OARs followed published data and clinical experience reported from other particle series, especially for CIRT. Dose constraints to the OARs for PT were maximum dose of 54 and 63 Gy(RBE) to the center and surface of the brainstem, respectively, and 60 Gy(RBE) to the optic pathway.18–21 Dose constraints to the OARs for CIRT were maximum dose of 30 Gy(RBE) to the surface of the brainstem and 40 Gy(RBE) to the optic pathways.22,23 All treatment plans were calculated with the Syngo-RT (Siemens AG Healthcare) planning system in use at CNAO since the beginning of clinical activity. A constant RBE value of 1.1 is applied for PT RBE-weighted dose calculation, while the Local Effect Model I provides CIRT RBE-weighted doses.24,25 Two to 3 beams were used for plan optimization. The dose was delivered with pencil beam scanning technique and synchrotron-based active energy selection with a scan step of 3 mm and 2 mm for PT and CIRT beams, respectively.26 The distance between subsequent iso-energy slices was 2 mm for both particles.
Follow-up Evaluation
Patients’ follow-ups with brain and head and neck MRI and clinical examination were planned every 3 months after the end of treatment for the first 2 years, every 6 months for the following 3 years, and then annually. Blood hormone assays and audiometric and visual examinations were performed every year. Early (up to 90 days after treatment) and late (after 90 days) adverse events were classified according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.03 grading system.27 Response to treatment was defined according to RECIST 1.1 (Response Evaluation Criteria in Solid Tumors).28
Statistical Analysis
Descriptive analyses were prepared with the use of medians, means, ranges, and interquartile ranges. Univariate and multivariate analyses were performed, to identify the impact of patient-, tumor-, and treatment-related factors on clinical outcomes. We used the chi-squared test and the Wilcoxon-rank test to assess differences in the distribution of categorical and continuous variables across CIRT and PT cohorts. The length of follow-up was calculated from the last date of treatment to the last follow-up visit. In-field progression-free interval was defined as the time interval between the last day of radiotherapy and the first diagnosis of LR or the last follow-up visit, in case no LR was found. OS was also calculated to investigate time to death. Survival curves were estimated by the Kaplan–Meier method and differences between curves evaluated by the log-rank test. To calculate differences in treatment on tumor outcome, multivariate proportional hazards Cox regression models were performed adjusting for other prognostic and confounding factors (patient, tumor, and treatment-related factors). Hazard ratios and 95% CIs from multivariate Cox models are presented. All P-values were set at 0.05, two-sided. In order to identify the most appropriate cutoff value for GTV, we considered receiver operating characteristic (ROC) analysis for local relapse. We choose the point on the ROC curve where Youden’s index is maximum, which is the point that maximizes both sensitivity and specificity.29 Statistical analyses were performed using SAS statistical software v9.02 for Windows.
Results
Patients and tumor characteristics are summarized in Table 1. Sixty-five patients were treated with CIRT, 70 patients with PT. Median follow-up was 49 months (range 6–87). The two cohorts of patients (PT vs CIRT) were statistically different in GTV volume, quality of resection, treatment phase (primary or recurrent disease regardless of number of resections), and visual field deficit at baseline evaluation. At the time of our analysis, 113 (84%) patients remained locally controlled: 51 (78%) receiving CIRT, 62 (89%) receiving PT. Overall 117 (87%) patients are still alive: 57 (88%) receiving CIRT, 60 (86%) PT. The choice of the type of radiation (protons or carbon ions) did not influence the outcome in LC (P = 0.15) and OS (P = 0.82). No treatment-related secondary malignancies were reported among patients included in this study.
Table 1.
Patients’ characteristics
| Total n (%) 135 Patients | CIRT Cohort n (%) 65 Patients | PT Cohort n (%) 70 Patients | P-value | |
