Abstract
In 2015, public awareness of Zika virus (ZIKV) rose in response to alarming statistics of infants with microcephaly being born to women who were infected with the virus during pregnancy, triggering global concern over these potentially devastating consequences. Although we have discovered a great deal about the genome and pathogenesis of this reemergent flavivirus since this recent outbreak, we still have much more to learn, including the nature of the virus-host interactions and mechanisms that determine its tropism and pathogenicity in the nervous system, which are in turn shaped by the continual evolution of the virus. Inevitably, we will find out more about the potential long-term effects of ZIKV exposure on the nervous system from ongoing longitudinal studies. Integrating clinical and epidemiological data with a wider range of animal and human cell culture models will be critical to understanding the pathogenetic mechanisms and developing more specific antiviral compounds and vaccines.
Keywords: microcephaly, Zika, neurodevelopment
INTRODUCTION
Zika virus (ZIKV) captured the world’s attention in 2015, almost 70 years after it was discovered in the Ziika Forest of Uganda (Dick et al. 1952). In the intervening years, it was not considered a major threat to human health, with only 13 naturally occurring infections in humans reported before 2007 (Wikan & Smith 2016). In 2007, there was an outbreak in the Federated States of Micronesia, with several dozen confirmed cases of patients presenting with a rash and fever (Lanciotti et al. 2008). By 2013, ZIKV had reached French Polynesia and was first associated with Guillain-Barre syndrome (GBS), an autoimmune disorder affecting the peripheral nervous system (PNS) (Cao-Lormeau et al. 2016). In early 2015, ZIKV was considered to be the likely cause of a large-scale outbreak in Brazil in which most people reported symptoms of a mild fever, rash, and/or arthralgia. However, the coincident rise in cases of microcephaly in Brazil during this outbreak led to concerns about the potential lethality of ZIKV and its devastating impact on the developing central nervous system (CNS) (Metsky et al. 2017). Although the immediate question of whether ZIKV caused microcephaly was resolved in the affirmative by early 2016, many questions remained. How did a fairly innocuous virus so quickly evolve into one of the most feared pathogens in the world? In the absence of microcephaly, are there other complications of fetal neurological development? And are there previously unrecognized effects on the adult nervous system? In this review, we focus on the neurological sequelae of ZIKV infection and how our understanding of the virus and its pathogenicity has evolved. We also outline the status of current efforts to prevent ZIKV infections, ameliorate its effects through targeted therapies, and identify functional mutations in the virus that can change the landscape of symptomatology and susceptibility. Finally, we discuss the challenges that lie ahead in identifying ZIKV-associated neurodevelopmental and cognitive impairments.
ZIKV AND MODEL SYSTEMS TO INVESTIGATE ZIKV INFECTION
ZIKV is a member of the Flavivirus genus in the Flaviviridae family. It was discovered independently by two researchers as part of a coordinated effort to track yellow fever virus and identify new viruses in the Ziika Forest of Uganda (Dick et al. 1952). ZIKV was first isolated from a sentinel rhesus monkey following a sustained fever. Serum taken from Rhesus 766 was subsequently injected into groups of mice either intraperitoneally or intracerebrally. All the mice injected intracerebrally showed an adverse reaction, whereas the mice receiving intraperitoneal injections remained healthy, providing the first evidence of ZIKV’s neurotropism in this species. Nonetheless, the few reports of human infections over the next six decades described relatively mild symptoms and did not raise any serious concerns.
After ZIKV reemerged as a potential pathogen for severe CNS developmental disorders in 2015, investigations rapidly proceeded along three parallel tracks: clinical case reports and epidemiological studies, animal models, and cell culture models. The primary focus was to investigate its putative causal role in microcephaly. Epidemiological evidence suggested that ZIKV infections in pregnant women may be linked to a rise in the number of cases of babies born with microcephaly in Brazil (Kleber de Oliveira et al. 2016). The first line of evidence came from reports revealing the presence of ZIKV in the brains of fetuses with microcephaly in women who were infected with ZIKV during pregnancy (Driggers et al. 2016, Mlakar et al. 2016), but it was unclear whether developing neurons and/or proliferating progenitor cells were direct targets of ZIKV. Human cell and animal models helped to definitively answer that question by showing that cortical neural progenitors are the direct target of ZIKV, which leads to cell cycle deficits and increased cell death (Li et al. 2016, Tang et al. 2016). These earliest studies were cited in the report by scientists at the Centers for Disease Control and Prevention declaring that ZIKV infections were causal for microcephaly (Rasmussen et al. 2016). ZIKV is an arthropod-borne virus and its primary mode of transmission is mosquitoes. However, once it was determined that ZIKV was responsible for neurodevelopmental disorders, the discovery that ZIKV can also be sexually transmitted (D’Ortenzio et al. 2016), as well as being vertically transmitted from mother to fetus, set it apart from other flaviviruses and further increased public concern.
Mutations and Evolution of ZIKV
The crystal structure of the mature virus was published in 2016, allowing for a better understanding of how the virus has evolved and how to design vaccines and targeted antibodies and small molecules to block replication (Sirohi et al. 2016). There are two major lineages of ZIKV, Asian and African, each of which has acquired numerous mutations over the past several decades (Beaver et al. 2018). The originally isolated strain from Rhesus 766 in Uganda has been referred to as MR766 and is still used today. The first detection of ZIKV in Asia occurred in 1966 in Malaysia and yielded the prototype Asian strain (P6-740) (Marchette et al. 1969). Sublineages of the Asian strain arising in 2007 in Micronesia (FSM) and 2013 in French Polynesia (H/PF/2013) have generated additional isolates from both human and mosquito, and nearly 200 isolates have been sequenced (Beaver et al. 2018). A systematic comparison between three contemporary strains and an ancestral strain isolated in 2010 identified a single serine-to-asparagine substitution, S139N, in the structural precursor membrane (prM) protein that may be responsible for the dramatic increase in neurovirulence, which likely occurred during the French Polynesia outbreak in 2013. Using a targeted gene editing gain-of-function approach, this substitution led to 100% lethality when injected into early postnatal mouse brains (Yuan et al. 2017).
A study separately mapping infection-related cases of microcephaly for two distinct waves of the ZIKV epidemic in Brazil from 2015 to 2016 showed a surprising lack of correlation between the estimated cases of ZIKV infection in pregnant women and reports of microcephaly in specific regions of Brazil (de Oliveira et al. 2017). Although the reason for this discrepancy is unclear, these surveillance-based analyses raise several interesting possibilities, including a potential resilience to either vertical transmission or neurological consequences based on previous exposure. This observation highlights the importance of ongoing epidemiological analysis to reveal trends in susceptibility and symptomatology and the emergence of functional mutations as well as a more accurate assessment of the number of asymptomatic cases (Mitchell et al. 2019).
Genomic Structure and Life Cycle
Similar in structure to other flaviviruses, ZIKV is a positive-sense, single-stranded RNA around 11,000 nucleotides long consisting of a single open reading frame (ORF) and 5′ and 3′ noncoding regions. The 5′ end contains a type 1 cap structure, and the 3′ untranslated region lacks a poly-A tail. Translation of the long ORF results in a large precursor polyprotein that is cleaved to produce three structural proteins as well as seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) (Figure 1). The life cycle and replication of ZIKV follow four basic steps: RNA translation into viral proteins, replication of viral RNA, assembly of viral particles in the endoplasmic reticulum (ER), and virion release from the host cell. NS5 protein is required to produce the negative-strand RNA needed for RNA synthesis (Garcia-Blanco et al. 2016). Other NS proteins are involved in the formation of replication complexes that associate with intracellular membranes, while NS3 and NS2B form a protease complex that is essential for replication (Li et al. 2017). The structural proteins consist of capsid (C), envelope (E), and prM proteins. The prM and E proteins attach to the host via transmembrane domains, and cleavage of prM is required for the packaging of infectious viral particles following replication. Although parts of the E protein were found to be structurally similar to other flaviviruses such as dengue, West Nile virus (WNV), and Japanese encephalitis, ZIKV has an overall more compact structure, which may contribute to its stability in a wider range of temperatures and bodily fluids, allowing for sexual transmission (Kostyuchenko et al. 2016). A recent study used a FLAG tag expression system to examine each protein and its subcellular localization in more detail (W. Hou et al. 2017). Nuclear localization signals (NLSs) were detected in the C, NS1, NS3, and NS5 proteins, with NS5 being exclusively localized to the nucleus. C and NS1 were localized to both cytoplasm and nucleus, and the remainder of the proteins, PrM, E, NS2A, NS2B, NS3, NS4A, and NS4B, were found only in the cytoplasm despite the NLSs detected in NS3. The C protein associates with at least three different organelles: the nucleoli in the nucleus and the lipid droplet and Golgi apparatus in the cytoplasm. NS2B and NS4A also associate with the Golgi apparatus. The other structural proteins, E and PrM, colocalize with the ER alongside the NS2A protein, suggesting a critical role in the synthesis of new viral proteins. These two structural proteins are often targeted for vaccine development (A. Li et al. 2018).
