Abstract
Summary
Background
Gout flares are driven by interleukin (IL)-1β. Dapansutrile inhibits the NLRP3 inflammasome and subsequent activation of IL-1β. In this study we aimed to investigate the safety and efficacy of orally administered dapansutrile in patients with a gout flare.
Methods
In this open-label, proof-of-concept, phase 2a trial, adult patients (aged 18–80 years) with a monoarticular monosodium urate crystal-proven gout flare were enrolled at an outpatient clinic in the Netherlands and sequentially assigned using a dose-adaptive design to receive 100 mg/day, 300 mg/day, 1000 mg/day, or 2000 mg/day oral dapansutrile for 8 days. The coprimary outcomes were change in patient-reported target joint pain from baseline to day 3 and from baseline to day 7, assessed in the per-protocol population (all patients who received at least 80% of the study drug and had no major protocol deviations). Safety was assessed in the intention-to-treat population. This trial is registered with the EU Clinical Trials Register, EudraCT 2016-000943-14, and is completed.
Findings
Between May 18, 2017, and Jan 21, 2019, 144 patients were assessed for eligibility, of whom 34 were enrolled and 29 were included in the per-protocol population (three patients were excluded due to receiving <80% of study drug and two had major protocol deviations): eight patients received 100 mg/day, seven received 300 mg/day, six received 1000 mg/day, and eight received 2000 mg/day. Between baseline and day 3, there was a mean reduction in patient-reported target joint pain of 52·4% (SD 32·94; p=0∙016) for the 100 mg/day group, 68·4% (34·29; p=0∙016) for the 300 mg/day group, 55·8% (44·90; p=0∙063) for the 1000 mg/day group, and 57·6% (38·72; p=0∙016) for the 2000 mg/day group. At day 7, there was a mean reduction of 82·1% (22·68; p=0∙031) for the 100 mg/day group, 84·2% (16·33; p=0∙016) for the 300 mg/day group, 68·9% (34·89; p=0∙031) for the 1000 mg/day group, and 83·9% (15·44; p=0∙008) for the 2000 mg/day group, compared to baseline. 25 (73·5%) of 34 patients reported a total of 45 treatment-emergent adverse events, most of which were metabolism and nutrition disorders (17 [37·8%]) and gastrointestinal disorders (ten [22·2%]). Two serious adverse events occurred during the study, admission to hospital because of worsening of gout flare at day 3, and admission to hospital because of coronary stenosis 18 days after the patient received their last dose; these were considered moderate in severity and unrelated to the study drug.
Interpretation
Dapansutrile is a specific NLRP3 inflammasome inhibitor with a satisfactory safety profile and efficacy in the reduction of target joint pain in this study. Future studies are needed to confirm the clinical potential of dapansutrile.
Introduction
Gout flares are excruciatingly painful, clinically evident episodes of acute joint inflammation induced by monosodium urate crystals.1,2 Flares are characterised by classic inflammatory symptoms—pain, redness, heat, swelling, and loss of function—each of which reduces quality of life in patients with gout.3 The first metatarsophalangeal, ankle, and knee joints are the joints most often affected during gout flares.4
Gout flares are driven by uptake of deposited monosodium urate crystals by macrophages and consequent activation of the NLRP3 inflammasome, which converts pro-caspase-1 into active caspase-1, leading to proteolytic cleavage of the inactive intracellular interleukin (IL)-1β precursor (pro-IL-1β) to mature, bioactive IL-1β.5 A pro-inflammatory stimulus is first needed for the transcription of the IL-1β gene, after which pro-IL-1β is rapidly converted into its active form by the NLRP3 inflammasome.6,7 Active IL-1β binds to IL-1 receptor 1 and induces a broad range of secondary inflammatory mediators including cytokines, prostaglandins, and chemokines, which lead to infiltration of neutrophils and monocytes and subsequent amplification of joint inflammation.6,8 A prompt interruption of this auto-inflammatory process is needed to achieve control of symptoms, which is the main goal in the management of gout flares.9,10
Current first-line oral therapies for gout flares include drugs with broad anti-inflammatory properties such as non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and colchicine; all associated with serious adverse events.11 Patients with gout often have comorbidities such as hypertension, diabetes, and cardiovascular or renal diseases,12–14 which can result in relative or absolute contraindications for NSAIDS, gluco corticoids, and colchicine. For example, more than 90% of patients with gout have at least one contraindication for NSAIDs, and colchicine use is contraindicated in more than 40% of patients with gout.12 In addition to contraindications, some patients are either intolerant or fail to respond to currently used anti-inflammatory drugs, further limiting the use of available oral therapies for the management of gout flares.12
IL-1β has a major role in inflammation induced by monosodium urate crystals;5,6,15 however, use of parenteral targeted therapies such as IL-1 receptor blockade and IL-1β neutralisation is limited.16 Both canakinumab, a monoclonal antibody against human IL-1β, and anakinra, a recombinant form of IL-1 receptor antagonist, have shown efficacy in randomised controlled trials in gout.17,18 However, these biological drugs have not been widely used because of the need for parenteral administration and their high cost.
Dapansutrile, a novel β-sulfonyl nitrile compound, is an orally active small molecule that selectively inhibits the NLRP3 inflammasome, as demonstrated in neutrophils and human monocyte-derived macrophages.19 Moreover, oral dapansutrile reduces joint inflammation in gouty arthritis induced by monosodium urate crystals in mice.20 A phase 1 trial has demonstrated that dapansutrile is safe in healthy humans at effective plasma levels greater than 100 times those needed to inhibit activation of the NLRP3 inflammasome in vitro.19 Taken together, these studies show that dapansutrile is an NLRP3 inhibitor that reduces IL-1β generation, and that it has pre-clinical and early clinical promise as an oral treatment for gout flares. Here we report the results of the first dose-adaptive, proof-of-concept, phase 2 study of dapansutrile with the following objectives: to assess the safety and tolerability of oral dapansutrile in patients with a gout flare, to assess the clinical activity of various doses in treating signs and symptoms resulting from a gout flare, and to assess dapansutrile-induced changes in inflammatory biomarkers.
Methods
Study design and participants
In this open-label, dose-adaptive, proof-of-concept, phase 2a trial, we enrolled patients with a diagnosis of monoarticular gout flare at an outpatient clinic in the southeast area of the Netherlands. Patients with gout were recruited and screened by the attending investigator and a specialised research nurse.
Adult patients (aged 18–80 years) with a diagnosis of monoarticular gout flare, defined as a clinically evident episode of an acute inflammation induced by monosodium urate crystals, were eligible for inclusion in the trial, including patients with a previous diagnosis of gout and those who had not previously been diagnosed.2 Confirmation was obtained by polarised light microscopic identification of only monosodium urate crystals in the punctate from the target joint (first metatarsophalangeal, ankle, or knee). Patients with comorbidities known to be associated with gout, including metabolic syndrome, hypertension, obesity, and diuretic use, were eligible for inclusion. Patients were ineligible if they used any chronic pain medication, took glucocorticoids within 4 weeks before screening, or took NSAIDs within 12 h before screening. Patients with a glomerular filtration rate less than 40 mL/min were also excluded. To ensure that the patient was in the initial acute phase of a gout flare, confirmation that the gout flare in the target joint began within 96 h before the baseline visit was required. The full inclusion and exclusion criteria are listed in the appendix (p 6).
Each patient provided written informed consent before enrolment, received instructions on the use of the study drug, recording of daily pain responses, and attendance of clinic follow-up visits, and was instructed that no other treatment for gout could be taken for the duration of the 8-day treatment period. If needed, paracetamol was allowed as a rescue medication, starting 12 h after the first dose of study drug for the duration of treatment. No other pain medications, steroids, or treatments for gout flare were allowed during the treatment period.
