Abstract
Background
The purpose of this analysis is to report long-term health-related quality of life (HRQoL) among brain tumor survivors treated with proton therapy (PRT) at a very young age.
Methods
Fifty-nine children <4 years old received PRT between 2000 and 2011. Forty families participated. HRQoL was assessed by child self-report (CSR; age ≥5) and parent proxy report (PPR; age 2+) using the PedsQL Core.
Results
The median age was 2.5 years (range, 0.3–3.8) at PRT and 9.1 years (5.5–18) at last follow-up. The most common diagnoses were ependymoma (n = 22) and medulloblastoma (n = 7). Median follow-up is 6.7 years (3–15.4). Follow-up mean CSR and PPR scores were: total core (78.4 and 72.9), physical (82.9 and 75.2), psychosocial (76.0 and 71.6), emotional (74.4 and 70.7), social (81.2 and 75.1), and school (72.4 and 69.9). Parent-reported HRQoL fell within a previously defined range for healthy children in 37.5% of patients, and for children with severe health conditions in 45% of patients. PPR HRQoL was stable from baseline to last follow-up among all domains except for social functioning. History of gastrostomy tube was significantly associated with poorer CSR and PPR HRQoL on multivariable analysis. Ninety percent of children functioned in a regular classroom, 14 (36%) used a classroom aid, 9 (23%) used an outside tutor, and 18 (46%) had an individualized education plan.
Conclusion
Long-term HRQoL among brain tumor survivors treated with PRT at a very young age is variable, with over a third achieving HRQoL levels commensurate with healthy children.
Key Points
1. One third of survivors reported long-term HRQoL scores comparable to those of healthy children.
2. Treatment for hydrocephalus or a feeding tube was associated with significantly lower HRQoL.
3. Total core HRQoL scores remained stable from baseline to last follow-up.
Keywords: brain tumor, pediatric, proton, quality of life, radiation
Importance of the Study.
Concern over radiation late effects and the resulting impact on quality of life is a driving factor in making treatment decisions for very young children with brain tumors. However, long-term quality of life outcome data are lacking in this population, particularly among a cohort of patients treated with modern radiation therapy techniques such as proton therapy. This is the first manuscript, to our knowledge, to report prospectively collected HRQoL data among pediatric brain tumor survivors treated with protons at the very young age of less than 4 years. Results are encouraging, with HRQoL scores remaining stable over time and approximately one third of the population reporting HRQoL scores comparable to those of healthy children, though a larger population reported HRQoL scores more similar to those of children with other severe health conditions. These results are important to help counsel families about treatment effects and in guiding treatment strategies specific to each child.
Radiation therapy is a critical component of the curative treatment for multiple pediatric brain tumors. However, radiation carries a risk for a myriad of late effects, including cognitive dysfunction, hormonal deficits, and hearing loss which can negatively and permanently impact the quality of life of survivors.1–3 Radiation is most feared among very young children, who are highly vulnerable to the adverse effects of radiotherapy. Treatment strategies focused on optimizing quality of life for brain tumor survivors may involve avoiding or delaying radiation in our youngest patients, sometimes even at the risk of compromising cancer cure.
The negative sequelae of radiation therapy in children are directly related to the volume of normal tissues exposed and the radiation dose received and are often inversely related to the age of the patient.4–8 Proton therapy (PRT) is an advanced radiotherapy approach now increasingly used for pediatric brain tumor patients in an effort to reduce the risk of radiation-associated adverse late effects due its unique dose deposition pattern resulting in markedly reduced radiation exposure to the normal brain compared with photon therapy.9 Dose modeling studies predict that the reduced radiation exposure to the surrounding brain with protons will translate to a reduced risk of cognitive dysfunction,10 and emerging clinical data among proton brain tumor survivors demonstrates favorable cognitive and health-related quality of life (HRQoL) outcomes.11–18
Very young children remain a unique population who are most sensitive to the adverse effect of radiotherapy and for whom the long-term impacts of radiotherapy on HRQoL remain poorly described. Few children below the age of 4 have been included in previous reports, and HRQoL outcomes specific to children exposed to cranial radiotherapy at this young age have not yet been described. Understanding quality of life outcomes among brain tumor survivors treated with contemporary radiotherapy techniques such as PRT at a very young age is critical for both providers and patient families when developing a treatment strategy for each child. The purpose of this analysis is to (i) report long-term HRQoL in pediatric brain tumor survivors treated with PRT at less than 4 years of age and how demographic and clinical variables affect them; and (ii) to compare HRQoL outcomes with published scores for healthy children and children with other chronic health conditions.
Methods
Patient Population
After institutional review board approval, pediatric brain tumor patients treated with PRT at Massachusetts General Hospital between 2004 and 2011 were offered enrollment onto a prospective longitudinal study of HRQoL assessment.15 Families who elected to participate signed informed consent for study participation. Patients and their families completed the Pediatric Quality of Life Inventory (PedsQL) core module, by child-self report (CSR; children ≥5 y) and parent proxy report (PPR; children ≥2 y). Assessments were completed at baseline, during treatment, and annually thereafter. Patients <4 years of age at the time of enrollment were selected for inclusion in this analysis.
