Abstract
Objective.
We examined whether associations between daily psychosocial stressor exposures and carotid artery intima medial thickness (IMT) may be stronger among those showing larger stress-related cardiovascular reactivity (CVR) during the course of daily living.
Methods.
474 healthy working adults (ages 30–54) collected ambulatory blood pressure (ABP) and recorded their daily experiences, using electronic diaries, during two 2-day periods over the course of a week. Measures of mean momentary Task Strain and Social Conflict were used as indices of stressor exposure, and partial regression coefficients linking momentary Strain and Conflict with ABP fluctuations were used as measures of CVR. IMT was assessed in the carotid arteries using B-mode ultrasound.
Results.
After covariate adjustment, associations between mean Task Strain exposure and IMT were significant among those high in CVR to Strain (for SBP, p = .006, for DBP, p = .011) but not among those low in Strain CVR. Similarly, associations involving mean Conflict exposure were significant among those high in CVR to Social Conflict (p < .001 for SBP, p=.001 for DBP) but not among low Social Conflict reactors. Significant moderation effects were more consistently shown for Task Strain than for Social Conflict, but the overall pattern of results was robust across two different types of statistical modeling procedures.
Conclusion.
Individual differences in CVR may moderate the effects of daily psychosocial stress on subclinical CVD among healthy employed adults. Using ecological momentary assessment (EMA) to measure stress exposure as well as stress reactivity may facilitate our ability to detect these effects.
Keywords: Cardiovascular disease, intima medial thickness, ambulatory blood pressure, ecological momentary assessment, job stress, psychological stress
Chronic psychosocial stress is increasingly recognized as a potential contributor to cardiovascular disease (CVD) (1). For example, work-related occupational stress is associated with a 30 % excess risk of CVD, on average (2) and chronic marital conflict has been linked with a 3-fold elevated risk of recurrent cardiac events (3). Exaggerated stress-related cardiovascular reactivity (CVR) is also associated with elevated CVD risk; in population samples, those showing the largest acute blood pressure (BP) changes in response to laboratory stressors show increased risk for accelerated atherosclerosis and hypertension incidence (4), as well as stroke (5) and cardiovascular mortality (6) later in life.
If the effects of chronic psychosocial stress on cardiovascular pathophysiology are transduced, in part, by their impact on hemodynamic changes, then the effects of stress on CVD risk could be moderated, in part, by individual differences in CVR, with relatively stronger effects among those who are more stress-reactive. This diathesis-stress hypothesis is a logical extension of the two literatures linking stress and CVR with CVD (7, 8), but it has rarely been tested (see (9, 10)). One of the challenges in testing a diathesis-stress hypothesis of CVD risk is that our field lacks the methods for assessing chronic stress and CVR using comparable metrics. Measures of psychosocial stress are typically assessed using questionnaire or interview assessments, in which participants are asked to report on recent role disruptions, while CVR is usually assessed in response to acute laboratory challenges. These two types of measures are conceptually dissimilar, and both are abstracted from the environments in which daily life stressors, and our responses to them, actually unfold.
Ecological momentary assessments (EMA), using behavioral and biological measurements collected in real time and in the natural environment, allow us to capture stress reactivity and chronic stress exposure using comparable metrics. Use of repeated assessments yield representative samples of ongoing stressor exposures, while measures of ambulatory blood pressure (ABP) allow us to examine the corresponding episodic adjustments in hemodynamic activity that may accompany ongoing stressor events. These approaches allow us to coordinate our measures of stress exposure and stress susceptibility, and to capture both constructs in a manner that is proximal to, rather than abstracted from our daily life experience (11).
Two models of chronic stressors will be examined in this study: The “job strain” model (12) characterizes as stressful those work environments that are high in psychological demands and low in control or decision latitude; such exposures have been linked with increased CVD risk (2). Moreover, we have shown that those who rate their ongoing daily activities (inside or outside of the workplace) as high in Demand or low in Latitude, using time-averaged EMA assessments, show an elevated risk for subclinical atherosclerosis, an effect that appears to be more robust than associations with traditional measures of job strain (13–15). Chronic social conflict has also been identified as a model of chronic stress, and has been shown to be associated with elevated risk of atherosclerosis and coronary heart disease in humans (16–18) as well as non-human primates (19). We have shown that mean momentary ratings of exposures to negative marital interactions during daily life are associated with increased subclinical atherosclerosis, effects that are stronger than those associated with traditional global assessments of marital quality (20).
In this study, we examine the diathesis-stress hypothesis for CVD risk using each of these two models of chronic psychosocial stress. We hypothesize that exposure to a high demand and low control environment (high “task strain”) may be associated with increases in carotid artery atherosclerosis in an employed adult sample, and that such effects may be stronger for those who demonstrate larger ABP elevations during high demand/low control episodes in the course of daily living. Similarly, we expect that the association between daily life social conflict exposures and carotid atherosclerosis may be stronger for those who demonstrate larger ABP elevations during social conflict episodes in daily life.
