Abstract
Background
Breast cancer treatment is based on estrogen receptors (ERs), progesterone receptors (PRs), and human epidermal growth factor receptor 2 (HER2). At the time of metastasis, receptor status can be discordant from that at initial diagnosis. The purpose of this study was to determine the incidence of discordance and its effect on survival and subsequent treatment in patients with breast cancer brain metastases (BCBM).
Methods
A retrospective database of 316 patients who underwent craniotomy for BCBM between 2006 and 2017 was created. Discordance was considered present if the ER, PR, or HER2 status differed between the primary tumor and the BCBM.
Results
The overall receptor discordance rate was 132/316 (42%), and the subtype discordance rate was 100/316 (32%). Hormone receptors (HR, either ER or PR) were gained in 40/160 (25%) patients with HR-negative primary tumors. HER2 was gained in 22/173 (13%) patients with HER2-negative primary tumors. Subsequent treatment was not adjusted for most patients who gained receptors—nonetheless, median survival (MS) improved but did not reach statistical significance (HR, 17–28 mo, P = 0.12; HER2, 15–19 mo, P = 0.39). MS for patients who lost receptors was worse (HR, 27–18 mo, P = 0.02; HER2, 30–18 mo, P = 0.08).
Conclusions
Receptor discordance between primary tumor and BCBM is common, adversely affects survival if receptors are lost, and represents a missed opportunity for use of effective treatments if receptors are gained. Receptor analysis of BCBM is indicated when clinically appropriate. Treatment should be adjusted accordingly.
Key Points
1. Receptor discordance alters subtype in 32% of BCBM patients.
2. The frequency of receptor gain for HR and HER2 was 25% and 13%, respectively.
3. If receptors are lost, survival suffers. If receptors are gained, consider targeted treatment.
Keywords: breast cancer, brain metastases, estrogen/progesterone/HER2 receptor discordance
Importance of the Study.
This study is important because BCBM are a common clinical problem and this study demonstrates that ER, PR, and HER2 discordance between the primary tumor and BM is also common. Survival is worse if receptors are lost, and gain of receptors represents an often missed opportunity to implement receptor-targeted therapies. When clinically appropriate, biopsy/resection of BCBM for receptor analysis should be considered.
Breast cancer (BC) is the second most common cancer worldwide and the most common cancer in women. Globally, over 2 million patients receive this diagnosis annually and over 600 000 die from the disease.1 In the United States alone, in 2019 an estimated 268 600 new patients will be diagnosed and approximately 41 760 deaths will occur from the disease.2 Tumor subtype, governed by receptor expression or lack thereof, is a key prognostic factor for recurrence and survival.3 There are 3 established immunohistochemical biomarkers: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). These can be combined into 4 main subtypes: hormone receptor (HR)–positive/HER2-negative; HR-positive/HER2-positive (triple positive); HR-negative/HER2-positive, and HR-negative/HER2-negative (triple negative).4 Initial treatment is predicated upon subtype at the time of initial diagnosis.5 At the time of recurrence, the subtype can be discordant (receptor expression changing from that established at initial diagnosis).6
Studies focused on comparison of receptor status between primary tumor versus metachronous extracranial metastases have reported receptor discordance rates for ER, PR, and HER2 of 10–56%, 25–49%, and 3–16%, respectively.7–12 Based on these data, current guidelines of the American Society of Clinical Oncology advise offering biopsy where feasible to patients with recurrence to evaluate receptor status.13
BC is the second most common cause of brain metastases (BM). About half of BCBM occur in HER2-positive patients, followed by triple negative and then HR (ER or PR)-positive patients.14 There is limited literature on the incidence of subtype discordance and conflicting literature regarding the impact of discordance on subsequent treatment and survival.15–22 The purpose of this study is to determine the incidence of subtype discordance and the impact of discordance on subsequent therapy and survival in patients with BCBM, and represents the largest effort in the literature to date on this subject.