|---|---|---|---|---|
| KPS | 0.8493 | |||
| ≤80 | 22 (16) | 11 (17) | 11 (16) | |
| 90–100 | 113 (84) | 54 (83) | 59 (84) | |
| Sex | 0.374 | |||
| Male | 82 (61) | 42 (65) | 40 (57) | |
| Female | 53 (39) | 23 (35) | 30 (43) | |
| Age, y, median (range) | 57 (13–81) | 58 (13–81) | 53 (17–81) | 0.1388 |
| Treatment | 0.019 | |||
| Primary | 107 (79) | 46 (71) | 61 (87) | |
| Recurrent | 28 (21) | 19 (29) | 9 (13) | |
| Aim of the treatment | 0.146 | |||
| Postoperative | 130 (96) | 61 (94) | 69 (99) | |
| Exclusive | 5 (4) | 4 (6) | 1 (1) | |
| Resection status | <0.0001 | |||
| Complete | 19 (14) | 0 (0) | 19 (27) | |
| Incomplete | 115 (85) | 64 (98) | 51 (73) | |
| Only biopsy | 1 (1) | 1 (2) | 0 (0) | |
| Surgical technique | 0.130 | |||
| Endoscopic endonasal | 112 (83) | 55 (84) | 57 (82) | |
| Other approach (transcranial) | 13 (10) | 5 (8) | 8 (11) | |
| Not known | 10 (7) | 5 (8) | 5 (7) | |
| Surgery (n) | 0.285 | |||
| 1 | 77 (57) | 34 (52) | 43 (61) | |
| >1 | 58 (43) | 31 (48) | 27 (39) | |
| Brainstem abutment and/or compression | 0.671 | |||
| Y | 31 (23) | 14 (22) | 17 (25) | |
| N | 103 (77) | 51 (78) | 52 (75) | |
| Not evaluated * | 1 | 0 (0) | 1 | |
| Optic pathway abutment and/or compression | 0.579 | |||
| Y | 11 (8) | 58 (89) | 64 (94) | |
| N | 123 (92) | 7 (11) | 4 (6) | |
| Not evaluated * | 1 | 0 (0) | 1 | |
| Visual defect | 0.016 | |||
| Y | 24 (18) | 17 (26) | 7 (10) | |
| N | 110 (81) | 48 (74) | 62 (89) | |
| Not evaluated | 1 (1) | 0 (0) | 1 (1) | |
| Diplopia | 0.5945 | |||
| Y | 55 (41) | 28 (43) | 27 (33) | |
| N | 80 (59) | 37 (57) | 47 (67) | |
| Hearing impairment | 0.5221 | |||
| Y | 62 (46) | 28 (43) | 34 (49) | |
| N | 73 (54) | 37 (57) | 36 (51) | |
| Pituitary dysfunction | 0.303 | |||
| N | 112 (82.9) | 51 (78.4) | 61 (87.1) | |
| Y (1 hormonal deficit) | 10 (7.4) | 7 (10.8) | 3 (4.3) | |
| Y (>1 hormonal deficits) | 13 (9.6) | 7 (10.8) | 6 (8.6) | |
| Cranial nerve deficit | 0.014 | |||
| Y | 58 (43) | 35 (54) | 23 (33) | |
| N | 77 (57) | 30 (46) | 47 (67) | |
| GTV, cm3, median (range) | 7 (0–99.3) | 13 (0.4–87.4) | 3.5 (0–99.3) | 0.0001 |
| Dose, median (range) | ‒ | 70.4 (70.4-70.4) | 74 (72–74) | ‒ |
Abbreviations: *Only CT imaging, CIRT: carbon ion radiotherapy, PT: proton therapy, Y: yes, N: no, GTV: gross target volume, KPS: Karnofsky performance status.
CIRT Cohort Clinical Outcome and Prognostic Factors
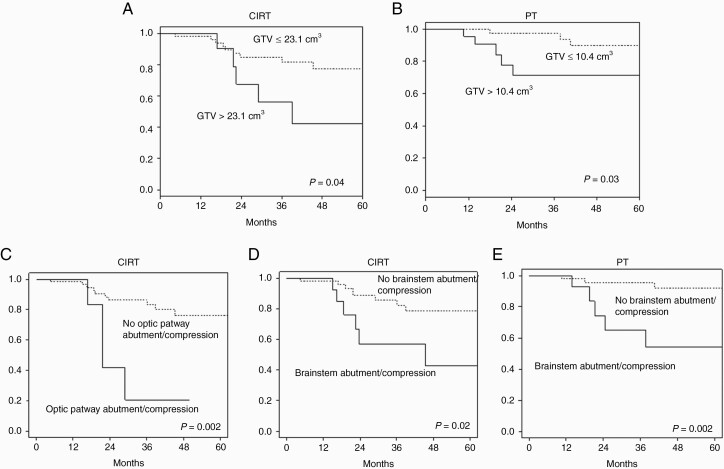
For the CIRT group, the 3-year and 5-year LC rates were 77% and 71%, respectively (Figure 1A). LR occurred in 14 of 65 patients. After further analysis the origin of recurrence was determined to be situated in close proximity to brainstem or optic chiasm (13 out of 14 LR, 92%), where dose coverage was compromised to spare the OARs. Nine patients developed distant relapse (included extracranial metastases and cranial recurrence outside radiation field): 4 cases occurred in the cervical spine, 2 cases in the nasal septum/cavity likely due to surgical seeding, and 3 cases of cranial recurrence were reported outside the radiation field. Three of these 9 patients had also LR. The 3-year and 5-year DPFS rates were 87% and 84%, respectively. The 3-year and 5-year OS rates were 90% and 82%, respectively (Figure 1B): 8 patients died, 6 patients for the disease (2 for LR and distant progression, 1 patient for distant progression) and 2 patients for disease/treatment non-related cause. In univariate analysis, the GTV extension had a major impact on LC and OS. The estimated 3-year LC rates were 81% and 56% for patients with GTV ≤23.1 cm3 and >23.1 cm3, respectively (P = 0.04); OS rates were 95% and 65% for patients with GTV ≤23.1 cm3 and >23.1 cm3, respectively (P > 0.0001) (Figure 2A). Likewise, optic pathways and brainstem compression had a significant (P = 0.002 and P = 0.02, respectively) negative impact on LC (Figure 2C, D) and, as a consequence of OAR sparing, target coverage (D95% of CTV-HR and GTV) was significantly lower (P < 0.001 and P < 0.0001, respectively) in patients with LR (Table 2). Sex, age, histology, and anatomic extension of the disease (upper/middle/lower clivus) had no influence on LC and OS.
Fig. 1.