Additionally, institutional review board approval was obtained for a cross-sectional HRQoL protocol whereby institutional records were retrospectively reviewed to identify patients treated in 2000–2011 who were less than 4 years of age at the time of radiotherapy, with no known tumor recurrence, and not previously enrolled in the prospective longitudinal HRQoL study. Additional inclusion criteria included a minimum of 3 years between radiotherapy and last follow-up, and primary language of English or Spanish. These patients were contacted through the mail and offered enrollment in an effort to expand the cohort. A consent for participation was included along with the study surveys in the mailed packet. Patients and their families who elected to participate completed the PedsQL core module, by CSR and PPR at a single timepoint in follow-up, and returned them in a prepaid envelope along with the signed consent statement.
HRQoL Assessments
The PedsQL is a widely used and validated pediatric HRQoL tool with a generic core scale suitable for use with both healthy populations and populations with acute and chronic health conditions such as cancer.19–21 The 23-item total core score reflects physical functioning (8 items), emotional functioning (5 items), social functioning (5 items), and school functioning (5 items). Physical, emotional, social, and school functioning are measured and reported as subscores, and the psychosocial health summary score is a mean of individual items from the emotional, social, and school subscales.19 Parents respond based on a 5-point Likert scale. All PedsQL scores are scaled from 0 to 100, with higher scores indicating better HRQoL. A school placement questionnaire inquiring about the type of school and use of any specialized education services was also completed.22
For all patients, baseline demographic and clinical treatment variables were collected. Socioeconomic status was estimated by using the median household income calculated by residential zip code.23
Statistics
Descriptive statistics for patient demographic, clinical, and treatment variables are reported. PedsQL total core scores and subscores are reported as mean and standard deviation for the cohort at baseline and follow-up. For the brain tumor cohort, CSR scores are compared with PPR scores using the paired t-test. Linear regression coefficient and Spearman correlation coefficient are used for univariate and multivariate analysis to evaluate the relationship of HRQoL scores to demographic and clinical variables. For patients with baseline and follow-up data, the mean and median HRQoL at baseline and last follow-up are compared using the paired t-test and Wilcoxon signed rank test, respectively.
HRQoL scores among the brain tumor survivors are also compared with published cohorts of healthy children and children with chronic health conditions, using a paired t-test. The population used for comparison was previously reported by Varni et al19 and included children aged 5–18 years and parents of children aged 2–18 years seen in outpatient primary care or specialty clinics such as orthopedics, rheumatology, cardiology, and diabetes.19 The 768 children and 1379 parents who either self-reported chronic health conditions or were designated as healthy were used for comparison in this analysis.19 In addition, PPR total core scores were used to identify brain tumor patients meeting previously established clinically meaningful cutoffs of the PedsQL to identify patients with HRQoL similar to healthy children versus those with mild, moderate, and major chronic health conditions.24
Responses to the school data questionnaire are presented. Data analysis was performed using SAS 9.4. All P-values are based on a two-sided hypothesis test with values less than 0.05 considered statistically significant.
Results
Patient Population
Fifty-nine eligible children received PRT for a brain tumor at <4 years of age between 2000 and 2011 and were without recurrent disease. A total of 40 children and their families participated in this study, including 18 of 22 patients enrolled on the prospective longitudinal HRQoL protocol who completed baseline and follow-up QoL assessments, and 22 of 37 patients who returned HRQoL assessments through the mail.
Patient, tumor, and treatment characteristics are detailed in Table 1. The median age at PRT was 2.5 years (range, 0.3‒3.8) and at last follow-up was 9.1 years (range, 5.5‒18). The most common diagnoses were ependymoma (n = 22) and medulloblastoma (n = 7), followed by craniopharyngioma (n = 4), atypical teratoid rhabdoid tumor (n = 3), primitive neuroectodermal tumor (n = 3), and glioblastoma (n = 1). Five of the medulloblastoma patients received craniospinal irradiation (CSI; median dose, 23.4 Gy relative biological effectiveness [RBE]; range, 18–36 GyRBE) and all patients received PRT to a median dose of 54 GyRBE to a boost or focal field.
Table 1.
Patient demographics and clinical variables
| n Median (range) | |
|---|---|
| Age at radiotherapy | 2.5 years (0.3–3.8) |
| Age at follow-up | 9.1 years (5.5–18.0) |
| Sex | |
| Male | 15 (37.5%) |
| Female | 25 (62.5%) |
| Race | |
| White | 37 (92.5%) |
| Black | 1 (2.5%) |
| Other | 2 (5%) |
| Income | $76K (32–151) |
| Histology | |
| Ependymoma | 22 (55%) |
| Medulloblastoma | 7 (17.5%) |
| Other | 11 (27.5%) |
| Shunt for hydrocephalus | |
| Yes | 13 (32.5%) |
| No | 27 (67.5%) |
| Gastrostomy tube | |
| Yes | 7 (17.5%) |
| No | 33 (82.5%) |
| Tracheostomy | |
| Yes | 2 (5%) |
| No | 38 (95%) |
| Chemotherapy | |
| Yes | 24 (60%) |
| No | 16 (40%) |
| High-dose or intrathecal chemotherapy | |
| Yes | 10 (25%) |
| No | 30 (75%) |
| Radiation type | |
| Supratentorial involved field | 12 (30%) |
| Infratentorial involved field | 23 (57.5%) |
| Craniospinal Irradiation | 5 (12.5%) |
| Total radiation dose | 54 Gy (50.4–57.6) |
HRQoL Outcomes
The median follow-up between treatment and last HRQoL assessment is 6.7 years (range, 3–15.4 y). The PedsQL total core score and subscores at last-follow-up according to CSR and PPR are listed in Table 2. Child- and parent-reported scores were highly correlated (correlation coefficient, 0.685–0.784, P < 0.001). Child-reported scores tended to be higher than parent-reported scores, a finding that was statistically significant for the total core score and physical, psychosocial, and social subscores (Supplementary Table 1).
