Abstract
Background and Aims
Despite recent progress in elucidating the molecular basis of secondary growth (cambial growth), the functional implications of this developmental process remain poorly understood. Targeted studies exploring how abiotic and biotic factors affect this process, as well as the relevance of secondary growth to fitness of annual dicotyledonous crop species under stress, are almost entirely absent from the literature. Specifically, the physiological role of secondary growth in roots has been completely neglected yet entails a unique array of implications for plant performance that are distinct from secondary growth in shoot tissue.
Scope
Since roots are directly responsible for soil resource capture, understanding of the fitness landscape of root phenotypes is important in both basic and applied plant biology. Interactions between root secondary growth, edaphic conditions and soil resource acquisition may have significant effects on plant fitness. Our intention here is not to provide a comprehensive review of a sparse and disparate literature, but rather to highlight knowledge gaps, propose hypotheses and identify opportunities for novel and agriculturally relevant research pertaining to secondary growth of roots. This viewpoint: (1) summarizes evidence from our own studies and other published work; (2) proposes hypotheses regarding the fitness landscape of secondary growth of roots in annual dicotyledonous species for abiotic and biotic stress; and (3) highlights the importance of directing research efforts to this topic within an agricultural context.
Conclusions
Secondary growth of the roots of annual dicots has functional significance with regards to soil resource acquisition and transport, interactions with soil organisms and carbon sequestration. Research on these topics would contribute significantly toward understanding the agronomic value of secondary growth of roots for crop improvement.
Keywords: Anatomy, cambial growth, carbon sequestration, drought, herbivory, nutrient stress, pathogens, roots, secondary growth, soil compaction, xylem
A FOCUS ON ROOTS FOR GLOBAL AGRICULTURE
Yield deficits caused by edaphic (i.e. relating to soil) stresses are primary causes of food insecurity in developing regions (Lynch, 2019). As the world’s population gains 2.3 billion people by the year 2050, primarily in food-insecure regions, it is estimated that food production will need to increase by 25–70 % to keep up with demand (Hunter et al., 2017). In areas where capital and infrastructure are lacking, the use of fertilizer and irrigation to mitigate yield losses is limited (World Bank, 2017). In contrast, in high-input agriculture, intensive fertilization and irrigation are unsustainable and cause massive environmental pollution (Woods et al., 2010). Global climate change is projected to intensify these issues by shifting precipitation patterns, augmenting evaporative demand and exacerbating soil erosion (Tebaldi and Lobell, 2008; St Clair and Lynch, 2010). Given that drought and nutrient deficiencies are difficult to sustainably mitigate, the development of crop cultivars with improved productivity under limited water and nutrient availability is one of the most pragmatic strategies in addressing these challenges (Lynch, 2007, 2019).
Since roots are directly responsible for acquisition and transport of water and nutrients, understanding of the fitness landscape (i.e. performance across an array of environments/selective pressures) of root phenotypes is key for the development of crop cultivars with improved soil resource capture. Significant genotypic variation for root anatomy has been reported in many crop species and has been shown to regulate the metabolic costs of soil exploration, axial and radial transport of soil resources, interactions with soil organisms and penetration of compacted soils (Lynch, 2018, 2019). Root anatomical phenes that are important for these processes include root cortical aerenchyma (Fan et al., 2003; Zhu et al., 2010a; Postma and Lynch, 2011a, b;Burton et al., 2013; Hu et al., 2014; Saengwilai et al., 2014; Chimungu et al., 2015; Galindo-Castañeda et al., 2018), cortical cell size (Chimungu et al., 2014a), cortical cell file number (Chimungu et al., 2014b), cortical senescence (Schneider et al., 2017a, b, 2018; Schneider and Lynch, 2018), metaxylem vessel size and number (Richards and Passioura, 1989), root hair length and density (Bates and Lynch, 2001; Yan et al., 2004; Zhu et al., 2005, 2010b; Vieira et al., 2007; Miguel et al., 2015), and secondary growth (Strock et al., 2018).
From a crop improvement perspective, breeding for root anatomical phenotypes related to yield under stress has advantages over brute-force yield selection since these phenes are under simpler genetic control than yield, and generally show less genotype × environment interaction (Lynch, 2019). While dramatic advances in genetic tools have occurred over the past few decades, breeding efforts are still constrained by limited knowledge of the fitness value of variation in root anatomy across diverse conditions (Passioura, 2002; Lynch, 2019). Secondary growth (cambial growth) of roots in dicotyledonous species is a prime example of an unexplored anatomical phene that probably has significant implications for soil resource acquisition and plant fitness under abiotic and biotic stress. Although secondary growth is not a novel research topic, the current knowledge base of this process is almost exclusively derived from studies of shoot tissue in arabidopsis and perennial tree species (Tomescue and Groover, 2019). While secondary growth of shoots has direct relevance to the timber, pulpwood and biofuel industries, secondary growth of roots may also have significant effects on the fitness and productivity of annual crop species within an agricultural context. Specifically, the implications of secondary growth for resource capture, axial transport, metabolic costs and interactions with soil organisms in roots of annual crops are important research foci that warrant investigation.
In this viewpoint, we explore what is presently known about secondary growth in roots within the context of agricultural production of annual dicot species. Our intention here is not to provide a comprehensive review of the limited literature concerning this process, but rather to highlight knowledge gaps, propose hypotheses and identify opportunities for novel and agriculturally relevant research pertaining to secondary growth of roots.
DEVELOPMENT OF SECONDARY GROWTH IN ROOTS
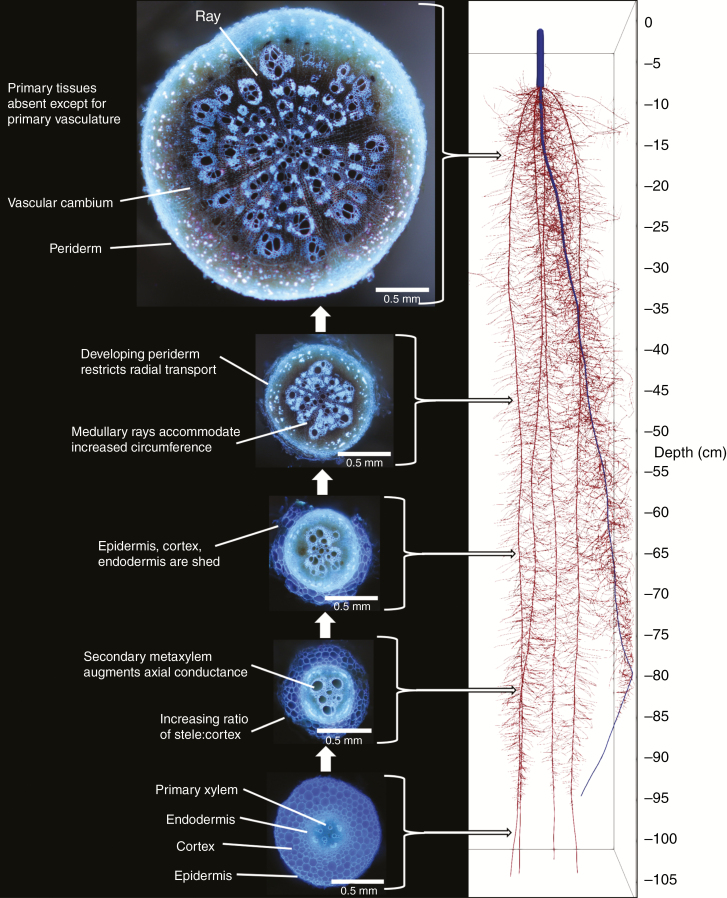
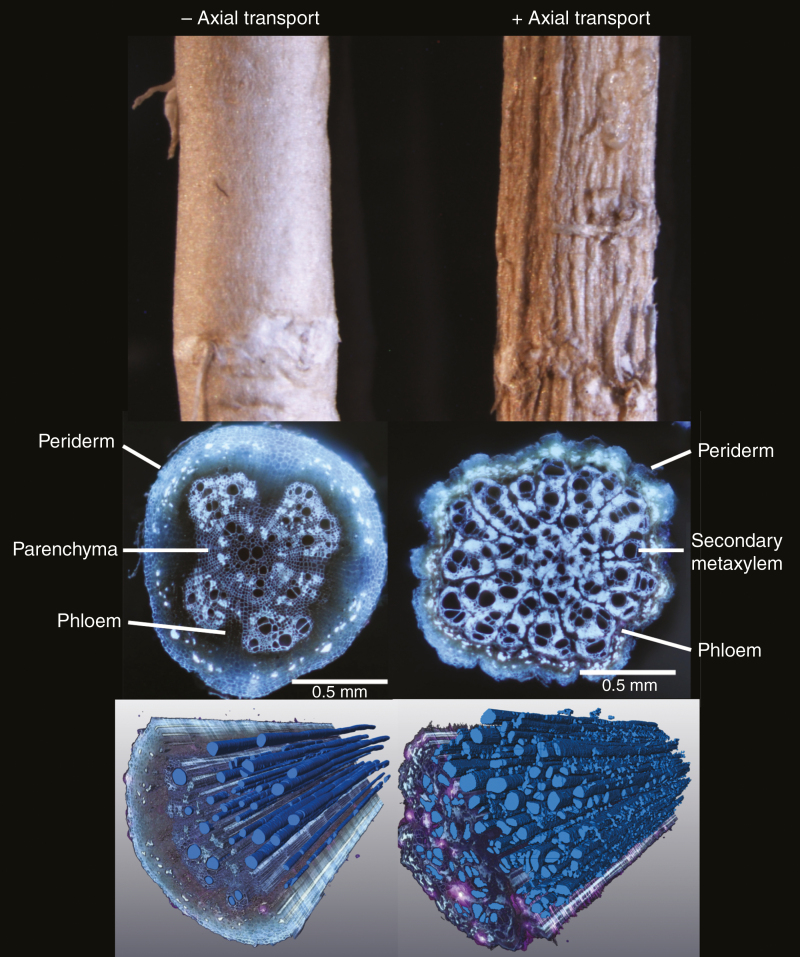
In dicotyledonous species, secondary growth is evident as the radial thickening of roots as they age (Fig. 1). On a finer scale, this increase in root diameter is defined by the rates and planes of cell division and differentiation that occur within two cylindrical meristems known as the vascular cambium and cork cambium (or phellogen) (Evert and Eichhorn, 2006). These cambia undergo periclinal cell divisions and differentiation of secondary tissue that ultimately cause the destruction of the epidermis, cortex and endodermis (Fig. 1) (Dickison, 2008). During this process, secondary metaxylem vessels are produced centripetally (inside) and phloem centrifugally (outside) to the vascular cambium, while a protective tissue called phellem is produced centrifugally to the cork cambium (Sanio, 1873; Larson, 1994; Evert and Eichhorn, 2006; Smetana et al., 2019). Overall, the bulk of root secondary thickening is driven by the production of secondary xylem elements and parenchyma centripetally to the vascular cambium (Dickison, 2008). To accommodate this increased thickness, the circumference of the vascular cambium expands through anticlinal cellular divisions oriented perpendicular to the surface of the root. As the vascular cambium increases in circumference, these anticlinal divisions add new radial cell files to produce vascular rays in the secondary tissue (Esau, 1965).
