Abstract
Background
We performed a multicenter retrospective observational study to investigate the impact of clinical–pathological features and therapeutic strategies on both the complications and survival of patients with bone metastases (BMs) from malignant melanoma.
Patients and Methods
A total of 305 patients with melanoma and radiological evidence of BMs were retrospectively enrolled from 19 Italian centers. All patients received conventional treatments in accordance with each own treating physician’s practice. Both univariate and multivariate models were used to explore the impact of melanoma features, including skeletal-related events (SREs), and different treatments on both overall survival (OS) and time-to-SREs. The chi-squared test evaluated the suitability of several parameters to predict the occurrence of SREs.
Results
Eighty-three percent of patients had metachronous BMs. The prevalent (90%) bone metastatic site was the spine, while 45% had involvement of the appendicular skeleton. Forty-seven percent experienced at least one SRE, including palliative radiotherapy (RT) in 37% of cases. No melanoma-associated factor was predictive of the development of SREs, although patients receiving early treatment with bone-targeted agents showed 62% lower risk and delayed time of SRE occurrence. Median OS from the diagnosis of bone metastasis was 10.7 months. The multivariate analysis revealed as independent prognostic factors the number of BMs, number of metastatic organs, baseline lactate dehydrogenase levels, and treatment with targeted therapy or immunotherapy. Subgroup analyses showed the best OS (median = 16.5 months) in the subset of patients receiving both immunotherapy and palliative RT.
Conclusion
Based on our results, patients undergoing immunotherapy and palliative RT showed an OS benefit suggestive of a possible additive effect. The apparent protective role of bone targeting agent use on SREs observed in our analysis should deserve prospective evaluation.
Keywords: melanoma, bone metastases, SREs, immunotherapy, bisphosphonates, denosumab
Introduction
Innovative therapies have improved the survival of patients with unresectable metastatic cutaneous melanoma (CM). However, the prognosis remains poor in those harboring negative prognostic factors, such as poor performance status (PS), high tumor burden, brain metastases, and high baseline levels of lactate dehydrogenase (LDH) (1, 2). Also, the genomic landscape of melanoma influences the clinical evolution as well as innate and acquired drug resistance, thus representing an up-growing field of interest (3).
The impact of bone disease (BD) in melanoma has been scarcely investigated. Data from clinical trials indicate that the skeleton is the fourth site of metastasis after lung, liver, and brain, that occurs in about 11–18% of patients (4, 5). Bone metastases (BMs) generate typical skeletal-related events (SREs), such as severe bone pain, pathological fractures, spinal cord compression, hypercalcemia, and need for radiotherapy (nRT) or surgery to the bone, and are common in breast, prostate, and lung cancers while being so far a clinical challenge in other malignancies like melanoma (6). Moreover, the potential beneficial effect of novel anti-melanoma agents on BMs and SREs is, at present, unclear (7).
Data collected from the SEER (Surveillance, Epidemiology and End Results) database and large series from individual medical centers demonstrated that BMs from CM are frequently observed in young patients and are associated with elevated LDH, while the prognosis is apparently poor and almost similar to that of patients developing brain or liver metastases (8). In addition, BMs occur in patients with CM, while they are rarely detected in those with mucosal, uveal, and acral melanoma. Furthermore, BMs are frequently revealed at diagnosis of the metastatic disease as involving single or multiple sites and frequently affect the axial skeleton.
The pathogenesis of BMs is regulated by the interplay among malignant cells, osteoclasts, osteoblasts, and immune cells. Epithelial tumor cells, indeed, produce high RANK-L (the receptor activator of nuclear factor-kB ligand) levels that engulf the bone metastatic sites, thus interfering with osteoclasts through the RANK receptor that is also expressed by cancer cells like melanoma and regulated by interferon (IFN)-γ (9). Systemic agents for the treatment of BMs include inhibitors of bone resorption and anabolic signals, namely, bone-targeting agents (BTAs), aimed at restoring the physiological bone turnover that results profoundly impaired in patients with skeletal colonization. Their use in breast, prostate, and lung cancers is currently well supported in various clinical settings (10), while their effective contribution in patients with BMs from melanoma is still debated. Recent studies reported the synergistic effect of RANK-L blockade combined with immune checkpoint inhibition in patients with BMs from melanoma, suggesting a potential clinical benefit from this strategy (11). Based on preliminary evidence (12, 13), clinical trials are currently ongoing and are aimed at exploring the overall therapeutic effect of denosumab with immunotherapy (NCT-03161756; EudraCT-2016-001925-15).
Growing interest in melanoma has also been oriented in combining an immune checkpoint inhibitor (ICI) with either systemic or local treatment to maximize the antitumor response. Ongoing clinical trials, for example, are exploring the association of targeted therapy and immunotherapy (14–16). They were supported by preclinical and translational studies showing that BRAF and MEK inhibition has immune-modulating effects that increase tumor T cell infiltration as well as tumor antigen exposition and PD-L1 expression (17, 18). Another attractive scenario is combining immunotherapy with radiotherapy (RT), the latter being often used in the case of BMs. The rationale is that tumor irradiation induces cell death, provoking local release of tumor-derived antigens that, in turn, promote T cell cross-priming by dendritic cells and long-term immunological memory (19). This priming of the immune system by RT can synergize with immunotherapy. Consequently, trials evaluating combinations of immunotherapy and RT have progressively increased in the last years, both in melanoma and other malignancies (20).
Herein, we completed a retrospective multicentric survey in an Italian melanoma population bearing BMs to investigate the potential impact of clinical–pathological features and the therapeutic strategies on survival.
Patients and Methods
Study Population
In this observational multicenter study, we retrospectively enrolled 305 patients with a diagnosis of melanoma and radiological evidence of BD. All patients received standard treatments in accordance with each own treating physician’s practice in 19 Italian Centers from November 1984 to March 2019. Clinical data were collected throughout the disease course and included features of melanoma, BM detection, access to systemic treatments (immunotherapy, target therapy, or chemotherapy), nRT, as well as date of first SRE and death. When available, other variables, including age, sex, melanoma primary site, histological parameters (e.g., histotype, Breslow, ulceration, number of mitoses, lymphocyte infiltrate, and nodal stage), BRAF/NRAS status, LDH levels, calcemia, time to appearance of BMs, presence of extraosseous metastases, and SRE type, were assessed. Specific information about the systemic treatments (e.g., drug sequences, objective responses, or time of disease progression) were not considered since these are out of the scope of this survey. All patients had written informed consent for clinical data collection and use for research purposes according to each own center’s rules. Ethical approval was not required for this study according to local legislation.
