Abstract
This study aimed to explore whether and how anti-lysyl oxidase (anti-LOX) combined with a vacuum device (VD) could promote penile lengthening and to evaluate the effect on erectile function. This study was performed on four groups of adult rats: control, anti-LOX, VD (negative pressure value of −300 mmHg), and anti-LOX + VD. Penile length was measured by a modified VD method and verified on exposed length data. Intracavernous pressure (ICP) and maximum ICP/mean arterial pressure (MAP) ratio were recorded to assess erectile function. For corpus cavernosum, LOX activity and concentrations of pyridinoline, desmosine, hydroxyproline, and elastin were analyzed; transmission electron microscope and Hart's elastin staining were performed to monitor microstructural changes. Anti-LOX and VD significantly lengthened the penis by 10.8% (3.75 mm) and 8.2% (2.48 mm) compared with the control group, respectively, while anti-LOX + VD achieved the longest penile size (40.58 ± 0.40 mm) which was 17.4% longer than the control group (34.58 ± 0.54 mm). After 1-week washout, no penile retraction was observed. Meanwhile, exposed penile length data confirmed that the penis in the anti-LOX + VD group was also significantly longer. Anti-LOX inhibited LOX activity to reduce pyridinoline level, which led the penile tunica albuginea remodeling. However, it had no effect on hydroxyproline, desmosine, and elastin levels. Moreover, anti-LOX had no impact on erectile function, which was determined by ICP and ICP/MAP ratio. These results suggest that anti-LOX elongates the penis by reducing pyridinoline, which induces tunica albuginea remodeling. This lengthening effect was more obvious when combined with a VD. All procedures had no impact on erectile function.
Keywords: anti-lysyl oxidase, crosslinking, penile lengthening, tunica albuginea remodeling, vacuum device
INTRODUCTION
Penile length is measured from the pubic bone to the tip of glans under the erectile or stretched condition.1 Penile growth is gradual from birth, reaching the peak growth between 12 and 16 years of age, which coincides with the maximal pubertal growth spurt.2 Throughout human history, penis has been considered central for power and identity, and penile growth is currently an important indicator for male sexual development.3,4 Penile size abnormality includes micropenis (normally formed but with a stretched length below 2.5 standard deviations of the normal median; <7.5 cm for adult men) and acquired penile retraction (Peyronie's disease, postinfection, posttrauma, and postpriapism),1,5,6,7 which cause considerable sexual dysfunction and psychological problems.7
Due to the ideology of “Phallocentrism” ,3 most patients with abnormal penile size suffer from a penile dysmorphic disorder6,7 and desire a larger penile size3 (a considerable challenge for urologists) to improve self-esteem or to satisfy partners. Postnatal testosterone treatment has been recommended for congenital micropenis;7,8,9 on the other hand, most patients seek reconstructive surgery after treatment, even under active psychological counseling.7 Some researchers reported that postnatal testosterone therapy simply advanced penile growth rather than enhancing the final length,10 while others suggested that prepubertal androgen exposure of the hypogonadotropic hypogonadal micropenis significantly reduced eventual penile size in adulthood.11,12 As a result, testosterone therapy remains debatable for congenital micropenis.7,13,14 The advertised noninvasive techniques for penile growth, such as the penile extender, penoscrotal rings, and botulinum toxin, are only supported by limited scientific evidence.6 Phalloplasty, such as penile suspensory ligament division, inverted V-Y plasty closure, and venous grafting for the corpora cavernosa may achieve optical enlargement effects, but the penis is not genuinely longer or bigger.1,3 On the contrary, because of poorly standardized indications and surgical methods,1,6 these techniques remain controversial6 and experimental.1,3 Therefore, more investigation is needed for suitable methods to lengthen the penis.
The tunica albuginea, which is mainly composed of thick collagen bundles and a few elastic fibers, forms the key structure that determines penile length during erection.15,16 Therefore, we speculated that some albuginea remodeling occurs during penile growth, and interference with this remodeling process may promote penile lengthening.
Lysyl oxidase (LOX) is an extracellular copper-dependent monoamine oxidase17 that catalyzes the crosslinking of collagen and elastin proteins into insoluble mature fibers, which are essential for the tensile strength and mechanical stability of collagen, as well as the elasticity of elastin.17,18,19,20 Our previous data showed that LOX levels decreased with age in the corpus cavernosum, as in other organs.21 In addition, inhibition or knockout of LOX prevented the crosslinking of collagen and elastin in the aorta, which significantly increased aortic diameter19 or led to aneurysmal dilatation.17,18 As the tunica albuginea and aorta are both composed mainly of collagen and elastin fibers, we investigated whether anti-LOX could lengthen the penis by inducing penile albuginea remodeling, similar to the effect of an increased aortic diameter.
In addition, continuous traction mechanically induced collagen remodeling and increased its extensibility,22,23 and the traction device was reported to increase penile length in some noncontrolled case studies.6,24,25 Therefore, we explored whether a potential lengthening effect from anti-LOX-induced penile albuginea remodeling may further be stretched by the force of a vacuum device.
MATERIALS AND METHODS
All experimental procedures were approved by the Animal Ethics Committee of West China Hospital, Sichuan University, Chengdu, China (No. 20160461A), and all rats were housed and cared under strict guidelines.
LOX in rats with different ages
Six Sprague–Dawley (SD) male rats (6 per group; Chengdu Dossy Experimental Animals, Co., Ltd., Chengdu, China) were sacrificed at 8, 12, 16, 24, and 48 weeks old. The penis was obtained and washed with cold phosphate-buffered saline (PBS; self-configuration) for the following Western blot and LOX activity analyses.
The protein expression of LOX was determined by a standard Western blot analysis. LOX activity was measured using an Amplite™ Fluorimetric LOX assay kit (AAT Bioquest Inc., Sunnyvale, CA, USA) according to the manufacturer's instructions (Supplementary Materials and Methods (57.3KB, pdf) ).
According to the section, rats (8 weeks of age) with the highest LOX protein expression and activity were selected for the further study.
Animals in adult age
Twenty-four SD adult male rats at 8 weeks of age (240–260 g) were randomly divided into four groups: (1) control group (gavaged with normal saline [NS]; Chengdu Qingshan Likang Pharmaceutical Co., Ltd., Chengdu, China); (2) anti-LOX group; (3) vacuum device (VD) group (aspiration with a negative pressure at −300 mmHg value); and (4) anti-LOX + VD group. Anti-LOX was performed by intragastric administration of a specific LOX inhibitor, β-aminopropionitrile (BAPN) fumarate (Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China), using a dose of 100 mg kg−1 day−1.26,27
The rats were anesthetized using a facemask first with 5.0% (v/v) isoflurane (Shenzhen RWD Life Science Co., Ltd., Shenzhen, China) for 3 min and then with 1.0% isoflurane.28 VD aspiration with a negative pressure of −300 mmHg (Chengdu Xin Wei Cheng Technology Co., Ltd., Chengdu, China) was performed twice (with a 2-min interval) daily, each session lasting 5 min, Monday to Friday.29 The total treatment duration (anti-LOX with or without VD aspiration) was 7 weeks. All paraphimoses were manually recovered.30 VD aspiration was stopped for 1 or 2 days when serious prepuce bleeding. These animals were used for the following analysis.
