Abstract
We present 4 cases of dorsal root ganglion stimulation lead fracture. In these cases, the surgical technique involved (1) traversing fascial layers for placement of leads via a Tuohy needle in the upper low back, (2) subcutaneous tunneling from the implantable pulse generator site to the lead puncture site without dissecting below the superficial fascial plane at the puncture site, and (3) connection of the lead/extension with the generator. All fractures occurred adjacent to the original lead puncture site. These cases suggest lead entrapment within the membranous fascial plane, with tension on a thin lead, is a mechanism underlying lead fracture.
Dorsal root ganglion stimulation (DRG-S) is a type of neuromodulation which has been shown to be effective in the treatment of complex regional pain syndrome and is showing promise in the treatment of low back pain as well as other pain syndromes.1–3 DRG-S system placement involves placing a lead over the DRG. The DRG-S lead is smaller in size compared to traditional spinal cord stimulation (SCS) leads; relative to SCS leads, DRG-S leads are 30% thinner (1.2 vs 0.9 mm in diameter). DRG-S has been found to be a safe and efficacious treatment; however, as with SCS, lead migration and lead fracture have been highlighted as potential complications.4,5 Lead fracture typically presents with loss of stimulation and readings of high impedance or “malfunction of lead” on the DRG-S system programmer. Lead fracture or disconnection is confirmed through imaging of the lead or checking lead impedances.5,6 We present 4 cases of lead fracture after DRG-S placement (Proclaim, Abbott, Chicago, IL). In these cases, the surgical technique involved (1) traversing the superficial and deep fascial layers for epidural placement of the DRG-S lead via a Tuohy needle in the upper low back, (2) subcutaneous burial of the implantable pulse generator (IPG) in the gluteal region, (3) subcutaneous tunneling from the IPG site to the lead puncture site without dissecting below the superficial fascial plane at the puncture site, and finally, (4) connection of the lead/extension with the IPG, as described in the literature.7,8
In these cases, the fractures were visible on fluoroscopy and found to be superficial, adjacent to the original lead puncture site at the skin. We describe a putative mechanism causing the fractures, as well as alterations to the surgical procedure, to mitigate similar events in the future. All patients described in this report provided Health Insurance Portability and Accountability Act (HIPAA) authorization and written informed consent for the publication of their case. The following descriptions describe the course of the cases at long-term follow-up after implantation. Each patient initially underwent a DRG-S trial and experienced >50% relief on the visual analog pain scale (VAS) with concomitant improvements in function before permanent implantation.
CASE DESCRIPTION NUMBER 1
The first case is a 46-year-old man with a history of L5-S1 laminectomy and discectomy with persistent low back and right leg pain who had DRG-S leads implanted at the bilateral T12 and right S1 levels.7 His VAS pain score improved from a baseline of 90/100 to 35/100 mm, and his Oswestry Disability Index (ODI) from 78 (crippled) to 28 (moderate disability). He continued to do well until 14 months postimplantation when his low back pain worsened. On interrogation of the DRG-S system with the programmer, his right T12 DRG-S lead demonstrated high impedance (>7000 Ω). The patient denied any events, such as trauma or falls, that might have affected the lead. A fluoroscopic image was obtained and identified a lead fracture. The location of the fracture was consistent with the original lead puncture site at the skin (Figure 1). The patient has since had his lead surgically revised and experienced subsequent restoration of pain relief from DRG-S therapy.
Figure 1.
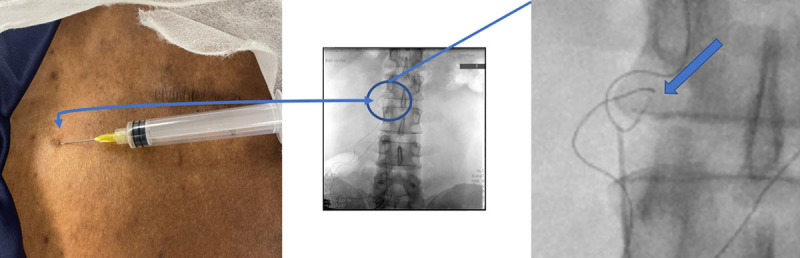
Lead fracture visible on fluoroscopy occurring at the region where skin puncture with the lead and the tunneled epidural catheter technique were performed. A 2-mm puncture site was used for the tunneling, without anchoring the lead. The needle tip location was consistent with the fracture site under fluoroscopy.
