Abstract
The aim of this study was to investigate the role of seminal plasma miR-210-3p in the impairment of semen quality caused by varicocele. This study included 102 patients whose semen quality was normal when they were diagnosed with varicocele. A 2-year follow-up for included patients was performed, and they were divided into Group A (semen quality became abnormal) and Group B (semen quality remained normal) according to the results of semen analysis during the follow-up. Semen parameters and seminal plasma miR-210-3p expression were investigated by semen analysis and quantitative real-time polymerase chain reaction, respectively. In vitro experiments with GC-2 cells were performed to explore the role of miR-210-3p in spermatogenic cells. The results of quantitative real-time polymerase chain reaction showed that the level of seminal plasma miR-210-3p in Group A was higher than that in Group B both after 2-year follow-up and when they were diagnosed with varicocele (both P < 0.01). Apoptosis and proliferation assays showed that miR-210-3p induces apoptosis of spermatogenic cells by promoting caspase-3 activation. In conclusion, our study indicated that seminal plasma miR-210-3p induces spermatogenic cell apoptosis by activating caspase-3 in patients with varicocele. Seminal plasma miR-210-3p may be a potential biomarker for predicting impaired semen quality caused by varicocele.
Keywords: biomarker, cleaved caspase-3, miR-210-3p, semen quality, varicocele
INTRODUCTION
Varicocele is defined as a collection of abnormally dilated and tortuous spermatic veins in the pampiniform plexus. In all adult males, the prevalence is approximately 15%.1 It is 35% in men with primary infertility and as much as 81% in men with secondary infertility.2 Previous studies have shown that varicocele has a negative impact on semen quality, sperm function, testicular histology, and reproductive hormones.3,4,5,6 Varicocele is widely considered to be the most common correctable cause of male infertility.7,8
It is obviously irrational if all patients with varicocele are recommended for invasive treatment in order to protect their fertility unless there is evidence of varicocele affecting testicular development or semen quality.9 At present, the evaluation of testicular development and semen quality in clinical practice mainly depends on measuring testicular volume and semen analysis. However, 11.7% of varicocele patients who were excluded from other causes of male infertility were completely normal in testicular volume and semen quality.10 In other words, existing clinical examination methods do not provide accurate information for developing optimal treatment timing. Therefore, it is very necessary to find biomarkers that can effectively predict testicular development or semen quality damage caused by varicocele.
MicroRNAs (miRNAs) are a class of small noncoding RNAs, approximately 19–23 nucleotides in length that regulate gene expression by inhibiting translation and reducing the stability of their target mRNAs.11 By affecting protein translation, miRNAs have been recognized as powerful regulators of key cellular processes such as proliferation, differentiation, and apoptosis.12 miRNAs play a vital role in the pathogenesis of many diseases, including various cancers and cardiovascular diseases.13,14,15 Recently, some studies have shown that biological fluids such as blood, semen, saliva, and vaginal secretions contain specific miRNAs.16,17,18 In addition, plasma and seminal plasma miRNAs are found to be very stable even under harsh conditions, such as boiling and high pH,19 making peripheral circulating miRNAs a potential new source of noninvasive biomarkers for cancer and other diseases.13,15,20
Studies have shown that the hypoxic environment in spermatogenic tissue caused by blood stasis in the testis is one of the most important causes of male infertility caused by varicocele.21,22 The stable expression of miR-210-3p in human hypoxic tissue can directly reflect the hypoxia of tissues and is an early biomarker for many tumor and nontumor diseases.13,14
In the present study, the aim was to evaluate the role of seminal plasma miR-210-3p in the impairment of semen quality caused by varicocele.
PARTICIPANTS AND METHODS
Study design and participants
The study was conducted from March 2016 to November 2018. Potentially eligible participants in this study were recruited from Tianjin Medical University General Hospital and the Second Hospital of Tianjin Medical University (Tianjin, China) from March 2016 to November 2016. They had to meet the following inclusion criteria: (1) clinical varicocele, (2) fertility needs, (3) normal ejaculation, (4) normal testicular volume and texture, and (5) normal semen quality. In order to eliminate interference from other factors that may affect the quality of semen, we had established the following exclusion criteria: (1) hormone disorder; (2) orchitis, epididymitis, or prostatitis; (3) testicular tumor, surgery, or trauma; (4) alcoholism or substance abuse; (5) occupational/environmental exposure; and (6) severe concomitant diseases such as liver failure and uremia. Given the lack of evidence that the testis or semen quality was impaired by varicocele when diagnosed with varicocele, these patients were advised to be followed up regularly. The purpose of follow-up was to detect early damage to testicular and semen quality. The follow-up interval was 6 months, and the follow-up included physical examination, testicular evaluation, and semen quality assessment. We conducted a 2-year follow-up of all eligible patients. Patients with impaired semen quality (any one of normal sperm morphology, sperm concentration, and sperm motility was abnormal) during follow-up were divided into Group A (n = 30), and all patients whose semen quality remained normal were Group B (n = 72). The control Group (n = 30) consisted of healthy adult males who had a need for semen analysis. They had no special medical history, and the results of semen analysis were completely normal.
All participants involved in our study joined us voluntarily, and informed consent was obtained from each of them. The study protocol was approved by the Internal Research Ethics Committee of the Tianjin Medical University General Hospital.