Kaplan–Meier curves of local control (LC) (A) and overall survival (OS) (B) for PT and CIRT.
Fig. 2.
Kaplan–Meier curves of local control (LC) probability after CIRT in case of GTV ≤23.1 cm3 versus volume >23.1 cm3 (A) and PT in case of GTV ≤10.4 cm3 versus volume >10.4 cm3 (B) and after CIRT in case optic pathway abutment/compression (C) or brainstem abutment/compression (D) and after PT in case of brainstem abutment/compression (E).
Table 2.
High risk clinical target volume (CTV HR) and gross tumor volume (GTV) coverage
| CIRT | n | Median Gy(RBE) | Lower Quartile | Upper Quartile | P-value | |
|---|---|---|---|---|---|---|
| D95 CTV HR | LC | 47 | 63 | 55 | 67 | <0.001 |
| D95 CTV HR | PD | 14 | 48.6 | 35.5 | 54.9 | |
| D98 GTV | LC | 47 | 61 | 52 | 67 | <0.001 |
| D95 GTV | 67 | 58 | 69 | |||
| D98 GTV | PD | 14 | 25.25 | 17.4 | 44.4 | |
| D95 GTV | 33.4 | 18.5 | 51.9 | |||
| PT | n | Median Gy(RBE) | Lower Quartile | Upper Quartile | P-value | |
| D95 CTV HR | (GTV0) LC | 19 | 70.4 | 68.9 | 71.1 | 0.002 |
| D95 CTV HR | (GTV+) LC | 41 | 68 | 66 | 71 | |
| D95 CTV HR | PD | 8 | 63.75 | 61.75 | 65.45 | |
| D98 GTV | LC | 41 | 67 | 62 | 69 | 0.0072 |
| D95 GTV | 70 | 65 | 72 | |||
| D98 GTV | PD | 8 | 59.6 | 32.4 | 63 | |
| D95 GTV | 63.2 | 40 | 67.3 |
Data available for 129 patients out of 135.
Abbreviations: LC: local control, PD: progression disease, D95: dose covering 95% of the volume, D98: dose covering 98% of the volume.
PT Cohort: Clinical Outcome and Prognostic Factors
For the PT group, the 3-year and 5-year LC rates were 89% and 84%, respectively (Figure 1a). LR occurred in 8 of 70 patients. After further analysis, the origin of LR was determined to be situated in close proximity to brainstem in 7 out of 8 (87 %). Two patients developed distant relapse in the nasal septum/cavity due to surgical seeding. One of them had also LR. The 3-year and 5-year DPFS rates were 98% and 96%, respectively. The 3-year and 5-year OS rates were 93% and 83%, respectively (Figure 1B): 10 patients died, 5 patients from the disease (LR and 1 of them had also distant progression) and 5 patients from disease/treatment non-related cause. In univariate analysis the cutoff of GTV influencing LC and OS was 10.4 cm3; the LC rates at 3 years were 94% and 71% for patients with GTV ≤10.4 cm3 and >10.4 cm3, respectively (P = 0.03) (Figure 2B). OS rates were 100% and 80% for patients with GTV ≤10.4 cm3 and >10.4 cm3, respectively (P < 0.0001). Moreover, brainstem compression had a significant (P = 0.002) negative impact on LC (Figure 2E). No correlation was found between LC and optic chiasm compression/abutment in PT group. Target coverage (D95% of CTV-HR and GTV) was significantly lower (P = 0.002 and P = 0.0072, respectively) in patients with LR (Table 2). The subgroup analysis on the 19 patients (27%) that underwent macroscopic complete surgery (GTV = 0) showed LC of 100% with almost significantly better prognosis compared with GTV >0 (P = 0.074 for LC and P = 0.079 for OS).
Toxicity Profile
We have assessed the potential correlation between the type of particle treatment and high grade late adverse events, but no CIRT or PT were predictors of grade (G) ≥3 late toxicity. Therefore, toxicity profile was analyzed for the entire cohort of patients. No high grade (G ≥3) acute toxicity was observed. Sixteen (12%) patients experienced high grade (13 patients G3 and 3 patients G4) late toxicity. Late toxicity profiles are summarized in Table 3. Forty-four (32.5%) patients experience brain or soft tissue alterations: 39 patients (30%) had temporal lobe injuries, and 4 patients (3%) had bone and/or soft tissue injuries. Brain injury was asymptomatic in 25 out of 39 patients (64 %), and 14 patients (36 %) developed moderate symptoms controlled by steroids. At least follow-up 16 out of 39 patients (41%) had a reduction/resolution of the altered contrast-enhancing area. The estimated 3-year high grade toxicity-free survival was 85% for CIRT and 91 % for PT.
Table 3.
Late toxicity profile for the entire cohort of patients (PT + CIRT)
| Patients | % | ||
|---|---|---|---|
| High grade late toxicity | No | 119 | 88 |
| Yes | 16 | 12 | |
| G3 | 13 | 10 | |
| G4 | 3 | 2 | |
| CTCAE high grade late toxicity | No | 119 | 88 |
| Ear | 8 | 6 | |
| G3 | 7 | 1 | |
| G4 | 1 | 3 | |
| Endocrine | 1 | 2 | |
| G3 | 1 | ||
| G4 | 0 | ||
| Eye | 4 | ||
| G3 | 2 | ||
| G4 | 2 | ||
| Nervous system disorders | 3 | ||
| G3 | 3 | ||
| G4 | 0 |
Abbreviations: PT: proton therapy, CIRT: carbon ion radiotherapy, CTCAE: Common Terminology Criteria for Adverse Events, G: grading.