CASE DESCRIPTION NUMBER 2
The second case is a 35-year-old woman with chronic vaginal and pelvic pain secondary to endometriosis, interstitial cystitis, and pelvic floor dysfunction who had DRG-S leads implanted at the bilateral L1 and S2 levels. With DRG-S, her VAS pain improved from 90/100 to 10/100 mm, and her modified ODI changed from 71 (crippled) to 13 (minimal disability). She continued to do well until 13 months postimplantation when after a fall, she experienced a partial return of her pain. On interrogation of her DRG-S system, her left L1 lead demonstrated high impedance (>7000 Ω). A visible lead fracture was noted on her lumbar X-ray, which was consistent with the location of the superficial skin incision (Figure 2A). The patient subsequently underwent lead revision, with a restoration of pain coverage by the DRG-S therapy.
Figure 2.
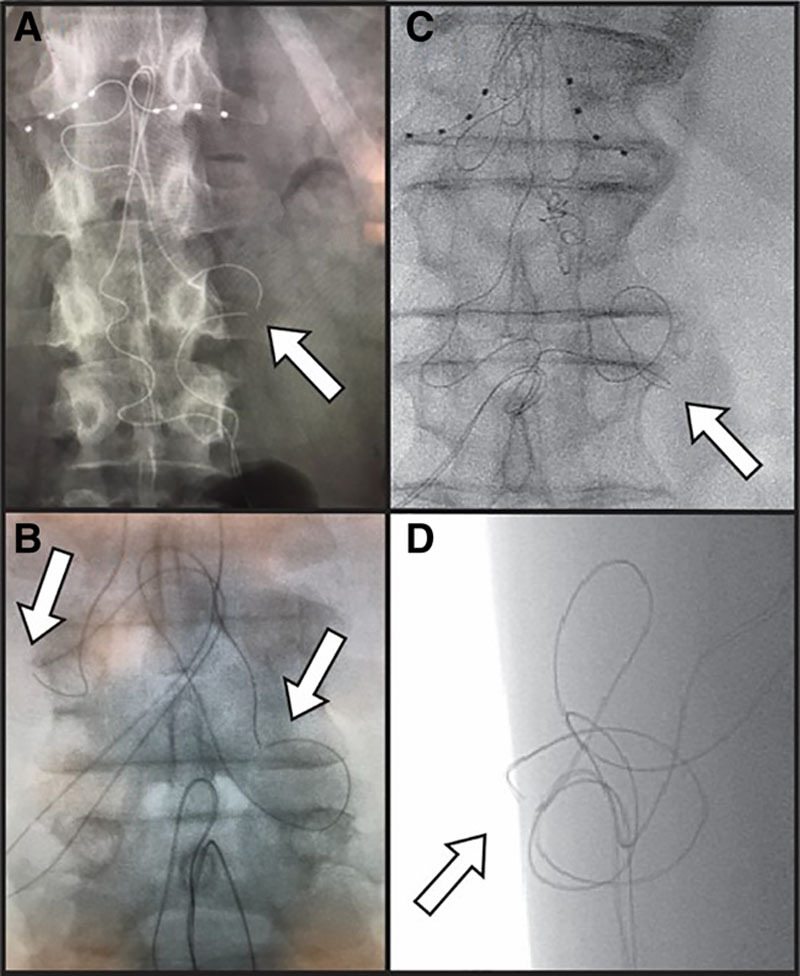
Visualized lead fractures demonstrated on fluoroscopy. A, A visible lead fracture of a right L1 lead. B, Visible lead fractures at the bilateral L1 DRG-S leads. C and D, Anteroposterior and lateral fluoroscopic views of lead fracture occurring in a superficial plane at the Tuohy needle entry point. The separation of the internal electrical components was visualized in all leads and all leads had intact outer lead sheaths at the time of revision. DRG-S indicates dorsal root ganglion stimulation.
CASE DESCRIPTION NUMBER 3
The third case is a 66-year-old woman with chronic pelvic pain who failed multiple interventional procedures and had a previous high-frequency SCS system that lost efficacy. She had DRG-S leads implanted at the bilateral L1 and S2 levels. After implantation, her pain improved from 90/100 to 30/100 mm on VAS, and her modified ODI improved from 55 to 18. She returned 18 months after implantation with worsening bilateral groin and labial pain. Interrogation of the DRG-S system revealed her bilateral L1 leads had an impedance of >7000 Ω, and a fluoroscopic image identified bilateral L1 lead fractures close to where the lead originally had punctured the skin (Figure 2B). She underwent surgical revision and had a restoration of the effectiveness of DRG-S therapy.
