Abstract
Medication overuse headache is estimated to affect 2% of the population, and is ranked in the top 20 most disabling disorders due to its high level of disability. Several therapies used in the treatment of acute migraine are thought to be associated with medication overuse headache, including opioids and triptans. With limited treatment options, it is critical to determine the risk profile of novel therapies prior to their widespread use. The current study explores the potential medication overuse risk of two novel therapeutic drug classes, namely the ditans: 5-HT1F receptor agonists, and the gepants: calcitonin gene-related peptide receptor antagonists, in a preclinical model of medication overuse. Persistent exposure of mice to the 5-HT1F agonist LY344864, but not olcegepant produced a significant reduction in hind paw and orofacial mechanical withdrawal thresholds as a surrogate readout of allodynia. In agreement, only LY344864 induced neuroplastic changes in trigeminal sensory afferents, increasing calcitonin gene-related peptide expression and basal trigeminal nociception. Our data highlight a differential medication overuse headache risk profile for the ditan and gepant classes of drugs that has important implications for their clinical use and patient education to help reduce the burden of medication overuse headache.
Keywords: migraine, headache: experimental models, headache: drug treatment, secondary headache, trigeminal ganglion
Several acute anti-migraine therapies increase the risk of developing medication overuse headache (MOH), a severely disabling condition. Saengjaroentham et al. report a potential MOH risk for novel 5-HT1F receptor agonists that have recently been approved by the FDA, but not for CGRP receptor antagonists.
Introduction
Headache disorders are common causes of disability, particularly chronic migraine, chronic cluster headache and medication overuse headache (MOH). These complex conditions represent a major challenge for healthcare services, with 30–50% of all tertiary headache clinic patients suffering from MOH (Bigal et al., 2008). Migraine is the most common disabling headache disorder, with over 1 billion sufferers globally (GBD 2016 Headache Collaborators, 2018), 2.5% of whom transition to a chronic state annually (Bigal et al., 2008). Patients with headache disorder biology appear particularly susceptible to the development of MOH (Bahra et al., 2003), whereby persistent overuse of their acute anti-migraine therapies for between 10 and 15 days per month significantly increases the risk of developing MOH (Headache Classification Committee of the International Headache Society, 2018). MOH, despite only affecting ∼2% of the population (Bigal et al., 2008) is considered one of the most disabling disorders (Global Burden of Disease Study 2013 Collaborators, 2015), resulting in a total of 9.5 million years lived with disability (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018) and a socioeconomic cost of €37 billion annually in the EU (Linde et al., 2012).
Several headache medications increase the potential risk for MOH when used to excess, including triptans (5-HT1B/1D receptor agonists), some non-steroidal anti-inflammatory drugs (NSAIDs) and opioids (Bigal et al., 2008). Thus, MOH appears to develop in genetically susceptible individuals (Cargnin et al., 2018) in response to a diverse array of agents. While there are no specific treatments, withdrawal of the causative agent normally improves the symptoms (Engelstoft et al., 2019); however, ∼40% relapse within 12 months. Given the broad spectrum of agents that can induce MOH it is critical to determine the relative MOH risk of novel anti-migraine therapies.
In the past 6 months lasmiditan (5-HT1F receptor agonist, ditan), ubrogepant and rimegepant [calcitonin-gene-related peptide (CGRP) receptor antagonists, gepants] received FDA approval. Lasmiditan, shares partial receptor affinity with selected triptans (Goadsby and Classey, 2003) that have an established MOH risk profile. Whereas, data from CGRP monoclonal antibodies (Tepper et al., 2019), and preliminary data on preventive action of gepants (Goadsby et al., 2019) suggest lower MOH risk profiles. Interestingly, several of the established and novel therapies share similar mechanisms, via the modulation of CGRP signalling (Durham and Russo, 2003; Labastida-Ramirez et al., 2020), a key neuropeptide in the pathophysiology of headache, making it difficult to predict potential MOH risk.
Therefore, the aim of the current study was to determine the potential MOH risk of ditans and gepants in an established preclinical model of MOH, whereby persistent exposure of rodents to specific therapeutic agents induces a state of mechanical hypersensitivity (De Felice et al., 2010) as a surrogate readout of allodynia observed in MOH patients (Lipton et al., 2019). This information is essential to inform clinical practice and permit patient education as to the potential risks of overuse of these novel compounds.