Penile length
On the last intervention day, body weights of all rats were measured and recorded. Penile length was measured by a modified 2.5 ml disposable syringe (inner diameter: 9.0 mm) with the following procedures (Figure 1a and 1b), the injection end of the syringe was connected to a negative-pressure device (Chengdu Xin Wei Cheng Technology Co., Ltd.), and the flanged end was placed over the penis and firmly pressed onto the pubis. After a unified vacuum device aspiration (−200 mmHg of 5 min for twice, with a 2-min interval), penile length was recorded, with head of the ruler close to syringe flanged end (Figure 1), as described previously.31 Following a 1-week washout period, penile length was measured once more using the same method to observe any penile retraction.
Figure 1.
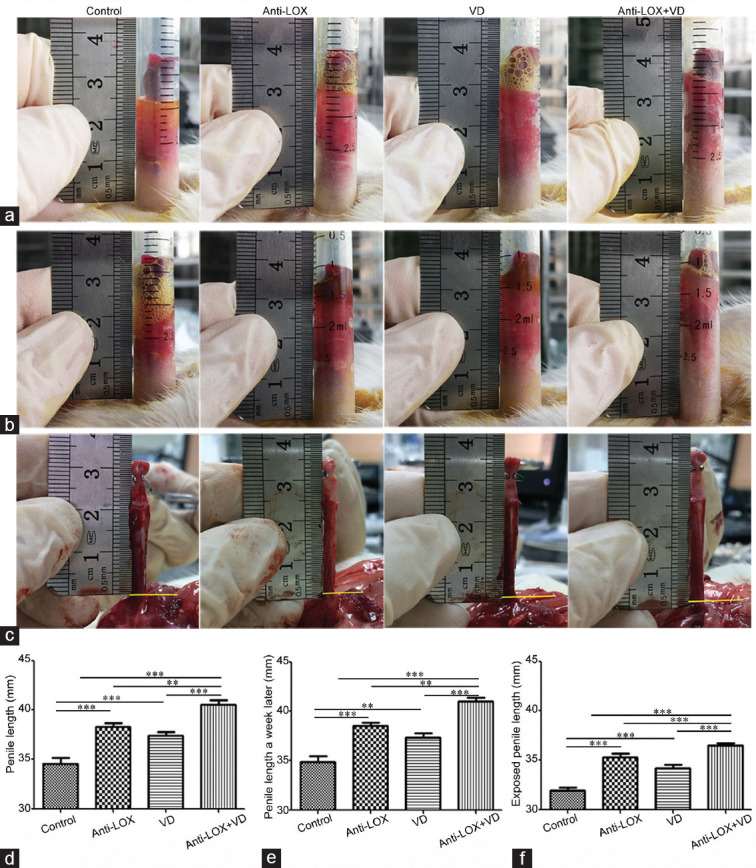
Anti-LOX combined with a VD induces penile lengthening (n = 6 per group). (a) Representative images of penile length. (b) Representative images of penile length after a 1-week washout period. For a and b, penile length was measured under unified vacuum device aspiration condition (measured at –200 mmHg for 5 min twice with a 2-min interval). (c) Exposed penile length; the length was measured from the junction of urethral bulb and corpus cavernosum (marked with yellow line), to tip of glans cartilage; the measurement errors of length data were corrected on recorded images. (d) Statistical analysis of penile length (P < 0.0001). (e) Statistical analysis of penile length after a 1-week washout period (P < 0.0001). (f) Statistical analysis for exposed penile length (P < 0.0001). Anti-LOX: intragastric administration of β-aminopropionitrile (BAPN) with a dose of 100 mg kg−1 day−1; VD: vacuum device aspiration with negative pressure of -300 mmHg. **P < 0.01, ***P < 0.0001. LOX: lysyl oxidase.
After erectile function assessment (described next), the exposed penile length was recorded. Briefly, the penis was totally exposed and vertically stretched with the glans cartilage clipped by vessel forceps, until rat's rump/back separated from the animal experimental table. The exposed length was measured from the junction of the urethral bulb and corpus cavernosum (marked with a yellow line in Figure 1), to the tip of the glans cartilage; the measurement errors of length data were corrected using recorded images (Figure 1). Meanwhile, dorsal–ventral and lateral–lateral diameters of the middle penis were measured (in the exposed stretched position) by a vernier caliper.
Erectile function assessment
Erectile function was assessed after a 1-week washout period from the last penile length measurement. Rats were kept on a heating pad at 38°C32 and anesthetized using a facemask first with 5.0% isoflurane for 3 min and then with 2.0% isoflurane. Following the exposure and intubation of the crus penile and carotid artery, the concentration of isoflurane was decreased and maintained at 0.9%.28,33 The cavernous nerve was stimulated electrically at 5.0 V, 20.0 Hz, pulse width of 5.0 ms, for 50 s.28,29,33 Intracavernous pressure (ICP) and arterial pressure were simultaneously monitored with a BL420 biofunctional experiment system (TME Technology Co. Ltd. Chengdu, China).28,33 The maximum ICP/mean arterial pressure (MAP) ratio was calculated and analyzed. After the erectile function measurement, the exposed penis was cut at the junction of the urethral bulb and corpus cavernosum and then the excised penile weight was measured. The glans was removed, and the remaining penis tissue was washed with PBS and cut into four (proximal, proximal-middle, distal-middle, and distal) sections. The harvested penile tissues were used for the following detections.
Component testing
The obtained penis was used for the detection of LOX activity as described previously.18,34 Total hydroxyproline (HYP) and total elastin level were assessed. The crosslinking level of collagen and elastin (detected by pyridinoline [PYD] and desmosines [DES], respectively). All of the procedures were performed according to the manufacturer's instructions (Supplementary Materials and Methods (57.3KB, pdf) ).
Transmission electron microscopy (TEM)
TEM was performed to determine microstructural changes to smooth muscle cells (SMCs), fibroblasts, collagen, and elastin in the tunica albuginea. A small piece (0.5 cm × 0.5 cm) of penile tunica albuginea was harvested (from the distal–middle penis), fixed in 3.0% glutaraldehyde (kindly provided by Chengdu Li Lai Biotechnology Co., Ltd., Chengdu, China), and sent to Chengdu Li Lai Biotechnology Co., Ltd. for analysis. Briefly, the tissues were postfixed in 1.0% osmium tetroxide (provided by Chengdu Li Lai Biotechnology Co., Ltd.), dehydrated in acetone (Guangzhou Sagene Biotech Co., Ltd., Guangzhou, China), and embedded in Epon 812 (Beijing Zhongjingkeyi Technology Co., Ltd., Beijing, China).35,36 Ultrathin sections were double stained with uranium acetate and citric acid (Beijing Zhongjingkeyi Technology Co., Ltd.) and examined using a H-600IV transmission electron microscope (Hitachi group, Tokyo, Japan).
Hart's elastin staining
Distal penile pieces were fixed in the formaldehyde solution (Pathology Platform, West China Hospital, Sichuan University, Chengdu, China), underwent routine dehydration and paraffin embedding, and cut into 5 μm sections (Leica RM2135, Wetzlar, Germany). As described previously,16,37 tissue slides were soaked in a 0.25% KMnO4 solution (Beijing Solarbio Science and Technology Co., Ltd., Beijing, China) for 5 min, cleaned in a 5.0% oxalic acid solution (Beijing Solarbio Science and Technology Co., Ltd.), and incubated overnight in resorcin–fuchsin working solution (Xiya Chemical Co., Ltd., Jinan, China). After washing, the samples were counterstained with Van Gieson's solution (Beijing Solarbio Science and Technology Co., Ltd.) for 1 min. The elastic fibers were stained blue-black to blue, and collagen was stained pink to red. To examine the cumulative area of elastic fibers in tunica albuginea and corpus spongiosum (locations at 0, 3, 6, and 9 o'clock),16,38 images at ×400 magnification were acquired by a Zeiss Axio Imager (ZEISS, Oberkochen, Germany) and analyzed with Image-Pro Plus version 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analyses
The data were analyzed by GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). Statistical differences among multiple groups were compared using the one-way ANOVA analysis, followed with a Tukey's test to compare all pairs of columns. For some groups, we performed the Student's t-test to obtain P values. P < 0.05 was considered statistically significant.