CASE DESCRIPTION NUMBER 4
The fourth case is a 78-year-old man with chronic low back pain and left-sided radicular pain who failed multiple interventional procedures and was deemed a poor surgical candidate by his surgeon. He had DRG-S leads implanted at the bilateral T12 DRGs for his low back pain and a left S1 lead for his left leg pain. After implantation, his pain improved from 85/100 to 30/100 mm on VAS, and his ODI improved from 68 to 18. He returned 13 months after implantation with worsening left-sided low back pain. Interrogation of the DRG-S system revealed his left T12 lead had an impedance of >7000 Ω, and a fluoroscopic image identified a lead fracture close to where the lead likely originally had punctured the skin (Figure 2C, D). He underwent surgical revision and had a restoration of the effectiveness of DRG-S therapy.
DISCUSSION
We described 4 cases of visible DRG-S lead fracture, which occurred adjacent to the puncture site at the skin. After DRG-S lead placement, the lead exits the epidural space and sequentially passes through the spinal ligaments, the erector spinae muscles, the deep thoracolumbar fascia (TLF), the deep adipose tissue (DAT), the membranous plane, superficial adipose tissue (SAT), and the skin. The membranous plane was previously referred to as the superficial, Scarpa’s, Camper’s, or Colle’s fascia. The subcutaneous tissue has since been renamed to the DAT and the SAT, separated by a membranous plane. This membranous plane surrounds the body, whereas the second layer, the deep fascia, surrounds the musculoskeletal system and separates it into fascial compartments.9–12 Together, the TLF, DAT, membranous plane, and SAT create a sliding system between the skin and the muscles, allowing changes in external and internal stimuli and muscular activity to be disconnected from direct transmission between the muscles and the skin.10 Fascial planes can respond to sensory input by contracting; by relaxing; or by adding, reducing, or changing their composition through fascial remodeling.13 In response to physiologic stress or injury, fibroblasts secrete collagen and other proteins making the fascia’s composition thicker and less extensible. These changes potentiate the fascia’s tensile strength but may cause restriction of elements passing through the fascia. In the case of DRG-S leads passing through the fascia, such changes may potentially anchor the lead at this point.
In our 4 cases, lead fractures occurred superficially, well above the deep fascial layer. The tunneled epidural implant technique we used involves standard DRG-S lead placement with an “S” tension loop (Figure 3A), and then driving a Tuohy needle from the IPG pocket, traversing the DAT, through the membranous layer, and through the SAT to the paramedian skin puncture site (Figure 3B).7 Thus, in its final position, the lead passes from the pocket through the DAT, and as it nears the puncture site, it passes through the membranous plane, and then dips back down into the DAT and TLF to the epidural space, causing a small section of the lead to be trapped above the membranous plane in the SAP (Figure 3C). This relatively acute bend may cause a focal area of tension and entrapment of the lead, potentially leading to friction and localized tension within the thin lead. In the cases we described, there was a clear separation of the fractured leads, indicating that the underlying wiring had separated, whereas, on explant, the lead casings were still intact. A similar phenomenon can be seen in focal nerve entrapments syndromes, as nerves traverse muscle, intermuscular septa, and fascia planes. This multiplanar course can cause an “internal stretch lesion” due to stress and tension on the nerve.14 These types of injuries are often difficult to diagnose and can explain why traditional nerve release procedures may be inadequate in treating focal nerve injuries and require dissection of the fascia layers.15
Figure 3.
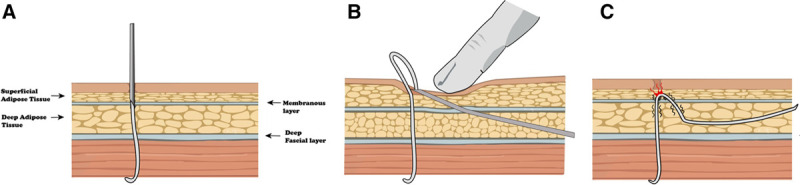
Previously used DRG-S lead placement approach. A, Planes of subcutaneous tissue with the Tuohy needle and lead placed. B, Tunneled epidural catheter technique for tunneling the lead to the pocket. Note the transection of the membranous layer. C, The lead is pulled between the membranous plane, potentially causing friction and focal areas of tension, ultimately damaging the lead.