Semen analysis
Semen samples were obtained in a private room by masturbation into a sterile glass vessel after 2–7 days of sexual abstinence and were allowed to liquefy for 30 min at 37°C. Routine semen analysis was performed by a computer-assisted semen analysis system (WLJY-9000; Beijing Weili New Century Science and Technology Development Co., Ltd., Beijing, China) according to the World Health Organization guidelines (1999).23 Evaluated semen parameters included semen volume, semen pH, sperm concentration, sperm motility, and proportion of normal sperm morphology. For all samples, at least 200 spermatozoa were analyzed to assess sperm morphology and concentration. Each semen sample was analyzed twice in succession, and the average was used as the final result. In order to increase the credibility of the semen analysis results, all processes were completed by two experienced laboratory researchers. During the research, internal quality control was performed to ensure that there was no significant difference between the results of the technicians.
Seminal plasma collection
The seminal plasma was obtained by centrifuging (1500 g for 10 min and then 12 000 g for 5 min) at 4°C within 2 h after sampling as described in a previous study.24 The supernatant (seminal plasma) obtained by the second centrifugation was carefully collected and immediately stored at −80°C until used for total RNA isolation.
Total RNA isolation
Total RNA isolation was performed as described in a previous study.25 The total RNA was isolated from 1 ml seminal plasma with the equal volume of TRIzol Reagent (Aidlab Biotechnologies Co., Ltd., Beijing, China) according to the manufacturer's protocol. Three steps of phenol/chloroform purification were performed to remove proteins and ensure that pure seminal plasma total RNA was collected. The purity of the total RNA was assessed using a Nano-Drop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) at 260 nm and 280 nm, and its concentration was checked using the optical density (OD)260.
Quantification of miR-210-3p
miR-210-3p was quantified as previously described with minor modification.25 RNA was reverse transcribed in a final volume of 20 μl containing 5 μl template RNA, 2 μl antisense loop primer (1 μmol l−1), 4 μl dNTPs, 4 μl 5× Hiscript Buffer, 1 μl Hiscript Reverse Transcriptase, 0.5 μl Ribonuclease Inhibitor, and 3.5 μl RNase-free water according to the manufacturer's instructions. The mix was incubated at 25°C for 5 min, 50°C for 15 min, 85°C for 5 min, and 4°C for 10 min. Subsequently, quantitative real-time polymerase chain reaction (qRT-PCR) was performed using an Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems, Waltham, MA, USA).
Each qRT-PCR (in 20 μl) included 4 μl reverse transcription product (1:10 dilution), 0.4 μl forward primer (0.2 μmol l−1), 0.4 μl reverse primer (0.2 μmol l−1), 10 μl SYBR Green Master Mix, 0.4 μl 50× ROX Reference Dye 2, and 4.8 μl RNase-free water. The reactions were incubated in a 96-well optical plate at 50°C for 2 min, 95°C for 10 min, and then subjected to 40 cycles at 95°C for 30 s and 60°C for 30 s. A melting curve was generated at the end of each run to ensure product uniformity. All reactions were run in triplicate, and the mean quantification cycle (Cq) was determined from the triplicate qRT-PCRs. U6 snRNA was chosen as the endogenous control gene to normalize the miR-210-3p content among different samples.26,27 The expression of miR-210-3p relative to U6 snRNA was determined by the 2−ΔCt method. The sequence of the primers is shown in Supplementary Table 1.
Supplementary Table 1.
Sequence of primers
| Name | Primer | Sequence |
|---|---|---|
| miR-210-3p | Forward | 5‘- TGCGCCTGTGCGTGTGACAGCG-3’ |
| Reverse | 5‘- CCAGTGCAGGGTCCGAGGTATT -3’ | |
| Loop | 5‘- GTCGTATCCAGTGCAGGGTCCGAGGT ATTCGCACTGGATACGAC TCAGCCGC-3’ | |
| U6 | Forward | 5‘- CGCTTCGGCAGCACATATAC-3’ |
| Reverse | 5‘- AAATATGGAACGCTTCACGA-3’ |
Cell proliferation and apoptosis analysis
GC-2 cell line is a mouse spermatocyte-derived cell line widely used for in vitro experiments.28,29 GC-2 cells were cultured in Dulbecco's modified Eagle's medium (Thermo Fisher Scientific) supplemented with 10% (v/v) fetal bovine serum (Thermo Fisher Scientific) and maintained at 37°C in a humidified atmosphere with 5% (v/v) CO2. The prepared cells were transfected with miR-210-3p mimics, miR-210-3p mimics negative control (NC), miR-210-3p inhibitor or miR-210-3p inhibitor NC. They were seeded into a 96-well plate with 1 × 104 cells per well. We used the qRT-PCR assays to assess the expression of miR-21-3p in GC-2 cells (1 × 106) of each group 24 h after transfection according to the above methods. After 120 h of culture, the proliferation potential of GC-2 cells was assessed by cell counting kit-8 (CCK-8) assay (No. BS350B; Biosharp, Beijing, China) according to the manufacturer's instructions. To investigate the role of miR-210-3p in apoptosis, GC-2 cells (1 × 106) were homogenized in lysis buffer (Beyotime, Shanghai, China) containing protease inhibitors. The eluate was centrifuged at 16 000 g for 10 min at 4°C, and 1× loading buffer was added after removing the supernatant. Protein concentration was determined by BCA protein concentration assay kit (No. P0010; Beyotime) according to the manufacturer's instructions. Twenty micrograms of protein was loaded onto each gel and electrotransferred onto a polyvinylidene fluoride membrane by standard procedures.30 Caspase-3 was detected using a rabbit anti-caspase-3 polyclonal antibody (1:600; Proteintech Group, Wuhan, China), followed by a mouse anti-rabbit secondary antibody (1:50 000; Boster Biological Technology, Wuhan, China). Horseradish peroxidase was used to label the secondary antibody, and the enhanced chemiluminescent substrate solution (Bioscience, Shanghai, China) was used for visualization. The band intensity was quantified relative to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH; AB-P-R 001; Xianzhi Biological, Hangzhou, China) by ImageQuant TL 7.0 software (GE Healthcare, Little Chalfont, UK).