Table 2.
Child and parent reported HRQoL for brain tumor and reference cohorts
| Child Self-Report | Parent Proxy Report | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Brain Tumor (N = 39) | Healthy Children (N = 401) | P- value | Chronically Ill (N = 367) | P- value | Brain Tumor N = 40 | Healthy Children (N = 717) | P- value* | Chronically Ill (N = 662) | P- value* | |
| Total Core | 78.4 (14.4)) | 83.00 (14.79) | 0.064 | 77.19 (15.53) | 0.642 | 72.9 (19.1) | 87.61 (12.33) | 0.0001 | 74.22 (18.40) | 0.660 |
| Physical | 82.9 (17.3) | 84.41 (17.26) | 0.602 | 77.36 (20.36) | 0.102 | 75.2 (23.1) | 89.32 (16.35) | 0.0001 | 73.28 (27.02) | 0.660 |
| Psychosocial | 76.0 (14.5) | 82.38 (15.51) | 0.014 | 77.10 (15.84) | 0.678 | 71.6 (18.7) | 86.58 (12.79) | 0.0001 | 74.80 (18.16) | 0.280 |
| Emotional | 74.4 (15.1) | 80.86 (19.64) | 0.047 | 76.40 (21.48) | 0.571 | 70.7 (19.5) | 82.64 (17.54) | 0.0001 | 73.05 (23.27) | 0.532 |
| Social | 81.2 (17.9) | 87.42 (17.18) | 0.032 | 81.60 (20.24) | 0.906 | 75.1 (23.0) | 91.56 (14.2) | 0.0001 | 79.77 (21.91) | 0.192 |
| School | 72.4 (19.6) | 78.63 (20.53) | 0.070 | 73.43 (19.57) | 0.755 | 69.9 (20.6) | 85.47 (17.6) | 0.0001 | 71.08 (23.99) | 0.761 |
* Brain tumor survivor and normal control cohort mean scores (SD) at last follow-up are compared with the paired t-test.
HRQoL at last follow-up for the brain tumor population is compared with HRQoL for the reference population in Table 2. For both CSR and PPR HRQoL, mean total core scores at last follow-up were similar to those of children with other chronic health conditions (78.4 vs 77.19, P = 0.642, and 72.9 vs 74.22, P = 0.660, respectively) and inferior to those of healthy children (78.4 vs 83.0, P = 0.064, and 72.9 vs 87.61, P < 0.001). The brain tumor population scores, for both CSR and PPR, were similar to those of children with other chronic health conditions in all domains. CSR scores were statistically inferior to healthy children in psychosocial, emotional, and social health. CSR scores of school functioning bordered on significance (72.4 vs 78.63, P = 0.070), and physical scores were similar (82.9 vs 84.41, P = 0.602). Parents of brain tumor survivors reported statistically significantly lower HRQoL than the parents of healthy children across all domains.
PPR total core scores for each patient were used to classify survivors into groups based on whether their scores were similar to healthy children (>83), or children with mild (>79–83), moderate (>77–79), or severe (≤77) health conditions as has been previously defined.24 Using these values, 15 parents (37.5%) reported HRQoL within the range of healthy children, while 7 (17.5%) parent reports fell within the range for mild to moderate health conditions, and 18 (45%) for severe chronic health conditions.
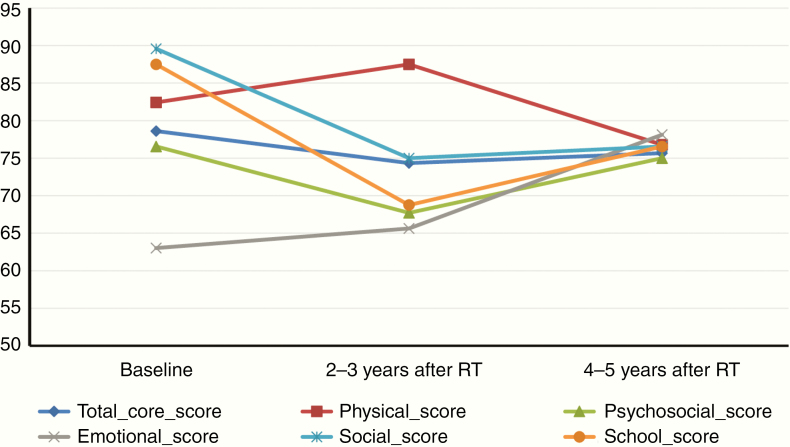
For the 18 patients with prospectively collected longitudinal data, the median follow-up was 7.0 years (range, 3.1–11 y). HRQoL scores were stable over time among all domains (Figure 1, Supplementary Table 2), with the exception of social score, median 89.6 at baseline and 75 at follow-up (P = 0.035).
Fig. 1.
Change in HRQoL over time among the 18 patients enrolled in the prospective longitudinal protocol. Change is represented by the median scores at baseline and in follow-up 2–3 years and 4–5 years after radiotherapy. Of note, baseline school functioning scores are available for only 8 patients due to the young age at the time of radiotherapy.
