Abstract
Background
Lymphocyte antigen 6 complex, locus K (LY6K) is a putative oncogene in various cancers. Elevated expression of LY6K is correlated with poor patient prognosis in glioblastoma (GBM). The aim of this study is to advance our understanding of the mechanism by which LY6K contributes to GBM tumor biology.
Methods
Bioinformatic data mining was used to investigate LY6K expression in relation to GBM clinical outcome. To understand the role of LY6K in GBM, we utilized patient-derived glioma stemlike cells (GSCs) and U87 cells and employed immunoblotting, immunofluorescent staining, radiation treatment, and orthotopic GBM xenograft models.
Results
Our results show that increased expression of LY6K inversely correlates with GBM patient survival. LY6K promotes tumorigenicity in GBM cells both in vitro and in vivo. The mechanism underlying this tumorigenic behavior is enhancement of extracellular signal-regulated kinase 1 and 2 (ERK1/2) signaling. Interestingly, we observed that tumor-promoting LY6K-ERK1/2 signaling is mediated by the interaction of LY6K with caveolin-1, rather than through oncogenic receptor tyrosine kinase–mediated signaling. Moreover, association of LY6K with the cell membrane is crucial for its tumorigenic functions. Finally, DNA methylation maintains LY6K silencing, and hypomethylation of the LY6K promoter increases its expression. In GSCs, ionizing radiation leads to demethylation of the LY6K promoter, thereby increasing LY6K expression and GSC resistance to radiation.
Conclusions
Our study highlights the importance of the contribution of LY6K to GBM tumor biology and suggests LY6K as a potential membrane target for treating GBM.
Keywords: LY6K, glioblastoma, ERK1/2, CAV-1, methylation
Key Points.
1. Elevated expression of LY6K correlates with poor patient survival.
2. LY6K enhances ERK1/2 signaling through interactions with caveolin-1.
3. LY6K promoter methylation status is a key determinant of LY6K expression and response to radiation.
Importance of the Study.
Although LY6K was previously linked to tumorigenicity in multiple types of cancers, including GBM, the underlying mechanistic basis by which LY6K mediates tumor-promoting signaling in GBM is unknown. Here, we illustrate a novel signaling mechanism by which membrane-anchored LY6K enhances ERK1/2 signaling through its association with caveolin-1 to facilitate GBM tumorigenicity. Additionally, DNA methylation contributes to LY6K expression, and irradiation induces LY6K expression by promoter demethylation, resulting in enhanced radiation resistance in GSCs. This is the first study to investigate the function and underlying mechanism of LY6K in GBM tumor biology. Our study highlights LY6K as a potential targetable protein to promote radiation sensitivity in GBM patients.
Glioblastoma (GBM) is the most malignant primary brain cancer, with extremely poor prognoses for patients.1 Intratumoral heterogeneity is a key contributor to GBM therapy resistance, which in turn promotes poor survival outcomes. Integrated genomic analysis of primary, isocitrate dehydrogenase wild-type (IDH-WT) GBM tumors led to their classification into 3 subtypes: proneural (PN), classical (CL), and mesenchymal (MES).2 Regardless of subtype classification, however, each tumor can comprise multiple cell subpopulations,3 including a small population of glioma stemlike cells (GSCs).4
Our previous gene expression profiling analysis resulted in classification of patient-derived GSCs into subtypes that phenotypically resemble GBM, with MES-like GSCs being more aggressive relative to PN-like GSCs.5,6 Of the 3000 differentially expressed genes between these subtypes, lymphocyte antigen 6 complex, locus K (LY6K) was among the most significant genes. LY6K is a member of LY6/urokinase-type plasminogen activator receptor family of proteins and has a glycosylphosphatidylinositol (GPI) anchor that results in its localization on the plasma membrane.7 In addition, LY6K is a cancer/testis antigen that is upregulated in several types of human cancers.8,9 In breast cancer, LY6K and LY6E mediate transforming growth factor (TGF)β–promoted tumor progression and are involved in helping cancer cells escape immune surveillance.10 Recently, a model based on alternative splicing events using a set of genes including LY6K was proposed to be a prognostic indicator for GBM.11 Moreover, a peptide vaccine that included an epitope for LY6K induced an immunoreactivity and cytotoxic T lymphocyte responses in GBM patients.12 The functional role of LY6K in GBM, however, has yet to be determined.
The mitogen-activated protein kinase (MAPK) pathway is among the most highly dysregulated signaling networks observed in GBM.13 For canonical MAPK pathway activation, a ligand such as epidermal growth factor (EGF) binds to its receptor tyrosine kinase (RTK) (eg, EGFR) and activates receptor-mediated signaling through dimerization and autophosphorylation.14 The phosphorylated residues create docking sites for adapter proteins, which then initiate various downstream signaling pathways, including RAS-mediated signaling. RAS activates RAF, which then activates MEK, which subsequently activates MAPK/ERK1/2 via phosphorylation. ERK1/2 activates various transcription factors, including some responsible for cell proliferation.15 These sequential activation steps enhance signal amplification, causing robust biological effects. Cancer cells are able to hijack these pathways, leading to the activation of ERK1/2-mediated oncogenic signaling, thereby promoting abnormal proliferation.14,16
Activation of the MAPK pathway is influenced by various factors within the cell, including caveolae. Caveolae are small membrane invaginations that have been linked to various cellular functions, such as signal transduction.17 The primary structural component of caveolae in tissues is caveolin-1 (CAV-1). In cancers, CAV-1 is known to have a stage-dependent role,18 in which it suppresses oncogenic signaling pathways early in tumor progression, but enhances these pathways in more advanced cancers.19 CAV-1 has been shown to be involved in modulating the MAPK signaling pathway,20 and EGF stimulation recruits CAV-1 to early endocytic compartments to promote MAPK signaling.21
In this study, we investigated the function and molecular mechanisms of LY6K in GBM. We show that elevated LY6K expression is a prognostic factor for GBM patients and increases tumorigenicity in GBM cells. We show that this effect relies upon LY6K-enhanced ERK1/2 signaling, which can be further modulated by EGF stimulation. Moreover, LY6K-enhanced ERK1/2 signaling results from the association of GPI-anchored LY6K with CAV-1. Finally, DNA methylation maintains LY6K silencing, and irradiation induces the expression of LY6K via promoter hypomethylation, thereby promoting GBM tumorigenicity and resistance to radiation therapy (RT).
Materials and Methods
Cell Culture
HEK293T cells and U87 glioma cells (ATCC) were cultured in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and 1% streptomycin. Patient-derived GSCs that were previously characterized5 were cultured in stem cell media containing DMEM/F12 (Invitrogen), supplemented with B27 (2%, Invitrogen), 1% penicillin, 1% streptomycin, heparin (5 μg/mL, Sigma-Aldrich), EGF (20 ng/mL), and basic fibroblast growth factor (20 ng/mL; PeproTech).