Statistical analyses
All quantitative data were expressed as mean ± standard deviation (s.d.), and the qualitative variables were described as counts and percentages. Chi-squared and t-tests were used to compare qualitative variables and independent data, respectively. The differences in semen parameters between Group A and Group B were evaluated by Kruskal–Wallis test. Mann–Whitney U test, Fisher's test, and analysis of variance (ANOVA) were used to analyze the expression level of seminal plasma miR-210-3p and the difference in the affected side of varicocele between groups. ANOVA was used to compare the difference in optical density value between groups at five time points (24 h, 48 h, 72 h, 96 h, and 120 h). At each time point, the optical density value of each group was compared with that of the other four groups. All statistical tests were two-sided, and P < 0.05 was considered statistically significant. Statistical analysis was performed by SPSS software version 20.0 (IBM SPSS Inc., Chicago, IL, USA).
RESULTS
Characteristics of the patients
A total of 157 potentially eligible participants were recruited in this study, of whom 55 patients were excluded because of prostatitis (n = 25), alcoholism or substance abuse (n = 7), severe chronic disease (n = 2), occupational/environmental exposure (n = 5), hormone disorder (n = 3), testicular trauma (n = 1), or did not complete all scheduled study procedures (n = 12). The remaining 102 patients (n = 41 from Tianjin Medical University General Hospital and n = 61 from the Second Hospital of Tianjin Medical University) were included in the final analysis. All baseline characteristics were similar between Group A and Group B (all P > 0.05; Table 1). The main semen parameters are presented in Supplementary Table 2.
Table 1.
Baseline characteristics of the patients
| Variables | Group A (n=30) | Group B (n=72) | P |
|---|---|---|---|
| Physical appearance | |||
| Age (year), mean±s.d. | 27.1±5.0 | 26.5±4.6 | 0.61a |
| BMI (kg m−2), mean±s.d. | 22.8±1.8 | 22.5±2.5 | 0.09a |
| Lifestyle | |||
| Smoking, n (%) | 15 (50.0) | 34 (47.2) | 0.80b |
| Alcohol consumption, n (%) | 18 (60.0) | 42 (58.3) | 0.88b |
| Ejaculation abstinence (day), mean±s.d. | 4.39±1.7 | 4.15±2.2 | 0.77a |
| Grade of varicocele (n) | |||
| 1 | 4 | 16 | 0.55b |
| 2 | 6 | 15 | |
| 3 | 20 | 41 | |
| Side of varicocele (n) | |||
| Unilateral left | 24 | 56 | 0.36b |
| Unilateral right | 5 | 16 | |
| Bilateral | 1 | 0 |
The differences in variables between Group A and Group B were evaluated by at-test model and bChi-squared model. Group A: semen quality became abnormal; Group B: semen quality remained normal. BMI: body mass index; s.d.: standard deviation
Supplementary Table 2.
The main semen parameters
| Semen parameters | When varicocele was diagnosed | After 2-year follow-up | ||||
|---|---|---|---|---|---|---|
| Group A (n=30) | Group B (n=72) | P | Group A (n=30) | Group B (n=72) | P | |
| pH | 7.5±0.1 | 7.6±0.1 | 0.77 | 7.4±0.1 | 7.5±0.2 | 0.78 |
| Semen volume (ml) | 2.9±1.1 | 2.8±1.3 | 0.68 | 3.0±1.0 | 2.9±1.2 | 0.45 |
| Normal sperm morphology (%) | 56.3±15.3 | 58.8±19.1 | 0.11 | 21.8±12.3 | 38.2±17.6 | 0.02 |
| Sperm concentration (106/ml) | 71.0±36.6 | 73.3±37.4 | 0.23 | 38.4±32.2 | 59.6±36.2 | <0.01 |
| Sperm motility (PR + NP) (%) | 57.7±16.5 | 57.9±18.7 | 0.18 | 21.9±11.6 | 43.6±13.1 | <0.001 |
The differences in semen parameters between Group A and Group B were evaluated by Kruskal–Wallis test. s.d.: standard deviation; PR: percentages of progressive; NP: nonprogressive; the values of semen parameters were presented by mean±s.d.