Statistical Analysis
Descriptive statistics were used for patient demographics and incidence of SREs. The chi-squared test analyzed the relationship of SRE occurrence with the clinical and pathological features of melanoma patients. Survival analyses were completed by the Kaplan–Meier method. Factors for analyses were identified a priori and included baseline clinical features and known prognostic factors for metastatic melanoma or BD. The univariate analysis (log-rank test) explored the clinical variables as prognostic factors for overall survival (OS) and time to SREs from BM diagnosis. Patients who did not experience a SRE were censored at the date of death or at the last follow-up visit for the analysis of time to develop SRE. All significant variables in the univariate model were used to build the multivariate analysis of survival using the Cox proportional hazards regression. Patients that were lost in follow-up were not considered for survival analyses. The statistics were completed with the Medcalc software (version 12.7.0.0). A p value < 0.05 was considered significant.
Results
Baseline Demographic Features
In total, 305 patients with cutaneous (n = 290), mucosal (n = 6), and uveal (n = 9) melanoma were enrolled in the study. The basal clinical and pathological features of the population are described in Table 1. Of note is that the median age at diagnosis was 56 years and 63.3% were male (n = 193). A BRAF mutation was documented in 59%, while NRAS mutation was present in 36% of patients. Almost 22% (n = 23/105) of patients had LDH levels >2 times the upper limit of the normal range (ULN) at the time of melanoma diagnosis.
TABLE 1.
Patient demographics and basal melanoma characteristics in the study population.
| Characteristics | Frequency | Percentage |
| Age at melanoma diagnosis (n = 305) | ||
| Median, 56 (range = 18–86) years | ||
| Sex (n = 305) | ||
| Male | 193 | 63.3 |
| Female | 112 | 36.7 |
| Primary site (n = 305) | ||
| Limbs | 82 | 26.9 |
| Head and neck | 39 | 12.8 |
| Trunk | 134 | 43.9 |
| Occult | 35 | 11.5 |
| Mucosa | 6 | 2.0 |
| Uvea | 9 | 3.0 |
| Histology (n = 239) | ||
| SSM | 88 | 36.8 |
| Nodular | 95 | 39.7 |
| Acral | 9 | 3.8 |
| Lentigo Maligna | 1 | 0.4 |
| Mucosal | 6 | 2.5 |
| Uveal | 9 | 3.8 |
| Other | 31 | 13.0 |
| Breslow depth (n = 239) | ||
| ≤1 mm | 28 | 11.7 |
| >1–2 mm | 48 | 20.1 |
| >2–4 mm | 73 | 30.5 |
| >4 mm | 90 | 37.7 |
| Ulceration (n = 239) | ||
| Present | 138 | 57.7 |
| Absent | 101 | 42.3 |
| Number of mitosis (n = 201) | ||
| ≤1 | 46 | 22.9 |
| >1 | 155 | 77.1 |
| Tumor-infiltrating lymphocytes (n = 169) | ||
| Brisk | 37 | 21.9 |
| Absent or not brisk | 132 | 78.1 |
| Lymph node mts (n = 263) | ||
| N0 | 133 | 50.6 |
| N+ | 130 | 49.4 |
| BRAF status (n = 285) | ||
| Mutated | 168 | 58.9 |
| Wild type | 117 | 41.1 |
| NRAS status (n = 59) | ||
| Mutated | 21 | 35.6 |
| Wild type | 38 | 64.4 |
| LDH levels (n = 105) | ||
| ≤2 × ULN | 82 | 78.1 |
| >2 × ULN | 23 | 21.9 |
LDH, lactate dehydrogenase; ULN, upper limit of normal; SSM, superficial spreading melanoma; mts, metastases.
The majority of patients (97%) had extraosseous metastases, which included 247 patients (81%) with more than three metastatic sites and 73 patients (24%) with brain metastases. The onset of BMs and the diagnosis of primary melanoma were mostly metachronous (83%). The prevalent bone metastatic site was the spine (90%), while only 45% of patients had involvement of the appendicular skeleton, including 10% of them with both axial and appendicular colonization. The majority of patients (61%, n = 183/301) showed less than five BMs, while 39% (n = 118) harbored more lesions. Calcium levels at the time of BM diagnosis were usually normal, whereas 37% of patients (n = 95/254) expressed LDH levels > 2 times the ULN.
Almost half of the patients received BTAs, including bisphosphonates (40.8%, n = 119/292) or denosumab (6.8%, n = 20). With regard to systemic treatments, 33% of patients (n = 96/291) received only targeted therapy, 39% (n = 114) only ICIs, and 20.6% (n = 60) both ICIs and targeted agents, whereas a minority (7%, n = 21) underwent only chemotherapy (CHT). Data are summarized in Table 2.
TABLE 2.
Characteristics of metastatic disease in the study population.
| Characteristics | Frequency | Percentage |
| Number of metastatic organs (n = 305) | ||
| <3 | 58 | 19.0 |
| ≥3 | 247 | 81.0 |
| Presence of extraosseous mts (n = 305) | ||
| No | 9 | 3.0 |
| Yes, without brain | 223 | 73.1 |
| Yes, with brain | 73 | 23.9 |
| Time of BM diagnosis (n = 305) | ||
| Synchronous | 50 | 16.4 |
| Metachronous | 255 | 83.6 |
| Sites of BM (n = 300) | ||
| Axial | 165 | 55 |
| Appendicular | 29 | 9.7 |
| Both | 106 | 35.3 |
| Number of BM (n = 301) | ||
| <5 | 183 | 60.8 |
| ≥5 | 118 | 39.2 |
| Calcaemia at BM diagnosis (n = 235) | ||
| ≤ULN | 218 | 92.8 |
| >ULN | 17 | 7.2 |
| LDH levels at BM diagnosis (n = 254) | ||
| ≤2 × ULN | 159 | 62.6 |
| >2 × ULN | 95 | 37.4 |
| Use of BTA (n = 292) | ||
| No | 153 | 52.4 |
| Bisphosphonates | 119 | 40.8 |
| Denosumab | 20 | 6.8 |
| Systemic treatment (n = 291) | ||
| Targeted therapy | 96 | 33.0 |
| Immunotherapy | 114 | 39.2 |
| Targeted and ICIs | 60 | 20.6 |
| Chemotherapy | 21 | 7.2 |
BTA, bone-targeting agents; BM, bone metastases; ICIs, immune checkpoint inhibitors; mts; metastases; LDH, lactate dehydrogenase; ULN, upper limit of normal.