RESULTS
LOX in rats with different ages
Western blot revealed that LOX expression in the corpus cavernosum was highest in the 8-week-old rats and then significantly decreased with age (P < 0.0001). LOX activity showed the same trend, with LOX activity highest at 8 weeks of age and then significantly declining (P < 0.0001) and finally reaching a relative stable level (P > 0.05; (Figure 2 and Supplementary Figure 1 (423.8KB, tif) )
Figure 2.
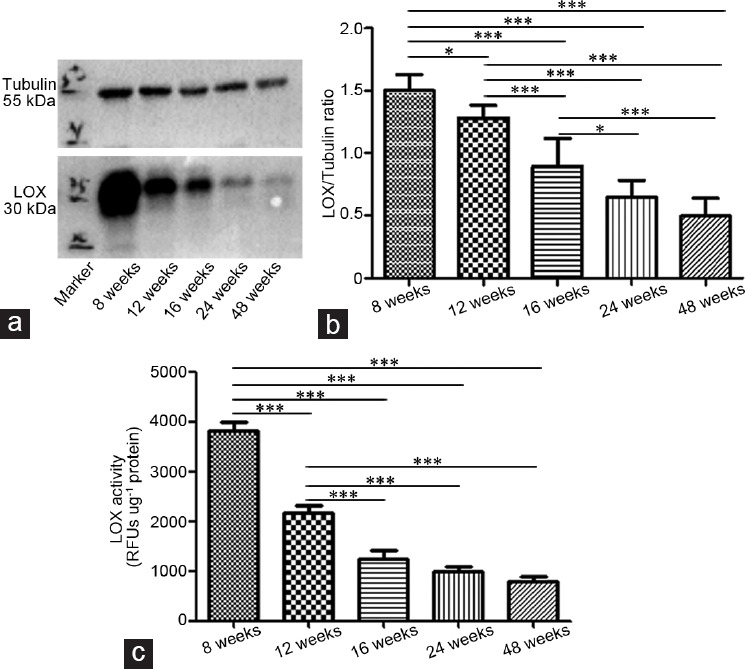
LOX variation in corpus cavernosum in rats with 8, 12, 16, 24, and 48 weeks, respectively (n = 6 per group). (a) Representative image from Western blot. (b) Statistical analysis revealed that LOX expression was the highest at 8 weeks of age, which was significantly decreased with age (P < 0.0001). (c) LOX activity was the highest at 8 weeks of age; it was then significantly declined (P < 0.0001) and finally attained a relative stable level (P > 0.05). *P < 0.05, ***P < 0.0001. LOX: lysyl oxidase; RFUs: relative fluorescent units.
Penile length
Penile length data were reported as mean ± standard error mean (s.e.m.). All rats showed similar primary penile length (P = 0.9186; Supplementary Table 1). After 7 weeks, anti-LOX and VD treatment significantly increased penile length to 38.33 ± 0.36 mm and 37.42 ± 0.33 mm (P < 0.0001), respectively, which was 10.8% (3.75 mm) and 8.2% (2.48 mm) longer than the control group (34.58 ± 0.54 mm), respectively. Interestingly, anti-LOX + VD produced the longest penis (40.58 ± 0.40 mm; P < 0.0001), which was 17.4% (6.00 mm), 5.9% (2.25 mm), and 8.4% (3.16 mm) longer than the control, anti-LOX, and VD groups, respectively (Figure 1, Supplementary Table 1 and 2).
Supplementary Table 1.
Data collection
| Control | Anti-LOX | VD | Anti-LOX + VD | P | |
|---|---|---|---|---|---|
| Primary penile length (mm) | 31.17±0.11 | 31.08±0.15 | 31.25±0.11 | 31.08±0.33 | 0.9186 |
| Penile length (mm) | 34.58±0.54 | 38.33±0.36*** | 37.42±0.33*** | 40.58±0.40*** | <0.0001 |
| Penile length a week later (mm) | 34.83±0.59 | 38.50±0.32*** | 37.33±0.48*** | 41.00±0.37*** | <0.0001 |
| Exposed penile length (mm) | 31.92±0.33 | 35.33±0.31*** | 34.17±0.38*** | 36.50±0.26*** | <0.0001 |
| Rats’ final weight (g) | 440.83±14.76 | 430.83±12.58 | 420.50±11.21 | 424.17±8.11 | 0.6467 |
| Excised penile weight (mg) | 290.17±4.05 | 295.15±3.92 | 293.03±4.49 | 297.73±3.39 | 0.5924 |
| Dorsal-ventral length (mm) | 3.48±0.06 | 3.56±0.11 | 3.59±0.10 | 3.59±0.05 | 0.7520 |
| Lateral-lateral length (mm) | 3.37±0.03 | 3.35±0.11 | 3.43±0.07 | 3.42±0.04 | 0.8263 |
| Basic ICP (mmHg) | 6.69±0.67 | 6.66±0.31 | 6.81±0.23 | 6.95±0.15 | 0.9511 |
| ICP (mmHg) | 67.06±5.13 | 67.48±4.60 | 72.55±2.76 | 74.44±1.97 | 0.4512 |
| MAP (mmHg) | 109.07±5.77 | 118.02±5.12 | 128.73±6.79 | 120.74±2.36 | 0.1017 |
| ICP/MAP ratio | 0.62±0.04 | 0.57±0.03 | 0.57±0.02 | 0.62±0.02 | 0.4297 |
P values were calculated by ANOVA analysis for the difference among the four groups. ***P<0.0001, difference compared with control group. ICP: intracavernous pressure; MAP: mean arterial pressure; LOX: lysyl oxidase; VD: vacuum device aspiration with negative pressure of -300 mmHg
Supplementary Table 2.
Penile length comparison in relative and percentage value ([longer−shorter]/shorter)
| Control | Anti-LOX | VD | Anti-LOX + VD | |
|---|---|---|---|---|
| Anti-LOX (mm, %) | 3.75 (10.8) | 0 | 0.91 (2.4) | 2.25 (5.9) |
| VD (mm, %) | 2.48 (8.2) | 0.91 | 0 | 3.16 (8.4) |
| Anti-LOX + VD (mm, %) | 6.00 (17.4) | 2.25 (5.9) | 3.16 (8.4) | 0 |
LOX: lysyl oxidase; VD: vacuum device
After a 1-week washout period, similar results were observed. The anti-LOX + VD group had the longest penis (41.00 ± 0.37 mm; P < 0.0001), which was 17.7% (6.17 mm), 6.5% (2.50 mm), and 9.8% (3.67 mm) longer than the control (34.83 ± 0.59 mm), anti-LOX (38.50 ± 0.32 mm), and VD (37.33 ± 0.48 mm) groups, respectively (Figure 1, Supplementary Table 1 and 3). For exposed length measured by stretched method, anti-LOX and VD significantly lengthened the penis to 35.33 ± 0.31 mm and 34.17 ± 0.38 mm (P < 0.0001), respectively, which was 10.7% (3.41 mm) and 7.1% (2.25 mm) longer than the control (31.92 ± 0.33 mm) group, respectively. Anti-LOX + VD resulted in the longest penis (36.50 ± 0.26 mm; P < 0.0001), which was 14.4% (4.58 mm), 3.3% (1.17 mm), and 6.8% (2.33 mm) longer than the control, anti-LOX, and VD groups, respectively (Figure 1, Supplementary Table 1 and 4).
