Abstract
Semen liquefaction is a proteolytic process where a gel-like ejaculated semen becomes watery due to the enzymatic activity of prostate-derived serine proteases in the female reproductive tract. The liquefaction process is crucial for the sperm to gain their motility and successful transport to the fertilization site in Fallopian tubes (or oviducts in animals). Hyperviscous semen or failure in liquefaction is one of the causes of male infertility. Therefore, the biochemical inhibition of serine proteases in the female reproductive tract after ejaculation is a prime target for novel contraceptive development. Herein, we will discuss protein components in the ejaculates responsible for semen liquefaction and any developments of contraceptive methods in the past that involve the liquefaction process.
Keywords: semen liquefaction, semenogelins, kallikrein-related peptidase, prostate-specific antigen, sperm motility, contraceptive, fertility
We propose inhibition of semen liquefaction has the potential to be developed as a non-hormonal contraceptive method.
Introduction
The fate of ejaculated spermatozoa in humans is very different from that in rodents. Male mice and rats ejaculate sperm and accessory gland secretions (e.g., seminal vesicle, prostate) directly into the uterus and produce a copulatory plug, which is not liquefied in vivo. In humans, however, the ejaculate is deposited in the anterior wall of the vagina, which later liquefies, and the sperm gain their motility to transport to the upper female reproductive tract for fertilization (reviewed in [1]).
In humans, the semen is a fluid conglomerate consisting of two major components: the cellular fraction (consisting of spermatozoa, migrating leucocytes, immature germ cells, and epithelial cells) and acellular fraction consisting of seminal plasma and extracellular vesicles (epididymosomes and prostasomes) (Figure 1) (reviewed in [2]). Human semen consists of approximately 2–5% spermatozoa and 98–95% seminal plasma, have a minimum volume of 2 mL, a pH of 7.2–8.0 and contain 200–500 million spermatozoa. The liquefaction process requires proteins present in the acellular fraction (seminal plasma) of the semen. Therefore, before describing the process, we will discuss necessary protein components present in the seminal plasma that are involved in the liquefaction process.
Figure 1.
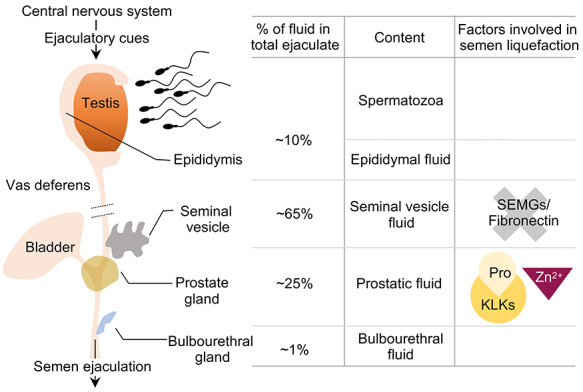
Fluid components in human ejaculate. The majority of semen is made up of seminal vesicle fluid (~65%; containing semenolgelins or SEMGs and fibronectin) and prostatic fluid (~25%; containing pro-kallikrein (Pro-KLK) enzymes and Zn2+). Epididymal fluid and testis make up to ~10% of the semen, while bulbourethral gland (mostly secretes mucinous proteins) is only 1%.
Seminal plasma
The seminal plasma is rich in sugars, glycans, lipids, inorganic ions, metabolites, cell-free DNA, microRNAs, peptides, and proteins, which are secreted from seminal vesicles, prostate, epididymis, and bulbourethral glands (reviewed in [3]). Seminal vesicles contribute to ~65% of the semen volume and are rich in semenogelins (SEMGs), fibronectin, prostaglandins, cytokines, and fructose, while the prostatic secretions are rich in proteolytic enzymes, citrate, and lipids and contribute to ~25% of the total volume of seminal fluid (Figure 1). The semen has an alkaline pH (7.2–8.0) from seminal vesicles and prostate secretions containing basic polyamines such as spermine, spermidine, and putrescine, which counteract the vaginal acidity and are important for sperm survival. Secretions from bulbourethral glands (contain mucins, galactose, sialic acid) contribute to ~1% of semen volume and act as lubricants enabling efficient sperm transfer (reviewed in [2]). The seminal plasma proteins play an important role in semen coagulation, sperm motility, capacitation, acrosome reaction, and immune activity suppression in the female reproductive tract (reviewed in [3]). Here, we will discuss the function of key factors present in the seminal plasma that are involved in the semen liquefaction process.
Seminal vesicle secretions: SEMGs
Semenogelin proteins (encoded by SEMG1 and SEMG2 genes) are secreted from seminal vesicles [4]. SEMG1 and SEMG2 are the two major proteins of the seminal coagulum and represent 20–40% of the seminal plasma proteins [5, 6]. SEMG1, a predominant 52 kDa protein, contains a single cysteine residue at position 239 (Cys239) and forms intermolecular disulfide bridges with the less abundant SEMG2 (exist as non-glycosylated 71 kDa and glycosylated 76 kDa) at Cys159 and Cys360 residues, resulting in high molecular weight complex SEMGs (reviewed in [7]). Upon ejaculation, semen immediately turns into a gelatinous meshwork of crosslinking SEMGs. As a result, sperm are entrapped within the seminal coagulum. The N-terminal fragment of SEMG1 was originally identified as the region of seminal plasma motility inhibitor (SPMI) [8, 9]. In addition, the C-terminal fragment of SEMG1 containing Cys239 (164–283 amino acids) was found to have significant inhibitory effects on both motility and progressive motility of intact live human spermatozoa [10]. Accordingly, O’Rand et al. [11] reported that recombinant human SEMG inhibits sperm progressive motility. In this context, a study by Yamakasi et al. [12] also indicated that patients with higher number of SEMG-unbound spermatozoa can achieve successful pregnancy, making total SEMG-unbound sperm count a relevant parameter for in vivo fertilization. Additionally, SEMG peptides are also involved in other biological functions such as increasing sperm hyaluronidase activity [13], antibacterial activity [14], hyperpolarization, and permeability of sperm plasma membrane [15].
Prostatic fluid: kallikreins
Prostate-specific antigen (also known as kallikrein-related peptidase 3 or KLK3), prostatic acid phosphatase, and prostate secretory protein of 94 amino acids (PSP94) are the three predominant proteins in the prostate fluid secreted by the prostate gland [16]. Tissue kallikrein-related peptidases (KLKs) are trypsin- and/or chymotrypsin-like serine proteases secreted by the prostatic epithelial cells. The KLK locus, the largest contiguous cluster of serine proteases, is localized on human chromosome 19 and encodes KLK1-15. Despite 36–77% homology among the 15 KLKs at the protein level, amino acid sequences surrounding the catalytic triad (His57, Asp102, and Ser195) are highly conserved among mammalian species (reviewed in [17]). Tissue KLKs are different from plasma kallikrein, which is a liver-derived protease, encoded by KLKB1 gene on human chromosome 4 [18]. Shaw and Diamandis [19] performed a comprehensive expression profiling of KLKs in human tissues and biological fluids and observed that the majority of KLKs (KLK1–5, 9, 11, 13–15) are expressed by the prostate and secreted into seminal plasma.
KLKs are initially synthesized as pre-pro-KLK proteins, and then the pre-peptides are removed during secretion. Pro-KLKs are inactive, and extracellular cleavage of their amino-terminal pro-peptide by proteolytic activation cascades governs the semen liquefaction mechanism. After secretion, the zymogen activation cascade is initiated by pro-KLK5, which undergoes autoactivation [20] and subsequently activates downstream pro-KLK2, 3, 7, 8, and 14 [17, 21] (Figure 2). Activated KLK14, in turn, also activates pro-KLK5 in a positive feedback loop [20]. Active KLK2 is a known activator of pro-KLK3 [22–25] (Figure 2). Additional prostatic KLKs such as KLK4 [26] and KLK14 [27, 28] have also been reported to activate pro-KLK3 and presumably aid in semen liquefaction. KLK14 is also involved in the activation of pro-KLK1 and KLK11 [27]. While their role in semen liquefaction is not completely known, KLK11 is expressed in intermediate amounts in seminal plasma at concentrations ranging from 2 to 37 μg/mL [29], compared to other KLKs (detailed below). Interestingly, KLK2 and KLK3 are only present in the primates [30, 31] and have no known orthologs among the rodent KLKs.
Figure 2.
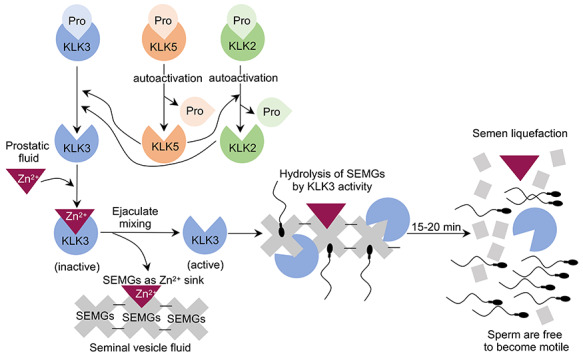
Signaling cascade of kallikrein 3 (KLK3) activation during liquefaction process. Pro-KLKs are secreted into the prostatic fluid. High concentration of Zn2+ in prostatic fluid inactivates KLK3 activity. After ejaculation, prostatic and seminal vesicle fluids are combined. SEMGs are available to sequester Zn2+ as SEMGs have higher affinity to Zn2+ compared to KLKs. Pro-KLK5 undergoes autocleavage to rid of pro-peptide sequences and autoactivates. Subsequently, KLK5 activates pro-KLK2 and 3. KLK2 also potentially activates pro-KLK3. Activated KLK3 then hydrolyzes SEMGs into smaller fractions. After hydrolysis, semen becomes liquefied and sperm gain their motility to transport to the upper female reproductive tract for fertilization.
In human body, prostate glands accumulate the highest levels of Zn2+ in prostatic epithelial cells in an androgen-dependent Zn2+ cellular uptake, which is mediated by specific zinc transporters [32]. The role of Zn2+ in liquefaction process will be discussed in the following section. In addition to KLKs, prostatic secretions are also rich in other proteins, such as prostatic acid phosphatase and zinc-α2-glycoprotein, which are involved in proteolytic cleavage of SEMGs [33], degradation of phospholipids [34], and lipid mobilization [35].
Semen liquefaction process
Semen liquefaction at the molecular level is characterized by progressive and site-specific cleavage of SEMGs into soluble low molecular weight proteins in the female reproductive tract [8, 36]. Human semen usually liquefies within ~15 to 20 min post-ejaculation [37] (Figure 3) and is a necessary step for further sperm processes related to fertilization, such as capacitation [38]. KLK3 is the major enzyme (staggering concentrations of 1290 μg/mL in seminal plasma [39]) that hydrolyzes SEMGs and fibronectin and liquefies semen coagulum facilitating sperm motility [8, 10, 36, 39–41]. KLK3 hydrolysis of SEMGs occurs preferentially at tyrosine, glutamine, and leucine and less commonly at other residues (histidine, aspartic acid, serine, and asparagine) [8, 41]. Other members of the KLK family participating in the process of semen liquefaction include KLK2 (concentration of 10–100 μg/mL), KLK5, and KLK14 (both enzymes ranging from 1 to 10 ng/mL) [19, 21, 23, 27, 42]. Active KLK2, 5, and 14 have been reported to cleave fibronectin and SEMGs in ex vivo and in vitro studies [21, 27, 28, 42–44]. Additionally, KLK6, 7, and 13 also exhibit catalytic activity toward fibronectin [45–47].
Figure 3.