Materials and methods
Animals
Adult male C57Bl6/J mice (n = 78; Charles River) aged 8 weeks, were maintained under standard animal husbandry conditions with food and water available ad libitum. All studies were ethically approved and conducted in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986 and are reported in agreement with the ARRIVE guidelines.
Drugs
LY344864-hydrochloride, a selective 5-HT1F receptor agonist, and olcegepant, a CGRP receptor antagonist were selected because of their commercial availability (Tocris) and prior in vivo use. Both compounds were dissolved in 2% dimethyl sulfoxide in saline and sumatriptan in saline. Drug doses were based on available literature demonstrating biological effects in rodents at these doses. Olcegepant and LY344864 were injected at a dose of 1 mg/kg and sumatriptan at 0.6 mg/kg. Drugs were administered intraperitoneally daily for 11 days for hind paw assessment of olcegepant and LY344864 (n = 10 per group), or for 17 days for orofacial sensory testing in response to LY344864 (n = 8 per group). A second study, established to assess orofacial sensory thresholds in response to olcegepant (n = 8 per group), was terminated early at 7 days due to the immediate suspension of all research during the COVID-19 pandemic.
Sensory testing
Mechanical withdrawal thresholds were assessed using von Frey filaments, as previously described using the up-down method in separate groups of mice (Moye et al., 2019). For the hind paw, following habituation, mice were tested to establish reliable baseline responses and then every second day following the onset of drug administration. Because of potential sensitization from excessive orofacial stimuli, this was conducted every 4–7 days following the establishment of stable baseline responses and the onset of drug administration. All testing was conducted at the same time of day under dim light (30–50 lx) to minimize variability. Graduated von Frey filaments (0.008–2 g) were then applied to the hind paw or periorbital region using the up-down method to calculate mechanical withdrawal thresholds (Chaplan et al., 1994).
Tissue processing
At the conclusion of testing, mice were perfused with heparinized phosphate-buffered saline, followed by 4% paraformaldehyde under terminal anaesthesia. The spinal cord was dissected, post-fixed for 1 h and cryoprotected, prior to sectioning at 30 µm on a cryostat. To determine the expression of CGRP or the immediate-early gene c-Fos in the trigeminocervical complex (TCC), the primary interface of peripheral trigeminal sensory afferents and the CNS, sections were then processed using standard immunohistochemical approaches and incubated with either anti-CGRP (Abcam; 1:2000) or anti-c-Fos (Millipore; 1:10 000) primary antibodies. Specific staining was confirmed by the omission of primary antibodies and the specificity of each antibody has been previously confirmed. The expression of CGRP immunoreactive fibres in the TCC was visualized using an appropriate secondary antibody, conjugated to Alexa Fluor® 568 and the number of c-Fos-positive nuclei in the TCC was visualized via 3,3′-diaminobenzidine (DAB), following appropriate amplification. Sections were then coverslipped prior to undergoing fluorescent or light microscopic analysis (Zeiss Axio Imager).
Statistical analysis
Sample sizes, calculated in G.Power, are based on previous studies (Moye et al., 2019), combined with a medium to high effect size (0.25–0.5), a probability of 0.05 and power of 0.8–0.9, resulting in an n of 8–10 for behavioural analysis, depending on the number of repeated measures. This additionally provided sufficient tissue for immunohistochemical analysis. Mice were initially counterbalanced into groups that were subsequently randomly assigned to an experimental grouping. All analysis was conducted blind to the experimental group, and all data are presented as the mean ± standard error of the mean (SEM) or median and interquartile ranges (IQR). All mice tested were included in the behavioural analysis and all analysis and graphs were generated in GraphPad Prism v.7. To assess hind paw and orofacial mechanical withdrawal thresholds the 50% withdrawal thresholds were compared across time via a two-way repeated measures ANOVA, comparing to vehicle control treated mice, followed by Sidak’s multiple comparison test exploring group differences at different time points where appropriate. Additionally, the integrated area under the curve (AUC) for each intervention across time was calculated for graphical representation. To assess CGRP and c-Fos expression n = 8 and 7 mice per group, respectively were included in the final analysis, based on those mice that had all appropriate tissue samples available for analysis following processing. The percentage area stained for CGRP (six sections across the TCC) in lamina I and II or the total number of c-Fos positive nuclei (nine sections across the TCC) in lamina I–V of the TCC were calculated. The groups were then compared via the Kruskal-Wallis test, followed by Dunn’s multiple comparison test where appropriate.