Supplementary Table 3.
Penile length a week later comparison in relative and percentage value ([longer−shorter]/shorter)
| Control | Anti-LOX | VD | Anti-LOX + VD | |
|---|---|---|---|---|
| Anti-LOX (mm, %) | 3.67 (10.5) | 0 | 1.17 (3.1) | 2.5 (6.5) |
| VD (mm, %) | 2.50 (7.2) | 1.17 (3.1) | 0 | 3.67 (9.8) |
| Anti-LOX + VD (mm, %) | 6.17 (17.7) | 2.50 (6.5) | 3.67 (9.8) | 0 |
Supplementary Table 4.
Exposed penile length comparison in relative and percentage value ([longer−shorter]/shorter)
| Control | Anti-LOX | VD | Anti-LOX + VD | |
|---|---|---|---|---|
| Anti-LOX (mm, %) | 3.41 (10.7) | 0 | 1.16 (3.0%) | 1.17 (3.3%) |
| VD (mm, %) | 2.25 (7.1) | 1.16 (3.0%) | 0 | 2.33 (6.8%) |
| Anti-LOX + VD (mm, %) | 4.58 (14.4) | 1.17 (3.3%) | 2.33 (6.8%) | 0 |
No significant difference was found among the treatment groups in final body weights (P = 0.6467) and dorsal–ventral (P = 0.7520) and lateral–lateral (P = 0.8263) penile diameters. Rats in the control group had a lower excised penis weight, but there was no significant difference (P = 0.5924) (Supplementary Table 1 and Supplementary Figure 2 (536.6KB, tif) ).
Erectile function assessment
There were no statistical differences in the ICP (P = 0.4512), MAP (P = 0.1017), and maximum ICP/MAP ratio (P = 0.4297) among the four groups (Figure 3 and Supplementary Table 1].
Figure 3.
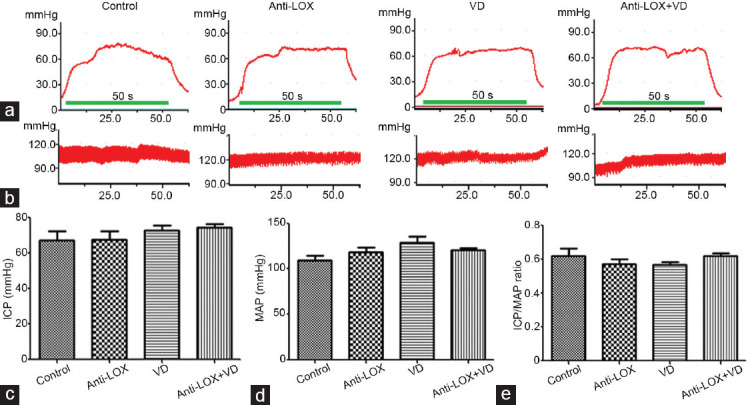
Anti-LOX combined with a VD does not impact normal erectile function (n = 6 per group). (a) Representative images of ICP under cavernous nerve stimulation. (b) Representative images of arterial pressure under cavernous nerve stimulation. (c) Statistical analysis of ICP (P = 0.4512). (d) Statistical analysis of mean arterial pressure (MAP; P = 0.1017). (e) Statistical analysis of ICP/MAP ratio (P = 0.4297). Anti-LOX: intragastric administration of β-aminopropionitrile (BAPN) with a dose of 100 mg kg−1 day−1; VD: vacuum device aspiration with negative pressure of -300 mmHg. LOX: lysyl oxidase; ICP: intracavernous pressure.
Component testing
The anti-LOX and anti-LOX + VD groups exhibited significantly lower (P = 0.0149) LOX activity than the control and VD groups. No difference was found between the control and VD groups (P = 0.8496) or between the anti-LOX and anti-LOX + VD groups (P = 0.6318) (Figure 4).
Figure 4.
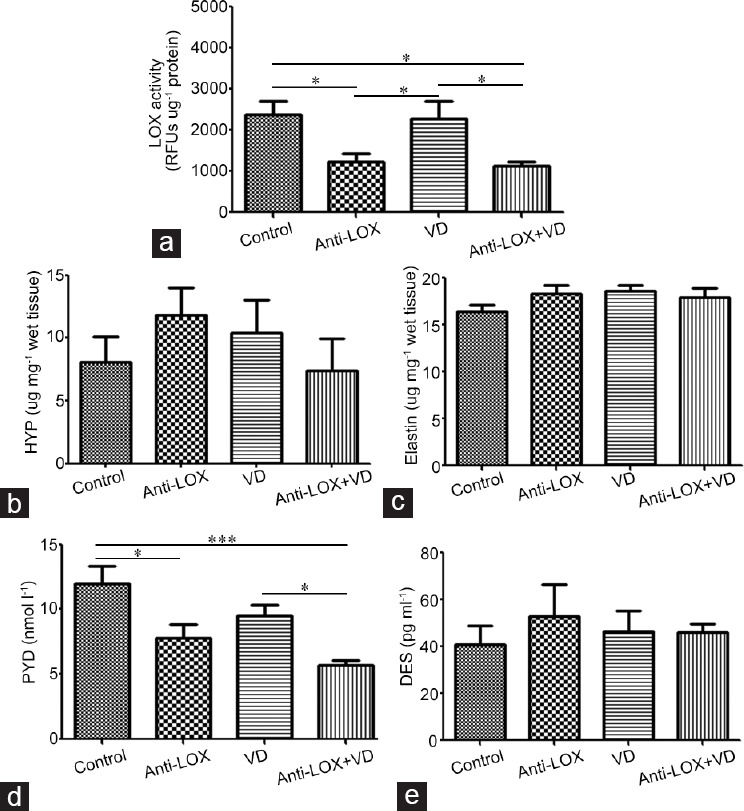
Biomarker analysis for corpus cavernosum. (a) anti-LOX and anti-LOX + VD groups exhibited significantly lower lysyl oxidase (LOX) activity than control and VD groups, respectively (P = 0.0149). (b) No significant difference was found on HYP (P = 0.5440) level. (c) No significant difference was found on total elastin content (P = 0.2228). (d) Anti-LOX group showed significantly lower PYD concentration than the control group (P = 0.0449), while anti-LOX + VD revealed significant lower level than the control (P = 0.0015) and −300 mmHg (P = 0.0015) groups. (e) No significant difference was found on DES concentration (P = 0.8226). n = 6 per group. Anti-LOX: intragastric administration of β-aminopropionitrile (BAPN) with a dose of 100 mg kg−1 day−1. VD: vacuum device aspiration with negative pressure of -300 mmHg. *P < 0.05, ***P < 0.0001. RFUs: relative fluorescent units; LOX: lysyl oxidase; HYP: hydroxyproline; PYD: pyridinoline; DES: desmosines.
No statistical differences were observed in HYP (P = 0.5440) or elastin (P = 0.2228) levels among the groups (Figure 4). On the other hand, the anti-LOX group exhibited a significantly lower (P = 0.0449) PYD concentration than the control group. The anti-LOX+VD group had a significantly lower (P = 0.0015) level of PYD compared with the control and VD groups (Figure 4). No statistical difference in DES levels was found (P = 0.8226; Figure 4).