Figure 1.
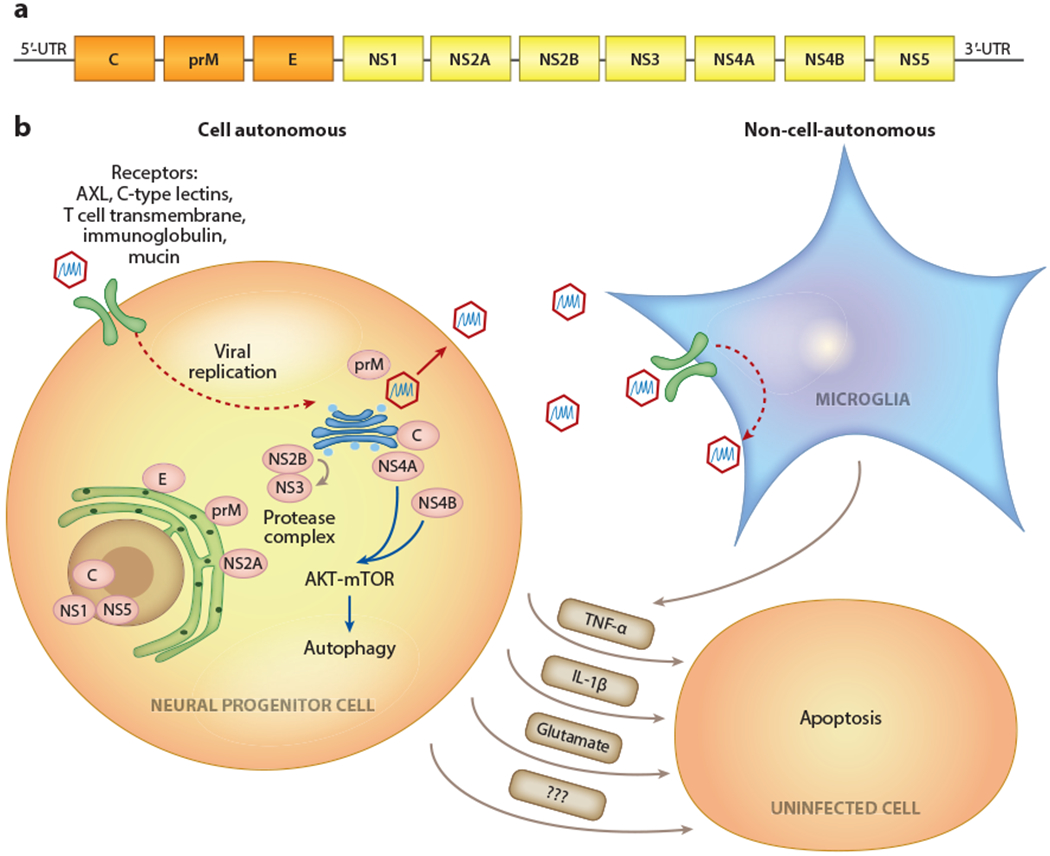
Cell-autonomous and non-cell-autonomous effects of ZIKV infection. (a) The ZIKV genome encodes three structural proteins (C,E, and prM; orange) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5;yellow). (b) At the subcellular localization of ZIKV proteins and associated organelles, the infection of neural progenitor cells can lead to decreased proliferation and apoptosis. Microglia and astrocytes can be productive viral reservoirs but are less susceptible to infection-mediated cell death. Non-cell-autonomous toxic factors released from infected cells can lead to bystander cell death in uninfected populations. Abbreviations: AKT, protein kinase B; C, capsid; E, envelope; IL-1β, interleukin 1 β; mTOR, mammalian target of rapamycin; prM, precursor membrane; TNF-α, tumor necrosis factor α; ZIKV, Zika virus.
Model Systems to Study the ZIKV Impact on the Nervous System
Neurotropic viruses present a considerable challenge to modeling infections in disease-relevant systems. Animal models can provide a physiological environment to interrogate the dynamics between the host organism and the virus, but mechanisms underlying infection and replication may not be fully shared across species, and there may be species-specific differences in susceptibility. Traditionally, the most commonly used human cell lines are not of a neuronal lineage, so they often cannot capture cell type–specific tropism. Recent advances in engineering mouse models that can be productively infected, as well as cellular reprogramming strategies that can generate a renewable resource of human neural cell types, have begun to fill in the gaps left by earlier model systems.
Two- and three-dimensional human cell culture systems.
The ZIKV outbreak coincided with the emergence of advanced models of neural function and brain development based on recently developed technology to generate human neural cell types from stem cells (Takahashi et al. 2007). By the time of the publicized outbreak in Brazil, there were well-established and robust protocols to generate human neural progenitor cells (hNPCs) and neurons from human pluripotent stem cells (hPSCs), including human embryonic stem cells and human induced pluripotent stem cells (hiPSCs). This advance in the stem cell field allowed for one of the earliest and most straightforward studies to determine whether hPSCs, hNPCs, and immature and/or mature neurons could be directly infected by ZIKV and, if so, what the effect would be on cell viability and function. Strikingly, initial observations indicated that ZIKV could directly infect hNPCs, leading to cell death and decreased neurogenesis (Tang et al. 2016). In parallel, more recent advances in the field of cellular reprogramming revealed the potential of these hPSCs to self-organize and form three-dimensional (3D) structures reminiscent of developing brain and biological systems (Kadoshima et al. 2013, Lancaster et al. 2013). Following these seminal discoveries, other groups focused on modeling specific regions and/or developmental time points to generate more homogenous structures that were better suited to model particular aspects of human brain function and development, including corticogenesis (Qian et al. 2016). Using hiPSC-derived 3D brain organoid models, several groups have shown that ZIKV can productively infect hNPC populations, leading to phenotypes reminiscent of microcephaly, which can then be used to screen effective drugs for protection (Dang et al. 2016, Gabriel et al. 2017, Watanabe et al. 2017, Zhou et al. 2017). These models have distinct advantages in allowing for the study of virus-host interactions at the cellular level using the most relevant human cell types and avoiding any confounds arising from species-specific differences in susceptibility (Figure 2).
Figure 2.
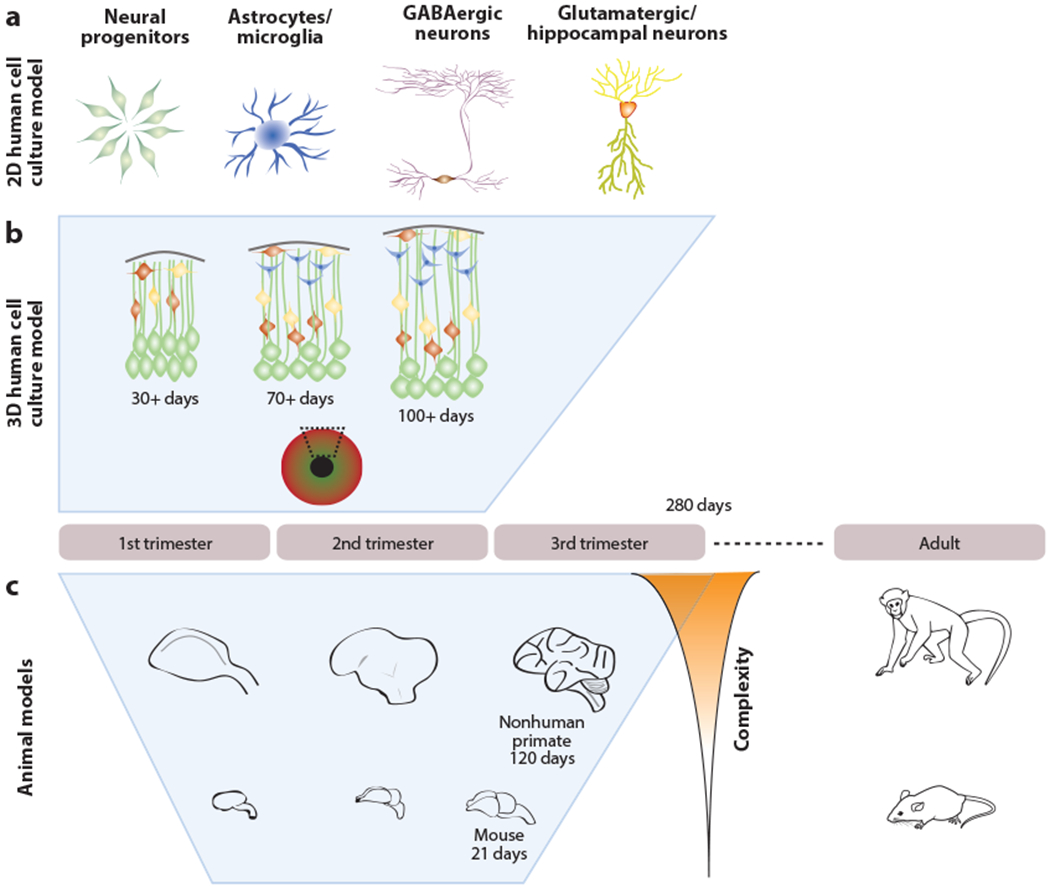
Models of disease using human cell culture and animals. Human induced pluripotent stem cells and animal models capture different properties of brain development and function. (a) 2D cultures of specific cell types can reveal cell type-specific tropism and mechanisms and can also be used to identify quantifiable phenotypes for drug screening and diagnostics. (b) 3D cerebral organoids can mirror morphological and transcriptional dynamics through the first two trimesters of pregnancy. (c) Animal models can be used to investigate the entire gestational period but have species-specific end points of neural complexity and different timescales that may limit the fidelity to human development. Behavioral effects and the impact of viral infections on the adult organism can be modeled using animals.