Data availability
All data are available upon reasonable request.
Results
Differential impact of persistent exposure to two novel anti-migraine therapies in the hind paw
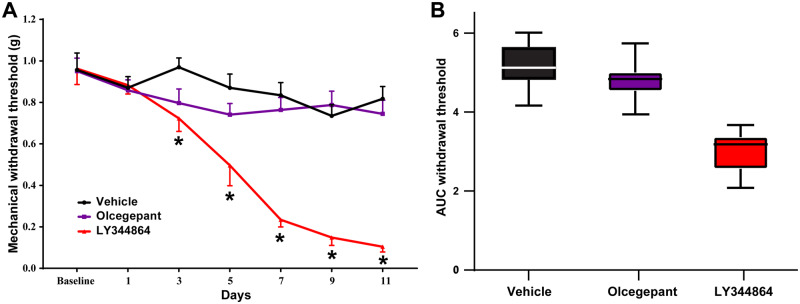
Persistent exposure of mice to sumatriptan [F(6,108) = 9.79, P ≤ 0.0001] and LY344864 [F(6,108) = 13.08, P ≤ 0.0001], but not olcegepant [F(6,108) = 0.84, P = 0.54] for 11 days induced a time-dependent reduction in mechanical withdrawal thresholds as compared to vehicle control treated mice (Fig. 1A, n = 10 per group). The AUC across the 11 days was 4.80 ± 0.33 and 5.17 ± 0.32 for olcegepant and vehicle control, respectively; however, this was reduced to 3.0 ± 0.33 for LY344864 (Fig. 1B).
Figure 1.
Persistent exposure to the 5-HT1F receptor agonist LY344864, but not olcegepant reduces hind paw mechanical withdrawal thresholds in mice. Repeated daily exposure of mice to LY344864 [1 mg/kg; F(6,108) = 13.08, P ≤ 0.0001], but not olcegepant [1 mg/kg; F(6,108) = 0.84, P = 0.54] induced a temporal reduction in hind paw mechanical withdrawal thresholds when compared to vehicle treated mice (A) as a preclinical readout of medication overuse-induced cutaneous allodynia. LY344864 reduced mechanical withdrawal thresholds from Day 3 [t(16.4) = 3.21, P ≤ 0.05] that remained significantly reduced across the 11 days, maximally at Day 11 [t(12.2) = 11.10, P ≤ 0.0001]. The integrated AUC was similar between vehicle (5.17 ± 0.32) and olcegepant (4.80 ± 0.33) groups, but reduced following persistent LY344864 exposure 3.0 ± 0.33 (B), highlighting a potential medication overuse headache risk profile for the 5-HT1F agonist ditan class of drugs. *P < 0.05, n = 10 mice per group.