Relationship of HRQoL to Clinical Variables
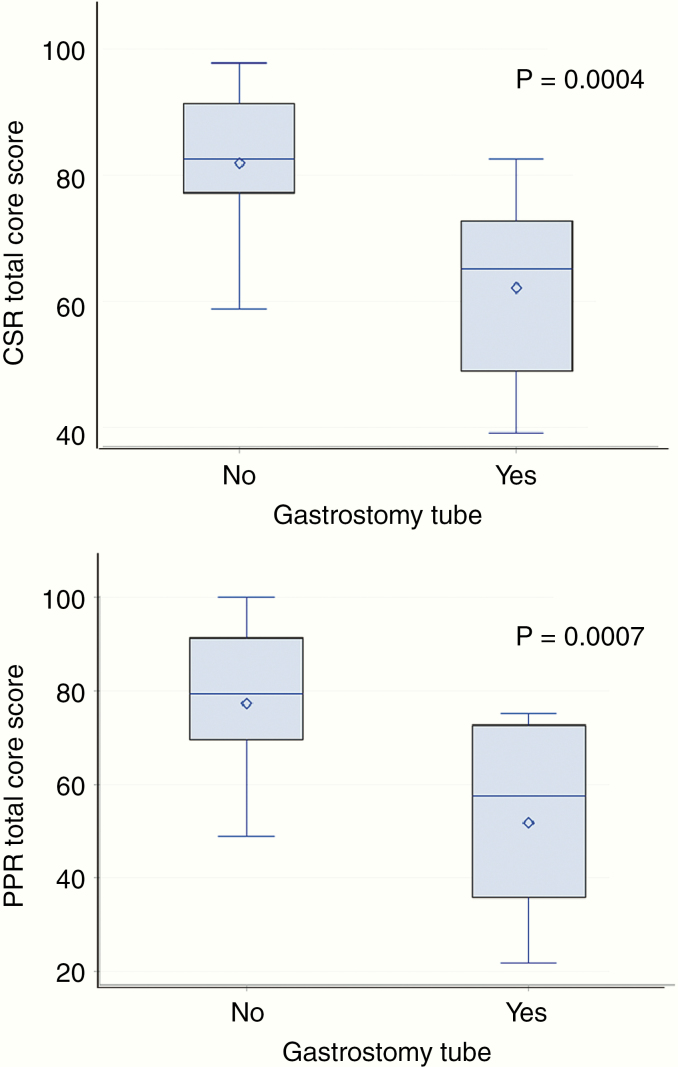
Univariate analyses of CSR and PPR HRQoL outcomes with all demographic and clinical variables are presented in Tables 3 and 4. A statistically significant relationship between both CSR and PPR HRQoL outcomes and the need for a gastrostomy tube (G-tube) at the time of diagnosis and treatment was found, with patients requiring a G-tube reporting significantly lower follow up HRQoL scores in the following domains: total core, physical, psychosocial, and social and school functioning (P < 0.01 for CSR and PPR scores among all domains). Mean (SD) total core score for patients with and without a history of G-tube are 62.1(14.6) versus 81.9 (11.8) for CSR and 51.8 (20.2) versus 77.3 (15.9) for PPR, P < 0.001 (Figure 2). The requirement for cerebrospinal fluid shunt due to hydrocephalus at the time of diagnosis was also significantly associated with poorer HRQoL scores, including PPR total core (P = 0.007) and physical (P = 0.008), psychosocial (P = 0.012), and social scores (P = 0.001) and CSR social score (P = 0.012). The receipt of chemotherapy was associated with poorer CSR total core score (P = 0.046) and psychosocial score (P = 0.052) but no relationship with PPR outcomes was found. There were no other significant associations between CSR or PPR HRQoL outcomes and other patient demographic and clinical variables, including: patient age, household income, tumor histology, tumor location, PRT dose, or whether PRT was involved field or craniospinal irradiation (CSI).
Table 3.