Tumorigenicity Assays
All animal experiments were conducted under an Institutional Animal Care and Use Committee‒approved protocol at Northwestern University in accordance with the National Institutes of Health and institution guidelines. For further details, see the Supplementary Material.
Bioinformatic Analyses
The Cancer Genome Atlas (TCGA) RNA-sequencing data of glioma were downloaded from FireBrowse (http://firebrowse.org/). For further details, see the Supplementary Material.
Lentiviral Plasmids and Infection
Commercial and constructed lentiviral plasmids used and methods of infection are provided in the Supplementary Material.
In Vitro Assays
Details regarding in vitro cell proliferation, limiting dilution, and signaling assays are included in the Supplementary Material.
Kyte–Doolittle Hydropathy Plot
A Kyte–Doolittle hydropathy plot for LY6K was generated with the ProtScale tool from ExPASy (https://web.expasy.org/protscale/). The full nascent amino acid sequence for LY6K was used as the input and the resulting plot was analyzed for hydrophobicity patterns typical of GPI-anchored proteins.
Coimmunoprecipitation, Immunoblotting, and Immunofluorescence
Details regarding protein assays for coimmunoprecipitation, immunoblot (IB), and immunofluorescence are provided in the Supplementary Materials.
Methylation Analyses and Radiation Treatment
Details regarding methylation analyses and RT are provided in the Supplementary Materials.
Statistics
Statistical analysis was carried out using Microsoft Excel and GraphPad Prism v5.0 for Windows. Analysis included one-way ANOVA with a Newman–Keuls post-hoc test and paired Student’s t-test. Log-rank tests were used to determine significance of Kaplan–Meier curves.
Results
Elevated Expression of LY6K Is Inversely Correlated with GBM Patient Survival
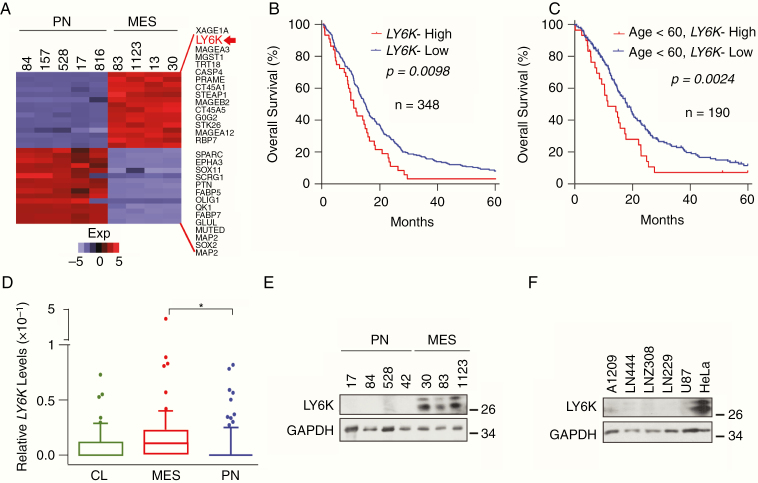
Analysis of our previously published gene expression profiling data between PN- and MES-like GSCs5 showed LY6K being among the most highly differentially expressed genes (Fig. 1A). Similar to other types of human cancers, the primary aberration in LY6K seen in gliomas was amplification/copy number gain (Supplementary Figure 1A), with no known mutations in gliomas (Supplementary Figure 1B). Multiple datasets showed that GBM patients with elevated LY6K expression had shorter survival compared with patients with lower levels of LY6K (Fig. 1B, Supplementary Figure 1C).8,22 Moreover, GBM patients under 60 years of age with lower levels of LY6K experienced relatively longer survival (Fig. 1C). LY6K expression was not prognostically significant when considering tumor IDH1 status, TP53 status, or sex (data not shown). Furthermore, MES GBM had relatively higher levels of LY6K compared with PN GBM, and grade IV gliomas have higher levels of LY6K than grade III (Fig. 1D, Supplementary Figure 1D). Consistent with our gene expression profiling data, LY6K protein expression was higher in MES-like GSCs relative to PN-like GSCs (Fig. 1E), and its expression in established glioma cell lines was nearly undetectable (Fig. 1F).
Fig. 1.
Elevated expression of LY6K is inversely correlates with GBM patient survival. (A) LY6K was among the top differentially expressed genes in MES-like GSCs, relative to PN-like GSCs. (B) LY6K expression correlated with poor patient survival in GBM samples in a five-year analysis of TCGA datasets. (C) Among all tested variables, only age (<60 y) correlated with poor survival in GBM patients in multivariate analyses with LY6K in TCGA datasets. (D) Expression of LY6K was higher in MES GBM samples, relative to CL or PN in TCGA datasets. (E, F) Immunoblot (IB) for LY6K expression in PN-like and MES-like GSCs (E) and GBM cell lines and HeLa cells (F). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was a loading control. Data are representative of 3 independent experiments with similar results. *P < 0.05.
LY6K Enhances GBM Cell Proliferation, Sphere-Forming Frequencies, and Tumorigenicity
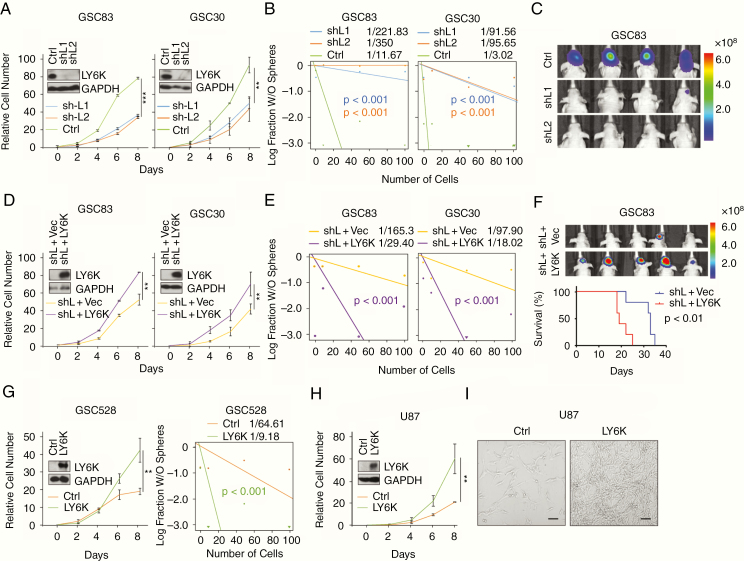
To analyze the tumor-promoting potential of LY6K in GSCs, we utilized short hairpin RNAs (shRNAs) to suppress LY6K expression in 2 MES-like GSC lines, GSC83 and GSC30, which have high endogenous expression of LY6K (Fig. 1E). In both cell lines, knockdown of LY6K significantly inhibited GSC proliferation (Fig. 2A), sphere-forming frequencies (Fig. 2B), and in vivo tumor growth measured by bioluminescent imaging (BLI) of mice or histological staining of mouse brain sections (Fig. 2C, Supplementary Figure 2A). Subsequent rescue of LY6K expression through exogenous expression of shRNA-resistant LY6K in these GSCs restored proliferation (Fig. 2D), sphere-forming frequencies (Fig. 2E), and in vivo tumor growth and animal survival (Fig. 2F, Supplementary Figure 2B). These data demonstrate the specificity of the tumor-promoting roles of LY6K expression in GSCs. To further characterize the effect of LY6K expression, we overexpressed LY6K in GSC528 and U87 cells that otherwise have undetectable levels of endogenous LY6K (Fig. 1E, F). As expected, exogenous expression of LY6K in these cells promoted cell growth and sphere-forming frequencies (Fig. 2G–I). Taken together, elevated expression of LY6K enhances tumorigenicity in vitro and in vivo.