TEM
In the tunica albuginea of the corpus cavernosum, collagen bundles in the control group displayed a regular arrangement, diameter, and shape, with abundant ground substance between these fibers. In contrast, fibrous bundles in the anti-LOX + VD group were loosely arranged in longitudinal sections. In cross sections, irregular collagen bundles were more obvious in the anti-LOX, VD, and anti-LOX + VD groups, with disorganized fibrous bundles exhibiting irregular shapes and less ground substance (Figure 5). In the tunica albuginea of the corpus cavernosum, fibroblasts with spindle cell nuclei were found among collagen fibers of all groups. Rich rough endoplasmic reticulum in the cytoplasm was observed in the control and anti-LOX groups, whereas dilated rough endoplasmic reticulum and swollen mitochondria were found in the VD and anti-LOX + VD groups. All smooth muscle cells (SMCs) exhibited spindle cell nuclei and homogeneously distributed chromatin; myofibrils had formed dense bodies, with well-structured mitochondria and rough endoplasmic reticulum in the cytoplasm, and macula densa beneath the cell membrane (Supplementary Figure 3 (368.8KB, tif) ).
Figure 5.
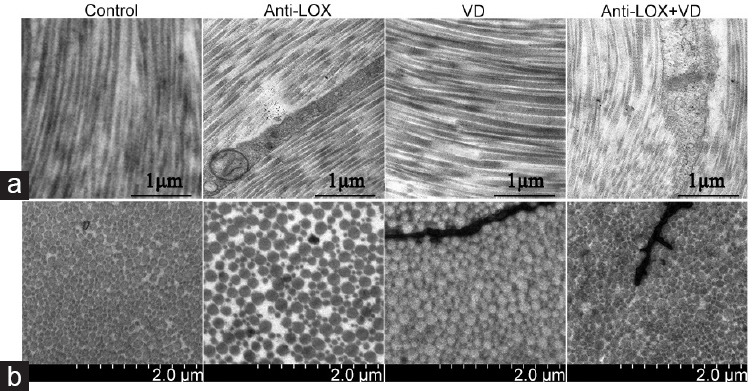
Transmission electron microscopy in tunica albuginea of corpus cavernosum (n = 2 per group). (a) In longitudinal section, anti-LOX + VD group showed loosely arranged collagen fibers (scale bars = 1 μm). (b) In cross section, remodeled appearances of collagen bundlea were more obvious in anti-LOX, VD, and anti-LOX + VD groups, they also showed irregular arranged collagen bundles and less ground substance, compared with the control group (scale bars = 2 μm). Anti-LOX: intragastric administration of β-aminopropionitrile (BAPN) with a dose of 100 mg kg−1 day−1. VD: vacuum device aspiration with negative pressure of -300 mmHg. LOX: lysyl oxidase.
Hart's elastin staining
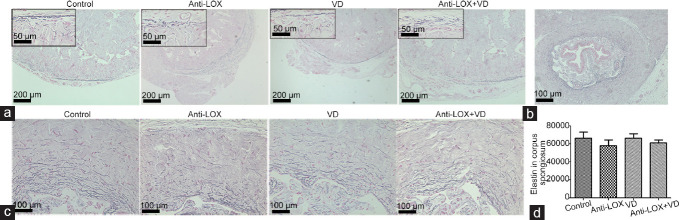
In the tunica albuginea, elastic fibers with regular size and length were tightly intertwined with collagen bundles. These fibers were abundant between the Buck's fascia and tunica albuginea (Figure 6). Corpus spongiosum contained abundant elastic fibers with a twisted appearance, and no significant difference was observed among the groups in the cumulative area (P = 0.6375; Figure 6).
Figure 6.
Hart's elastin staining in rat models (n = 6 per group). (a) Tunica albuginea showed blue-black to blue elastic fibers, which was abundant between Buck's fascia and tunica (at ×50 magnification, scale bars = 200 μm), representative elastic fibers on the upper left box (at ×400 magnification, scale bars = 50 μm); elastic fibers with regular shapes were tightly intertwined with collagen bundles along tunica albuginea. (b) Representative elastic fibers of corpus spongiosum at ×100 magnification (scale bars = 100 μm). Corpus spongiosum revealed substantially abundant elastic fibers with a tortuous appearance. (c) Elastic fibers of corpus spongiosum at 0 o'clock (at ×200 magnification, scale bars = 100 μm). (d) Cumulative area of elastic fibers in corpus spongiosum (0, 3, 6, and 9 o'clock at ×200 magnification), with insignificant difference found (P = 0.1865). Anti-LOX: intragastric administration of β-aminopropionitrile (BAPN) with a dose of 100 mg kg−1 day−1; VD: vacuum device aspiration with negative pressure of -300 mmHg. LOX: lysyl oxidase.
DISCUSSION
Penile length is determined by the tunica albuginea, which is mainly composed by collagen bundles and elastic fibers.15,16 LOX can catalyze the crosslinking of collagen and elastin proteins into insoluble mature fibers.17,18,19 Our results have revealed that both the protein expression and activity of LOX sharply decreased with age to relative stable levels. We remodeled the tunica albuginea by anti-LOX and investigated this effect on penile lengthening in adult rats.
All groups showed a similar primary penile length before the treatment. After 7 weeks of treatment, anti-LOX significantly lengthened the penis by 10.8%. We speculated that anti-LOX remodeled the penile tunica albuginea by inhibiting the crosslinking of collagen and elastin, thereby reducing the tensile strength of collagen and elasticity of elastin.17,18,19
Chronic traction has been previously applied to lengthen limbs using the Ilizarov technique.39 Mechanical stressing restructured collagen and allowed its stretching in Dupuytren's disease tissue,23,40 while the traction device was reported to lengthen the penis in some noncontrolled case studies.6,24,25 A recent animal study also showed that the traction device and VD can exert a tissue remodeling effect to correct penile curvature.41 Our study revealed that VD (−300 mmHg) significantly increased penile length by 8.2% compared with the controls. Possible explanations for this increase include continuous traction-induced collagen realignment,22,42 stimulation of fibroblasts42 and soft cellular proliferation,40,43 or tissue growth.6,40
We combined anti-LOX with VD and investigated their effects on penile lengthening. Our results showed that anti-LOX + VD achieved the longest penile length, which was 17.4%, 5.9%, and 8.4% longer than the control, anti-LOX, and VD groups, respectively. We further speculated that anti-LOX induced incomplete crosslinked collagen and elastin, and the remodeled tunica albuginea was unable to bear the extra drag force of VD.18,44 As a result, penile lengthening was enhanced when anti-LOX was combined with a VD force.
A 1–2 cm length gain has been considered a success.1 The current and prevalent invasive penile suspensory ligament division can lengthen the penis by 1.5–2 cm, and penile disassembly and rib cartilage transplant can achieve a short-term increase of 2–3 cm, that is, a 12.5%–16.7% and 16.7%–25% increase, respectively (assuming a human stretched normal penile length of 12 cm).5 In comparison, our noninvasive anti-LOX + VD method has lengthened the penis by 17.4% and has achieved effects to that of the invasive lengthening phalloplasty techniques.1,3 Considering −150 to −300 mmHg is acceptable for human VD,45,46 we found that −300 mmHg + anti-LOX exhibited a larger penile lengthening effect than −200 mmHg + anti-LOX (unpublished data); therefore, we selected the pressure of −300 mmHg.