Fig. 1.
Root cross-sections highlighting the spatiotemporal progression of secondary growth along the length of axial roots. Secondary growth shifts the physiological role of the root from resource capture to axial transport of water and nutrients. Images of cross-sections from roots of common bean (Phaseolus vulgaris). All cross-sections are at the same scale. Scale bars = 0.5 mm.
Although radial thickening is most obvious in older root segments, this process is initiated during early development in procambial cells just prior to the end of elongation, where the last tracheary elements of the primary xylem mature (Eames and MacDaniels, 1947; Esau, 1965). In roots of arabidopsis, the cambium originates from procambial cells sandwiched between primary xylem and phloem, as well as from cells surrounding the primary xylem and phloem (pericycle) (Chaffey et al., 2002; Wunderling et al., 2017). Recently, it has been shown that secondary growth is specifically initiated around early protophloem sieve element cell files of the procambial tissue of the root (Miyashima et al., 2019), and cells with a xylem identity act as an organizer of secondary growth to direct adjacent vascular cambium cells to divide and function as stem cells (Smetana et al., 2019).
For dicotyledonous root and tuber crops such as cassava (Manihot esculenta), potato (Solanum tuberosum) (stem tuber) and sweet potato (Ipomoea batatas), root secondary growth and starch deposition are the chief components underlying harvestable agronomic yield (Duque and Villordon, 2019). Secondary development of these storage organs is initiated similarly to that in roots of other dicots, with secondary metaxylem elements and parenchyma being produced centripetally, and phloem centrifugally, to a vascular cambium. As secondary growth progresses, however, secondary cambia begin to develop around clusters (or strands) of xylem elements and parenchyma cells (McCormick, 1916). Cambial strips that are unassociated with vascular tissues can also develop within the secondary parenchyma and contribute to increases in girth of the tuber. Most of the cells differentiating from these secondary cambia at this later stage of tuber development are thin-walled, starch-filled, storage parenchyma (McCormick, 1916; Wilson and Lowe, 1973). Non-tuberous roots distributed throughout the rest of the root system continue to undergo typical woody secondary growth characterized by a heavily lignified stele, vascular rays, a limited amount of secondary phloem and a well-developed periderm (Wilson and Lowe, 1973). Although root and tuber crops make up a significant portion of carbohydrates consumed globally (FAO, 1998), relative to their importance as a food source, research attention devoted to the physiological response, as well as the genetic, hormonal and molecular controls of tuber formation under drought and nutrient deficiency, is limited (Villordon et al., 2014; Duque and Villordon, 2019).
While secondary growth is present in seed plants and Isoetes, most monocot species do not have the capacity for secondary growth (Tomlinson and Zimmermann, 1969; Gifford and Foster, 1989). Although some species have evolved a lateral meristem, most species of monocots lack a typical vascular cambium. It is believed that monocots lost the capacity for secondary growth when they lost their procambial cells (between xylem and phloem tissues) in the shift from primary vascular bundles to a closed anatomy (Ragni and Greb, 2018). In monocotyledonous tuber crops such as yams (Dioscorea sp.) as well as arborescent monocots (Asparagales) that exhibit secondary thickening, the novel monocot cambium functions by producing secondary vascular bundles embedded within the ground tissue (Tomlinson and Zimmerman, 1969; Carlquist, 2012a; Raman et al., 2014; Jura-Morawiec et al., 2015; Tomescu and Groover, 2019).
REGULATION OF ROOT SECONDARY GROWTH
Although secondary growth is regulated to determine the physical structure of a given species that has evolved for a specific environment, a significant level of plasticity in the meristematic activity of the vascular cambium exists to respond to variable growth conditions (Brewer et al., 2013). In most plants, activity of the vascular cambium is indeterminate, suggesting that the process of secondary growth is regulated by homeostatic mechanisms (Tomescue and Groover, 2019). Savidge (1993) provides a useful perspective of the geometry of secondary growth along the length of a stem or root where he describes it as an, ‘inverted cone, which volume expands exponentially with time’. Thamm et al. (2019) further expounds upon this by showing with quantitative data that growth of the cone fits a simple mathematical model of allometric exponential growth, implying co-ordination between the expansion in diameter of the cone with secondary growth and elongation of the cone with primary growth.
The mechanistic control of a complex developmental process such as secondary growth is challenging to disentangle, especially considering that the regulatory pathways are integrated with other developmental processes and the phytohormones that regulate secondary growth have numerous downstream targets and complex spatiotemporal distributions (Ragni and Greb, 2018). While some common components controlling secondary growth exist across dicots, these hormonal signals are also part of broader regulatory networks that are governed by a genetic framework specific to each species. Hormones involved in promoting cambial activity include auxin (Thimann and Skoog, 1933; Snow, 1935), strigolactones (Agusti et al., 2011), gibberellins (Bjorklund et al., 2007) and cytokinin (Ragni and Greb, 2018; Miyashima et al., 2019). Flow of auxin through the meristematic cells maintains cambial identity and the polarity of cambial cells (Dengler, 2001), and initiates cambial activity, as is shown in studies where auxin flow above a certain threshold is required for cambial reactivation following dormancy (Snow, 1935; Avery et al., 1937; Savidge and Wareing, 1981; De Groote and Larson, 1984; Smetana et al., 2019). Although auxin is one of the best characterized hormones for long-distance signalling and its role in linking primary and secondary growth was shown almost a century ago (Thimann and Skoog, 1933; Snow, 1935), auxin signalling at the level of cambial cells and how cambial activity integrates with the many other growth processes regulated by auxin is largely unknown (Smetana et al., 2019).
Gibberellins have a synergistic effect with auxin on cambial divisions (Wareing et al., 1964; Little and Savidge, 1987), and have been shown to stimulate polar auxin transport at the cambium (Bjorklund et al., 2007). Isolated application of gibberellins also directly stimulates cambial activity in angiosperm trees, herbaceous species and some conifers (Wareing et al., 1964; Little and Savidge, 1987). Gibberellins have a common transcriptome with auxin, sharing many transcripts that relate to cell growth (Bjorklund et al., 2007).
The function of strigolactones in positively regulating cambial activity is conserved among species and it has been shown that they interact strongly with the auxin signalling pathway, but alone are also sufficient for cambium stimulation (Agusti et al., 2011). Given that strigolactones also promote rhizoid elongation in moss, liverworts and stoneworts (Delaux et al., 2012), it is hypothesized that the primary role of strigolactones may be to modify plant architecture for optimization of nutrient uptake as plants transitioned to life on land (Brewer et al., 2013). Further evidence for this is found in the 100 000-fold increase in strigolactone levels under phosphate stress (Yoneyama et al., 2012), as well as the positive effect that strigolactone exudates have on hyphal branching of arbuscular mycorrhizae (Akiyama et al., 2005).
Cytokinin signalling has been shown to promote the expression of mobile transcription factors known as PEAR proteins. PEAR proteins activate genes that promote secondary growth, and their expression is concentrated at protophloem sieve elements. Additionally, PEAR proteins promote the transcription of HD-ZIP III proteins which inhibit PEAR proteins in a negative feedback loop. This negative feedback between PEAR and HD-ZIP III forms a distinct boundary of the zone of cell division that comprises the cambium (Miyashima et al., 2019).
We are just beginning to untangle the genetic and hormonal networks that control secondary growth, and more research attention in elucidating the hormonal and transcription factors that modify secondary growth under different environmental conditions in agriculturally relevant species is warranted.
SECONDARY GROWTH OF ROOTS VERSUS SHOOTS
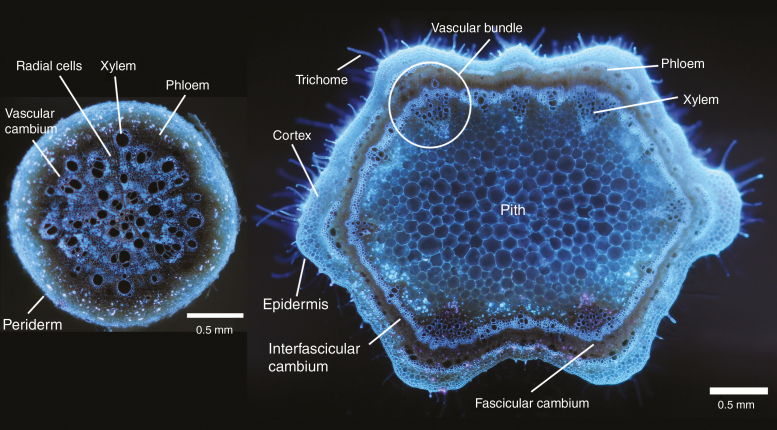
While extrapolation of observations of secondary growth in the shoot to the root is tempting, coalescing perspectives of secondary growth across these organs is problematic. Roots and shoots have been subject to different selective pressures throughout the evolution of terrestrial plants (Bastos et al., 2016). Functionally, roots must penetrate the soil matrix to compete for and acquire resources with heterogeneous spatiotemporal availabilities in an environment that is rife with plant pathogens and herbivores. Additionally, roots form symbiotic partnerships with soil organisms such as rhizobia and mycorrhizae as well as secreting exudates that modify the soil environment surrounding this organ. Although above-ground tissue is similarly subject to pathogens and herbivores, the primary function of stem tissue is not for resource acquisition, but rather in axial transport of resources and structural support of leaves and reproductive organs. Consequently, the anatomical arrangement of primary and secondary tissues is distinct in both shoots and roots (Fig. 2). Shoot tissue of annual dicots is characterized by having a proportionally thin cortex with xylem and phloem organized into vascular bundles encircling a central pith composed of parenchyma. As the stem develops, an interfascicular cambium (occurring between the vascular bundles) is established connecting these bundles and creating secondary stem anatomy (Altamura et al., 2001; Agusti et al., 2011). Contrastingly, roots have proportionally more cortex in their primary anatomy than shoots and, in the place of the pith, xylem vessels are centrally arranged and surrounded by phloem tissue in a bundle of vasculature referred to as the stele (Fig. 2). Vascular bundles are absent in the roots and, as secondary growth progresses, the vascular cambium produces new xylem and phloem continuously throughout the entire circumference of the vascular cambium. While current evidence suggests that the regulatory mechanisms of secondary growth are common to both root and shoot tissue, anatomical and developmental differences between shoots and roots obscure the translation of observations on the functional implications of secondary growth between these two organs and highlight the need for research specific to roots.
Fig. 2.
Comparison of root and shoot cross-sections highlighting differences in the anatomical arrangement of primary and secondary tissues of annual dicots. Shoot tissue is characterized by having a thin cortex, vascular bundles encircling a central pith with a cambium alternating between fascicular and interfascicular regions. Contrastingly, roots have their vasculature arranged into a central stele with a vascular cambium that produces new xylem and phloem continuously throughout the entire circumference. Images of cross-sections are from root and stem tissue of common bean (Phaseolus vulgaris). All cross-sections are at the same scale. Scale bars = 0.5 mm.