Bone Disease and Development of SREs
As shown in Supplementary Figure S1A, 47% (n = 137/291) of patients developed at least one SRE, which included nRT in 37% (n = 109), fractures in 12% (n = 35), spinal cord injury in 7% (n = 21), and surgery in 3% (n = 8), while hypercalcemia occurred in a single case (< 1%). Thirty-four patients (12%) experienced two or more SREs.
The next set of analyses (Table 3) explored the features of melanoma patients who experienced SREs. The median age was 59 years and 65% were male. The majority of them showed metachronous BMs (87%) located in the axial skeleton (56%) as well as LDH levels ≤ 2 times the ULN (67%) and normal calcemia (89%) at the time of BM detection. Notably, 66% of patients had less than five BMs, while extraosseous metastases occurred in about 95% of patients. Among patients who experienced at least one SRE, almost 54% were previously treated with BTAs such as bisphosphonates.
TABLE 3.
Patient demographics and BM characteristics in the population who experienced SREs.
| Characteristics | Frequency | Percentage |
| Age at BM diagnosis (n = 137) | ||
| Median, 59 years | ||
| Sex (n = 137) | ||
| Male | 89 | 65.0 |
| Female | 48 | 35.0 |
| BRAF status (n = 131) | ||
| Mutated | 71 | 54.2 |
| Wild type | 60 | 45.8 |
| NRAS status (n = 27) | ||
| Mutated | 11 | 40.7 |
| Wild type | 16 | 59.3 |
| LDH levels at BM diagnosis (n = 109) | ||
| ≤2 × ULN | 73 | 67.0 |
| >2 × ULN | 36 | 33.0 |
| Calcaemia at BM diagnosis (n = 104) | ||
| ≤ ULN | 93 | 89.4 |
| > ULN | 11 | 10.6 |
| BM and melanoma diagnosis (n = 137) | ||
| Synchronous | 18 | 13.1 |
| Metachronous | 119 | 86.9 |
| SRE and BM diagnosis (n = 129) | ||
| Synchronous | 38 | 29.5 |
| Metachronous | 91 | 70.5 |
| Localization of BM (n = 133) | ||
| Axial | 74 | 55.6 |
| Appendicular | 17 | 12.8 |
| Both | 42 | 31.6 |
| Number of BM (n = 134) | ||
| <5 | 89 | 66.4 |
| ≥5 | 45 | 33.6 |
| Presence of extraosseous mts (n = 137) | ||
| No | 7 | 5.1 |
| Yes | 130 | 94.9 |
| Use of BTA (n = 137) | ||
| No | 63 | 46 |
| Bisphosphonates | 67 | 48.9 |
| Denosumab | 7 | 5.1 |
BM, bone metastases; BTA, bone-targeting agents; LDH, lactate dehydrogenase; mts, metastases; SRE, skeletal-related events; ULN, upper limit of normal.
Neither clinical parameters nor tumor-related variables correlated with SREs (data not shown). However, patients who were early treated with BTAs showed a minor SRE occurrence with respect to the untreated patients [20% vs. 39.6%; odds ratio (OR) = 0.38, 95% confidence interval (CI) = 0.2–0.72, p = 0.003] (Supplementary Figure S1B). Moreover, the univariate analysis (Table 4) investigated those factors putatively associated with longer time to SRE development and revealed a correlation with the axial localization of BMs [hazard ratio (HR) = 0.61, 95% CI = 0.33–1.13, p = 0.05] and with previous use of BTAs (HR = 0.41, 95% CI = 0.26–0.66, p = 0.001). However, only the use of BTAs before the development of SREs was confirmed as an independent prognostic factor (p = 0.003).
TABLE 4.
Univariate and multivariate analyses of factors associated with time to SRE in patients with BM from melanoma.
| Factors | Effect tested | Univariate analysis | Multivariate analysis | ||||
| HR | 95% CI | p | HR | 95% CI | p | ||
| Demographics | |||||||
| Age (years) | ≤55 vs. >55 | 1.22 | 0.86–1.74 | 0.25 | |||
| Sex | Male vs. Female | 1.13 | 0.80–1.61 | 0.48 | |||
| Baseline melanoma characteristics | |||||||
| Histology | SSM vs. Nodular | 0.96 | 0.61–1.50 | 0.85a | |||
| Mucosal vs. Nodular | 0.95 | 0.23–3.94 | |||||
| Acral vs. Nodular | 1.42 | 0.47–4.34 | |||||
| Uveal vs. Nodular | 0.50 | 0.17–1.43 | |||||
| Others vs. Nodular | 0.81 | 0.34–1.93 | |||||
| BRAF genotype | V600 vs. Wild type | 0.74 | 0.52–1.07 | 0.10 | |||
| NRAS genotype | Mutated vs. Wild type | 1.37 | 0.65–2.90 | 0.33 | |||
| Characteristics present at BM diagnosis | |||||||
| Time of diagnosis | Synchronous vs. Metachronous | 0.75 | 0.47–1.20 | 0.27 | |||
| Localization of BM | Axial vs. Extra-axial | 0.61 | 0.33–1.13 | 0.05 | 0.73 | 0.36–1.48 | 0.39 |
| Number of BM | <5 vs. ≥5 | 0.98 | 0.68–1.41 | 0.91 | |||
| Calcaemia | ≤ULN vs. >ULN | 0.70 | 0.28–1.72 | 0.35 | |||
| LDH levels | ≤2× ULN vs. > 2× ULN | 1.00 | 0.66–1.52 | 1.00 | |||
| Treatment and SRE | |||||||
| Use of BTA before SRE | Yes vs. No | 0.41 | 0.26–0.66 | 0.001 | 0.43 | 0.24–0.75 | 0.003 |
| Systemic treatment | Targeted/ICIs vs. CHT | 1.12 | 0.51–2.44 | 0.78 | |||
BM, bone metastases; BTA, bone-targeting agents; CHT, chemotherapy; ICIs, immune checkpoint inhibitors; LDH, lactate dehydrogenase; SSM, superficial spreading melanoma; SRE, skeletal-related events; ULN, upper limit of normal. aLogrank test. Bold values are indicate to p < 0.05.