Methods
Our multinational (n = 3), multi-institutional (n = 18) consortium created an institutional review board–approved retrospective database of 2473 evaluable patients with newly diagnosed BCBM treated between January 1, 2006 and December 31, 2017 using Research Electronic Data Capture (REDCap) software hosted at the University of Minnesota. All patients had newly diagnosed BM, which we arbitrarily defined as those receiving treatment within 2 months of the diagnosis of BCBM. Patients with recurrent BCBM and those with leptomeningeal metastases were excluded. Of these 2473 patients, 521 underwent craniotomy for resection of the BCBM. In 2019, each institution updated its REDCap data with receptor status of the resected BCBM. Receptor analysis was available in 316/521 (61%) patients. ER/PR was defined as positive if >1% of cells stained positive and HER2 was defined as positive if 3+ stained positive or if fluorescent in situ hybridization (FISH) was >2.0. Discordance was considered present if ER or PR or HER2 status differed between the primary tumor and the BCBM. The overall receptor discordance rate was defined as the number of patients who had any receptor (ER, PR, or HER2) differ in the BM compared with the receptors in the primary tumor. The subtype discordance rate was defined as the number of patients who had their tumor subtype (triple positive, triple negative, ER or PR positive and HER2 negative, ER and PR negative and HER2 positive) differ in the BM compared with the tumor subtype in the primary tumor. The type of hormonal therapy and HER2-targeted therapy and the dates the patient received those therapies were collected. The criterion for receiving a given treatment was receipt of one or more doses of that treatment.
Median survival (MS) was calculated in months from date of BCBM diagnosis using the Kaplan–Meier method. Survival curves were compared using standard log-rank tests, and time from primary diagnosis to BM was compared using Wilcoxon rank-sum tests.
Results
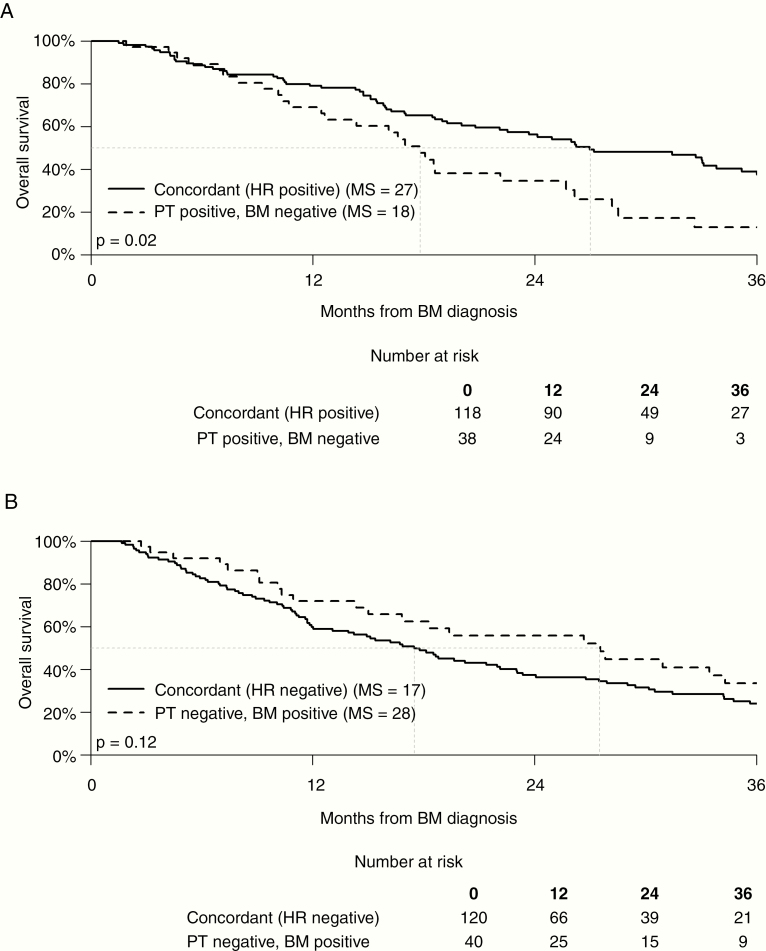
Patient characteristics are shown in Table 1. The majority of patients (59%) had solitary BM. The tumor subtypes of both the primary tumor and BM were roughly evenly distributed across the 4 subtypes. The receptor discordance rate (change in at least 1 of the 3 receptors) was 132/316 (42%). The overall rate of subtype discordance (receptor discordance leading to change in subtype classification) was 100/316 (32%). Table 1 also shows that the HR (ER or PR) gain occurred in 40 of 160 patients (25%) with HR-negative primary tumors, and HER2 was gained in 22 of 173 patients (13%) with HER2-negative primary tumors. The HR was lost in 38 of 156 patients (24%) with HR-positive primary tumors and HER2 was lost in 10 of 143 patients (7%) with HER2-positive primary tumors. Fig. 1 shows the Kaplan–Meier curves comparing MS for patients with concordant versus discordant HR: the MS for HR-positive patients with concordant BM (27 mo) was significantly longer than that for patients who had HR-positive primary tumors and discordant HR-negative BM (18 mo) (P = 0.02). The MS for HR-negative patients with concordant brain metastases (17 mo) was shorter than that for patients who had HR-negative primary tumors and discordant HR-positive brain metastases (28 mo), but the difference did not reach statistical significance (P = 0.12).
