Abstract
Reactive oxygen species (ROS) production is a by-product of mitochondrial activity and is necessary for the acquisition of the capacitated state, a requirement for functional spermatozoa. However, an increase in oxidative stress, due to an abnormal production of ROS, has been shown to be related to loss of sperm function, highlighting the importance of an accurate detection of sperm ROS, given the specific nature of this cell. In this work, we tested a variety of commercially available fluorescent probes to detect ROS and reactive nitrogen species (RNS) in human sperm, to define their specificity. Using both flow cytometry (FC) and fluorescence microscopy (FM), we confirmed that MitoSOX™ Red and dihydroethidium (DHE) detect superoxide anion (as determined using antimycin A as a positive control), while DAF-2A detects reactive nitrogen species (namely, nitric oxide). For the first time, we also report that RedoxSensor™ Red CC-1, CellROX® Orange Reagent, and MitoPY1 seem to be mostly sensitive to hydrogen peroxide, but not superoxide. Furthermore, mean fluorescence intensity (and not percentage of labeled cells) is the main parameter that can be reproducibly monitored using this type of methodology.
Keywords: flow cytometry, fluorescent probes, human spermatozoa, oxidative stress, reactive oxygen species
INTRODUCTION
From the various reasons contributing to statistics regarding male infertility,1 defective sperm function has been considered the major cause,2 and in turn, oxidative stress (OS) is a big contributor to this impaired function, stemming from the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS).
Spermatozoa produce both cytosolic and mitochondrial superoxide and ROS-related hydrogen peroxide (H2O2).3,4 RNS are also produced, namely nitric oxide (NO) synthesized by NO synthase (NOS) and peroxynitrite (ONOO−).5,6,7 The overproduction of ROS/RNS has been associated with membrane damage, loss of motility, premature capacitation and the acrosome reaction, abnormal morphology, impaired oocyte-sperm fusion, and apoptosis, among others.8,9 DNA damage is also a critical by-product of OS that can ultimately result in fertilization problems, miscarriage, and abnormalities of the offspring.10,11,12 Moreover, another consequence of ROS production is lipid peroxidation,8,9 compromising the integrity of the sperm membrane, impairing motility, and decreasing viability.13 The increasing interest in understanding the involvement of ROS/RNS production in male infertility highlights the need for improved detection systems to measure accurately the content of ROS/RNS, mostly using fluorescent probes.
One of the staple reagents is MitoSOX™ Red, a probe comprising dihydroethidium bound to the triphenylphosphonium (TPP) cation that directs it specifically to the mitochondria.14 Once inside the mitochondria, dihydroethidium will react with O2•-, producing another molecule, 2-hydroxyethidium.15 In a reduced state, this compound emits blue fluorescence. However, on oxidation, it will intercalate with DNA, emitting red fluorescence.16 MitoSOX™ Red has been widely used for the detection of mitochondrial O2•- in human spermatozoa.14,17,18,19 Dihydroethidium (DHE) can also be used by itself to detect ROS. Lacking the TPP cation, DHE is confined to the cytoplasm20 and is commonly used to detect cytosolic O2•- (originating from the nicotinamide adenine dinucleotide phosphate [NADPH]-oxidase 5 [NOX5] system).14,20,21 De Iuliis et al.22 first reported the use of this probe in human spermatozoa. On a different note, RedoxSensor™ Red CC-1 is used as an indicator of the oxidative activity in living cells.23 For more general ROS detection, CellROX® Orange Reagent has been used, and on oxidation, it produces orange/red fluorescence in the cytosol.24 Another commonly used probe is MitoPY1, also including the TPP cation, which is selectively targeted toward the mitochondria.25 This probe derives from boronate26 and has been used in the constitution of probes highly sensitive to H2O2.27 On interaction with H2O2, boronate is converted to phenol and emits green fluorescence.25 None of these three probes (RedoxSensor™ Red CC-1, CellROX® Orange Reagent, and MitoPY1) has been previously tested in human spermatozoa. Finally, for the detection of RNS, DAF-2 DA is the standard probe. Once inside the cell, DAF-2 DA will be hydrolyzed through the activity of cytosolic esterases, preventing its exit from the cell. By reacting with NO•, DAF-2 is converted to its fluorescent triazole derivative (DAF-2T), that emits green fluorescence.28 Our goal was to compare these commercially available fluorescent probes, to determine their specificity towards certain reactive species.
MATERIALS AND METHODS
Reagents and media
Unless stated otherwise, all reagents were from Sigma–Aldrich (St. Louis, MO, USA) and all probes were from Molecular Probes/Invitrogen (Eugene, OR, USA). Two sperm suspension media were used: Sperm Preparation Medium (SPM - Medicult-Origio, Jyllinge, Denmark) and a PBS supplemented medium.29
Sperm sample collection, processing, and analysis
All sperm samples were kindly provided by the Reproductive Medicine Unit at the University Hospitals of Coimbra, Coimbra, Portugal. The patients undergoing fertility treatments signed informed consent forms authorizing the use of the remaining sample, and all human material was used in accordance with the appropriate ethical and Internal Review Board (IRB) guidelines provided by the University Hospitals of Coimbra, which approved the study. The semen samples were obtained after 3–5 days of sexual abstinence and the spermiogram was performed according to the World Health Organization Guidelines (WHO, 2010),30 including parameters such as concentration, motility, and morphology. After analysis, the samples were prepared by density gradient centrifugation as described previously,31 which allowed sperm segregation from seminal plasma and round cells, and this was also confirmed visually, although no specific method to remove leukocytes was used. Finally, the spermatozoa were incubated at least for 3 h in Sperm Preparation Medium to allow capacitation to occur.
Flow cytometry (FC)
For all FC experiments, a cell suspension of 5 × 106 sperm ml−1 (in a volume of 500 μl) was analyzed using a BD FACSCalibur (Becton Dickinson, Franklin Lakes, NJ, USA) flow cytometer with an argon laser that performs with an excitation wavelength of 488 nm coupled with the following emission filters: 530/30 band pass (FL-1 channel/green), 585/42 band pass (FL-2 channel/red) (Supplementary Figure 1 (853.8KB, tif) ).