To determine whether the lengthened penes would retract or shrink, we remeasured penile length after a 1-week washout period. Significantly longer penes were still present in the anti-LOX + VD group than in other groups, showing that penises had not retracted to previous sizes. However, a longer washout period is needed to determine the long-term effects. Compared with the conventional method of stretched penile measurement, the measurement of penile length with a syringe is simple, less prone to interindividual variation and more reproducible.31 As accurate penile measurement is paramount, we also recorded exposed penile length as a second method, verified the lengthening effect and eliminated the potential impact of a stretched prepuce. In the current study, anti-LOX and anti-LOX + VD significantly increased penile length by 10.7% and 14.4%, respectively, compared with controls. In addition, anti-LOX with or without VD aspiration had no effects on penile dorsal–ventral and lateral–lateral diameters, but they slightly increased excised penile weight, although without significant difference.
BAPN is a lathyrogen that forms a covalent adduct with LOX and directly inhibits the LOX enzyme. Our study suggested that BAPN (anti-LOX) directly inhibited LOX activity, which was consistent with previous studies.19,20 Previous studies found that anti-LOX inhibited the crosslinking of collagen and elastin proteins,18,20,44,47 but had no effect on total collagen (determined by HYP) or elastin protein concentrations.19,48 The inhibition efficiency of anti-LOX has been estimated using PYD and DES levels, the mature crosslinking forms of collagen and elastin, respectively.19,20
In the present study, anti-LOX significantly reduced PYD concentrations. We also observed microstructural remodeling of the tunica albuginea using TEM. Examination of longitudinal sections showed that anti-LOX + VD induced loosely arranged collagen fibers, whereas cross sections exhibited more obvious remodeling, with collagen fibrils arranged in irregular shapes and less ground substance between the fibrils. We propose that the delayed collagen crosslinking resulted in albuginea remodeling, which decreased its tensile strength and its ability to withstand extra drag force,17,19,47 ultimately leading to lengthening of the penis.
In addition, anti-LOX had no impact on DES levels. Hart's elastin staining revealed elastic fibers with regular shapes that were tightly intertwined with collagen bundles along the tunica albuginea. To eliminate the influence of abundant elastic fibers between the Buck's fascia and tunica albuginea (the irregular shape of the tunica albuginea made it difficult to analyze the cumulative area of elastic fibers), we further analyzed the cumulative area of elastic fibers in the corpus spongiosum, which had a relatively fixed geometry and substantially more fibers. No significant difference was observed for the cumulative areas among the groups. This might result from the fact that newly crosslinked DES was too small to detect any difference,19 or that BAPN could not completely inhibit LOX activity.18,19
People who pursued an enlarged penis must pay close attention to erectile function. In this study, increased penile length was achieved with no differences in ICP and the ICP/MAP ratio, which suggested that erectile function was not impacted. In addition, SMCs exhibited a normal microstructure by TEM, suggesting that anti-LOX with or without VD had no harmful effects on normal erectile function. The intragastric administration of BAPN had no impact on MAP, supporting previous findings.49,50
In general, no serious side effects were observed, while minor cases such as edema, petechial, and ecchymosis were common.30 Prepuce bleeding was found when VD was combined with anti-LOX; however, bleeding could stop spontaneously. However, suspension of the aspiration procedure for 1 or 2 days was needed for some cases. Our results demonstrated that anti-LOX combined with a VD could significantly increase penile length without impairing normal erectile function.
However, this study has several limitations. First, we showed that anti-LOX combined with a VD lengthened the penis by remodeling the tunica albuginea in healthy adult rats. However, penile lengthening therapy was only recommended for abnormal penis sizes, so results from such an animal model may be more convincing. Second, the penile growth spurt occurs during puberty, and whether the anti-LOX-induced lengthening effect would be more efficient during this period requires further investigation. Third, no significant differences were found in penile dorsal–ventral and lateral–lateral diameters of the exposed stretched penile status, and a more accurate method was needed to investigate the influence on penile circumference. Most importantly, anti-LOX with BAPN has been employed to induce thoracic aortic dissection in rodent models, and systemic effects after intragastric administration of BAPN could not be ruled out in the current study. Therefore, other methods to inhibit LOX activity or more targeted modes of drug administration (such as topical testosterone/dihydrotestosterone gel) will be necessary to overcome systemic responses. Meanwhile, we just described (though not clarified with statistics) the side effects in the penis; therefore, we think that it is necessary to analyze the systematic responses and pay more attention to these complications. However, this was just an animal research, whether anti-LOX accompanied with a VD can be applied to lengthen human penis remained to be supported by further strong ethical, basic, and clinical evidence.
CONCLUSIONS
Anti-LOX significantly increased the penile length. This effect was more prominent when combined with a VD. We propose that anti-LOX inhibited LOX activity to reduce pyridinoline levels, which suppressed collagen crosslinking and induced penile tunica albuginea remodeling. In addition, anti-LOX had no effect on normal erectile function, even combined with a VD.
AUTHOR CONTRIBUTIONS
TL, FDF, and CJW carried out the experiments. TL, FDF, and FQ contributed to the statistical analysis, interpretation of data, and the manuscript preparation. TL, FDF, and JHY participated in the article screening, experiment design, and critically revising the manuscript. JHY and RW conceived of this study and supervised the experiments and the manuscript drafting. All authors read and approved the final manuscript
COMPETING INTERESTS
All authors declared no competing interests.
Western Blot bands in supplemental data. Different bands from gel (a), (b), and (c). LOX: lysyl oxidase.
Analysis for rats in different groups. (a) No significant difference was found on rat's final weight (P = 0.6467). (b) Control groups revealed insignificantly lower excised penile weight than other three groups (P = 0.9206, n = 6 per group). (c) No significant difference was found on rat's dorsal–ventral (P = 0.7520). (d) No significant difference was found on rat's lateral–lateral (P = 0.8263) diameters. Anti-lysyl oxidase: intragastric administration of ß-aminopropionitrile with a dose of 100 mg kg-1 day-1. VD: aspiration with negative pressure of -300 mmHg.
Transmission electron microscopy in tunica albuginea of corpus cavernosum (n = 2 per group). (a) Fibroblasts with spindle cell nuclei were found among collagen fibers in all groups. Rough endoplasmic reticulum was dilated and mitochondria was swelled in VD and anti-LOX + VD groups (scale bars = 1 µm). (b) Smooth muscle cell which exhibited spindle cell nuclei and homogeneous distributed chromatin, with myofibrils formed dense bodies and well-structured mitochondria rough endoplasmic reticulum found in cytoplasm (scale bars = 1 µm). Anti-lysyl oxidase: intragastric administration of ß-aminopropionitrile with a dose of 100 mg kg-1 day-1. VD: aspiration with negative pressure of -300 mmHg. ▴: mitochondrial structures, ★: rough endoplasmic reticulum, ♦: macular densa beneath cell membrane, ◾: myofibrils formed dense bodies.