Fig. 2.
LY6K enhances GBM cell proliferation, sphere-forming frequencies, and tumorigenicity. (A–C) In MES-like GSC83 and GSC30, knockdown of LY6K suppressed cell proliferation (A), sphere-forming frequencies (B), and in vivo tumor growth (C). (D–F) Rescuing LY6K in GSC83 and GSC30 with knockdown of endogenous LY6K restored cell proliferation (D), sphere-forming frequencies (E), and in vivo tumor growth and survival (F). For (F) Top, BLI. Bottom, Kaplan–Meier survival analysis. (G) Overexpression of exogenous LY6K in GSC528 increased cell proliferation (left) and sphere-forming frequencies (right). (H) Overexpression of LY6K in U87 cells increased cell proliferation. (I) Bright-field phase contrast representative images showing cell growth at day 8 of proliferation assay from (H). Scale bar in (I) is 100 μm. Insets in (A), (D), (G), (H): IB for LY6K and GAPDH (loading control) in indicated GSCs. Data are representative of 2–3 independent experiments with similar results. **P < 0.03, ***P < 0.01.
LY6K Promotes ERK1/2 Activation in GBM Cells
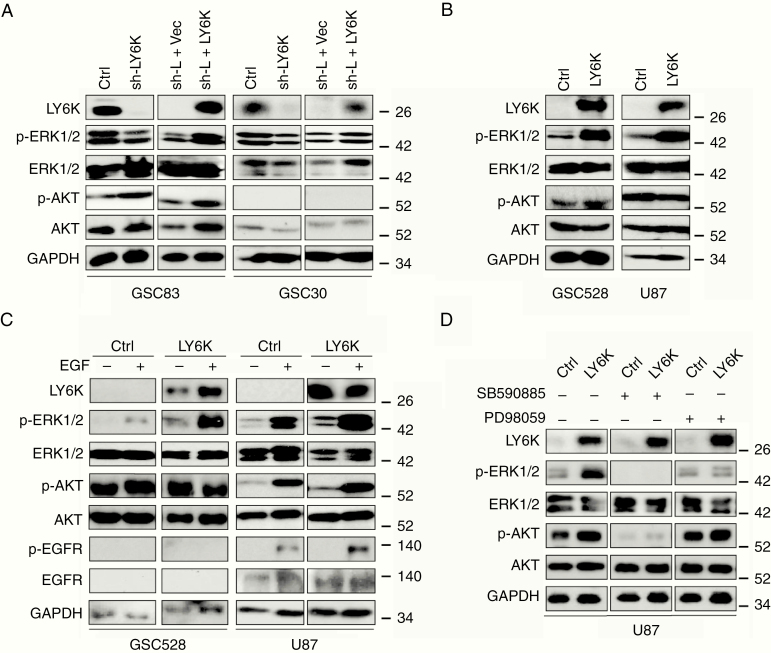
To determine the mechanisms underlying the pro-tumor effects of LY6K expression, we examined downstream signaling mediators known to be aberrantly activated in GBM,2,23 including phosphorylated (p)-AKT, p-ERK1/2, p-GSK3β, p-SMAD2, p-SRC, and p-STAT3 (Supplementary Figure 3A). Of these, only p-ERK1/2 levels consistently displayed marked decreases when endogenous LY6K was knocked down in GSC83 or GSC30 cells (Fig. 3A). Subsequent re-expression of LY6K in these cells rescued p-ERK1/2 levels. Consistent with these findings, in GSC528 and U87 cells, which have undetectable endogenous expression of LY6K, exogenous expression of LY6K markedly increased p-ERK1/2 levels (Fig. 3B). For both aforementioned experiments, no appreciable alteration in levels of p-AKT was observed (Fig. 3A, B), indicating that LY6K selectively induces ERK1/2 signaling in GSCs.
Fig. 3.
LY6K promotes ERK1/2 activation in GBM cells. (A) IB. LY6K knockdown decreased pERK1/2 levels in GSC83 (left) and GSC30 (right) cells, while subsequent expression of exogenous LY6K restored p-ERK1/2 levels. (B) IB. Exogenous expression of LY6K enhanced p-ERK1/2 in GSC528 (left) and U87 cells (right) with otherwise undetectable levels of LY6K. (C) IB. EGF further increased LY6K-enhanced p-ERK1/2 levels in GSC528 and U87 cells with exogenous LY6K expression. No notable changes in p-EGFR/EGFR were observed. (D) IB. RAF inhibition by SB590885 eliminated p-ERK1/2 expression, while MEK inhibition by PD98059 blocked LY6K-enhanced ERK1/2 signaling. For all IB, p-AKT/AKT were used as nonspecific proteins and GAPDH was a loading control. Data are representative of three independent experiments with similar results.
Given that ERK1/2 activation is known to be a downstream effect of EGFR signaling in GBM,13 we examined EGFR as a potential upstream initiator of LY6K-enhanced ERK1/2 activation. In GSC528 and U87 cells, which have undetectable or low levels of endogenous EGFR expression respectively, p-ERK1/2 levels increased in response to LY6K overexpression (Fig. 3C). Upon EGF stimulation, cells overexpressing LY6K showed further enhancement of p-ERK1/2 levels. However, no accompanying changes in p-EGFR levels were observed, suggesting that EGFR may not be responsible for LY6K-enhanced ERK1/2 activation (Fig. 3C). To further understand the mechanistic basis of LY6K action, we treated cells with RAF and MEK inhibitors. Treatment with a RAF inhibitor, SB590885, eliminated p-ERK1/2 levels, regardless of LY6K expression. However, MEK inhibition with PD98059 blocked LY6K-enhanced p-ERK1/2 only. Thus, MEK inhibition causes cells to retain basal p-ERK1/2 levels (Fig. 3D and Supplementary Figure 3C). Taken together, these data indicate that the observed modulation of ERK1/2 signaling is likely due to other dynamic changes occurring at the membrane, without involvement of EGFR. Therefore, we examined other RTKs that are frequently activated in GBM, including platelet derived growth factor receptor alpha, 11C-methyl-l-methionine (c-MET), and Axl.23 Modulation of LY6K expression did not affect activation of these RTKs (Supplementary Figure 3D), thus indicating that LY6K activation of p-ERK1/2 is unlikely to be related to RTK signaling in GBM cells.