Factors Associated With Overall Survival
At the time of data lock, 182 patients were deceased and 108 were still alive, while the other 15 resulted lost in follow-up. The median OS was 10.7 months (Supplementary Figure S2). Table 5 describes data from both univariate and multivariate analyses of the factors potentially correlated with prognosis. The univariate analysis revealed a worse prognosis in males (p = 0.03) and in patients with melanoma of the trunk (p = 0.05) as well as a number of five or more BMs (p < 0.0001), high LDH levels at metastatic BD diagnosis (p < 0.0001), and evidence of three or more metastatic sites (p = 0.0027), or brain metastasis (p = 0.0002). In addition, patients who received new agents (targeted therapy and/or immunotherapy) showed OS improvement with respect to those treated with CHT (p < 0.0001). In addition, neither detrimental impact on OS was determined by the occurrence of SREs, nor in the case of multiple SREs (data not shown). The multivariate analysis confirmed as positive strong independent prognostic factors less than five skeletal metastasis (HR = 0.56, 95% CI = 0.39–0.79, p = 0.0013; Supplementary Figure S3), limited LDH values (HR = 0.49, 95% CI = 0.34–0.71, p = 0.0001), less than three metastatic sites (HR = 0.58, 95% CI = 0.34–0.99, p = 0.047), and treatments with targeted agents and/or ICIs (HR = 0.32, 95% CI = 0.17–0.58, p = 0.0002).
TABLE 5.
Univariate and multivariate analyses of baseline factors associated with OS in patients with BM from melanoma.
| Factors | Effect tested | Univariate analysis | Multivariate analysis | ||||
| HR | 95% CI | p | HR | 95% CI | p | ||
| Demographics | |||||||
| Age (years) | ≤55 vs. >55 | 0.75 | 0.56–1.01 | 0.07 | |||
| Sex | Male vs. Female | 1.41 | 1.05–1.89 | 0.03 | 1.43 | 0.97–2.10 | 0.08 |
| Baseline melanoma characteristics | |||||||
| Histology | SSM vs. Nodular | 0.61 | 0.42–0.89 | ||||
| Mucosal vs. Nodular | 1.62 | 0.45–5.87 | |||||
| Acral vs. Nodular | 0.56 | 0.23–1.34 | 0.07a | ||||
| Uveal vs. Nodular | 0.95 | 0.38–2.39 | |||||
| Others vs. Nodular | 0.79 | 0.34–1.85 | |||||
| Melanoma site | Limbs vs. Trunk | 0.68 | 0.47–0.97 | 0.87 | 0.56–1.35 | 0.54 | |
| Others vs. Trunk | 1.09 | 0.70–1.71 | 0.05a | 1.08 | 0.69–1.69 | 0.73 | |
| Occult vs. Trunk | 0.65 | 0.41–1.03 | 0.75 | 0.41–1.38 | 0.36 | ||
| BRAF genotype | V600 vs. Wild type | 1.21 | 0.89–1.64 | 0.22 | |||
| NRAS genotype | Mutated vs. Wild type | 0.87 | 0.48–1.55 | 0.65 | |||
| Characteristics present at BM diagnosis | |||||||
| Time of diagnosis | Synchronous vs. Metachronous | 1.08 | 0.73–1.59 | 0.68 | |||
| Localization of BM | Axial vs. Extra-axial | 1.20 | 0.76–1.89 | 0.46 | |||
| Number of BM | <5 vs. ≥5 | 0.47 | 0.34–0.64 | <0.0001 | 0.56 | 0.39–0.79 | 0.0013 |
| Calcaemia | ≤ULN vs. >ULN | 0.54 | 0.23–1.26 | 0.06 | |||
| LDH levels | ≤2 × ULN vs. >2 × ULN | 0.42 | 0.30–0.60 | <0.0001 | 0.49 | 0.34–0.71 | 0.0001 |
| Characteristics of metastatic disease | |||||||
| Number of metastatic organs | <3 vs. ≥3 | 0.55 | 0.40–0.77 | 0.0027 | 0.58 | 0.34–0.99 | 0.047 |
| Presence of visceral mts | Yes with Brain vs. Yes w/o Brain | 1.79 | 1.23–2.62 | 0.0002a | 1.33 | 0.88–2.01 | 0.18 |
| No vs. Yes w/o Brain | 0.42 | 0.21–0.87 | 0.58 | 0.13–2.71 | 0.49 | ||
| Treatment and SRE | |||||||
| Use of BTA | Yes vs. No | 0.80 | 0.60–1.06 | 0.13 | |||
| SRE occurrence | Yes vs. No | 0.85 | 0.64–1.14 | 0.28 | |||
| Systemic treatment | Targeted/ICIs vs. CHT | 0.24 | 0.10–0.57 | <0.0001 | 0.32 | 0.17–0.58 | 0.0002 |
BM, bone metastases; BTA, bone targeting agents; CHT, chemotherapy; ICIs, immune checkpoint inhibitors; LDH, lactate dehydrogenase; mts, metastases; SSM, superficial spreading melanoma; SRE, skeletal-related events; ULN, upper limit of normal; w/o, whitout. aLogrank test. Bold values are indicate to p < 0.05.