Differential impact of persistent exposure to two novel anti-migraine therapies in the orofacial dermatome
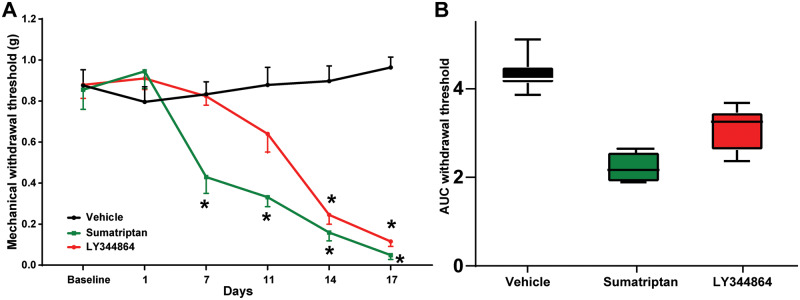
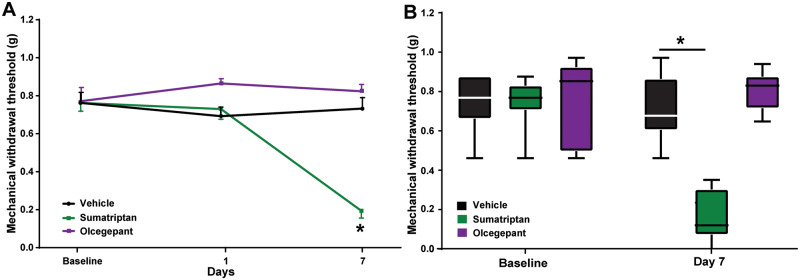
Having determined a differential MOH risk for LY344864 and olcegepant, we next sought to confirm these results in the orofacial dermatome (n = 8 per group). Given the longer interval between sensory testing in the face, mice were exposed to sumatriptan (0.6 mg/kg), LY344864 (1 mg/kg), or vehicle control for 17 days following the establishment of basal sensory thresholds; however, for olcegepant the study was terminated early after 7 days due to the COVID-19 pandemic. Persistent exposure of mice to sumatriptan [F(5,70) = 17.86, P ≤ 0.0001] and LY344864 [F(5,70) = 17.15, P ≤ 0.0001] for 17 days induced a time-dependent reduction in orofacial mechanical withdrawal thresholds as compared to vehicle control treated mice (Fig. 2A). The AUC across the 17 days was 4.3 ± 0.3 for vehicle control treated mice and 2.3 ± 0.30 and 3.10 ± 0.28 for the sumatriptan and LY344864 groups, respectively (Fig 2B). In a separate cohort, persistent exposure of mice to daily olcegepant for 7 days did not alter orofacial mechanical withdrawal thresholds when compared to vehicle treated mice [F(2,28) = 1.38, P = 0.27; Fig. 3A and B], despite a clear reduction in response to sumatriptan [F(2,28) = 21.94, P ≤ 0.0001; Fig. 3A and B]. Because of the reduced duration, no AUC calculations were conducted.
Figure 2.
Persistent exposure to the 5-HT1F receptor agonist LY344864 reduces orofacial mechanical withdrawal thresholds in mice. Repeated daily exposure of mice to LY344864 [F(5,70) = 17.15, P ≤ 0.0001] and sumatriptan [F(5,70) = 17.86, P ≤ 0.0001] induced a temporal reduction in orofacial mechanical withdrawal thresholds (A) as a preclinical readout of medication overuse-induced cephalic allodynia, when compared to vehicle treated mice. LY344864 reduced mechanical withdrawal thresholds from Day 14 [t(84) = 6.93, P ≤ 0.0001] that remained significantly reduced across the 17 days, maximally at Day 17 [t(84) = 9.02, P ≤ 0.0001]. Sumatriptan reduced mechanical withdrawal thresholds from Day 7 [t(84) = 4.09, P ≤ 0.001] that remained significantly reduced across the 17 days, maximally at Day 17 [t(84) = 9.28, P ≤ 0.0001]. The integrated AUC for vehicle treated mice was 4.3 ± 0.32, compared to 3.10 ± 0.28 for LY344864 and 2.3 ± 0.30 for sumatriptan (B), highlighting a potential medication overuse headache risk profile for the 5-HT1F agonist ditan class of drugs that is similar to the related triptans that are known to increase the risk of medication overuse headache in migraineurs. *P < 0.05, n = 8 mice per group.
Figure 3.
Persistent exposure to the CGRP receptor antagonist olcegepant has no effect on orofacial mechanical withdrawal thresholds in mice. In an additional cohort of mice that had to be terminated early (Day 7) due to the COVID-19 pandemic, repeated daily exposure of mice to sumatriptan [F(2,28) = 21.94, P ≤ 0.0001] but not olcegepant [F(2,28) = 1.38, P = 0.27] reduced orofacial mechanical withdrawal thresholds (A) out to Day 7 (B). *P < 0.05, n = 8 mice per group.