CAV-1 Mediates LY6K-Enhanced p-ERK1/2 in GBM Cells
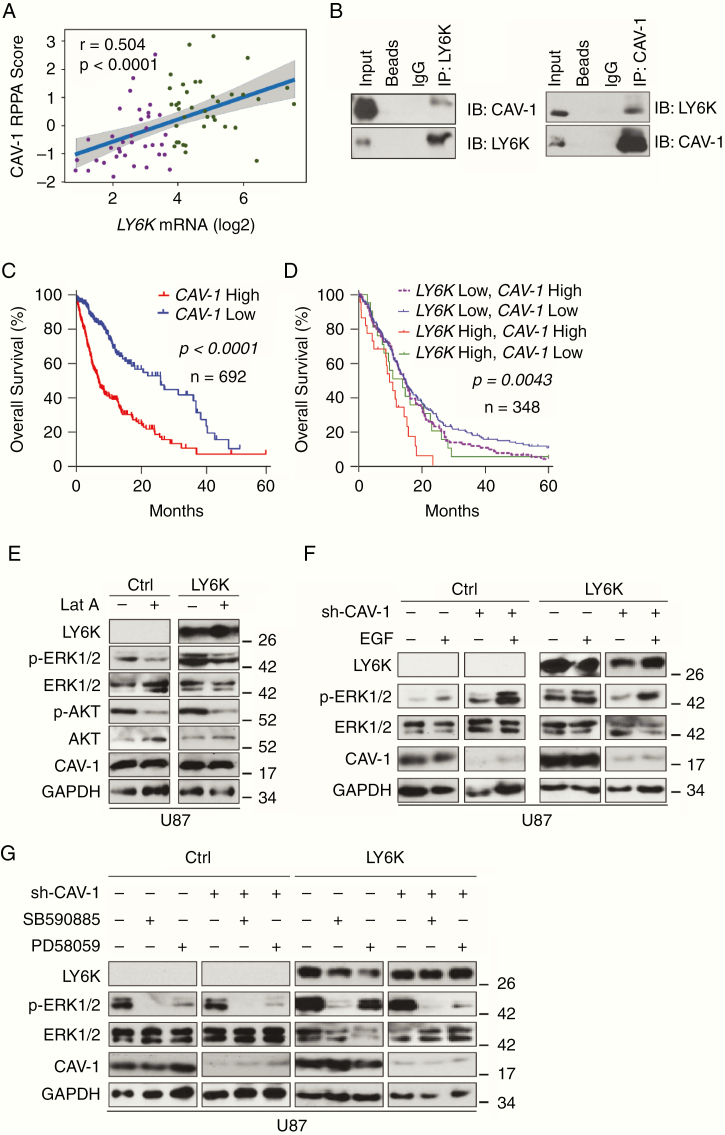
Since LY6K is a GPI-anchored membrane protein, we analyzed additional membrane proteins that may be involved in LY6K-enhanced p-ERK1/2 activation. Reverse phase protein array (RPPA) analysis24 identified caveolin-1 (CAV-1) as a potential interacting protein (Fig. 4A). CAV-1 is a major component of caveolae, which are small, 50–100 nm membrane invaginations that are important for a plethora of cellular functions, including signal transduction.17 Caveolae are found in the ordered lipid rafts of the plasma membrane, which also house GPI-anchored proteins.25 LY6 family members are known to utilize CAV-1–mediated endocytosis to influence cell signaling.26 As CAV-1 is upregulated in GBM and has tumor-promoting roles in advanced cancers,18,19 we hypothesized that CAV-1 is involved in LY6K-enhanced ERK1/2 signaling. Reciprocal immunoprecipitation-immunoblot analysis showed that LY6K can associate with CAV-1 (Fig. 4B). Additionally, high expression of CAV-1 (Fig. 4C) and coexpression of CAV-1 with LY6K (Fig. 4D) inversely correlated with glioma patient survival.
Fig. 4.
CAV-1 mediates LY6K-enhanced p-ERK1/2 in GBM cells. (A) RPPA analysis. CAV-1 is the only protein whose expression significantly correlated with LY6K. (B) Reciprocal coimmunoprecipitation analysis. Exogenous LY6K associated with CAV-1. (C-D) Kaplan–Meier survival analysis of TCGA datasets. High levels of CAV-1 correlated with poor survival in TCGA GBM + LGG dataset (C). Co-expression of LY6K and CAV-1 correlated with poor survival in TCGA GBM dataset (D). (E) IB. Latrunculin A treatment decreased LY6K-enhanced p-ERK1/2 levels. (F) IB. CAV-1 knockdown decreased LY6K-enhanced p-ERK1/2, even in the presence of EGF. (G) IB. RAF inhibition by SB590885 eliminated p-ERK1/2 levels, while MEK inhibition by PD58059 blocked LY6K-enhanced p-ERK1/2 only but retained basal p-ERK1/2 levels. Knockdown of CAV-1 suppressed even basal levels of LY6K-enhanced pERK1/2. For all IB, U87 cells with indicated modifications were used. p-AKT/AKT were used as nonspecific proteins and GAPDH was a loading control. Data in B and E–G are representative of three independent experiments with similar results.
To further assess membrane dynamics, we treated U87 cells with latrunculin A, a toxin that disrupts proper membrane formation by inhibiting actin polymerization, thereby interfering with CAV-1 distribution.27 As expected in treatment with latrunculin A, the level of p-ERK1/2 markedly decreased, even in the presence of LY6K (Fig. 4E), indicating that membrane polymerization and proper CAV-1 distribution are necessary for LY6K function.
To further investigate the role of CAV-1 in LY6K-enhanced ERK1/2 signaling, we suppressed CAV-1 expression through shRNA-mediated knockdown. CAV-1 knockdown had negligible effects on p-ERK1/2 levels in control U87 cells but caused an appreciable decrease in p-ERK1/2 levels in cells overexpressing LY6K (Fig. 4F). In the presence of LY6K, CAV-1 expressing cells showed strong enhancement of p-ERK1/2 in response to EGF stimulation. However, in cells with CAV-1 knocked down, EGF stimulation only partially increased LY6K-enhanced p-ERK1/2 levels (Fig. 4F). Moreover, CAV-1 knockdown eliminated LY6K-enhanced p-ERK1/2 upon inhibition of RAF or MEK (Fig. 4G).