Univariate analysis of parent proxy-reported HRQoL
| Parameter (numbers) | Parent-Proxy Reported PedsQL | |||||
|---|---|---|---|---|---|---|
| Total Core Score | Physical Score | Psychosocial Score | Emotional Score | Social Score | School Score | |
| Distinction* or Correlation** Coefficient (P Value) | ||||||
| Age** | 0.003 (0.99) | 0.06 (0.73) | -0.03 (0.84) | -0.07 (0.67) | 0.07 (0.66) | -0.10 (0.55) |
| Sex: F vs M* 25/15 | 3.0 (0.64) | 7.5 (0.33) | 0.8 (0.89) | 1.4 (0.83) | -0.4 (0.96) | -1.2 (0.87) |
| Race: Other vs Wh.* (3/37) | 13.1 (0.26) | 15.3 (0.28) | 12.1 (0.29) | 10.1 (0.40) | 12.5 (0.37) | 12.7 (0.31) |
| Income** | 0.06 (0.72) | 0.01 (0.93) | 0.08 (0.63) | -0.07 (0.69) | 0.07 (0.68) | 0.19 (0.25) |
| Ependymoma vs Other* (22/18) | 1.5 (0.81) | 0.8 (0.92) | 2.1 (0.73) | 5.3 (0.41) | -0.3 (0.97) | -0.1 (0.99) |
| Location: PF vs ST* 28/12 | -2.1 (0.75) | 1.1 (0.89) | -3.9 (0.56) | -6.2 (0.37) | -5.2 (0.52) | 1.1 (0.88) |
| Gastric tube* (33/7) | 25.5 (<0.001) | 31.6 (<0.001) | 22.2 (0.003) | 5.7 (0.49) | 35.0 (<0.001) | 25.9 (0.002) |
| EVD/shunt* 27/13 | 17.1 (0.007) | 20.2 (0.008) | 15.6 (0.012) | 7.6 (0.25) | 24.3 (0.001) | 13.0 (0.06) |
| Chemotherapy* 16/24 | 2.6 (0.68) | 1.1 (0.88) | 3.4 (0.58) | 1.5 (0.82) | 7.2 (0.35) | 0.1 (0.99) |
| IT/High dose* chemotherapy 30/10 | 1.7 (0.81) | 0.3 (0.97) | 2.8 (0.69) | 4.2 (0.56) | -2.7 (0.76) | 3.6 (0.64) |
| Involved field vs CSI* 5/35) | -4.3 (0.65) | -4.5 (0.69) | -4.0 (0.66) | -5.3 (0.58) | -9.3 (0.40) | 1.2 (0.90) |
| PRT dose** | 0.198 (0.22) | 0.203 (0.21) | 0.176 (0.28) | 0.279(0.08) | 0.073 (0.66) | 0.149 (0.36) |
Relationship assessed using the paired T-test for categorical variables*, and Pearson correlation coefficient for continuous variables**. Statistical significance measures are highlighted in bold.
Abbreviations: F, female; M, male; PF, Posterior Fossa, ST, Supratentorial, EVD, extraventricular drain; IT, intrathecal; CSI, craniospinal irradiation; PRT, proton therapy
Table 4.
Univariate analysis of child self-reported HRQoL
| Parameter (numbers) | Child Self-Reported PedsQL | |||||
|---|---|---|---|---|---|---|
| Total Core Score | Physical Score | Psychosocial Score | Emotional Score | Social Score | School Score | |
| Distinction* or Correlation Coefficient** (P value) | ||||||
| Age** | 0.01 (0.97) | -0.03 (0.87) | 0.03 (0.87) | 0.06 (0.71) | 0.18 (0.28) | -0.15 (0.38) |
| Sex*: F vs M 25/15 | 3.1 (0.53) | 1.1 (0.85) | 4.1 (0.40) | 3.5 (0.50) | 5.7 (0.34) | 3.1 (0.64) |
| Race*: Other vs Wh. (3/37) | 5.8 (0.51) | 9.5 (0.37) | 3.8 (0.67) | 6.1 (0.50) | 6.0 (0.59) | -0.8 (0.95) |
| Income** | 0.05 (0.78) | 0.06 (0.73) | 0.04 (0.83) | -0.03 (0.88) | -0.02 (0.93) | 0.11 (0.51) |
| Ependymoma vs Other* (22/18) | 1.9 (0.68) | 5.9 (0.30) | -0.2 (0.97) | -2.2 (0.65) | -2.0 (0.73) | 3.6 (0.57) |
| Location: PF vs ST* 28/12 | -0.5 (0.92) | 4.2 (0.50) | -3.1 (0.56) | -3.4 (0.53) | -3.5 (0.59) | -2.4 (0.74) |
| Gastric tube* (33/7) | 19.8 (<0.001) | 24.3 (<0.001) | 17.4 (0.003) | 7.9 (0.21) | 22.3 (0.002) | 22.0 (0.006) |
| EVD/shunt * 27/13 | 6.3 (0.20) | 5.6 (0.34) | 6.6 (0.18) | 1.3 (0.80) | 15.0 (0.012) | 3.6 (0.60) |
| Chemotherapy* 16/24 | 9.3 (0.046) | 9.5 (0.09) | 9.1 (0.05) | 6.9 (0.16) | 9.2 (0.12) | 11.3 (0.08) |
| IT/High dose chemotherapy* 30/10 | 4.9 (0.36) | 7.6 (0.23) | 3.4 (0.53) | 1.8 (0.74) | 2.2 (0.74) | 6.2 (0.39) |
| Involved field vs CSI* (35/5) | -2.6 (0.71) | -4.0 (0.64) | -1.9 (0.79) | 3.0 (0.68) | 2.1 (0.81) | -10.8 (0.26) |
| RT dose** | 0.044 (0.79) | 0.133 (0.42) | -0.018 (0.91) | 0.059 (0.72) | -0.156 (0.34) | 0.057 (0.73) |
Relationship assessed using the paired t-test for categorical variables,* and Pearson correlation coefficient for continuous variables.** Statistical significance measures are highlighted in bold.
Abbreviations: PF, posterior fossa, ST, supratentorial, EVD, extraventricular drain; IT, intrathecal; CSI, craniospinal irradiation; PRT, proton therapy.
Fig. 2.
Comparison of long-term follow-up child self-reported (top) and parent proxy-reported (bottom) PedsQL total core score according to whether the patient required a gastrostomy tube at time of diagnosis or treatment. Upper and lower bars represent maximum and minimum scores, diamond is mean, horizontal line is median, shaded box is interquartile range. Scores are compared with the ANOVA method.