ACKNOWLEDGMENTS
This work was supported by grants from the National Nature Science Foundation of China (No. 81871147 and No. 81671453) and Sichuan Science and Technology Program (No. 2018SZ0019 and No. 2018TJPT0018).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Ghanem H, Glina S, Assalian P, Buvat J. Position paper: management of men complaining of a small penis despite an actually normal size. J Sex Med. 2013;10:294–303. doi: 10.1111/j.1743-6109.2012.02725.x. [DOI] [PubMed] [Google Scholar]
- 2.Tomova A, Deepinder F, Robeva R, Lalabonova H, Kumanov P, et al. Growth and development of male external genitalia: a cross-sectional study of 6200 males aged 0 to 19 years. Arch Pediatr Adolesc Med. 2010;164:1152–7. doi: 10.1001/archpediatrics.2010.223. [DOI] [PubMed] [Google Scholar]
- 3.Vardi Y. Is penile enlargement an ethical procedure for patients with a normal-sized penis? Eur Urol. 2006;49:609–11. doi: 10.1016/j.eururo.2005.12.053. [DOI] [PubMed] [Google Scholar]
- 4.Nugteren HM, Balkema GT, Pascal AL, Schultz WC, Nijman JM, et al. Penile enlargement: from medication to surgery. J Sex Marital Ther. 2010;36:118–23. doi: 10.1080/00926230903554453. [DOI] [PubMed] [Google Scholar]
- 5.Colombo F, Casarico A. Penile enlargement. Curr Opin Urol. 2008;18:583–8. doi: 10.1097/MOU.0b013e32830fe427. [DOI] [PubMed] [Google Scholar]
- 6.Oderda M, Gontero P. Non-invasive methods of penile lengthening: fact or fiction? BJU Int. 2011;107:1278–82. doi: 10.1111/j.1464-410X.2010.09647.x. [DOI] [PubMed] [Google Scholar]
- 7.Kayes O, Shabbir M, Ralph D, Minhas S. Therapeutic strategies for patients with micropenis or penile dysmorphic disorder. Nat Rev Urol. 2012;9:499–507. doi: 10.1038/nrurol.2012.150. [DOI] [PubMed] [Google Scholar]
- 8.Bin-Abbas B, Conte FA, Grumbach MM, Kaplan SL. Congenital hypogonadotropic hypogonadism and micropenis: effect of testosterone treatment on adult penile size why sex reversal is not indicated. J Pediatr. 1999;134:579–83. doi: 10.1016/s0022-3476(99)70244-1. [DOI] [PubMed] [Google Scholar]
- 9.Ma YM, Wu KJ, Dang Q, Shi Q, Gao Y, et al. Testosterone regulates keratin 33B expression in rat penis growth through androgen receptor signaling. Asian J Androl. 2014;16:817–23. doi: 10.4103/1008-682X.129935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welsh M, MacLeod DJ, Walker M, Smith LB, Sharpe RM. Critical androgen-sensitive periods of rat penis and clitoris development. Int J Androl. 2010;33:e144–52. doi: 10.1111/j.1365-2605.2009.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Husmann DA, Cain MP. Microphallus: eventual phallic size is dependent on the timing of androgen administration. J Urol. 1994;152:734–9. doi: 10.1016/s0022-5347(17)32694-0. [DOI] [PubMed] [Google Scholar]
- 12.McMahon DR, Kramer SA, Husmann DA. Micropenis: does early treatment with testosterone do more harm than good? J Urol. 1995;154:825–9. [PubMed] [Google Scholar]
- 13.Becker D, Wain LM, Chong YH, Gosai SJ, Henderson NK, et al. Topical dihydrotestosterone to treat micropenis secondary to partial androgen insensitivity syndrome (PAIS) before, during, and after puberty – a case series. J Pediatr Endocrinol Metab. 2016;29:173–7. doi: 10.1515/jpem-2015-0175. [DOI] [PubMed] [Google Scholar]
- 14.Shen R, Lin MC, Sadeghi F, Swerdloff RS, Rajfer J, et al. Androgens are not major down-regulators of androgen receptor levels during growth of the immature rat penis. J Steroid Biochem Mol Biol. 1996;57:301–13. doi: 10.1016/0960-0760(95)00283-9. [DOI] [PubMed] [Google Scholar]
- 15.Hsu GL, Brock G, von Heyden B, Nunes L, Lue TF, et al. The distribution of elastic fibrous elements within the human penis. Br J Urol. 1994;73:566–71. doi: 10.1111/j.1464-410x.1994.tb07645.x. [DOI] [PubMed] [Google Scholar]
- 16.Akkus E, Carrier S, Baba K, Hsu GL, Padma-Nathan H, et al. Structural alterations in the tunica albuginea of the penis: impact of Peyronie's disease, ageing and impotence. Br J Urol. 1997;79:47–53. doi: 10.1046/j.1464-410x.1997.26511.x. [DOI] [PubMed] [Google Scholar]
- 17.Maki JM. Inactivation of the lysyl oxidase gene lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–9. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- 18.Remus EW, O'Donnell RE, Jr, Rafferty K, Weiss D, Joseph G, et al. The role of lysyl oxidase family members in the stabilization of abdominal aortic aneurysms. Am J Physiol Heart Circ Physiol. 2012;303:H1067–75. doi: 10.1152/ajpheart.00217.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruel A, Ortoft G, Oxlund H. Inhibition of cross-links in collagen is associated with reduced stiffness of the aorta in young rats. Atherosclerosis. 1998;140:135–45. doi: 10.1016/s0021-9150(98)00130-0. [DOI] [PubMed] [Google Scholar]
- 20.López B. Role of lysyl oxidase in myocardial fibrosis: from basic science to clinical aspects. Am J Physiol Heart Circ Physiol. 2010;299:H1–9. doi: 10.1152/ajpheart.00335.2010. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi K, Fong KS, Mercier F, Boyd CD, Csiszar K, et al. Comparative immunocytochemical localization of lysyl oxidase (LOX) and the lysyl oxidase-like (LOXL) proteins: changes in the expression of LOXL during development and growth of mouse tissues. J Mol Histol. 2004;35:845–55. doi: 10.1007/s10735-004-2340-1. [DOI] [PubMed] [Google Scholar]
- 22.Balestrini JL, Billiar KL. Equibiaxial cyclic stretch stimulates fibroblasts to rapidly remodel fibrin. J Biomech. 2006;39:2983–90. doi: 10.1016/j.jbiomech.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 23.Tarlton JF, Meagher P, Brown RA, McGrouther DA, Bailey AJ, et al. Mechanical stress in vitro induces increased expression of MMPs 2 and 9 in excised Dupuytren's disease tissue. J Hand Surg Br. 1998;23:297–302. doi: 10.1016/s0266-7681(98)80044-2. [DOI] [PubMed] [Google Scholar]
- 24.Gontero P, Di Marco M, Giubilei G, Bartoletti R, Pappagallo G, et al. A pilot phase-II prospective study to test the 'efficacy' and tolerability of a penile-extender device in the treatment of 'short penis'. BJU Int. 2009;103:793–7. doi: 10.1111/j.1464-410X.2008.08083.x. [DOI] [PubMed] [Google Scholar]
- 25.Nikoobakht M, Shahnazari A, Rezaeidanesh M, Mehrsai A, Pourmand G. Effect of penile-extender device in increasing penile size in men with shortened penis: preliminary results. J Sex Med. 2011;8:3188–92. doi: 10.1111/j.1743-6109.2009.01662.x. [DOI] [PubMed] [Google Scholar]
- 26.Brasselet C, Durand E, Addad F, Al Haj Zen A, Smeets MB, et al. Collagen and elastin cross-linking: a mechanism of constrictive remodeling after arterial injury. Am J Physiol Heart Circ Physiol. 2005;289:H2228–33. doi: 10.1152/ajpheart.00410.2005. [DOI] [PubMed] [Google Scholar]