The GPI-Anchor Domain of LY6K Is Necessary for LY6K-Enhanced ERK1/2 Signaling and Tumorigenicity
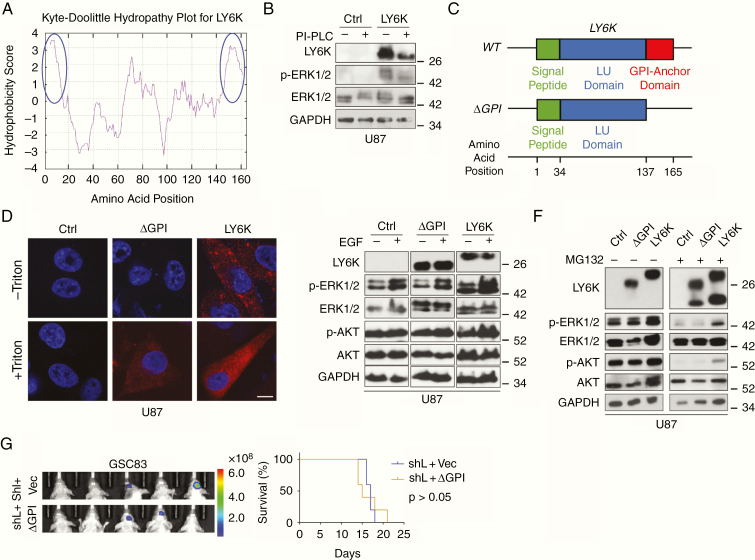
Similar to other members of the LY6 family, LY6K is predicted to have a GPI anchor.28 We confirmed this prediction by analyzing a Kyte–Doolittle hydropathy plot (Fig. 5A), which indicated that the LY6K amino acid sequence is consistent with GPI-anchored proteins. Furthermore, we experimentally corroborated this finding by treating cells with phosphatidylinositol-phospholipase C (PI-PLC), an enzyme that specifically cleaves GPI anchors, thus releasing GPI-anchored proteins from the membrane.28 When U87 cells overexpressing LY6K were treated with PI-PLC, we observed decreased levels of LY6K as well as p-ERK1/2 (Fig. 5B). To further demonstrate the necessity of membrane association of LY6K in ERK1/2 activation, we treated cells with mannosamine hydrochloride (mann-HCl). Mann-HCl inhibits incorporation of GPI anchors into their respective proteins, thereby causing these proteins to accumulate along the secretory pathway instead of localizing to the membrane.29 Treatment with mann-HCl resulted in a notable shift in molecular weight of LY6K, due to the loss of the GPI anchor, and decreased p-ERK1/2 levels (Supplementary Figure 4A). Thus, inhibition of LY6K membrane association abrogates the role of LY6K in ERK1/2 activation.
Fig. 5.
The GPI-anchor domain of LY6K is necessary for LY6K-enhanced ERK1/2 signaling and tumorigenicity. (A) Kyte–Doolittle Hydropathy Plot analysis. LY6K fits the typical hydrophobicity profile for GPI-anchored proteins (see blue circles marking strong hydrophobic regions at N- and C-termini). (B) IB. Treatment with PI-PLC decreased molecular weight of decreased levels LY6K and showed accompanying suppression of p-ERK1/2 levels. (C) Schematic of the three domains of the transcribed sequence for LY6K-WT (signal peptide, LU domain, and GPI-anchor domain) and the constructed mutant LY6K-ΔGPI, which lacks the GPI-anchor domain. The predicted amino acid position corresponding to each domain is depicted below. (D) Immunofluorescent staining. Unlike the LY6K-ΔGPI mutant, LY6K-WT is present on the cell membrane. LY6K-ΔGPI could not be visualized in the absence of membrane permeabilization (-Triton). (E) IB. Expression of LY6K-WT enhanced p-ERK1/2 levels, whereas LY6K-ΔGPI failed to do so, even in the presence of EGF. (F) IB. Proteasomal inhibitor MG132 reduced LY6K-enhanced p-ERK1/2. (G) GBM xenograft experiments. In GSC83 cells with knockdown of endogenous LY6K, subsequent expression of LY6K-ΔGPI mutant failed to restore tumorigenicity. Left, BLI images. Right, Kaplan-Meier survival analysis. For all IB, U87 cells with indicated modifications were used. p-AKT/AKT were used as nonspecific proteins and GAPDH was a loading control. Scale bar in D is 10 μm. Data in B and D-G are representative from two to three independent experiments with similar results.
To complement these pharmacological manipulations, we generated U87 cells that stably express a mutant LY6K, LY6K-ΔGPI, which lacks the GPI anchor domain (Fig. 5C). We determined proper subcellular localization of LY6K based on the presence of the GPI anchor by using immunofluorescent staining. In the absence of triton-mediated membrane permeabilization, LY6K was undetectable in both control and LY6K-ΔGPI-expressing cells, whereas strong LY6K expression was seen in LY6K-WT-expressing cells (Fig. 5D). Moreover, LY6K-WT-expressing cells also showed clear colocalization between LY6K and CAV-1, while control and LY6K-ΔGPI-expressing cells showed no colocalization between LY6K and CAV-1 (Supplementary Figure 4B). However, in the presence of triton-mediated membrane permeabilization, LY6K expression was detected in both LY6K-ΔGPI- or LY6K-WT-expressing cells. These data indicate that deletion of the GPI anchor domain prevents LY6K from localizing to the membrane. We then examined p-ERK1/2 signaling in these cells. Compared with LY6K-WT, U87 cells expressing the LY6K-ΔGPI mutant failed to enhance p-ERK1/2 relative to control, even in the presence of EGF stimulation (Fig. 5E). Together, these data suggest that the GPI anchor and proper membrane localization are crucial for LY6K-enhanced ERK1/2 signaling.
Additionally, proteasome inhibition attenuates ERK1/2 signaling and reduces cell proliferation30 by suppressing degradation of dual specificity phosphatases (eg, MAPK-phosphatase-1). Moreover, we observed that total levels of LY6K increased when GSC528 cells were subjected to EGF stimulation (see Fig. 3C), suggesting an effect on LY6K protein stability. To determine whether proteasome inhibition modulates LY6K-enhanced ERK1/2 activation, we treated U87 cells expressing a control vector, LY6K-ΔGPI, or LY6K-WT with a proteasome inhibitor, MG132 (Fig. 5F). While treatment with MG132 markedly reduced p-ERK1/2 levels in all conditions (Fig. 5F), cells expressing a control vector or LY6K-ΔGPI showed more pronounced reduction of p-ERK1/2 relative to cells expressing LY6K-WT (Fig. 5F, right). Of note, MG132 treatment resulted in the appearance of different sizes of LY6K, suggesting that LY6K requires processing prior to its maturation as a functional protein.