This study was approved by the ethical review board BEBO NL58629.056.16 (Groningen, Netherlands) and the institutional ethics committee (Venlo, Netherlands) in accordance with the principles of the Declaration of Helsinki and Dutch legislation. The ethics committee and Dutch authorities prohibited the inclusion of a placebo arm. The original clinical trial protocol and statistical analysis plan are available in the appendix (pp 15–80, 81–108). The original protocol was amended to allow the inclusion of an additional group to establish the lowest dose with clinical efficacy, and various inclusion criteria were adjusted to increase the rate of enrolment. A summary of all amendments made to the protocol are available in the appendix (pp 3–4).
Procedures
Dapansutrile capsules (100 mg each) were self-administered during the treatment period beginning at the baseline visit (with the first dose taken under the supervision of site personnel) and continuing up to and including day 7. To determine dosing, a responder-type adaptive dosing scheme was developed for the data monitoring committee and described in the protocol (appendix p 5). This included a responder analysis for a 60% or greater reduction in symptoms at 3 days and 7 days after first administration of dapansutrile, after which the dose for the subsequent group of patients was determined. As this responder analysis was intended to be used only by the data monitoring committee, it does not form part of the final analysis of the study.
The first group of patients was assigned to receive 1000 mg/day dapansutrile. After responder analysis was conducted by the data monitoring committee, the subsequent group of patients received 2000 mg/day. Following responder analysis of the 2000 mg/day group, a dose de-escalation was performed, with the subsequent group of patients receiving 300 mg/day and the final group receiving 100 mg/day. Patients in the 1000 mg/day group took 500 mg dapansutrile twice per day,20 patients in the 2000 mg/day group took 500 mg four times per day, patients in the 300 mg/day took 200 mg in the morning and 100 mg in the evening, and patients in the 100 mg/day group took 100 mg once per day. Patients were asked to score their pain score at the same time as taking the drug: immediately after breakfast and then after dinner.
Patients were screened for eligibility at the baseline visit, returned to the study clinic on days 3, 7, and 14, and were contacted by telephone on day 35 for additional follow-up. Safety assessments were conducted at each visit and consisted of monitoring the occurrence of adverse events, assessing changes in physical examination findings at days 0 and 14, electrocardiogram, vital signs, and laboratory tests consisting of sodium, potassium, creatinine, liver function, haemoglobin, leucocyte counts, platelet counts, and urinalysis. Plasma concentrations of dapansutrile were measured at baseline and at each clinic visit.
Joint pain intensity, general disability, and walking disability were scored in a patient diary on a visual analogue scale (VAS) from 0 mm to 100 mm before treatment initiation, at 2 h, 4 h, 6 h, and 12 h after the first dose (day 0), twice daily on days 1–7 at the same time as taking the drug, and during the study visit on day 14.21 Patient-reported global evaluation of treatment at day 7 was scored on a 0–4 Likert scale (0=poor, 1=fair, 2=good, 3=very good, 4=excellent).22 For comparison with previous published work, responders were defined post-hoc as individuals who reached a pain reduction of 50% or greater at day 3.23
The investigator-assessed Index Joint Score consisted of four measures—tenderness (0=none, 1=mild, 2=moderate, 3=severe), swelling (0=none, 1=mild, 2=moderate, 3=severe), redness (absent or present), and heat (absent or present). Additionally, all signs of inflammation at day 3 and day 7 were compared to baseline and scored on a 0–4 Likert scale (0=no change, 1=slight improvement, 2=major improvement, 3=fully normalised). The investigator-assessed Global Rating of Disease summarises the overall perceived status of the patient’s symptoms by answering the following question: “Considering all of the patient’s signs and symptoms, how well are they doing?” Results were assessed on a 5-point Likert scale (1=poor, 2=fair, 3=good, 4=very good, 5=excellent).
Inflammatory variables assessed were haematology, C-reactive protein (CRP), serum amyloid A (SAA), and circulating cytokines, which were measured in plasma on the Ella platform (Protein Simple, San Jose, CA, USA) using multiplex cartridges for IL-1β, IL-6, and tumour necrosis factor (TNF)α, or specific Quantikine kits (R&D Systems, Minneapolis, MI, USA) for total IL-18 and IL-18 binding protein (IL-18BP).
Peripheral blood mononuclear cells (PBMCs) were isolated from drawn blood by gradient centrifugation using Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden). PBMCs were suspended in Roswell Park Memorial Institute 1640 medium supplemented with 50 mg/mL gentamicin, 2 mM L-glutamine, and 1 mM pyruvate, and were either cultured without or stimulated with lipopolysaccharide (10 ng/mL) with or without monosodium urate crystals (300 μg/mL) for 24 h. Supernatants were removed and assayed for cytokines. The cells were then lysed with 0·5% Triton X-100 in water to measure intracellular cytokines. For analysis of supernatants and lysates from PBMCs, ELISAs specific for pro-IL-1β, mature IL-1β, and IL-6 (R&D Systems) were used.
Outcomes
The coprimary outcomes were the change in patient-reported target joint pain from baseline to day 3 (in the evening) and from baseline to day 7 (in the evening; if the patient did not record a VAS measurement in the evening on day 7, the morning VAS measurement was used).
The main secondary outcome was patient-reported global evaluation of treatment at day 7. Other secondary outcomes were investigator-assessed Index Joint Score and Global Rating of Disease, patient-reported general disability score and walking disability score, and blood concentrations of SAA, CRP, and inflammatory cytokines in addition to white blood cell counts, monocyte counts, and neutrophil counts.
In a post-hoc analysis we also assessed the responder rate, defined as the percentage of patients achieving a 50% or greater pain reduction at day 3 (as previously reported23), based on a historical control of standard of care (eg, naproxen and prednisolone).24
Adverse events were classified according to MedDRA System Organ Class preferred terms.
Statistical analysis
A total of eight patients per group was selected as the minimum group size to ascertain preliminary clinical efficacy based on the inherent variability in the onset of signs and symptoms seen at the clinical centre and the evolution of gout flares over time, and to allow for a robust safety database. This sample size was not designed to allow drop-outs. Therefore, the protocol was adjusted for the discretionary replacement of any patient who did not complete dosing up to the day 7 visit.
All efficacy analyses were performed in the per-protocol population, and safety data were reported for the intention-to-treat population. The per-protocol population consisted of all patients who took at least 80% of the intended dose of dapansutrile capsules up to and including day 3 and had no major protocol violations, as determined by the medical monitor. Baseline characteristics were analysed by descriptive methods (mean [SD], median [IQR], and percentage). Differences from baseline to day 3 and day 7 within each group were assessed with a non-parametric paired test (Wilcoxon matched-pairs signed rank test; GraphPad Prism 5). To evaluate differences between groups, non-parametric tests (Kruskal Wallis or Mann-Whitney test; GraphPad Prism 5) were used. Correlations between clinical and inflammatory parameters were evaluated using Spearman’s ρ correlations calculated in IBM SPSS Statistics 25. All tests were performed two-sided and p values less than 0·05 were considered significant.
This trial is registered with the EU Clinical Trials Register, EudraCT 2016-000943-14, and is completed.
Role of the funding source
The main study funder (Olatec Therapeutics) was involved in the study design, data analysis, data interpretation, and writing of the report. US National Institutes of Health and The Interleukin Foundation had no role in the study. All authors had access to the full study data and the sponsor and investigators share responsibility for the decision to submit for publication.