Methods
Participants
Participants were drawn from the Adult Health and Behavior Project – Phase 2 (AHAB-II), a study of CVD risk in a sample of clinically healthy, employed adults (ages 30–54; employed 25 or more hours per week) in the Pittsburgh, Pennsylvania metropolitan area. AHAB-II participants were recruited between March 2008 and October 2011 through mass mailings using voter registration and other public domain lists. Individuals were excluded if they (a) had a history of cardiovascular disease, schizophrenia or bipolar disorder, chronic hepatitis, renal failure, neurological disorder, lung disease requiring drug treatment, or stage 2 hypertension (SBP/DBP ≥ 160/100 mm Hg); (b) consumed excessive quantities of alcohol (≥5 portions 3–4 times per week); (c) used fish-oil supplements (because of the requirements for another substudy); (d) were prescribed medications with autonomic effects or used insulin, glucocorticoid, antiarrhytmic, antihypertensive, lipid-lowering, psychotropic, or prescription weight loss medications; (e) were pregnant; or (f) were shift workers. (21).
The study was approved by the University of Pittsburgh Institutional Review Board, and all participants were administered informed consent before enrollment. Participants received compensation up to $410, depending on compliance with the protocol.
Procedure
Participants completed 7 laboratory visits, the first 3 of which are relevant to this report. During V1, participants were administered a medical history, a demographic questionnaire, and a fasting blood draw to assess biological risk factors for CVD (see below). During V2, participants were trained to use an automated ABP monitor (Oscar 2 oscillometric monitor, Suntech Medical, Inc., Morrisville, NC) (22), a self-report electronic diary using a personal digital assistant (PDA; Palm Z22 with Satellite Forms software), a multichannel accelerometry-based physical activity monitor used to calculate minute-by-minute energy expenditure in metabolic equivalent units (METS) (Sensewear Pro3 armband device, Body Media, Pittsburgh, PA) (21, 23), and additional devices. Following V2, participants underwent ambulatory monitoring during two 2-day periods over the course of a week, with a data collection schedule involving 3 working days and one nonworking day for each person. During waking hours on each of these monitoring days, ABP was assessed hourly, and each hourly assessment was followed by the completion of a PDA- administered questionnaire. Physical activity was monitored on a continuous basis during the waking day throughout the monitoring week. Monitoring equipment was set aside at bedtime, was reapplied upon awakening, and was returned at V3 one week later. Clinic blood pressure (BP) was assessed at Visits 2 and 3 using a mercury sphygmomanometer and standard methods (24). Carotid artery ultrasonography was administered on one occasion in conjunction with V3, V4 or V6 for the assessment of intima medial thickness (IMT), a marker of carotid artery atherosclerosis. All sessions were scheduled within 2–3 months after study enrollment.
Measures
Carotid Artery Atherosclerosis
B-mode ultrasound assessments were conducted at the University of Pittsburgh Ultrasound Research Laboratory using an Acuson Sonoline Antares high-resolution duplex scanner (Acuson-Siemens, Malvern, PA). Trained, certified sonographers identified the borders of the intima and medial layers of the left and right carotid artery. Distances between these interfaces were measured using digitized images from the right and left common carotid artery (far wall and near wall), from the right and left carotid bulb, and from the right and left internal carotid artery. Studies were read using the Automated Measurement System (Goreborg University, Gothenburg, Sweden) using an automated edge detection algorithm (25). Measures of IMT (mm) at each of these eight locations were averaged to create a mean IMT score. Two outliers (>4 SDs above the mean) were winsorized (set equal to a value 4 SDs from the mean-- 1.11 mm). To reduce remaining skewness, we applied log transformation to the resulting distribution.
Ambulatory Blood Pressure
ABP was assessed hourly across the waking day during 3 working days and one nonworking day over the course of a week. Some participants (n = 40) completed more than 4 monitoring days at the request of the researchers due to initial problems with some of their assessments. We deleted all ABP observations associated with error codes (e.g., leaky cuff, low battery) and those deemed to be in a non-physiologic range, using previously published criteria (26). Systolic (SBP) and diastolic (DBP) readings were used. Heart rate readings were not included here, as we have not previously found heart rate reactivity to be a consistent predictor of carotid artery atherosclerosis (27, 28).
Ambulatory Diary Measures
After each hourly ABP cuff inflation, participants completed a 43-item electronic diary on the handheld PDA (see above). The diary included a number of multi-item scales assessing current mood, stressor exposures, and social interaction characteristics (see below). Across both working and nonworking days, all valid ABP observations that were followed (within 15 minutes) by a diary entry were used in the analyses described here.