No brainstem or spinal cord complications were observed in this series. All of 3 cases of G4 toxicity were expected: in 2 patents, the optic nerve was very close to the GTV (causing visual field deficit at the baseline); in a third case, the patient was affected by preexistent unilateral important hearing deficit. In all of these cases, the involved OARs were sacrificed with patients’ consent, and efforts were made to spare the contralateral optic nerve and cochlea.
Discussion
Outcome
To our knowledge, this is the largest single institution prospective study describing results of dual particle radiation treatments (protons or carbon ions) of SBC. In our series of 135 patients treated with PT or CIRT, 5-year LC rate were 84% and 71%, respectively, in accordance with other published series of SBC treated with particle radiotherapy (Table 4). The gap between these results in LC could be explained by patients’ selection and imbalances between PT and CIRT population. In the era of personalized medicine, the choice of particle has been tailored in a customized way based on tumor amount of “gross” disease after surgery, patterns of macroscopic disease extension and relationship with critical organs, particular histopathology aggressive features, and toxicity risks related to patients, to post-surgical anatomic changes, and to complications (eg, dural fistula). CIRT has been the treatment of choice in the presence of unfavorable tumor characteristics, thus probably negatively influencing LC rate.
Table 4.
Published series of skull base chordomas treated with particle radiotherapy
| Study (Institution) | Radiation Type | RT Dose (GyRBE) | Patients (number) | Follow-up Months (median) | GTV | LC (%) | OS (%) |
|---|---|---|---|---|---|---|---|
| Hug, 1999; LLMUC4 | Ph + P | TD 71.9 median (66.6–79.2, range) Dfp: 1.8 | 33 | 32.2 | 9%: 0 to ≤15 mL 12%: >15 to ≤25 mL 79%: >25 mL | 3-y: 67 5-y: 59 | 3-y: 87 5-y: 79 |
| Munzenrider, 1999; HCL-MGH10 | Ph + P | TD: 66–83 range Dpf: 1.8–1.92 | 169 | 41 | NR | 5-y: 73 10-y: 54 | 5-y: 80 10-y: 54 |
| Noel, 2005; CPO40 | Ph + P | TD: 67 median (60–71, range) Dpf: 1,8-2 | 100 (1993–2002) | 31 | 23 cm3 (median) | 4-y: 53 | 4-y: 90 |
| Mizoe, 2009 (NIRS)41 | C | TD: 48–60.8 range Dpf: 3–3.8 | 33 | 53 (mean) | NR | 5-y: 85 10-y: 64 | 5-y: 88 10-y: 67 |
| Uhl, 2014 (GSI)9 | C | TD: 60 median (54–70, range) Dpf: 3 | 155 | 38 | NR | 3-y: 82 5-y: 72 10-y: 54 | 3-y: 95 5-y: 85 10-y: 75 |
| Weber, 2016 (PSI)11 | P | TD: 72.5 mean Dpf: 1,8-2 | 151 | 50 (mean) | 35.4 cm3 (mean) | 5-y: 75.8 7-y: 70.9 | 7-y: 72.9 |
| Fung, 2018 (CPO)12 | Ph + P | TD 68.4–73.8 range Dpf: 1,8 | 106 (2006–2012) | 61 | 25 cm3 (mean) | 4-y: 78.3 5-y: 75.1 | 4-y: 90.2 5-y: 88.3 |
| Present study, CNAO | P or C | P: TD: 74 median (72–74, range) Dpf: 1,8-2 C: TD: 70,4 Dpf: 4,4 | 135 70 P 65 C | 44 | 7 cm3 (median) P: 3.5 cm3 (median) C: 12.9 cm3 (median) | P: 3-y: 89 5-y: 84 C: 3-y: 77 5-y: 71 | P: 3-y: 93 5-y: 83 C: 3-y: 90 5-y: 82 |
Abbreviations: C, carbon ion therapy; Gy, gray; LC, local control; OS, overall survival; P, proton therapy; Ph: photon therapy; RBE, relative biological effectiveness; TD: total dose; Dpf: dose per fraction. NR: not reported; GTV: gross tumor volume; CTV: clinical target volume; GSI: Society for Heavy Ion Research (Darmstadt, Germany); NIRS: National Institute for Radiologic Sciences (Chiba, Japan); HCL-MGH = Harvard Cyclotron Laboratory and Massachusetts General Hospital (Bost, USA); LLUMC = Loma Linda University Medical Center (Loma Linda, USA); PSI = Paul Scherrer Institut (Villigen, Switzerland); CPO = Centre de Protontherapie d’Orsay (Orsay France); CNAO: National Center for Oncological Hadrontherapy (Pavia, Italy).