Results
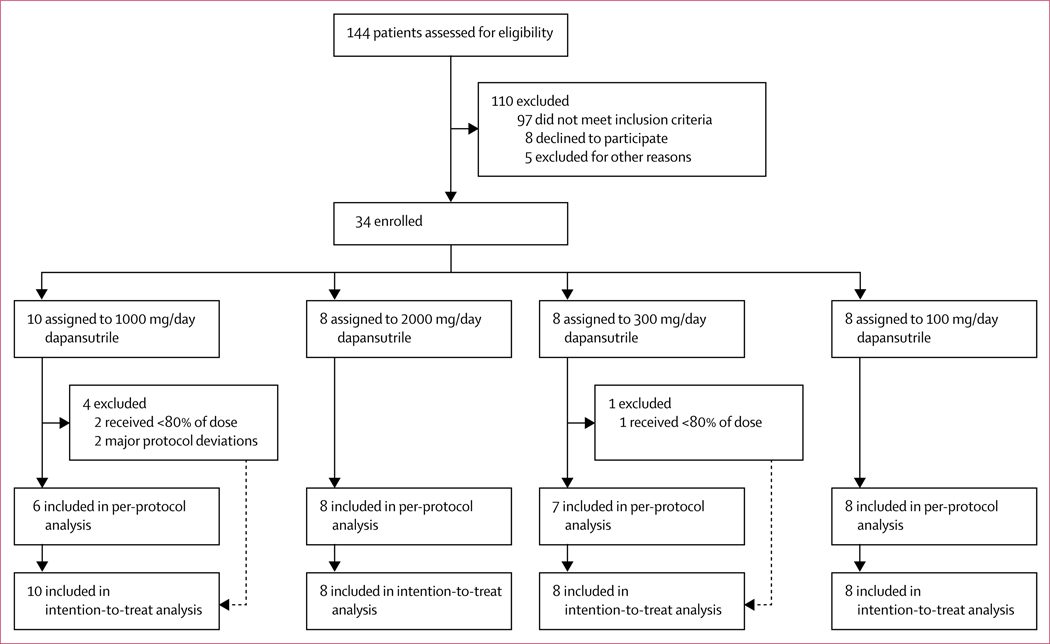
Between May 18, 2017, and Jan 21, 2019, 144 patients were assessed for eligibility. After exclusion of 110 patients who were ineligible or declined to participate, 34 eligible patients were enrolled in the study and were sequentially assigned to the four treatment groups: the first ten patients received 1000 mg/day and the subsequent eight patients received 2000 mg/day, after which a dose de-escalation was performed with the next group of patients receiving 300 mg/day (n=8) and the final group receiving 100 mg/day (n=8; figure 1, appendix p 5). The de-escalation was stopped at 100 mg/day as this dose seemed to be less efficacious in reduction of pain and inflammation than the other doses.
Figure 1:
Trial profile
Five individuals were excluded from the analysis: three patients received less than 80% of the study drug (two in the 1000 mg/day group and one in the 300 mg/day group) and two patients experienced a major deviation from the protocol (both in the 1000 mg/day group; figure 1). The per-protocol population therefore consisted of 29 patients: eight receiving 100 mg/day, seven receiving 300 mg/day, six receiving 1000 mg/day, and eight receiving 2000 mg/day. The per-protocol population consisted of 28 men and one woman, with first metatarsophalangeal (69%), ankle (24%), or knee (7%) as the target joint (table 1). We observed no differences in the use of paracetamol as rescue medication for gout between groups throughout the study (appendix p 7).
Table 1:
Baseline characteristics (per-protocol population)
| 100 mg (n=8) | 300 mg (n=7) | 1000 mg (n=6) | 2000 mg (n=8) | All groups (n=29) | |
|---|---|---|---|---|---|
| General characteristics | |||||
| Age, years | |||||
| Mean (SD) | 62·9 (8·6) | 62·1 (12·2 | 66·7 (11·6) | 50·9 (11·B) | 60·2 (12·1) |
| Median (IQR) | 64·0 (55·8–69·0) | 60·0 (54·5–70·5) | 6B·0 (59·3–74·5) | 46·5 (41·3–63·3) | 62·0 (50·0–70·0) |
| Sex | |||||
| Male | 8 (l00%) | 7 (100%) | 6 (100%) | 7 (87·5%) | 2B (96·6%) |
| Female | 0 | 0 | 0 | 1 (12·5%) | 1 (3·4%) |
| BMI, kg/m2 | |||||
| Mean (SD) | 28·5 (2·0) | 28·l (3·5) | 28·7 (3·3) | 29·1 (4·0) | 2B·6 (3·1) |
| Median (IQR) | 28·7 (27·4–29·9 | 27·7 (27·1–29·4) | 29·0 (27·9–30·7) | 27·7 (26·2–32·1) | 28·l (26·7–30·9 |
| SBP, mm Hg | |||||
| Mean (SD) | 136·6 (11·4) | 142·0 (16·5) | 128·7 (15·4) | 149·3 (17·3) | 139·8 (16·2) |
| Median (IQR) | 134·0 (130·0–139·3) | 136·0 (135·0–147·0) | 129·5 (122·3–136·0) | 141·5 (137·5–159·8) | 136·0 (131·0–145·0) |
| DBP, mm Hg | |||||
| Mean (SD) | 83·5 (10·0) | 90·6 (9·3) | 80·5 (5·0) | 92·3 (16·1) | 87·0 (11·7) |
| Median (IQR) | 82·5 (76·0–88·8) | 90·0 (84·5–96·0) | 80·0 (78·5–80·0) | 86·0 (79·0–104·0) | 82·0 (79·0–94·0) |
| Duration from gout flare to treatment, h | |||||
| Mean (SD) | 63·1 (16·9) | 33·3 (15·9) | 58·3 (20·7) | 45·0 (29·2) | 49·9 (23·6) |
| Median (IQR) | 66·5 (53·5–74·5) | 34·0 (15·0–51·0) | 53·0 (42·0–83·0) | 35·5 (23·5–69·5) | 52·0 (31·0–68·0) |
| Affected joint | |||||
| MTP1 | 5 (62·5%) | 4 (57·1%) | 4 (66·7%) | 7 (87·5%) | 20 (69·0%) |
| Ankle | 3 (37·5%) | 3 (42·9%) | 1 (16·7%) | 0 | 7 (24·1%) |
| Knee | 0 | 0 | 1 (16·7%) | 1 (12·5%) | 2 (6·9%) |
| Laboratory measurements | |||||
| Serum uric acid, pmol/L | |||||
| Mean (SD) | 399 (108) | 439 (159) | 403 (217) | 444 (54) | 422 (134) |
| Median (IQR) | 440 (298–465) | 500 (295–535) | 420 (263–518) | 460 (413–483) | 450 (330–500) |
| CRP, mg/L | |||||
| Mean (SD) | 30·5 (63·8) | 19·6 (16·3) | 22·8 (31·2) | 11·1 (8·6) | 20·9 (36·4) |
| Median (IQR) | 8·8 (3·9–14·5) | 15·0 (7·1–33·0) | 10·1 (7·7–17·0) | 10·8 (4·4–14·5) | 11·0 (5·9–17·0) |
| SAA, mg/L | |||||
| Mean (SD) | 228·5 (614·9) | 49·3 (63·9) | 96·2 (192·7) | 15·3 (14·1) | 99·0 (330·9) |
| Median (IQR) | 10·1 (3·0–25·2) | 29·7 (11·3–51·0) | 23·2 (8·5·30·6) | 10·9 (7·0–19·2) | 16·3 (6·7–29·7) |