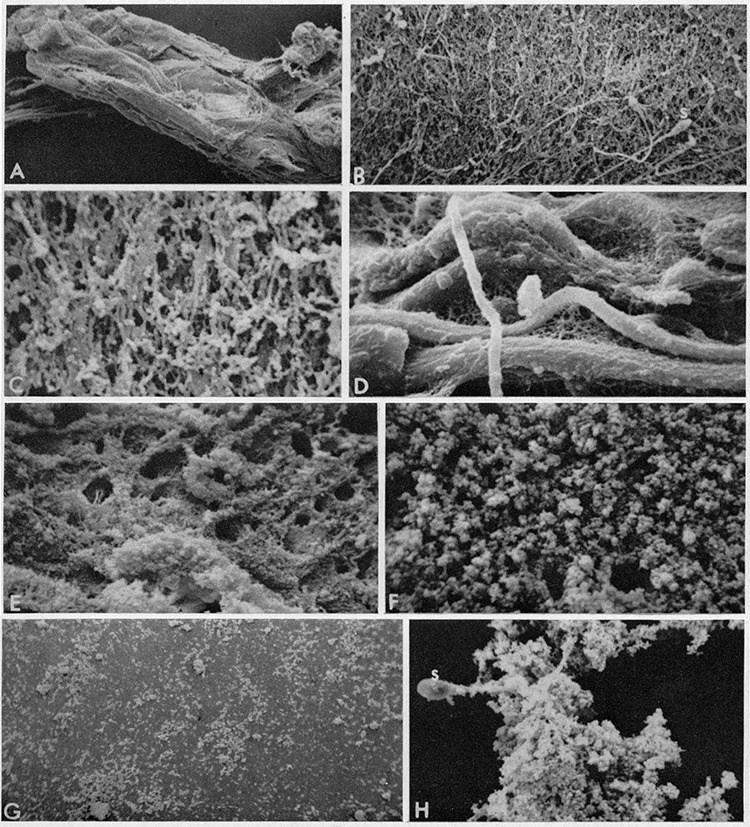
Scanning images of human semen before and after liquefaction. The samples were fixed at (A–D) 3 min, (E–F) 6 min, and (G–H) 15 min after ejaculation. Images in (G–H) were taken from samples immediately after liquefaction. (A) 30× magnification, (B) 600×, (C) 3000×, (D) 2875×, (E) 1200×, (F) 3100×, and (H) 1200×. S, spermatozoon. (A–C) and (E–H) normally liquefying; (D) slowly liquefying. Reprint of original images with permission from [37].
Semen liquefaction is also regulated by endogenous inhibitors such as Zn2+ (Figure 2) as well as protein C inhibitor (PCI). Prostatic KLKs are inactivated by allosteric reversible binding of Zn2+ in the seminal plasma (reviewed in [2]). Numerous studies have reported the ability of Zn2+ to inhibit KLK2 [43], KLK3 [8, 41, 48], KLK5 [21], and KLK14 [28] activities. Moreover, the inhibitory effect of Zn2+ on KLK3, 5, and 14 activities is reversible by SEMGs [21, 28, 48].
Once the ejaculation cue is triggered, SEMG-containing seminal vesicle secretions and prostatic fluid enriched with Zn2+ and KLKs are mixed with the sperm-enriched epididymal fluid to form a coagulum that entraps spermatozoa. Upon ejaculation, SEMGs sequester Zn2+ ions from KLKs as SEMGs possess a higher affinity to Zn2+, leading to KLK disinhibition and activation of the proteolytic cascade resulting in semen liquefaction (reviewed in [2]). Therefore, KLKs in concert with SEMGs regulate semen coagulation and liquefaction in a Zn2+-dependent manner. In addition to Zn2+, PCI has been shown to form a complex with SEMGs and KLKs to inhibit activities of KLKs in the seminal plasma [43, 49]. However, biological contribution of PCI in human semen liquefaction is widely unknown and requires further investigations.
Factors affecting semen liquefaction
Genetic variations of genes involved in the liquefaction process as well as biochemical disruption could lead to liquefaction defect. This includes factors affecting the production and activity of KLKs, SEMGs, Zn2+, endogenous protease inhibitors, and other pathological conditions in male accessory organs (Table 1). As liquefaction process takes place in the female reproductive tract, local production of KLKs, endogenous protease inhibitors, and pathological conditions in the female tract could also be contributing factors for liquefaction defect. In clinical settings, the liquefaction time is of diagnostic importance if more than 1 h elapses without any change in the semen consistency [50]. Any defects in the liquefaction process can lead to impaired semen liquefaction and ~12% of infertility patients have the symptom of non-liquefied semen [51]. Here, we describe possible factors contributing to, or conditions resulting in defective semen liquefaction.
Table 1.
Physiological functions of proteins in male accessory gland secretions potentially involved in regulating fertility in humans and rodents.
| Protein | Gene (human) | Gene (mouse) | Function | Phenotypes when mutated, overexpressed, or genetically ablated |
|---|---|---|---|---|
| Seminal vesicles | ||||
| SEMG1 | SEMG1 | Svs2 | SEMG1 forms intermolecular disulfide bridges with SEMG2 resulting in high molecular weight coagulum upon ejaculation [7]. Inhibits sperm motility [10, 11]. SVS2 is a known decapacitation factor and maintains sperm motility and sperm cholesterol levels, prevents spontaneous sperm capacitation, and is essential for sperm survival in the mouse uterus [66, 68] | SEMG1 variants (rs147894843, rs2301366) associated with infertility [57, 63]. Elevated SEMG1 precursor reported in oligozoospermic men [64]. Svs2−/− mice are subfertile with defects in copulatory plug formation [68] |
| SVS7 | SVS7 | Pate4 | SVS7 in mouse is essential for copulatory plug formation in vivo [69]. SVS7 enhances mouse sperm motility in vitro [70] | No known mutation/phenotype reported in humans. Pate4−/− mice are subfertile with defects in copulatory plug formation [69] |
| SERPINE2/PN1 | SERPINE2/PN1 | Serpine2/Pn1 | Serine protease inhibitor acts as a decapacitation factor [82]. Inhibits protein tyrosine phosphorylation and sperm capacitation [82] | Elevated PN1 levels in semen of men displaying seminal dysfunction [85]. Pn1−/− mice are infertile due to altered seminal protein composition and defects in copulatory plug formation [85] |
| SPINK3/SPINK1 | SPINK3/SPINK1 | Spink3/Spink1 | Serine protease inhibitor prevents premature acrosomal reaction and protects sperm in the uterine environment in mice [89, 90] | No known mutation/phenotype involving fertility reported in humans or mice |
| SPINKL | No known ortholog | Spinkl | Serine protease inhibitor acts as decapacitation factor and enhances sperm motility in mice [97] | No known mutation/phenotype involving fertility reported in humans or mice |
| Testis and epididymis | ||||
| EPPIN | SPINLW1 | No known ortholog | Localized on the sperm surface. Modulates KLK3 activity and acts as decapacitating factor [10, 78, 79]. EPPIN-bound SEMG1 crucial for SEMGs degradation and initiation of progressive sperm motility [11] | SPINLW1 upregulated in caput epididymis of non-obstructive azoospermic patients [80]. rs11594 variant associated with increased risk of idiopathic male infertility in Chinese–Han population [81]. |
| Epididymis | ||||
| SPINK2 | SPINK2 | Spink2 | Serine protease inhibitor protects sperm against protease activity during spermatogenesis | Homozygous SPINK2 mutation leads to azoospermia in men [86]. Decreased SPINK2 expression in azoospermic infertile men [87]. Spink2 mutant mice have elevated serine protease activity and exhibit impaired fertility [88]. Spink2−/− mice are azoospermic and infertile [86] |
| SPINK5 | SPINK5 | Spink5 | Serine protease inhibitor inhibits KLK5, 7, and 14 activities in corneocytes and regulates desquamation process [91, 92] | No known mutation/phenotype reported involving fertility in humans or mice |
| SPINK13 | SPINK13 | Spink13 | Serine protease inhibitor. Essential for acrosomal integrity, sperm maturation, and fertility in rats [96] | No known mutation/phenotype reported in humans. Spink13 knockdown rats demonstrate premature acrosomal reaction and reduced fertility [96] |
| Prostate gland | ||||
| KLK1 | KLK1 | Klk1/mGK6 | Serine protease | Low level observed in SHV samples [58]. No known mutation/phenotype reported involving fertility in mice |
| KLK2/hK2 | KLK2 | No known ortholog | Serine protease cleaves fibronectin and SEMGs [42, 43]. Activator of pro-KLK3 [22–25]. Inhibited by Zn2+ [43] | Low KLK2 seminal levels observed in men with abnormal liquefaction and SHV [58]. SNP (rs2664155) associated with male infertility [61] |
| KLK3/PSA | KLK3 | No known ortholog | Serine protease. Major enzyme hydrolyzes SEMGs and fibronectin and liquefies semen coagulum facilitating sperm motility [8, 10, 36, 39–41]. Inhibited by Zn2+ [8, 41, 48] | Low KLK3 level observed in men with SHV [55, 57] and abnormal liquefaction [58]. Reduced sperm motility observed in men with low seminal KLK3 levels [59]. SNPs (rs266881, rs174776, rs1810020, rs266875, rs35192866) associated with male infertility [60] |
| KLK4 | KLK4 | Klk4 | Serine protease activates pro-KLK3 [26] | No known mutation/phenotype reported involving fertility in humans or mice |
| KLK5 | KLK5 | Klk5 | Serine protease. Initiates liquefaction cascade by activating downstream pro-KLK2, 3, 7, 8 and 14 [17, 21]. Cleaves fibronectin and SEMGs [21, 44]. Inhibited by Zn2+ [21] | Low level observed in SHV samples [58]. No known mutation/phenotype reported involving fertility in mice |
| KLK6 | KLK6 | Klk6 | Serine protease exhibits catalytic activity towards fibronectin [46] | Low level observed in SHV samples in humans [58]. No known mutation/phenotype reported involving fertility in mice |
| KLK7 | KLK7 | Klk7 | Serine protease exhibits catalytic activity towards fibronectin [47] | KLK7 (rs1654526) SNP associated with SHV in humans [57]. Low level observed in SHV samples in humans [58]. No known mutation/phenotype reported involving fertility in mice |
| KLK8 | KLK8 | Klk8 | Serine protease | Low level observed in SHV samples [58]. No known mutation/phenotype reported involving fertility in mice |
| KLK10 | KLK10 | Klk10 | Serine protease | Low level observed in SHV samples [58]. No known mutation/phenotype reported involving fertility in mice |
| KLK12 | KLK12 | Klk12 | Serine protease | KLK12 (rs61742847) SNP associated with SHV [57]. No known mutation/phenotype reported involving fertility in mice |
| KLK13 | KLK13 | Klk13 | Serine protease exhibits catalytic activity towards fibronectin [44] | Low seminal levels observed in men with abnormal liquefaction and SHV [58]. No known mutation/phenotype reported involving fertility in mice |
| KLK14 | KLK14 | Klk14 | Serine protease. Activates pro-KLK1, 3, 5 and 11 [20, 27, 28]. Cleaves fibronectin and SEMGs [27, 28]. Inhibited by Zn2+ [28] | Low seminal levels observed in men with clinically delayed liquefaction, SHV, and asthenospermia [28, 58]. KLK14 inhibition by ACTG9 delays semen liquefaction [27]. No known mutation/phenotype reported in mice involving fertility |
| TGM4 | TGM4 | Tgm4 | A prostate-specific autoantigen plays a critical role in male reproduction and catalyzes the formation of N-ε-(γ-glutamyl)lysine cross-bridges between SEMGs in humans [72] and SVS proteins in mice [75], respectively | TGM4 autoantibodies are detected in subfertile adult male patients with autoimmune polyendocrine syndrome type 1, caused by mutations in autoimmune regulator (AIRE) gene [74]. Tgm4−/− mice are subfertile with defects in copulatory plug formation and seminal fluid viscosity [75]. Aire−/− mice develop TGM4 autoantibodies, compromised TGM4 secretion, prostatitis, and exhibit subfertility [74]. |
Semen hyperviscosity
According to WHO criteria, viscosity can be assessed in semen by observing the length of the thread formed by gently aspirating semen and allowing it to drop by gravity after 1-h incubation at room temperature [50]. A normal sample leaves the pipette in small discrete drops, while in cases of semen hyperviscosity (SHV), the drop will form a thread greater than 2 cm long [50]. Based on the thread length, SHV can be further classified into mild (2–4 cm), moderate (4–6 cm), and severe SHV (≥6 cm) [52].