Levels of seminal plasma miR-210-3p in Group A and Group B
To determine whether the level of seminal plasma miR-210-3p was associated with impaired semen quality, we compared the difference in seminal plasma miR-210-3p between Group A (semen quality became abnormal), Group B (semen quality remained normal), and the control group. The qRT-PCR results showed that the expression of seminal plasma miR-210-3p in Group A was almost twice as high as that in Group B (P < 0.01), and both Group A and Group B values were higher than those in the control group (both P < 0.01; Figure 1b) after 2-year follow-up. Further, we compared the differences in seminal plasma miR-210-3p levels between the three groups at the time that varicocele was diagnosed.
Figure 1.
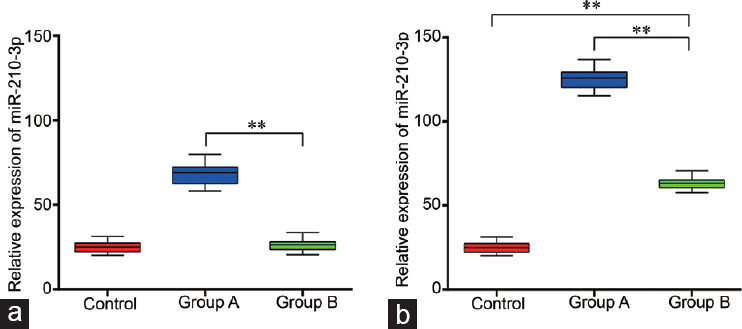
The expression of seminal plasma miR-210-3p, (a) when varicocele was diagnosed, and (b) after 2-year follow-up. Group A: 30 patients with impaired semen quality during follow-up; Group B: 72 patients whose semen quality remained normal; Control group: 30 healthy adult males. Statistical test: one-way analysis of variance, **P < 0.01; the box is the 25th–75th centiles, the line is the median and the whiskers is the 5th–95th centiles.
Our results showed that the expression of seminal plasma miR-210-3p in Group A was higher than that in Group B (2.6 times, P < 0.01), but it was very similar between control group and Group B (P = 0.11; Figure 1a).
Stability of seminal plasma miR-210-3p expression
To observe the variation in seminal plasma miR-210-3p among varicocele patients (n = 6) and healthy males (n = 6), we collected the semen specimens on the 1st, 8th, and 15th days of 1 month from 12 individuals and quantified the relative expression levels of seminal plasma miR-210-3p by qRT-PCR as previously described.20,24,31 As with our previous study,32 there was no significant difference in the relative expression levels of seminal plasma miR-210-3p among the three samples (P = 0.12 for varicocele and P = 0.09 for control; Figure 2).
Figure 2.
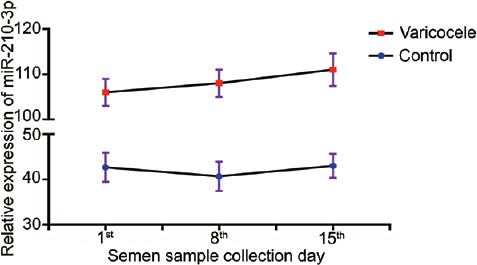
Fluctuations of seminal plasma miR-210-3p level in patients with varicocele (n = 6) and healthy control (n = 6). Statistical test: one-way analysis of variance; results are reported as mean ± standard deviation, and all results are representative of three independent experiments.
Our results indicated that the level of seminal plasma miR-210-3p was stable in both varicocele patients and healthy males and had the trait of becoming a biomarker.
miR-210 induces apoptosis of germ cells by promoting caspase-3 activation
To investigate the role of miR-210-3p in the apoptosis and proliferation, we transfected miR-210-3p mimics and inhibitors into GC-2 cells. The transfection with miR-210-3p mimics and inhibitors resulted in a statistically significant change of miR-210-3p expression compared with controls (P < 0.01; Figure 3). CCK8 results showed that the number of cells in the sample treated with miR-210-3p mimics was lower than that in other treatment groups or the control (P < 0.05; Figure 4), and the number of cells in the sample treated with miR-210-3p inhibitor was the highest among the control and all treatment groups (P < 0.05) at the five time points, while there was no significant difference (P > 0.05) between the other three groups (group mimics NC, group inhibitor NC, and group control) at the five time points. Western blot was used to quantify the detectable amount of caspase-3 proteins. The results showed that cleaved caspase-3 was increased in the miR-210-3p mimics group compared with the other groups (P < 0.01), and the miR-210-3p inhibitor group was the lowest in all groups (P < 0.01; Figure 5). There was no significant difference between the detectable amount of the other three groups (group control, group mimics NC, and group inhibitor NC; P > 0.05). There was also no significant difference in the amount of the five groups (group control, miR-210-3p mimics, mimics NC, miR-210-3p inhibitor, and inhibitor NC) of pro-caspase-3 (P > 0.05).
Figure 3.