Flow cytometry data were analyzed using the FlowJo® (FlowJo LLC, Ashland, OR, USA) software and 200 000 events were acquired per assay. Mean fluorescence intensity (MFI) and percentage of labeled cells (PLC) were both considered, with controls to assure that only sperm cells (and not debris, leukocytes, or other round cells) were being gated and analyzed.30 The concentration and incubation time for each probe was as follows: MitoSOX™ Red (1 μmol l−1, 15 min), DHE (50 μmol l−1, 15 min), RedoxSensor™ Red CC-1 (3 μmol l−1, 10 min), CellROX® Orange Reagent (1 μmol l−1, 30 min), MitoPY1 (10 μmol l−1, 40 min; Tocris Bioscience, Bristol, UK), and DAF-2 DA (1 μmol l−1, 40 min). As positive controls for the probes against ROS, we used three different conditions: antimycin A (79 μmol l−1), H2O2 (0.006% [v/v]; Merck Millipore, Billerica, MA, USA), and H2O2 (0.006% [v/v]) + EDTA (100 μmol l−1; Bio-Rad; Hercules, CA, USA). This last control was to determine if there would be an increase in the levels of H2O2 without the production of cytosolic O2•-, since EDTA would prevent NOX5 activation by chelating calcium (Ca2+). Finally, to clarify if DAF-2 DA (Merck Millipore) is sensitive to NO•, spermine (Spermine NONOate; 50 μmol l−1) was used.31,32,33 We used only samples with high viability (over 95% viability at the start), and attempted to monitor simultaneously sperm viability in the same samples, by performing pilot experiments with MitoSOX and Sytox Green. However, at least in our hands, the presence of the viability staining significantly interfered with the MitoSOX signal (unpublished data). This is clearly an important point to address in the future.
Statistical analyses
Statistical analysis was performed with IBM SPSS Software, version 21 (Armonk, NY, USA). All variables were checked for normal distribution through the Shapiro–Wilk normality test. In this work, when the data presented a nonnormal distribution, only nonparametric tests were applied. Therefore, when comparing two dependent groups, we used the Wilcoxon test. When the data had a normal distribution, the Student's t-test was used. Normally distributed data were presented as mean ± standard deviation (s.d.) and nonparametric data were represented through box and whisker plots as quartiles Q1, Q2 (median), and Q3. P ≤ 0.05 was considered statistically significant.
RESULTS
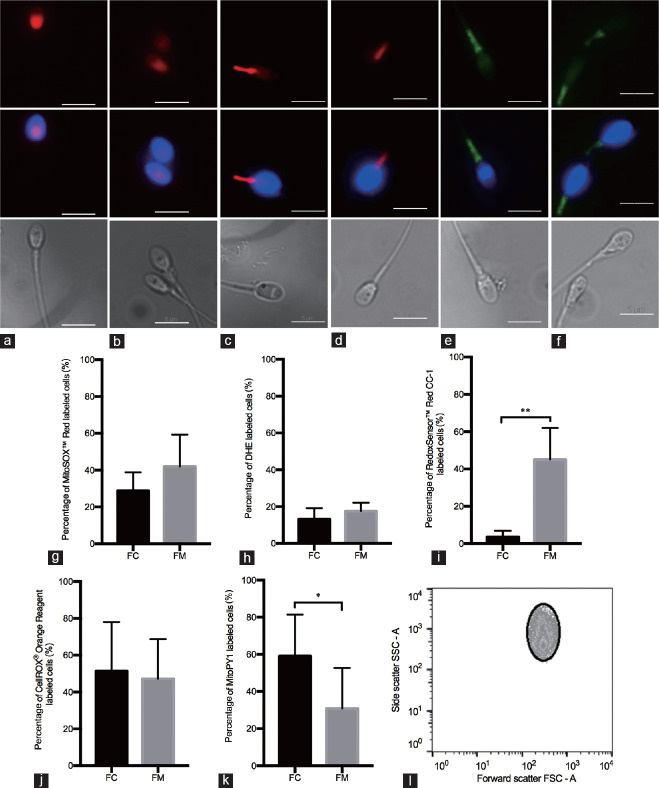
To characterize each probe, the percentage of labeled cells in each condition is crucial and we determined if this parameter changed when monitored via flow cytometry or when manually counted using fluorescence microscopy. Staining representative images for all probes are shown in Figure 1a–1f. MitoSOX™ Red exhibits, in most cases, red fluorescence in the head region of the cell (Figure 1a), and, as expected, similar results were obtained for DHE (Figure 1b). A prevalent staining of the midpiece was observed when using RedoxSensor™ Red CC-1 (Figure 1c), and for CellROX® Orange Reagent, orange/red fluorescence was also seen exclusively in the midpiece (Figure 1d). MitoPY1, a probe targeted to the mitochondria, also emits green fluorescence in the midpiece (Figure 1e).25 Finally, DAF-2 DA revealed green labeling in the sperm midpiece (Figure 1f).
Figure 1.
(a–f) Representative images of spermatozoa labeled with fluorescent probes against ROS/RNS. In the first row, (a) MitoSOX™ Red (1 μmol l−1) and (b) DHE (50 μmol l−1) showed red fluorescence in the sperm head; (c) RedoxSensor™ Red CC-1 (3 μmol l−1) stained mostly the midpiece of the cell with red fluorescence, with some cases labeling the head as well; (d) CellROX® Orange Reagent (1 μmol l−1) labeled only the midpiece with red fluorescence, while (e) MitoPY1 (10 μmol l−1) and (f) DAF-2 DA (1 μmol l−1) labeled the same area in green fluorescence. Sperm nucleus were labeled with Hoechst (blue fluorescence, middle row). Phase contrast images in gray show the entire sperm cell (bottom row). Scale bars = 5 μm. (g–k) Percentage of labeled cells obtained through flow cytometry (FC) versus fluorescence microscopy (FM). Percentage of labeled cells for the fluorescent probes: (g) MitoSOX™ Red (n= 5), (h) DHE (n = 4), (i) RedoxSensor™ Red CC-1 (n = 4), (j) CellROX® Orange Reagent (n = 5) and (k) MitoPY1 (n = 5). All data present a Gaussian distribution; therefore, the Student's t-test was the statistical test performed. Data are presented as mean ± standard deviation. *P < 0.05, **P < 0.01. (l) Dot plot representation of the gate set to exclude nonsperm-specific events. ROS: reactive oxygen species; RNS: reactive nitrogen species.