Prognostic Factors: General Considerations
In the era of personalized treatment, the importance of prognostic factor analysis is well established, leading to various types of therapeutic strategies and allowing to focus efforts on critical or high risk of recurrence subgroup of patients. However, as reported in a recent review,32 some prognostic factors have consistently been reported to be of predictive value (relationship with OARs, GTV, dose target coverage), whereas others have only been sporadically discussed. Classic clinical factors such as older and younger age are shown to be significant poor prognostic factors, but the results are often controversial.33,34 Some authors found that female sex was a significant poor prognostic factor, but other studies reported opposite results.10,35 We did not find correlation between outcome and these patients’ characteristics. Moreover, except for Jahangiri and colleagues, who identified tumor localization in the middle and lower third of the clivus as other risk factors for recurrence, no relation between the site of residual tumor and LC was reported in our study.36
Gross Tumor Volume
Regarding the influence of the residual tumor volume after surgery, the recurrence rate for SBC was markedly higher in patients with a partial resection than in those with a total resection.37,38 Many series have reported residual tumor volume to be a prognostic factor of LC.30,39 In several proton centers, reported experiences of patients with SBC have associated GTV with tumor control at cut-points of 25 mL and 28 mL.11,12,20,34 Considering carbon ion, Uhl and colleagues reported a significantly better LC rate when the boost PTV (including the GTV plus 2 mm of margins) was <75 mL.9
Likewise, in our series, a smaller GTV is associated with a more favorable outcome. Patients treated with PT without residual tumor (GTV not identified) volume after surgery showed a 100% LC rate after 5 years. In PT and CIRT cohorts, volumes of GTV influencing LC and OS were 10.4 cm3 and 23.1 cm3, respectively, presumably due to higher average GTV in patients who underwent CIRT. In the CIRT group, the estimated 3-year LC rates were 81% and 56% for patients with GTV ≤23.1 cm3 and >23.1 cm3, respectively; while in the PT group, 3-year LC rates were 94% and 71% for patients with GTV ≤10.4 cm3 and >10.4 cm3, respectively. Maximal safe resection in a highly specialized center for skull base surgery is the first goal to minimize this unfavorable prognostic factor for LC. Furthermore, the findings in our analysis data permit us to address more precisely the choice of particle based on GTV values. For the next future GTV >10.4 cm3 will be considered the first cut-point to address the patient preferentially to CIRT (balanced with other prognostic and toxicity risk factors), while GTV >23.1 cm3 will be considered for higher priority CIRT choice.
Prognostic Factors: Relationship with Organs at Risk
Every surgical effort must be attempted to remove, whenever possible, any tumor extension lying along critical structures (mainly optic nerves, optic chiasm, and brainstem). Although PT and CIRT can achieve a steep dose gradient to healthy tissue, critical structures’ dose constraints required to be taken into account.3 Different studies have identified optic apparatus and/or brainstem compression as one of the major prognostic factors for patients’ outcome, especially in PT series.4,11 Not surprisingly, in our series, brainstem compression alone in PT and optic apparatus and brainstem compression in CIRT had significant negative impacts for LC (87% and 92% of LR in the PT and CIRT cohorts, respectively). Brainstem and optic pathways dose constraints are significantly lower than therapeutic effective dose, consequently a significant percentage of target volume, especially GTV, may receive inadequate curative dose in patients with brainstem and/or optic pathways compression/abutment. In patients with brainstem and optic pathways compression/abutment and no feasibility for maximal safe resection, we usually discuss with referral neurosurgeon the feasibility of personalized combined strategy consisting of debulking-space surgery aimed to eliminate or however minimize abutment/compression. The relaxation of dose constraints for brainstem and optic pathways adopted in our institution since January 2019, particularly for CIRT (as detailed below), could permit further reduction of the impact of this prognostic factor for the future in patients treated in our institution.
Dose Level and Target Coverage
The presence of low-dose regions and dose inhomogeneity within the GTV is one primary reason for LR, and underdosing a portion of tumor may increase the risk of LR.40 In the French proton experience, dose delivered to 90% of the GTV, the minimum dose to GTV, and volume of GTV covered by the 95% isodose line (V95) were significantly associated with local failure.3
In 1999, Terahara and colleagues reported an evaluation of target dose coverage and LC in SBC treated with PT.31 In their experience, minimum dose to the target was a significant predictor for LC without finding any relation between prescribed dose and LC. Authors commented that portions of the target were underdosed in order to meet constraints to critical normal structures. This caused a significant heterogeneity in treatment plans: a lower probability of LC in a low-dose region cannot be compensated by higher doses delivered to the rest of the target volume.31
Our results emphasize the importance of adequate coverage of the target volume, which is a quantitative factor also inherently related to relationship between GTV and OARs. Due to target underdosages aimed at OAR sparing, target coverage (D95% of the CTV HR and D95% and D98% of the GTV) is significantly lower in patients that had LR. On the other hand, these findings confirm further the significant importance to deliver effectively a high dose level to obtain higher probability of LC, therefore our data analysis doesn’t support at the moment any changes in radiation dose adopted, especially for CIRT, for which dose concepts are more influenced by many features. For what concerns the specific case of CIRT, the approach followed at our institution was to take advantage of the long-term experience, in terms of LC and toxicity outcomes, of Japanese centers.14,41 Nevertheless, a different RBE model is used for RBE-weighted dose calculation in Europe and in Japan, potentially affecting the dose delivered to the patient if not accounted for. The applied prescription dose value is the result of a dose conversion study carried out at the beginning of clinical activity at CNAO,42,43 while brainstem and optic chiasm constraints were taken from the Japanese experience without correction, thus following a highly conservative approach.44 Recent evaluation of clinical outcomes showed that over-sparing of OARs could have compromised LC in specific patient groups.22 Therefore, efforts were directed toward an RBE model update and relaxation of constraints, particularly for the brainstem and optic pathways, with the goal of improving target coverage and therefore LC, without expected increase in tissue toxicity.44
Toxicity
Late high-grade (G3-G4) toxicity rates varied between 6–8.1% and 4.1–6% in PT and CIRT series, respectively.3,8,11,20 We reported 12% of high-grade late toxicity after SBC irradiation, considering both PT and CIRT cohort.