| eGFR (MDRD), mL/min per 173m2 | |||||
| Mean (SD) | 68·8 (9·2) | 59·6 (9·5) | 70·0 (11·1) | 83·4 (9·7) | 70·9 (12·8) |
| Median (IQR) | 68·6 (65·6–70·0) | 59·1 (56·7–65·0) | 75·0 (67·3–75·9) | 83·3 (75·3–92·3) | 71·2 (64·8–76·5) |
| HbAlc, mmol/mol | |||||
| Mean (SD) | 40·3 (6·0) | 39·9 (7·0) | 38·2 (11·1) | 35·9 (6·2) | 38·5 (7·4) |
| Median (IQR) | 38·0 (36·5–44·0) | 40·0 (34·0–45·5) | 31·5 (31·0–46·3) | 35·0 (32·5–36·3) | 36·0 (33·0–44·0) |
| Comorbidities | |||||
| Hypertension | 6 (75·0%) | 4 (57·1%) | 5 (83·3%) | 5 (62·5%) | 20 (69·0%) |
| Cardiovascular diseases | 2 (25·0%) | 4 (57·1%) | 4 (66·7%) | 1 (12·5%) | 11 (37·9%) |
| Diabetes | 2 (25·0%) | 1 (14·3%) | 2 (33·3%) | 0 | 4 (13·8%) |
| Clinical scores | |||||
| Pain score | |||||
| Mean (SD) | 64·1 (9·6) | 70·6 (5·9) | 82·2 (14·8) | 68·9 (9·5) | 70·7 (11·6) |
| Median (IQR) | 63·0 (57·0–73·5) | 71·0 (68·0–74·0) | 82·5 (73·0–95·0) | 70·5 (51·0–80·0) | 71·0 (61·0–76·0) |
| General disability | |||||
| Mean (SD) | 62·8 (18·1) | 63·6 (27·8) | 60·7 (24·5) | 68·3 (16·2) | 64·0 (20·7) |
| Median (IQR) | 65·5 (50·0–77·0) | 73·0 (43·0–78·0) | 66·0 (52·0–75·0) | 72·5 (53·0–77·5) | 71·0 (52·0–76·0) |
| Walking disability | |||||
| Mean (SD) | 71·9 (18·5) | 70·3 (14·7) | 79·5 (31·4) | 73·0 (20·1) | 73·4 (20·5) |
| Median (IQR) | 79·5 (65·0–82·5) | 71·0 (60·0–80·0) | 91·5 (75·0–100·0) | 80·5 (61·5–88·5) | 79·0 (60·0–88·0) |
BMI=body-mass index. CRP=C-reactive protein. DBP=diastolic blood pressure. eGFR=estimated glomerular filtration rate. HbA1c=glycated haemoglobin. MDRD=modification of diet in renal disease. MTP1=first metatarsophalangeal. SAA=serum amyloid A. SBP=systolic blood pressure.
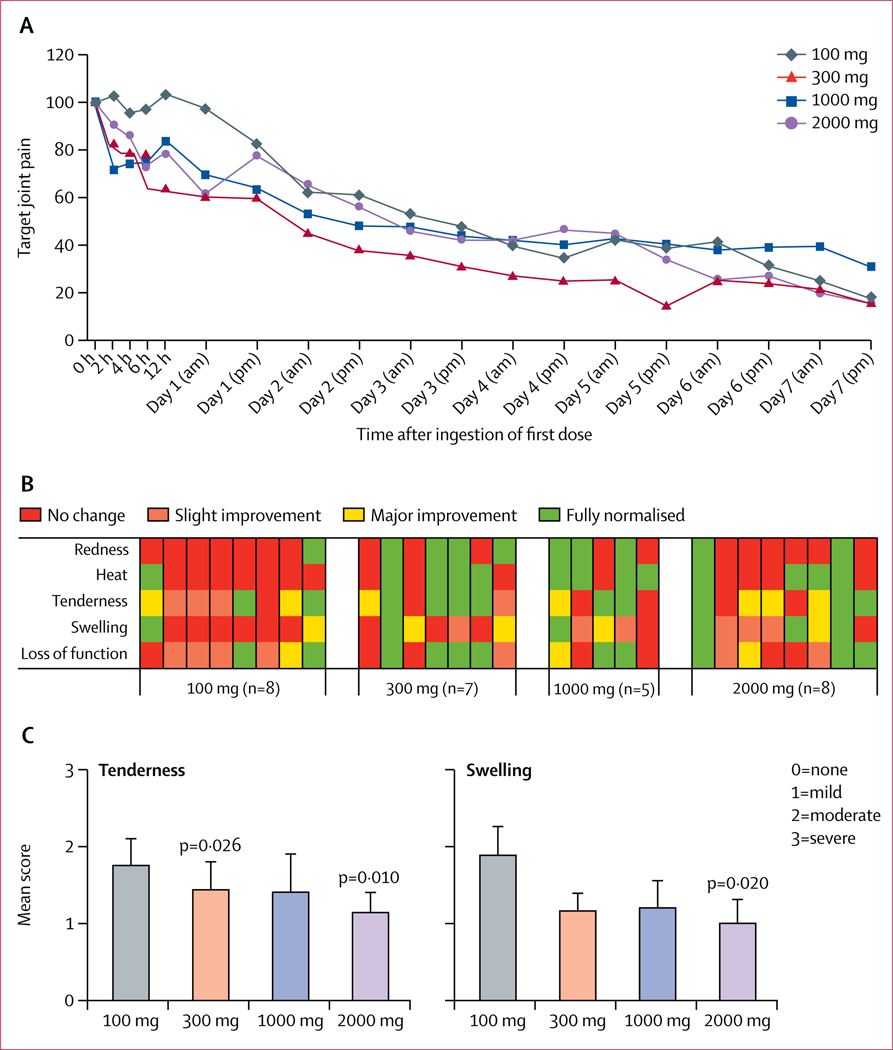
Target joint pain markedly decreased between baseline and day 3, and between baseline and day 7, in all four dose groups without between-group differences (figure 2A). Wilcoxon matched-pairs signed rank tests, two-tailed for differences between baseline and day 3, showed a mean reduction of 52·4% (SD 32·94; p=0∙016) for the 100 mg/day group, 68·4% (34·29; p=0∙016) for the 300 mg/day group, 55·8% (44·90; p=0∙063) for the 1000 mg/day group, and 57·6% (38·72; p=0∙016) for the 2000 mg/day group. At day 7, there was a mean reduction of 82·1% (22·68; p=0∙031) for the 100 mg/day group, 84·2% (16·33; p=0∙016) for the 300 mg/day group, 68·9% (34·89; p=0∙031) for the 1000 mg/day group, and 83·9% (15·44; p=0∙008) for the 2000 mg/day group. Between-group differences were not statistically significant. Results for the intention-to-treat population are shown in the appendix (p 9).
Figure 2: Target joint pain and joint inflammation.
A) Target joint pain for all timepoints. Patients scored target joint pain on a 0–100 mm VAS scale twice daily. Data are presented as mean target joint pain in comparison with the pain score at baseline, which was set at 100%.
(B) Investigator-assessed signs of inflammation. At day 3 a score graded from no change to slight improvement, major improvement, or fully normalised was given for the target joint for all five components of inflammation. Each column represents one individual (one patient in the 1000 mg/day group missed the day 3 visit).
(C) Investigator-scored tenderness at day 3 (mean ± SEM; 0=no pain, 1=mild pain, 2=moderate pain, 3=severe pain), and investigator-scored swelling in target joint at day 3 (mean ± SEM; 0=no swelling, 1=mild swelling, 2=moderate swelling, 3=severe swelling). VAS=visual analogue scale.
Although a large variation in patient-reported global evaluation of treatment was observed within each group, among patients in the 100 mg/day treatment group, three (38%) of eight scored “poor”, the highest frequency reported for any group. In the group taking 2000 mg/day, 3 out of 8 individuals scored ‘excellent’, which is the highest frequency reported for any group.