Mouse models.
Because virus-host interactions are modulated by physiological states and ongoing biological processes, it is also critical to model ZIKV infections in an intact environment in vivo. However, most adult wild-type strains of mice are resistant to ZIKV infections. To engineer susceptible mice, investigators targeted the interferon pathway, which is known to mediate flavivirus infections and antiviral responses in rodents. The first successful models used interferon-α/β receptor-deficient mice (A129) (Dowall et al. 2016) or other immunocompromised mice that either produced minimal interferons or lacked the interferon receptor (Aliota et al. 2016, Lazear et al. 2016). Although initial studies in mice used immune-compromised models, recent studies have shown productive ZIKV infections in immunocompetent mice (Gorman et al. 2018) and that ZIKV infections of neonatal mice can be fatal (S. Li et al. 2018). It had also been shown that ZIKV targets STAT2 for degradation to disrupt the host immune response in human cells but not in mouse cells (Grant et al. 2016). Building on this, a mouse model with a homozygous knockout (KO) of Stat2 was also vulnerable to ZIKV infection and exhibited a similar neurotropism as observed in humans (Tripathi et al. 2017).
Organotypic embryonic mouse brain slice cultures have also been used to study local interactions among cell populations in a preparation that retains some features of neural circuitry (Rosenfeld et al. 2017). Similar to what had been observed in human brain organoids, ZIKV infections resulted in dysregulated neurogenesis, aberrant migration, and apoptosis in infected and neighboring cells (Rosenfeld et al. 2017). Although the slice cultures lose some critical properties of intact physiological systems, this preparation revealed how the spread of infection could potentially be limited by non-cell-autonomous cytotoxicity of adjacent cells.
Nonhuman primate and other animal models.
Although mouse models were instrumental in generating the early data needed to determine that ZIKV was causal for microcephaly, primate models are often better proxies for specific features of human physiology. A recent study in nonhuman primates revealed that infection during early pregnancy results in microcalcifications and vascular abnormalities, in addition to cell death of neural progenitor cells (NPCs) (Martinot et al. 2018). Furthermore, placenta-specific magnetic resonance imaging of rhesus monkeys following infection of pregnant females revealed severely compromised oxygen delivery and a clear maternal-placental-fetal inflammatory response, although no microcephaly was observed (Hirsch et al. 2018). Similar to studies in humans, pregnant rhesus macaques took longer to clear viremia than nonpregnant animals (Nguyen et al. 2017). Supporting evidence for sexual transmission was provided by a marmoset model showing that ZIKV was present in bodily fluids such as semen, urine, and saliva, and ZIKV RNA was detectable for up to two weeks post infection, despite viremia lasting only about one week and an absence of symptoms (Chiu et al. 2017). Recently, other models have been developed in piglets (Darbellay et al. 2017, Wichgers Schreur et al. 2018), which recapitulate many features of neurodevelopmental pathology, including microcephaly, when infected in utero, and olive baboons (Papio anubis), which may prove to be another useful nonhuman primate model that could be more amenable to in vivo monitoring of placental integrity and maternal-fetal immune responses (Gurung et al. 2018). A chicken embryo model revealed dose-dependent ZIKV-induced developmental abnormalities that included reduced brain volume and microcephaly; this model has several advantages based on the extensive literature of developmental biology and virology in this system (Goodfellow et al. 2016).
PATHOPHYSIOLOGY OF ZIKV INFECTION IN THE NERVOUS SYSTEM
Neurogenesis Deficits
As investigators joined forces to determine whether ZIKV was causal for microcephaly, the first study reported in early 2016 that the original MR766 strain could directly target hNPCs derived from hiPSCs (Tang et al. 2016). Subsequently, studies using more recent clinical isolates confirmed similar productive infection and death of NPCs in 2D cultures as well as premature differentiation, aberrant expression of centrosomal proteins, and disrupted centrosomal structure (Gabriel et al. 2017). Disruption of the centrosome and spindle positioning was also observed in HeLa cells after ZIKV infection (Wolf et al. 2017). Adherens junctions, a connective structure formed among NPCs, appear to be vulnerable to ZIKV infection, which leads to their degradation and reduced mouse NPC proliferation in an NS2A-dependent fashion (Yoon et al. 2017). Together, these studies suggest a mechanistic similarity to genetic causes of microcephaly. In vivo, ZIKV infection at embryonic day 12.5 in IFN-deficient mice showed reduced numbers of NPCs and neurons as well as fewer blood vessels in the brain, retina, and placenta at embryonic day 15.5 (Garcez et al. 2018). Recently, a study using both human postmortem tissue and mouse models showed that ZIKV led to ER stress and an unfolded protein response that resulted in a net loss of neurons by affecting neurogenesis (Gladwyn-Ng et al. 2018). Collectively, it appears that there are both direct and indirect mechanisms that contribute to overall restrictions on neurogenesis.
Neuronal Developmental Deficits
To map pathology in the developing brain following in utero exposure to ZIKV, one study characterized CNS lesions in stillborn or newborn babies who died within 48 h of birth (Chimelli et al. 2017). Postmortem tissue pathology revealed that early gestational infection directly infects neuroglial elements, with secondary consequences arising from associated ischemia. Although there was a wide spectrum of histopathology, all samples had some degree of calcification and destructive lesions with agyria. Much of the focus has been on the severe consequences resulting from exposure to ZIKV in early pregnancy, but we still do not know the full range of pathology that falls below the threshold for microcephaly or the extent to which later-stage infections may impact the developing brain, which indeed appears to occur. In several animal models, postnatal ZIKV infections led to various developmental complications, including sustained structural and functional changes in the brain (Mavigner et al. 2018), delayed brain atrophy (Nem de Oliveira Souza et al. 2018), and transient seizure activity (van der Linden et al. 2016). One of the most critical outstanding questions about ZIKV-mediated pathophysiology is how infections in the pregnant mother or developing child, which may go undiagnosed, could lead to long-lasting effects on nervous system function later in life. More long-term animal studies may provide the answer.
Deficits in the Mature Nervous System
ZIKV infection in the adult population has been linked to GBS, transverse myelitis, meningoencephalitis, peripheral neuropathy, and a host of ophthalmological complications (Acosta-Ampudia et al. 2018). Based on these associations, a key question was whether the PNS was a direct target of infection or if the pathology was indirectly due to inflammatory responses, as was suggested by case reports of sensory neuropathy that developed weeks after the initial infection (Martinez et al. 2017). In CNS and PNS myelinating cultures from Ifnar1 KO mice, all CNS cells were vulnerable to ZIKV infection, especially oligodendrocytes, but infection rates of PNS cells were much lower (Cumberworth et al. 2017). A study using both hiPSCs and mouse models showed that ZIKV could directly infect PNS cell populations, especially neurons in vivo and in vitro (Oh et al. 2017). Schwann cells from Ifnar1 KO mice also appeared to be vulnerable, exhibiting disrupted myelin sheath, induced ER stress, and cell death after ZIKV infection (Volpi et al. 2018). A recent imaging study of ZIKV-infected adults who had reported neurological symptoms revealed reduced gray matter volume in specific motor-associated cortical regions, raising alarm about the potential long-term impact on the adult CNS (Bido-Medina et al. 2018).
In both the developing fetus and adults, ZIKV exhibits tropism for components of the visual system (de Paula Freitas et al. 2017). Injection of ZIKV in Ifnar1 KO adult mice induced conjunctivitis, and viral RNA was detected in the intraocular fluid (Miner et al. 2016). Another study found retinopathy and the presence of viral RNA in retinal cells following in utero or early postnatal exposure to ZIKV but minimal effects following adult exposure, which suggests a selective vulnerability before the retina-blood barrier is fully established (Zhao et al. 2017). Examination of eye tissue from fetuses with congenital Zika syndrome revealed the expression of NS2B in the retina, choroid, and optic nerve as well as muscle derived from the neuroectoderm (Fernandez et al. 2017). Müller cells, the principal glial cells in the retina, were also found to be permissive to ZIKV and exhibited a proinflammatory response with the activation of many pathways upon infection (S. Zhu et al. 2017), which could be partly ameliorated by blocking p38 mitogen-activated protein kinase activation. As with most neurological sequelae, ophthalmic complications appear to be more severe during fetal development (Agrawal et al. 2018).