Finally, to assess the importance of the GPI anchor of LY6K on GBM tumorigenicity, we conducted in vivo xenograft experiments using GSC83 cells with knockdown of endogenous LY6K and subsequent exogenous expression of either LY6K-ΔGPI or a control vector (Fig. 5G). Unlike LY6K-WT (see Fig. 2F), exogenous expression of LY6K-ΔGPI failed to rescue LY6K-enhanced GSC intracranial tumor growth and overall survival of tumor-bearing animals relative to controls. This highlights the importance of the GPI anchor for LY6K-mediated GBM tumorigenicity.
Promoter Methylation Contributes to LY6K Gene Expression and Modulates GBM Cell Response to Radiation
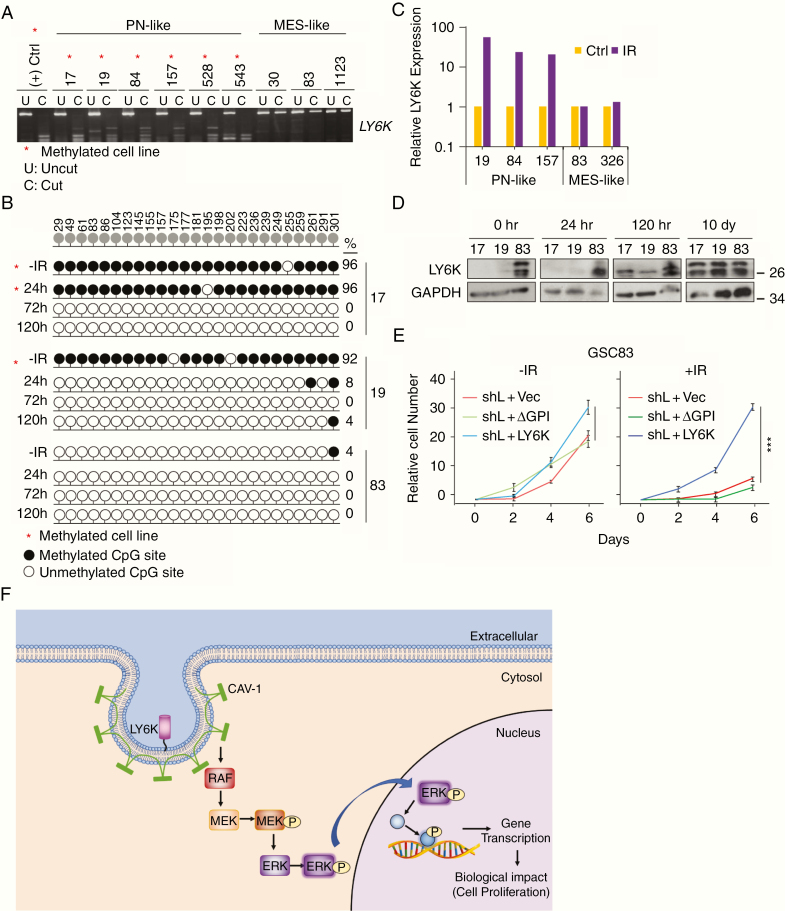
Cancer/testis antigens are epigenetically silenced in non-reproductive tissues, but can become aberrantly activated during cancer.31 Since LY6K is a cancer/testis antigen, we investigated whether LY6K expression is silenced by DNA methylation in GBM. Characterization of the gene locus of LY6K revealed a cytosine-phosphate-guanine (CpG) island along its promoter region (Supplementary Figure 5A). Analysis of these CpG sites using our published 450K Methylation Array dataset32 revealed that PN-like GSCs have significantly higher levels of methylation relative to MES-like GSCs, which is consistent with PN-like GSCs having relatively lower levels of LY6K expression (Supplementary Figure 5B, left). We validated these results by using combined bisulfite and restriction analysis (CoBRA), which revealed that LY6K is hypermethylated (cut) in PN-like GSCs and hypomethylated (uncut) in MES-like GSCs (Fig. 6A, Supplementary Figure 5C and 5D). These methylation profiles were further confirmed at single-base resolution with bisulfite sequencing analysis. Over 90% of CpG sites in PN-like GSCs were methylated, while less than 10% of CpG sites in MES-like GSCs were methylated (Fig. 6B). Interestingly, this differential methylation appears to be limited to GSCs, as methylation analysis of TCGA GBM samples showed high levels of methylation for all probes examined, regardless of GBM subtype (Supplementary Figure 5B, right).
Fig. 6.
Promoter methylation contributes to LY6K gene expression and modulates GBM cell response to radiation. (A) CoBRA. LY6K was methylated in PN-like GSCs and was unmethylated in MES-like GSCs. DNA incubated with S-adenosyl methionine was used as a positive control. (B). Bisulfite sequencing analysis. In the absence of IR, PN-like GSC17 and GSC19 had high levels of methylation of the LY6K gene promoter, while GSC83 had relatively low DNA methylation. Within 72h or 24h after 2 Gy IR, the methylation profile switched to be much more MES-like in both GSC17 and GSC19, respectively. The methylation profile for GSC83 remained stable, regardless of IR. Top row indicates position of each CG site along the LY6K promoter. Filled circles indicate methylated CG sites. Clear circles indicate unmethylated CG sites. Percentage of CG sites methylated is shown on the right. Base pair location is listed on top. (C) Analysis of DNA expression array data of GSCs treated with 2 Gy IR.6 IR induced 20 and 50-fold higher LY6K expression in PN-like GSCs, whereas LY6K expression remained stable in IR-treated MES-like GSCs. (D) IB. IR-induced LY6K protein expression in PN-like GSC17 and GSC19 increases with time, whereas LY6K expression in IR-treated MES-like GSC83 remained stable. GAPDH was a loading control. (E) Cell proliferation assay. In GSC83 cells with knockdown of endogenous LY6K, only expression of LY6K-WT increased cell proliferation and resistance to IR. (F) Schematic depiction of how membrane-anchored LY6K associates with CAV-1 to enhance ERK1/2 signaling, thereby promoting GBM cell proliferation and tumorigenicity. Red asterisks (*) in A and B indicate methylated cell lines. Data in A-B and D-E are representative from two to three independent experiments with similar results. *P < 0.05, ***P < 0.001.