MEASURING ROOT SECONDARY GROWTH
Although studies directly focused on the functional effects of secondary growth in roots are lacking, numerous reports on root mass density and specific root length have indirectly alluded to shifts in secondary growth of root systems under stress. For example, the relationship between specific root length and nutrient acquisition under low soil fertility has been widely reported in many taxa including soybean (Glycine max L.) (Zhou et al., 2016), common bean (Phaseolus vulgaris) (Strock et al., 2018), tomato (Solanum lycopersicum) (Basirat et al., 2011), rapeseed (Brassica napus) (Lyu et al., 2016), temperate pastures (Hill et al., 2006) and perennial tree species (Ostonen et al., 2007; Laliberté et al., 2015; Kramer-Walter et al., 2016). Certainly, metrics such as specific root length provide a useful characterization of plant response to soil resource availability and whole-plant economics (Reich et al., 1998; Wright and Westoby, 1999; Comas and Eissenstat, 2004; Ostonen et al., 2007) but, while measures of specific root length and root mass density may be suggestive of the extent of secondary growth, drawing conclusions about secondary growth solely from these aggregate metrics is imprudent. The underlying components of specific root length (root diameter and root tissue density) have been shown to vary independently of one another, and in many cases there may be no relationship between these subsidiary parameters (Ostonen et al., 2007; Kramer-Walter et al., 2016). In other words, ratios of root length and root mass taken across the root system fail to resolve if changes in root diameter are due to modification of secondary growth or shifts in the proportion of root classes having distinct properties, including radial diameter, specific root length and secondary growth. Consequently, for studies directly addressing questions on the topic of root secondary growth, it is critical that measures of root length and mass be differentiated by root class and age.
Since many studies focus on elucidating the genetic, hormonal and molecular controls of secondary growth, experimental results often consist of qualitative assessment of mutant phenotypes determined from histochemical staining and labelling (Miyashima et al., 2019; Smetana et al., 2019; Zhang et al., 2019). However, for studies focused on the detection of environmental or genetic effects on cambial growth in roots, quantitative comparison of secondary development is essential. Although destructive, direct quantification of anatomical features is necessary. While root diameter is symptomatic of secondary growth and is rapidly quantifiable, the cross-sectional anatomy of the root provides the best opportunity to precisely define variation in this developmental process. The coarseness of root diameter as a measure of secondary growth is highlighted in the observation that roots with similar diameters may have very different root tissue densities, probably stemming from differences in root secondary growth and the proportion of primary and secondary tissues (Ostonen et al., 2007; Kramer-Walter et al., 2016).
Appropriate anatomical measures for quantifying secondary growth in young roots include the ratio of primary to secondary tissue, i.e. the stele area internal to the vascular cambium compared with the cortical tissue external to the vascular cambium in a root cross-section. In older root cross-sections where the epidermis and cortex have been completely shed, quantification of the abundance and size of secondary metaxylem vessels in anatomical cross-sections can provide an estimate of secondary growth (Strock et al., 2018; Zhang et al., 2019). Allocation to the proportion of different secondary cell types such as secondary metaxylem, parenchyma and periderm may shift significantly with treatment, species, genotype, location in the root system and local edaphic conditions. These modifications of anatomy that occur with secondary growth are also indirectly quantifiable through in situ measures of axial conductance along a root segment using a hydraulic head (Strock, 2019,), neutron radiography with deuterated water (Zarebanadkouki et al., 2016; Ahmed et al., 2018) or a pressure probe (Meunier et al., 2018). Shifts in radial transport resulting from secondary development of roots can be indirectly observed by using a pitman chamber (Hu et al., 2014), pressure probe (Steudle and Boyer, 1985) or rapid neutron tomography with deuterated water (Zarebanadkouki et al., 2019).
METABOLIC AND CONSTRUCTION COSTS OF ROOT SECONDARY GROWTH
In general, secondary growth of roots benefits plants by augmenting axial water transport (Valenzuela-Estrada et al., 2008; Strock, 2019), providing mechanical support for the growing shoot, and increasing resistance to edaphic herbivores and pathogens (Eissenstat, 1992; Valenzuela-Estrada et al., 2008) (Fig. 3). While the constitutive nature of secondary growth in dicot roots is suggestive that this process affords some level of increased fitness in most settings, in some environments secondary development of roots may encumber overall plant growth. Radial expansion increases the metabolic and construction costs of a root segment. Given that roots are heterotrophic and can consume >50 % of daily photosynthate production, the allocation of resources to radial expansion of roots as opposed to competing resource demands may be counterproductive in some cases (Strock et al., 2018). Ma et al. (2018) suggest that the significant costs of the root system have pressured plants to evolve thinner roots since first emerging in land ecosystems. The consequence of secondary growth and increased metabolic costs per length of root can be especially pronounced under nutrient stress, in which plants allocate even more of their daytime net carbon assimilation to the root system than non-stressed plants (van Der Werf et al., 1988; Lambers et al., 1996; Nielsen et al., 1998, 2001). For example, Nielsen et al. (1998) found that under phosphorus deficit, maintenance respiration accounts for 90 % of total root respiration in P. vulgaris.
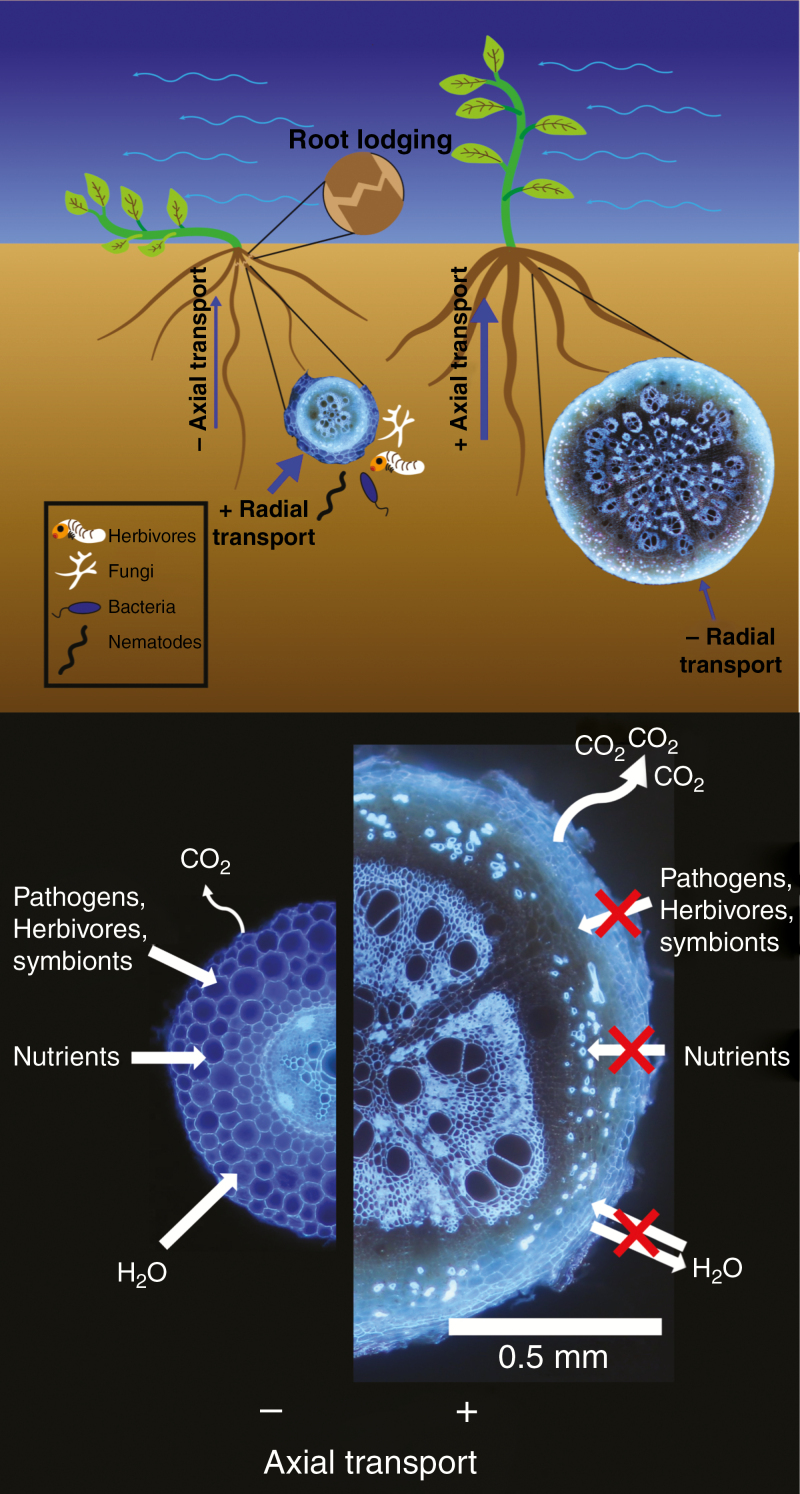
Fig. 3.
Diagram summarizing the relationships between secondary growth of roots and root lodging, soil resource acquisition, metabolic costs and interactions with soil organisms. Images of cross-sections from roots of common bean (Phaseolus vulgaris).
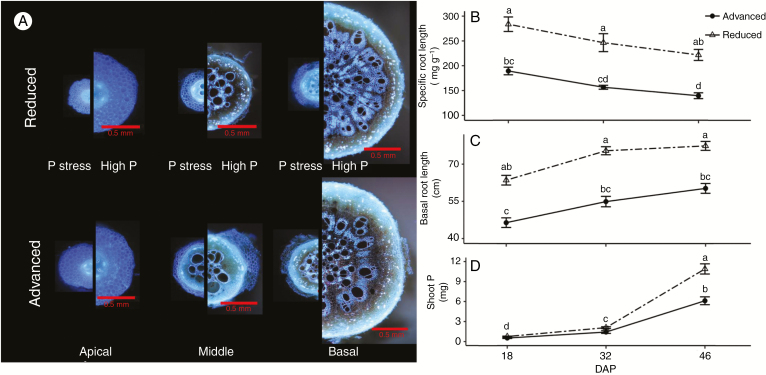
Suppression of root secondary growth reduces the metabolic (i.e. carbon, nutrient and energy) costs of producing and maintaining root length, and has been proposed to be an adaptive strategy to improve the metabolic efficiency of soil exploration (Lynch 1995, 2007; Lynch and Brown, 2008; De la Riva and Lynch, 2010; Strock et al., 2018). Allocation of resources to greater total root length and soil exploration rather than radial thickening of roots is especially important for exploration of soil domains where growth-limiting resources are localized. Even small changes in the density of root tissue can have significant effects on the soil volume explored per unit of carbon invested (Ma et al., 2018). Under phosphorus deficit, where diffusion of phosphate through the soil is outpaced by plant uptake, suppression of secondary growth is associated with greater root elongation, increased soil exploration and greater phosphorus acquisition (Fig. 4) (Strock et al., 2018).
Fig. 4.