Calcitonin-gene-related peptide expression in the trigeminocervical complex
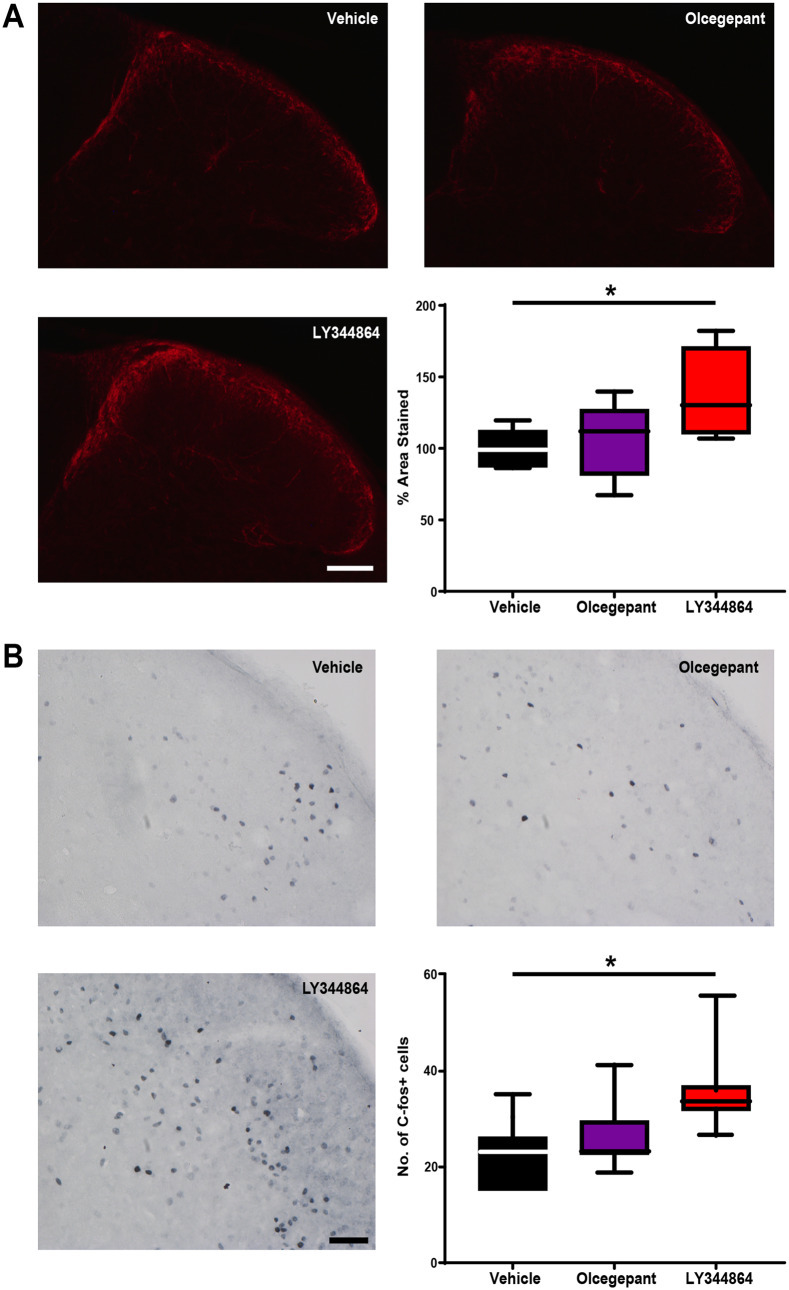
CGRP is a key neuropeptide involved in the pathophysiology of headache and a potential biomarker for chronic migraine and MOH. Preclinically, increased CGRP expression in these trigeminal sensory afferents is a reliable marker of neuroplastic changes following the induction of MOH (De Felice et al., 2010). Following 11 days of drug exposure there was a significant increase in the percentage area stained with CGRP in the TCC [H(2) = 7.22, P ≤ 0.05, n = 8 per group]. Mice persistently exposed to LY344864 had significantly increased CGRP expression when compared to vehicle control mice [median (IQR): 130 (110–170) versus 99 (87–112), Z = 2.62, P ≤ 0.05; Fig. 4A]. There was no significant difference between vehicle control and olcegepant treated mice [median (IQR): 99 (87–112) versus 112 (81–127), Z = 0.78, P = 0.99].