General disability and walking disability declined in all groups during treatment with dapansutrile without differences between groups (appendix p 8).
At day 3, patients receiving 100 mg/day demonstrated less normalisation of signs of inflammation than the other dose groups, as assessed by the study nurse (figure 2B). Investigator-assessed Index Joint Scores for tenderness and swelling at day 3 showed an apparent dose-dependent improvement, with significant improvement in patients taking 2000 mg/day for tenderness (p=0·010) and swelling (p=0·020); differences between groups were not statistically significant (figure 2C). For joint tenderness the difference between baseline and day 3 was also significant for the 300 mg cohort (p=0·026). Additionally, General Rating of Disease scores showed lower wellbeing on day 3 in patients receiving 100 mg/day than in those receiving 300 mg/day (p=0·044; appendix p 9). At day 7, we did not observe any apparent differences in scores for joint inflammation between dose groups.
The post-hoc analysis of responder rate, defined as the percentage of patients who achieved a 50% or greater pain reduction on day 3, was 75% (six of eight patients) in the 2000 mg/day group, 67% (four of six) in the 1000 mg/day group, 71% (five of seven) in the 300 mg/day group, and 50% (four of eight) in the 100 mg/day group. Additionally, patient-reported pain relief for the 100 mg/day group did not start to change from baseline until about 24 h after treatment (figure 2A).
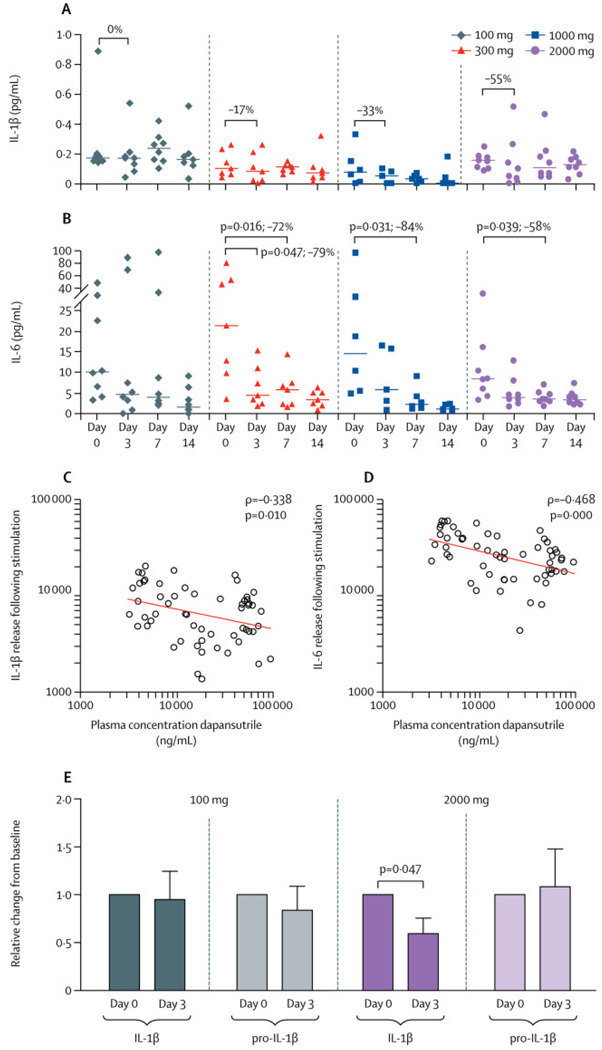
Although plasma IL-1β levels appeared to decrease, there was no significant difference from baseline to day 3 or to day 7 (figure 3A). However, plasma IL-6 levels were significantly reduced at day 7 in patients receiving 2000 mg/day, 1000 mg/day, and 300 mg/day, but not in those receiving 100 mg/day (figure 3B). There were no significant changes in plasma concentrations of TNFα, total IL-18, and IL-18BP during treatment with dapansutrile (appendix p 11). Total white blood cell counts were increased at baseline and declined during treatment with dapansutrile owing to a reduction in monocytes and neutrophils, and SAA was significantly reduced at day 7 in patients receiving 2000 mg/day, 1000 mg/day, and 300 mg/day (appendix p 10).
Figure 3: Plasma cytokine levels and release.
At all timepoints (baseline, day 3, day 7, and day 14) edetic acid plasma was collected and plasma IL-1β (A) and IL-6 (B) determined by Ella (Protein Simple). Data are shown as scatterplots with median for each group. Differences within each group between baseline and day 3 or day 7 were tested by Wilcoxon matched-pairs signed rank test. Plasma concentrations of dapansutrile at day 3 and day 7 for all individuals in the study were correlated to IL-1β (C) and IL-6 (D) release from stimulated peripheral blood mononuclear cells at the same timepoints. Spearman’s correlation was performed to analyse the correlation between the variables. Unstimulated peripheral blood mononuclear cellsfrom individuals taking 2000 mg/day and 100 mg/day were lysed and intracellular mature IL-1β and pro-IL-1β were measured by specific ELISA (E). Data are shown as mean ± SEM of relative change from baseline (value set at 1). One extreme outlier in each group was removed based on Grubb’s test. p values were calculated using Wilcoxon matched-pairs signed rank test for differences between day 0 and day 3. IL=interleukin.
IL-1β secretion upon stimulation was significantly reduced in PBMCs from patients receiving 1000 mg/day and patients receiving 2000 mg/day. For the cohort receiving 2000 mg/day, a 44% reduction of the median IL-1β secretion was observed (p=0·023); for the cohort receiving 1000 mg/day: a 45% reduction of the median was observed (p=0·031); differences were not statistically significant for cohorts receiving 300 mg/day and 100 mg/day. A similar pattern was observed for IL-6 (appendix p 12). For the 2000 mg/day cohort, a 24% reduction of the median was observed (p=0·039) between basline and day 7, but no significant differences were observed for the other cohorts. There was an inverse correlation between plasma levels of dapansutrile and cytokine release from PBMCs at day 3 and 7: higher plasma levels of dapansutrile significantly correlated with lower IL-1β and IL-6 production, which is consistent with NLRP3 inhibition (figure 3C, D). Correlations between clinical variables and inflammatory markers are shown in the appendix (p 13).
In unstimulated PBMCs, intracellular concentrations of processed IL-1β showed a reduction of 41% in the mean and were significantly decreased at day 3 compared with baseline (p=0·047) for the 2000 mg/day dose group, whereas no decrease was observed for the 100 mg/day group (figure 3E). These findings are consistent with the mechanism of action of dapansutrile (inhibition of the processing of pro-IL-1β into active IL-1β).19
Dapansutrile was well tolerated at all doses and no pathological changes were reported in metabolic, physiological, or haematological measurements for any dose group. 25 (73·5%) of 34 patients reported a total of 45 treatment-emergent adverse events (table 2). Most adverse events were related to metabolism and nutrition (17 [37·8%]) and gastrointestinal disorders (ten [22·2%]). All but two of the adverse events related to metabolism and nutrition (decreased appetite and hyponatraemia) were instances of gout or gout flare. Gastrointestinal adverse events included one instance of constipation, three of diarrhoea, three of flatulence, two of abnormal gastrointestinal sounds, and one instance of nausea. Only four (8·9%) adverse events were infections. Two serious adverse events were reported during the study, admission to hospital because of worsening of gout flare at day 3, and admission because of coronary stenosis 18 days after receiving the final dose (appendix p 14); these were considered moderate in severity and unrelated to dapansutrile use by the safety monitoring board. The pharmacokinetic profile of dapansutrile is summarised in the appendix (p 14).