When comparing flow cytometry and manual fluorescence microscopy counting, there was no difference for MitoSOX™ Red (Figure 1g) and DHE (Figure 1h). Figure 1i shows that there are statistically significant differences for RedoxSensor™ Red CC-1. This did not happen with CellROX® Orange (Figure 1j), but was the case for MitoPY1 (Figure 1k). These differences found with RedoxSensor™ Red CC-1 and MitoPY1 are, in great part, subjective, owing to the operators and their ability to identify correctly labeled cells (i.e., notably what intensity of staining against the background constitutes positive staining). Therefore, throughout this work, we favored MFI over the percentage of labeled cells (regardless of the method used). Nonetheless, graphs showing the percentage of labeled cells (obtained through flow cytometry, and not manual counting) were also included, to ultimately better characterize the probes.
For MitoSOX™ Red, our data show a statistically significant increase in both MFI (Figure 2a–2c) and PLC (Figure 2d–2f) in all control conditions, compared with the initial condition, MitoSOX™ Red only. Regarding DHE, similarly, the use of positive controls increased the levels of MFI (Figure 2g–2i) and PLC (Figure 2j–2l), compared with the initial condition (only DHE), and this increase was statistically significant throughout.
Figure 2.
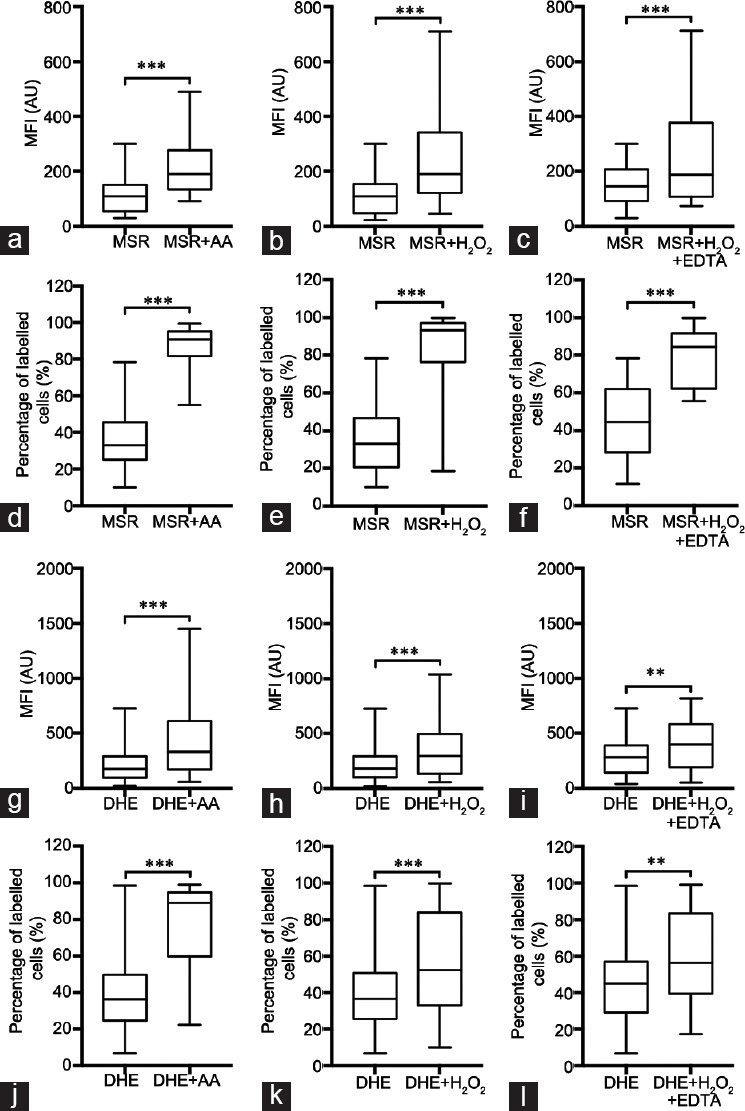
Assessment of MFI and PLC in the sperm populations labeled with MitoSOX™ Red and DHE. (a–c) MFI values were obtained through FC for MitoSOX™ Red and compared to the positive controls: (a) MSR versus MSR + AA (n =33), (b) MSR versus MSR + H2O2 (n = 31) and (c) MSR versus MSR + H2O2 + EDTA (n = 15); (d–f) PLC values were obtained through FC for MitoSOX™ Red and compared to the positive controls: (d) MSR versus MSR + AA (n = 33), (e) MSR versus MSR + H2O2 (n = 31) and (f) MSR versus MSR + H2O2 + EDTA (n = 15); (g–i) MFI values were obtained through FC for DHE and compared to the positive controls: (g) DHE versus DHE + AA (n = 37), (h) DHE versus DHE + H2O2 (n = 36) and (i) DHE versus DHE + H2O2 + EDTA (n = 19); (j–l) PLC values were obtained through FC for DHE and compared to the positive controls: (j) DHE versus DHE + AA (n = 37), (k) DHE versus DHE + H2O2 (n = 36) and (l) DHE versus DHE + H2O2 + EDTA (n = 19). All data present a non-Gaussian distribution; therefore, the Wilcoxon test was performed. Data are presented as quartiles, Q1, Q2 (median) and Q3. **P < 0.01, ***P < 0.001. MSR: MitoSOX™ Red; AA: antimycin A; DHE: dihydroethidium; FC: flow cytometry; EDTA: ethylenediamine tetra acetic acid; MFI: mean fluorescence intensity; PLC: percentage of labeled cells.