Temporal lobe necrosis is one of the most dreaded late adverse events in high-dose proton therapy for SBC.12 In several reports on particle therapy for skull chordomas and chondrosarcomas, it constitutes the most frequent normal tissue damage compared with optic nerve, optic chiasm, or brainstem toxicities.45 Santoni and colleagues reported 10.4% of clinical and radiological MRI changes consistent with radionecrosis after proton and photon therapy; 7 patients of 10 developed G3 late clinical symptoms such as seizure. In our series, no G3-G4 radionecrosis was reported, while 13% of patients developed moderate symptoms controlled by steroids.46 In a recent Japanese publication by Koto, asymptomatic radiation-induced brain injury was found in 28% of patients treated with CIRT. Symptomatic radionecrosis ≥G2 was reported in 8% of cases.47 In our study, asymptomatic white-matter injuries in the temporal lobe (radionecrosis G1) were noted in 20% of the patients. Temporal lobe lesions occurred after a median of 25 months after treatment and were confined to the high-dose region of the PT and CIRT plans. In all patients, MRI changes remained stable or resolved spontaneously.
Potential bias of the study could be: absence of histopathological review, inhomogeneity between treatment groups, or required larger number of patients due to the rareness of disease.
Conclusions
Five-year LC rates achieved after postoperative PT and CIRT were similar to those reported in the literature. Brainstem and/or optic apparatus compression, residual tumor volume, and target coverage were major prognostic factors. The estimated 3-year high-rate grade toxicity-free survival was >85% for CIRT and PT. In the era of personalized medicine, prognostic factors should address strategy in the optimal multimodality treatment of such a rare and challenging disease as SBC.
Dual particle choice treatment with consequent different RBE and different types of biological damages on tumor cells offers already a dual profile of radiation dose personalized on patients’ profile based on prognostic factors.
Furthermore, we can consider a prospective trial to investigate further personalization of treatment approach, based on these recognized prognostic factors and new possible factors as biomolecular/histopathologic and radiomic factors.
Funding
There are no funding sources to disclose.
Acknowledgments
S. Stacchiotti (Istituto Nazionale Tumori, Milan, Italy); D. Mazzatenta, E. Pasquini, M. Zoli (Bellaria Hospital, Bologna, Italy); F. Zenga (University of Turin, Italy); F. Doglietto (University of Brescia, Italy); D. Locatelli, P. Castelnuovo, M. Turri-Zanoni (Ospedale di Circolo e Fondazione Macchi, Varese, Italy); P. Ferroli, F. Acerbi, M. Schiariti, A. Perin, F. Prada, M. Saini, A. Saladino (Istituto Neurologico “Carlo Besta,” Milan, Italy); P. Cappabianca, L. M. Cavallo, D. Solari (Federico II University, Naples, Italy), G. B. Lasio, D. Milani, G. Colombo (Humanitas Clinical and Research Center, Rozzano, Milan, Italy); R. Galzio, M. Benazzo (Policlinico San Matteo, Pavia, Italy); M. Locatelli (Ospedale Maggiore Policlinico, Milan, Italy); L. Lauretti, A. Pompucci (Policlinico Universitario A. Gemelli, Rome, Italy). C.N.A.O. Foundation: E. Villani, A. Facoetti, A. Ferent.
Conflict of interest statement.
The authors declare no conflict of interest.
Authorship statement:
AI EDI GR conceived and designed the analysis.
AI EDI GR SM SG GV analyzed and interpreted data collected.
AI EDI GR wrote the first draft of the article.
SM SG contributed to critical revision of the article.
AI EDI GR SM GV MRF BV VV MB SR RP AB contributed to data collection.
SG conducted statistical analysis.
All authors contributed toward subsequent revisions and approved the submitted manuscript.