Figure 4.
Persistent exposure to the 5-HT1F receptor agonist LY344864, but not olcegepant induces neuroplastic changes in trigeminal sensory afferents. (A) Repeated daily exposure of mice to LY344864, but not olcegepant induced an increased expression of CGRP in the trigeminal sensory afferents synapsing on the superficial dorsal horn of the TCC. The % area stained for CGRP in lamina I and II was significantly increased across all groups [H(2) = 7.22, P < 0.05]. There was no significant increase following olcegepant administration when compared to vehicle control treated mice [median (IQR): 99 (87–112) versus 112 (81–127), Z = 0.78, P = 0.99]. LY344864 significantly increased the AUC when compared to vehicle control mice [median (IQR): 130 (110–70) versus 99 (87–112), Z = 2.62, P < 0.05]. (B) Repeated daily exposure of mice to LY344864, but not olcegepant induced an increase in the number of c-Fos positive neuronal nuclei in lamina I to V of the trigeminocervical complex. The number of c-Fos positive nuclei was significantly increased across all groups [H(2) = 7.40, P < 0.05]. There was no significant increase following olcegepant administration when compared to vehicle control treated mice [median (IQR): 22 (22–29) versus 23 (15–26), Z = 0.78, P = 0.99]. LY344864 significantly increased the number of c-Fos positive nuclei when compared to vehicle control [median (IQR): 33 (32–36) versus 23 (15–26), Z = 2.59, P < 0.05]. Highlighting underlying neuroplastic changes in trigeminal sensory afferents and their increased basal activity as a surrogate readout of increased trigeminal nociception. *P < 0.05, n = 8 mice per group for A and n = 7 mice per group for B. Scale bar = 100 µm and 50 µm for A and B, respectively.
C-Fos neuronal activation in the trigeminocervical complex
Trigeminal sensory afferents expressing CGRP synapse on second order neurons in the TCC, giving rise to the trigeminothalamic tract that conveys nociceptive information from the head. Increased expression of the immediate early gene c-Fos in the TCC is an established readout of increased trigeminal nociception (Harriott et al., 2019). Following 11 days of drug exposure there was a significant increase in the number of c-Fos-positive cells in the TCC [H(2) = 7.40, P ≤ 0.05, n = 7 per group]. Mice persistently exposed to LY344864 had a significantly increased number of c-Fos-positive cells when compared to vehicle control [median (IQR): 33 (32–36) versus 23 (15–26), Z = 2.59, P ≤ 0.05; Fig. 4B]. There was no significant difference between vehicle control and olcegepant treated mice [median (IQR): 23 (15–26) versus 22 (22–29), Z = 0.78, P = 0.99; Fig. 4B].
Discussion
The results demonstrate a differential potential MOH risk profile for gepants: CGRP receptor antagonists, and ditans: 5-HT1F receptor agonists. While the 5-HT1F receptor agonist LY344864 induced a significant reduction in mechanical withdrawal thresholds, olcegepant showed no reduction. This selective MOH risk of a ditan is supported by increased expression of CGRP in trigeminal sensory afferents and neuronal activation (c-Fos) in the TCC.
Our data are in agreement with an established MOH risk for the 5-HT1B/1D receptor agonists: triptans. It is further supported by a potential beneficial effect of blocking CGRP signalling via monoclonal antibodies (Tepper et al., 2019) and preliminary data for the gepants (Goadsby et al., 2019), with no evidence of MOH. While the pathophysiology of MOH remains to be fully characterized it is interesting that several drugs, including NSAIDs (Vellani et al., 2017) and triptans (Durham and Russo, 2003), which are known to block CGRP release, increase MOH risk. Herein our data suggest that the ditans (Labastida-Ramirez et al., 2020) may have a similar impact, a common effect of which being increased CGRP expression in trigeminal sensory afferents. While our results, and that from patients (Goadsby et al., 2019; Tepper et al., 2019), suggest that blockade of the CGRP receptor does not. Interestingly, CGRP expressing trigeminal afferents consistently express Nav1.9 (Bonnet et al., 2019), with known roles in orofacial neuropathic pain (Luiz et al., 2015), and recently linked to MOH (Bonnet et al., 2019). As such, persistent exposure to drugs that can act presynaptically to alter primary sensory afferent neuropeptide expression and receptor function, may lead to a state of increased evoked activity and neurotransmitter/neuropeptide release. Clinically, this is supported by the ability of CGRP-targeted antibodies to reduce attack frequency and acute medication use in MOH patients (Tepper et al., 2019).