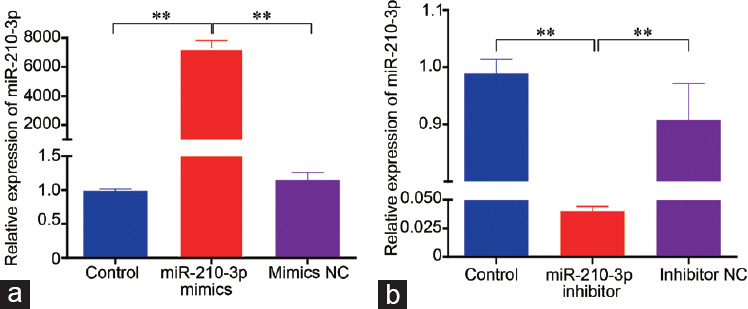
Transfection efficiency of miR-210-3p mimics and inhibitor in GC-2 cells. (a) miR-210-3p mimics. (b) miR-210-3p inhibitor. Time-point: 24 h after transfection; number of cells: 1 × 106; NC: negative control; statistical test: one-way analysis of variance, **P < 0.01; results are reported as mean ± standard deviation and all results are representative of three independent experiments, n = 3 per group.
Figure 4.
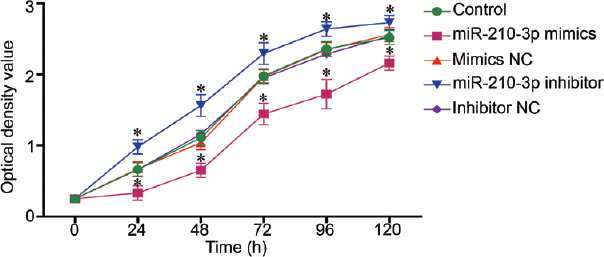
Cell proliferation was measured by cell counting kit-8 assay. Optical density: 450 nm; NC: negative control; statistical test: one-way analysis of variance, *P < 0.05 (significant differences at 5 time points: 24 h, 48 h, 72 h, 96 h, and 120 h), the indicated group versus control group; results were reported as mean ± standard deviation, and all results are representative of three independent experiments.
Figure 5.
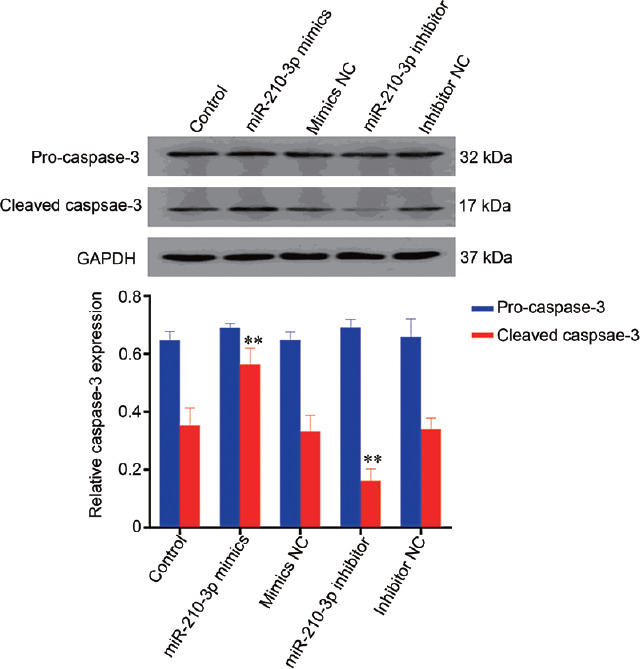
Western blot analysis of caspase-3 proteins at 96 h after cell transfection. NC: negative control; statistical test: one-way analysis of variance, **P < 0.01; results are reported as mean ± standard deviation and all results are representative of three independent experiments, n = 3 per group. GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
DISCUSSION
Early screening for impaired spermatogenic function in patients with varicocele plays a key role in protecting their fertility.33 At present, assessment of varicocele patients is based on medical history, physical examination, Doppler ultrasound, and semen analysis. However, none of these means can directly and effectively evaluate the spermatogenic status of patients with varicocele. Our results indicated that seminal plasma miR-210-3p was increased in varicocele patients with impaired semen quality (P < 0.01), which helps develop optimal treatment timing.
In previous studies, the stability of seminal plasma miRNAs as a biomarker has been widely discussed.20,24,28,34 Wang et al.24 evaluated the stability of miRNA (miR-9, miR-106b, and miR-202) expression by repeated freezing and thawing of seminal plasma and incubation at room temperature and 4°C for different durations. They found that these miRNAs are sufficiently stable in seminal plasma. Wu et al.,20 who analyzed the expression levels of seminal plasma miRNAs (miR-19b and let-7a) on the 1st, 8th, and 15th days of 1 month from 6 subjects by qPCR, concluded that the seminal plasma miRNAs were stable and their expression levels were consistent in individuals. Consistent with previous studies, our study confirmed that seminal plasma miR-210-3p was fairly stable both in varicocele patients and healthy males. The stability of expression made miR-210-3p one of the characteristics of an ideal biomarker.
A recent study confirmed that seminal plasma miR-192a can be used to predict the spermatogenic status of patients with nonobstructive azoospermia after varicocele repair. Zhi et al.28 found that the expression level of seminal plasma miR-192a in patients who successfully ejaculated spermatozoa after varicocele repair was higher than that in patients who unsuccessfully ejaculated spermatozoa (P < 0.001). In another recent study, Barcelo et al.34 used differential high-throughput miRNA profiling techniques for miRNA quantitative PCR plates to analyze differences in seminal plasma exosomes in azoospermic and fertile individuals. Their findings have revealed altered levels of miRNAs in seminal plasma exosomes that may be involved in the process of azoospermia origin and testicular spermatogenesis. Previous studies have confirmed the value of miRNAs as biomarkers for evaluating spermatogenic status from different perspectives. However, information on whether miRNAs can be used as a potential biomarker to evaluate early impaired semen quality in patients with varicocele is still lacking. Our study confirms that miR-210-3p can predict impaired semen quality in patients with varicocele.