When using RedoxSensor™ Red CC-1 and acquiring MFI, the control with antimycin A showed no differences (Figure 3a), and only the controls H2O2 and H2O2+ EDTA showed a statistically significant increase (Figure 3b and 3c). However, in terms of PLC, there were statistically significant differences for all the controls compared with the initial condition (Figure 3d–3f).
Figure 3.
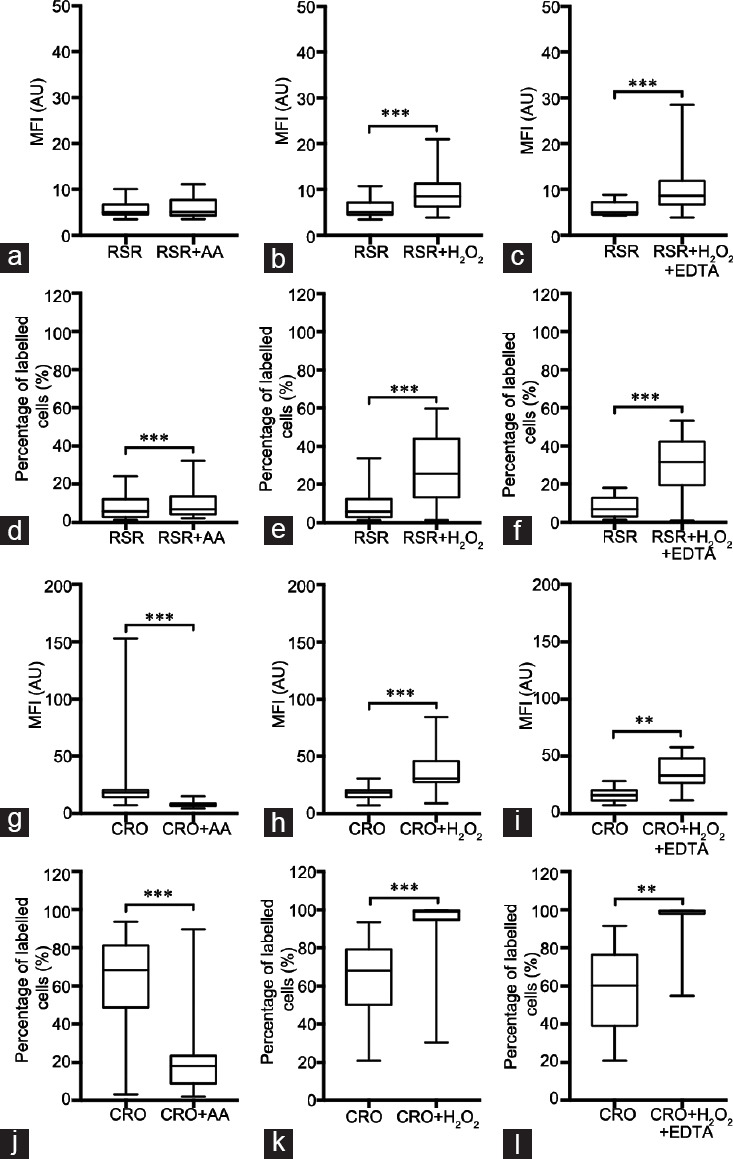
Assessment of MFI and PLC in the sperm populations labeled with RedoxSensor™ Red CC-1 and CellROX® Orange Reagent. (a–c) MFI values were obtained through FC for RedoxSensor™ Red CC-1 and compared to the positive controls: (a) RSR CC-1 versus RSR + AA (n = 29), (b) RSR versus RSR + H2O2 (n = 31) and (c) RSR versus RSR + H2O2 + EDTA (n = 18); (d–f) PLC values were obtained through FC for RedoxSensor™ Red CC-1 and compared to the positive controls: (d) RSR versus RSR + AA (n = 29), (e) RSR versus RSR + H2O2 (n = 31) and (f) RSR versus RSR + H2O2 + EDTA (n = 18); (g–i) MFI values were obtained through FC for CellROX® Orange Reagent and compared to the positive controls: (g) CRO versus CRO + AA (n = 31), (h) CRO versus CRO + H2O2 (n = 33) and (i) CRO versus CRO + H2O2 + EDTA (n = 13); (j–l) PLC values were obtained through FC for CRO and compared to the positive controls: (j) CRO versus CRO + AA (n = 31), (k) CRO versus CRO + H2O2 (n = 33) and (l) CRO versus CRO + H2O2 + EDTA (n = 13). All data present a non-Gaussian distribution; therefore, the Wilcoxon test was performed. Data are presented as quartiles, Q1, Q2 (median) and Q3. **P < 0.01, ***P < 0.001. RSR: RedoxSensor™ Red CC-1; CRO: CellROX® Orange Reagent; AA: antimycin A; FC: flow cytometry; EDTA: ethylenediamine tetra acetic acid; MFI: mean fluorescence intensity; PLC: percentage of labeled cells.
For CellROX® Orange FC, the results show a statistically significant decrease in MFI upon the use of antimycin A (Figure 3g), while, on the other hand, both H2O2 and H2O2+ EDTA produced a statistically significant increase (Figure 3h and i). The exact same pattern with the different controls was seen when using PLC (Figure 3j–3l).
With MitoPY, the use of antimycin A had no effect when using MFI (Figure 4a), while a statistically significant increase was seen following the addition of H2O2 and H2O2+ EDTA, (Figure 4b and 4c). Interestingly, the use of antimycin A significantly decreased the percentage of labeled cells (Figure 4d), as did both the addition of H2O2 and H2O2+ EDTA (Figure 4e and 4f). The results using these two controls were similar to those described for RedoxSensor™ Red CC-1.
Figure 4.