Reduced root secondary growth improves primary root growth, soil exploration and phosphorus (P) capture. (A) Cross-sections of basal roots of two common bean (Phaseolus vulgaris) genotypes with contrasting root secondary growth (‘advanced’ and ‘reduced’ secondary growth) under P stress [12 ppm available P from Mehlich-3 (ICP)]. All cross-sections are at the same scale. (B–D) Specific root length, axial root length of basal roots and total shoot P, respectively, for common bean genotypes with contrasting levels of secondary growth (‘advanced’ and ‘reduced’) at 18, 32 and 46 days of growth under P stress [12 ppm available P from Mehlich-3 (ICP)]. Error bars represent ± s.e. of the mean. Letters denote differences at α ≤ 0.05. Comparisons are made across all time points. Modified from Strock et al. (2018) (www.plantphysiol.org; Copyright American Society of Plant Biologists).
In contrast to phosphorus, water is highly mobile in the soil and, under prolonged drought, shallow horizons are the first to dry in most agricultural soils, leading to greater water availability at depth (Lynch, 2013). Similarly, nitrogen in the form of nitrate is leached through the soil profile with irrigation or rainfall events, and is often localized in deeper soil horizons over time (Lynch and Wojciechowski, 2015; Thorup-Kristensen and Kirkegaard, 2016). We propose that suppression of secondary growth in roots may also be beneficial for acquisition of these limiting resources by affording greater resources to exploration of deep soil domains.
Reallocation of resources within a plant is a hallmark adaptive response to nutrient stress (Fohse et al., 1988), and further investigation into the effect of nutrient and water limitation on secondary growth of roots is warranted. While the influence of radial thickening of roots on metabolic and construction costs is obvious, because secondary growth affects multiple aspects of root function, we believe that the utility of suppressing this developmental process is likely to be limited to specific environments (Fig. 3). While thin roots with a low tissue density are more metabolically efficient in soil exploration, thin roots have less hydraulic conductance, root longevity and ability to penetrate strong soils (Bengough et al., 2006). Beyond roots, investigation into the modification of secondary growth in shoots is also worthwhile and may also have important implications on the plant adaptive response to resource limitation and the balance of internal resource demands.
RADIAL AND AXIAL TRANSPORT AND ROOT SECONDARY GROWTH
In root systems, secondary growth shifts the physiological role of a root segment from resource capture to axial transport of water and nutrients (Fig. 3) (McCully, 1999; Steudle, 2000; Strock et al., 2018). Specifically, as the epidermis, cortex and endodermis are destroyed, the heavily lignified and suberized secondary tissue restricts radial transport of water and nutrients (Fig. 3) (Guo et al., 2008; Rewald et al., 2011). In addition to hydrophobic effects of drying mucilage (Carminati and Vetterlein, 2013), the increase in these secondary cell wall compounds also serves to decrease the absorptive capacity of roots as they age (Bouma et al., 2001; Volder et al., 2005). This inhibition of radial transport is especially important under drought where hydraulically isolating older root segments from drying surface horizons prevents leakage and air seeding through interconduit pit membranes, thereby preserving the hydraulic integrity of the vasculature (Sperry and Saliendra, 1994; Hacke and Sperry, 2001; Zwieniecki et al., 2002; Cuneo et al., 2016).
While radial transport is restricted, axial transport is augmented during secondary growth through the production of phloem and secondary metaxylem elements, with the efficiency of axial conductance increasing with vessel diameter (Zimmerman, 1983; Tyree et al., 1994; Hacke et al., 2017; Strock et al., 2018). Just as with nutrient deficiency, plants can modify the secondary growth rate of their roots in response to water availability. In situations of water stress where transpirational demand is reduced, the activity of the vascular cambium may be suppressed and production of secondary metaxylem halted (Zimmermann and Brown, 1974). For example, several species of Prunus suppress secondary growth and secondary metaxylem size of roots in response to water stress (Ljubojevic et al., 2018). In contrast, species that are adapted to arid environments, such as the xeric tree Acacia tortilis, may be capable of maintaining cambial activity even under water deficit (Al-Mefarrej, 2014). Nevertheless, these observations of cambial activity under water stress are made in perennial species which have different constraints and opportunities with regards to drought adaptation than annual species. Ultimately, the effect of water availability on secondary growth in annual crops is likely to be dependent upon species adaptation to drought and integration of the plant vasculature with other components of plant water relations such as phenology, shoot architecture, leaf morphology and distribution of root length in the soil. Overall, we hypothesize that alteration of secondary growth and the production of secondary metaxylem vessels in roots may be integral to adaptive strategies of either drought avoidance or drought escape (Vadez et al., 2013, 2014).
Although increasing rates of water and nutrient transport are required for continued growth of the shoot, under terminal drought, reducing secondary growth in roots may help to meter water uptake and desiccation of root tips for sustained soil exploration and water capture later in the season (Richards and Passioura, 1989; Lynch et al., 2014; Vadez, 2014: Strock, 2019). This inhibition of secondary growth may integrate into a strategy of drought avoidance by ensuring suppressed, but consistent, conductance of water in dry environments (Vadez et al., 2013, 2014). Contrastingly, species that maintain or increase root secondary growth under drought may demonstrate a strategy of drought escape where increased axial conductance aids in maximizing water capture in rapidly drying soil, especially in species with a short phenology (Vadez et al., 2013, 2014). Alternatively, it may be that species that maintain cambial activity under drought have other physiological adaptations that enable greater secondary growth and increased axial transport of water.
While reduction of axial conductance may integrate with certain strategies of drought adaptation, roots with reduced secondary growth and few, small secondary xylem vessels require a larger water potential gradient between the soil and atmosphere to function, thereby limiting the utility of this response to situations of water deficit. As an example, the reduced conductance efficiency of narrow metaxylem vessels in desert trees is thought to be one of the major limitations to the distribution of these species in wetter environments (Pockman and Sperry, 2000). Consequently, wet, humid conditions with a low water potential gradient between soil and the atmosphere would favour plants with increased secondary growth (Tyree et al., 1994; Purushothaman et al., 2013). Considering these hydrophysical principles of axial conductance, we propose that by restraining the transport of water and nutrients, the advantage of repressing secondary growth of roots would probably be limited to dry environments, while roots with greater secondary growth would afford greater water and nutrient transport for growth in humid environments.
In addition to the effects of secondary xylem vessels on axial transport, we hypothesize that other components of secondary root tissue may also have significant effects on water transport and plant performance under drought (Fig. 5). The xylem parenchyma surrounding secondary xylem vessels may provide some capacity for water storage to help buffer declines in soil water potential, as well as play a role in nocturnal refilling of cavitated vessels (Nobel and Jordan, 1983; Stiller et al., 2005). Tracheids are a secondary tissue that may provide an alternative pathway for water transport in drier soils when larger vessel elements have cavitated (Carlquist, 2012b). Phloem is essential for conductance of photosynthates to support continued growth of the root system, but, presently, the literature does not address how alteration of phloem capacity may influence plant fitness under edaphic stress. We hypothesize that shifts in phloem capacity would have an influence on root:shoot allocation, an important adaptive response to both drought and low soil fertility. When integrated across an entire root system, small shifts in the arrangement and proportions of these secondary tissues may have significant effects on root growth as well as water transport and utilization under drought. Similarly, alteration in the arrangement of secondary tissues in the shoot is also likely to have important implications on plant–water relations and adaptive response to drought stress.
Fig. 5.
Example of intraspecific variation for secondary development of roots in common bean (Phaseolus vulgaris). Differences in allocation to the proportion of different secondary cell types such as secondary metaxylem, parenchyma and periderm results in roots contrasting in radial and axial conductance capacity. Modified from Strock (2019).
ROOT SECONDARY GROWTH AND BIOPHYSICAL INTERACTIONS
One of the obvious physiological advantages that secondary growth has afforded land plants is structural support of growing shoot tissue (Gerrienne et al., 2011; Hoffman and Tomescu, 2013). As the canopy and above-ground biomass develop, roots must correspondingly increase in thickness and tensile strength to resist lodging under strong winds. Root lodging has dramatic effects on harvest quality and yields, and, while root architecture is known to affect vulnerability to lodging, the influence of root anatomy has yet to be explored. Specifically, for dicot crop species, we hypothesize that roots with increased secondary growth would be likely to provide greater resilience from root lodging than thinner roots with a lower tensile strength (Fig. 3).
In addition to the physical strains that roots experience from forces applied to above-ground tissues, soil strength is another influential physical constraint to root growth (Bengough et al., 2006). While secondary growth may not be directly relevant to the penetration of root apices into the soil matrix, heterogeneous radial pressure from the soil may have significant impact on the orientation of periclinal divisions (Louveaux and Hamant, 2013; Sampathkumar et al., 2014). This would be a concern especially in vertisols and other soils having a high content of expansive clay minerals that undergo dramatic expansions and contractions with varying moisture content. Although not yet examined in roots, the effect of similar biomechanical stresses on secondary growth in shoots is evidenced in the production of tension wood. Increased cambial proliferation and altered chemical composition of cell walls in the metaxylem are characteristic symptoms of asymmetrical forces applied to stem tissue (Andersson-Gunneras et al., 2003; Love et al., 2009). We hypothesize that similar anatomical shifts may occur in roots under physical stress that could affect their function.
ROOT SECONDARY GROWTH AND INTERACTIONS WITH SOIL ORGANISMS
We propose that secondary growth in roots may have important consequences for resistance to biotic stress and root longevity, but few studies address this interaction. The paucity of literature on the interface between root anatomy and soil biota may be related to difficulties with sampling and assessing the extent of damage by these organisms in the field. Extrapolation of observations of biotic stressors on shoot tissues to roots is imprudent, as root herbivores and pathogens have their own distinctive ecology within the soil matrix (Johnson et al., 2016). Many of these sub-terranean organisms have substantial economic impacts on agricultural production, with yield and biomass deficits being just as extensive as those caused by above-ground pests (Brown and Gange, 1990). Damage by soil organisms is also exacerbated by drought and nutrient deficiency (Zvereva and Kozlov, 2012), so understanding the interactions between biotic stress, abiotic stress and secondary growth of roots has significant relevance for global agriculture.
Most studies on biotic stress focus on shifts in biomass allocation, gene expression and production of secondary metabolites, but we advocate for research attention to also be directed to the relationship between root anatomy and soil organisms (Strock et al., 2019). There are many sub-terranean species that specialize in colonizing and feeding on the cortex, phloem, metaxylem vessels or surface of the root (Brown and Gange, 1990). Galindo-Castañeda et al. (2019) showed that root cortical anatomy has significant impacts on the ability for both symbiotic and pathogenic fungi to colonize maize roots and, similarly, we hypothesize that secondary growth in dicot roots would probably affect colonization by these organisms. As the heavily lignified and suberized periderm is developed during secondary growth, not only is radial transport of water and nutrients restricted, but the periderm also plays a significant role in protecting the root from attack by edaphic herbivores and pathogens (Eissenstat, 1992; Guo et al., 2008; Valenzuela-Estrada et al., 2008; Rewald et al., 2011). Deposition of suberin in this secondary tissue has been shown to be a key component in inhibiting penetration of hyphae by soil pathogens such as Phytophthora (Lulai and Corsini, 1998; Valenzuela-Estrada et al., 2011; Machado et al., 2013), and in Malus domestica the intensity of pathogen colonization in roots was closely linked to the senescence and loss of the cortex (Emmet et al., 2014).