- 27.Mercier N, Kakou A, Challande P, Lacolley P, Osborne-Pellegrin M. Comparison of the effects of semicarbazide and beta-aminopropionitrile on the arterial extracellular matrix in the Brown Norway rat. Toxicol Appl Pharmacol. 2009;239:258–67. doi: 10.1016/j.taap.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Wu C, Fu F, You X, Gao L, et al. Isoflurane inhalation anesthesia should be a new requirement in intracavernosal pressure detection-the gold standard of erectile function assessment. Sci Rep. 2017;7:14949. doi: 10.1038/s41598-017-15020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan J, Lin H, Li P, Zhang R, Luo A, et al. Molecular mechanisms of vacuum therapy in penile rehabilitation: a novel animal study. Eur Urol. 2010;58:773–80. doi: 10.1016/j.eururo.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Yuan J, Westney OL, Wang R. Design and application of a new rat-specific vacuum erectile device for penile rehabilitation research. J Sex Med. 2009;6:3247–53. doi: 10.1111/j.1743-6109.2009.01500.x. [DOI] [PubMed] [Google Scholar]
- 31.Ozbey H, Temiz A, Salman T. A simple method for measuring penile length in newborns and infants. BJU Int. 1999;84:1093–4. doi: 10.1046/j.1464-410x.1999.00366.x. [DOI] [PubMed] [Google Scholar]
- 32.Ning C, Wen J, Zhang Y, Dai Y, Wang W, et al. Excess adenosine A2B receptor signaling contributes to priapism through HIF-1alpha mediated reduction of PDE5 gene expression. FASEB J. 2014;28:2725–35. doi: 10.1096/fj.13-247833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Wang S, Qin F, Zhu M, You X, et al. Reduction in Peyronie's-like plaque size using a vacuum erection device in a rat model of Peyronie's disease via the TGF-beta/SMAD signalling pathway. Andrologia. 2018;50:e13051. doi: 10.1111/and.13051. [DOI] [PubMed] [Google Scholar]
- 34.van Boxtel AL, Kamstra JH, Fluitsma DM, Legler J. Dithiocarbamates are teratogenic to developing zebrafish through inhibition of lysyl oxidase activity. Toxicol Appl Pharmacol. 2010;244:156–61. doi: 10.1016/j.taap.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 35.Fang K, Xing Z, Su J. The Characterization of nano-materials by transmission electron microscope. Mod Sci Instrum. 2003;2:15–7. [Google Scholar]
- 36.Fang Z, Qiu M, Wang C. Light, scanning and transmission electron microscopical observation of the gill structure of swordtail, Xiphophorus helleri. J Chin Electron Micr Soc. 2004;23:553–9. [Google Scholar]
- 37.Zhou F, Li GY, Gao ZZ, Liu J, Liu T, et al. The TGF-beta1/Smad/CTGF pathway and corpus cavernosum fibrous-muscular alterations in rats with streptozotocin-induced diabetes. J Androl. 2012;33:651–9. doi: 10.2164/jandrol.111.014456. [DOI] [PubMed] [Google Scholar]
- 38.Lu YL, Shen ZJ, Wang H, Chen SW, Zhou XL, et al. Ultrastructural changes of penile tunica albuginea in diabetic rats. Asian J Androl. 2004;6:365–8. [PubMed] [Google Scholar]
- 39.Lue TF, El-Sakka AI. Lengthening shortened penis caused by Peyronie's disease using circular venous grafting and daily stretching with a vacuum erection device. J Urol. 1999;161:1141–4. [PubMed] [Google Scholar]
- 40.Levine LA, Rybak J. Traction therapy for men with shortened penis prior to penile prosthesis implantation: a pilot study. J Sex Med. 2011;8:2112–7. doi: 10.1111/j.1743-6109.2011.02285.x. [DOI] [PubMed] [Google Scholar]
- 41.Lin H, Liu C, Wang R. Effect of penile traction and vacuum erectile device for Peyronie's disease in an animal model. J Sex Med. 2017;14:1270–6. doi: 10.1016/j.jsxm.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Bailey AJ, Tarlton JF, Van der Stappen J, Sims TJ, Messina A. The continuous elongation technique for severe Dupuytren's disease. A biochemical mechanism. J Hand Surg Br. 1994;19:522–7. doi: 10.1016/0266-7681(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 43.Levine LA, Newell M, Taylor FL. Penile traction therapy for treatment of Peyronie's disease: a single-center pilot study. J Sex Med. 2008;5:1468–73. doi: 10.1111/j.1743-6109.2008.00814.x. [DOI] [PubMed] [Google Scholar]
- 44.Kurihara T, Shimizu-Hirota R, Shimoda M, Adachi T, Shimizu H, et al. Neutrophil-derived matrix metalloproteinase 9 triggers acute aortic dissection. Circulation. 2012;126:3070–80. doi: 10.1161/CIRCULATIONAHA.112.097097. [DOI] [PubMed] [Google Scholar]
- 45.Yang XL, Yang Y, Fu FD, Wu CJ, Qin F, et al. Optimal pressure in penile rehabilitation with a vacuum erection device: evidence based on a rat model. Asian J Androl. 2019;21:1–6. doi: 10.4103/aja.aja_7_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oakley N, Moore KT. Vacuum devices in erectile dysfunction: indications and efficacy. BJU Int. 1998;82:673–681. doi: 10.1046/j.1464-410x.1998.00823.x. [DOI] [PubMed] [Google Scholar]
- 47.Jia LX, Zhang WM, Zhang HJ, Li TT, Wang YL, et al. Mechanical stretch-induced endoplasmic reticulum stress, apoptosis and inflammation contribute to thoracic aortic aneurysm and dissection. J Pathol. 2015;236:373–83. doi: 10.1002/path.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herchenhan A, Uhlenbrock F, Eliasson P, Weis M, Eyre D, et al. Lysyl oxidase activity is required for ordered collagen fibrillogenesis by tendon cells. J Biol Chem. 2015;290:16440–50. doi: 10.1074/jbc.M115.641670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berry CL, Greenwald SE, Menahem N. Effect of beta-aminopropionitrile on the static elastic properties and blood pressure of spontaneously hypertensive rats. Cardiovasc Res. 1981;15:373–81. doi: 10.1093/cvr/15.7.373. [DOI] [PubMed] [Google Scholar]
- 50.Gao L, Wu C, Fu F, You X, Ma X, et al. Effect of lysyl oxidase (LOX) on corpus cavernous fibrosis caused by ischaemic priapism. J Cell Mol Med. 2018;22:2018–22. doi: 10.1111/jcmm.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Western Blot bands in supplemental data. Different bands from gel (a), (b), and (c). LOX: lysyl oxidase.
Analysis for rats in different groups. (a) No significant difference was found on rat's final weight (P = 0.6467). (b) Control groups revealed insignificantly lower excised penile weight than other three groups (P = 0.9206, n = 6 per group). (c) No significant difference was found on rat's dorsal–ventral (P = 0.7520). (d) No significant difference was found on rat's lateral–lateral (P = 0.8263) diameters. Anti-lysyl oxidase: intragastric administration of ß-aminopropionitrile with a dose of 100 mg kg-1 day-1. VD: aspiration with negative pressure of -300 mmHg.
Transmission electron microscopy in tunica albuginea of corpus cavernosum (n = 2 per group). (a) Fibroblasts with spindle cell nuclei were found among collagen fibers in all groups. Rough endoplasmic reticulum was dilated and mitochondria was swelled in VD and anti-LOX + VD groups (scale bars = 1 µm). (b) Smooth muscle cell which exhibited spindle cell nuclei and homogeneous distributed chromatin, with myofibrils formed dense bodies and well-structured mitochondria rough endoplasmic reticulum found in cytoplasm (scale bars = 1 µm). Anti-lysyl oxidase: intragastric administration of ß-aminopropionitrile with a dose of 100 mg kg-1 day-1. VD: aspiration with negative pressure of -300 mmHg. ▴: mitochondrial structures, ★: rough endoplasmic reticulum, ♦: macular densa beneath cell membrane, ◾: myofibrils formed dense bodies.