Impact of Melanoma-Dedicated Treatments on Overall Survival
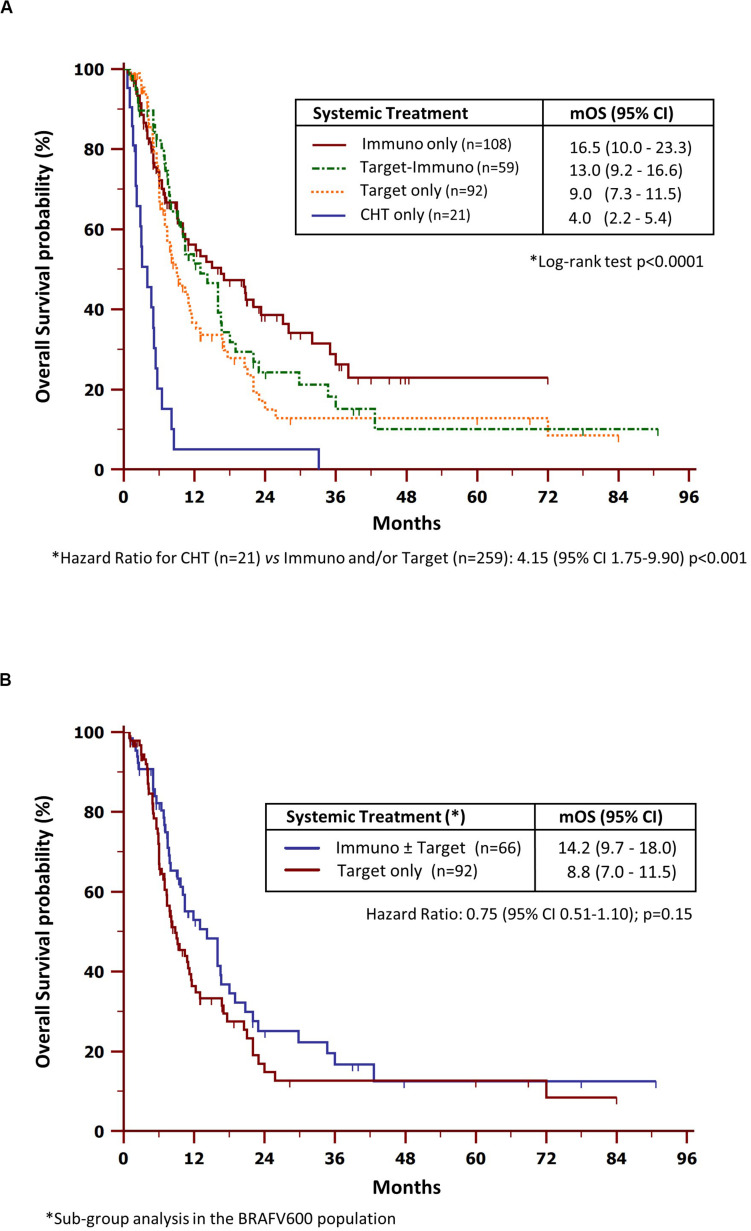
The next set of analyses explored the role of systemic treatments and RT on OS. As shown in Figure 1A, patients receiving CHT alone underwent worsened survival as compared to those treated with new agents, including ICIs and/or targeted drugs (HR = 4.15, CI = 1.75–9.90, p < 0.0001). In detail, the median OS (mOS) were 16.5 (95% CI = 10.0–23.3), 13.0 (95% CI = 9.2–16.6), 9.0 (95% CI = 7.3–11.5) and 4.0 months (95% CI = 2.2-5.4) in patients comprehensively treated with (i) ICIs only, (ii) ICIs and targeted therapy, (iii) targeted therapy only, or (iv) CHT only, respectively.
FIGURE 1.
Overall survival by systemic treatment in the study population (A) and BRAF-mutated population (B). mOS, median overall survival; CHT, chemotherapy.
Further investigations were dedicated to the BRAF-mutated population (Figure 1B). The median OS in patients who received at least one ICI (14.2 months, 95% CI = 9.7–18.0) resulted increased with respect to those treated with targeted therapy alone (8.8 months, 95% CI = 7.0–11.5), although the differences were not statistically significant (HR = 0.75, 95% CI = 0.51–1.10, p = 0.15).
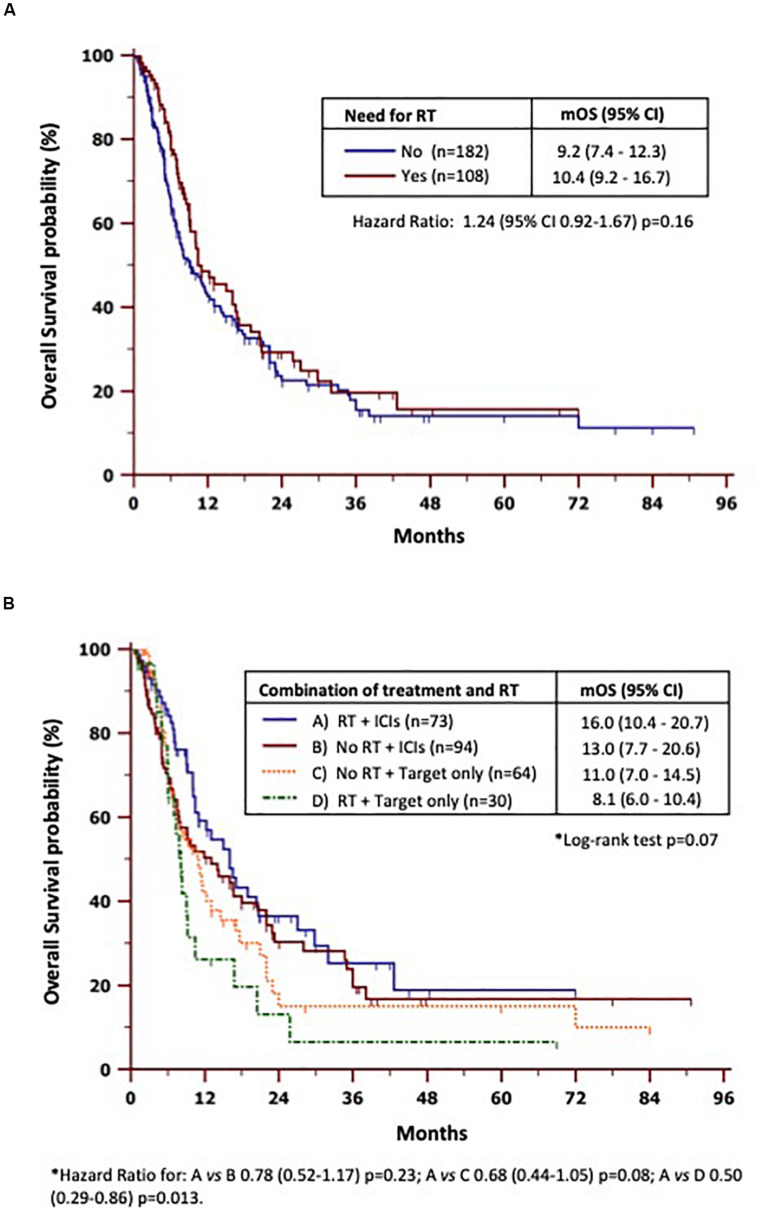
Regarding bone-specific treatments (Supplementary Figure S4), patients who received BTAs showed only a modest benefit in terms of mOS as compared to the untreated ones (11 vs. 9 months), but not statistically significant differences were found (HR = 0.80, 95% CI = 0.60–1.06, p = 0.13). On the other hand, mOS was quite similar between patients who underwent RT (Figure 2A) vs. those who were never treated (10.4 vs. 9.2 months). We also verified (Figure 2B) whether or not different combinations of RT with new therapies interfered with survival. To this purpose, we divided the study population into four groups based on the treatment received: (A) ICIs and RT (n = 73); (B) ICIs without RT (n = 94); (C) targeted therapy only without ICIs (n = 64); and (D) targeted therapy plus RT and never ICIs (n = 30). We found that patients in group A achieved the best mOS (16 months, 95% CI = 10.4–20.7) with respect to either group B (13 months; HR = 0.78, 95% CI = 0.52–1.17, p = 0.23), group C (11 months; HR = 0.68, 95% CI = 7.0–14.5, p = 0.08), or group D (8.1 months; HR = 0.5, 95% CI = 0.29–0.86, p = 0.013).