While the primary tissues of the seedling root system are especially vulnerable to damage by soil organisms at establishment (Fowler and Wilson, 1971), as the growing season progresses in temperate systems, many soil organisms become stratified with soil depth. Increased distribution of organisms into deeper soil strata may be a response to greater variability in moisture and temperature at the soil surface later in the season. However, we hypothesize that associations between roots and soil organisms are inhibited in older secondary root tissue, and root pathogens and symbionts maintain their relationships with advancing root apices as they extend deeper into the soil (Goldson and French, 1983).
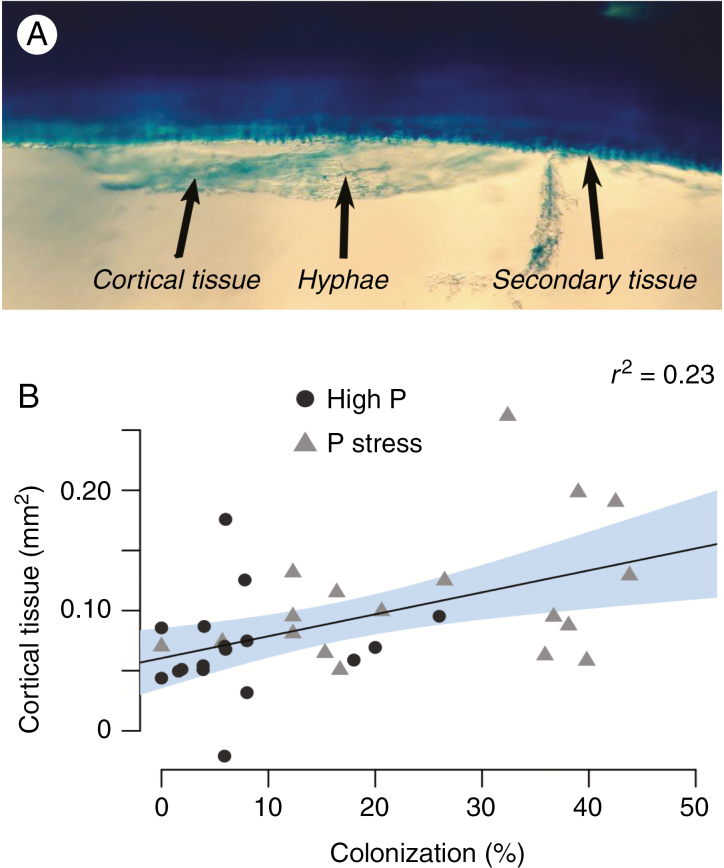
Although secondary growth may reduce root vulnerability to soil pathogens and herbivores, it also impairs associations with beneficial mycorrhizae. In Vitis ssp., root age significantly affects formation of arbuscular mycorrhizae, where younger roots have more arbuscules and older roots have more vesicles and/or spores (Vukicevich et al., 2019). Under phosphorus deficit, secondary growth of P. vulgaris roots is suppressed, thereby prolonging associations with arbuscular mycorrhizae (Strock et al., 2018) (Fig. 6). Valenzuela-Estrada et al. (2008) also observed in Vaccinium ssp. that roots with greater radial growth had less mycorrhizal colonization.
Fig. 6.
Reduced root secondary growth preserves cortical tissue and arbuscular mycorrhizal associations that colonize the cortex. (A) Cortical tissue containing fungal hyphae being shed during secondary growth in common bean (Phaseolus vulgaris), visualized with a stereomicroscope (Nikon, Tokyo, Japan) after clearing roots in 10 % KOH and staining with a 5 % ink–vinegar solution (Vierheilig et al., 1998). (B) The relationship between mean cortical tissue abundance in cross-sections of basal roots in common bean and the mean percentage colonization for roots grown under high phosphorus and phosphorus stress (n = 36). Mycorrhizal colonization was quantified using the magnified intersections method (McGonigle et al., 1990). Modified from Strock et al. (2018) (www.plantphysiol.org; Copyright American Society of Plant Biologists).
SECONDARY GROWTH AND ROOT EXUDATES
In addition to the physical inhibition of root colonization, we suggest that chemical signalling via root exudates may also be affected by the deposition of secondary tissues. The composition and quantity of root exudates have well-documented effects on the biotic activity of the rhizosphere such as altering microbial community structure and stimulating fungal spore germination, as well as nematode egg hatch (Khan et al., 1964; Sasse et al., 2018). Nevertheless, our understanding of exudate interactions with the rhizosphere is limited in both spatial and temporal dimensions (Micallef et al., 2009). While non-uniform exudation along the length of roots has been reported in wheat (Triticum sp.) (Semenov et al., 1999), maize (Zea mays) (McCully and Canny, 1985; Doan et al., 2017; Voothuluru et al., 2018) and wild oat (Avena barbata) (Jaeger et al., 1999), root exudation has not been examined along this dimension and at this scale in dicot root systems. It has been generally observed (in monocot species) that the majority of root exudation is localized at the root tip (Canarini et al., 2019), and we propose that in dicot species, deposition of secondary tissues would cause a similar decline in root exudation in older segments of root. Broadly, longitudinal studies have observed a decline in exudation of organic compounds over time in tomato (S. lycopersicum) (Rovira, 1959), clover (Trifolium subterraneum) (Rovira, 1959) and alfalfa (Medicago sativa) (Hamlen et al., 1972), and genotypic variation has been observed in arabidopsis (Micallef et al., 2009). However, the influence of secondary development on the process of root exudation has not been directly assessed. Additionally, we must point out that many studies on exudates only focus on the diffusible and water-soluble compounds, neglecting the contribution of epidermal, cortical and endodermal tissues shed from the root by secondary growth that probably have significant contributions to rhizosphere processes. For example, Griffin et al. (1976) found that axenic peanut roots (Arachis hypogaea) grown in nutrient solution shed 1.5 mg of tissue per gram of dry root tissue each week, which was believed to be an underestimate. Ultimately, we hypothesize that spatiotemporal shifts in exudation as roots undergo secondary growth may in turn alter the spatiotemporal distribution and structure of bacterial, fungal, nematode and insect communities in the rhizosphere. This merits research attention.
ROOT SECONDARY GROWTH UNDER HYPOXIC CONDITIONS
In addition to the aforementioned suggestions for research on nutrient stress, water stress, compacted soils and biotic factors, we propose that monitoring root secondary growth under hypoxic conditions and under Mn toxicity merits attention. Hypoxic soil conditions resulting from flooding may also affect root secondary growth, with the response likely to be dependent on species, root age, duration of hypoxia and other soil conditions (Kozlowski and Pallardy, 2002). We hypothesize that increased lignification and suberization of secondary tissues in dicots may aid in reducing O2 loss to hypoxic soils as deposition of lignin and suberin in exodermal tissue of monocot species has been shown to reduce radial O2 loss (Ejiri and Shiono, 2019). We advise that research focused on this topic should carefully consider the methodology for measuring secondary growth since root diameter may be affected by hypertrophy of the tissue (Kozlowski, 1984). In addition to the effects of O2 starvation under hypoxic conditions, manganes (Mn) is reduced in poorly drained soils, often increasing Mn bioavailability to the point of toxicity (Fernando and Lynch, 2015). Since suberized and lignified secondary tissues may prevent radial O2 loss to hypoxic soils, we suggest that they may also inhibit entry of reduced Mn from the soil (Ejiri and Shiono, 2019). Manganese toxicity may have important effects on meristematic activity of the vascular and cork cambiums in roots since it can inhibit primary growth of roots by suppressing auxin levels and reducing cell divisions at the apical meristem (Zhao et al., 2017). Manganese has an especially strong effect on cell divisions in young root segments as it is taken up via an active transport system in epidermal cells (Marschner, 1995; Pittman, 2005). Transport of Mn from the root apex to older root segments may similarly disrupt cell divisions in the vascular and cork cambia throughout the length of the root.
ROOT SECONDARY GROWTH AND CARBON SEQUESTRATION
In addition to studies exploring environmental effects and adaptive significance of secondary growth, we would like to highlight opportunities that exist in understanding how modification of this process in roots may be a valuable tool for the sequestration of atmospheric CO2. Carbon storage in soils has important ramifications for climate change since soils are the largest reservoir of carbon in terrestrial ecosystems, containing three times more carbon than the vegetation they support (Post et al., 1982). Secondary growth of trees is a major sink of atmospheric CO2 in terrestrial ecosystems (Thamm et al., 2019), and carbon from roots has a 2.4 times longer residence time in the soil than carbon derived from shoot tissue (Rasse et al., 2005). Accordingly, carbon sequestration into soil is primarily a function of plant allocation to root growth and the vertical distribution of those roots in the soil (Jobbagy and Jackson, 2000). The relevance of root secondary growth for CO2 sequestration is further highlighted in the meta-analysis by Poirier et al. (2018) where root suberin content was identified as one of the most influential promoters of soil organic matter stabilization. Greater research in understanding the potential that root secondary growth has for carbon storage below-ground will only become more critical as climate change progresses.
RESEARCH PROSPECTS
Research opportunities exist to understand the effects of various edaphic conditions on the rate of secondary growth, as this developmental process is likely to be responsive to environmental cues to adapt root anatomy to local conditions. Along with increasing our knowledge of how these abiotic and biotic factors affect this developmental process, we believe that understanding the adaptive significance and functional implications of modifying secondary growth of roots is of even greater importance. For example, how does suppressing or accelerating the rate of root secondary growth affect foraging for limiting soil resources, axial and radial transport of water, nutrients and photosynthates, and resistance or colonization by soil organisms?
Presently, the majority of published work on root secondary growth is fragmented across diverse research disciplines and often lies tangential to the foci of the present perspective. Because root secondary growth is directly associated with the physical interface between plants and the soil, future research on this topic will probably require collaborative efforts between those with expertise in physiology, genetics, pathology, entomology and soil science. In continuing work on secondary growth in roots, it is important to recognize that the fitness impacts of root secondary growth may shift with edaphic conditions and may have fitness trade-offs with other root and shoot phenes that limit its utility for plant productivity in certain environments. Ultimately, for fruitful manipulation of secondary growth in crop breeding programmes, we need to understand the contribution of this developmental process to water and nutrient capture under a diversity of conditions, and subsequently understand the fitness landscape in a broader context with other plant traits. The potentially important yet poorly understood interactions of root secondary growth with abiotic and biotic stress call for greater research attention to this topic, both for a better understanding of a fundamental process in plant development and for practical applications in plant breeding and agronomy.