Given the need for clinical confirmation, our study is strengthened by the use of sumatriptan. It is well established that the triptans induce mechanical hypersensitivity in rodents and increase the risk of progression to MOH in subjects with an underlying headache condition, when used to excess. As such, the behavioural effects and underlying mechanistic actions of LY344864 parallel sumatriptan, resulting in comparative sensitization and neuroplastic changes. These neuroplastic changes, including increased CGRP expression, can outlast sumatriptan withdrawal in rodents and persist after normalization of sensory thresholds, creating a state of ‘latent sensitization’. It is a limitation of our current study that we did not explore potential latent sensitization. Further, having identified that olcegepant did not induce MOH-like phenotypes in mice it would have been interesting to test the ability of gepants to block MOH induction. A recent report demonstrated that ubrogepant could prevent bright light-induced mechanical hypersensitivity is sumatriptan-induced latently sensitized mice (Navratilova et al., 2020), suggesting that at the very least gepants may be effective for established MOH.
As the 5-HT1F receptor agonist lasmiditan, and the CGRP antagonists ubrogepant and rimegepant have been approved by the FDA in the past 6 months, these molecules will shortly join the anti-migraine therapeutic toolkit. While it is clear that lasmiditan has specific advantages over the triptans with respect to cardiovascular risk factors (Shapiro et al., 2019), our data suggest that both classes of drugs confer a comparable MOH risk. It further suggests that gepants may demonstrate a more favourable MOH risk profile.
Understanding the MOH risk is critical, since MOH places a severe burden on healthcare services (Westergaard et al., 2014), individuals (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018) and the wider economy (Linde et al., 2012). Our data now provide a rationale for understanding the potential risk of their overuse and should inform patient education to avoid such excessive exposure and potential increased risk of developing chronic headache and MOH.
Funding
This work was supported by the Medical Research Council (MR/P006264/1), the Wellcome Trust (Synaptopathies; 104033), FP7 project EUROHEADPAIN (no. 602633), the Migraine Trust and the NIH (no. DA40688). C.S. received PhD funding from the Development and Promotion of Science and Technology Talents Project (DPST) and the Royal Thai Government.
Competing interests
A.A.P. reports research funding from Amgen. P.J.G. reports grants and personal fees from Amgen and Eli-Lilly and Company, grant from Celgene, and personal fees from Alder Biopharmaceuticals, Allergan, Autonomic Technologies Inc., Biohaven Pharmaceuticals Inc., Clexio, Electrocore LLC, eNeura, Impel Neuropharma, MundiPharma, Novartis, Teva Pharmaceuticals, Trigemina Inc., WL Gore, and personal fees from MedicoLegal work, Massachusetts Medical Society, Up-to-Date, Oxford University Press, and Wolters Kluwer; and a patent magnetic stimulation for headache assigned to eNeura without fee. P.R.H. reports honoraria for educational and advisory purposes from Allergan, Eli-Lilly, Novartis and TEVA as well as research funding from Amgen and Eli-Lilly. All other authors report no competing interests.