Task Strain
Participants were encouraged to “Think about mental and physical activity in the past 10 minutes.” The three-item Task Demand scale (i.e., “Required working hard?” “Required working fast?” and “Juggling several tasks at once?”) and the two-item Decisional Control scale (i.e., “Could change activity if you chose to?” and “Choice in scheduling this activity?”) followed; these items were derived from the Job Content Questionnaire (JCQ) (29), a standard instrument used for the assessment of job strain. Participants responded to each using a 6-point Likert scale (NO! No no yes Yes YES!) and scores ranging from 1–6 were averaged across items for each scale. Re-test reliability and criterion validity for these scales have been previously described (14).
According to the job strain model, conditions that are high in Demand and low in Control are hypothesized to be particularly harmful. One traditional approach for capturing this ordinal interaction effect involves designating a high strain group, using sample-specific median splits across each of these two component scales, and contrasting this group with the remainder of the sample (30–33). Using this approach, we created momentary measures of Task Strain by assigning a score of “1” to observations above the sample median (score of 3) for Demand and less than or equal to the sample median (score of 4) for Control. A score of “0” was assigned to all other periods. Similarly, we characterized individuals as high on exposure to mean Task-related strain when their average ratings during the monitoring period were above the sample median for Demand (again, a score of 3) and less than or equal to the sample median for Control (again, a score of 4). As with the momentary scores, these measures were dichotomized, so each individual was characterized as being high or low on Task Strain exposure.
Social Conflict
During each hourly assessment, participants were also administered several items inquiring about their most recent social interaction. To capture proximal effects most likely to affect current ABP, we extracted only responses pertaining to interactions reported to be occurring at the time of, or within the 10-minute period preceding the cuff inflation. Social Conflict scores for these recent interactions were assessed using a 2-item scale (i.e., “Someone treated you badly?” “Someone in conflict with you?”). Item responses [NO! No no yes Yes YES!] were scored 1–6 and were averaged across items (momentary conflict) and across all observations (mean momentary conflict). Validation data for this scale have been previously reported (20, 34, 35).
Time-Varying Covariates
The hourly diary contained a number of additional items measuring behavioral factors known to correlate with BP, to generate time-varying covariates in the assessment of CVR, including a) current posture, b) physical activity during past 10 minutes, c) perceived temperature, d) consumption of a meal, snack, alcoholic drink, or caffeine in the past hour, and e) speaking (yes or no) during ABP assessment (36). Participants also initiated a diary report whenever they smoked a cigarette, used to calculate number of cigarettes smoked in the hour prior to each ABP.
As an additional time-varying covariate, we obtained an objective measure of recent physical activity using the Sensewear Pro 3 (see above). Average METS for the 10-minute period prior to each ABP reading were extracted.
Demographic and Risk Factor (Person-level) Covariates
For IMT analyses, we used age, sex, race/ethnicity, and education as demographic covariates, and we included clinic SBP, low density lipoprotein (LDL) and high density lipoprotein (HDL) cholesterol, glucose, insulin, waist circumference, and smoking status as biological and behavioral risk factor covariates. Race/ethnicity was recoded as a dummy variable (white vs. nonwhite), and highest education was recoded into 4 categories: high school or less, associate degree or some college, college degree, advanced degree. Two clinic blood BP readings were taken during V2 and 3, and clinic SBP was calculated as the average value across all four readings. A standard lipid panel (total cholesterol, HDL, and LDL) was taken from the blood drawn at V2, along with a fasting serum glucose assay via standard colorimetry, and insulin concentration by radioimmunoassay. Waist circumference was assessed in cm at V1, and smoking status was self-reported and coded as current smoker vs. not.
Data Analysis
The interaction between chronic stress exposure and acute stress reactivity was modeled using two different approaches: a 2-step approach using multi-level modeling (step one) and general linear modeling (step 2), and a one-step approach using multilevel structural equation modeling (MSEM).
For the two-step approach, we first regressed momentary ABP (SBP and DBP in separate models) on momentary measures of stress, adjusting for the time-varying covariates listed above (level 1 effects; SAS Proc Mixed). From this first model, we extracted a random effects partial regression coefficient for each subject as an estimate of CVR. In a second model, we regressed IMT on this coefficient along with a measure of chronic stress exposure and an interaction term (stress exposure × stress reactivity), adjusted for person-level covariates (level 2 effects; SAS Proc GLM).