Multivariable analysis was performed including the variables history of hydrocephalus, G-tube, and receipt of chemotherapy. On multivariable analysis (MVA), the need for a G-tube was independently associated with inferior CSR and PPR scores among all domains except for emotional score (P < 0.02 for CSR and PPR total core score, physical score, psychosocial score, social score and school). The use of a G-tube was more common in patients with a history of hydrocephalus (G-tube was utilized in 30% of patients with hydrocephalus and 5% of patients without hydrocephalus, P = 0.09). After accounting for the G-tube in the MVA, history of hydrocephalus remained significantly associated with PPR emotional score (P = 0.046) only. Receipt of chemotherapy was no longer associated with CSR outcomes on MVA, but a trend for an association with inferior PPR total core score was seen (P = 0.063).
Five patients with medulloblastoma who ranged in age from 3.1 to 3.9 years at the time of PRT received CSI. Four patients received a CSI dose of 18–27 Gy. For these 4 patients CSR and PPR total core scores ranged 78.3–98.7 and 48.9–95.7, respectively. One patient received a dose of 36 Gy and total core CSR and PPR scores were 43.5 and 52.2, respectively. None of these patients required a G-tube. All patients received chemotherapy and a cumulative boost dose, delivered to either the entire posterior fossa or an involved field, of 54 GyRBE.
School Placement
Thirty-nine patients (97.5%) completed the school data questionnaire. Children were age appropriate for elementary school (n = 27), middle school (n = 9), or high school (n = 4) at the time of completion. Thirty-two (82%) survivors attended a public school and 35 (90%) functioned in a regular classroom. Three patients (8%), all of whom received craniospinal irradiation, described their classroom as a special education classroom and one patient reported unknown. Fourteen patients (36%) utilized a classroom aid, 9 (23%) an outside tutor, and 18 (46%) an individualized education plan.
Discussion
To our knowledge this is the first study to report long-term and prospectively collected HRQoL data among a population of brain tumor survivors treated with PRT at less than 4 years of age. The findings are encouraging, with over one third of parents reporting quality of life scores in follow-up similar to those of healthy children and HRQoL scores remaining stable over time in the longitudinally tested population. Mean child and parent HRQoL scores among the brain tumor population at last follow-up were similar to the reference population of children with other chronic health conditions, and inferior to a reference population of healthy children in all domains with the exception of child-reported physical score. The majority of children functioned in a regular school, with slightly less than half of patients using an individualized education plan and approximately one third of patients using a classroom aid.
Parent-reported HRQoL was consistently lower than CSR, though the two were well correlated. Previous studies of HRQoL among pediatric cancer patients have similarly reported that parent proxy-reported outcomes tend to be consistently lower than CSR scores.15,25–28 In contrast, among the healthy pediatric population, parents tend to report higher HRQoL than their children.29 This is evident in the data presented here, as there is a greater magnitude of difference between the parent-reported outcomes for the study population and healthy reference population than is seen for the child-reported outcomes. Though the mean HRQoL scores for the whole group are lower than those of the healthy children, some patients did report excellent long-term quality of life and over one third of the population had parent-reported HRQoL scores similar to other healthy children without chronic health conditions.24
Our results have demonstrated that quality of life outcomes for young children treated with radiotherapy can be highly variable, despite a relatively uniform treatment in terms of radiation dose received and patient age. We found no statistically significant association between HRQOL outcomes and PRT dose or whether CSI or involved field radiotherapy was used, though only 5 patients received CSI in this cohort and 3 of these children were later enrolled in special education services. Previous work in a larger population of predominantly older patients has demonstrated an association between receipt of CSI and inferior HRQOL, along with an association between poorer HRQOL and tumor histologies such as medulloblastoma, primitive neuroectodermal tumor, and germ cell tumors often treated with CSI.15 Among the 5 medulloblastoma patients treated with CSI in this cohort, HRQoL scores for each patient ranged from 46.7 to 98.9. The child with the poorest outcomes received 36 Gy CSI with a whole posterior fossa boost to 54 Gy due to leptomeningeal disease at presentation, while the remaining patients received lower CSI doses, often with involved field boost. The higher radiation dose delivered to the supratentorial brain from the combination of greater CSI dose and whole posterior fossa boost would be expected to lead to inferior neurocognitive outcomes in this patient4,8,30 and may be related to the poor HRQoL reported. Still, HRQoL outcomes remained variable among the other patients with similar PRT doses, suggesting patients may have a unique susceptibility to late effects of treatment, or this may represent variability in baseline functional status.
Among the patients treated with focal PRT, variability in tumor location may also play a role, as this can influence whether regions of the brain expected to lead to long-term late effects such as cognitive dysfunction or endocrine deficiency are exposed to radiation therapy. Further analyses evaluating more specific radiation dosimetric statistics to HRQoL outcomes among all patients in this analysis are under way.
The clinical variables most strongly and consistently associated with poorer HRQoL outcomes in long-term follow-up were the requirement for a feeding tube and treatment for hydrocephalus. A history of hydrocephalus has been associated with poor neurocognitive outcomes among brain tumor survivors in previous reports,4,31,32 which may be the underlying cause of the association with poor quality of life seen here. A history of hydrocephalus was more common among patients who required a G-tube, and on multivariable analysis it was the necessity for a G-tube that remained significantly associated with long-term HRQoL. Approximately 18% of the patients in this study required a feeding tube, which is consistent with the incidence of swallowing dysfunction requiring enteral nutrition among pediatric brain tumor patients in other reports.33,34 The necessity for a feeding tube most often reflects severe acute illness or cranial nerve dysfunction, the latter of which may be permanent and continue to negatively impact quality of life in survivorship. To our knowledge the association between requirement for a feeding tube and long-term quality of life among brain tumor survivors is a novel finding. It is our hypothesis that the underlying illness or neurologic dysfunction leading to the requirement for a feeding tube is what is truly associated with poor quality of life outcomes. Nutritional support remains an important goal in pediatric cancer care and it is unlikely that the feeding tube itself would negatively impact long-term quality of life.