SHV has a prevalence of 12–32% in men with fertility problems [52–54]. SHV negatively impacts semen quality and sperm motility because of the sperm-trapping effect of hyperviscous semen [53, 55]. Biochemical analysis of rheological properties of semen indicated the presence of highly organized peptide cores complexed with oligosaccharide chains and disulfide bonds in hyperviscous semen compared to normal samples [56]. Gopalkrishnan et al. [54] found that in semen samples with abnormal viscosity, the sperm count, motility, and chromatin integrity were significantly decreased when compared to controls with normal semen viscosity [54]. The etiology of SHV has often been attributed to male accessory gland infection, increased levels of leukocytes, and inflammation. Therefore, the composition of human seminal plasma is important in understanding the physiology of reproduction, and any alterations in seminal plasma may explain molecular mechanisms in some cases of infertility. The following section will discuss genetic variations of KLK enzymes that may contribute to SHV conditions in men and result in defective semen liquefaction.
KLK mutations
Genetic factors may also influence the viscosity of seminal fluid. KLK3 level was significantly lower in SHV samples when compared to samples with normal viscosity [55, 57] suggesting the association between prostatic enzymes and semen viscosity. In a recent study, genetic variation within KLK locus was found to be associated with SHV [57]. KLK7 (rs1654526) and KLK12 (rs61742847) polymorphisms are significantly associated with SHV, while genetic variation in KLK3 and KLK15 was found to be three times higher in SHV samples than in controls [57]. Emami et al. [58] reported a possible role of KLKs in the pathogenesis of delayed semen liquefaction and SHV. Lower concentrations of KLK2, 3, 13, and 14 in men with abnormal liquefaction and KLK1, 2, 5–8, 10, 13, and 14 in individuals with SHV semen were observed [58]. In agreement with these findings, men with low concentration of seminal KLK3 have reduced sperm motility [59]. Accordingly, KLK14 levels are significantly lower in individuals with clinically delayed liquefaction and in asthenospermic infertile men [28]. In addition, targeted inhibition of KLK14 activity by the pharmacological inhibitor ACTG9 (based on serum KLK3 inhibitor α1-anti-chymotrypsin) in seminal plasma considerably delays semen liquefaction [28].
Gupta et al. [60] sequenced KLK3 gene in 875 infertile and 290 fertile men and identified a total of 28 substitutions in KLK3 coding region. Of 28 KLK3 substitutions, 5 SNPs (rs266881, rs174776, rs1810020, rs266875, and rs35192866) appear to be strong risk factors for male infertility, while 1 SNP (c.206 + 235 T > C) is protective [60]. Variations in other KLKs have also been correlated with male infertility. Lee and Lee [61] performed genotypic association analysis in 218 non-obstructive azoospermic and 220 fertile controls and showed that a SNP in the KLK2 intron 1 (+255 G > A, rs2664155) was associated with male infertility. Savblom et al. [62] also reported the association of SNPs in KLK3 and KLK2 with concentrations of KLK3 and KLK2, respectively, in seminal plasma and serum. These studies indicate that genetic variations in KLK2 and 3 could also directly affect their enzymatic activity and hence semen liquefaction, ultimately affecting fertility.
SEMG mutations
Genetic alterations of SEMG1 variant rs147894843 is involved in altered proteolytic activity, which may affect semen quality and liquefaction leading to infertility in men [57]. In a recent study, the association between SEMG variants and male infertility was examined [63]. In Chinese–Han male population, the SEMG1 variant rs2301366 was associated with abnormal semen parameters such as semen volume, sperm concentration, sperm number per ejaculate, and sperm motility and more susceptible to infertility [63]. In another study, a negative correlation between sperm motility and the proportion of SPMI (of SEMG sequence)-bound spermatozoa was also found in male subjects including infertile normozoospermics, asthenozoospermics, and oligozoospermics [12]. These findings suggest that SEMGs remained on the sperm surface post-liquefaction might account for impaired sperm motility in infertile men. In a functional proteomic analysis of seminal plasma proteins, SEMG1 isoform b pre-pro-protein levels were elevated in oligozoospermic men with abnormal sperm morphology when compared to donors with normal sperm count and morphology [64]. Interestingly, Thacker et al. [65] analyzed the major proteins in the semen from fertile and infertile men and found that infertile men lacked SEMG2 precursor showing unique differences in the semen profile. Therefore, SEMGs are crucial for liquefaction process, and mutations in SEMGs may have a profound impact on sperm function that goes beyond liquefaction process.
In mice, seminal vesicle secretion 2 (SVS2), an ortholog of human SEMG1, is a major seminal vesicle secreted protein that acts as a decapacitation factor and controls sperm motility [66]. SVS2 maintains sperm cholesterol levels and prevents spontaneous sperm capacitation in the uterus [67]. In the oviduct, the removal of SVS2 from the sperm’s surface induces a decrease in cholesterol from the sperm membrane, thereby resulting in the ability of sperm to fertilize the eggs [67]. Svs2−/− mice are subfertile due to copulatory plug formation defect [68]. Additionally, SVS2 has been demonstrated to protect sperm against the spermicidal uterine environment as the sperm from Svs2−/− mice were killed in the uterine cavity and failed to reach the eggs in the oviduct [68]. SVS7, also known as PATE4 (prostate and testis expression 4), is another major seminal vesicle secreted protein essential for copulatory plug formation [69]. SVS7 has been shown to enhance mouse sperm motility in vitro [70], and Svs7−/− mice exhibit subfertility due to defects in copulatory plug formation ([69], reviewed in [71]).
In addition to SEMGs and SVSs, transglutaminases (TGMs) could also potentially involve in the enzymatic complex during liquefaction. In humans, SEMGs are important substrates for TGM [72]. TGM catalyze protein crosslinking by formation of N-ε-(γ-glutamyl)lysine cross-bridges between lysine and glutamine residues of donor and acceptor proteins respectively (reviewed in [73]). TGM4 is a prostate-specific autoantigen and plays a critical role in male reproduction. TGM4 autoantibodies are detected in subfertile adult men, and these patients elicit an autosomal recessive disorder caused by mutations in the autoimmune regulator (AIRE) gene [74]. Accordingly, Aire−/− mice develop TGM4 autoantibodies, have compromised TGM4 secretion, prostatitis, and are subfertile [74]. In mice, the copulatory plug formation is mediated by TGM4, which catalyzes the formation of N-ε-(γ-glutamyl)lysine cross-bridges between SVS proteins ([75], reviewed in [71]). Tgm4−/− male mice are subfertile due to faulty copulatory plug formation and seminal fluid viscosity [75]. Several glutamine and lysine residues in SVS proteins 1–4 serve as substrates and are target sites for TGM4 cross-linking and may aid in copulatory plug formation, reviewed in [71]. These findings indicate that functional SEMGs, SVSs, and TGM4 are required for normal male fertility in humans and mice.
Prostatectomy
If KLK3 is the key executor of semen liquefaction process, a loss of KLK3 production due to surgical removal of prostate glands (prostatectomy) would result in a liquefaction defect and ultimately male infertility. In patients with localized prostate cancer, radical prostatectomy is performed, which involves the removal of the entire prostate gland, the seminal vesicles, and the vas deferens. As the continuity of the genital tract is disrupted, seminal emission and ejaculation is lost (anejaculation), leading to obstructive azoospermia, but spermatogenesis normally persists in these patients. Nerve sparing radical prostatectomy may also result in erectile dysfunction [76]. Therefore, it is difficult to determine the absolute requirement of prostate-derived KLK3 in human reproduction. Or it is also possible that KLKs from other tissues (i.e., the female reproductive tract) can also contribute to the liquefaction process. This possibility will be discussed in a later section.
Enzymatic activity of endogenous protease inhibitors from male accessory glands
Although endogenous protease inhibitors are not directly shown to be involved in semen liquefaction, studies suggest that their activity may affect functions of SEMGs and KLKs. Therefore, the importance of these endogenous protease inhibitors will be discussed briefly below.
Human epididymal protease inhibitor
Epididymal protease inhibitor (EPPIN) plays a critical role in sperm function and male fertility. EPPIN is a serine protease inhibitor containing both Kunitz-type and whey acidic protein (WAP)-type four disulfide core protease inhibitor consensus sequences and is expressed in testis and epididymis [77]. In the ejaculate coagulum, EPPIN is localized on the sperm surface and bound to SEMG1 (at Cys239) where it acts as a decapacitating factor by modulating the activity of KLK3 [10, 78, 79]. EPPIN-bound SEMG1 is critical for the degradation of SEMGs during semen liquefaction and for the initiation of progressive sperm motility in vivo [11]. EPPIN gene (SPINLW1; serine protease inhibitor-like with Kunitz and WAP domains 1) is significantly upregulated in the caput epididymis of infertile men with non-obstructive azoospermia compared to fertile patients [80]. Genetic variants of the EPPIN are also reported to be associated with idiopathic male infertility. In the Chinese–Han population, Ding et al. [81] reported the association of EPPIN variant rs2231829 with decreased risk of idiopathic infertility, while variant rs11594 increased the risk of idiopathic male infertility with abnormal semen parameters such as semen volume, sperm concentration, and sperm motility. However, there are no differences in risk for these genotypes among men with normal semen parameters, suggesting that men with different EPPIN variants have either an elevated or a reduced frequency of abnormal sperm parameters [81].
Serpin peptidase inhibitor, clade E, member 2
Serpin peptidase inhibitor, clade E, member 2 (SERPINE2) is also known as Kunitz-type or protease nexin-1 (PN1) and is highly expressed in seminal vesicles [82]. SERPINE2 has broad protease inhibitor activity against serine proteases (such as thrombin, plasminogen activators, trypsin, and plasmin) and has been demonstrated to block protein tyrosine phosphorylation and inhibit sperm capacitation [82]. Protein tyrosine phosphorylation is essential for sperm functions such as motility, capacitation, hyperactivation, acrosome reaction, and fertilization [83, 84]. Pn1−/− mice are infertile due to altered seminal protein composition, which leads to inadequate semen coagulation and deficient vaginal plug formation upon copulation [85]. On the other hand, abnormally high PN1 levels were reported in semen of men with seminal vesicle dysfunction when compared to seminal plasma from fertile men who had low PN1 levels, indicating that controlled extracellular proteolytic activity is important for fertility in humans [85]. However, it remains unclear if SERPINs are involved in the liquefaction process in mammals.