Microglia and Non-Cell-Autonomous Effects
In addition to cell-autonomous effects on NPCs in the brain, non-cell-autonomous effects might also contribute to ZIKV pathology. Apoptosis was observed in both infected and noninfected cortical cells taken from a 20-week-old fetus (Ho et al. 2017). Cell culture studies were the first to demonstrate that ZIKV induces apoptosis in a non-cell-autonomous way through the release of cytotoxic factors, such as tumor necrosis factor α, interleukin 1 β, and glutamate (Olmo et al. 2017), and that blocking GluN2B via ifenprodil was able to reduce NPC death. Other sources of non-cell-autonomous effects and viral reservoirs that could exert long-term consequences are glial cells and microglia. Microglia are the resident macrophage cells in the CNS, and they appear to be a direct target of ZIKV infection based on the examination of human fetal brain tissue from terminated pregnancies (Lum et al. 2017) and hiPSC-derived microglia (Mesci et al. 2018a). Conditioned media alone, taken from infected primary microglia isolated from newborn mice, can inhibit the proliferation of NPCs (Wang et al. 2018), and microglia infection led to the subsequent infection of NPCs when cocultured, resulting in increased NPC death (Mesci et al. 2018a). Interestingly, the infection of NPCs by microglia was blocked by a US Food and Drug Administration–approved drug for hepatitis C, sofosbuvir. Another study examining the cell type–specific effects of ZIKV in hiPSC-derived populations observed direct infection of hNPCs, astrocytes, and microglia-like cells, but caspase-induced apoptosis was only observed in hNPCs (Muffat et al. 2018). Together, these studies suggest that a subset of cell types in the nervous system have an enhanced vulnerability to ZIKV and collectively contribute to the pathology.
MECHANISMS UNDERLYING ZIKV INFECTION AND PATHOGENESIS IN THE NERVOUS SYSTEM
Receptors
ZIKV infections occur following an interaction between the surface receptors in the host cell and viral surface glycoproteins. Several families of proteins have been suggested as possible entry receptors, including AXL family receptor tyrosine kinases, C-type lectins, and T cell TIM (transmembrane, immunoglobulin, and mucin) (Poland et al. 2018). Initially, the AXL receptor was implicated based on the expression pattern and tropism of ZIKV as well as the role of receptor tyrosine kinases in other flavivirus infections (Poland et al. 2018). However, a targeted study to evaluate its role in ZIKV cast doubt on the prevailing hypothesis that AXL was a critical entry point in the brain. Mice with an either heterozygous or homozygous KO of the gene encoding the AXL receptor were not protected against intracerebral injections of ZIKV, demonstrating that the Axl receptor is not a requisite factor for viral entry (Wang et al. 2017). Currently, the role of AXL in mediating viral entry remains controversial, with some studies showing its requirement for the infection of a human fibroblast cell line (HT1080) (Persaud et al. 2018) and others showing that AXL is not a viral entry receptor but rather enhances infection by suppressing ZIKV-induced activation of type 1 interferon genes (Chen et al. 2018). A role for AXL in the host immune response would help explain and reconcile earlier studies showing it to be dispensable in Axl KO mouse models and the effectiveness of interferon alpha receptor blocking antibody treatments (McFadden et al. 2018).
Gene Expression Dysregulation
The first reports of transcriptional dysregulation in hNPCs following ZIKV infection revealed differentially expressed genes related to cell cycle dynamics, transcription, and protein localization (Tang et al. 2016, Zhang et al. 2016). To examine whether ZIKV could be interacting with genetic risk factors in the host that could contribute to microcephaly, one group compared gene expression profiles in hNPCs infected with ZIKV to three mouse models of microcephaly (Ghouzzi et al. 2017) and identified p53 activation as a point of convergence. p53 was further confirmed to be a hub of a ZIKV-activated gene network, and the structural C protein was shown to interact with MDM2, a protein involved in the p53 apoptosis pathway (Teng et al. 2017). In neurospheres derived from hiPSCs, over 500 genes were differentially expressed following ZIKV infection, many of which were enriched for previously identified gene ontology categories related to cell cycle, differentiation, and cellular stress pathways. In addition, this study also revealed a significant involvement of genes related to RNA processing, microRNA biogenesis, and ribosomal proteins (Garcez et al. 2017).
Functional RNA
There is an increasing appreciation for the role of RNA modifications, including m6Amethylation, in cellular function as well as the potential for RNA-binding proteins to mediate interactions between host cells and flaviviruses. In the ZIKV genome, the methyltransferase of NS5 caps viral RNA, allowing for the initiation of translation, while host methyltransferases further methylate viral genomic RNA, allowing for replication (Goertz et al. 2017). The ability of NS5 to methylate host RNA is less clear. A methylated 5′ cap also allows viral RNA to escape detection as exogenous RNA and exploit the intracellular machinery in the host cell for translation. This function of NS5 and its structural similarity to other flaviviral methyltransferases thus make it an attractive target for antiviral drugs. Patterns of m6A methylation differ between the Asian and African strains, but the significance of these differences is not yet known. In the African MR766 isolate, overall m6A methylation levels are negatively correlated with viral replication (Lichinchi et al. 2016). And YTHDF2, a member of the YTHDF family of m6A reader proteins, promotes degradation of ZIKV RNA, similar to its functional role in many mammalian cells (Du et al. 2016). These host-dependent RNA processes suggest a potentially more stable target for therapeutics that would be less susceptible to viral mutations.
G-quadruplexes (G4s) are 4+ contiguous runs of 2+ guanine nucleotides within a short span that form tetrads and folds that can regulate mRNA splicing, translation, and transcription. In recent years, their detection in viruses and their potential regulatory role in the viral life cycle have made these structures a new target of antiviral therapy (Ruggiero & Richter 2018). There are several conserved G4 sequences across Flaviviridae genomes and a ZIKV-specific G4 sequence near the 3′ end of the genome (Fleming et al. 2016). Although promising as a potential target of drugs and small molecules, it is challenging to ensure selectivity for viral rather than host cell G4 sites, and we need a better understanding of the role of these metastructures in RNA regulation and viral replication.
The Musashi family of proteins (MSI) are RNA-binding proteins that are highly expressed in NPCs and have consensus binding sites in the 3′ untranslated region of both African and Asian lineage strains of ZIKV (Chavali et al. 2017). MSI1 directly binds to ZIKV and is critical for ZIKV replication. ZIKV binding ofMSI1 reduces its interaction with microcephalin (MCPH1), a developmentally regulated gene that has been implicated in microcephaly, and CDK6, a regulator of cell cycle and proliferation. ZIKV also appears to target microRNAs, which were depleted in Aedes aegypti mosquitoes, resulting in a disruption of their target immune-related transcripts (Saldana et al. 2017). As an RNA virus, ZIKV may not only induce direct viral-mediated effects that alter cell function but also compete with host RNA-binding proteins to disrupt ongoing posttranscriptional modifications.
Autophagy and Cell Stress Responses
Autophagy appears to play both proviral and antiviral roles in complex host-virus interactions in a cell type-specific manner. For example, NS4A and NS4B have been shown to work together to inhibit protein kinase B–mammalian target of rapamycin (AKT-mTOR) and autophagy pathways to influence human fetal NPC function (Liang et al. 2016). Inhibition of AKT-mTOR also has a proviral role in ZIKV replication and vertical transmission (Chiramel & Best 2017). However, in human umbilical vein endothelial cells, ZIKV induces autophagy that serves to limit viral replication (Peng et al. 2018). Consistent with this finding, KO of Atg16/1, an essential autophagy gene, improved placental and fetal outcomes in a mouse model for ZIKV vertical transmission (Cao et al. 2017).
Similar to the context-dependent role of autophagy, ZIKV has a differential effect on stress granule formation depending on the cellular state (Amorim et al. 2017). Stress granules are large aggregates of stalled translation preinitiation complexes. Under conditions of oxidative stress, phosphorylation of eIF2a inhibits protein synthesis, inducing the formation of stress granules, which restrict the bioavailability of intracellular machinery for translation. In response to viral entry, the formation of stress granules reflects a defensive strategy within the host to reduce viral replication, and many viruses actively suppress the stress response and stress granule assembly. Some research groups found that ZIKV blocks stress granule formation in a phospho-eIF2a-dependent manner (Amorim et al. 2017), while others showed that ZIKV blocks both phosphorylation of eIF2a and stress granule assembly, independent of suppressing host cell protein translation (Roth et al. 2017). Complicating the picture further, another group reported that ZIKV infection induced the phosphorylation of eIF2a and inhibition of translation in addition to the inhibition of stress granule formation (S. Hou et al. 2017). There are several factors that may contribute to these apparently conflicting data, including differences in the host cell type, ZIKV strain, and states of oxidative stress induced by different stressors. These results may also reflect the complex biological responses of host cells to viral infections and have important implications in designing rational treatments.