In this dataset, both child- and parent-reported HRQoL scores were each lowest in the school domain. The school assessment includes questions about whether the child finds it hard to concentrate, forgets things, has trouble keeping up in school, or misses school due to not feeling well or for a doctor’s appointment. Low school scores are likely reflective, at least in part, of the well-recognized effect of radiotherapy on cognitive functioning, processing speed, and attention. Neuropsychiatric test scores are not part of this analysis, but previous studies have correlated cognitive functioning and HRQoL outcomes.15 Low school scores may also reflect time away from school secondary to routine clinic visits for patients who traveled for PRT or chronic health conditions in these patients at risk for endocrine and other late effects. In the longitudinal analysis, school scores were stable from baseline to last follow-up; however, this analysis is limited given that baseline scores were only available in 8 children on account of age at the time of treatment and that most children were not in school at this age.
This analysis is of high value due to the young age of the population at the time of PRT and the robust HRQoL data presented with up to 15 years of follow-up. Eighteen patients (45%) participated through a longitudinal prospective protocol, and 22 additional patients (55%) completed surveys through the mail for cross-sectional data collection in an expanded cohort. A favorable 60% response rate for the mailed surveys was achieved, though selection bias among families electing to participate may have impacted the results in unknown ways. Due to the nature of study design and historical context of patients treated with up to 15 years of follow-up, baseline quality of life data are not available for all patients and are limited to parent proxy reports due to the patients’ young age at treatment. Small sample size remains a weakness of this study due to the rarity of the target population reported.
Due to the scarcity of HRQoL and educational attainment data among pediatric brain tumor survivors of this very young age group, we are unable to make appropriate comparisons to outcomes for patients treated with historical radiation techniques or with surgery or chemotherapy alone. Though outcomes from this small and heterogeneous patient population may not be generalizable to all young brain tumor patients, these data may serve as a useful tool to help counsel families about potential long-term quality of life after proton treatment. Further analyses focused on evaluating how clinical and treatment variable impact HRQoL outcomes may be helpful in guiding treatment strategies specific to these young children.
In conclusion, these data demonstrate HRQoL outcomes among very young children receiving PRT for a brain tumor are highly variable and significantly associated with severity of neurologic injury at the time of diagnosis and treatment, as indicated by the negative association of outcomes with a history of requiring G-tube placement or treatment for hydrocephalus. Overall, HRQoL outcomes are similar to patients’ with other benign chronic health conditions, although over a third of patients report quality of life scores similar to healthy children’s. The risks of late effects remains despite the use of PRT, but there is hope for very good quality of life and functional status long term among even our youngest patients.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number P01CA021239. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This project was also supported by the Federal Share of program income earned by Massachusetts General Hospital on C06 CA059267, Proton Therapy Research and Treatment Center.
Conflict of interest statement. Dr Torunn Yock has received research funding from Protom, IBA, Elekta, and Mim (in-kind funding, not monetary).
Authorship statement. Study design: BE, NT, KK, TY. Data collection: BE, NT, SM, TY, ML, SG, EW. Data analysis and interpretation: BE, SG, NT, TY, KK, SM, DE. Manuscript preparation and review: BE, SG, NT, ML, SG, EW, KK, DE, SM, TY.
Supplementary Material
References
- 1. Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101(13):946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Packer RJ, Sposto R, Atkins TE, et al. Quality of life in children with primitive neuroectodermal tumors (medulloblastoma) of the posterior fossa. Pediatr Neurosci. 1987;13(4):169–175. [DOI] [PubMed] [Google Scholar]
- 3. Schulte F, Brinkman TM, Li C, et al. Social adjustment in adolescent survivors of pediatric central nervous system tumors: a report from the Childhood Cancer Survivor Study. Cancer. 2018;124(17): 3596–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Conklin HM, Li C, Xiong X, Ogg RJ, Merchant TE. Predicting change in academic abilities after conformal radiation therapy for localized ependymoma. J Clin Oncol. 2008;26(24):3965–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Copeland DR, deMoor C, Moore BD 3rd, Ater JL. Neurocognitive development of children after a cerebellar tumor in infancy: a longitudinal study. J Clin Oncol. 1999;17(11):3476–3486. [DOI] [PubMed] [Google Scholar]
- 6. Merchant TE, Kiehna EN, Li C, Xiong X, Mulhern RK. Radiation dosimetry predicts IQ after conformal radiation therapy in pediatric patients with localized ependymoma. Int J Radiat Oncol Biol Phys. 2005;63(5):1546–1554. [DOI] [PubMed] [Google Scholar]
- 7. Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5(7):399–408. [DOI] [PubMed] [Google Scholar]