FUNDING
This work was supported by the USAID Feed the Future Innovation Laboratory for Climate Resilient Beans and the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch project 4732
ACKNOWLEDGEMENTS
We thank Kathleen Brown, Meredith Hanlon and Hannah Schneider for their comments on the manuscript. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
LITERATURE CITED
- Agusti J, Herold S, Schwarz M, et al. . 2011. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proceedings of the National Academy of Sciences, USA 108: 20242–20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed MA, Zarebanadkouki M, Meunier F, Javaux M, Kaestner A, Carminati A. 2018. Root type matters: measurement of water uptake by seminal, crown, and lateral roots in maize. Journal of Experimental Botany 69: 1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama K, Matsuzaki K, Hayashi H. 2005. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827. [DOI] [PubMed] [Google Scholar]
- Al-Mefarrej HA. 2014. Cambial activity in Acacia tortilis subsp tortilis is highest during the hottest and driest month. IAWA Journal 35: 138–154. [Google Scholar]
- Altamura MM, Possenti M, Matteucci A, Baima S, Ruberti I, Morelli G. 2001. Development of the vascular system in the inflorescence stem of Arabidopsis. New Phytologist 151: 381–389. [Google Scholar]
- Andersson-Gunneras S, Hellgren JM, Bjorklund S, Regan S, Moritz T, Sundberg B. 2003. Asymmetric expression of a poplar ACC oxidase controls ethylene production during gravitational induction of tension wood. The Plant Journal 34: 339–349. [DOI] [PubMed] [Google Scholar]
- Avery GS, Burkholder PR, Creighton HB. 1937. Production and distribution of growth hormone in shoots of Aesculus and Malus, and its probable role in stimulating cambial activity. American Journal of Botany 24: 51–58. [Google Scholar]
- Basirat M, Malboobi MA, Mousavi A, Asgharzadeh A, Samavat S. 2011. Effects of phosphorous supply on growth, phosphate distribution and expression of transporter genes in tomato plants. Australian Journal of Crop Science 5: 537–543. [Google Scholar]
- Bastos CL, Tamaio N, Angyalossy V. 2016. Unravelling roots of lianas: a case study in Sapindaceae. Annals of Botany 118: 733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates TR, Lynch JP. 2001. Root hairs confer a competitive advantage under low phosphorus availability. Plant and Soil 236: 243–250. [Google Scholar]
- Bengough AG, Bransby MF, Hans J, McKenna SJ, Roberts TJ, Valentine TA. 2006. Root responses to soil physical conditions; growth dynamics from field to cell. Journal of Experimental Botany 57: 437–447. [DOI] [PubMed] [Google Scholar]
- Bjorklund S, Antti H, Uddestrand I, Moritz T, Sundberg B. 2007. Cross-talk between gibberellin and auxin in development of Populus wood: gibberellin stimulates polar auxin transport and has a common transcriptome with auxin. The Plant Journal 52: 499–511. [DOI] [PubMed] [Google Scholar]
- Bouma TJ, Yanai RD, Elkin AD, Hartmond U, Flores-Alva DE, Eissenstat DM. 2001. Estimating age-dependent costs and benefits of roots with contrasting life span: comparing apples and oranges. New Phytologist 150: 685–695. [Google Scholar]
- Brewer PB, Koltai H, Beveridge CA. 2013. Diverse roles of strigolactones in plant development. Molecular Plant 6: 18–28. [DOI] [PubMed] [Google Scholar]
- Brown VK, Gange AC. 1990. Insect herbivory below ground. Advances in Ecological Research 20: 1–44. [Google Scholar]
- Burton AL, Brown KM, Lynch JP. 2013. Phenotypic diversity of root anatomical and architectural traits in Zea species. Crop Science 53: 1042–1055. [Google Scholar]
- Canarini A, Kaiser C, Merchant A, Richter A, Wanek W. 2019. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Frontiers in Plant Science 10: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlquist S. 2012a. Monocot xylem revisited: new information, new paradigms. Botanical Review 78: 87–153. [Google Scholar]
- Carlquist S. 2012b. How wood evolves: a new synthesis. Botany-Botanique 90: 901–940. [Google Scholar]
- Carminati A, Vetterlein D. 2013. Plasticity of rhizosphere hydraulic properties as a key for efficient utilization of scarce resources. Annals of Botany 112: 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffey N, Cholewa E, Regan S, Sundberg B. 2002. Secondary xylem development in Arabidopsis: a model for wood formation. Physiologia Plantarum 114: 594–600. [DOI] [PubMed] [Google Scholar]
- Chimungu JG, Brown KM, Lynch JP. 2014a. Large root cortical cell size improves drought tolerance in maize. Plant Physiology 166: 2166–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimungu JG, Brown KM, Lynch JP. 2014b. Reduced root cortical cell file number improves drought tolerance in maize. Plant Physiology 166: 1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimungu JG, Loades KW, Lynch JP. 2015. Root anatomical phenes predict root penetration ability and biomechanical properties in maize (Zea mays). Journal of Experimental Botany 66: 3151–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comas LH, Eissenstat DM. 2004. Linking fine root traits to maximum potential growth rate among 11 mature temperate tree species. Functional Ecology 18: 388–397. [Google Scholar]
- Cuneo IF, Knipfer T, Brodersen CR, McElrone AJ. 2016. Mechanical failure of fine root cortical cells initiates plant hydraulic decline during drought. Plant Physiology 172: 1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroote DK, Larson PR. 1984. Correlations between net auxin and secondary xylem development in young Populus deltoides. Physiologia Plantarum 60: 459–466. [Google Scholar]
- De la Riva LM, Lynch JP. 2010. Root etiolation as a strategy for phosphorus acquisition in common bean . Master’s dissertation, Pennsylvania State University. [Google Scholar]
- Delaux PM, Xie X, Timme RE, et al. . 2012. Origin of strigolactones in the green lineage. New Phytologist 195: 857–871. [DOI] [PubMed] [Google Scholar]
- Dengler NG. 2001. Regulation of vascular development. Journal of Plant Growth Regulation 20: 1–13. [Google Scholar]
- Dickison WC. 2008. Integrative plant anatomy. San Diego: Academic Press. [Google Scholar]
- Doan TH, Doan TA, Kangas MJ, et al. . 2017. A low-cost imaging method for the temporal and spatial colorimetric detection of free amines on maize root surfaces. Frontiers in Plant Science 8: 1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque LO, Villordon A. 2019. Root branching and nutrient efficiency: status and way forward in root and tuber crops. Frontiers in Plant Science 10: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames AJ, MacDaniels LH. 1947. An introduction to plant anatomy. New York: McGraw-Hill Book Company, Inc. [Google Scholar]
- Eissenstat DM. 1992. Costs and benefits of constructing roots of small diameter. Journal of Plant Nutrition 15: 763–782. [Google Scholar]
- Ejiri M, Shiono K. 2019. Prevention of radial oxygen loss is associated with exodermal suberin along adventitious roots of annual wild species of Echinochloa. Frontiers in Plant Science 10: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett B, Nelson EB, Kessler A, Bauerle TL. 2014. Fine-root system development and susceptibility to pathogen colonization. Planta 239: 325–340. [DOI] [PubMed] [Google Scholar]
- Esau K. 1965. Vascular differentiation in plants. New York: Holt, Rinehart and Winston. [Google Scholar]
- Evert RF, Eichhorn SE. 2006. Esau’s plant anatomy: Meristems, cells, and tissues of the plant body—their structure, function and development, 3rd edn. Hoboken: John Wiley & Sons. [Google Scholar]
- Fan MS, Zhu JM, Richards C, Brown KM, Lynch JP. 2003. Physiological roles for aerenchyma in phosphorus-stressed roots. Functional Plant Biology 30: 493–506. [DOI] [PubMed] [Google Scholar]
- FAO . 1998. Storage and processing of root and tubers in the tropics.Rome: Food and Agriculture Organization of the United Nations. [Google Scholar]
- Fernando D, Lynch JP. 2015. Mn phytotoxicity: new light on an old problem. Annals of Botany, 116: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fohse D, Claassen N, Jungk A. 1988. Phosphorus efficiency of plants. 1. External and internal P requirement and P uptake efficiency of different plant species. Plant and Soil 110: 101–109. [Google Scholar]
- Fowler RF, Wilson LF. 1971. White grub populations, Phyllophaga spp., in relation to damaged red pine seedlings in Michigan and Wisconsin plantations (Coleoptera: Scarabaeidae). The Michigan Entomologist 4: 23–28. [Google Scholar]
- Galindo-Castañeda T, Brown KM, Lynch JP. 2018. Reduced root cortical burden improves growth and grain yield under low phosphorus availability in maize. Plant, Cell & Environment 41: 1579–1592. [DOI] [PubMed] [Google Scholar]
- Galindo-Castañeda T, Brown KM, Kuldau GA, et al. . 2019. Root cortical anatomy is associated with differential pathogenic and symbiotic fungal colonization in maize. Plant, Cell & Environment 42: 2999–3014. [DOI] [PubMed] [Google Scholar]
- Gerrienne P, Gonez P. 2011. Early evolution of life cycles in embryophytes: a focus on the fossil evidence of gametophyte/sporophyte size and morphological complexity. Journal of Systematics and Evolution 49: 1–16. [Google Scholar]
- Goldson SL, French RA. 1983. Age-related susceptibility of lucerne to Sitona weevil, Sitona discoideus Gyllenahl (Coleoptera: Curculionidae), larvae and the associated patterns of adult infestation. New Zealand Journal of Agricultural Research 26: 251–255. [Google Scholar]
- Gifford EM, Foster AS. 1989. Morphology and evolution of vascular plants. New York: W.H. Freedman and Company. [Google Scholar]
- Griffin GJ, Hale MG, Shay FJ. 1976. Nature and quantity of sloughed organic-matter produced by roots of axenic peanut plants. Soil Biology & Biochemistry, 8: 29–32. [Google Scholar]
- Guo DL, Xia MX, Wei X, Chang WJ, Liu Y, Wang ZQ. 2008. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytologist 180: 673–683. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS. 2001. Functional and ecological xylem anatomy. Perspectives in Plant Ecology, Evolution and Systematics 4: 97–115. [Google Scholar]
- Hacke UG, Spicer R, Schreiber SG, Plavcová L. 2017. An ecophysiological and developmental perspective on variation in vessel diameter. Plant, Cell & Environment 40: 831–845. [DOI] [PubMed] [Google Scholar]
- Hamlen RA, Lukezic FL, Bloom JR. 1972. Influence of age and stage of development on neutral carbohydrate components in root exudates from alfalfa plants grown in a gnotobiotic environment. Canadian Journal of Plant Science 52: 633–642. [Google Scholar]
- Hill JO, Simpson RJ, Moore AD, Chapman DF. 2006. Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant and Soil, 286: 7–19. [Google Scholar]
- Hoffman LA, Tomescu AM. 2013. An early origin of secondary growth: Franhueberia gerriennei gen. et sp. nov. from the Lower Devonian of Gaspé (Quebec, Canada). American Journal of Botany 100: 754–763. [DOI] [PubMed] [Google Scholar]