Virus–Host Protein–Protein Interactions
Viral replication and survival typically rely on co-opting intracellular machinery of the host organism and evading immune responses designed to detect and suppress exogenous pathogens. The use of unbiased screens and bioinformatic strategies to identify novel genes, proteins, and pathways involved in viral replication may lead to targeted development of new treatment strategies. For example, protein–protein interactions can be profiled in an unbiased way using affinity purification–mass spectrometry followed by analytical pipelines to identify key molecular pathways essential biological processes (Morris et al. 2014). Using one such strategy, a recent study did a comparative analysis between dengue virus and ZIKV and identified several overlapping protein–protein interactions between virus and host (Shah et al. 2018). The PAF1C complex is involved in chromatin modification and transcriptional elongation and was among the most highly conserved interactions between the two viruses and their host cells. Functional validation subsequently suggested a model in which both dengue virus and ZIKV NS5 bind to PAF1C to block its interaction with interferon-stimulated genes, thereby suppressing the host immune response. Another shared mechanism appears to be mediated by an NS4A interaction with the SEC61 translocon complex in both mosquito and human cells that is required for early stages of viral replication. Although conserved mechanisms can reveal druggable targets for pan-flavivirus antiviral strategies, it is also important to identify virus-specific interactions that could lead to distinct pathologies, such as microcephaly associated with ZIKV In this same study, ZIKV NS4A showed a specific interaction with ANKLE2 (Shah et al. 2018). Mutations in ANKLE2 are known to cause autosomal recessive microcephaly in humans, and previous studies showed conserved function of this gene in other species. Thus, large-scale, unbiased screens can be used not only to identify divergent and convergent mechanisms underlying viral replication and pathology but also to discover specific targets for drug development.
Genetics and Twin Studies
One of the best predictors of the extent of fetal pathology is the time point during pregnancy when the infection occurs, with early fetal development appearing to be a critical window for ZIKV-associated dysregulation. However, not all women who were infected in early pregnancy went on to develop obvious complications in fetal development. Using primary human fetal cell lines, an Asian strain of ZIKV selectively reduced differentiation in two out of three lines, which could be due to differences in the innate immune response via interferons, cytokines, and complements (McGrath et al. 2017). In an early case report of dizygotic twins in which only one of the two infants was born with microcephaly (van der Linden et al. 2017), a partially compromised placental barrier was proposed. In a more comprehensive recent study of twins, hNPCs generated from dizygotic discordant twins showed different levels of ZIKV replication and proliferation following infection (Caires-Junior et al. 2018). Gene-expression analysis of these hNPCs revealed over 60 genes that were differentially expressed prior to infection, including FOXG1, which has been implicated in microcephaly, and LHX2, which is involved in early neural development and Wnt signaling. These results suggest that there may be a genetic component of resistance or vulnerability that has yet to be identified.
DIAGNOSTICS, VACCINE AND DRUG DEVELOPMENT, AND OTHER ONGOING EFFORTS
Diagnostics
Because most infected adults remain asymptomatic, it is essential from a public health standpoint to have accurate and affordable methods for diagnosis available in all regions of active transmission. Protein biomarkers are another potential target for increasing the accuracy and availability of diagnostics to differentiate among closely related viruses. A recent study showed that the protein array tested was approximately 90% effective in distinguishing between ZIKV and dengue virus using serum from infected patients and uninfected controls (Song et al. 2018). Moving toward field-based diagnostics, one group recently described the development of a paper microfluidic chip that relies on reverse transcription loop–mediated isothermal amplification and imaging that could be performed by a smartphone (Kaarj et al. 2018).
Vaccine Development
Vaccines are proven to be effective in protecting us from various viral infections. In response to the ZIKV pandemic, rapid progress since 2015 has resulted in the identification of several promising candidates for vaccines (Abbink et al. 2018). Currently, there are over 30 vaccine candidates in development, ranging from preclinical studies to phase 1 clinical trials, with the most advanced candidates being DNA vaccines, whole inactivated ZIKV, and vectored vaccines (Poland et al. 2018). The initial target for vaccines was an antibody-mediated disruption of the E protein, based on successful strategies employed for other flaviviruses. Recent efforts have expanded to include the prM protein (Nambala & Su 2018) as well as the polyprotein of prM, E, and NS1 (A. Li et al. 2018). Recombinant vesicular stomatitis virus expressing full-length prM and either full-length or truncated E proteins was shown to be effective in preventing infection in Ifnar−/− mice within 3 days of exposure to ZIKV (Emanuel et al. 2018). The effectiveness of these vaccines in humans remains to be demonstrated.
Drug Development
Among the most important advancements to accompany induced pluripotent stem cell (iPSC) technology is the ability to conduct higher throughput screens of potential drug candidates in relevant human cell types. Animal models are still essential to evaluate drug efficacy in an intact, physiologically relevant biological system, but they are inefficient to employ at a large scale and are better suited to focused, hypothesis-driven or validation experiments. In contrast, although generating and maintaining human neurons derived from iPSCs are costly, these methods allow for large quantities of cells to be produced, which can then be evaluated in parallel in an unbiased manner if there is a clear and robust phenotype that can be used as a readout for drug efficacy. Since the first studies published showing a clear effect of ZIKV on the proliferation and survival of hNPCs, several therapeutic candidates have been identified that either suppress viral replication or ameliorate its consequences on NPCs. Using a drug-repurposing screen of over 6,000 compounds, one of the first drug-screening studies identified niclosamide as an effective antiviral drug that could inhibit ZIKV replication and a pan-caspase inhibitor, emricasan, as protecting NPCs against cell death (Xu et al. 2016). Similar large-scale screening studies revealed that hippeastrine hydrobromide eliminates infection from NPCs in culture and can suppress active infection in the mouse brain, whereas amodiaquine dihydrochloride dihydrate can suppress infection in vitro, and both can reverse transcriptional dysregulation (Zhou et al. 2017). Sofosbuvir, which has received much attention as a clinically approved antiviral for hepatitis C, was shown to inhibit ZIKV replication and block vertical transmission in an immunodeficient mouse model (Ferreira et al. 2017, Mesci et al. 2018b, Sacramento et al. 2017).
Hypothesis-driven drug discovery based on the structure and function of the ZIKV genome has led to the discovery that the synthetic peptide Z2, derived from the stem region of ZIKV envelope protein, disrupts the integrity of ZIKV viral membrane and, importantly, can penetrate the placenta, as intraperitoneal injections in mice inhibit vertical transmission (Yu et al. 2017). Silvestrol is another compound that was shown to have a robust antiviral effect on other RNA viruses such as Ebola and corona- and picornaviruses by disrupting translation via inhibition of eIF4A, and it was also effective against two strains of ZIKV (Elgner et al. 2018). Despite the controversy over its functional role in mediating viral entry or modulating the host immune response, specific targeting of the AXL receptor has led to the identification of new antiviral compounds that block AXL dimerization (Sarukhanyan et al. 2018). Rational design will be further facilitated by the development of 3D cell culture models that better recapitulate the target biological system, such as cerebral organoids that capture morphological and transcriptional features of early fetal brain development (Qian et al. 2017, Watanabe et al. 2017).
Ongoing Efforts to Identify Submicrocephaly and Long-Term Outcomes
The Centers for Disease Control and Prevention partnered with Brazil’s Ministry of Health to identify infants and children up to two years old who were born with ZIKV-related microcephaly in the Zika Outcomes and Development in Infants and Children (ZODIAC) study. Data collection in the ZODIAC study was completed in late 2017, and a subsequent report documented a host of ongoing behavioral and developmental problems related to congenital ZIKV infection, including chronic seizures, sleep disorders, and motor dysfunction (Satterfield-Nash et al. 2017). The Zika in Infants and Pregnancy study is a large-scale prospective study that began in 2016 and is currently underway to identify up to 10,000 pregnant women in regions with active ZIKV transmission and to track the status of the women through pregnancy and the health of the fetus and newborn for at least one year. This type of study is urgently needed to identify emergent pathology in women infected with ZIKV whose babies do not present with fetal microcephaly. Animal and cell culture models have revealed a spectrum of cellular phenotypes in response to ZIKV infections, many of which provide independent support for the potential for neural pathology aside from microcephaly. 3D models will be particularly informative in suggesting the types of pathology we may see in developing fetuses who do not meet the criteria for microcephaly, and it is critical to be able to perform longitudinal studies in developing children following in utero exposure to ZIKV. We know from twin studies that there may be differences in fetal exposure as well as susceptibility to ZIKV. Furthermore, we need to look beyond gross developmental aberrations and begin to evaluate more subtle differences that may emerge in brain connectivity and function, which could lead to earlier behavioral interventions (Adams Waldorf et al. 2018, Walker et al. 2019).