Table 2:
Total adverse events (intention-to-treat population)
| 100 mg/day | 300 mg/day | 1000 mg/day | 2000 mg/day | All groups | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients (n=8) | Events (n=7) | Patients (n=8) | Events (n=6) | Patients (n=10) | Events (n=20) | Patients (n=8) | Events (n=12) | Patients (n=34) | Events (n=45) | ||
| Any serious adverse events | 6 (75·0%) | 7 (100%) | 4 (50.0%) | 6 (100%) | 8 (80·0%) | 20 (100%) | 7 (87·5%) | 12 (100%) | 25 (73 5%) | 45 (100%) | |
| Gastrointestinal disorders | 0 | 0 | 0 | 0 | 4 (40·0%) | 6 (30·0%) | 4 (50·0%) | 4 (333%) | 8 (23 5%) | 10 (22·2%) | |
| Constipation | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12.5%) | 1 (8·3%) | 1 (2·9%) | 1 (2·2%) | |
| Diarrhoea | 0 | 0 | 0 | 0 | 3 (30·0%) | 3 (15·0%) | 0 | 0 | 3 (8·8%) | 3 (67%) | |
| Flatulence | 0 | 0 | 0 | 0 | 2 (20·0%) | 2 (10·0%) | 1 (12·5%) | 1 (8·3%) | 3 (8·8%) | 3 (6·7%) | |
| Abnormal gastrointestinal sounds | 0 | 0 | 0 | 0 | 0 | 0 | 2 (25·0%) | 2 (16·7%) | 2 (5·9%) | 2 (4·4%) | |
| Nausea | 0 | 0 | 0 | 0 | 1 (10·0%) | 1 (5·0%) | 0 | 0 | 1 (2·9%) | 1 (2·2%) | |
| Infections and infestations | 0 | 0 | 1 (12·5%) | 1 (16·7%) | 2 (20·0%) | 2 (10·0%) | 1 (12·5%) | 1 (8·3%) | 4 (11·8%) | 4 (8·9%) | |
| Bronchitis | 0 | 0 | 0 | 0 | 1 (10·0%) | 1 (5·0%) | 0 | 0 | 1 (2·9%) | 1 (2·2%) | |
| Influenza | 0 | 0 | 1 (12·5%) | 1 (16·7%) | 0 | 0 | 0 | 0 | 1 (2·9%) | 1 (2·2%) | |
| Myringitis | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12·5%) | 1 (8·3%) | 1 (2·9%) | 1 (2·2%) | |
| Nasopharyngitis | 0 | 0 | 0 | 0 | 1 (10·0%) | 1 (5·0%) | 0 | 0 | 1 (2·9%) | 1 (2·2%) | |
| Injury, poisoning, and procedural complications | 1 (12·5%) | 1 (14·3%) | 0 | 0 | 1 (10·0%) | 1 (5·0%) | 0 | 0 | 2 (5·9%) | 2 (4·4%) | |
| Joint injury | 1 (12·5%) | 1 (14·3%) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2·9%) | 1 (2·2%) | |
| Post-procedural myocardial Infarction | 0 | 0 | 0 | 0 | 1 (10·0%) | 1 (5·0%) | 0 | 0 | 1 (2·9%) | 1 (2·2%) | |
| Investigations | 0 | 0 | 1 (12·5%) | 2 (33·3%) | 1 (10·0%) | 1 (5·0%) | 0 | 0 | 2 (5·9%) | 3 (6·7%) | |
| Increased C-reactive protein | 0 | 0 | 1 (12·5%) | 1 (16·7%) | 0 | 0 | 0 | 0 | 1 (2·9%) | 1 (2·2%) | |
| Increased serum amyloid A | 0 | 0 | 1 (12·5%) | 1 (16·7%) | 0 | 0 | 0 | 0 | 1 (2·9%) | 1 (2·2%) | |
| protein | |||||||||||
| Weight decreased | 0 | 0 | 0 | 0 | 1 (10·0%) | 1 (5·0%) | 0 | 0 | 1 (2·9%) | 1 (2·2%) | |
| Metabolism and nutrition disorders | 5 (62·5%) | 6 (85·7%) | 3 (37·5%) | 3 (50·0%) | 5 (50·0%) | 6 (30·0%) | 2 (25·0%) | 2 (16·7%) | 15 (44·1%) | 17 (37·8%) | |
| Decreased appetite | 0 | 0 | 0 | 0 | 1 (10·0%) | 1 (5·0%) | 0 | 0 | 1 (2·9%) | 1 (2·2%) | |
| Gout | 5 (62·5%) | 6 (85·7%) | 3 (37·5%) | 3 (50·0%) | 4 (40·0%) | 4 (20·0%) | 2 (25·0%) | 2 (16·7%) | 14 (41·2%) | 15 (33·3%) | |
| Hyponatraemia | 0 | 0 | 0 | 0 | 1 (10·0%) | 1 (5·0%) | 0 | 0 | 1 (2·9%) | 1 (2·2%) | |
| Musculoskeletal and connective | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12·5%) | 1 (8·3%) | 1 (2·9%) | 1 (2·2%) | |
| tissue disorders | |||||||||||
| Arthritis | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12·5%) | 1 (8·3%) | 1 (2·9%) | 1 (2·2%) | |
| Nervous system disorders | 0 | 0 | 0 | 0 | 3 (30·0%) | 3 (15·0%) | 1 (12·5%) | 2 (16·7%) | 4 (11·8%) | 5 (11·4%) | |
| Dizziness | 0 | 0 | 0 | 0 | 1 (10·0%) | 1 (5·0%) | 0 | 0 | 1 (2·9%) | 1 (2·2%) | |
| Dysgeusia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12·5%) | 1 (8·3%) | 1 (2·9%) | 1 (2·2%) | |
| Headache | 0 | 0 | 0 | 0 | 2 (20·0%) | 2 (10·0%) | 1 (12·5%) | 1 (8·3%) | 3 (8·8%) | 3 (6·7%) | |
| Respiratory, thoracic, and mediastinal disorders | 0 | 0 | 0 | 0 | 1 (10·0%) | 1 (5·0%) | 1 (12·5%) | 1 (8·3%) | 2 (5·9%) | 2 (4·4%) | |
| Dyspnoea | 0 | 0 | 0 | 0 | 1 (10·0%) | 1 (5·0%) | 0 | 0 | 1 (2·9%) | 1 (2·2%) | |
| Rhinorrhoea | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12·5%) | 1 (8·3%) | 1 (2·9%) | 1 (2·2%) | |
| Skin and subcutaneous tissue disorders | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12·5%) | 1 (8·3%) | 1 (2·9%) | 1 (2·2%) | |
| Hyperhidrosis | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12·5%) | 1 (8·3%) | 1 (2·9%) | 1 (2·2%) | |
| Adverse events are listed according to MedDRA System Organ Class preferred terms. | |||||||||||
Adverse events are listed according to MedDRA System Organ Class preferred terms.
Discussion
In this open-label, dose-adaptive, proof-of-concept, phase 2a study, oral dapansutrile demonstrated a marked target joint pain reduction in all dose groups. Additionally, a reduction in investigator-assessed joint tenderness and swelling was observed at day 3. The observed reductions in target joint pain and inflammation were accompanied by a significant decline in plasma IL-6, but not the NLRP3-independent cytokine TNFα. Ex vivo studies in cultured cells from the patients suggested that these effects were mediated by inhibition of the NLRP3 inflammasome. Moreover, all doses of dapansutrile were well tolerated with few adverse events related to the study drug.