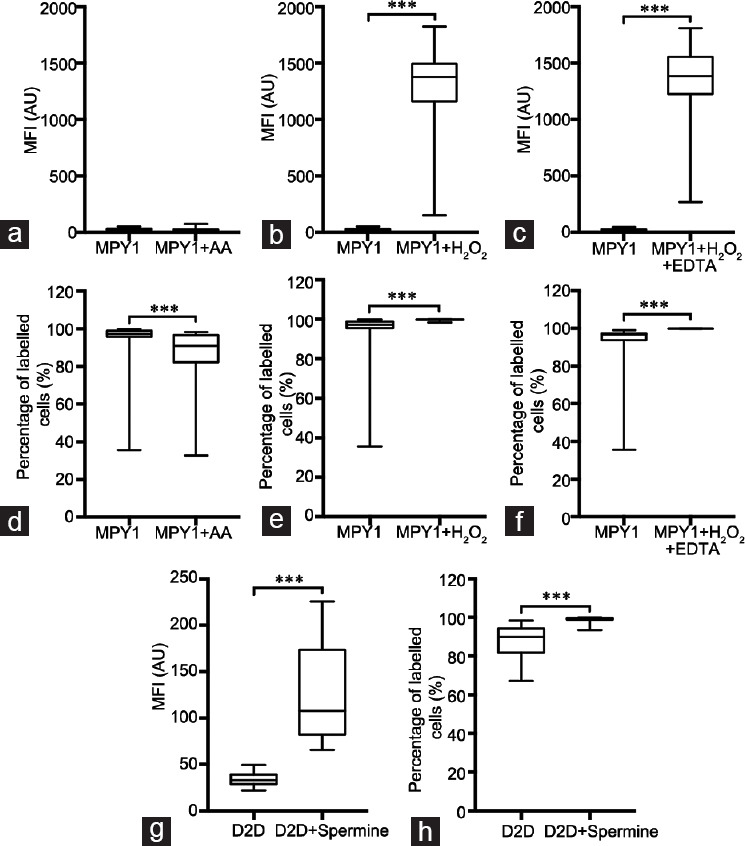
Assessment of MFI and percentage of labeled cells in the sperm populations labeled with MitoPY1 and DAF-2 DA. (a–c) MFI values were obtained through FC for MitoPY1 and compared to the positive controls: (a) MPY1 versus MPY1 + AA (n = 28), (b) MPY1 versus MPY1 + H2O2 (n = 30) and (c) MPY1 versus MPY1 + H2O2 + EDTA (n = 14); (d–f) PLC values were obtained through FC for MitoPY1 and compared to the positive controls: (d) MPY1 versus MPY1 + AA (n = 28), (e) MPY1 versus MPY1 + H2O2 (n = 30) and (f) MitoPY1 versus MPY1 + H2O2 + EDTA (n = 14); (g) MFI and (h) PLC values were obtained through FC for DAF-2 DA and compared to the positive control: (g) D2D versus D2D + Spermine (n = 28), (h) D2D versus D2D + Spermine (n = 28). All data present a non-Gaussian distribution; therefore, the Wilcoxon test was performed. Data are presented as quartiles, Q1, Q2 (median) and Q3. ***P < 0.001. MPY1: MitoPY1; D2D: DAF-2 DA; AA: antimycin A; FC: flow cytometry; EDTA: ethylenediamine tetra acetic acid; MFI: mean fluorescence intensity; PLC: percentage of labeled cells.
Finally, with DAF-2 DA, we could observe an increase in both parameters when adding spermine as a control (Figure 4g and 4h).
DISCUSSION
Although unexpected, the nuclear staining observed when using MitoSOX™ Red (probe specifically targeted toward the mitochondria), has been previously reported, particularly when using antimycin A as a control,20 and may have several speculative explanations. However, other studies with different cell types with a higher cytoplasmic volume and organelle structure that is very different from the specific nature of spermatozoa from different species have demonstrated mitochondrial staining.16,34,35,36 Regardless, through flow cytometric experiments, we can suggest that MitoSOX™ Red is sensitive to both mitochondrial O2•-, which agrees with the literature,20 and with H2O2, which does not.27 While the results might lead us to believe that this probe is also sensitive to cytosolic O2•-, there were no differences observed between the addition of H2O2 (control used to increase the production of H2O2 as well as cytosolic O2•-) versus H2O2+ EDTA (control used to increase the production of H2O2 only), suggesting that such detection is unlikely, although it cannot be ruled out (data not shown).
Similarly, our results suggest that DHE is also sensitive to mitochondrial O2•- and H2O2. While others have stated that this probe is not specific for H2O2, they do acknowledge that it might react with H2O2.27 However, the data do not seem to support the hypothesis that DHE can be used to detect cytosolic O2•- specifically, since there were no statistically significant differences between the use of H2O2 versus H2O2+ EDTA (data not shown). Owing to the characteristics of this probe, namely the lack of the TPP cation, it would be expected that DHE is only sensitive to cytosolic O2•-. A possible explanation to why DHE detects mitochondrial O2•- might be that this ROS can leave the mitochondria through the mitochondrial voltage-dependent anion channels (VDACs).37 At any rate, in our hands, results with MitoSOX™ Red and DHE were basically the same, suggesting that owing to the structure of the male gamete, a specific mitochondrial targeting of this DHE-related probes might be more difficult than with other cell types.
According to the literature, RedoxSensor™ Red CC-1 has been used both for the assessment of the oxidative activity23,38,39 and for the detection of ROS, although of no species in particular.40,41,42,43 From the data obtained, we suggest that RedoxSensor™ Red CC-1 is sensitive to H2O2. Regarding cytosolic O2•-, we have demonstrated that RedoxSensor™ Red CC-1 is not sensitive to it, owing to the lack of statistically significant differences when comparing the addition of H2O2 with that of H2O2+ EDTA (data not shown). For mitochondrial O2•-, the absence of a statistically significant increase in MFI when adding antimycin A (compared with the condition with the probe only) confirms that this probe does not target mitochondrial O2•-.
In the case of CellROX® Orange Reagent, the noticeable increase in signals in the conditions where H2O2 was added and the decrease with the use of antimycin A allow us to conclude that CellROX® Orange Reagent is oxidized by H2O2 and is not specific toward mitochondrial O2•-. Furthermore, despite its localization in the cytosol, our results do not show a specificity toward cytosolic O2•-. Although the literature available is scarce, CellROX® Orange Reagent has been previously used in different cell types from various species for the detection of intracellular ROS44,45,46,47 and was also used in a study performed in adipocytes derived from human stem cells specifically to detect H2O2,24 which supports our results.