- 8. Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23(24):5511–5519. [DOI] [PubMed] [Google Scholar]
- 9. Eaton BR, Yock T. The use of proton therapy in the treatment of benign or low-grade pediatric brain tumors. Cancer J. 2014;20(6):403–408. [DOI] [PubMed] [Google Scholar]
- 10. Merchant TE, Hua CH, Shukla H, Ying X, Nill S, Oelfke U. Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer. 2008;51(1):110–117. [DOI] [PubMed] [Google Scholar]
- 11. Pulsifer MB, Sethi RV, Kuhlthau KA, MacDonald SM, Tarbell NJ, Yock TI. Early cognitive outcomes following proton radiation in pediatric patients with brain and central nervous system tumors. Int J Radiat Oncol Biol Phys. 2015;93(2):400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pulsifer MB, Duncanson H, Grieco J, et al. Cognitive and adaptive outcomes after proton radiation for pediatric patients with brain tumors. Int J Radiat Oncol Biol Phys. 2018;102(2):391–398. [DOI] [PubMed] [Google Scholar]
- 13. Yock TI, Bhat S, Szymonifka J, et al. Quality of life outcomes in proton and photon treated pediatric brain tumor survivors. Radiother Oncol. 2014;113(1):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yock TI, Yeap BY, Ebb DH, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol. 2016;17(3):287–298. [DOI] [PubMed] [Google Scholar]
- 15. Kuhlthau KA, Pulsifer MB, Yeap BY, et al. Prospective study of health-related quality of life for children with brain tumors treated with proton radiotherapy. J Clin Oncol. 2012;30(17):2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antonini TN, Ris MD, Grosshans DR, et al. Attention, processing speed, and executive functioning in pediatric brain tumor survivors treated with proton beam radiation therapy. Radiother Oncol. 2017;124(1):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamran SC, Goldberg SI, Kuhlthau KA, et al. Quality of life in patients with proton-treated pediatric medulloblastoma: results of a prospective assessment with 5-year follow-up. Cancer. 2018;124(16):3390–3400. [DOI] [PubMed] [Google Scholar]
- 18. Kahalley LS, Douglas Ris M, Mahajan A, et al. Prospective, longitudinal comparison of neurocognitive change in pediatric brain tumor patients treated with proton radiotherapy versus surgery only. Neuro Oncol. 2019;21(6):809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Varni JW, Seid M, Kurtin PS. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812. [DOI] [PubMed] [Google Scholar]
- 20. Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94(7):2090–2106. [DOI] [PubMed] [Google Scholar]
- 21. Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhat SR, Goodwin TL, Burwinkle TM, et al. Profile of daily life in children with brain tumors: an assessment of health-related quality of life. J Clin Oncol. 2005;23(24):5493–5500. [DOI] [PubMed] [Google Scholar]
- 23. Geronimus AT, Bound J. Use of census-based aggregate variables to proxy for socioeconomic group: evidence from national samples. Am J Epidemiol. 1998;148(5):475–486. [DOI] [PubMed] [Google Scholar]
- 24. Huang IC, Thompson LA, Chi YY, et al. The linkage between pediatric quality of life and health conditions: establishing clinically meaningful cutoff scores for the PedsQL. Value Health. 2009;12(5):773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levi RB, Drotar D. Health-related quality of life in childhood cancer: discrepancy in parent-child reports. Int J Cancer Suppl. 1999;12:58–64. [DOI] [PubMed] [Google Scholar]
- 26. Parsons SK, Barlow SE, Levy SL, Supran SE, Kaplan SH. Health-related quality of life in pediatric bone marrow transplant survivors: According to whom? Int J Cancer Suppl. 1999;12:46–51. [DOI] [PubMed] [Google Scholar]
- 27. Penn A, Lowis SP, Stevens MC, et al. Family, demographic and illness-related determinants of HRQL in children with brain tumours in the first year after diagnosis. Pediatr Blood Cancer. 2009;53(6):1092–1099. [DOI] [PubMed] [Google Scholar]
- 28. Yoo HJ, Ra YS, Park HJ, et al. Agreement between pediatric brain tumor patients and parent proxy reports regarding the Pediatric Functional Assessment of Cancer Therapy–Childhood Brain Tumor Survivors questionnaire, version 2. Cancer. 2010;116(15):3674–3682. [DOI] [PubMed] [Google Scholar]
- 29. Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3(6):329–341. [DOI] [PubMed] [Google Scholar]
- 30. Moxon-Emre I, Bouffet E, Taylor MD, et al. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol. 2014;32(17): 1760–1768. [DOI] [PubMed] [Google Scholar]
- 31. Brinkman TM, Krasin MJ, Liu W, et al. Long-term neurocognitive functioning and social attainment in adult survivors of pediatric CNS tumors: results from the St Jude lifetime cohort study. J Clin Oncol. 2016;34(12):1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Di Pinto M, Conklin HM, Li C, Merchant TE. Learning and memory following conformal radiation therapy for pediatric craniopharyngioma and low-grade glioma. Int J Radiat Oncol Biol Phys. 2012;84(3):e363–e369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Newman LA, Boop FA, Sanford RA, Thompson JW, Temple CK, Duntsch CD. Postoperative swallowing function after posterior fossa tumor resection in pediatric patients. Childs Nerv Syst. 2006;22(10):1296–1300. [DOI] [PubMed] [Google Scholar]
- 34. Lee WH, Oh BM, Seo HG, et al. One-year outcome of postoperative swallowing impairment in pediatric patients with posterior fossa brain tumor. J Neurooncol. 2016;127(1):73–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.