The tunneled epidural catheter technique, with or without a superficial stab incision at the skin, creates less tissue trauma, requires less suturing, and may potentially decrease infection risk. However, because of the potential risk of lead fracture, we have modified our implantation technique to abandon the approach above. As was demonstrated in these 4 cases, we believe the transection of membranous, or superficial fascial, plane can potentially contribute to lead fracture. Our updated implant technique utilizes an incision and anchoring of leads deep to the membranous plane, at all thoracolumbar levels. When passing the tunneling device, it should be performed toward the IPG pocket, as passing from the pocket to a smaller, superficial incision may still result in the lead crossing through the membranous plane. See Figure 4 for an ideal lead position.
Figure 4.
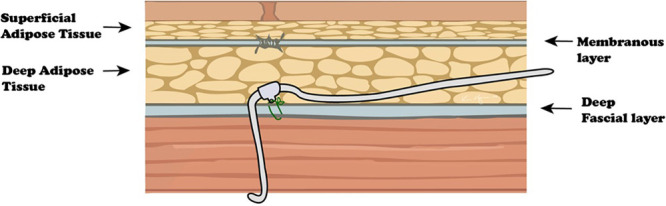
Passing the tunneling device deep to the membranous layer may avoid entrapment within the fascial plane.
Lead fracture occurring in DRG-S not only results in loss of pain relief, but also leads to revision, replacement, or explantation of the device. Revision of DRG-S leads can be challenging technically, given the possible presence of adhesions, which increases the risk of further complications.
LIMITATIONS
Our study design is limited by the fact that we are only describing 4 cases with limited long-term follow-up. Large prospective studies with long-term follow-up, in vivo dynamic biomechanics analyses, and real-world registry data could be helpful to elucidate the robustness of the proposed anchoring technique in mitigating future lead fracture risks. Our leads were all at the upper lumbar/lower thoracic region, and a larger pool of subjects could identify if the distance to the generator pocket plays a role. Additionally, DRG-S was used off-label in the present cases, since the only approved indication in the United States is complex regional pain syndrome. However, off-label use of DRG-S is common,16 and retrospective series have specifically described the use of DRG-S for low back pain1 and pelvic pain.17
CONCLUSIONS
We described 4 cases of DRG-S lead fracture that we believe to be partially related to the technical component of the implant, which resulted in short lead sections experiencing tension due to fascial plane contraction. These cases suggest entrapment within the membranous plane of a thin DRG-S lead may be one of the potential causes for lead fracture, and thus, modification of the DRG-S implant technique should be considered to avoid interactions of the lead and this plane. Ongoing longitudinal surveillance of complications with DRG-S systems may help corroborate our observations.
DISCLOSURES
Name: Kenneth B. Chapman, MD.
Contribution: This author helped prepare the manuscript draft with important intellectual input from Kiran V. Patel, Noud van Helmond, and George C. Chang Chien.
Conflicts of Interest: None.
Name: Kiran V. Patel, MD.
Contribution: This author helped edit the manuscript and approved the final version manuscript.
Conflicts of Interest: Kiran V. Patel is a consultant and educator for Abbott Neuromodulation.
Name: Noud van Helmond, MD.
Contribution: This author helped edit the manuscript and approved the final version manuscript.
Conflicts of Interest: None.
Name: George C. Chang Chien, DO.
Contribution: This author helped edit the manuscript and approved the final version manuscript.
Conflicts of Interest: None.
This manuscript was handled by: Mark C. Phillips, MD.
Footnotes
GLOSSARY
- DAT =
- deep adipose tissue
- DRG-S =
- dorsal root ganglion stimulation
- HIPAA =
- Health Insurance Portability and Accountability Act
- IPG =
- implantable pulse generator
- ODI =
- Oswestry Disability Index
- SAP =
- superior articular process
- SAT =
- superficial adipose tissue
- SCS =
- spinal cord stimulation
- TLF =
- thoracolumbar fascia
- VAS =
- visual analog pain scale
Funding: None.