Serine protease inhibitor Kazal-type
Serine protease inhibitor Kazal-type 2 (SPINK2) is an acrosomal protein localized in the human mature spermatozoa [86]. Comparative gene expression profiling of infertile men diagnosed with azoospermia showed that SPINK2 expression was decreased fourfold compared with fertile men [87]. Genetic variation of SPINK2 is also reported to be associated with male infertility, where homozygous SPINK2 mutation leads to azoospermia while haploinsufficiency can result in oligozoospermia [86]. In mice, SPINK2 is expressed in the germ cells and epididymis where it protects the developing sperm against protease activity during spermatogenesis [88]. Spink2 mutant mice exhibit significantly impaired fertility accompanied by elevated serine protease activity [88], while Spink2−/− mice are azoospermic and infertile [86].
SPINK3 is a seminal vesicle-secreted protease inhibitor, which binds to the plasma membrane of the mouse sperm and appears to have protective function against protease activity in the uterus [89]. In addition, SPINK3 prevents the premature acrosomal reaction of the sperm until fertilization through reduction in endogenous nitric oxide [90].
Although the role of human SPINKs in semen liquefaction is unknown, SPINK5 is known to specifically inhibit KLK5, 7, and 14 activities in corneocytes and regulate desquamation process [91, 92]. The absence of SPINK5-mediated inhibition of KLK5, 7, and 14 is implicated in Netherton syndrome, a severe skin disorder with impaired keratinization and hair malformation [91–93]. However, it is unclear if Spink5−/− mice are fertile [93]. Similarly, SPINK6 is a potent inhibitor of KLK2, 4, 5, 6, 7, 12, 13, and 14 and plays an important role in skin barrier homeostasis [94, 95]. In the context of semen liquefaction, preventing the activation of KLKs by SPINK5 and 6 could potentially lead to liquefaction defects.
Epididymal specific protein, SPINK13 is associated with the sperm membrane and essential for acrosomal integrity, sperm maturation, and fertility in rats [96]. A related protease inhibitor, SPINKL (SPINK-like) is another seminal vesicle-secreted protease inhibitor reported to prevent premature sperm capacitation in mice [97]. Nevertheless, the results obtained from these rodent and human studies highlight the importance of a balance between proteases and their regulation by inhibitors, which may disrupt liquefaction by suppressing protease activities of KLK in the semen.
Endogenous proteases and protease inhibitors in the female reproductive tract
After ejaculation, semen is exposed to numerous secretory proteins (including proteinases and proteinase inhibitors) from the female reproductive tract. The distribution of KLKs in female reproductive tract varies widely (Table 2) [19, 98]. Immunohistochemical (IHC) studies revealed the expression of KLK5, 6, 11, 12, and 13 in the vaginal stratified squamous epithelium, cervical mucus-secreting epithelium, glandular epithelium of Fallopian tubes, and endometrium [98]. Additionally, in an earlier study using enzyme-linked immunosorbent assay (ELISA) performed in adult tissue, Shaw and Diamandis [19] detected the presence of multiple KLKs in the vagina (KLK1, 5–14), cervix (KLK1, 4–6, 8, 11–14), uterus (KLK1, 4, 6, 9, 11–14), Fallopian tube (KLK1, 6, 7, 9–14), and ovary (KLK1, 6–8, 10, 11, 14) at varying concentrations. Cervical–vaginal fluid (CVF) hydrates the mucosa of the vagina and ectocervix. CVF contains large amounts of both endogenous proteases and protease inhibitors [99, 100]. In the CVF, multiple KLKs (excluding KLK2, 4, and 9) are also detected using ELISA and proteomic analyses [99, 100]. The presence of KLKs in the CVF is thought to be a combined secretory action by the tissues and glands in the female reproductive tract. Secretory protein levels of KLK11–13 in the CVF are remarkably high and only exceeded by KLK2 and KLK3 levels in seminal plasma. In addition, endogenous inhibitors such as α2-macroglobulin, SERPINs, and SPINKs that regulate KLK activity have also been detected in the CVF [99, 100].
Table 2.
Protease and protease inhibitors in the female reproductive tract.
| Region | Description |
|---|---|
| Serine protease | |
| Vagina | KLK1, 5–14 detected by ELISA [19] |
| KLK5, 6, 11, 12, 13 expression detected using IHC in the vaginal stratified squamous epithelium [98] | |
| Estradiol decreased KLK6, 10, and 11 levels in vaginal epithelial cells [98] | |
| Cervix | KLK1, 4–6, 8, 11–14 detected by ELISA [19] |
| KLK5, 6, 11, 12, 13 expression detected using IHC in the mucus-secreting epithelium [98] | |
| KLK5 and 12 cleave MUC4 and 5B in the endocervical epithelium leading to collagen remodeling [98] | |
| Estradiol upregulated KLK4, 5, and 8 expression in ectocervical cells [101] | |
| Uterus | KLK1, 4, 6, 9, 11–14 detected by ELISA [19] |
| KLK5, 6, 11, 12, 13 expression detected using IHC in the glandular epithelial cells of the endometrium [98] | |
| KLK1 expression is upregulated in endometrium during mid-menstrual cycle [102] | |
| Fallopian tube | KLK1, 6, 7, 9–14 detected by ELISA [19] |
| KLK5, 6, 11, 12, 13 expression detected using IHC in the glandular epithelium [98] | |
| Ovary | KLK1, 6–8, 10, 11, 14 detected by ELISA [19] |
| CVF | KLK1, 3, 5–8, 10–15 detected using ELISA and proteomic analyses [99, 100] |
| Progesterone increases KLK5–7, 11, and 12 levels in the CVF [98] | |
| Serine protease inhibitor | |
| CVF | SPINK5 is specific inhibitor of KLK5, 7, and 14 activities [100] |
| SPINK6 is a potent inhibitor of KLK2, 4, 5, 6, 7, 12, 13, and 14 activities | |
| Estradiol upregulates SPINK5 and SPINK6 expression in ectocervical cells [101] |
In the female reproductive tract, KLK expression is regulated by female steroid hormones [98, 101]. Progesterone appears to stimulate KLK expression as levels of KLK5–7, 11, and 12 in CVF peaked after ovulation and positively correlated with the levels of progesterone [98]. In contrast, estradiol (E2) treatment decreased the concentrations of KLK6, 10, and 11 in a vaginal epithelium cell line [98], but increased KLK4, 5, and 8 in ectocervical cells [101]. Moreover, KLK1 is expressed at high concentrations in human endometrium during mid-menstrual cycle when circulating E2 is elevated [102]. Similarly, Klk1 expression in mouse and rat uteri is stimulated by E2 [103]. In a recent study, Li et al. [101] reported that the expression of KLKs (KLK4,5, and 8) and proteinase inhibitors SPINK5 and SPINK6 in human ectocervical cells is regulated by E2 in an estrogen receptor (ESR1)-dependent manner. Additionally, cell type-specific deletion of Esr1 in the epithelial cells of the female reproductive tract (Wnt7aCre/+Esr1f/f mice) severely reduces the expression of uterine Klk genes (Klk1 and Klk1b) [101]. Although the anatomy between human and mice reproductive tract is different, the contribution of the female factor found in the Wnt7aCre/+Esr1f/f mice provides fundamental evidence that the exposure of post-ejaculated semen to the suboptimal microenvironment in the female reproductive tract leads to faulty liquefaction and subsequently causes a fertility defect. Therefore, it is possible that an imbalance between proteases and protease inhibitors due to abnormal estrogen signaling within the female reproductive environment may disrupt liquefaction, which could be one of the reasons for unexplained infertility observed in humans.
Mucins (MUC) are the primary glycoproteins comprising cervical mucus and are thought to influence sperm transport through the cervix and uterus as they allow sperm penetrance. Apart from contributing to activation of semen liquefaction cascade, KLK5 is also responsible for digestion of collagen and modification of mucins [98]. MUC4 and 5B are the major mucins in the endocervical epithelium and are cleaved by both KLK5 and 12 in vitro [98]. Therefore, collective proteolytic action of KLKs from the seminal plasma and secretions from the female reproductive tract are crucial for normal semen liquefaction, sperm release, and transport to the site of fertilization in Fallopian tubes.
Development of inhibitors for semen liquefaction
Current contraceptive technologies fail to meet the needs for all women. Hormonal methods of contraception, including oral contraceptive pills (OCPs), dermal patches, injections, and implants, are highly effective and reversible. However, a critical drawback of hormonal contraceptives arises from concerns over the long-term effects of hormones on patient health [104]. For instance, estrogen-containing OCPs have been linked to an increased risk of venous thrombosis [105], breast cancer [106], among other pathologies [107, 108]. Uterine bleeding is also a common reason given for women to discontinue progestin-only regimens [109]. The current over-the-counter contraceptives (condoms and spermicides) are associated with high failure rates [110]. In addition, usage of spermicides can damage vaginal and cervical mucosa increasing the risk of viral infection in women [111–113]. Therefore, there is a need for new non-hormonal vaginal contraceptives for women that can be used on demand. As mentioned above, pathophysiology, genetic inhibition, or biochemical inhibition of liquefaction process negatively impacts fertility in humans. Therefore, blocking KLK3 activity remains the prime candidate for the development of new contraceptives as it would prevent semen liquefaction and sperm transport in the female reproductive tract, potentially leading to clinical use.
Inhibition of key components regulating the coagulation and liquefaction has been previously assessed in vitro using pan-serine protease inhibitors. Early studies by Matsuda et al. [114] demonstrated that treatment of human ejaculates with proteinase inhibitor, Fusan (6-amidino-2-naphtyl-6-guanidinobenzoate dihydrochloride), for 30 min inhibited liquefaction (Figure 4A–D), caused solidification of semen, and completely inhibited sperm motility. Other studies focused on the use of commercially available synthetic serine protease inhibitors [such as 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF) and phenylmethylsulfonyl fluoride (PMSF)], heavy metal cations (Zn2+ and Hg2+), and heavy metal chelator 1,10-phenanthroline to partially or completely inhibit KLK3 activity in vitro [8, 115]. However, these inhibitors have never been tested in in vivo models until recently.
Figure 4.

Treatment of pan-serine protease inhibitors in (A–D) human ejaculate and (E–F) female mice. (A) Human spermatozoa after liquefaction (336× magnification). Inhibition of liquefaction after Fusan treatment: (B) 1 mM Fusan (400×), (C) 10 mM Fusan (200×), (D) 10 mM Fusan (400×). (E, F) Female mice were transcervically treated with AEBSF before mating, and semen was collected ~8 h after mating. (E) Seminal volume was severely reduced in female mice treated with AEBSF compared to saline (Vehicle). (F) Total sperm number in the oviduct is significantly reduced in AEBSF-treated mice. (G) A lack of SEMG2 cleavage in the uteri from female mice treated with AEBSF. *P < 0.05, unpaired t-test. Reprint of original (A)–(D) images with permission from [114]. Images (E, F) were modified with permission from [101] under the Creative Commons Attribution License.