RT is the first line of treatment for GBM patients,33 and RT is known to alter cancer cell epigenomes.34 Therefore, we tested whether ionizing radiation (IR) alters LY6K promoter methylation and expression. IR markedly increased LY6K expression in PN-like GSCs,6 whereas minimal changes were found in IR-treated MES-like GSCs (Fig. 6C). We subjected PN-like GSC17 and GSC19, and MES-like GSC83 to 2 Gy IR and subsequently isolated genomic DNA at 24 h, 72 h, and 120 h after IR. IR-treated PN-like GSCs showed markedly decreased CpG island methylation (Fig. 6B) and induction of LY6K protein expression (Fig. 6D). For GSC17 cells, the changes in methylation and protein expression were only apparent 72 h after IR, while GSC19 showed changes within 24 h (Fig. 6B, 6D). We also observed changes in cellular morphology in response of IR in PN-like GSCs (Supplementary Figure 5E). In contrast, MES-like GSC83 showed no changes in promoter methylation (Fig. 6B), LY6K expression (Fig. 6D), or cell morphology (Supplementary Figure 5E). Notably, 10 days after IR, expression of LY6K was comparable between PN-like and MES-like GSCs (Fig. 6D).
Finally, using MES-like GSC83 cells, we determined the effect of modifying LY6K expression on response to IR. Knockdown of endogenous LY6K and subsequent expression of LY6K-WT rendered GSC83 cells resistant to 2 Gy IR. However, cells with LY6K knockdown and subsequent expression of LY6K-ΔGPI or a control vector showed sensitivity to the same treatment (Fig. 6E). These data are consistent with our previous finding that MES-like GSCs are resistant to IR in vitro,5 and suggest that upregulation of LY6K in MES-like GSCs contributes to GBM RT resistance. Taken together, these results indicate that LY6K promoter methylation is especially important for GSC subpopulations, particularly with respect to modulating GSC response to RT.
Discussion
Sustained cell proliferation resulting from aberrant signal transduction is a hallmark of cancer.16 This study details functional and phenotypic effects associated with the aberrant expression of LY6K, a previously undescribed contributor to GBM biology. Previous studies have shown elevated expression of LY6K and its family members in other human cancers,35 including breast,10 esophageal,9 and lung8 cancers. In breast cancer, LY6K and LY6E are required for TGFβ signaling and promote drug resistance and cancer cell escape from immune surveillance.10 Here, we show for the first time that LY6K expression correlates with poor prognosis for GBM patients and that increased expression of LY6K promotes pro-tumor phenotypes in GBM cells in vitro and in vivo. This is especially relevant, given recent evidence showing the utility of alternative splicing events of LY6K as a prognostic indication of GBM patient survival.11 Mechanistically, we report that membrane-associated LY6K interacts with CAV-1 to enhance ERK1/2 signaling and subsequently promotes GBM cell proliferation (Fig. 6F).
Although ERK1/2 activation is often a result of RTK-stimulated signaling, we found no evidence that any RTK examined is responsible for LY6K-enhanced ERK1/2 activation. Rather, LY6K enhances ERK1/2 activity through its association with CAV-1. Our results are also consistent with previous studies indicating that CAV-1 transport from the cell surface to early endocytic vesicles activates ERK1/2 signaling.21 Given the ability of CAV-1 to switch from having a tumor-suppressive role to a tumor-promoting role depending on cancer stage,18 we examined the role of CAV-1 in LY6K-mediated ERK1/2 signaling. Regardless of EGFR expression, EGF stimulation increased p-ERK1/2 levels in the presence of LY6K. However, knockdown of CAV-1 suppressed this increase. Additionally, RAF inhibition eliminated detectable p-ERK1/2 levels, while MEK inhibition blocked LY6K-enhanced p-ERK1/2 only but retained basal p-ERK1/2 levels. However, knockdown of CAV-1 in the presence of LY6K suppressed even the basal levels, effectively eliminating all ERK1/2 activity. These results suggest that LY6K interacts with CAV-1 to induce ERK1/2 signaling and that EGF stimulation further enhances LY6K-CAV-1-mediated ERK1/2 activation.
Given that EGF is known to recruit CAV-1 to endocytic compartments and subsequently activate MAPK signaling,21 it is possible that LY6K acts as a mediator of this recruitment. Moreover, other members of the LY6 family are also known to utilize caveolin-mediated internalization to modulate cell signaling.26 Indeed, pharmacologic inhibition of proper membrane formation or CAV-1 knockdown dampened LY6K- enhanced ERK1/2 activity. Furthermore, genetic ablation of the GPI-anchor domain of LY6K caused improper localization of LY6K and abolished LY6K-enhancement of p-ERK1/2, even in the presence of EGF. Moreover, GPI anchor ablation resulted in decreased cell proliferation in vitro and suppressed tumor growth in xenograft experiments. These data suggest that proper membrane localization of LY6K is critical for its role in enhancing ERK1/2 signaling and promoting tumorigenic phenotypes in GBM. Although our results indicate that membrane-anchored LY6K and CAV-1 interact to promote ERK1/2 signaling, future studies are warranted to illuminate the precise mechanism by which LY6K and CAV-1 interact. As both proteins are localized to lipid rafts,26 it is also plausible that other proteins in the lipid bilayer, or perhaps the lipids themselves, are helping to facilitate this interaction.
A clinically relevant finding in this study is that LY6K promoter methylation maintains its silencing and contributes to RT responses in GBM. LY6K is a member of the cancer/testis antigen family of proteins, which can become aberrantly expressed in cancers originating from non-reproductive tissues through modulation of DNA methylation profiles. Our DNA methylation analysis32 revealed that the LY6K gene promoter is hypermethylated in PN-like GSCs, but hypomethylated in MES-like GSCs. LY6K promoter methylation results in LY6K silencing, such that PN-like GSCs express very low levels of LY6K. Moreover, RT is the first line of treatment in the standard care of GBM patients. RT reduced LY6K promoter methylation and induced LY6K expression in PN-like GSCs, at both the gene and protein levels. Consistent with these results, MES-like GSCs with either knockdown of endogenous LY6K or knockdown and subsequent expression of GPI-deficient LY6K showed sensitivity to RT. In contrast, MES-like GSCs with knockdown and subsequent expression of full-length LY6K showed resistance to RT and maintained the high growth potential observed in non-IR conditions.
In conclusion, our study shows that elevated expression of LY6K is an important contributor to GBM tumor biology and functions by activating the MAPK pathway via interactions with CAV-1. Furthermore, our results indicated that targeting LY6K-CAV1-ERK1/2 signaling in GBM may be a valuable therapeutic strategy, especially in GBM tumors that are resistant to RT.
Supplementary Material
Funding
All Shared Resources at the Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine that contribute to this study were supported by an NIH NCI Cancer Center grant P30CA060553. The Northwestern Nervous System Tumor Bank is supported by an NIH NCI P50CA221747. This work was supported by US NIH grants NS095634 (S.Y.C.), F31 CA232630 (N.S), CA813991 (I.N.), NS095642 (C.D.J.), NS102669 (C.H.), T32 fellowship CA070085 (A.A.A.), NIH LRP award L32 MD010147 (A.A.A.), and Lou and Jean Malnati Brain Tumor Institute at Northwestern Medicine (S-Y.C., B.H.). S-Y.C. is a Zell Scholar at Northwestern University. Fishel Family Fellowship in Cancer Research, the Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine (N.S.).