Table 1.
Patient characteristics
| Overall (N = 316) | |
|---|---|
| Median age, y, at BM diagnosis (dx) (Q1, Q3) | 54 (46, 62) |
| Female | 312 (99%) |
| Ethnicity | |
| Not Hispanic or Latino | 284 (90%) |
| Hispanic or Latino | 17 (5%) |
| Unknown/not reported | 15 (5%) |
| Race | |
| White | 231 (73%) |
| Black or African American | 33 (10%) |
| Asian | 13 (4%) |
| American Indian/Alaska Native | 2 (1%) |
| More than one race | 2 (1%) |
| Unknown/not reported | 35 (11%) |
| Number of BM at initial BM dx | |
| 1 | 187 (59%) |
| 2 | 57 (18%) |
| 3 | 23 (7%) |
| 4 | 11 (3%) |
| 5 | 15 (5%) |
| 6 | 5 (2%) |
| 7 | 5 (2%) |
| >7 | 13 (3%) |
| Extracranial metastases at BM dx | 187 (59%) |
| KPS at BM dx | |
| <70 | 16 (5%) |
| 70 | 42 (13%) |
| 80 | 82 (26%) |
| 90 | 98 (31%) |
| 100 | 29 (9%) |
| Unknown | 49 (16%) |
| Subtype of primary tumor | |
| HR-positive/HER2-negative | 88 (28%) |
| HR-positive/HER2-positive | 68 (22%) |
| HR-negative/HER2-positive | 75 (24%) |
| Triple negative | 85 (27%) |
| Subtype of BM | |
| HR-positive/HER2-negative | 74 (23%) |
| Triple positive | 84 (27%) |
| HR-negative/HER2-positive | 71 (22%) |
| Triple negative | 87 (28%) |
| Complete receptor concordance | |
| ER, PR, and HER2 concordant | 184 (58%) |
| ER, PR, or HER2 discordant | 132 (42%) |
| ER concordance Concordant negative | 140 (44%) |
| Concordant positive | 108 (34%) |
| Primary-negative, BM-positive | 30 (9%) |
| Primary-positive, BM-negative | 38 (12%) |
| PR concordance | |
| Concordant negative | 180 (57%) |
| Concordant positive | 54 (17%) |
| Primary-negative, BM-positive | 30 (9%) |
| Primary-positive, BM-negative | 52 (16%) |
| Hormone receptor concordance (ER and PR) | |
| Concordant negative | 120 (38%) |
| Concordant positive | 118 (37%) |
| Primary-negative, BM-positive | 40 (13%) |
| Primary-positive, BM-negative | 38 (12%) |
| HER2 concordance | |
| Concordant negative | 151 (48%) |
| Concordant positive | 133 (42%) |
| Primary-negative, BM-positive | 22 (7%) |
| Primary-positive, BM-negative | 10 (3%) |
Fig. 1.
PT: primary tumor.