Viral Vector Engineering
Although the consequences of ZIKV infection can be devastating, particularly in its effect on the developing CNS, it is also possible to exploit this RNA virus, which is so highly neurotropic and selective for proliferating cells, for therapeutic applications. A provocative study has shown that ZIKV was highly selective for patient-derived glioblastoma stem cells over differentiated glioma cells in vitro and was also effective in targeting tumors in a mouse model of glioblastoma (Z. Zhu et al. 2017). This is an exciting area of research that could potentially lead to a novel approach to cancer treatment in the mature nervous system.
Microbiota
Currently, there is a surge of research devoted to understanding how the microbiota contributes to the homeostatic regulation of physiological functions as well as its role in an immunological response to a perturbagen. Oral antibiotics have been shown to increase the susceptibility to and the severity of symptoms in WNV, dengue, and ZIKV (Thackray et al. 2018). However, topical antibiotics have been shown to enhance the host immune response in a microbiota-independent manner (Gopinath et al. 2018). There is also an active area of research investigating the role of the gut microbiota in the most prominent vectors of flavivirus transmission (Taracena et al. 2018). Although we are still in the early stages, this area of research is proving to be an important dimension of understanding the dynamics of virus-host interactions and potential differences in susceptibility and reactivity among the population.
CONCLUSION
Since the onset of the ZIKV epidemic in the Americas, several critical lessons have been learned. Of primary importance is the biological insight into the causal role of ZIKV in the emergence of severe developmental and neurological disorders and the public health campaign that was implemented based on that knowledge. Importantly, there was also recognition across the scientific community of the urgency of these studies and a willingness to share data and work together to publish studies in a timely manner. As a result of this increased research focused on ZIKV, the number of studies continues to rise even as the media spotlight begins to wane. Given that virus–host interactions are a dynamic phenomenon, subject to evolving with acquired viral mutations, continued efforts are needed to understand the biology of this particular flavivirus and why it can lead to such devastating consequences in a subset of the population. Perhaps the most pressing concern currently is the fact that ZIKV-related neurological complications can also emerge during later stages of development, even without a confirmed diagnosis of ZIKV infection or long after the virus is detectable. This suggests that there may be an unrecognized population of children who could be subject to long-term effects following in utero exposure, and all children born to mothers who were infected during pregnancy should continue to be monitored over the long-term. Because we still do not know the full spectrum of consequences that could arise following ZIKV exposure, we must continue to be vigilant and work toward better diagnostics, preventative vaccines, and a better understanding of the basic mechanisms of its pathogenesis.
ACKNOWLEDGMENTS
Research in the authors’ laboratories was supported by the US National Institutes of Health (R35NS097370 and U19AI131130 to G.-L.M., R21MH118037 to K.M.C., and R37NS047344 and U19MH106434 to H.S.).
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Abbink P, Stephenson KE, Barouch DH. 2018. Zika virus vaccines. Nat. Rev. Microbiol 16:594–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Ampudia Y, Monsalve DM, Castillo-Medina LF, Rodriguez Y, Pacheco Y, et al. 2018. Autoimmune neurological conditions associated with Zika virus infection. Front. Mol. Neurosci 11:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams Waldorf KM, Olson EM, Nelson BR, Little ME, Rajagopal L. 2018. The aftermath of Zika: need for long-term monitoring of exposed children. Trends Microbiol. 26:729–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal R, Oo HH, Balne PK, Ng L, Tong L, Leo YS. 2018. Zika virus and the eye. Ocul. Immunol. Inflamm 26:654–59 [DOI] [PubMed] [Google Scholar]
- Aliota MT, Caine EA, Walker EC, Larkin KE, Camacho E, Osorio JE. 2016. Characterization of lethal Zika virus infection in AG129 mice. PLOS Negl. Trop. Dis 10:e0004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim R, Temzi A, Griffin BD, Mouland AJ. 2017. Zika virus inhibits eIF2 α-dependent stress granule assembly. PLOS Negl. Trop. Dis 11:e0005775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JT, Lelutiu N, Habib R, Skountzou I. 2018. Evolution of two major Zika virus lineages: implications for pathology, immune response, and vaccine development. Front. Immunol 9:1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bido-Medina R, Wirsich J, Rodriguez M, Oviedo J, Miches I, et al. 2018. Impact of Zika virus on adult human brain structure and functional organization. Ann. Clin. Transl. Neurol 5:752–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caires-Junior LC, Goulart E, Melo US, Araujo BHS, Alvizi L, et al. 2018. Discordant congenital Zika syndrome twins show differential in vitro viral susceptibility of neural progenitor cells. Nat. Commun 9:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Parnell LA, Diamond MS, Mysorekar IU. 2017. Inhibition of autophagy limits vertical transmission of Zika virus in pregnant mice. J. Exp. Med 214:2303–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, et al. 2016. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387:1531–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavali PL, Stojic L, Meredith LW, Joseph N, Nahorski MS, et al. 2017. Neurodevelopmental protein Musashi-1 interacts with the Zika genome and promotes viral replication. Science 357:83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yang YF, Yang Y, Zou P, Chen J, et al. 2018. AXL promotes Zika virus infection in astrocytes by antagonizing type I interferon signalling. Nat. Microbiol 3:302–9 [DOI] [PubMed] [Google Scholar]
- Chimelli L, Melo ASO, Avvad-Portari E, Wiley CA, Camacho AHS, et al. 2017. The spectrum of neuropathological changes associated with congenital Zika virus infection. Acta Neuropathol. 133:983–99 [DOI] [PubMed] [Google Scholar]
- Chiramel AI, Best SM. 2017. Role of autophagy in Zika virus infection and pathogenesis. Virus Res. 254:34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CY, Sanchez-San Martin C, Bouquet J, Li T, Yagi S, et al. 2017. Experimental Zika virus inoculation in a new world monkey model reproduces key features of the human infection. Sci. Rep 7:17126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberworth SL, Barrie JA, Cunningham ME, Gomes de Figueiredo DP, Schultz V, et al. 2017. Zika virus tropism and interactions in myelinating neural cell cultures: CNS cells and myelin are preferentially affected. Acta Neuropathol. Commun 5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, et al. 2016. Evidence of sexual transmission of Zika virus. N. Engl. J. Med 374:2195–98 [DOI] [PubMed] [Google Scholar]
- Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, et al. 2016. Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell 19:258–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbellay J, Cox B, Lai K, Delgado-Ortega M, Wheler C, et al. 2017. Zika virus causes persistent infection in porcine conceptuses and may impair health in offspring. EBioMedicine 25:73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira WK, de Franca GVA, Carmo EH, Duncan BB, de Souza Kuchenbecker R, Schmidt MI. 2017. Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: a surveillance-based analysis. Lancet 390:861–70 [DOI] [PubMed] [Google Scholar]
- de Paula Freitas B, Ventura CV, Maia M, Belfort R Jr. 2017. Zika virus and the eye. Curr. Opin. Ophthalmol 28:595–99 [DOI] [PubMed] [Google Scholar]
- Dick GW, Kitchen SF, Haddow AJ. 1952. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg 46:509–20 [DOI] [PubMed] [Google Scholar]
- Dowall SD, Graham VA, Rayner E, Atkinson B, Hall G, et al. 2016. A susceptible mouse model for Zika virus infection. PLOS Negl. Trop. Dis 10:e0004658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jaaskelainen AJ, et al. 2016. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N. Engl. J. Med 374:2142–51 [DOI] [PubMed] [Google Scholar]
- Du H, Zhao Y, He J, Zhang Y, Xi H, et al. 2016. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun 7:12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgner F, Sabino C, Basic M, Ploen D, Grunweller A, Hildt E. 2018. Inhibition of Zika virus replication by silvestrol. Viruses 10:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel J, Callison J, Dowd KA, Pierson TC, Feldmann H, Marzi A. 2018. A VSV-based Zika virus vaccine protects mice from lethal challenge. Sci. Rep 8:11043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez MP, Parra Saad E, Ospina Martinez M, Corchuelo S, Mercado Reyes M, et al. 2017. Ocular histopathologic features of congenital Zika syndrome. JAMA Ophthalmol. 135:1163–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira AC, Zaverucha-do-Valle C, Reis PA, Barbosa-Lima G, Vieira YR, et al. 2017. Sofosbuvir protects Zika virus-infected mice from mortality, preventing short- and long-term sequelae. Sci. Rep 7:9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AM, Ding Y, Alenko A, Burrows CJ. 2016. Zika virus genomic RNA possesses conserved G-quadruplexes characteristic of the Flaviviridae family. ACS Infect. Dis 2:674–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel E, Ramani A, Karow U, Gottardo M, Natarajan K, et al. 2017. Recent Zika virus isolates induce premature differentiation of neural progenitors in human brain organoids. Cell Stem Cell 20:397–406.e5 [DOI] [PubMed] [Google Scholar]