Conflict of interest statement. The authors declare no conflict of interest.
Authorship statement: Conceptualization and study supervision, B.H., S-Y.C. Methodology, development, and investigation, N.G.S., T.H. Data analysis and interpretation, T.H., A.A.A., X.S., X.W., R.P.P., provided reagents and consultation, C.D.J., C.M.H., C.W.B., I.N. Writing, review, and editing of the manuscript: N.G.S., B.H., S-Y.C. All authors edited and reviewed the manuscript.
References
- 1. Aldape K, Brindle KM, Chesler L, et al. Challenges to curing primary brain tumours. Nat Rev Clin Oncol. 2019;16(8):509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Q, Hu B, Hu X, et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 2017;32(1):42–56 e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neftel C, Laffy J, Filbin MG, et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell. 2019;178(4):835–849 e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev. 2015;29(12):1203–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mao P, Joshi K, Li J, et al. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci U S A. 2013;110(21):8644–8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Minata M, Audia A, Shi J, et al. Phenotypic plasticity of invasive edge glioma stem-like cells in response to ionizing radiation. Cell Rep. 2019;26(7):1893–1905 e1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loughner CL, Bruford EA, McAndrews MS, Delp EE, Swamynathan S, Swamynathan SK. Organization, evolution and functions of the human and mouse Ly6/uPAR family genes. Hum Genomics. 2016;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luo L, McGarvey P, Madhavan S, Kumar R, Gusev Y, Upadhyay G. Distinct lymphocyte antigens 6 (Ly6) family members Ly6D, Ly6E, Ly6K and Ly6H drive tumorigenesis and clinical outcome. Oncotarget. 2016;7(10):11165–11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ishikawa N, Takano A, Yasui W, et al. Cancer-testis antigen lymphocyte antigen 6 complex locus K is a serologic biomarker and a therapeutic target for lung and esophageal carcinomas. Cancer Res. 2007;67(24):11601–11611. [DOI] [PubMed] [Google Scholar]
- 10. AlHossiny M, Luo L, Frazier WR, et al. Ly6E/K Signaling to TGFβ promotes breast cancer progression, immune escape, and drug resistance. Cancer Res. 2016;76(11):3376–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen X, Zhao C, Guo B, Zhao Z, Wang H, Fang Z. Systematic profiling of alternative mrna splicing signature for predicting glioblastoma prognosis. Front Oncol. 2019;9:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kikuchi R, Ueda R, Saito K, et al. A pilot study of vaccine therapy with multiple glioma oncoantigen/glioma angiogenesis-associated antigen peptides for patients with recurrent/progressive high-grade glioma. J Clin Med. 2019;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov. 2014;13(12):928–942. [DOI] [PubMed] [Google Scholar]
- 15. Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 2005;6(11):827–837. [DOI] [PubMed] [Google Scholar]
- 16. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 17. Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Caveolae and signalling in cancer. Nat Rev Cancer. 2015;15(4):225–237. [DOI] [PubMed] [Google Scholar]
- 18. Parat MO, Riggins GJ. Caveolin-1, caveolae, and glioblastoma. Neuro Oncol. 2012;14(6):679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goetz JG, Lajoie P, Wiseman SM, Nabi IR. Caveolin-1 in tumor progression: the good, the bad and the ugly. Cancer Metastasis Rev. 2008;27(4):715–735. [DOI] [PubMed] [Google Scholar]
- 20. Mineo C, James GL, Smart EJ, Anderson RG. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J Biol Chem. 1996;271(20):11930–11935. [DOI] [PubMed] [Google Scholar]
- 21. Pol A, Lu A, Pons M, Peiró S, Enrich C. Epidermal growth factor-mediated caveolin recruitment to early endosomes and MAPK activation. Role of cholesterol and actin cytoskeleton. J Biol Chem. 2000;275(39):30566–30572. [DOI] [PubMed] [Google Scholar]
- 22. Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. [DOI] [PubMed] [Google Scholar]
- 23. Brennan CW, Verhaak RG, McKenna A, et al. ; TCGA Research Network The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bowman RL, Wang Q, Carro A, Verhaak RG, Squatrito M. GlioVis data portal for visualization and analysis of brain tumor expression datasets. Neuro Oncol. 2017;19(1):139–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mayor S, Riezman H. Sorting GPI-anchored proteins. Nat Rev Mol Cell Biol. 2004;5(2):110–120. [DOI] [PubMed] [Google Scholar]
- 26. Özhan G, Sezgin E, Wehner D, et al. Lypd6 enhances Wnt/β-catenin signaling by promoting Lrp6 phosphorylation in raft plasma membrane domains. Dev Cell. 2013;26(4):331–345. [DOI] [PubMed] [Google Scholar]
- 27. Mundy DI, Machleidt T, Ying YS, Anderson RG, Bloom GS. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J Cell Sci. 2002;115(Pt 22):4327–4339. [DOI] [PubMed] [Google Scholar]
- 28. Lee PY, Wang JX, Parisini E, Dascher CC, Nigrovic PA. Ly6 family proteins in neutrophil biology. J Leukoc Biol. 2013;94(4):585–594. [DOI] [PubMed] [Google Scholar]
- 29. Lisanti MP, Field MC, Caras IW, Menon AK, Rodriguez-Boulan E. Mannosamine, a novel inhibitor of glycosylphosphatidylinositol incorporation into proteins. EMBO J. 1991;10(8):1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cirit M, Grant KG, Haugh JM. Systemic perturbation of the ERK signaling pathway by the proteasome inhibitor, MG132. PLoS One. 2012;7(11):e50975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5(8):615–625. [DOI] [PubMed] [Google Scholar]
- 32. Pangeni RP, Zhang Z, Alvarez AA, et al. Genome-wide methylomic and transcriptomic analyses identify subtype-specific epigenetic signatures commonly dysregulated in glioma stem cells and glioblastoma. Epigenetics. 2018;13(4):432–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang T, Kim CK, Alvarez AA, et al. MST4 phosphorylation of ATG4B regulates autophagic activity, tumorigenicity, and radioresistance in glioblastoma. Cancer Cell. 2017;32(6):840–855 e848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Antwih DA, Gabbara KM, Lancaster WD, Ruden DM, Zielske SP. Radiation-induced epigenetic DNA methylation modification of radiation-response pathways. Epigenetics. 2013;8(8):839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Upadhyay G. Emerging role of lymphocyte antigen-6 family of genes in cancer and immune cells. Front Immunol. 2019;10:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.