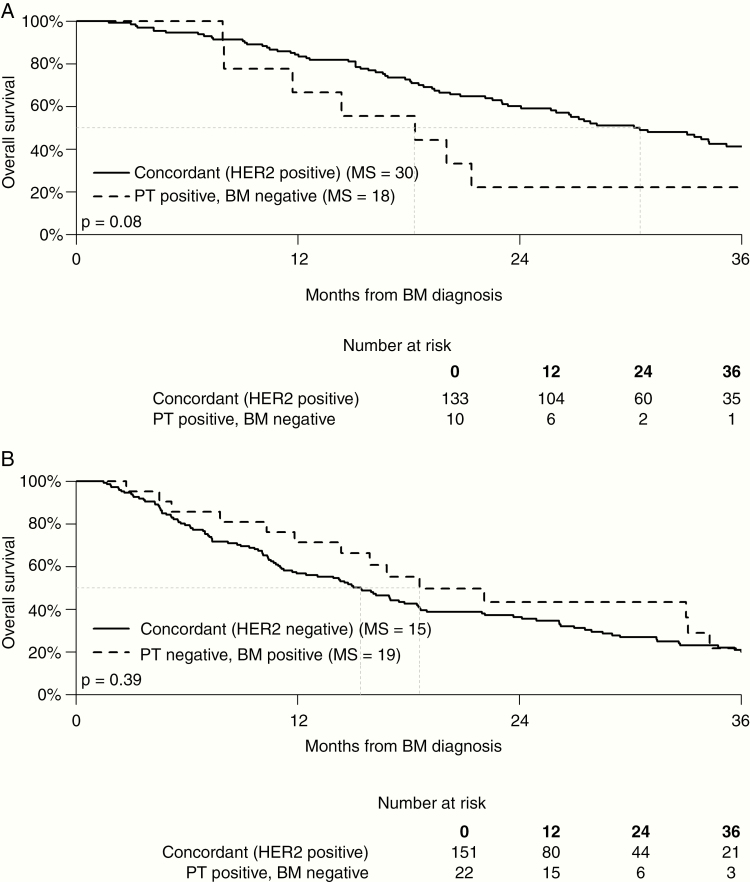
Fig. 2 shows that the Kaplan–Meier curves comparing MS for HER2-positive patients with concordant BM (30 mo) was longer than that for patients with HER2-positive primary tumors and discordant HER2-negative BM (18 mo), but the difference did not reach statistical significance (P = 0.08). The MS for patients with HER2-negative primary tumors and concordant BM (15 mo) was shorter than that for patients with HER2-negative primary tumors and discordant HER2-positive brain metastases (19 mo), but the difference did not reach statistical significance (P = 0.39).
Fig. 2.
PT: primary tumor.
Table 2 shows survival by primary tumor subtype and BM subtype. MS for patients whose primary tumor was HR-positive/HER2-negative (n = 88) and who were concordant in their BCBM (n = 55) was 18 months, in contrast to 33 months for the 14 discordant patients who gained HER2 expression, becoming HR-positive/HER2-positive. MS for patients whose primary tumor was HR-positive/HER2-positive (n = 68) and were concordant in their BCBM (n = 47) was 40 months, but only 18 months in the 18 discordant patients who lost ER/PR expression but maintained HER2 expression. MS for patients whose primary tumor was HR-negative/HER2-positive (n = 75) and who were concordant in their BCBM (n = 50) was 24 months compared with 33 months for discordant patients who gained ER/PR expression (n = 18), becoming HR-positive/HER2-positive. MS for patients whose primary tumor was triple negative (n = 85) and who were concordant in their BCBM (n = 64) was 11 months compared with 15 months for discordant patients who gained ER/PR expression (n = 14), becoming HR-positive/HER2-negative.
Table 2.
Discordance rate and survival by initial tumor subtype
| Primary Subtype | N (% of total) | Metastasis Subtype | N (% of primary subtype) | MS, months |
|---|---|---|---|---|
| HRpos/HER2neg | 88 (28) | Concordant | 55 (62) | 18 |
| HRpos/HER2pos | 14 (16) | 33 | ||
| HRneg/HER2pos | 1 (1) | NA | ||
| Triple negative | 18 (20) | 17 | ||
| HRpos/HER2pos2HER | 68 (22) | Concordant | 47 (69) | 40 |
| HRpos/HER2neg | 2 (3) | NA | ||
| HRneg/HER2pos | 18 (26) | 18 | ||
| Triple negative | 1 (1) | NA | ||
| HRneg/HER2pos | 75 (24) | Concordant | 50 (67) | 24 |
| HRpos/HER2neg | 3 (4) | NA | ||
| HRpos/HER2pos | 18 (24) | 33 | ||
| Triple negative | 4 (5) | NA | ||
| Triple negative | 85 (27) | Concordant | 64 (75) | 11 |
| HRpos/HER2neg | 14 (6) | 15 | ||
| HRpos/HER2pos | 5 (6) | NA | ||
| HRnegHER2pos | 2 (2) | NA | ||
| Overall | 316 | Concordant | 216 (68) | 22 |
| Discordant | 100 (32) | 20 |
HR considered positive if either ER or PR was positive.
The time from primary diagnosis to BM was analyzed. There was no significant difference in this time between patients with concordant versus discordant receptor status.
Table 3 shows the subsequent treatment for discordant patients. Among the 40 patients whose ER or PR status changed from negative to positive, 33 (82%) did not receive hormonal therapy after the diagnosis of BCBM, and 15/22 (68%) patients whose HER2 status changed from negative to positive did not receive HER2-targeted therapy after diagnosis of BCBM.
Table 3.