- Garcez PP, Nascimento JM, de Vasconcelos JM, Madeiro da Costa R, Delvecchio R, et al. 2017. Zika virus disrupts molecular fingerprinting of human neurospheres. Sci. Rep 7:40780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcez PP, Stolp HB, Sravanam S, Christoff RR, Ferreira J, et al. 2018. Zika virus impairs the development of blood vessels in a mouse model of congenital infection. Sci. Rep 8:12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Blanco MA, Vasudevan SG, Bradrick SS, Nicchitta C. 2016. Flavivirus RNA transactions from viral entry to genome replication. Antiviral Res. 134:244–49 [DOI] [PubMed] [Google Scholar]
- Ghouzzi VE, Bianchi FT, Molineris I, Mounce BC, Berto GE, et al. 2017. ZIKA virus elicits P53 activation and genotoxic stress in human neural progenitors similar to mutations involved in severe forms of genetic microcephaly and p53. Cell Death Dis. 8:e2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwyn-Ng I, Cordon-Barris L, Alfano C, Creppe C, Couderc T, et al. 2018. Stress-induced unfolded protein response contributes to Zika virus-associated microcephaly. Nat. Neurosci 21:63–71 [DOI] [PubMed] [Google Scholar]
- Goertz GP, Abbo SR, Fros JJ, Pijlman GP. 2017. Functional RNA during Zika virus infection. Virus Res. 254:41–53 [DOI] [PubMed] [Google Scholar]
- Goodfellow FT, Tesla B, Simchick G, Zhao Q, Hodge T, et al. 2016. Zika virus induced mortality and microcephaly in chicken embryos. Stem Cells Dev. 25:1691–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath S, Kim MV, Rakib T, Wong PW, van Zandt M, et al. 2018. Topical application of aminoglycoside antibiotics enhances host resistance to viral infections in a microbiota-independent manner. Nat. Microbiol 3:611–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman MJ, Caine EA, Zaitsev K, Begley MC, Weger-Lucarelli J, et al. 2018. An immunocompetent mouse model of Zika virus infection. Cell Host Microbe 23:672–85.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A, Ponia SS, Tripathi S, Balasubramaniam V, Miorin L, et al. 2016. Zika virus targets human STAT2 to inhibit Type I interferon signaling. Cell Host Microbe 19:882–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung S, Preno AN, Dubaut JP, Nadeau H, Hyatt K, et al. 2018. Translational model of Zika virus disease in baboons. J. Virol 92:e00186–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch AJ, Roberts VHJ, Grigsby PL, Haese N, Schabel MC, et al. 2018. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat. Commun 9:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CY, Ames HM, Tipton A, Vezina G, Liu JS, et al. 2017. Differential neuronal susceptibility and apoptosis in congenital Zika virus infection. Ann. Neurol 82:121–27 [DOI] [PubMed] [Google Scholar]
- Hou S, Kumar A, Xu Z, Airo AM, Stryapunina I, et al. 2017. Zika virus hijacks stress granule proteins and modulates the host stress response. J. Virol 91:e00474–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W, Cruz-Cosme R, Armstrong N, Obwolo LA, Wen F, et al. 2017. Molecular cloning and characterization of the genes encoding the proteins of Zika virus. Gene 628:117–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaarj K, Akarapipad P, Yoon JY. 2018. Simpler, faster, and sensitive Zika virus assay using smartphone detection of loop-mediated isothermal amplification on paper microfluidic chips. Sci. Rep 8:12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoshima T, Sakaguchi H,Nakano T, Soen M, Ando S, et al. 2013. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. PNAS 110:20284–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber de Oliveira W, Cortez-Escalante J, De Oliveira WT, do Carmo GM, Henriques CM, et al. 2016. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy—Brazil, 2015. Morb. Mortal. Wkly. Rep 65:242–47 [DOI] [PubMed] [Google Scholar]
- Kostyuchenko VA, Lim EX, Zhang S, Fibriansah G, Ng TS, et al. 2016. Structure of the thermally stable Zika virus. Nature 533:425–28 [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, et al. 2013. Cerebral organoids model human brain development and microcephaly. Nature 501:373–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kosoy OL, Laven JJ,Velez JO, Lambert AJ, et al. 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis 14:1232–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, et al. 2016A mouse model of Zika virus pathogenesis. Cell Host Microbe 19:720–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Yu J, Lu M, Ma Y, Attia Z, et al. 2018. A Zika virus vaccine expressing premembrane-envelope-NS1 polyprotein. Nat. Commun 9:3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xu D, Ye Q, Hong S,Jiang Y, et al. 2016. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell 19:120–26 [DOI] [PubMed] [Google Scholar]
- Li S, Armstrong N, Zhao H, Hou W, Liu J, et al. 2018. Zika virus fatally infects wild type neonatal mice and replicates in central nervous system. Viruses 10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Brecher M, Deng YQ, Zhang J, Sakamuru S, et al. 2017. Existing drugs as broad-spectrum and potent inhibitors for Zika virus by targeting NS2B-NS3 interaction. Cell Res. 27:1046–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q, Luo Z, Zeng J, Chen W, Foo SS, et al. 2016. Zika virus NS4A and NS4B proteins deregulate Akt-mTOR signaling in human fetal neural stem cells to inhibit neurogenesis and induce autophagy. Cell Stem Cell 19:663–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichinchi G, Zhao BS, Wu Y, Lu Z, Qin Y, et al. 2016. Dynamics of human and viral RNA methylation during Zika virus infection. Cell Host Microbe 20:666–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum FM, Low DK, Fan Y, Tan JJ, Lee B, et al. 2017. Zika virus infects human fetal brain microglia and induces inflammation. Clin. Infect. Dis 64:914–20 [DOI] [PubMed] [Google Scholar]
- Marchette NJ, Garcia R, Rudnick A. 1969. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am. J. Trop. Med. Hyg 18:411–15 [DOI] [PubMed] [Google Scholar]
- Martinez ARM, Costa MCM, Novaes MAC, Lima HC, Nucci A, Franca MC Jr. 2017A novel phenotype of ZIKV-related neurological disease: sensory neuronopathy. Muscle Nerve 57:E100–1 [DOI] [PubMed] [Google Scholar]
- Martinot AJ, Abbink P, Afacan O, Prohl AK, Bronson R, et al. 2018. Fetal neuropathology in Zika virus-infected pregnant female rhesus monkeys. Cell 173:1111–22.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavigner M, Raper J, Kovacs-Balint Z, Gumber S, O’Neal JT, et al. 2018. Postnatal Zika virus infection is associated with persistent abnormalities in brain structure, function, and behavior in infant macaques. Sci. Transl. Med 10:eaao6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden MJ, Mitchell-Dick A, Vazquez C, Roder AE, Labagnara KF, et al. 2018A fluorescent cell-based system for imaging Zika virus infection in real-time. Viruses 10:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath EL, Rossi SL, Gao J, Widen SG, Grant AC, et al. 2017. Differential responses of human fetal brain neural stem cells to Zika virus infection. Stem Cell Rep. 8:715–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesci P, Macia A, LaRock CN, Tejwani L, Fernandes IR, et al. 2018a. Modeling neuro-immune interactions during Zika virus infection. Hum. Mol. Genet 27:41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesci P, Macia A, Moore SM, Shiryaev SA, Pinto A, et al. 2018b. Blocking Zika virus vertical transmission. Sci. Rep 8:1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsky HC, Matranga CB, Wohl S, Schaffner SF, Freije CA, et al. 2017. Zika virus evolution and spread in the Americas. Nature 546:411–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, Sene A, Richner JM, Smith AM, Santeford A, et al. 2016. Zika virus infection in mice causes panuveitis with shedding of virus in tears. Cell Rep. 16:3208–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PK, Mier-y-Teran-Romero L, Biggerstaff BJ, Delorey MJ, Aubry M, et al. 2019. Reassessing serosurvey-based estimates of the symptomatic proportion of Zika virus infections. Am. J. Epidemiol 188:206–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, et al. 2016. Zika virus associated with microcephaly. N. Engl. J. Med 374:951–58 [DOI] [PubMed] [Google Scholar]
- Morris JH, Knudsen GM, Verschueren E,Johnson JR, Cimermancic P, et al. 2014. Affinity purification-mass spectrometry and network analysis to understand protein-protein interactions. Nat. Protocols 9:2539–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muffat J, Li Y, Omer A, Durbin A, Bosch I, et al. 2018. Human induced pluripotent stem cell-derived glial cells and neural progenitors display divergent responses to Zika and dengue infections. PNAS 115:7117–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambala P, Su WC. 2018. Role of Zika virus prM protein in viral pathogenicity and use in vaccine development. Front. Microbiol 9:1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nem de Oliveira Souza I,Frost PS,Franca JV,Nascimento-Viana JB,Neris RLS,et al. 2018Acute and chronic neurological consequences of early-life Zika virus infection in mice. Sci. Transl. Med 10:eaar2749. [DOI] [PubMed] [Google Scholar]