FIGURE 2.
Subset analyses of overall survival by radiotherapy need (A) and combinations of radiotherapy and new therapies (B). mOS, median overall survival; RT, radiotherapy.
Discussion
The development of BMs frequently occurs in cancer with a negative impact on the quality of life and survival. Appropriate algorithms for the management of BD mainly derive from extensive prospective trials with patients harboring tumors that frequently metastasize to the skeleton, such as breast, prostate, lung, and kidney cancers (21). Otherwise, a definite evidence-based strategy for the treatment of BMs from other malignancies endowed with lower osteotropic propensity does not exist, while apparently innovative drugs such as cilengitide failed to provide satisfactory results to be applied for routine clinical practice (22, 23). Particularly, the impact of BD in melanoma has been poorly investigated, as well as the potential therapeutic strategies such as either RT or BTA use. Moreover, another unanswered question from recent registrative clinical trials in melanoma concerns the possible impact of ICIs or targeted agents in patients with BM.
The present study was aimed at exploring retrospectively the characteristics of melanoma patients bearing BD. As a result of the available literature to the topic, ours appears as a large retrospective study investigating the features of BMs in melanoma. The definition of the effective incidence of BMs in melanoma, however, is out of the scope of the study and, therefore, was not investigated.
The baseline demographic data in our melanoma population demonstrated that BMs occurred primarily in males with prevalent involvement of the axial skeleton. Almost all patients (97%) showed extraosseous metastases. Among them, 83% developed metachronous bone and visceral metastases, while in 13% of them BMs were discovered in consequence of a SRE occurrence. The frequency of patients harboring a BRAF mutation (59%) was almost in line with that observed in the general melanoma population. On the other hand, the relative higher frequency observed for mutated NRAS (35.6%) should not be considered as a major propensity of these patients in developing BMs since the NRAS mutational status was available only in less than 20% of cases.
Other studies describing a modest incidence of bone involvement at diagnosis of metastatic melanoma probably reflect the fact that the BD was not properly suspected and investigated at diagnosis. However, one-fourth (23.9%) of melanoma patients from our series showed a brain involvement which is instead reported in about 40–60% of the general melanoma population (24). This apparent difference from our data may probably imply specific molecular mechanisms which critically drive the osteotropism of melanoma cells like those affecting the SDF1/CXCR7/CXCR4 pathway, as recently proven (25, 26).
The median survival from the onset of BM was 10.7 months, but our analysis revealed that patients receiving innovative therapies including ICIs and/or targeted agents showed a better prognosis (9.0–16.5 months) than those undergoing CHT (4.0 months). The patients analyzed in this study (n = 290) apparently showed a lower OS with respect to those enrolled in recent phase 3 clinical trials with new agents resulting in 5-year survival rates higher than 50%. However, it is noteworthy that our survey refers to the time from BM diagnosis because the information relative to the time of a comprehensive diagnosis of metastatic disease was not available. The lack of a control group without BMs also restrains the possibility of exploring the real impact of the BD in the general melanoma prognosis. Moreover, our real-world experience includes patients with clinical features that are generally excluded from clinical trials, including a suboptimal PS or brain involvement, thus reducing the possibility of a direct comparison between various case studies.
The data from large clinical trials with recent 5-year survival updates and pooled analyses defined major negative prognostic factors in metastatic melanoma that included elevated LDH levels, poor PS, and three or more metastatic sites. Despite the lack of information regarding the PS of our population, our multivariate model was in line with these results, while revealing for the first time that an elevated number of BM (five or more skeletal lesions) significantly harms the survival. Additionally, the mutational status of melanoma patients bearing BM did not apparently influence OS, although a positive trend was seen in BRAF-mutated patients treated with immunotherapy (14.2 months) as compared to those treated with targeted therapy alone (8.8 months). These results, however, are conditioned by an a priori selection bias since the clinician therapeutic decisions were driven by individual prognostic factors; therefore, they should not lead to considering a superiority of immunotherapy in this setting.
SREs are major complications of BMs that restrain the quality of life in cancer patients. The 2-year cumulative incidence of SREs in breast, prostate, and lung tumors ranges from 41 to 54%, and more than one half of patients develop a SRE at cancer diagnosis or thereafter. In the majority of these tumors, SREs dramatically impact on cancer-specific survival (27, 28). Similarly to previous studies (29–31), we observed that at least half of patients with BMs developed a SRE, whose nRT predominantly occurred in 37% of the studied population, while 12% of these patients experienced more than a single SRE. Moreover, in the majority of patients (85%), SREs followed the diagnosis of BMs, with about 50% of patients experiencing one SRE within 1 year from diagnosis.
No melanoma-associated factors were predictive for SRE development in our cohort, although we observed that those receiving primary treatment with BTAs showed a 62% reduced risk of experiencing SREs and delayed time to their occurrence. This apparent protective effect of BTAs in reducing the risk of SREs sounds very impressive if compared to other osteotropic tumors whose relative risk reduction ranges from 15 to 30% (32, 33). However, it is almost difficult to speculate on the possible more potent effect of BTAs in the treatment of melanoma BMs due to the retrospective nature of our study. Finally, a possible effect of BTAs on OS was not demonstrated in our melanoma population. Other studies investigated the efficacy of combining immunotherapy with anti-RANK-L monoclonal antibodies in this setting of patients, providing encouraging though weak results that undoubtedly require further investigation (11). Based on our data, the use of BTAs should be thus suggested at the time of BM detection since it reduces the incidence and delays the development of SREs.
In a different fashion from other tumors, SREs had no impact on the survival of our melanoma patients bearing BMs, including the nRT that was the most recurrent complication. The modest effect of RT on prognosis was previously demonstrated since the majority of patients showed lower survival and required further treatments to stabilize the skeletal complications (34). However, relevant data from our study concerns the protective effect in terms of survival for patients receiving RT and immunotherapy, independently of targeted agents. Therefore, it is conceivable that the OS benefit observed in these patients could have balanced the worse prognosis of those combining RT and targeted therapy without ICIs.
Benefits derived from the combination of RT and immunotherapy have been widely described as abscopal effect in melanoma (35) as well as in other malignancies (36) and reflects the regression of non-irradiated metastatic lesions at a distance from the primary site of irradiation. The putative synergic effect of RT and immunotherapy gained by our population is also suggested by the similar effect of immunotherapy vs. targeted therapy in the absence of RT, although both a defect of enrollment and radiological imaging as well as the retrospective nature of the analysis are probably limiting factors in our study. However, this combined effect also results from restoration of the immune cell activity in their systemic anticancer effect. In addition, it has been demonstrated that T cells may protect against bone loss (37), while IFN-γ counterbalances the osteoclastogenesis by interfering with RANK signaling (38). Finally, osteoblasts are also influenced by local T-helper 2 cells through parathormone (PTH) production (39), and a role of plasmacytoid dendritic cells has also been described (40).