MitoPY1 also proved to be sensitive toward H2O2. This confirms the literature available since MitoPY1 has been previously used specifically for the detection of H2O2 in various cell types from different species,25,34,35,48 including mouse spermatozoa26 but never in human spermatozoa. We were also able to conclude that MitoPY1 does not detect mitochondrial O2•-, owing to the lack of response in both parameters upon use of antimycin A, nor cytosolic O2•-, since there were no statistically significant differences between MitoPY1 + H2O2 and MitoPY1 + H2O2+ EDTA (data not shown).
Finally, FC experiments with DAF-2 DA allowed to conclude that it is specific for NO•. This kind of labeling has been previously shown49 in bovine spermatozoa and human gametes.28 The present results corroborate all the reports mentioned, enhancing the conclusion that DAF-2 DA labels the sperm midpiece in the presence of NO•.
CONCLUSIONS
With the present work, we show that MitoSOX™ Red, CellROX® Orange Reagent, DHE, and MitoPY1 can be employed for the detection of ROS with each probe showing distinct specificities toward superoxide (MitoSOX™ Red and DHE), hydrogen peroxide (CellROX® Orange Reagent and MitoPY1), or both (MitoSOX™ Red and DHE) while DAF-2 DA can be used to detect RNS. The use of RedoxSensor™ Red CC-1, CellROX® Orange Reagent, and MitoPY1 in human spermatozoa is reported for the first time. We note that MFI (and not PLC) is the main parameter that can be reproducibly monitored by this type of methodology. In this work, it was, however, impossible in practice to distinguish mitochondrial from cytosolic contributions. Although MitoSOX™ Red and DHE detect H2O2, these probes should not be used only for this specific purpose. All the ROS probes tested, MitoSOX™ Red and MitoPY1 are those we advise to be used for the accurate detection of superoxide and hydrogen peroxide in human spermatozoa, respectively, owing to the clarity of the results when observing/analyzing the cells, whether through flow cytometry (where we can, together with the control conditions, see a clear specificity toward the respective ROS probe) or fluorescence microscopy (we can easily distinguish labeled cells from nonlabeled as well as observe the expected cell region emitting fluorescence).
In future, it would be interesting to correlate the content of ROS/RNS detected by these probes with functional cellular parameters that are known to be affected by oxidative stress (such as capacitation, the acrosome reaction, DNA damage, among others) to infer gamete quality through ROS/RNS content.
AUTHOR CONTRIBUTIONS
JRS, APS, and SER designed the study. SER and FGM performed experiments and data analysis and contributed equally to this work. TAS and APS provided all human sperm samples and performed basic sperm analysis, and AP provided flow cytometer expertise. All authors critically analyzed the data. SER, FGM, APS, and JRS wrote the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
Representative dot plots obtained through FC for the sperm populations labeled with the probes against ROS/RNS. (a) MitoSOX™ Red, (b) DHE, (c) MitoPY1 and (d) DAF-2 DA. FC experiments, were conducted using a BD FACSCalibur (Becton Dickinson; NJ, USA) flow cytometer with an argon laser that performs with an excitation wavelength of 488 nm coupled with the following emission filters: 530/30 band pass (FL-1 channel/green), 585/42 band pass (FL-2 channel/red). The voltage used for each detector was as follows: FSC – 0 V, SSC – 381 V, FL1 – 605 V and FL2 – 510 V. The compensation used was FL1- 0.6% FL2 and FL2 – 45.3% FL1. The obtained data were analyzed using the FlowJo® (FlowJo LLC, Ashland, OR, USA) software, as shown here. FC: flow cytometry; ROS: reactive oxygen species; RNS: reactive nitrogen species; DHE: dihydroethidium.
ACKNOWLEDGMENTS
We would like to thank to all the members of the Biology of Reproduction and Stem cell research group (CNC) for helpful discussions. SER is supported by the Portuguese funding agency for science and technology (PD/BD/128237/2016 – PhD Programme in Experimental Biology and Biomedicine). CNC is funded by FEDER, through Programa Operacional Factores de Competitividade – COMPETE 2020 and National funds via FCT under the project POCI-01-0145-FEDER-007440. This work was also partially funded by the European Regional Development Fund (ERDF), through the Centro 2020 Regional Operational Programme: project CENTRO-01-0145-FEDER-000012-HealthyAging2020, the COMPETE 2020 – Operational Programme for Competitiveness and Internationalisation, and the Portuguese national funds via FCT – Foundation for Science and Technology I.P.: project POCI-01-0145-FEDER-007440 and UID/NEU/04539/2019.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Jarow JP, Sharlip ID, Belker AM, Lipshultz LI, Sigman M, et al. Best practice policies for male infertility. J Urol. 2002;167:2138–44. [PubMed] [Google Scholar]
- 2.Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, et al. Population study of causes, treatment, and outcome of infertility. Br Med J (Clin Res Ed) 1985;291:1693–7. doi: 10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Heal. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goossens E, Tournaye H. Spermatogenesis. In: De Jonge CJ, Barratt CL, editors. The Sperm Cell: Production, Maturation, Fertilization, Regeneration. Cambridge: Cambridge University Press; 2017. p. 1. [Google Scholar]
- 5.Buzadzic B, Vucetic M, Jankovic A, Stancic A, Korac A, et al. New insights into male (in)fertility: the importance of NO. Br J Pharmacol. 2015;172:1455–67. doi: 10.1111/bph.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.du Plessis SS, Hagenaar K, Lampiao F. The in vitro effects of melatonin on human sperm function and its scavenging activities on NO and ROS. Andrologia. 2010;42:112–6. doi: 10.1111/j.1439-0272.2009.00964.x. [DOI] [PubMed] [Google Scholar]