Glossary
- CGRP =
calcitonin-gene-related peptide
- MOH =
medication overuse headache
- TCC =
trigeminocervical complex
References
- Bahra A, Walsh M, Menon S, Goadsby PJ. Does chronic daily headache arise de novo in association with regular use of analgesics? Headache 2003; 43: 179–90. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache 2008; 48: 1157–68. [DOI] [PubMed] [Google Scholar]
- Bonnet C, Hao J, Osorio N, Donnet A, Penalba V, Ruel J, et al. Maladaptive activation of Nav1.9 channels by nitric oxide causes triptan-induced medication overuse headache. Nat Commun 2019; 10: 4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnin S, Viana M, Sances G, Tassorelli C, Terrazzino S. A systematic review and critical appraisal of gene polymorphism association studies in medication-overuse headache. Cephalalgia 2018; 38: 1361–73. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- De Felice M, Ossipov MH, Wang R, Lai J, Chichorro J, Meng I, et al. Triptan-induced latent sensitization: a possible basis for medication overuse headache. Ann Neurol 2010; 67: 325–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham PL, Russo AF. Stimulation of the calcitonin gene-related peptide enhancer by mitogen-activated protein kinases and repression by an antimigraine drug in trigeminal ganglia neurons. J Neurosci 2003; 23: 807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelstoft IMS, Carlsen LN, Munksgaard SB, Nielsen M, Jensen RH, Bendtsen L. Complete withdrawal is the most feasible treatment for medication-overuse headache: a randomized controlled open-label trial. Eur J Pain 2019; 23: 1162–70. [DOI] [PubMed] [Google Scholar]
- GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018; 17: 954–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386: 743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby PJ, Classey JD. Evidence for serotonin (5-HT)1B, 5-HT1D and 5-HT1F receptor inhibitory effects on trigeminal neurons with craniovascular input. Neuroscience 2003; 122: 491–8. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Dodick DW, Trugman JM, Finnegan M, Lakkis H, Lu KF, et al. Orally administered atogepant was efficacious, safe, and tolerable for the prevention of migraine: results from a phase 2b/3 Study. Neurology 2019; 92: S17.001. 31068157 [Google Scholar]
- Harriott AM, Strother LC, Vila-Pueyo M, Holland PR. Animal models of migraine and experimental techniques used to examine trigeminal sensory processing. J Headache Pain 2019; 20: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- Labastida-Ramirez A, Rubio-Beltran E, Haanes K, Chan KY, Garrelds I, Kovalchin J, et al. Lasmiditan Inhibits calcitonin gene-related peptide release in the rodent trigeminovascular system. Pain 2020; 161: 1092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde M, Gustavsson A, Stovner LJ, Steiner TJ, Barre J, Katsarava Z, et al. The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol 2012; 19: 703–11. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Fanning KM, Buse DC, Martin VT, Hohaia LB, Adams AM, et al. Migraine progression in subgroups of migraine based on comorbidities: results of the CaMEO Study. Neurology 2019; 93: e2224–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luiz AP, Kopach O, Santana-Varela S, Wood JN. The role of Nav1.9 channel in the development of neuropathic orofacial pain associated with trigeminal neuralgia. Mol Pain 2015; 11: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moye LS, Tipton AF, Dripps I, Sheets Z, Crombie A, Violin JD, et al. Delta opioid receptor agonists are effective for multiple types of headache disorders. Neuropharmacology 2019; 148: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Oyarzo J, Dodick DW, Banerjee P, Porreca F. Efficacy of Ubrogepant in a Preclinical Model of Medication Overuse Headache. Neurology 2020; 94 (15 Suppl): S58.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RE, Hochstetler HM, Dennehy EB, Khanna R, Doty EG, Berg PH, et al. Lasmiditan for acute treatment of migraine in patients with cardiovascular risk factors: post-hoc analysis of pooled results from 2 randomized, double-blind, placebo-controlled, phase 3 trials. J Headache Pain 2019; 20: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper SJ, Diener HC, Ashina M, Brandes JL, Friedman DI, Reuter U, et al. Erenumab in chronic migraine with medication overuse: subgroup analysis of a randomized trial. Neurology 2019; 92: e2309–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani V, Moschetti G, Franchi S, Giacomoni C, Sacerdote P, Amodeo G. Effects of NSAIDs on the release of calcitonin gene-related peptide and prostaglandin E2 from rat trigeminal ganglia. Mediators Inflamm 2017; 2017: 9547056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard ML, Hansen EH, Glumer C, Olesen J, Jensen RH. Definitions of medication-overuse headache in population-based studies and their implications on prevalence estimates: a systematic review. Cephalalgia 2014; 34: 409–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available upon reasonable request.