Dose selection in each group was determined by an adaptive dosing scheme, and after successful treatment of gout flares with doses of 1000 mg/day and 2000 mg/day, a dose de-escalation procedure resulted in patients receiving 300 mg/day and 100 mg/day to ascertain the lowest dose with clinical efficacy. Although the mean percentage change in target joint pain at day 3 was similar among groups, only four (50%) of eight patients in the low-dose (100 mg/day) group achieved 50% or greater pain reduction at day 3. Moreover, a rapid reduction in target joint pain was observed in the three higher-dose groups, whereas the 100 mg/day group showed no change in the first 24 h after treatment. Additionally, at day 3, the investigator-assessed scores for joint inflammation showed a significant reduction in joint tenderness and swelling in patients receiving 2000 mg/day, whereas patients receiving the lowest dose did not show significant reductions. In line with these findings, no reduction in IL-6 was observed in the 100 mg/day group. Taken together, the 100 mg/day group showed less efficacy in treating gout flares than higher dosages.
A notable aspect of this study is the evaluation of circulating cytokines during treatment with dapansutrile. As expected, we observed no change in plasma TNFα, an NLRP3-independent cytokine, during treatment with oral dapansutrile. Although differences in plasma IL-1β were not statistically significant, plasma IL-6 showed a significant decrease. Both in this study and in a larger cohort of 500 healthy Dutch volunteers, plasma IL-1β and IL-6 concentrations significantly correlate, which validates plasma IL-6 as a reliable marker for plasma IL-1β concentrations.25 As the NLRP3 inflammasome also cleaves the IL-18 precursor, plasma levels of IL-18 were evaluated throughout the study. Plasma IL-18 levels have been reported to be higher in patients with gout than in healthy controls;26 however, gout flares are generally not considered to be mediated by IL-18.27 In this study, baseline concentrations of total IL-18 were low compared to levels reported in IL-18-mediated diseases such as macro phage activation syndrome28 and did not change during treatment with dapansutrile.
The proposed mechanism of action of dapansutrile is further supported by our ex vivo assays with isolated PBMCs. Plasma levels of dapansutrile correlated negatively with release of IL-1β and IL-6 from isolated PBMCs upon stimulation. Consistent with the mechanism of action of NLRP3 inhibition, dapansutrile did not affect the level of pro-IL-1β from baseline to day 3, whereas there was a significant reduction of nearly 50% in processed IL-1β in cells from patients treated with 2000 mg/day at the same timepoint. This reduction was not observed in the 100 mg/day group.
Previously, NLRP3-independent processing of the IL-1β precursor by neutrophil-derived serine proteases was reported to have a role in gout flares.29 Although dapansutrile does not directly influence extracellular processing by neutrophil elastase and proteinase-3, we observed a decrease in circulating neutrophils during treatment with dapansutrile, possibly resulting in reduced neutrophil infiltration into the affected joint. Moreover, dapansutrile inhibits caspase-1 in freshly obtained human neutrophils, resulting in decreased release of IL-1β.19 Both mechanisms probably contribute to reduced processing of IL-1β by neutrophils during gout flares in addition to NLRP3 inhibition in myeloid cells.
The general safety of dapansutrile in these small groups was acceptable, even at the highest dose of 2000 mg/day. There were no changes in assessed safety variables, such as metabolic, biochemical, and haematological measurements, and urinalysis, and we observed no serious adverse events related to dapansutrile treatment.
The pain reduction of 56–68% at day 3 in patients receiving 300 to 2000 mg/day dapansutrile are similar to the 65% reduction with NSAIDs and 61% reduction with prednisolone at timepoint 66 h, as previously reported.24 This efficacy and safety profile is also similar to that reported for the IL-1 inhibitors canakinumab and anakinra.17,18 Moreover, the rapid decline in target joint pain observed in the canakinumab trials (a reduction of approximately 22% after 6 h) was also seen with the three higher-dose groups of dapansutrile (a mean reduction of 28% after 6 h in patients taking 2000 mg/day). Compared with currently available treatment options, the combination of its efficacy, safety profile, and oral administration makes dapansutrile a promising treatment option for patients with gout and multiple comorbidities.
A limitation of this study is the small numbers of patients in each group, which were chosen to ascertain preliminary clinical efficacy. The small numbers of patients resulted in variation in the type of joints involved, concentrations of acute-phase proteins at baseline, and duration of flare before start of treatment. These differences could affect the interpretation of dose-response effects. Another explanation for the lack of dose response could be saturation of NLRP3 inhibition with doses above 300 mg/day. Further studies are needed to define the optimal dose for dapansutrile. Another limitation of this study is the lack of a placebo control. However, adding an untreated control group was not ethically justified when effective treatments for gout flares are available. We therefore used a dose de-escalation schedule to determine a dose with inadequate clinical efficacy. Bellamy and colleagues30 described the natural history of gout flares over 7 days in a similar cohort of 11 individuals, providing a comparison for new agents. They described a clinically relevant and statistically significant improvement in joint pain, tenderness, heat, swelling, and erythema as early as day 4 after enrolment in the study. Moreover, two patients withdrew from the study on day 4 because of severe persistent pain and only three patients noted resolution of their pain during the study period.30 Therefore, the early decrease in target joint pain and decreased joint inflammation observed at day 3 in this study are likely to be due to the efficacy of dapansutrile. As the primary objective of this proof-of-concept study was to establish safety and an estimate of the clinical effect of various doses, we did not include an active comparator. Nevertheless, a larger randomised trial with a comparator approved by the Food and Drug Administration or European Medicines Agency is warranted to further explore the potential of dapansutrile for the treatment of gout flares. A key strength of our study is the minimal use of exclusion criteria, which increases the generalisability of our results. The joint distribution, baseline pain scores, and comorbidities seen in our patients are as expected and similar to those seen in other studies.4 Our findings could therefore have implications for a larger population of patients with gout.
In conclusion, dapansutrile reduced target joint pain, and joint and systemic inflammation, in individuals with a monoarticular gout flare and had an acceptable safety profile in this proof-of-concept study. This novel NLRP3 inflammasome inhibitor has substantial potential for further development for the treatment of gout flares and other NLRP3-mediated diseases.
Supplementary Material
Research in context.
Evidence before this study
Gout flares are driven by uptake of deposited monosodium urate crystals by macrophages and consequent activation of the NLRP3 inflammasome; this leads to maturation of interleukin (IL)-1β, which causes fulminant joint inflammation. Dapansutrile is a novel NLPR3 inflammasome inhibitor that has been safely administered in humans using oral and topical formulations. When administered orally, dapansutrile reduced joint inflammation in gouty arthritis induced by monosodium urate crystals in mice and in a broad range of other translational animal models. We searched PubMed (without date or language restrictions) for articles containing the terms “gout” and “NLRP3 inhibition” or “NLRP3 inhibitor”, which returned 51 articles. We also searched ClinicalTrials.gov using the term “NLRP3”, with “gout” as the indication. We did not find, in either database, any clinical study reporting the efficacy and safety of a selective and specific NLRP3 inflammasome inhibitor in patients with gout. The 51 studies identified used in vitro or in vivo models and included reviews. A phase 1 trial has demonstrated that dapansutrile is safe in healthy humans at effective plasma concentrations greater than 100 times those needed to inhibit activation of the NLRP3 inflammasome in vitro.
Added value of this study
To our knowledge, this is the first phase 2 trial of oral dapansutrile in humans. We demonstrated the efficacy of dapansutrile in the treatment of gout flares by showing a reduction in joint pain and inflammatory biomarkers, providing insights into the mechanism of action of the drug, including confirmation of NLRP3 inhibition in peripheral blood mononuclear cells isolated from individuals receiving dapansutrile.