- 7.du Plessis SS, McAllister DA, Luu A, Savia J, Agarwal A, et al. Effects of H2O2 exposure on human sperm motility parameters, reactive oxygen species levels and nitric oxide levels. Andrologia. 2010;42:206–10. doi: 10.1111/j.1439-0272.2009.00980.x. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez JG, Touchstone JC, Blasco L, Storey BT. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl. 1987;8:338–48. doi: 10.1002/j.1939-4640.1987.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 9.Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil. 1987;81:459–69. doi: 10.1530/jrf.0.0810459. [DOI] [PubMed] [Google Scholar]
- 10.Henkel R, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, et al. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil Steril. 2004;81:965–72. doi: 10.1016/j.fertnstert.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 11.Ozmen B, Koutlaki N, Youssry M, Diedrich K, Al-Hasani S. DNA damage of human spermatozoa in assisted reproduction: origins, diagnosis, impacts and safety. Reprod Biomed Online. 2007;14:384–95. doi: 10.1016/s1472-6483(10)60883-8. [DOI] [PubMed] [Google Scholar]
- 12.Zorn B, Vidmar G, Meden-Vrtovec H. Seminal reactive oxygen species as predictors of fertilization, embryo quality and pregnancy rates after conventional in vitro fertilization and intracytoplasmic sperm injection. Int J Androl. 2003;26:279–85. doi: 10.1046/j.1365-2605.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 13.Jones R, Mann T, Sherins R. Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil Steril. 1979;31:531–7. doi: 10.1016/s0015-0282(16)43999-3. [DOI] [PubMed] [Google Scholar]
- 14.Aitken RJ, Smith TB, Lord T, Kuczera L, Koppers AJ, et al. On methods for the detection of reactive oxygen species generation by human spermatozoa: analysis of the cellular responses to catechol oestrogen, lipid aldehyde, menadione and arachidonic acid. Andrology. 2013;1:192–205. doi: 10.1111/j.2047-2927.2012.00056.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, et al. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–68. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 16.Mukhopadhyay P, Rajesh M, Hasko G, Hawkins BJ, Madesh M, et al. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc. 2007;2:2295–301. doi: 10.1038/nprot.2007.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amaral S, Redmann K, Sanchez V, Mallidis C, Ramalho-Santos J, et al. UVB irradiation as a tool to assess ROS-induced damage in human spermatozoa. Andrology. 2013;1:707–14. doi: 10.1111/j.2047-2927.2013.00098.x. [DOI] [PubMed] [Google Scholar]
- 18.Marques M, Sousa AP, Paiva A, Almeida-Santos T, Ramalho-Santos J. Low amounts of mitochondrial reactive oxygen species define human sperm quality. Reproduction. 2014;147:817–24. doi: 10.1530/REP-13-0644. [DOI] [PubMed] [Google Scholar]
- 19.Sousa MI, Amaral S, Tavares RS, Paiva C, Ramalho-Santos J. Concentration-dependent Sildenafil citrate (Viagra) effects on ROS production, energy status, and human sperm function. Syst Biol Reprod Med. 2014;60:72–9. doi: 10.3109/19396368.2013.867380. [DOI] [PubMed] [Google Scholar]
- 20.Koppers AJ, De Iuliis GN, Finnie JM, McLaughlin EA, Aitken RJ. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocrinol Metab. 2008;93:3199–207. doi: 10.1210/jc.2007-2616. [DOI] [PubMed] [Google Scholar]
- 21.Musset B, Clark RA, DeCoursey TE, Petheo GL, Geiszt M, et al. NOX5 in human spermatozoa: expression, function, and regulation. J Biol Chem. 2012;287:9376–88. doi: 10.1074/jbc.M111.314955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Iuliis GN, Wingate JK, Koppers AJ, Mclaughlin EA, Aitken RJ. Definitive evidence for the nonmitochondrial production of superoxide anion by human spermatozoa. J Clin Endocrinol Metab. 2006;91:1968–75. doi: 10.1210/jc.2005-2711. [DOI] [PubMed] [Google Scholar]
- 23.Morado S, Cetica P, Beconi M, Thompson JG, Dalvit G. Reactive oxygen species production and redox state in parthenogenetic and sperm-mediated bovine oocyte activation. Reproduction. 2013;145:471–8. doi: 10.1530/REP-13-0017. [DOI] [PubMed] [Google Scholar]
- 24.Kang T, Lu W, Xu W, Anderson L, Bacanamwo M, et al. MicroRNA-27 (miR-27) targets prohibitin and impairs adipocyte differentiation and mitochondrial function in human adipose-derived stem cells. J Biol Chem. 2013;288:34394–402. doi: 10.1074/jbc.M113.514372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickinson BC, Lin VS, Chang CJ. Preparation and use of MitoPY1 for imaging hydrogen peroxide in mitochondria of live cells. Nat Protoc. 2013;8:1249–59. doi: 10.1038/nprot.2013.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray JE, Starmer J, Lin VS, Dickinson BC, Magnuson T. Mitochondrial hydrogen peroxide and defective cholesterol efflux prevent in vitro fertilization by cryopreserved inbred mouse sperm. Biol Reprod. 2013;89:17, 1–12. doi: 10.1095/biolreprod.113.109157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purdey MS, Connaughton HS, Whiting S, Schartner EP, Monro TM, et al. Boronate probes for the detection of hydrogen peroxide release from human spermatozoa. Free Radic Biol Med. 2015;81:69–76. doi: 10.1016/j.freeradbiomed.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Roessner C, Paasch U, Glander HJ, Grunewald S. Activity of nitric oxide synthase in mature and immature human spermatozoa. Andrologia. 2010;42:132–7. doi: 10.1111/j.1439-0272.2009.01005.x. [DOI] [PubMed] [Google Scholar]