Li et al. [101] showed that AEBSF effectively inhibited semen liquefaction in vivo using a mouse model as there was a lack of SEMG2 cleavage in semen collected from uterus. This semen liquefaction blockade by AEBSF treatment caused severe reduction of sperm transport to the oviduct compared to vehicle treatment in vivo (Figure 4E–G). As a proof-of-concept to determine whether protease inhibitors could potentially be used as a contraceptive, we performed a study using a pan-serine protease inhibitor (AEBSF). We showed that AEBSF (1) effectively and reversibly reduced fecundity in female mice; (2) acted as spermicide and inhibited sperm motility, resulting in a decreased fertilization in vivo and in vitro; and (3) was significantly less damaging to the vaginal epithelium (compared to N9) when treated for 10 min or three consecutive days in vivo in mice [116]. This review is preceding the report on our AEBSF study in this Special Issue. Despite inhibitory capacity of AEBSF, their application as therapeutic agent is hampered due to a lack of selectivity. Therefore, increased interest for development of a highly potent and selective inhibitor toward KLK3 activity that would cause blockade of semen liquefaction and sperm transport within the female reproductive tract will have potential pharmaceutical utility as a novel contraceptive.
Before moving on to the selective inhibitor for KLK3, it is important to note that anti-EPPIN was also developed as a male contraceptive; however, the goal was to decrease sperm motility and not inhibition of the semen liquefaction. Briefly, a study in Macaca radiata monkeys immunized with recombinant human EPPIN showed an effective and reversible male infertility without hormone disruption [117]. Treatment of human spermatozoa with anti-EPPIN antibodies inhibited EPPIN–SEMG1 interaction and significantly decreased sperm motility [11]. Furthermore, anti-EPPIN antibodies have been demonstrated to inhibit human sperm acrosomal reaction, reduce intracellular Ca2+ concentration, and does not alter tyrosine phosphorylation of sperm proteins [118]. The use of sperm surface EPPIN as a non-hormonal contraceptive target has led to the development of small organic compounds that could substitute for SEMG1 or anti-EPPIN antibodies and provide a reversible, short-lived pharmacological alternative. In this regard, EP055 is a 1,3,5-triazine compound that targets EPPIN on the surface of sperm and inhibits motility [119]. Intravenous infusion of EP055 in male macaques demonstrated plasma half-life of 11 min and the drug being retained in semen for up to 78 h followed by recovery of sperm motility [119]. Although EPPIN modulates the hydrolysis of SEMGs by KLK3, it is not an effective inhibitor of KLK3 activity [120, 121]. Therefore, mode of action of anti-EPPIN will be different than that of semen liquefaction inhibition.
Development of small molecule(s) inhibitors specifically for KLK3
KLK3 is an ideal target for the development of small-molecule inhibitors targeting its enzymatic activity that would allow development of non-invasive contraception technologies (Figure 5). Most of the studies involved in generation of small molecule inhibitors of KLK3 were focused on their usage in targeted treatment of prostate cancer (Table 3). The first KLK3 inhibitors reported in the literature used a homology model derived from porcine KLK to design and synthesize β-lactam analogs, which showed promising inhibitory activity with an IC50 (inhibitor maximal inhibitory concentration) as low as 226 nM [122]. To obtain mechanistic insights into the inhibition of KLK3, Singh et al. [123, 124] showed that β-lactam based inhibitors compete with KLK3 substrates and form a stable covalent complex at the catalytic Ser189 residue in a time-dependent manner. Other strategies include the use of azapeptides, which target both cysteine and serine proteases and effectively inhibit KLK3 activity with the Ki (inhibition constant) as low as 500 nM [125].
Figure 5.

Using of specific KLK3 inhibitors as a novel contraceptive method to block semen liquefaction process. KLK3 inhibitor will potentially attenuate semen liquefaction process as its activity will be specific to KLK3 and does not affect other KLKs or other serine proteases in semen as well as in the female reproductive tract.
Table 3.
Summary of key KLK3 inhibitors reported in the literature.
| Type | Relevance | Agent | Description/pharmacological data/therapeutic impact |
|---|---|---|---|
| β-lactam analogs | Unclear | 2-azetidinone | IC50 = 226 nM [122] |
| Prostate cancer | Benzoxazinone derivatives | Ki = 300 nM. 30 times more selective compared to chymotrypsin (Ki = 8.5 μM) [126] | |
| Triazole derivatives | Ki = 500 nM. 10 times more selective compared to chymotrypsin (Ki = 5.4 μM) [126] | ||
| Cysteine and serine protease inhibitors | Prostate cancer | Azapeptides | Ki = 500 nM [125] |
| Heavy metal cations | Semen liquefaction | Zn2+ | Inhibits KLK3 activity at 10 mM [8] |
| IC50 = 20 μM [41] | |||
| Hg2+ | Inhibits KLK3 activity at 10 mM [8] | ||
| IC50 = 150 μM [41] | |||
| Cu2+ | IC50 = 150 μM | ||
| Cd2+ | IC50 = 200 μM | ||
| Co2+ | IC50 = 500 μM | ||
| Heavy metal chelator | Semen liquefaction | 1,10-phenanthroline | Inhibits KLK3 activity at 50 mM [8] |
| Pan-serine protease inhibitors | Semen liquefaction | PMSF | Inhibits KLK3 activity at 5 mM [8] |
| AEBSF | Inhibits KLK3 activity at 5 mM [8] | ||
| Prostate cancer | PMSF | Inhibits KLK3 activity at 20 mM [115] | |
| AEBSF | Inhibits KLK3 activity at 10 mM [115] | ||
| Peptide aldehyde inhibitor | Prostate cancer | Z-SSKLL-H | Ki = 6.5 μM [128] |
| Peptidyl boronic acid inhibitor | Prostate cancer | Z-SSKL(boro)L | Ki = 65 nM. 60 times more selective compared to chymotrypsin (Ki = 3.9 μM). Reduction in free and total KLK3 serum levels in human prostate cancer xenografts produced in nude mice upon intravenous administration of 33 mg/kg dose for two cycles of three consecutive days/5 days [128] |
| Z-SSKn(boro)L | Ki = 48.4 nM. Norleucine substitution of Z-SSKL(boro)L [129] | ||
| Ahx-FSQn(boro)Bpg | Ki = 72 nM. Eight times more selective compared to chymotrypsin (Ki = 580 nM). Reduction in free and total KLK3 serum levels in human prostate cancer xenografts produced in nude mice upon intravenous administration of 10 mg/kg dose for three cycles of five consecutive days/week [130] | ||
| RNA aptamer | Prostate cancer | Not applicable | Synthetic RNA molecules (92 mer) selected from pools of random-sequence oligonucleotides to specifically bind active KLK3 [131] |
Using high-throughput screening of chemical libraries, Koistinen et al. [126] screened 49 920 compounds to identify small drug-like molecules and pinpointed two compounds inhibiting KLK3-activity in a dose-dependent manner in human umbilical vein endothelial cells [126]. These two active compounds contain either benzoxazinone or triazole derivatives and exhibit potent KLK3 inhibition with IC50 of 300–500 nM but lack selectivity toward KLK3 activity [126]. In addition, triazole derivatives have also been identified to inhibit other KLKs such as KLK5, 7, and 14, as well as matriptase (a transmembrane serine protease) [127]. Therefore, the non-selective mechanism of serine protease inhibition by β-lactam analogs and azapeptides severely limited their development as therapeutic drugs as they possess off-target inhibitory activity.
LeBeau et al. generated a series of small-molecule active-site inhibitors of KLK3 using peptide aldehydes and boronic acid-based inhibitors containing Ser-Ser-Lys-Leu-Gln peptide present in SEMG2 (a natural specific substrate for KLK3) as a template. Peptide aldehyde inhibitors showed specific proteolytic activity toward KLK3 with more than 20-fold specificity than that of chymotrypsin but had a Ki of 6.5 μM [128]. Boronic acid modification led to the development of more potent inhibitors with high specificity and a Ki of 65 nM, 100-fold lower than the aldehyde-modified peptide inhibitors [128]. In vivo evaluation of this inhibitor through intravenous injection at a dose of 33 mg/kg for two cycles of three consecutive days in human prostate cancer xeno-grafted mice led to significant reduction in KLK3 serum levels (free KLK3 levels by 35% and total KLK3 levels by 30%), however, had minimal effect on tumor growth [128]. Subsequent modification of this inhibitor by introducing the non-natural amino acid nor-leucine (Nle) exhibited potent KLK3 inhibition with a Ki of 48 nM [129].
To enhance selectivity of KLK3, Kostova et al. generated a peptidyl boronic acid-based selective KLK3 inhibitor containing a bromopropylglycine group, which had a Ki of 72 nM and eightfold selectivity over chymotrypsin. Systemic administration of this compound at a dose of 10 mg/kg for three cycles of five consecutive days in nude mice with human prostate cancer xenografts showed minimal effect on tumor growth but led to significant reduction in serum KLK3 levels [130]. Other novel KLK3-targeting therapeutic strategies involve the use of RNA aptamers, synthetic nucleic acid molecules, selected from pools of random oligonucleotides via the systematic evolution of ligands by exponential enrichment (SELEX) process to specifically target active KLK3 [131]. However, the selectivity of RNA aptamers on KLK3 activity has not yet been tested in vivo.
To date, numerous endogenous inhibitors of KLK3 activity with physiological significance ranging from metal ions (Zn2+) to proteinase inhibitors (SERPINs) have been reported, but none have been employed for the development of contraceptives to inhibit semen liquefaction in vivo. Additionally, the development of selective KLK3 inhibitors was focused for targeted treatment of prostate cancer. Although numerous small molecule and peptides to inhibit KLK3 activity have been developed, they may not be suitable for contraceptive purposes because the activity is not entirely specific to KLK3 but also bind to a great variety of proteases. An unusual feature of SERPINs is their ability to often inhibit non-target cysteine proteases, i.e., cross-class inhibition [132]. In addition, peptide inhibitors are often pH-dependent, thus may not withstand the relatively low pH in vaginal microenvironment [133].
Conclusion
Human semen liquefaction is a post-ejaculation proteolytic process that changes semen from a gel-like coagulum to a watery consistency (liquefied) and is mainly governed by SEMGs and prostate-derived KLK enzymatic activities. The blockade of semen liquefaction prevents sperm migration in the female reproductive tract and is an unexplored target for both male and female contraception. Inhibition of semen liquefaction can be achieved by using molecules that can stabilize SEMGs (preventing hydrolysis), local delivery of exogenous metal ions (Zn2+), overexpression of endogenous protease inhibitors (SERPINs/SPINKs), or administration of synthetic serine protease inhibitors. Of the numerous key molecules involved in the liquefaction cascade, targeting KLK activities (i.e., KLK2, 3, 5, and 14) is a viable option due to the fact that these KLKs are produced specifically in the prostate gland, hence, providing a localized target for the development of a non-hormonal contraceptive.