Timing of targeted treatment by concordance status
| Concordant Negative, (N = 120) | Concordant Positive (N = 118) | Primary − BM + (N = 40) | Primary + BM - (N = =38) | |
|---|---|---|---|---|
| Hormonal therapy | ||||
| Before BM only | 4 (3%) | 29 (25%) | 3 (8%) | 9 (24%) |
| After BM only | 1 (1%) | 8 (7%) | 1 (2%) | 1 (3%) |
| Both before and after BM | 1 (1%) | 33 (28%) | 2 (5%) | 8 (21%) |
| No hormonal therapy | 113 (94%) | 44 (37%) | 33 (82%) | 17 (45%) |
| Timing not reported | 1 (1%) | 4 (3%) | 1 (2%) | 3 (8%) |
| Concordant Negative (N = 151) | Concordant Positive (N = 133) | Primary − BM+ (N = 22) | Primary + BM− (N = 10) | |
| HER2 therapy | ||||
| Before BM only | 7 (5%) | 21 (16%) | 1 (5%) | 3 (30%) |
| After BM only | 6 (4%) | 7 (5%) | 3 (14%) | 1 (10%) |
| Both before and after BM | 4 (3%) | 62 (47%) | 3 (14%) | 1 (10%) |
| No HER2 therapy | 133 (88%) | 37 (28%) | 15 (68%) | 5 (50%) |
| Timing not reported | 1 (1%) | 6 (5%) | 0 (0%) | 0 (0%) |
Table 4 shows a comparison of ER, PR, and HER2 discordance rates between the primary breast tumor and BM in our data with 8 published studies. Supplementary Table 1 shows survival by era and primary treatment for BCBM patients. Supplementary Table 2 shows a list of the clinical data obtained in our REDCap database. Supplementary Figure 1 shows a diagram from the Consolidated Standards of Reporting Trials (CONSORT) for our study.
Table 4.
Comparison of ER, PR, and HER2 discordance rates between primary breast tumor and BM
| Author | Year | Discordance Rate | ||
|---|---|---|---|---|
| ER (%) | PR (%) | HER2 (%) | ||
| Yonemori | 2008 | 4/24 (17) | 1/24 (4) | 3/24 (13) |
| Hoefnagel | 2012 | 6/44 (14) | 16/44 (36) | 1/44 (2) |
| Omoto | 2010 | 4/21 (19) | 4/21 (19) | 4/21 (19) |
| Brogi | 2011 | 6/37 (16) | 8/37 (22) | 2/40 (5) |
| Duchnowski | 2012 | 35/120 (29) | 34/119 (29) | 17/119 (14) |
| Bachmann | 2013 | 7/22 (32) | 9/24 (38) | 4/24 (17) |
| Shen | 2015 | 10/35 (29) | 7/34 (21) | 1/36 (3) |
| Thomson | 2016 | 3/41 (7.3) | 1/41 (2) | 6/41 (15) |
| Pooled rates | 75/344 (21) | 80/344 (23) | 41/344 (12) | |
| Current study | 2020 | 68/316 (22) | 82/316 (26) | 32/316 (10) |
Discussion
Survival and our ability to predict survival for BCBM patients are improving.23–25 Receptor discordance between the primary tumor and the BM may impact survival. This study represents the single largest series to investigate the incidence of receptor discordance in this patient population. Furthermore, this work both shows the prognostic relevance to receptor discordance and highlights the often missed opportunity to implement effective targeted therapies when receptors are gained.
Discordance Rates
Two independent meta-analyses have investigated receptor discordance between the primary breast cancer and metastases.7,26 Aurilio reviewed 48 articles published between 1983 and 2011 from which ER, PR, and HER2 discordance was analyzed in 4200, 2739, and 2987 tumors, respectively.7 The discordance rates for ER, PR, and HER2 receptors were 20%, 33%, and 8%, respectively. Schrijver reviewed 39 articles published between 1986 and 2016 from which ER, PR, and HER2 discordance was analyzed in 1948, 1730, and 2440 tumors, respectively.26 They reported the direction of change (positive-to-negative or negative-to-positive) and metastasis location-specific differences. The positive-to-negative conversion rates for ER, PR, and HER2 were 22.5%, 49.4%, and 21.3%, respectively. The negative-to-positive conversion rates for ER, PR, and HER2 were 21.5%, 15.9%, and 9.5%, respectively. Furthermore, Schrijver found that ER discordance was more common in brain (20.8%) and bone (29.3%) than in liver (14.3%) metastases. PR discordance was more common in bone (42.7%) and liver (47.0%) than in brain (23.3%) metastases. There was no significant difference in HER2 discordance between brain, bone, and liver metastases. Both meta-analyses concluded that large prospective studies are needed to determine the impact of receptor discordance on treatment and survival. Meanwhile, reassessing receptor status in metastases was strongly encouraged.