References
- 1. Smoll NR, Gautschi OP, Radovanovic I, Schaller K, Weber DC. Incidence and relative survival of chordomas: the standardized mortality ratio and the impact of chordomas on a population. Cancer. 2013;119(11):2029–2037. [DOI] [PubMed] [Google Scholar]
- 2. Flanagan AM, Yamaguchi T. Chordoma. In: Fletcher CDM, Bridge JA, Pancras CW, Mertens F, eds. World Health Organization (WHO) Classification of Tumours of Soft Tissue and Bone. Pathology and Genetics. Lyon, France: IARC Press; 2013:328–329. [Google Scholar]
- 3. Noël G, Feuvret L, Ferrand R, Boisserie G, Mazeron JJ, Habrand JL. Radiotherapeutic factors in the management of cervical-basal chordomas and chondrosarcomas. Neurosurgery. 2004;55(6):1252–1260; discussion 1260. [DOI] [PubMed] [Google Scholar]
- 4. Hug EB, Loredo LN, Slater JD, et al. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J Neurosurg. 1999;91(3):432–439. [DOI] [PubMed] [Google Scholar]
- 5. Samii A, Gerganov VM, Herold C, et al. Chordomas of the skull base: surgical management and outcome. J Neurosurg. 2007;107(2):319–324. [DOI] [PubMed] [Google Scholar]
- 6. Walcott BP, Nahed BV, Mohyeldin A, Coumans JV, Kahle KT, Ferreira MJ. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012;13(2):e69–e76. [DOI] [PubMed] [Google Scholar]
- 7. Stacchiotti S, Sommer J; Chordoma Global Consensus Group . Building a global consensus approach to chordoma: a position paper from the medical and patient community. Lancet Oncol. 2015;16(2):e71–e83. [DOI] [PubMed] [Google Scholar]
- 8. Schulz-Ertner D, Karger CP, Feuerhake A, et al. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int J Radiat Oncol Biol Phys. 2007;68(2):449–457. [DOI] [PubMed] [Google Scholar]
- 9. Uhl M, Mattke M, Welzel T, et al. Highly effective treatment of skull base chordoma with carbon ion irradiation using a raster scan technique in 155 patients: first long-term results. Cancer. 2014;120(21):3410–3417. [DOI] [PubMed] [Google Scholar]
- 10. Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol. 1999;175(Suppl 2):57–63. [DOI] [PubMed] [Google Scholar]
- 11. Weber DC, Malyapa R, Albertini F, et al. Long term outcomes of patients with skull-base low-grade chondrosarcoma and chordoma patients treated with pencil beam scanning proton therapy. Radiother Oncol. 2016;120(1):169–174. [DOI] [PubMed] [Google Scholar]
- 12. Fung V, Calugaru V, Bolle S, et al. Proton beam therapy for skull base chordomas in 106 patients: a dose adaptive radiation protocol. Radiother Oncol. 2018;128(2):198–202. [DOI] [PubMed] [Google Scholar]
- 13. Durante M, Loeffler JS. Charged particles in radiation oncology. Nat Rev Clin Oncol. 2010;7(1):37–43. [DOI] [PubMed] [Google Scholar]
- 14. Mizoe JE. Review of carbon ion radiotherapy for skull base tumors (especially chordomas). Rep Pract Oncol Radiother. 2016;21(4):356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanai T, Endo M, Minohara S, et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44(1):201–210. [DOI] [PubMed] [Google Scholar]
- 16. Tuan J, Vischioni B, Fossati P, et al. Initial clinical experience with scanned proton beams at the Italian National Center for Hadrontherapy (CNAO). J Radiat Res. 2013;54(Suppl 1):i31–i42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vogin G, Wambersie A, Koto M, et al. A step towards international prospective trials in carbon ion radiotherapy: investigation of factors influencing dose distribution in the facilities in operation based on a case of skull base chordoma. Radiat Oncol. 2019;14(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mayo C, Yorke E, Merchant TE. Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S36–S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Debus J, Hug EB, Liebsch NJ, et al. Brainstem tolerance to conformal radiotherapy of skull base tumors. Int J Radiat Oncol Biol Phys. 1997;39(5):967–975. [DOI] [PubMed] [Google Scholar]
- 20. Ares C, Hug EB, Lomax AJ, et al. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int J Radiat Oncol Biol Phys. 2009;75(4):1111–1118. [DOI] [PubMed] [Google Scholar]
- 21. Mayo C, Martel MK, Marks LB, Flickinger J, Nam J, Kirkpatrick J. Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S28–S35. [DOI] [PubMed] [Google Scholar]
- 22. Molinelli S, Bonora M, Magro G, et al. RBE-weighted dose in carbon ion therapy for ACC patients: impact of the RBE model translation on treatment outcomes. Radiother Oncol. 2019;141:227–233. [DOI] [PubMed] [Google Scholar]
- 23. Hasegawa A, Mizoe JE, Mizota A, Tsujii H. Outcomes of visual acuity in carbon ion radiotherapy: analysis of dose-volume histograms and prognostic factors. Int J Radiat Oncol Biol Phys. 2006;64(2):396–401. [DOI] [PubMed] [Google Scholar]
- 24. Scholz M, Kellerer AM, Kraft-Weyrather W, Kraft G. Computation of cell survival in heavy ion beams for therapy. The model and its approximation. Radiat Environ Biophys. 1997;36(1):59–66. [DOI] [PubMed] [Google Scholar]
- 25. Krämer M, Scholz M. Treatment planning for heavy-ion radiotherapy: calculation and optimization of biologically effective dose. Phys Med Biol. 2000;45(11):3319–3330. [DOI] [PubMed] [Google Scholar]
- 26. Mirandola A, Molinelli S, Vilches Freixas G, et al. Dosimetric commissioning and quality assurance of scanned ion beams at the Italian National Center for Oncological Hadrontherapy. Med Phys. 2015;42(9):5287–5300. [DOI] [PubMed] [Google Scholar]
- 27. Program, CTE. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0.http://evs.nci.nih.gov/ftp1/CTCAE. Accessed January 16, 2011.