- Hu B, Henry A, Brown KM, Lynch JP. 2014. Root cortical aerenchyma inhibits radial nutrient transport in maize (Zea mays). Annals of Botany 113: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MC, Smith RG, Schipanski ME, Atwood LW, Mortensen DA. 2017. Agriculture in 2050: recalibrating targets for sustainable intensification. Bioscience 67: 385–390. [Google Scholar]
- Jaeger CH 3rd, Lindow SE, Miller W, Clark E, Firestone MK. 1999. Mapping of sugar and amino acid availability in soil around roots with bacterial sensors of sucrose and tryptophan. Applied and Environmental Microbiology 65: 2685–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobbagy EG, Jackson RB. 2000. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecological Applications 10: 423–436. [Google Scholar]
- Johnson SN, Erb M, Hartley SE. 2016. Roots under attack: contrasting plant responses to below- and aboveground insect herbivory. New Phytologist 210: 413–418. [DOI] [PubMed] [Google Scholar]
- Jura-Morawiec J, Tulik M, Iqbal M. 2015. Lateral meristems responsible for secondary growth of the monocotyledons: a survey of the state of the art. Botanical Review 81: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AM, Ahmed A, Saxena SK. 1964. Attractiveness of excised roots of plants to root-knot nematode Meloidogyne incognita (kofoid + white) chitwood. Indian Journal of Experimental Biology, 2: 208-&. [Google Scholar]
- Kozlowski TT. 1984. Plant-responses to flooding of soil. Bioscience 34: 162–167. [Google Scholar]
- Kozlowski TT, Pallardy SG. 2002. Acclimation and adaptive responses of woody plants to environmental stresses. Botanical Review 68: 270–334. [Google Scholar]
- Kramer-Walter KR, Bellingham PJ, Millar TR, Smissen RD, Richardson SJ, Laughlin DC. 2016. Root traits are multidimensional: specific root length is independent from root tissue density and the plant economic spectrum. Journal of Ecology 104: 1299–1310. [Google Scholar]
- Laliberté E, Lambers H, Burgess TI, Wright SJ. 2015. Phosphorus limitation, soil-borne pathogens and the coexistence of plant species in hyperdiverse forests and shrublands. New Phytologist 206: 507–521. [DOI] [PubMed] [Google Scholar]
- Lambers H, Stulen I, vanderWerf A. 1996. Carbon use in root respiration as affected by elevated atmospheric O2. Plant and Soil 187: 251–263. [Google Scholar]
- Larson PR. 1994. The vascular cambium: development and structure. Berlin: Springer. [Google Scholar]
- Little CHA, Savidge RA. 1987. The role of plant-growth regulators in forest tree cambial growth. Plant Growth Regulation 6: 137–169. [Google Scholar]
- Ljubojevic M, Maksimovic I, Lalic B, et al. . 2018. Environmentally-related cherry root cambial plasticity. Atmosphere 9: 16. [Google Scholar]
- Louveaux M, Hamant O. 2013. The mechanics behind cell division. Current Opinion in Plant Biology 16: 774–779. [DOI] [PubMed] [Google Scholar]
- Love J, Björklund S, Vahala J, Hertzberg M, Kangasjärvi J, Sundberg B. 2009. Ethylene is an endogenous stimulator of cell division in the cambial meristem of Populus. Proceedings of the National Academy of Sciences, USA 106: 5984–5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lulai EC, Corsini DL. 1998. Differential deposition of suberin phenolic and aliphatic domains and their roles in resistance to infection during potato tuber (Solanum tuberosum L.) wound-healing. Physiological and Molecular Plant Pathology 53: 209–222. [Google Scholar]
- Lynch J. 1995. Root architecture and plant productivity. Plant Physiology 109: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2007. Roots of the second green revolution. Australian Journal of Botany 55: 493–512. [Google Scholar]
- Lynch JP. 2013. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Annals of Botany 112: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2018. Rightsizing root phenotypes for drought resistance. Journal of Experimental Botany 69: 3279–3292. [DOI] [PubMed] [Google Scholar]
- Lynch JP. 2019. Root phenotypes for improved nutrient capture: an underexploited opportunity for global agriculture. New Phytologist 223: 548–564. [DOI] [PubMed] [Google Scholar]
- Lynch JP, Brown KM. 2008. Root strategies for phosphorus acquisition. In: White PJ, Hammond JP, eds. The ecophysiology of plant–phosphorus interactions. Dordrecht: Springer, 83–116 [Google Scholar]
- Lynch JP, Wojciechowski T. 2015. Opportunities and challenges in the subsoil: pathways to deeper rooted crops. Journal of Experimental Botany 66: 2199–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, Chimungu JG, Brown KM. 2014. Root anatomical phenes associated with water acquisition from drying soil: targets for crop improvement. Journal of Experimental Botany 65: 6155–6166. [DOI] [PubMed] [Google Scholar]
- Lyu Y, Tang HL, Li HG, et al. . 2016. Major crop species show differential balance between root morphological and physiological responses to variable phosphorus supply. Frontiers in Plant Science 7: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ZQ, Guo DL, Xu XL, et al. . 2018. Evolutionary history resolves global organization of root functional traits. Nature 555: 94–97. [DOI] [PubMed] [Google Scholar]
- Machado A, Pereira H,Teixeira RT. 2013. Anatomy and development of the endodermis and phellem of Quercus suber L. roots. Microscopy and Microanalysis 19: 525–534. [DOI] [PubMed] [Google Scholar]
- Marschner H. 1995. Rhizosphere pH effects on phosphorus nutrition. In: Genetic Manipulation of Crop Plants to Enhance Integrated Nutrient Management in Cropping Systems-1. Phosphorus: Proceedings of an Fao-Icrisat Expert Consultancy Workshop: 107–115. [Google Scholar]
- McCormick JC. 1916. Notes on the anatomy of the young tuber of Ipomoea batatas (Lam.). Botanical Gazette, 61: 388–398. [Google Scholar]
- McCully ME. 1999. Roots in soil: unearthing the complexities of roots and their rhizospheres. Annual Review of Plant Physiology and Plant Molecular Biology 50: 695–718. [DOI] [PubMed] [Google Scholar]
- McCully ME, Canny MJ. 1985. Localisation of translocated 14C in roots and root exudates of field-grown maize. Physiologia Plantarum 65: 380–392. [Google Scholar]
- McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. 1990. A new method which gives an objective-measure of colonization of roots by vesicular arbuscular mycorrhizal fungi. New Phytologist, 115: 495–501. [DOI] [PubMed] [Google Scholar]
- Meunier F, Zarebanadkouki M, Ahmed MA, Carminati A, Couvreur V, Javaux M. 2018. Hydraulic conductivity of soil-grown lupine and maize unbranched roots and maize root–shoot junctions. Journal of Plant Physiology 227: 31–44. [DOI] [PubMed] [Google Scholar]
- Micallef SA, Shiaris MP, Colón-Carmona A. 2009. Influence of Arabidopsis thaliana accessions on rhizobacterial communities and natural variation in root exudates. Journal of Experimental Botany 60: 1729–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel MA, Postma JA, Lynch JP. 2015. Phene synergism between root hair length and basal root growth angle for phosphorus acquisition. Plant Physiology 167: 1430–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashima S, Roszak P, Sevilem I, et al. . 2019. Mobile PEAR transcription factors integrate positional cues to prime cambial growth. Nature 565: 490–494. [DOI] [PubMed] [Google Scholar]
- Nielsen KL, Bouma TJ, Lynch JP, Eissenstat DM. 1998. Effects of phosphorus availability and vesicular-arbuscular mycorrhizas on the carbon budget of common bean (Phaseolus vulgaris). New Phytologist 139: 647–656. [Google Scholar]
- Nielsen KL, Eshel A, Lynch JP. 2001. The effect of phosphorus availability on the carbon economy of contrasting common bean (Phaseolus vulgaris L.) genotypes. Journal of Experimental Botany 52: 329–339. [PubMed] [Google Scholar]
- Nobel PS, Jordan PW. 1983. Transpiration stream of desert species – resistances and capacities for a C-3, a C-4, and a CAM plant. Journal of Experimental Botany 34: 1379–1391. [Google Scholar]
- Ostonen I, Puttsepp U, Biel C, et al. . 2007. Specific root length as an indicator of environmental change. Plant Biosystems 141: 426–442. [Google Scholar]
- Passioura JB. 2002. Environmental biology and crop improvement. Functional Plant Biology 29: 537–546. [DOI] [PubMed] [Google Scholar]
- Pittman JK. 2005. Managing the manganese: molecular mechanisms of manganese transport and homeostasis. New Phytologist 167: 733–742. [DOI] [PubMed] [Google Scholar]
- Pockman WT, Sperry JS. 2000. Vulnerability to xylem cavitation and the distribution of Sonoran Desert vegetation. American Journal of Botany 87: 1287–1299. [PubMed] [Google Scholar]
- Poirier V, Roumet C, Munson AD. 2018. The root of the matter: linking root traits and soil organic matter stabilization processes. Soil Biology & Biochemistry 120: 246–259. [Google Scholar]
- Post WM, Emanuel WR, Zinke PJ, Stangenberger AG. 1982. Soil carbon pools and world life zones. Nature 298: 156–159. [Google Scholar]
- Postma JA, Lynch JP. 2011. Root cortical aerenchyma enhances the growth of maize on soils with suboptimal availability of nitrogen, phosphorus, and potassium. Plant Physiology 156: 1190–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma JA, Lynch JP. 2011. Theoretical evidence for the functional benefit of root cortical aerenchyma in soils with low phosphorus availability. Annals of Botany 107: 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman R, Zaman-Allah M, Mallikarjuna N, Pannirselvam R, Krishnamurthy L, Gowda CLL. 2013. Root anatomical traits and their possible contribution to drought tolerance in grain legumes. Plant Production Science 16: 1–8. [Google Scholar]
- Ragni L, Greb T. 2018. Secondary growth as a determinant of plant shape and form. Seminars in Cell & Developmental Biology 79: 58–67. [DOI] [PubMed] [Google Scholar]
- Raman V, Galal AM, Avula B, Sagi S, Smillie TJ, Khan IA. 2014. Application of anatomy and HPTLC in characterizing species of Dioscorea (Dioscoreaceae). Journal of Natural Medicines 68: 686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasse DP, Rumpel C, Dignac MF. 2005. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant and Soil 269: 341–356. [Google Scholar]
- Reich PB, Tjoelker MG, Walters MB, Vanderklein DW, Buschena C. 1998. Close association of RGR, leaf and root morphology, seed mass and shade tolerance in seedlings of nine boreal tree species grown in high and low light. Functional Ecology, 12: 327–338. [Google Scholar]
- Rewald B, Ephrath JE, Rachmilevitch S. 2011. A root is a root is a root? Water uptake rates of Citrus root orders. Plant, Cell & Environment 34: 33–42. [DOI] [PubMed] [Google Scholar]
- Richards RA, Passioura JB. 1989. A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain-fed environments. Australian Journal of Agricultural Research 40: 943–950. [Google Scholar]
- Rovira AD. 1959. Root excretions in relation to the rhizosphere effect. Plant and Soil 11: 53–64. [Google Scholar]
- Saengwilai P, Nord EA, Chimungu JG, Brown KM, Lynch JP. 2014. Root cortical aerenchyma enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiology 166: 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathkumar A, Yan A, Krupinski P, Meyerowitz EM. 2014. Physical forces regulate plant development and morphogenesis. Current Biology 24: R475–R483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanio C. 1873. Anatomie der gemeinen Kiefer (Pinus silvestris L.) II. Entwickelungsgeschichte der Holzzellen. Jahrbucher fur Wissenschaftliche Botanik 9: 50–128. [Google Scholar]