One of the immediate possibilities is the use of specific KLK3 inhibitors that were previously developed for prostate cancer patients. KLK3 in the seminal plasma is secreted at extremely high concentration, relative to other KLKs, and is the key executor enzyme involved in semen liquefaction. Rather than using a pan inhibitor of KLK gene family, which could potentially lead to non-intended effects, studies focusing on the development of small drug-like molecules specifically inhibiting seminal KLK3 activity would prove useful in the development of novel non-steroidal, over-the-counter contraceptive with improved efficiency.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1. Suarez SS, Pacey AA. Sperm transport in the female reproductive tract. Hum Reprod Update 2006; 12:23–37. [DOI] [PubMed] [Google Scholar]
- 2. Verze P, Cai T, Lorenzetti S. The role of the prostate in male fertility, health and disease. Nat Rev Urol 2016; 13:379–386. [DOI] [PubMed] [Google Scholar]
- 3. Drabovich AP, Saraon P, Jarvi K, Diamandis EP. Seminal plasma as a diagnostic fluid for male reproductive system disorders. Nat Rev Urol 2014; 11:278–288. [DOI] [PubMed] [Google Scholar]
- 4. Lilja H, Lundwall A. Molecular cloning of epididymal and seminal vesicular transcripts encoding a semenogelin-related protein. Proc Natl Acad Sci U S A 1992; 89:4559–4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lilja H, Abrahamsson PA, Lundwall A. Semenogelin, the predominant protein in human semen. Primary structure and identification of closely related proteins in the male accessory sex glands and on the spermatozoa. J Biol Chem 1989; 264:1894–1900. [PubMed] [Google Scholar]
- 6. Malm J, Hellman J, Magnusson H, Laurell CB, Lilja H. Isolation and characterization of the major gel proteins in human semen, semenogelin I and semenogelin II. Eur J Biochem 1996; 238:48–53. [DOI] [PubMed] [Google Scholar]
- 7. Robert M, Gagnon C. Semenogelin I: a coagulum forming, multifunctional seminal vesicle protein. Cell Mol Life Sci 1999; 55:944–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robert M, Gibbs BF, Jacobson E, Gagnon C. Characterization of prostate-specific antigen proteolytic activity on its major physiological substrate, the sperm motility inhibitor precursor/semenogelin I. Biochemistry 1997; 36:3811–3819. [DOI] [PubMed] [Google Scholar]
- 9. Robert M, Gagnon C. Purification and characterization of the active precursor of a human sperm motility inhibitor secreted by the seminal vesicles: identity with semenogelin. Biol Reprod 1996; 55:813–821. [DOI] [PubMed] [Google Scholar]
- 10. Mitra A, Richardson RT, O'Rand MG. Analysis of recombinant human semenogelin as an inhibitor of human sperm motility. Biol Reprod 2010; 82:489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Rand MG, Widgren EE, Beyler S, Richardson RT. Inhibition of human sperm motility by contraceptive anti-eppin antibodies from infertile male monkeys: effect on cyclic adenosine monophosphate. Biol Reprod 2009; 80:279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamasaki K, Yoshida K, Yoshiike M, Shimada K, Nishiyama H, Takamizawa S, Yanagida K, Iwamoto T. Relationship between semenogelins bound to human sperm and other semen parameters and pregnancy outcomes. Basic Clin Androl 2017; 27:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mandal A, Bhattacharyya AK. Sperm hyaluronidase activation by purified predominant and major basic human seminal coagulum proteins. Hum Reprod 1995; 10:1745–1750. [DOI] [PubMed] [Google Scholar]
- 14. Edstrom AM, Malm J, Frohm B, Martellini JA, Giwercman A, Morgelin M, Cole AM, Sorensen OE. The major bactericidal activity of human seminal plasma is zinc-dependent and derived from fragmentation of the semenogelins. J Immunol 2008; 181:3413–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoshida K, Krasznai ZT, Krasznai Z, Yoshiike M, Kawano N, Yoshida M, Morisawa M, Toth Z, Bazsane ZK, Marian T, Iwamoto T. Functional implications of membrane modification with semenogelins for inhibition of sperm motility in humans. Cell Motil Cytoskeleton 2009; 66:99–108. [DOI] [PubMed] [Google Scholar]
- 16. Lilja H, Abrahamsson PA. Three predominant proteins secreted by the human prostate gland. Prostate 1988; 12:29–38. [DOI] [PubMed] [Google Scholar]
- 17. Prassas I, Eissa A, Poda G, Diamandis EP. Unleashing the therapeutic potential of human kallikrein-related serine proteases. Nat Rev Drug Discov 2015; 14:183–202. [DOI] [PubMed] [Google Scholar]
- 18. Koumandou VL, Scorilas A. Evolution of the plasma and tissue kallikreins, and their alternative splicing isoforms. PLoS One 2013; 8:e68074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shaw JL, Diamandis EP. Distribution of 15 human kallikreins in tissues and biological fluids. Clin Chem 2007; 53:1423–1432. [DOI] [PubMed] [Google Scholar]
- 20. Brattsand M, Stefansson K, Lundh C, Haasum Y, Egelrud T. A proteolytic cascade of kallikreins in the stratum corneum. J Invest Dermatol 2005; 124:198–203. [DOI] [PubMed] [Google Scholar]
- 21. Michael IP, Pampalakis G, Mikolajczyk SD, Malm J, Sotiropoulou G, Diamandis EP. Human tissue kallikrein 5 is a member of a proteolytic cascade pathway involved in seminal clot liquefaction and potentially in prostate cancer progression. J Biol Chem 2006; 281:12743–12750. [DOI] [PubMed] [Google Scholar]
- 22. Kumar A, Mikolajczyk SD, Goel AS, Millar LS, Saedi MS. Expression of pro form of prostate-specific antigen by mammalian cells and its conversion to mature, active form by human kallikrein 2. Cancer Res 1997; 57:3111–3114. [PubMed] [Google Scholar]
- 23. Lovgren J, Rajakoski K, Karp M, Lundwall A, Lilja H. Activation of the zymogen form of prostate-specific antigen by human glandular kallikrein 2. Biochem Biophys Res Commun 1997; 238:549–555. [DOI] [PubMed] [Google Scholar]
- 24. Vaisanen V, Lovgren J, Hellman J, Piironen T, Lilja H, Pettersson K. Characterization and processing of prostate specific antigen (hK3) and human glandular kallikrein (hK2) secreted by LNCaP cells. Prostate Cancer Prostatic Dis 1999; 2:91–97. [DOI] [PubMed] [Google Scholar]
- 25. Takayama TK, Carter CA, Deng T. Activation of prostate-specific antigen precursor (pro-PSA) by prostin, a novel human prostatic serine protease identified by degenerate PCR. Biochemistry 2001; 40:1679–1687. [DOI] [PubMed] [Google Scholar]
- 26. Takayama TK, McMullen BA, Nelson PS, Matsumura M, Fujikawa K. Characterization of hK4 (prostase), a prostate-specific serine protease: activation of the precursor of prostate specific antigen (pro-PSA) and single-chain urokinase-type plasminogen activator and degradation of prostatic acid phosphatase. Biochemistry 2001; 40:15341–15348. [DOI] [PubMed] [Google Scholar]
- 27. Emami N, Diamandis EP. Human kallikrein-related peptidase 14 (KLK14) is a new activator component of the KLK proteolytic cascade. Possible function in seminal plasma and skin. J Biol Chem 2008; 283:3031–3041. [DOI] [PubMed] [Google Scholar]
- 28. Emami N, Deperthes D, Malm J, Diamandis EP. Major role of human KLK14 in seminal clot liquefaction. J Biol Chem 2008; 283:19561–19569. [DOI] [PubMed] [Google Scholar]
- 29. Luo LY, Shan SJ, Elliott MB, Soosaipillai A, Diamandis EP. Purification and characterization of human kallikrein 11, a candidate prostate and ovarian cancer biomarker, from seminal plasma. Clin Cancer Res 2006; 12:742–750. [DOI] [PubMed] [Google Scholar]
- 30. Karr JF, Kantor JA, Hand PH, Eggensperger DL, Schlom J. The presence of prostate-specific antigen-related genes in primates and the expression of recombinant human prostate-specific antigen in a transfected murine cell line. Cancer Res 1995; 55:2455–2462. [PubMed] [Google Scholar]
- 31. Diamandis EP, Yousef GM, Olsson AY. An update on human and mouse glandular kallikreins. Clin Biochem 2004; 37:258–260. [DOI] [PubMed] [Google Scholar]
- 32. Franklin RB, Milon B, Feng P, Costello LC. Zinc and zinc transporters in normal prostate and the pathogenesis of prostate cancer. Front Biosci 2005; 10:2230–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lilja H. A kallikrein-like serine protease in prostatic fluid cleaves the predominant seminal vesicle protein. J Clin Invest 1985; 76:1899–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tanaka M, Kishi Y, Takanezawa Y, Kakehi Y, Aoki J, Arai H. Prostatic acid phosphatase degrades lysophosphatidic acid in seminal plasma. FEBS Lett 2004; 571:197–204. [DOI] [PubMed] [Google Scholar]
- 35. Hirai K, Hussey HJ, Barber MD, Price SA, Tisdale MJ. Biological evaluation of a lipid-mobilizing factor isolated from the urine of cancer patients. Cancer Res 1998; 58:2359–2365. [PubMed] [Google Scholar]
- 36. Mattsson JM, Ravela S, Hekim C, Jonsson M, Malm J, Narvanen A, Stenman UH, Koistinen H. Proteolytic activity of prostate-specific antigen (PSA) towards protein substrates and effect of peptides stimulating PSA activity. PLoS One 2014; 9:e107819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zaneveld LJ, Tauber PF, Port C, Propping D, Schumacher GF. Scanning electron microscopy of the human, guinea-pig and rhesus monkey seminal coagulum. J Reprod Fertil 1974; 40:223–225. [DOI] [PubMed] [Google Scholar]
- 38. Lamirande E, Yoshida K, Yoshiike TM, Iwamoto T, Gagnon C. Semenogelin, the main protein of semen coagulum, inhibits human sperm capacitation by interfering with the superoxide anion generated during this process. J Androl 2001; 22:672–679. [PubMed] [Google Scholar]
- 39. Wang TJ, Rittenhouse HG, Wolfert RL, Lynne CM, Brackett NL. PSA concentrations in seminal plasma. Clin Chem 1998; 44:895–896. [PubMed] [Google Scholar]
- 40. Lilja H. Structure and function of prostatic- and seminal vesicle-secreted proteins involved in the gelation and liquefaction of human semen. Scand J Clin Lab Invest Suppl 1988; 191:13–20. [PubMed] [Google Scholar]
- 41. Malm J, Hellman J, Hogg P, Lilja H. Enzymatic action of prostate-specific antigen (PSA or hK3): substrate specificity and regulation by Zn(2+), a tight-binding inhibitor. Prostate 2000; 45:132–139. [DOI] [PubMed] [Google Scholar]
- 42. Deperthes D, Frenette G, Brillard-Bourdet M, Bourgeois L, Gauthier F, Tremblay RR, Dube JY. Potential involvement of kallikrein hK2 in the hydrolysis of the human seminal vesicle proteins after ejaculation. J Androl 1996; 17:659–665. [PubMed] [Google Scholar]
- 43. Lovgren J, Airas K, Lilja H. Enzymatic action of human glandular kallikrein 2 (hK2). Substrate specificity and regulation by Zn2+ and extracellular protease inhibitors. Eur J Biochem 1999; 262:781–789. [DOI] [PubMed] [Google Scholar]