The data that are focused on receptor discordance specifically in BC patients with BM are much more limited. The combined sample size of the 8 retrospective reports in the Schrijver meta-analysis which included BM was 344.9,15,17,18,20,22,27,28 Our sample (n = 316 in this single report) is comparable in size and the discordance rates are also similar. See Tables 1 and 4. Regarding the direction of conversion, our data showed that lower positive-to-negative conversion rates for ER, PR, and HER2 were 38/316 (12%), 52/316 (16%), and 10/316 (10%), respectively, compared with the pooled all-site data in the Schrijver meta-analysis detailed above. The negative-to-positive conversion rates for ER, PR, and HER2 were 30/316 (9%), 30/316 (9%), and 22/316 (7%) and were also lower than the pooled all-site data in the Schrijver meta-analysis.
Effect of Discordance on Survival
One prospective study analyzed the effect of discordance on survival and found no significant association between overall survival and discordance (median overall survival was 27.6 and 30.2 mo in the concordant and discordant groups, respectively); however, that study included all sites of metastases, not just BM.29 In contrast, one retrospective series of patients who underwent craniotomies for BCBM between 2002 and 2014 reported 21/37 had receptor data available and 11/21 had conversion of at least one receptor from positive to negative.11 In that study, MS for patients with concordant receptor status versus discordant (change from positive to negative) was 31 and 19 months (P = 0.18), respectively. Our results were similar, in that MS for patients with concordant receptor status versus discordant (change from positive to negative) was 27 versus 18 months (P = 0.02) for ER/PR and 30 versus 18 months (P = 0.08) for HER2.
Effect of Discordance Discovery on Treatment
A few small retrospective studies have identified a change in management in 12–20% of patients when there was a gain in receptor status.30–32 Our results were similar in that only 18% of patients who gained estrogen or progesterone receptors (change from negative to positive) and only 32% of patients who gained HER2 received the indicated targeted therapy.
Physicians involved in the care of patients with BCBM need to be cognizant of the possibility of discordance, and when craniotomy is not clinically appropriate, we should develop other strategies to assess receptor status of BCBM (eg, liquid biopsy in either blood or even CSF) to help provide much-needed information to more effectively treat our patients. If an extracranial metastasis has been previously biopsied, a craniotomy is not indicated unless otherwise necessary for relief of mass effect.
Possible Explanations for Discordance
The many possible explanations for receptor discordance in BC include: (i) inaccuracy of the immunohistochemical staining varies33; (ii) different sampling methods (fine needle aspiration vs core biopsy vs surgical resection of the tumor) may contribute to discrepant receptor results6; (iii) intratumor and intertumor heterogeneity are more commonly seen with improved sequencing technology34; (iv) clonal genome evolution can cause discordance14,35–37; (v) newly acquired biological characteristics in the tumor microenvironment facilitate metastases38; and (vi) treatment can alter receptor status, as discussed below.
Effect of Treatment on Discordance
Intervening treatment between primary and metastasis may also explain loss of receptor expression either through a direct effect16,17,20 or via clonal expression. For example, selective eradication of ER/PR positive cells by hormonal therapy could select for a population of ER/PR negative cells that could later metastasize.39 Timmer et al reported that among 7 ER-positive patients treated with antihormonal therapy, the BM they developed were ER negative in all, and 5 of the 7 exhibited a negative conversion of the PR, whereas in patients without anti-estrogen treatment, only 1 of 10 had an ER conversion.21 Neoadjuvant chemotherapy can result in a significant reduction in the expression of ER and Ki-67 index,40 but the same group reported no significant change in PR or HER2.41 To our knowledge, no study has reported, as ours does, the percent of patients who actually had treatment added or omitted after discordance was discovered.
Limitations
Limitations of this study include the retrospective nature of the database, although there is no reason to believe that selection bias, inherent in all retrospective studies, would influence concordance/discordance status. Secondly, although this is the largest analysis of the concordance/discordance status of BC metastases ever reported (N = 316), some of the discordant subsets are relatively small (Table 1). Thirdly, no central review of pathology was performed, as it was not feasible in a retrospective study with 18 institutions spanning 3 countries. Fourth, retrospective data cannot be used to quantify the impact of a change in systemic therapy after diagnosis of BCBM on survival.