Implications of all the available evidence
Dapansutrile has substantial potential for further development for treatment of gout flares and other chronic diseases in which the NLRP3 inflammasome is implicated, such as type 2 diabetes, atherosclerosis, heart failure, multiple sclerosis, and Alzheimer’s disease, among others.
Acknowledgments
This study was funded by Olatec Therapeutics. The study was also supported by grants from the US National Institutes of Health (AI-15614, to CAD) and the Interleukin Foundation (also to CAD). VK was supported by an MSc–PhD grant from the Radboud University Medical Center (Nijmegen, Netherlands). MCPC was supported by a grant from the Dutch Arthritis Foundation (12-02-303). LABJ is supported by a Competitiveness Operational Programme grant from the Romanian Ministry of European Funds (P_37_762, MySMIS 103587). We thank the study participants and Daniëlle Poeth and Dorine Baselmans (VieCuri Medical Center, Venlo, Netherlands) for their contributions to the conduct of the study. We also acknowledge Robert Barrow for his help in initiating the study and Amy Poshusta for her contributions to data analysis and editing of the report (Olatec Therapeutics, New York, NY, USA).
Funding
Olatec Therapeutics, US National Institutes of Health, and Interleukin Foundation.
Footnotes
Declaration of interests
TLThAJ has received speaker fees from Grünenthal and ad hoc consultant advisory board fees from AbbVie, BMS, Celgene, Grünenthal, Janssen, Menarini, Novartis, and Sanofi Genzyme, and is a member of a clinical advisory board for Olatec Therapeutics (no fee). CAD is head of Olatec Therapeutics’ scientific advisory board and receives personal compensation for this role, and has equity in the company. LABJ is a member of Olatec Therapeutics’ scientific advisory board and has received personal fees from Olatec Therapeutics both related and unrelated to the present study to act as a scientific advisor. CM is the director of Olatec Therapeutics’ Innovative Science Program and has equity in the company. CLS and DBS are employees of Olatec Therapeutics. All other authors declare no competing interests.
Data sharing
Study data will be shared upon reasonable request to damaris.skouras@olatec.com.
References
- 1.Dalbeth N, Choi HK, Joosten LAB, et al. Gout. Nat Rev Dis Primers 2019; 5: 69. [DOI] [PubMed] [Google Scholar]
- 2.Bursill D, Taylor WJ, Terkeltaub R, et al. Gout, Hyperuricaemia and Crystal-Associated Disease Network (G-CAN) consensus statement regarding labels and definitions of disease states of gout. Ann Rheum Dis 2019; 78: 1592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandratre P, Roddy E, Clarson L, Richardson J, Hider SL, Mallen CD. Health-related quality of life in gout: a systematic review. Rheumatology (Oxford) 2013; 52: 2031–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roddy E. Revisiting the pathogenesis of podagra: why does gout target the foot? J Foot Ankle Res 2011; 4: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nat New Biol 2006; 440: 237–41. [DOI] [PubMed] [Google Scholar]
- 6.Dinarello CA. How interleukin-1β induces gouty arthritis. Arthritis Rheum 2010; 62: 3140–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busso N, Ea HK. The mechanisms of inflammation in gout and pseudogout (CPP-induced arthritis). Reumatismo 2011; 63: 230–37. [DOI] [PubMed] [Google Scholar]
- 8.Busso N, So A. Mechanisms of inflammation in gout. Arthritis Res Ther 2010; 12: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis 2017; 76: 29–42. [DOI] [PubMed] [Google Scholar]
- 10.Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken) 2012; 64: 1447–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wechalekar MD, Vinik O, Moi JH, et al. The efficacy and safety of treatments for acute gout: results from a series of systematic literature reviews including Cochrane reviews on intraarticular glucocorticoids, colchicine, nonsteroidal antiinflammatory drugs, and interleukin-1 inhibitors. J Rheumatol Suppl 2014; 92: 15–25. [DOI] [PubMed] [Google Scholar]
- 12.Keenan RT, O’Brien WR, Lee KH, et al. Prevalence of contraindications and prescription of pharmacologic therapies for gout. Am J Med 2011; 124: 155–63. [DOI] [PubMed] [Google Scholar]
- 13.Bardin T, Richette P. Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med 2017; 15: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richette P, Clerson P, Perissin L, Flipo RM, Bardin T. Revisiting comorbidities in gout: a cluster analysis. Ann Rheum Dis 2015; 74: 142–47. [DOI] [PubMed] [Google Scholar]
- 15.So A, Dumusc A, Nasi S. The role of IL-1 in gout: from bench to bedside. Rheumatology (Oxford) 2018; 57(suppl 1): i12–19. [DOI] [PubMed] [Google Scholar]
- 16.Pascart T, Norberciak L, Ea HK, Graf S, Guggenbuhl P, Liote F. Difficult-to-treat gout flares: eligibility for interleukin-1 inhibition in private practice is uncommon according to current EMA approval. Rheumatology (Oxford) 2019; 58: 2181–87. [DOI] [PubMed] [Google Scholar]
- 17.Schlesinger N, Alten RE, Bardin T, et al. Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active-controlled, double-blind trials and their initial extensions. Ann Rheum Dis 2012; 71: 1839–48. [DOI] [PubMed] [Google Scholar]
- 18.Janssen CA, Oude Voshaar MAH, Vonkeman HE, et al. Anakinra for the treatment of acute gout flares: a randomized, double-blind, placebo-controlled, active-comparator, non-inferiority trial. Rheumatology (Oxford) 2019; 58: 1344–52. [DOI] [PubMed] [Google Scholar]
- 19.Marchetti C, Swartzwelter B, Gamboni F, et al. OLT1177, a beta-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc Natl Acad Sci USA 2018; 115: e1530–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchetti C, Swartzwelter B, Koenders MI, et al. NLRP3 inflammasome inhibitor OLT1177 suppresses joint inflammation in murine models of acute arthritis. Arthritis Res Ther 2018; 20: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med 1996; 27: 485–89. [DOI] [PubMed] [Google Scholar]
- 22.Singh JA, Taylor WJ, Dalbeth N, et al. OMERACT endorsement of measures of outcome for studies of acute gout. J Rheumatol 2014; 41: 569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum 2010; 62: 1060–68. [DOI] [PubMed] [Google Scholar]
- 24.Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet 2008; 371: 1854–60. [DOI] [PubMed] [Google Scholar]
- 25.Ter Horst R, Jaeger M, Smeekens SP, et al. Host and environmental factors influencing individual human cytokine responses. Cell 2016; 167: 1111–24.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavalcanti NG, Marques CD, Lins ELTU, et al. Cytokine profile in gout: inflammation driven by IL-6 and IL-18? Immunol Invest 2016; 45: 383–95. [DOI] [PubMed] [Google Scholar]
- 27.Inokuchi T, Moriwaki Y, Tsutsui H, et al. Plasma interleukin (IL)-18 (interferon-gamma-inducing factor) and other inflammatory cytokines in patients with gouty arthritis and monosodium urate monohydrate crystal-induced secretion of IL-18. Cytokine 2006; 33: 21–27. [DOI] [PubMed] [Google Scholar]
- 28.Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol 2013; 4: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joosten LA, Crisan TO, Azam T, et al. Alpha-1-anti-trypsin-Fc fusion protein ameliorates gouty arthritis by reducing release and extracellular processing of IL-1β and by the induction of endogenous IL-1Ra. Ann Rheum Dis 2016; 75: 1219–27. [DOI] [PubMed] [Google Scholar]
- 30.Bellamy N, Downie WW, Buchanan WW. Observations on spontaneous improvement in patients with podagra: implications for therapeutic trials of non-steroidal anti-inflammatory drugs. Br J Clin Pharmacol 1987; 24: 33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.