Blood stasis in spermatic cord leads to hypoxia of spermatogenic tissue is one of the more important pathophysiological mechanisms of varicocele-induced male infertility.35 Spermatogenic hypoxia is thought to be due to the venous pressure of the internal spermatic vein exceeding the testicular arteriolar pressure, which adversely affects testicular function through the hypoxia-inducible factor (HIF) pathway. In addition, increased hydrostatic pressure causes reflux of adrenal and renal metabolites into the internal spermatic vein, which then enter the testes causing contraction of the testicular arterioles, resulting in hypoxia and impaired spermatogenesis.36 Hypoxia-inducible factor 1 alpha (HIF-1α) is a unique marker of tissue hypoxia and is stably expressed in hypoxic tissues.37 Studies have confirmed a significant increase in the expression of HIF-1α in the testis and epididymis of patients with varicocele.38 HIF-1α expression is increased in hypoxia and binds to the 400 bp hypoxia response element upstream of the promoter of miR-210-3p, thereby promoting the expression of miR-210-3p. At the same time, miR-210-3p inhibits the expression of HIF-1α hydrolase and increases the level of HIF-1α, forming a positive feedback between the two.39,40 Caspase is a family of cysteine proteases that specifically cleaves aspartate and play an important role in the process of cell apoptosis.41 Our results indicate that the upregulation of miR-210-3p does not affect the detectable amount of the caspase-3 but its activation (maturation). Thus, we speculate that miR-210-3p upregulation induces spermatogenic cell apoptosis by promoting caspase-3 activation.
However, the relationship between miR-210 upregulation and apoptosis seems to be still somewhat controversial. Many previous studies have supported the upregulation of miR-210 to induce apoptosis.42,43,44,45 Lv et al.42 aimed to explore the underlying mechanisms of hypoxia-induced miR-210 effects on mouse GC-2 cells. The results indicated that hypoxia-induced miR-210 upregulation stimulated the activation of the apoptosis signaling pathway and contributed to the apoptosis of GC-2 cells by targeting Kruppel-like factor 7. Chen et al.43 used lithium chloride and pilocarpine to induce epileptic activity, aiming to reveal its underlying mechanisms. The authors found that a miR-210 inhibitor inhibited the apoptosis induced by epileptic activity, thereby indirectly revealing that miR-210 upregulation induces apoptosis. On the contrary, some literature has also reported the negative effect of miR-210 upregulation on apoptosis.46,47 Wang et al.46 have conducted a study to investigate the impact of miR-210 on cardiac stem cells against hypoxia-induced injury. They demonstrated that miR-210 upregulation can attenuate the apoptosis of cardiac stem cells under hypoxic condition through regulating its target genes caspase-8-associated protein 2 (Casp8ap2)/Caspase 8 and protein tyrosine phosphatase nonreceptor type 2 (PTPN2).
There are some limitations in our study. First, varicocele is only one of the causes of male infertility, and future studies are needed to evaluate the association between seminal plasma miR-210-3p and more male infertility diseases. Second, it is necessary to combine more useful seminal plasma miRNAs to establish a predictive model for more accurate prediction of the spermatogenic status of patients with varicocele. Third, studies of larger sample sizes and longer follow-up times are needed in the future to confirm the relationship between seminal plasma miR-210-3p and semen quality in patients with varicocele. In addition, it is interesting that Group B has a higher miR-210-3p expression than control group after 2-year follow-up, but not at the beginning of the study. Whether these patients will develop impaired semen quality in the future is impossible to conclude currently. We will further design related studies for this topic for further exploration.
CONCLUSION
In summary, seminal plasma miR-210-3p may induce spermatogenic cell apoptosis by activating caspase-3 in patients with varicocele. Seminal plasma miR-210-3p could be a novel and noninvasive biomarker for predicting impaired semen quality caused by varicocele, which can be used to develop optimal treatment timing. However, a large-scale prospective study is required to confirm this finding.