Conclusions
Receptor and subtype discordance between primary breast cancers and brain metastases is common. When discordance was found, subsequent treatment was not adjusted for most patients who gained receptors, nonetheless MS improved but did not reach statistical significance. Receptor gain thus represents an often missed opportunity to implement potentially effective therapies in discordant BC patients who gain ERs/PRs or HER2. This is important because survival for these patients is much improved than in the past. In contrast, loss of receptor adversely affects survival and should influence decisions regarding the relative merits of continuing receptor-targeted treatments. We recommend biopsy/resection and subtype analysis on brain metastasis tissue whenever feasible and clinically appropriate. If discordance is found, change of treatment should be considered.
Funding
This work was supported by National Institutes of Health (NIH) grant #UL1TR002494 from the National Center for Advancing Translational Sciences (NCATS); NIH grant #P30 CA77598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and the NCATS.
Conflict of interest statement. The authors have no conflict of interest related to this work.
Authorship statement. All authors contributed to data collection, analysis, and writing of the manuscript. The design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication was solely the responsibility of the authors and does not necessarily represent the official views of the funders/sponsors (National Center for Research Resources or the NIH).
Supplementary Material
Acknowledgments
Database support and management: Susan Lowry, database programmer/analyst and REDCap administrator, Biostatistical Design and Analysis Center, Clinical and Translational Science Institute (CTSI), University of Minnesota, 717 Delaware St SE, Room 140–21, Minneapolis, MN 55414. Paul Sperduto and Ryan Shanley had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. Bray FG, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;0:1–31. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 3. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast cancer. Nature. 2012;490(7418):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldhirsch A, Winer EP, Coates AS, et al. ; Panel members Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amin MB, Edge S, Greene F, et al (eds). AJCC Cancer Staging Manual. 8th edition. Chicago, IL: Springer International Publishing: American Joint Commission on Cancer; 2017. [Google Scholar]
- 6. McAnena PF, McGuire A, Ramli A, et al. Breast cancer subtype discordance: impact on post-recurrence survival and potential treatment options. BMC Cancer. 2018;18(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aurilio G, Disalvatore D, Pruneri G, et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur J Cancer. 2014;50(2):277–289. [DOI] [PubMed] [Google Scholar]
- 8. Lindström LS, Karlsson E, Wilking UM, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012;30(21):2601–2608. [DOI] [PubMed] [Google Scholar]
- 9. Hoefnagel LD, van de Vijver MJ, van Slooten HJ, et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2010;12(5):R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gong Y, Han EY, Guo M, Pusztai L, Sneige N. Stability of estrogen receptor status in breast carcinoma: a comparison between primary and metastatic tumors with regard to disease course and intervening systemic therapy. Cancer. 2011;117(4):705–713. [DOI] [PubMed] [Google Scholar]
- 11. Jung J, Lee SH, Park M, et al. Discordance in ER, PR, and HER2 between primary breast cancer and brain metastasis. J Neurooncol. 2018;137:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thompson AM, Jordan LB, Quinlan P, et al. ; Breast Recurrence in Tissues Study Group Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS). Breast Cancer Res. 2010;12(6):R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Poznak C, Somerfield MR, Bast RC, et al. Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2015;33(24):2695–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Venur VA, Leone JP. Targeted therapies for brain metastases from breast cancer. Int J Mol Sci 2016;17:1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brogi E, Murphy CG, Johnson ML, et al. Breast carcinoma with brain metastases: clinical analysis and immunoprofile on tissue microarrays. Ann Oncol. 2011;22(12):2597–2603. [DOI] [PubMed] [Google Scholar]
- 16. Broom RJ, Tang PA, Simmons C, et al. Changes in estrogen receptor, progesterone receptor and Her-2/neu status with time: discordance rates between primary and metastatic breast cancer. Anticancer Res. 2009;29(5):1557–1562. [PubMed] [Google Scholar]
- 17. Duchnowska R, Dziadziuszko R, Trojanowski T, et al. ; Polish Brain Metastasis Consortium Conversion of epidermal growth factor receptor 2 and hormone receptor expression in breast cancer metastases to the brain. Breast Cancer Res. 2012;14(4):R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Omoto Y, Kurosumi M, Hozumi Y, et al. Immunohistochemical assessment of primary breast tumors and metachronous brain metastases, with particular regard to differences in the expression of biological markers and prognosis. Exp Ther Med. 2010;1(4):561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shao MM, Liu J, Vong JS, et al. A subset of breast cancer predisposes to brain metastasis. Med Mol Morphol. 2011;44(1):15–20. [DOI] [PubMed] [Google Scholar]