- 28. Shanbhogue AK, Karnad AB, Prasad SR. Tumor response evaluation in oncology: current update. J Comput Assist Tomogr. 2010;34(4):479–484. [DOI] [PubMed] [Google Scholar]
- 29. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. [DOI] [PubMed] [Google Scholar]
- 30. Potluri S, Jefferies SJ, Jena R, et al. Residual postoperative tumour volume predicts outcome after high-dose radiotherapy for chordoma and chondrosarcoma of the skull base and spine. Clin Oncol (R Coll Radiol). 2011;23(3):199–208. [DOI] [PubMed] [Google Scholar]
- 31. Terahara A, Niemierko A, Goitein M, et al. Analysis of the relationship between tumor dose inhomogeneity and local control in patients with skull base chordoma. Int J Radiat Oncol Biol Phys. 1999;45(2):351–358. [DOI] [PubMed] [Google Scholar]
- 32. Zou MX, Lv GH, Zhang QS, Wang SF, Li J, Wang XB. Prognostic factors in skull base chordoma: a systematic literature review and meta-analysis. World Neurosurg. 2018;109:307–327. [DOI] [PubMed] [Google Scholar]
- 33. Choy W, Terterov S, Kaprealian TB, et al. Predictors of recurrence following resection of intracranial chordomas. J Clin Neurosci. 2015;22(11):1792–1796. [DOI] [PubMed] [Google Scholar]
- 34. Noël G, Habrand JL, Jauffret E, et al. Radiation therapy for chordoma and chondrosarcoma of the skull base and the cervical spine. Prognostic factors and patterns of failure. Strahlenther Onkol. 2003;179(4):241–248. [DOI] [PubMed] [Google Scholar]
- 35. Halperin EC. Why is female sex an independent predictor of shortened overall survival after proton/photon radiation therapy for skull base chordomas? Int J Radiat Oncol Biol Phys. 1997;38(2):225–230. [DOI] [PubMed] [Google Scholar]
- 36. Jahangiri A, Chin AT, Wagner JR, et al. Factors predicting recurrence after resection of clival chordoma using variable surgical approaches and radiation modalities. Neurosurgery. 2015;76(2):179–185; discussion 185. [DOI] [PubMed] [Google Scholar]
- 37. Amit M, Na’ara S, Binenbaum Y, et al. Treatment and outcome of patients with skull base chordoma: a meta-analysis. J Neurol Surg B Skull Base. 2014;75(6):383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Samii A, Gerganov VM, Herold C, et al. Chordomas of the skull base: surgical management and outcome. J Neurosurg. 2007;107(2):319–324. [DOI] [PubMed] [Google Scholar]
- 39. McDonald MW, Linton OR, Moore MG, Ting JY, Cohen-Gadol AA, Shah MV. Influence of residual tumor volume and radiation dose coverage in outcomes for clival chordoma. Int J Radiat Oncol Biol Phys. 2016;95(1):304–311. [DOI] [PubMed] [Google Scholar]
- 40. Noël G, Feuvret L, Calugaru V, et al. Chordomas of the base of the skull and upper cervical spine. One hundred patients irradiated by a 3D conformal technique combining photon and proton beams. Acta Oncol. 2005;44(7):700–708. [DOI] [PubMed] [Google Scholar]
- 41. Mizoe JE, Hasegawa A, Takagi R, Bessho H, Onda T, Tsujii H. Carbon ion radiotherapy for skull base chordoma. Skull Base. 2009;19(3):219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fossati P, Molinelli S, Matsufuji N, et al. Dose prescription in carbon ion radiotherapy: a planning study to compare NIRS and LEM approaches with a clinically-oriented strategy. Phys Med Biol. 2012;57(22):7543–7554. [DOI] [PubMed] [Google Scholar]
- 43. Molinelli S, Magro G, Mairani A, et al. Dose prescription in carbon ion radiotherapy: How to compare two different RBE-weighted dose calculation systems. Radiother Oncol. 2016;120(2):307–312. [DOI] [PubMed] [Google Scholar]
- 44. Dale JE, Molinelli S, Vitolo V, et al. Optic nerve constraints for carbon ion RT at CNAO - Reporting and relating outcome to European and Japanese RBE. Radiother Oncol. 2019;140:175–181. [DOI] [PubMed] [Google Scholar]
- 45. Pehlivan B, Ares C, Lomax AJ, et al. Temporal lobe toxicity analysis after proton radiation therapy for skull base tumors. Int J Radiat Oncol Biol Phys. 2012;83(5):1432–1440. [DOI] [PubMed] [Google Scholar]
- 46. Santoni R, Liebsch N, Finkelstein DM, et al. Temporal lobe (TL) damage following surgery and high-dose photon and proton irradiation in 96 patients affected by chordomas and chondrosarcomas of the base of the skull. Int J Radiat Oncol Biol Phys. 1998;41(1):59–68. [DOI] [PubMed] [Google Scholar]
- 47. Koto M, Hasegawa A, Takagi R, et al. Risk factors for brain injury after carbon ion radiotherapy for skull base tumors. Radiother Oncol. 2014;111(1):25–29. [DOI] [PubMed] [Google Scholar]