AUTHOR CONTRIBUTIONS
YWX carried out substantial contributions to conception, experiments, data acquisition, data analysis, data interpretation, drafting the manuscript, and statistical analysis. NJO, YXS, XHW, JQK, and YJY carried out substantial contributions to conception, data analysis, and statistical analysis. XQL and YGC carried out design, supervision, and critical revision of the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This study was supported by Tianjin Natural Science Foundation of China (No. 17JCQNJC11900).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Greenberg SH, Lipshultz Li, Wein AJ. Experience with 425 subfertile male patients. J Urol. 1978;119:507–10. doi: 10.1016/s0022-5347(17)57531-x. [DOI] [PubMed] [Google Scholar]
- 2.Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril. 1993;59:613–6. [PubMed] [Google Scholar]
- 3.Damsgaard J, Joensen UN, Carlsen E, Erenpreiss J, Blomberg Jensen M, et al. Varicocele is associated with impaired semen quality and reproductive hormone levels: a study of 7035 healthy young men from six European countries. Eur Urol. 2016;70:1019–29. doi: 10.1016/j.eururo.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Wang YJ, Zhang RQ, Lin YJ, Zhang RG, Zhang WL, et al. Relationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysis. Reprod Biomed Online. 2012;25:307–14. doi: 10.1016/j.rbmo.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Abdelrahim F, Mostafa A, Hamdy A, Mabrouk M, el-Kholy M, et al. Testicular morphology and function in varicocele patients: pre-operative and post-operative histopathology. Br J Urol. 1993;72:643–7. doi: 10.1111/j.1464-410x.1993.tb16225.x. [DOI] [PubMed] [Google Scholar]
- 6.Mostafa T, Anis TH, Ghazi S, El-Nashar AR, Imam H, et al. Reactive oxygen species and antioxidants relationship in the internal spermatic vein blood of infertile men with varicocele. Asian J Androl. 2006;8:451–4. doi: 10.1111/j.1745-7262.2006.00172.x. [DOI] [PubMed] [Google Scholar]
- 7.Charny CW, Baum S. Varicocele and infertility. JAMA. 1968;204:1165–8. [PubMed] [Google Scholar]
- 8.Gat Y, Bachar GN, Zukerman Z, Belenky A, Gornish M. Varicocele: a bilateral disease. Fertil Steril. 2004;81:424–9. doi: 10.1016/j.fertnstert.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Jungwirth A, Diemer T, Kopa Z, Krausz C, Minhas S, et al. Male Infertility. European Association of Urology Guidelines. 2019. [Last accessed on 2019 Jul 14]. Available from: https://uroweb.org/guideline/male-infertility/#5 .
- 10.World Health Organization. The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. Fertil Steril. 1992;57:1289–93. [PubMed] [Google Scholar]
- 11.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–73. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 12.Pasquinelli AE, Hunter S, Bracht J. MicroRNAs: a developing story. Curr Opin Genet Dev. 2005;15:200–5. doi: 10.1016/j.gde.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Tsang VH, Dwight T, Benn DE, Meyer-Rochow GY, Gill AJ, et al. Overexpression of miR-210 is associated with SDH-related pheochromocytomas, paragangliomas, and gastrointestinal stromal tumours. Endocr Relat Cancer. 2014;21:415–26. doi: 10.1530/ERC-13-0519. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, Zuo J. Emerging roles of miR-210 and other non-coding RNAs in the hypoxic response. Acta Biochim Biophys Sin. 2014;46:220–32. doi: 10.1093/abbs/gmt141. [DOI] [PubMed] [Google Scholar]
- 15.Bittel DC, Kibiryeva N, Marshall JA, O'Brien JE. MicroRNA-421 dysregulation is associated with tetralogy of fallot. Cells. 2014;3:713–23. doi: 10.3390/cells3030713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson EK, Lubenow H, Ballantyne J. Identification of forensically relevant body fluids using a panel of differentially expressed microRNAs. Anal Biochem. 2009;387:303–14. doi: 10.1016/j.ab.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 17.Bandiera S, Hatem E, Lyonnet S, Henrion-Caude A. microRNAs in diseases: from candidate to modifier genes. Clin Genet. 2010;77:306–13. doi: 10.1111/j.1399-0004.2010.01370.x. [DOI] [PubMed] [Google Scholar]
- 18.Belleannee C. Extracellular microRNAs from the epididymis as potential mediators of cell-to-cell communication. Asian J Androl. 2015;17:730–6. doi: 10.4103/1008-682X.155532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–8. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu W, Hu Z, Qin Y, Dong J, Dai J, et al. Seminal plasma microRNAs: potential biomarkers for spermatogenesis status. Mol Hum Reprod. 2012;18:489–97. doi: 10.1093/molehr/gas022. [DOI] [PubMed] [Google Scholar]
- 21.Sheehan MM, Ramasamy R, Lamb DJ. Molecular mechanisms involved in varicocele-associated infertility. J Assist Reprod Genet. 2014;31:521–6. doi: 10.1007/s10815-014-0200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pastuszak AW, Wang R. Varicocele and testicular function. Asian J Androl. 2015;17:659–67. doi: 10.4103/1008-682X.153539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 4th ed. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 24.Wang C, Yang C, Chen X, Yao B, Yang C, et al. Altered profile of seminal plasma microRNAs in the molecular diagnosis of male infertility. Clin Chem. 2011;57:1722–31. doi: 10.1373/clinchem.2011.169714. [DOI] [PubMed] [Google Scholar]