- 44. Michael IP, Sotiropoulou G, Pampalakis G, Magklara A, Ghosh M, Wasney G, Diamandis EP. Biochemical and enzymatic characterization of human kallikrein 5 (hK5), a novel serine protease potentially involved in cancer progression. J Biol Chem 2005; 280:14628–14635. [DOI] [PubMed] [Google Scholar]
- 45. Kapadia C, Ghosh MC, Grass L, Diamandis EP. Human kallikrein 13 involvement in extracellular matrix degradation. Biochem Biophys Res Commun 2004; 323:1084–1090. [DOI] [PubMed] [Google Scholar]
- 46. Ghosh MC, Grass L, Soosaipillai A, Sotiropoulou G, Diamandis EP. Human kallikrein 6 degrades extracellular matrix proteins and may enhance the metastatic potential of tumour cells. Tumour Biol 2004; 25:193–199. [DOI] [PubMed] [Google Scholar]
- 47. Ramani VC, Haun RS. The extracellular matrix protein fibronectin is a substrate for kallikrein 7. Biochem Biophys Res Commun 2008; 369:1169–1173. [DOI] [PubMed] [Google Scholar]
- 48. Jonsson M, Linse S, Frohm B, Lundwall A, Malm J. Semenogelins I and II bind zinc and regulate the activity of prostate-specific antigen. Biochem J 2005; 387:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Suzuki K, Kise H, Nishioka J, Hayashi T. The interaction among protein C inhibitor, prostate-specific antigen, and the semenogelin system. Semin Thromb Hemost 2007; 33:46–52. [DOI] [PubMed] [Google Scholar]
- 50. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed. WHO; 2010. [PubMed] [Google Scholar]
- 51. Wilson VB, Bunge RG. Infertility and semen non-liquefaction. J Urol 1975; 113:509–510. [DOI] [PubMed] [Google Scholar]
- 52. Elia J, Delfino M, Imbrogno N, Capogreco F, Lucarelli M, Rossi T, Mazzilli F. Human semen hyperviscosity: prevalence, pathogenesis and therapeutic aspects. Asian J Androl 2009; 11:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gonzales GF, Kortebani G, Mazzolli AB. Hyperviscosity and hypofunction of the seminal vesicles. Arch Androl 1993; 30:63–68. [DOI] [PubMed] [Google Scholar]
- 54. Gopalkrishnan K, Padwal V, Balaiah D. Does seminal fluid viscosity influence sperm chromatin integrity? Arch Androl 2000; 45:99–103. [DOI] [PubMed] [Google Scholar]
- 55. EL S, Malm J, Giwercman A. Visco-elasticity of seminal fluid in relation to the epididymal and accessory sex gland function and its impact on sperm motility. Int J Androl 2004; 27:94–100. [DOI] [PubMed] [Google Scholar]
- 56. Mendeluk G, Gonzalez Flecha FL, Castello PR, Bregni C. Factors involved in the biochemical etiology of human seminal plasma hyperviscosity. J Androl 2000; 21:262–267. [PubMed] [Google Scholar]
- 57. Marques PI, Fonseca F, Carvalho AS, Puente DA, Damiao I, Almeida V, Barros N, Barros A, Carvalho F, Azkargorta M, Elortza F, Osorio H et al. . Sequence variation at KLK and WFDC clusters and its association to semen hyperviscosity and other male infertility phenotypes. Hum Reprod 2016; 31:2881–2891. [DOI] [PubMed] [Google Scholar]
- 58. Emami N, Scorilas A, Soosaipillai A, Earle T, Mullen B, Diamandis EP. Association between kallikrein-related peptidases (KLKs) and macroscopic indicators of semen analysis: their relation to sperm motility. Biol Chem 2009; 390:921–929. [DOI] [PubMed] [Google Scholar]
- 59. Ahlgren G, Rannevik G, Lilja H. Impaired secretory function of the prostate in men with oligo-asthenozoospermia. J Androl 1995; 16:491–498. [PubMed] [Google Scholar]
- 60. Gupta N, Sudhakar DVS, Gangwar PK, Sankhwar SN, Gupta NJ, Chakraborty B, Thangaraj K, Gupta G, Rajender S. Mutations in the prostate specific antigen (PSA/KLK3) correlate with male infertility. Sci Rep 2017; 7:11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee SH, Lee S. Genetic association study of a single nucleotide polymorphism of kallikrein-related peptidase 2 with male infertility. Clin Exp Reprod Med 2011; 38:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Savblom C, Hallden C, Cronin AM, Sall T, Savage C, Vertosick EA, Klein RJ, Giwercman A, Lilja H. Genetic variation in KLK2 and KLK3 is associated with concentrations of hK2 and PSA in serum and seminal plasma in young men. Clin Chem 2014; 60:490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wu J, Dong X, Liu K, Xia Y, Wang X, Shen O, Ding X, Zhang J. Association of semenogelin (SEMG) gene variants in idiopathic male infertility in Chinese-Han population. J Toxicol Environ Health A 2019; 82:928–934. [DOI] [PubMed] [Google Scholar]
- 64. Sharma R, Agarwal A, Mohanty G, Jesudasan R, Gopalan B, Willard B, Yadav SP, Sabanegh E. Functional proteomic analysis of seminal plasma proteins in men with various semen parameters. Reprod Biol Endocrinol 2013; 11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thacker S, Yadav SP, Sharma RK, Kashou A, Willard B, Zhang D, Agarwal A. Evaluation of sperm proteins in infertile men: a proteomic approach. Fertil Steril 2011; 95:2745–2748. [DOI] [PubMed] [Google Scholar]
- 66. Kawano N, Yoshida M. Semen-coagulating protein, SVS2, in mouse seminal plasma controls sperm fertility. Biol Reprod 2007; 76:353–361. [DOI] [PubMed] [Google Scholar]
- 67. Araki N, Trencsenyi G, Krasznai ZT, Nizsaloczki E, Sakamoto A, Kawano N, Miyado K, Yoshida K, Yoshida M. Seminal vesicle secretion 2 acts as a protectant of sperm sterols and prevents ectopic sperm capacitation in mice. Biol Reprod 2015; 92:8. [DOI] [PubMed] [Google Scholar]
- 68. Kawano N, Araki N, Yoshida K, Hibino T, Ohnami N, Makino M, Kanai S, Hasuwa H, Yoshida M, Miyado K, Umezawa A. Seminal vesicle protein SVS2 is required for sperm survival in the uterus. Proc Natl Acad Sci U S A 2014; 111:4145–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Noda T, Fujihara Y, Matsumura T, Oura S, Kobayashi S, Ikawa M. Seminal vesicle secretory protein 7, PATE4, is not required for sperm function but for copulatory plug formation to ensure fecundity. Biol Reprod 2019; 100:1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Luo CW, Lin HJ, Chen YH. A novel heat-labile phospholipid-binding protein, SVS VII, in mouse seminal vesicle as a sperm motility enhancer. J Biol Chem 2001; 276:6913–6921. [DOI] [PubMed] [Google Scholar]
- 71. Noda T, Ikawa M. Physiological function of seminal vesicle secretions on male fecundity. Reprod Med Biol 2019; 18:241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Peter A, Lilja H, Lundwall A, Malm J. Semenogelin I and semenogelin II, the major gel-forming proteins in human semen, are substrates for transglutaminase. Eur J Biochem 1998; 252:216–221. [DOI] [PubMed] [Google Scholar]
- 73. Lorand L, Iismaa SE. Transglutaminase diseases: from biochemistry to the bedside. FASEB J 2019; 33:3–12. [DOI] [PubMed] [Google Scholar]
- 74. Landegren N, Sharon D, Shum AK, Khan IS, Fasano KJ, Hallgren A, Kampf C, Freyhult E, Ardesjo-Lundgren B, Alimohammadi M, Rathsman S, Ludvigsson JF et al. . Transglutaminase 4 as a prostate autoantigen in male subfertility. Sci Transl Med 2015; 7:292ra101. [DOI] [PubMed] [Google Scholar]
- 75. Dean MD. Genetic disruption of the copulatory plug in mice leads to severely reduced fertility. PLoS Genet 2013; 9:e1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Burnett AL, Aus G, Canby-Hagino ED, Cookson MS, D'Amico AV, Dmochowski RR, Eton DT, Forman JD, Goldenberg SL, Hernandez J, Higano CS, Kraus S et al. . Erectile function outcome reporting after clinically localized prostate cancer treatment. J Urol 2007; 178:597–601. [DOI] [PubMed] [Google Scholar]
- 77. Richardson RT, Sivashanmugam P, Hall SH, Hamil KG, Moore PA, Ruben SM, French FS, O'Rand M. Cloning and sequencing of human Eppin: a novel family of protease inhibitors expressed in the epididymis and testis. Gene 2001; 270:93–102. [DOI] [PubMed] [Google Scholar]
- 78. Wang Z, Widgren EE, Sivashanmugam P, O'Rand MG, Richardson RT. Association of eppin with semenogelin on human spermatozoa. Biol Reprod 2005; 72:1064–1070. [DOI] [PubMed] [Google Scholar]
- 79. Wang Z, Widgren EE, Richardson RT, O'Rand MG. Characterization of an eppin protein complex from human semen and spermatozoa. Biol Reprod 2007; 77:476–484. [DOI] [PubMed] [Google Scholar]
- 80. Dube E, Hermo L, Chan PT, Cyr DG. Alterations in gene expression in the caput epididymides of nonobstructive azoospermic men. Biol Reprod 2008; 78:342–351. [DOI] [PubMed] [Google Scholar]
- 81. Ding X, Zhang J, Fei J, Bian Z, Li Y, Xia Y, Lu C, Song L, Wang S, Wang X. Variants of the EPPIN gene affect the risk of idiopathic male infertility in the Han-Chinese population. Hum Reprod 2010; 25:1657–1665. [DOI] [PubMed] [Google Scholar]
- 82. Lu CH, Lee RK, Hwu YM, Chu SL, Chen YJ, Chang WC, Lin SP, Li SH. SERPINE2, a serine protease inhibitor extensively expressed in adult male mouse reproductive tissues, may serve as a murine sperm decapacitation factor. Biol Reprod 2011; 84:514–525. [DOI] [PubMed] [Google Scholar]
- 83. Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995; 121:1129–1137. [DOI] [PubMed] [Google Scholar]
- 84. Kwon WS, Rahman MS, Pang MG. Diagnosis and prognosis of male infertility in mammal: the focusing of tyrosine phosphorylation and phosphotyrosine proteins. J Proteome Res 2014; 13:4505–4517. [DOI] [PubMed] [Google Scholar]
- 85. Murer V, Spetz JF, Hengst U, Altrogge LM, Agostini A, Monard D. Male fertility defects in mice lacking the serine protease inhibitor protease nexin-1. Proc Natl Acad Sci U S A 2001; 98:3029–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kherraf ZE, Christou-Kent M, Karaouzene T, Amiri-Yekta A, Martinez G, Vargas AS, Lambert E, Borel C, Dorphin B, Aknin-Seifer I, Mitchell MJ, Metzler-Guillemain C et al. . SPINK2 deficiency causes infertility by inducing sperm defects in heterozygotes and azoospermia in homozygotes. EMBO Mol Med 2017; 9:1132–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rockett JC, Patrizio P, Schmid JE, Hecht NB, Dix DJ. Gene expression patterns associated with infertility in humans and rodent models. Mutat Res 2004; 549:225–240. [DOI] [PubMed] [Google Scholar]
- 88. Lee B, Park I, Jin S, Choi H, Kwon JT, Kim J, Jeong J, Cho BN, Eddy EM, Cho C. Impaired spermatogenesis and fertility in mice carrying a mutation in the Spink2 gene expressed predominantly in testes. J Biol Chem 2011; 286:29108–29117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ou CM, Tang JB, Huang MS, Sudhakar Gandhi PS, Geetha S, Li SH, Chen YH. The mode of reproductive-derived Spink (serine protease inhibitor Kazal-type) action in the modulation of mammalian sperm activity. Int J Androl 2012; 35:52–62. [DOI] [PubMed] [Google Scholar]