- 20. Thomson AH, McGrane J, Mathew J, et al. Changing molecular profile of brain metastases compared with matched breast primary cancers and impact on clinical outcomes. Br J Cancer. 2016;114(7):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Timmer M, Werner JM, Röhn G, et al. Discordance and conversion rates of progesterone-, estrogen-, and HER2/neu-receptor status in primary breast cancer and brain metastasis mainly triggered by hormone therapy. Anticancer Res. 2017;37(9):4859–4865. [DOI] [PubMed] [Google Scholar]
- 22. Yonemori K, Tsuta K, Shimizu C, et al. Immunohistochemical profiles of brain metastases from breast cancer. J Neurooncol. 2008;90(2):223–228. [DOI] [PubMed] [Google Scholar]
- 23. Sperduto PW, Kased N, Roberge D, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012;82(5):2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sperduto PW, Kased N, Roberge D, et al. Summary report on the Graded Prognostic Assessment (GPA): an accurate and facile diagnosis-specific tool to estimate survival, guide treatment and stratify clinical trials for patients with brain metastases. J Clin Onc 2012;30:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sperduto PW, Mesko S, Li J, et al. Beyond an updated Graded Prognostic Assessment (Breast GPA): A prognostic index and trends in treatment and survival in breast cancer brain metastases from 1985 to today. Int J Radiat Oncol Biol Phys 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schrijver WAME, Suijkerbuijk KPM, van Gils CH, van der Wall E, Moelans CB, van Diest PJ. Receptor conversion in distant breast cancer metastases: a systematic review and meta-analysis. J Natl Cancer Inst. 2018;110(6):568–580. [DOI] [PubMed] [Google Scholar]
- 27. Bachmann C, Grischke EM, Staebler A, Schittenhelm J, Wallwiener D. Receptor change-clinicopathologic analysis of matched pairs of primary and cerebral metastatic breast cancer. J Cancer Res Clin Oncol. 2013;139(11):1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shen Q, Sahin AA, Hess KR, et al. Breast cancer with brain metastases: clinicopathologic features, survival, and paired biomarker analysis. Oncologist. 2015;20(5):466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Amir E, Miller N, Geddie W, et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol. 2012;30(6):587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. St Romain P, Madan R, Tawfik OW, Damjanov I, Fan F. Organotropism and prognostic marker discordance in distant metastases of breast carcinoma: fact or fiction? A clinicopathologic analysis. Hum Pathol. 2012;43(3):398–404. [DOI] [PubMed] [Google Scholar]
- 31. Zidan J, Dashkovsky I, Stayerman C, Basher W, Cozacov C, Hadary A. Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br J Cancer. 2005;93(5):552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simmons C, Miller N, Geddie W, et al. Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Ann Oncol. 2009;20(9):1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Allred DC. Commentary: hormone receptor testing in breast cancer: a distress signal from Canada. Oncologist. 2008;13(11):1134–1136. [DOI] [PubMed] [Google Scholar]
- 34. Venur VA, Cohen JV, Brastianos PK. Targeting molecular pathways in intracranial metastatic disease. Front Oncol. 2019;9:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brastianos PK, Carter SL, Santagata S, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5(11):1164–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shipitsin M, Campbell LL, Argani P, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11(3):259–273. [DOI] [PubMed] [Google Scholar]
- 37. Navin N, Kendall J, Troge J, et al. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472(7341):90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chambers AF, Naumov GN, Vantyghem SA, Tuck AB. Molecular biology of breast cancer metastasis. Clinical implications of experimental studies on metastatic inefficiency. Breast Cancer Res. 2000;2(6):400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kurbel S. Selective reduction of estrogen receptor (ER) positive breast cancer occurrence by estrogen receptor modulators supports etiological distinction between ER positive and ER negative breast cancers. Med Hypotheses. 2005;64(6):1182–1187. [DOI] [PubMed] [Google Scholar]
- 40. Chatterjee S, Saha A, Arun I, et al. Correlation of clinicopathological outcomes with changes in IHC4 status after NACT in locally advanced breast cancers: do pre-NACT ER/PR status act as better prognosticators? Breast Cancer (Dove Med Press). 2015;7:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Agrawal S, Banswal L, Saha A, et al. Progesterone receptors, pathological complete response and early outcome for locally advanced breast cancer—a single centre study. Indian J Surg Oncol. 2016;7(4):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.