- Nguyen SM, Antony KM, Dudley DM, Kohn S, Simmons HA, et al. 2017. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLOS Pathog. 13:e1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y, Zhang F, Wang Y, Lee EM, Choi IY, et al. 2017. Zika virus directly infects peripheral neurons and induces cell death. Nat. Neurosci 20:1209–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmo IG, Carvalho TG, Costa VV, Alves-Silva J, Ferrari CZ, et al. 2017. Zika virus promotes neuronal cell death in a non-cell autonomous manner by triggering the release of neurotoxic factors. Front. Immunol 8:1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Liu B, Yves TD, He Y, Wang S, et al. 2018. Zika virus induces autophagy in human umbilical vein endothelial cells. Viruses 10:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud M, Martinez-Lopez A, Buffone C, Porcelli SA, Diaz-Griffero F. 2018. Infection by Zika viruses requires the transmembrane protein AXL, endocytosis and low pH. Virology 518:301–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland GA, Kennedy RB, Ovsyannikova IG,Palacios R, Ho PL, Kalil J. 2018. Development of vaccines against Zika virus. Lancet Infect. Dis 18:E211–19 [DOI] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Jacob F, Song H, Ming GL. 2017. Using brain organoids to understand Zika virus-induced microcephaly. Development 144:952–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, et al. 2016. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165:1238–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. 2016. Zika virus and birth defects-reviewing the evidence for causality. N. Engl.J. Med 374:1981–87 [DOI] [PubMed] [Google Scholar]
- Rosenfeld AB, Doobin DJ, Warren AL, Racaniello VR, Vallee RB. 2017. Replication of early and recent Zika virus isolates throughout mouse brain development. PNAS 114:12273–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth H, Magg V, Uch F, Mutz P, Klein P, et al. 2017. Flavivirus infection uncouples translation suppression from cellular stress responses. MBio 8:e02150–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero E, Richter SN. 2018. G-quadruplexes and G-quadruplex ligands: targets and tools in antiviral therapy. Nucleic Acids Res. 46:3270–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacramento CQ, de Melo GR, de Freitas CS, Rocha N, Hoelz LV, et al. 2017. The clinically approved antiviral drug sofosbuvir inhibits Zika virus replication. Sci. Rep 7:40920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldana MA, Etebari K, Hart CE, Widen SG, Wood TG, et al. 2017Zika virus alters the microRNA expression profile and elicits an RNAi response in Aedes aegypti mosquitoes. PLOS Negl. Trop. Dis 11:e0005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarukhanyan E, Shityakov S, Dandekar T. 2018. In silico designed Axl receptor blocking drug candidates against Zika virus infection. ACS Omega 3:5281–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterfield-Nash A, Kotzky K,Allen J, Bertolli J,Moore CA, et al. 2017. Health and development at age 19-24 months of 19 children who were born with microcephaly and laboratory evidence of congenital Zika virus infection during the 2015 Zika virus outbreak—Brazil, 2017. Morb. Mortal. Wkly. Rep 66:1347–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PS, Link N, Jang GM, Sharp PP, Zhu T, et al. 2018. Comparative flavivirus-host protein interaction mapping reveals mechanisms of dengue and Zika virus pathogenesis. Cell 175(7):1931–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, et al. 2016. The 3.8 Å resolution cryo-EM structure of Zika virus. Science 352:467–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Rho HS, Pan J, Ramos P, Yoon KJ, et al. 2018. Multiplexed biomarker panels discriminate Zika and dengue virus infection in humans. Mol. Cell Proteom 17:349–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–72 [DOI] [PubMed] [Google Scholar]
- Tang H, Hammack C, Ogden SC, Wen Z, Qian X, et al. 2016. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 18:587–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taracena ML, Bottino-Rojas V, Talyuli OAC, Walter-Nuno AB, Oliveira JHM, et al. 2018. Regulation of midgut cell proliferation impacts Aedes aegypti susceptibility to dengue virus. PLOS Negl. Trop. Dis 12:e0006498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y, Liu S, Guo X, Liu S,Jin Y, et al. 2017. An integrative analysis reveals a central role of P53 activation via MDM2 in Zika virus infection induced cell death. Front. Cell Infect. Microbiol 7:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray LB, Handley SA, Gorman MJ, Poddar S, Bagadia P, et al. 2018. Oral antibiotic treatment of mice exacerbates the disease severity of multiple flavivirus infections. Cell Rep. 22:3440–53.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi S, Balasubramaniam VR, Brown JA, Mena I, Grant A, et al. 2017. A novel Zika virus mouse model reveals strain specific differences in virus pathogenesis and host inflammatory immune responses. PLOS Pathog. 13:e1006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden V, Pessoa A, Dobyns W, Barkovich AJ,van der Linden H Jr., et al. 2016. Description of 13 infants born during October 2015-January 2016 with congenital Zika virus infection without microcephaly at birth—Brazil. Morb. Mortal. Wkly. Rep 65:1343–48 [DOI] [PubMed] [Google Scholar]
- van der Linden V,van der Linden H Jr., Leal MC, Rolim Filho EL,van der Linden A, et al. 2017. Discordant clinical outcomes of congenital Zika virus infection in twin pregnancies. Arq. Neuropsiquiatr 75:381–86 [DOI] [PubMed] [Google Scholar]
- Volpi VG, Pagani I, Ghezzi S, Iannacone M, D’Antonio M, Vicenzi E. 2018. Zika virus replication in dorsal root ganglia explants from interferon receptor1 knockout mice causes myelin degeneration. Sci. Rep 8:10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CL, Little ME, Roby JA, Armistead B, Gale M Jr., et al. 2019. Zika virus and the nonmicrocephalic fetus: why we should still worry. Am. J. Obstet. Gynecol 220:45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu J, Zhou R, Ding X, Zhang Q, et al. 2018. Zika virus infected primary microglia impairs NPCs proliferation and differentiation. Biochem. Biophys. Res. Commun 497:619–25 [DOI] [PubMed] [Google Scholar]
- Wang ZY, Wang Z, Zhen ZD, Feng KH, Guo J, et al. 2017. Axl is not an indispensable factor for Zika virus infection in mice. J. Gen. Virol 98:2061–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Buth JE, Vishlaghi N, de la Torre-Ubieta L, Taxidis J, et al. 2017. Self-organized cerebral organoids with human-specific features predict effective drugs to combat Zika virus infection. Cell Rep. 21:517–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichgers Schreur PJ, van Keulen L, Anjema D, Kant J, Kortekaas J. 2018. Microencephaly in fetal piglets following in utero inoculation of Zika virus. Emerg. Microbes Infect 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikan N, Smith DR. 2016. Zika virus: history of a newly emerging arbovirus. Lancet Infect. Dis 16:e119–26 [DOI] [PubMed] [Google Scholar]
- Wolf B, Diop F, Ferraris P, Wichit S, Busso C, et al. 2017. Zika virus causes supernumerary foci with centriolar proteins and impaired spindle positioning. Open Biol. 7:160231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Lee EM, Wen Z, Cheng Y, Huang WK, et al. 2016. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat. Med 22:1101–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KJ, Song G, Qian X, Pan J, Xu D, et al. 2017. Zika-virus-encoded NS2A disrupts mammalian cortical neurogenesis by degrading adherens junction proteins. Cell Stem Cell 21:349–58.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Deng YQ, Zou P, Wang Q, Dai Y, et al. 2017. A peptide-based viral inactivator inhibits Zika virus infection in pregnant mice and fetuses. Nat. Commun 8:15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Huang XY, Liu ZY, Zhang F, Zhu XL, et al. 2017. A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science 358:933–36 [DOI] [PubMed] [Google Scholar]
- Zhang F, Hammack C, Ogden SC, Cheng Y, Lee EM, et al. 2016. Molecular signatures associated with ZIKV exposure in human cortical neural progenitors. Nucleic Acids Res. 44:8610–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Yang M, Azar SR, Soong L, Weaver SC, et al. 2017. Viral retinopathy in experimental models of Zika infection. Investig. Ophthalmol. Vis. Sci 58:4355–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Tan L, Cederquist GY, Fan Y, Hartley BJ, et al. 2017. High-content screening in hPSC-neural progenitors identifies drug candidates that inhibit Zika virus infection in fetal-like organoids and adult brain. Cell Stem Cell 21:274–83.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Luo H, Liu H, Ha Y, Mays ER, et al. 2017. p38MAPK plays a critical role in induction of a proinflammatory phenotype of retinal Muller cells following Zika virus infection. Antiviral Res. 145:70–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Gorman MJ,McKenzie LD, Chai JN, Hubert CG, et al. 2017. Zika virus has oncolytic activity against glioblastoma stem cells. J. Exp. Med 214:2843. [DOI] [PMC free article] [PubMed] [Google Scholar]