- Sasse J, Martinoia E, Northen T. 2018. Feed your friends: do plant exudates shape the root microbiome? Trends in Plant Science 23: 25–41. [DOI] [PubMed] [Google Scholar]
- Savidge RA. 1993. Formation of annual rings in trees. In: Rensing L, ed. Oscillations and morphogenesis. New York: Marcel Dekker, 343–363. [Google Scholar]
- Savidge RA, Wareing PF. 1981. Plant-growth regulators and the differentiation of vascular elements. In: Barnett JR, ed. Xylem cell development. London: Castle House Publications, 196– 235. [Google Scholar]
- Schneider HM, Lynch JP. 2018. Functional implications of root cortical senescence for soil resource capture. Plant and Soil 423: 13–26. [Google Scholar]
- Schneider HM, Postma JA, Wojciechowski T, Kuppe C, Lynch JP. 2017a. Root cortical senescence improves growth under suboptimal availability of N, P, and K. Plant Physiology 174: 2333–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider HM, Wojciechowski T, Postma JA, et al. . 2017. b. Root cortical senescence decreases root respiration, nutrient content and radial water and nutrient transport in barley. Plant, Cell & Environment 40: 1392–1408. [DOI] [PubMed] [Google Scholar]
- Schneider HM, Wojciechowski T, Postma JA, Brown KM, Lynch JP. 2018. Ethylene modulates root cortical senescence in barley. Annals of Botany 122: 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov AM, van Bruggen AHC, Zelenev VV. 1999. Moving waves of bacterial populations and total organic carbon along roots of wheat. Microbial Ecology 37: 116–128. [DOI] [PubMed] [Google Scholar]
- Smetana O, Makila R, Lyu M, et al. . 2019. High levels of auxin signaling define the stem-cell organizer of the vascular cambium. Nature 565: 485–489. [DOI] [PubMed] [Google Scholar]
- Snow R. 1935. Activation of cambial growth by pure hormones. Nature 135: 876–876. [Google Scholar]
- Sperry JS, Saliendra NZ. 1994. Intra-plant and inter-plant variation in xylem cavitation in Betula occidentalis. Plant, Cell & Environment 17: 1233–1241. [Google Scholar]
- St Clair SB, Lynch JP. 2010. The opening of Pandora’s Box: climate change impacts on soil fertility and crop nutrition in developing countries. Plant and Soil 335: 101–115. [Google Scholar]
- Steudle E. 2000. Water uptake by roots: effects of water deficit. Journal of Experimental Botany 51: 1531–1542. [DOI] [PubMed] [Google Scholar]
- Steudle E, Boyer JS. 1985. Hydraulic resistance to radial water flow in growing hypocotyl of soybean measured by a new pressure–perfusion technique. Planta 164: 189–200. [DOI] [PubMed] [Google Scholar]
- Stiller V, Sperry JS, Lafitte R. 2005. Embolized conduits of rice (Oryza sativa, Poaceae) refill despite negative xylem pressure. American Journal of Botany 92: 1970–1974. [DOI] [PubMed] [Google Scholar]
- Strock CF. 2019. Functional implications of root architectural and anatomical phenes for soil resource capture. PhD Thesis, Pennsylvania State University; (Unpublished). [Google Scholar]
- Strock CF, Morrow de la Riva L, Lynch JP. 2018. Reduction in root secondary growth as a strategy for phosphorus acquisition. Plant Physiology 176: 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strock CF, Schneider HM, Galindo-Castañeda T, et al. . 2019. Laser ablation tomography for visualization of root colonization by edaphic organisms. Journal of Experimental Botany 70: 5327–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebaldi C, Lobell DB. 2008. Towards probabilistic projections of climate change impacts on global crop yields. Geophysical Research Letters 35: 6. [Google Scholar]
- Thamm A, Sanegre-Sans S, Paisley J, et al. . 2019. A simple mathematical model of allometric exponential growth describes the early three-dimensional growth dynamics of secondary xylem in Arabidopsis roots. Royal Society Open Science 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann KV, Skoog F. 1933. Studies on the growth hormones of plants. III. The inhibition action of growth substance on bud development. Proceedings of the National Academy of Sciences, USA 19: 714–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorup-Kristensen K, Kirkegaard J. 2016. Root system-based limits to agricultural productivity and efficiency: the farming systems context. Annals of Botany 118: 573–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomescu AMF, Groover AT. 2019. Mosaic modularity: an updated perspective and research agenda for the evolution of vascular cambial growth. New Phytologist 222: 1719–1735. [DOI] [PubMed] [Google Scholar]
- Tomlinson PB, Zimmerman, MH. 1969. Vascular anatomy of monocotyledons with secondary growth – an introduction. Journal of the Arnold Arboretum 50: 159–159. [Google Scholar]
- Tyree MT, Davis SD, Cochard H. 1994. Biophysical perspectives of xylem evolution – is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction. IAWA Journal 15: 335–360. [Google Scholar]
- Vadez V. 2014. Root hydraulics: the forgotten side of roots in drought adaptation. Field Crops Research 165: 15–24. [Google Scholar]
- Vadez V, Kholova J, Zaman-Allah M, Belko N. 2013. Water: the most important ‘molecular’ component of water stress tolerance research. Functional Plant Biology 40: 1310–1322. [DOI] [PubMed] [Google Scholar]
- Valenzuela-Estrada LR, Bryla DR, Hoashi-Erhardt WK, Moore PP, Forge TA. 2012. Root traits associated with phytophthora root rot resistance in Red Raspberry. X International Rubus and Ribes Symposium 946: 283–287. [Google Scholar]
- Valenzuela-Estrada LR, Vera-Caraballo V, Ruth LE, Eissenstat DM. 2008. Root anatomy, morphology, and longevity among root orders in Vaccinium corymbosum (Ericaceae). American Journal of Botany 95: 1506–1514. [DOI] [PubMed] [Google Scholar]
- Van Der Werf A, Kooijman A, Welschen R, Lambers H. 1988. Respiratory energy costs for the maintenance of biomass for growth and for ion uptake in roots of Carex diandra and Carex acutiformis. Physiologia Plantarum 72: 483–491. [Google Scholar]
- Vieira RF, Jochua CN, Lynch JP. 2007. Method for evaluation of root hairs of common bean genotypes. Pesquisa Agropecuaria Brasileira 42: 1365–1368. [Google Scholar]
- Vierheilig H, Coughlan AP, Wyss U, Piche Y. 1998. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Applied and Environmental Microbiology 64: 5004–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villordon AQ, Ginzberg I, Firon N. 2014. Root architecture and root and tuber crop productivity. Trends in Plant Science 19: 419–425. [DOI] [PubMed] [Google Scholar]
- Volder A, Smart DR, Bloom AJ, Eissenstat DM. 2005. Rapid decline in nitrate uptake and respiration with age in fine lateral roots of grape: implications for root efficiency and competitive effectiveness. New Phytologist 165: 493–501. [DOI] [PubMed] [Google Scholar]
- Voothuluru P, Braun DM, Boyer JS. 2018. An in vivo imaging assay detects spatial variability in glucose release from plant roots. Plant Physiology 178: 1002–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukicevich E, Lowery DT, Eissenstat D, Hart M. 2019. Changes in arbuscular mycorrhizal fungi between young and old Vitis roots. Symbiosis 78: 33–42. [Google Scholar]
- Wareing PF, Hanney CEA, Digby J. 1964. The role of endogenous hormones in cambial activity and xylem differentiation. In: Zimmermann MH, ed. The formation of wood in forest trees. New York: Academic Press, 323–344. [Google Scholar]
- Wilson LA, Lowe SB. 1973. The anatomy of the root system in West Indian sweet potato (Ipomoea batatas (L.) Lam.) cultivars. Annals of Botany, 37: 633–643 [Google Scholar]
- Woods J, Williams A, Hughes JK, Black M, Murphy R. 2010. Energy and the food system. Philosophical Transactions of the Royal Society B: Biological Sciences 365: 2991–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank . 2017. World development indicators 2017. Washington, DC:World Bank. [Google Scholar]
- Wright IJ, Westoby M. 1999. Differences in seedling growth behaviour among species: trait correlations across species, and trait shifts along nutrient compared to rainfall gradients. Journal of Ecology, 87: 85–97. [Google Scholar]
- Wunderling A, Ben Targem M, Barbier de Reuille P, Ragni L. 2017. Novel tools for quantifying secondary growth. Journal of Experimental Botany 68: 89–95. [DOI] [PubMed] [Google Scholar]
- Yan XL, Liao H, Beebe SE, Blair MW, Lynch JP. 2004. QTL mapping of root hair and acid exudation traits and their relationship to phosphorus uptake in common bean. Plant and Soil 265: 17–29. [Google Scholar]
- Yoneyama K, Xie X, Kim HI, et al. . 2012. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 235: 1197–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarebanadkouki M, Meunier F, Couvreur V, Cesar J, Javaux M, Carminati A. 2016. Estimation of the hydraulic conductivities of lupine roots by inverse modelling of high-resolution measurements of root water uptake. Annals of Botany 118: 853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarebanadkouki M, Trtik P, Hayat F, Carminati A, Kaestner A. 2019. Root water uptake and its pathways across the root: quantification at the cellular scale. Scientific Reports 9: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Eswaran G, Alonso-Serra J, et al. . 2019. Transcriptional regulatory framework for vascular cambium development in Arabidopsis roots. Nature Plants 5: 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JJ, Wang WY, Zhou HK, et al. . 2017. Manganese toxicity inhibited root growth by disrupting auxin biosynthesis and transport in Arabidopsis. Frontiers in Plant Science 8: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Du YL, Ahmed S, et al. . 2016. Genotypic differences in phosphorus efficiency and the performance of physiological characteristics in response to low phosphorus stress of soybean in Southwest of China. Frontiers in Plant Science 7: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JM, Kaeppler SM, Lynch JP. 2005. Mapping of QTL controlling root hair length in maize (Zea mays L.) under phosphorus deficiency. Plant and Soil 270: 299–310. [Google Scholar]
- Zhu J, Brown KM, Lynch JP. 2010a. Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant, Cell & Environment 33: 740–749. [DOI] [PubMed] [Google Scholar]
- Zhu JM, Zhang CC, Lynch JP. 2010b. The utility of phenotypic plasticity of root hair length for phosphorus acquisition. Functional Plant Biology 37: 313–322. [Google Scholar]
- Zimmerman MH. 1983. Xylem structure and the ascent of sap. New York: Springer. [DOI] [PubMed] [Google Scholar]
- Zimmerman MH, Brown CL. 1974. Trees, structure, and function, 2nd edn. Berlin: Springer. [Google Scholar]
- Zvereva EL, Kozlov MV. 2012. Sources of variation in plant responses to belowground insect herbivory: a meta-analysis. Oecologia 169: 441–452. [DOI] [PubMed] [Google Scholar]
- Zwieniecki MA, Thompson MV, Holbrook NM. 2002. Understanding the hydraulics of porous pipes: tradeoffs between water uptake and root length utilization. Journal of Plant Growth Regulation 21: 315–323. [Google Scholar]