- 90. Zalazar L, Saez Lancellotti TE, Clementi M, Lombardo C, Lamattina L, De Castro R, Fornes MW, Cesari A. SPINK3 modulates mouse sperm physiology through the reduction of nitric oxide level independently of its trypsin inhibitory activity. Reproduction 2012; 143:281–295. [DOI] [PubMed] [Google Scholar]
- 91. Tartaglia-Polcini A, Bonnart C, Micheloni A, Cianfarani F, Andre A, Zambruno G, Hovnanian A, D'Alessio M. SPINK5, the defective gene in netherton syndrome, encodes multiple LEKTI isoforms derived from alternative pre-mRNA processing. J Invest Dermatol 2006; 126:315–324. [DOI] [PubMed] [Google Scholar]
- 92. Deraison C, Bonnart C, Lopez F, Besson C, Robinson R, Jayakumar A, Wagberg F, Brattsand M, Hachem JP, Leonardsson G, Hovnanian A. LEKTI fragments specifically inhibit KLK5, KLK7, and KLK14 and control desquamation through a pH-dependent interaction. Mol Biol Cell 2007; 18:3607–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Descargues P, Deraison C, Bonnart C, Kreft M, Kishibe M, Ishida-Yamamoto A, Elias P, Barrandon Y, Zambruno G, Sonnenberg A, Hovnanian A. Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nat Genet 2005; 37:56–65. [DOI] [PubMed] [Google Scholar]
- 94. Meyer-Hoffert U, Wu Z, Kantyka T, Fischer J, Latendorf T, Hansmann B, Bartels J, He Y, Glaser R, Schroder JM. Isolation of SPINK6 in human skin: selective inhibitor of kallikrein-related peptidases. J Biol Chem 2010; 285:32174–32181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Fischer J, Wu Z, Kantyka T, Sperrhacke M, Dimitrieva O, Koblyakova Y, Ahrens K, Graumann N, Baurecht H, Reiss K, Schroder JM, Proksch E et al. . Characterization of Spink6 in mouse skin: The conserved inhibitor of kallikrein-related peptidases is reduced by barrier injury. J Invest Dermatol 2014; 134:1305–1312. [DOI] [PubMed] [Google Scholar]
- 96. Ma L, Yu H, Ni Z, Hu S, Ma W, Chu C, Liu Q, Zhang Y. Spink13, an epididymis-specific gene of the Kazal-type serine protease inhibitor (SPINK) family, is essential for the acrosomal integrity and male fertility. J Biol Chem 2013; 288:10154–10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lin MH, Lee RK, Hwu YM, Lu CH, Chu SL, Chen YJ, Chang WC, Li SH. SPINKL, a Kazal-type serine protease inhibitor-like protein purified from mouse seminal vesicle fluid, is able to inhibit sperm capacitation. Reproduction 2008; 136:559–571. [DOI] [PubMed] [Google Scholar]
- 98. Shaw JL, Petraki C, Watson C, Bocking A, Diamandis EP. Role of tissue kallikrein-related peptidases in cervical mucus remodeling and host defense. Biol Chem 2008; 389:1513–1522. [DOI] [PubMed] [Google Scholar]
- 99. Shaw JL, Smith CR, Diamandis EP. Proteomic analysis of human cervico-vaginal fluid. J Proteome Res 2007; 6:2859–2865. [DOI] [PubMed] [Google Scholar]
- 100. Zegels G, Van Raemdonck GA, Coen EP, Tjalma WA, Van Ostade XW. Comprehensive proteomic analysis of human cervical-vaginal fluid using colposcopy samples. Proteome Sci 2009; 7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Li S, Garcia M, Gewiss RL, Winuthayanon W. Crucial role of estrogen for the mammalian female in regulating semen coagulation and liquefaction in vivo. PLoS Genet 2017; 13:e1006743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Clements J, Mukhtar A, Ehrlich A, Yap B. Glandular kallikrein gene expression in the human uterus. Braz J Med Biol Res 1994; 27:1855–1863. [PubMed] [Google Scholar]
- 103. Rajapakse S, Yamano N, Ogiwara K, Hirata K, Takahashi S, Takahashi T. Estrogen-dependent expression of the tissue kallikrein gene (Klk1) in the mouse uterus and its implications for endometrial tissue growth. Mol Reprod Dev 2007; 74:1053–1063. [DOI] [PubMed] [Google Scholar]
- 104. Peipert JF, Gutmann J. Oral contraceptive risk assessment: a survey of 247 educated women. Obstet Gynecol 1993; 82:112–117. [PubMed] [Google Scholar]
- 105. Gronich N, Lavi I, Rennert G. Higher risk of venous thrombosis associated with drospirenone-containing oral contraceptives: a population-based cohort study. CMAJ 2011; 183:E1319–E1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. La Vecchia C, Altieri A, Franceschi S, Tavani A. Oral contraceptives and cancer: an update. Drug Saf 2001; 24:741–754. [DOI] [PubMed] [Google Scholar]
- 107. Cushman M, Kuller LH, Prentice R, Rodabough RJ, Psaty BM, Stafford RS, Sidney S, Rosendaal FR, Women’s Health Initiative I. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004; 292:1573–1580. [DOI] [PubMed] [Google Scholar]
- 108. Group ECW Hormones and cardiovascular health in women. Hum Reprod Update 2006; 12:483–497. [DOI] [PubMed] [Google Scholar]
- 109. Huber LR, Hogue CJ, Stein AD, Drews C, Zieman M, King J, Schayes S. Contraceptive use and discontinuation: findings from the contraceptive history, initiation, and choice study. Am J Obstet Gynecol 2006; 194:1290–1295. [DOI] [PubMed] [Google Scholar]
- 110. Trussell J. Contraceptive failure in the United States. Contraception 2011; 83:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Roddy RE, Cordero M, Cordero C, Fortney JA. A dosing study of nonoxynol-9 and genital irritation. Int J STD AIDS 1993; 4:165–170. [DOI] [PubMed] [Google Scholar]
- 112. Raymond EG, Chen PL, Luoto J, Spermicide TG. Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: a randomized trial. Obstet Gynecol 2004; 103:430–439. [DOI] [PubMed] [Google Scholar]
- 113. Niruthisard S, Roddy RE, Chutivongse S. The effects of frequent nonoxynol-9 use on the vaginal and cervical mucosa. Sex Transm Dis 1991; 18:176–179. [DOI] [PubMed] [Google Scholar]
- 114. Matsuda Y, Oshio S, Yazaki T, Umeda T, Akihama S. The effect of some proteinase inhibitors on liquefaction of human semen. Hum Reprod 1994; 9:664–668. [DOI] [PubMed] [Google Scholar]
- 115. Zhang WM, Leinonen J, Kalkkinen N, Stenman UH. Prostate-specific antigen forms a complex with and cleaves alpha 1-protease inhibitor in vitro. Prostate 1997; 33:87–96. [DOI] [PubMed] [Google Scholar]
- 116. Barton BE, Rock JK, Willie AM, Harris EA, Finnerty RM, Herrera GG, Anamthathmakula P, Winuthayanon W. Serine protease inhibitor disrupts sperm motility leading to reduced fertility in female mice. Biol Reprod 2020:ioaa049. doi: 10.1093/biolre/ioaa049. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. O'Rand MG, Widgren EE, Sivashanmugam P, Richardson RT, Hall SH, French FS, VandeVoort CA, Ramachandra SG, Ramesh V, Jagannadha RA. Reversible immunocontraception in male monkeys immunized with eppin. Science 2004; 306:1189–1190. [DOI] [PubMed] [Google Scholar]
- 118. Zhang J, Ding X, Bian Z, Xia Y, Lu C, Wang S, Song L, Wang X. The effect of anti-eppin antibodies on ionophore A23187-induced calcium influx and acrosome reaction of human spermatozoa. Hum Reprod 2010; 25:29–36. [DOI] [PubMed] [Google Scholar]
- 119. O'Rand MG, Hamil KG, Adevai T, Zelinski M. Inhibition of sperm motility in male macaques with EP055, a potential non-hormonal male contraceptive. PLoS One 2018; 13:e0195953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wang Z, Widgren EE, Richardson RT, Orand MG. Eppin: A molecular strategy for male contraception. Soc Reprod Fertil Suppl 2007; 65:535–542. [PubMed] [Google Scholar]
- 121. O'Rand MG, Widgren EE, Hamil KG, Silva EJ, Richardson RT. Functional studies of EPPIN. Biochem Soc Trans 2011; 39:1447–1449. [DOI] [PubMed] [Google Scholar]
- 122. Adlington RM, Baldwin JE, Becker GW, Chen B, Cheng L, Cooper SL, Hermann RB, Howe TJ, McCoull W, McNulty AM, Neubauer BL, Pritchard GJ. Design, synthesis, and proposed active site binding analysis of monocyclic 2-azetidinone inhibitors of prostate specific antigen. J Med Chem 2001; 44:1491–1508. [DOI] [PubMed] [Google Scholar]
- 123. Singh P, Williams SA, Shah MH, Lectka T, Pritchard GJ, Isaacs JT, Denmeade SR. Mechanistic insights into the inhibition of prostate specific antigen by beta-lactam class compounds. Proteins 2008; 70:1416–1428. [DOI] [PubMed] [Google Scholar]
- 124. Singh P, LeBeau AM, Lilja H, Denmeade SR, Isaacs JT. Molecular insights into substrate specificity of prostate specific antigen through structural modeling. Proteins 2009; 77:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Huang X, Knoell CT, Frey G, Hazegh-Azam M, Tashjian AH Jr, Hedstrom L, Abeles RH, Timasheff SN. Modulation of recombinant human prostate-specific antigen: activation by Hofmeister salts and inhibition by azapeptides. Appendix: Thermodynamic interpretation of the activation by concentrated salts. Biochemistry 2001; 40:11734–11741. [DOI] [PubMed] [Google Scholar]
- 126. Koistinen H, Wohlfahrt G, Mattsson JM, Wu P, Lahdenpera J, Stenman UH. Novel small molecule inhibitors for prostate-specific antigen. Prostate 2008; 68:1143–1151. [DOI] [PubMed] [Google Scholar]
- 127. Tan X, Furio L, Reboud-Ravaux M, Villoutreix BO, Hovnanian A, El Amri C. 1,2,4-Triazole derivatives as transient inactivators of kallikreins involved in skin diseases. Bioorg Med Chem Lett 2013; 23:4547–4551. [DOI] [PubMed] [Google Scholar]
- 128. LeBeau AM, Singh P, Isaacs JT, Denmeade SR. Potent and selective peptidyl boronic acid inhibitors of the serine protease prostate-specific antigen. Chem Biol 2008; 15:665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. LeBeau AM, Banerjee SR, Pomper MG, Mease RC, Denmeade SR. Optimization of peptide-based inhibitors of prostate-specific antigen (PSA) as targeted imaging agents for prostate cancer. Bioorg Med Chem 2009; 17:4888–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kostova MB, Rosen DM, Chen Y, Mease RC, Denmeade SR. Structural optimization, biological evaluation, and application of peptidomimetic prostate specific antigen inhibitors. J Med Chem 2013; 56:4224–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Jeong S, Han SR, Lee YJ, Lee SW. Selection of RNA aptamers specific to active prostate-specific antigen. Biotechnol Lett 2010; 32:379–385. [DOI] [PubMed] [Google Scholar]
- 132. Swanson R, Raghavendra MP, Zhang W, Froelich C, Gettins PG, Olson ST. Serine and cysteine proteases are translocated to similar extents upon formation of covalent complexes with serpins. Fluorescence perturbation and fluorescence resonance energy transfer mapping of the protease binding site in CrmA complexes with granzyme B and caspase-1. J Biol Chem 2007; 282:2305–2313. [DOI] [PubMed] [Google Scholar]
- 133. Fox CA, Meldrum SJ, Watson BW. Continuous measurement by radio-telemetry of vaginal pH during human coitus. J Reprod Fertil 1973; 33:69–75. [DOI] [PubMed] [Google Scholar]