Conclusion
In conclusion, we identified the number of BM as a novel prognostic factor in metastatic melanoma and observed that both reduced risk and delay in SRE development occur in patients early treated with BTAs. Despite the SREs not impacting on the survival of melanoma patients with BM, those receiving immunotherapy and requiring palliative RT obtained a major extent of benefit in terms of OS. Further prospective studies are thus needed to understand the effective role of immunotherapy in melanoma patients with BD. However, our preliminary observation suggests that palliative RT on symptomatic BMs may potentially reinforce the immune response and T cell activity in patients treated with ICIs, while the complementary activity of BTAs requires further investigation.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MT, MM, VS, MP, AM, LP, FMo, LD, MG, AI, PQ, VF, RM, MCT, ER, ON, MO, AC, SQ, GP, JP, PA, MV, SS, PF, RB, GR, PC, and GS contributed to the provision of study materials or patients. AT did the data collection. FMa contributed to the statistical analysis. FMa and MT did the data interpretation and manuscript writing. All authors are accountable for all aspects of the work, gave final approval of the manuscript, and contributed to revising the manuscript critically.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was funded by the “Precision Medicine” project.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01652/full#supplementary-material
(A) Percentage of patients experiencing different skeletal related events (SREs) in the study population. (B) Incidence of SREs according to the use of bone-targeted agents (BTAs). nRT, need for radiotherapy; ∗∗p < 0.01.
Kaplan–Meier overall survival estimate in the study population (n = 290). mOS, median overall survival.
Overall survival by number of bone metastases (BM). mOS, median overall survival; LDH, lactate dehydrogenase.
Overall survival according to the use of bone-targeted agents (BTAs) mOS, median overall survival.
References
- 1.Schadendorf D, Long GV, Stroiakovski D, Karaszewska B, Hauschild A, Levchenko E, et al. Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur J Cancer. (2017) 82:45–55. 10.1016/j.ejca.2017.05.033 [DOI] [PubMed] [Google Scholar]
- 2.Petrelli F, Ardito R, Merelli B, Lonati V, Cabiddu M, Seghezzi S, et al. Prognostic and predictive role of elevated lactate dehydrogenase in patients with melanoma treated with immunotherapy and BRAF inhibitors: a systematic review and meta-analysis. Melanoma Res. (2018) 29:1–12. 10.1097/cmr.0000000000000520 [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. (2015) 161:1681–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selby HM, Sherman RS, Pack GT. A roentgen study of bone metastases from melanoma. Radiology. (1956) 67:224–8. 10.1148/67.2.224 [DOI] [PubMed] [Google Scholar]
- 5.Tas F. Metastatic behavior in melanoma: timing, pattern, survival, and influencing factors. J Oncol. (2012) 2012:647684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zekri J, Marples M, Taylor D, Kandukurti K, McParland L, Brown JE. Complications of bone metastases from malignant melanoma. J Bone Oncol. (2017) 8:13–7. 10.1016/j.jbo.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smyth MJ, Yagita H, McArthur GA. Combination anti-CTLA-4 and anti-RANKL in metastatic melanoma. J Clin Oncol. (2014) 34:e104–6. 10.1200/jco.2013.51.3572 [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Rahman O. Clinical correlates and prognostic value of different metastatic sites in patients with malignant melanoma of the skin: a SEER database analysis. J Dermatolog Treat. (2017) 29:176–81. 10.1080/09546634.2017.1360987 [DOI] [PubMed] [Google Scholar]
- 9.Chu GC-Y, Chung LWK. RANK-mediated signaling network and cancer metastasis. Cancer Metastasis Rev. (2014) 33:497–509. 10.1007/s10555-013-9488-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Oronzo S, Coleman R, Brown JE, Silvestris F. Metastatic bone disease: pathogenesis and therapeutic options: up-date on bone metastasis management. J Bone Oncol. (2018) 15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angela Y, Haferkamp S, Weishaupt C, Ugurel S, Becker JC, Oberndörfer F, et al. Combination of denosumab and immune checkpoint inhibition: experience in 29 patients with metastatic melanoma and bone metastases. Cancer Immunol Immunother. (2019) 68:1187–94. 10.1007/s00262-019-02353-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahern E, Harjunpää H, Barkauskas D, Allen S, Takeda K, Yagita H, et al. Co-administration of RANKL and CTLA4 antibodies enhances lymphocyte-mediated antitumor immunity in mice. Clin Cancer Res. (2017) 23:5789–801. 10.1158/1078-0432.ccr-17-0606 [DOI] [PubMed] [Google Scholar]
- 13.Ahern E, Smyth MJ, Dougall WC, Teng MWL. Roles of the RANKL-RANK axis in antitumour immunity–implications for therapy. Nat Rev Clin Oncol. (2018) 15:676–93. 10.1038/s41571-018-0095-y [DOI] [PubMed] [Google Scholar]
- 14.Sullivan RJ, Hamid O, Gonzalez R, Infante JR, Patel MR, Hodi FS, et al. Atezolizumab plus cobimetinib and vemurafenib in BRAF-mutated melanoma patients. Nat Med. (2019) 25:929–35. 10.1038/s41591-019-0474-7 [DOI] [PubMed] [Google Scholar]
- 15.Ascierto PA, Ferrucci PF, Fisher R, Del Vecchio M, Atkinson V, Schmidt H, et al. Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat Med. (2019) 25:941–6. [DOI] [PubMed] [Google Scholar]
- 16.Long GV, Lebbe C, Atkinson V, Mandalà M, Nathan PD, Aranceet A, et al. The anti–PD-1 antibody spartalizumab (S) in combination with dabrafenib (D) and trametinib (T) in previously untreated patients (pts) with advanced BRAF V600–mutant melanoma: updated efficacy and safety from parts 1 and 2 of COMBI-I. JCO. (2020) 38:57 10.1200/jco.2020.38.5_suppl.57 [DOI] [Google Scholar]
- 17.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. (2012) 18:1386–94. 10.1158/1078-0432.ccr-11-2479 [DOI] [PubMed] [Google Scholar]