- 29.Amaral A, Paiva C, Baptista M, Sousa AP, Ramalho-Santos J. Exogenous glucose improves long-standing human sperm motility, viability, and mitochondrial function. Fertil Steril. 2011;96:848–50. doi: 10.1016/j.fertnstert.2011.07.1091. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization; 2010. p. 1. [Google Scholar]
- 31.Sousa AP, Amaral A, Baptista M, Tavares R, Caballero Campo P, et al. Not all sperm are equal: functional mitochondria characterize a subpopulation of human sperm with better fertilization potential. PLoS One. 2011;6:e18112. doi: 10.1371/journal.pone.0018112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Champlin DT, Truman JW. Ecdysteroid coordinates optic lobe neurogenesis via a nitric oxide signaling pathway. Development. 2000;127:3543–51. doi: 10.1242/dev.127.16.3543. [DOI] [PubMed] [Google Scholar]
- 33.Ortiz P, Stoos BA, Hong NJ, Boesch DM, Plato CF, et al. High-salt diet increases sensitivity to NO and eNOS expression but not NO production in THALs. Hypertension. 2003;41:682–7. doi: 10.1161/01.HYP.0000047872.07864.20. [DOI] [PubMed] [Google Scholar]
- 34.Adesina SE, Kang BY, Bijli KM, Ma J, Cheng J, et al. Targeting mitochondrial reactive oxygen species to modulate hypoxia-induced pulmonary hypertension. Free Radic Biol Med. 2015;87:36–47. doi: 10.1016/j.freeradbiomed.2015.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boal F, Timotin A, Roumegoux J, Alfarano C, Calise D, et al. Apelin-13 administration protects against ischaemia/reperfusion-mediated apoptosis through the FoxO1 pathway in high-fat diet-induced obesity. Br J Pharmacol. 2016;173:1850–63. doi: 10.1111/bph.13485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A. 2006;103:15038–43. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fogg VC, Lanning NJ, Mackeigan JP. Mitochondria in cancer: at the crossroads of life and death. Chin J Cancer. 2011;30:526–39. doi: 10.5732/cjc.011.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma PK, Dwarakanath BS, Varshney R. Radiosensitization by 2-deoxy-D-glucose and 6-aminonicotinamide involves activation of redox sensitive ASK1-JNK/p38MAPK signaling in head and neck cancer cells. Free Radic Biol Med. 2012;53:1500–13. doi: 10.1016/j.freeradbiomed.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Tellado M, Alvarez G, Dalvit G, Cetica P. The conditions of ovary storage affect the quality of porcine oocytes. Adv Reprod Sci. 2014;2:57–67. [Google Scholar]
- 40.Camello-Almaraz MC, Pozo MJ, Murphy MP, Camello PJ. Mitochondrial production of oxidants is necessary for physiological calcium oscillations. J Cell Physiol. 2006;206:487–94. doi: 10.1002/jcp.20498. [DOI] [PubMed] [Google Scholar]
- 41.Haigh CL, Brown DR. Prion protein reduces both oxidative and non-oxidative copper toxicity. J Neurochem. 2006;98:677–89. doi: 10.1111/j.1471-4159.2006.03906.x. [DOI] [PubMed] [Google Scholar]
- 42.Lopes AS, Lane M, Thompson JG. Oxygen consumption and ROS production are increased at the time of fertilization and cell cleavage in bovine zygotes. Hum Reprod. 2010;25:2762–73. doi: 10.1093/humrep/deq221. [DOI] [PubMed] [Google Scholar]
- 43.Wakefield SL, Lane M, Schulz SJ, Hebart ML, Thompson JG, et al. Maternal supply of omega-3 polyunsaturated fatty acids alter mechanisms involved in oocyte and early embryo development in the mouse. Am J Physiol Endocrinol Metab. 2008;294:E425–34. doi: 10.1152/ajpendo.00409.2007. [DOI] [PubMed] [Google Scholar]
- 44.Criscuolo C, Procaccini C, Meschini MC, Cianflone A, Carbone R, et al. Powerhouse failure and oxidative damage in autosomal recessive spastic ataxia of Charlevoix-Saguenay. J Neurol. 2015;262:2755–63. doi: 10.1007/s00415-015-7911-4. [DOI] [PubMed] [Google Scholar]
- 45.Eid AA, Gosier JL, Primus CM, Hammond BD, Susin LF, et al. In vitro biocompatibility and oxidative stress profiles of different hydraulic calcium silicate cements. J Endod. 2014;40:255–60. doi: 10.1016/j.joen.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fucikova J, Moserova I, Truxova I, Hermanova I, Vancurova I, et al. High hydrostatic pressure induces immunogenic cell death in human tumor cells. Int J Cancer. 2014;135:1165–77. doi: 10.1002/ijc.28766. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez-Sanchez E, Perez MJ, Nytofte NS, Briz O, Monte MJ, et al. Protective role of biliverdin against bile acid-induced oxidative stress in liver cells. Free Radic Biol Med. 2016;97:466–77. doi: 10.1016/j.freeradbiomed.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 48.Guo H, Aleyasin H, Howard SS, Dickinson BC, Lin VS, et al. Two-photon fluorescence imaging of intracellular hydrogen peroxide with chemoselective fluorescent probes. J Biomed Opt. 2013;18:106002–17. doi: 10.1117/1.JBO.18.10.106002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reyes R, Vázquez ML, Delgado NM. Detection and bioimaging of nitric oxide in bovine oocytes and sperm cells. Syst Biol Reprod Med. 2004;50:303–9. doi: 10.1080/01485010490448471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative dot plots obtained through FC for the sperm populations labeled with the probes against ROS/RNS. (a) MitoSOX™ Red, (b) DHE, (c) MitoPY1 and (d) DAF-2 DA. FC experiments, were conducted using a BD FACSCalibur (Becton Dickinson; NJ, USA) flow cytometer with an argon laser that performs with an excitation wavelength of 488 nm coupled with the following emission filters: 530/30 band pass (FL-1 channel/green), 585/42 band pass (FL-2 channel/red). The voltage used for each detector was as follows: FSC – 0 V, SSC – 381 V, FL1 – 605 V and FL2 – 510 V. The compensation used was FL1- 0.6% FL2 and FL2 – 45.3% FL1. The obtained data were analyzed using the FlowJo® (FlowJo LLC, Ashland, OR, USA) software, as shown here. FC: flow cytometry; ROS: reactive oxygen species; RNS: reactive nitrogen species; DHE: dihydroethidium.