- 25.Hu L, Wu C, Guo C, Li H, Xiong C. Identification of microRNAs predominately derived from testis and epididymis in human seminal plasma. Clin Biochem. 2014;47:967–72. doi: 10.1016/j.clinbiochem.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Mao Q, Zhang L, Guo Y, Sun L, Liu S, et al. Identification of suitable reference genes for BDV-infected primary rat hippocampal neurons. Mol Med Rep. 2016;14:5587–94. doi: 10.3892/mmr.2016.5959. [DOI] [PubMed] [Google Scholar]
- 27.Jurcevic S, Olsson B, Klinga-Levan K. Validation of suitable endogenous control genes for quantitative PCR analysis of microRNA gene expression in a rat model of endometrial cancer. Cancer Cell Int. 2013;13:45. doi: 10.1186/1475-2867-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhi EL, Liang GQ, Li P, Chen HX, Tian RH, et al. Seminal plasma miR-192a: a biomarker predicting successful resolution of nonobstructive azoospermia following varicocele repair. Asian J Androl. 2018;20:396–9. doi: 10.4103/aja.aja_8_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang X, Yin L, Zhang N, Han F, Liu WB, et al. Bisphenol A induced male germ cell apoptosis via IFNβ-XAF1-XIAP pathway in adult mice. Toxicol Appl Pharmacol. 2018;355:247–56. doi: 10.1016/j.taap.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Kim B. Western blot techniques. Methods Mol Biol. 2017;1606:133–9. doi: 10.1007/978-1-4939-6990-6_9. [DOI] [PubMed] [Google Scholar]
- 31.Zhao K, Chen Y, Yang R, Bai Y, Li C, et al. miR-424/322 is downregulated in the semen of patients with severe DNA damage and may regulate sperm DNA damage. Reprod Fertil Dev. 2015;28:1598–607. doi: 10.1071/RD15052. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Zhang Y, Yang Y, Liu X, Chen Y. Seminal plasma miR-210-3p is a biomarker for screening dyszoospermia caused by varicocele. Andrologia. 2019;51:e13244. doi: 10.1111/and.13244. [DOI] [PubMed] [Google Scholar]
- 33.Fernando N, Leonard JM, Paulsen CA. The role of varicocele in male fertility. Andrologia. 1976;8:1–9. doi: 10.1111/j.1439-0272.1976.tb01636.x. [DOI] [PubMed] [Google Scholar]
- 34.Barcelo M, Mata A, Bassas L, Larriba S. Exosomal microRNAs in seminal plasma are markers of the origin of azoospermia and can predict the presence of sperm in testicular tissue. Hum Reprod. 2018;33:1087–98. doi: 10.1093/humrep/dey072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JD, Jeng SY, Lee TH. Increased expression of hypoxia-inducible factor-1alpha in the internal spermatic vein of patients with varicocele. J Urol. 2006;175:1045–8. doi: 10.1016/S0022-5347(05)00417-9. [DOI] [PubMed] [Google Scholar]
- 36.Cho CL, Esteves SC, Agarwal A. Novel insights into the pathophysiology of varicocele and its association with reactive oxygen species and sperm DNA fragmentation. Asian J Androl. 2016;18:186–93. doi: 10.4103/1008-682X.170441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–75. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang K, Wang Z, Wang H, Fu Q, Zhang H, et al. Hypoxia-induced apoptosis and mechanism of epididymal dysfunction in rats with left-side varicocele. Andrologia. 2016;48:318–24. doi: 10.1111/and.12449. [DOI] [PubMed] [Google Scholar]
- 39.Corn PG. Hypoxic regulation of miR-210: shrinking targets expand HIF-1's influence. Cancer Biol Ther. 2008;7:265–7. doi: 10.4161/cbt.7.2.5745. [DOI] [PubMed] [Google Scholar]
- 40.Huang X, Le QT, Giaccia AJ. MiR-210--micromanager of the hypoxia pathway. Trends Mol Med. 2010;16:230–7. doi: 10.1016/j.molmed.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–42. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- 42.Lv JX, Zhou J, Tong RQ, Wang B, Chen XL, et al. Hypoxiainduced miR210 contributes to apoptosis of mouse spermatocyte GC2 cells by targeting Kruppellike factor 7. Mol Med Rep. 2019;19:271–9. doi: 10.3892/mmr.2018.9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L, Zheng H, Zhang S. Involvement of upregulation of miR-210 in a rat epilepsy model. Neuropsychiatr Dis Treat. 2016;12:1731–7. doi: 10.2147/NDT.S108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng Z, Geng H, Cheng Y, Dong N, Ning F, et al. Effects of MiR-210 on proliferation, apoptosis and invasion abilities of esophageal cancer cells. J BUON. 2018;23:814–9. [PubMed] [Google Scholar]
- 45.Chen Y, Li H. Alkannin protects human renal proximal tubular epithelial cells from LPS-induced inflammatory injury by regulation of microRNA-210. Biomed Pharmacother. 2018;108:1679–85. doi: 10.1016/j.biopha.2018.09.102. [DOI] [PubMed] [Google Scholar]
- 46.Wang B, Gu TX, Yu FM, Zhang GW, Zhao Y. Overexpression of miR-210 promotes the potential of cardiac stem cells against hypoxia. Scand Cardiovasc J. 2018;52:367–71. doi: 10.1080/14017431.2019.1567932. [DOI] [PubMed] [Google Scholar]
- 47.Costales MG, Haga CL, Velagapudi SP, Childs-Disney JL, Phinney DG, et al. Small molecule inhibition of microRNA-210 reprograms an oncogenic hypoxic circuit. J Am Chem Soc. 2017;139:3446–55. doi: 10.1021/jacs.6b11273. [DOI] [PMC free article] [PubMed] [Google Scholar]