- 18.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. (2013) 19:1225–31. 10.1158/1078-0432.ccr-12-1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grassberger C, Ellsworth SG, Wilks MQ, Keane FK, Loeffler JS. Assessing the interactions between radiotherapy and antitumour immunity. Nat Rev Clin Oncol. (2019) 16:729–45. 10.1038/s41571-019-0238-9 [DOI] [PubMed] [Google Scholar]
- 20.Tang J, Yu JX, Hubbard-Lucey VM, Neftelinov ST, Hodge JP, Lin Y. Trial watch: the clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat Rev Drug Discov. (2018) 17:854–5. 10.1038/nrd.2018.210 [DOI] [PubMed] [Google Scholar]
- 21.Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J. ESMO guidelines working group. Bone health in cancer patients: ESMO clinical practice guidelines. Ann Oncol. (2014) 25(Suppl. 3):iii124–37. 10.1093/annonc/mdu103 [DOI] [PubMed] [Google Scholar]
- 22.Bäuerle T, Komljenovic D, Merz M, Berger MR, Goodman SL, Semmler W. Cilengitide inhibits progression of experimental breast cancer bone metastases as imaged noninvasively using VCT, MRI and DCE-MRI in a longitudinal in vivo study. Int J Cancer. (2010) 128:2453–62. 10.1002/ijc.25563 [DOI] [PubMed] [Google Scholar]
- 23.Tucci M, Stucci S, Silvestris F. Does cilengitide deserve another chance? Lancet Oncol. (2014) 15:e584–5. 10.1016/s1470-2045(14)70462-0 [DOI] [PubMed] [Google Scholar]
- 24.Zhang D, Wang Z, Shang D, Yu J, Yuan S. Incidence and prognosis of brain metastases in cutaneous melanoma patients: a population-based study. Melanoma Res. (2018) 29:77–84. 10.1097/cmr.0000000000000538 [DOI] [PubMed] [Google Scholar]
- 25.Tucci M, Mannavola F, Passarelli A, Stucci LS, Cives M., Silvestris F. Exosomes in melanoma: a role in tumor progression, metastasis and impaired immune system activity. Oncotarget. (2018) 9:20826–37. 10.18632/oncotarget.24846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mannavola F, Tucci M, Felici C, Passarelli A, D’Oronzo S, Silvestris F. Tumor-derived exosomes promote the in vitro osteotropism of melanoma cells by activating the SDF-1/CXCR4/CXCR7 axis. J Transl Med. (2019) 17:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oster G, Lamerato L, Glass AG, Richert-Boe KE, Lopez A, Chung K, et al. Natural history of skeletal-related events in patients with breast, lung, or prostate cancer and metastases to bone: a 15-year study in two large US health systems. Support Care Cancer. (2013) 21:3279–86. 10.1007/s00520-013-1887-3 [DOI] [PubMed] [Google Scholar]
- 28.Ulas A, Bilici A, Durnali A, Tokluoglu S, Akinci S, Silay K, et al. Risk factors for skeletal-related events (SREs) and factors affecting SRE-free survival for nonsmall cell lung cancer patients with bone metastases. Tumour Biol. (2015) 37:1131–40. 10.1007/s13277-015-3907-z [DOI] [PubMed] [Google Scholar]
- 29.Barnes M, Tiwana MS, Kiraly A, Hutchison M, Olson RA. Incidence of distal bone metastases in patients treated for palliative radiotherapy and associations with primary tumour types. J Bone Oncol. (2015) 4:107–9. 10.1016/j.jbo.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawai AT, Martinez D, Saltus CW, Vassilev ZP, Soriano-Gabarró M, Kaye JA. Incidence of skeletal-related events in patients with castration-resistant prostate cancer: an observational retrospective cohort study in the US. Prostate Cancer. (2019) 2019:5971615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka R, Yonemori K, Hirakawa A, Kinoshita F, Takahashi N, Hashimoto J, et al. Risk factors for developing skeletal-related events in breast cancer patients with bone metastases undergoing treatment with bone-modifying agents. Oncologist. (2016) 21:508–13. 10.1634/theoncologist.2015-0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong MHF, Stockler MR, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. (2012) 2:CD003474. [DOI] [PubMed] [Google Scholar]
- 33.James ND, Pirrie SJ, Pope AM, Barton D, Andronis L, Goranitiset I, et al. Clinical outcomes and survival following treatment of metastatic castrate-refractory prostate cancer with Docetaxel alone or with Strontium-89, zoledronic acid, or both: the trapeze randomized clinical trial. JAMA Oncol. (2016) 2:493–9. 10.1001/jamaoncol.2015.5570 [DOI] [PubMed] [Google Scholar]
- 34.Bostel T, Förster R, Schlampp I, Wolf R, Serras AF, Mayer A, et al. Stability, prognostic factors and survival of spinal bone metastases in malignant melanoma patients after palliative radiotherapy. Tumori. (2015) 102:156–61. 10.5301/tj.5000382 [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol. (2018) 11:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. (2018) 18:313–22. 10.1038/nrc.2018.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stucci S, Tucci M, Passarelli A, Silvestris F. Avβ3 integrin: pathogenetic role in osteotropic tumors. Crit Rev Oncol Hematol. (2015) 96:183–93. 10.1016/j.critrevonc.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 38.D’Oronzo S, Stucci S, Tucci M, Silvestris F. Cancer treatment-induced bone loss (CTIBL): pathogenesis and clinical implications. J Control Release. (2015) 41:798–808. 10.1016/j.ctrv.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 39.Cafforio P, Savonarola A, Stucci S, De Matteo M, Tucci M, Brunetti AE, et al. PTHrP produced by myeloma plasma cells regulates their survival and pro-osteoclast activity for bone disease progression. J Bone Miner Res. (2013) 29:55–66. 10.1002/jbmr.2022 [DOI] [PubMed] [Google Scholar]
- 40.Tucci M, Passarelli A, Mannavola F, Felici C, Stucci LS, Cives M, et al. Immune system evasion as hallmark of melanoma progression: the role of dendritic cells. Front Oncol. (2019) 5:1148. 10.3389/fonc.2019.01148 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Percentage of patients experiencing different skeletal related events (SREs) in the study population. (B) Incidence of SREs according to the use of bone-targeted agents (BTAs). nRT, need for radiotherapy; ∗∗p < 0.01.
Kaplan–Meier overall survival estimate in the study population (n = 290). mOS, median overall survival.
Overall survival by number of bone metastases (BM). mOS, median overall survival; LDH, lactate dehydrogenase.
Overall survival according to the use of bone-targeted agents (BTAs) mOS, median overall survival.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.