Conflicts of Interest: See Disclosures at the end of the article.
REFERENCES
- 1.Chapman KB, Groenen PS, Patel KV, Vissers KC, van Helmond N. T12 dorsal root ganglion stimulation to treat chronic low back pain: a case series. Neuromodulation. 2020; 23:203–212 [DOI] [PubMed] [Google Scholar]
- 2.Kallewaard JW, Edelbroek C, Terheggen M, Raza A, Geurts JW. A prospective study of dorsal root ganglion stimulation for non-operated discogenic low back pain. Neuromodulation. 2020; 23:196–202 [DOI] [PubMed] [Google Scholar]
- 3.Morgalla MH, Fortunato M, Lepski G, Chander BS. Dorsal Root Ganglion Stimulation (DRGS) for the treatment of chronic neuropathic pain: a single-center study with long-term prospective results in 62 cases. Pain Physician. 2018; 21:E377–E387 [PubMed] [Google Scholar]
- 4.Sivanesan E, Bicket MC, Cohen SP. Retrospective analysis of complications associated with dorsal root ganglion stimulation for pain relief in the FDA MAUDE database. Reg Anesth Pain Med. 2019; 44:100–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg. 2004; 100:254–267 [DOI] [PubMed] [Google Scholar]
- 6.Kumar K, North R, Taylor R, et al. Spinal cord stimulation vs. conventional medical management: a prospective, randomized, controlled, multicenter study of patients with failed back surgery syndrome (PROCESS Study). Neuromodulation. 2005; 8:213–218 [DOI] [PubMed] [Google Scholar]
- 7.van Velsen V, van Helmond N, Levine ME, Chapman KB. Single-incision approach to implantation of the pulse generator and leads for dorsal root ganglion stimulation: a case report. A A Pract. 2018; 10:23–27 [DOI] [PubMed] [Google Scholar]
- 8.Verrills P. Krames E, Hunter PP, Rezai AR. Dorsal root ganglion stimulation for pain control. Neuromodulation: Comprehensive Textbook of Principles, Technologies, and Therapies. 2018, Amsterdam: Elsevier, 683–692 [Google Scholar]
- 9.Lancerotto L, Stecco C, Macchi V, Porzionato A, Stecco A, De Caro R. Layers of the abdominal wall: anatomical investigation of subcutaneous tissue and superficial fascia. Surg Radiol Anat. 2011; 33:835–842 [DOI] [PubMed] [Google Scholar]
- 10.Chopra J, Rani A, Rani A, Srivastava AK, Sharma PK. Re-evaluation of superficial fascia of anterior abdominal wall: a computed tomographic study. Surg Radiol Anat. 2011; 33:843–849 [DOI] [PubMed] [Google Scholar]
- 11.Wendell-Smith CP. Fascia: an illustrative problem in international terminology. Surg Radiol Anat. 1997; 19:273–277 [DOI] [PubMed] [Google Scholar]
- 12.Willard FH. Vleeming A, Mooney V, Stoeckart R. The muscular, ligamentous, and neural structure of the lumbosacrum and its relationship to low back pain. Movement, Stability & Lumbopelvic Pain. 2007, Amsterdam: Elsevier, 5–45 [Google Scholar]
- 13.Schleip R, Gabbiani G, Wilke J, et al. Fascia is able to actively contract and may thereby influence musculoskeletal dynamics: a histochemical and mechanographic investigation. Front Physiol. 2019; 10:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stecco A, Pirri C, Stecco C. Fascial entrapment neuropathy. Clin Anat. 2019; 32:883–890 [DOI] [PubMed] [Google Scholar]
- 15.Choi PJ, Nwaogbe C, Iwanaga J, Georgiev GP, Oskouian RJ, Tubbs RS. The Deep fascia of the forearm and the ulnar nerve: an anatomical study. Cureus. 2018; 10:e2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter CW, Sayed D, Lubenow T, et al. DRG FOCUS: a multicenter study evaluating dorsal root ganglion stimulation and predictors for trial success. Neuromodulation. 2019; 22:61–79 [DOI] [PubMed] [Google Scholar]
- 17.Hunter CW, Yang A. Dorsal root ganglion stimulation for chronic pelvic pain: a case series and technical report on a novel lead configuration. Neuromodulation. 2019; 22:87–95 [DOI] [PubMed] [Google Scholar]