For the one step approach, we performed these level 1 and level 2 analyses simultaneously, using MSEM (MPLus), treating CVR as a latent variable. Once again, separate models were run for ambulatory SBP and DBP. The investigator specifies both regression equations at once, and the software simultaneously solves these equations using matrix algebra. Because it does not require us to extract random effects estimates, which are thought to be prone to error, this one-step approach may have greater precision than the two-step analysis. On the other hand, the two-step approach allows us to model autocorrelation effects at level 1 (not available within the MPlus MSEM framework) and it also facilitates our ability to plot the pattern of effects.
We looked for results that were statistically significant for both the one-step and the two-step approaches. We also evaluated whether our findings replicated across each of the two models of psychosocial stress (Task Strain and Social Conflict). We ran each analysis separately using measures of ambulatory SBP and DBP. For each analysis, we also ran the Level 2 models in two ways: first, using demographic covariates only, and second, using demographic and risk factor covariates.
Results
Sample Characteristics
Four hundred ninety-four participants completed ambulatory monitoring data collection as part of the AHAB-2 study. Nine of these were missing IMT data, 8 were missing Sensewear data, and 3 were missing risk factor data, yielding a sample size of 474 with complete data available on each measure. Fifty-three percent of the sample was female, and 18 % was nonwhite, with a mean age of 43. The sample was generally well educated (73 % with bachelor’s or graduate degree). Biological risk factors for CVD were generally in the normal range, and 13 % of the sample were current smokers. Mean IMT was .64 mm (original units; see Table 1). An average of 50 paired ambulatory monitoring observations (ABP and diary entry) per person were available for analysis in this sample.
Table 1.
Descriptive Statistics, AHAB-2 sample (N=474)
| Characteristic | Mean | Std Dev | % |
|---|---|---|---|
| Age (year) | 42.8 | 7.31 | |
| Sex | |||
| Men | 47.3 | ||
| Race | |||
| White | 81.9 | ||
| Black | 15.9 | ||
| Asian | 1.5 | ||
| Multi-racial | 0.4 | ||
| Other | 0.4 | ||
| Education | |||
| HS diploma or below | 5.7 | ||
| Some college, no degree | 21.7 | ||
| College degree | 38.6 | ||
| Graduate degree | 34.0 | ||
| Clinic SBP (mmHg) | 115.2 | 10.77 | |
| Clinic DBP (mmHg) | 72.6 | 7.75 | |
| Hypertension1 | 3.2 | ||
| LDL (mg/dL) | 123.0 | 31.90 | |
| HDL (mg/dL) | 56.0 | 15.02 | |
| Smoking Status | |||
| Current Smoker | 12.9 | ||
| GLU (mg/dL) | 98.3 | 11.22 | |
| Waist Circumference (cm) | 90.3 | 14.03 | |
| Insulin (mIU/L) | 12.5 | 6.28 | |
| IMT | 0.64 | 0.12 |
SBP≥ 140 and/or DBP≥ 90
Time Varying Covariates for Level 1 models
We simultaneously examined each of the time-varying covariates described above as correlates of ABP, in level 1 models with a spatial power autocorrelation pattern and one random effects parameter (intercept). Most of these covariates were significantly and independently associated with momentary fluctuations in SBP and DBP, as expected (results available upon request).
Between-person Covariates for Level 2 Models
We examined the demographic and risk factor correlates of mean carotid artery IMT in level 2 models. Examining demographic covariates alone, there were significant independent effects of age, race and sex (p for each < .001; with older adults, nonwhites, and men showing larger mean IMT). Examining demographic and risk factor covariates, there were significant independent effects of age, race, clinic SBP, LDL cholesterol, HDL cholesterol, and waist circumference (p for each < .030). Each of these variables was associated in the predicted direction with mean IMT (i.e., all of these risk factors save for HDL were associated with increases in IMT; results available upon request).
Task Strain Effects: Level 1 Model
Observations were identified as high in Task Strain based upon momentary scores for Task Demand and Decision Latitude (see above). Using the criteria employed here, 14 participants showed no periods of momentary Task Strain over the course of the monitoring period. Because we were concerned that differences in CVR may not be accurately estimated in this group, these participants were excluded, yielding a sample size of 460. Within the remaining sample, an average of 50 paired observations (ABP and diary entry) per person were available for analysis, and 30% of the observations, for the average person, were rated as high on Task Strain (range= 2–95 %).
Momentary SBP was regressed on momentary Task Strain, after adjusting for all of the time-varying covariates. We specified 2 random effects in this fully adjusted model (intercept and Task Strain). Results revealed a significant independent association between momentary Task Strain and SBP (b=.71, t (22E3) =3.13 p=.002). Constraining the Task Strain term to be a fixed effect (Random intercept only) significantly reduced model fit (χ2 = 38.93, df = 2, p < 0.001), suggesting significant individual differences in SBP reactivity to Task Strain in this sample. The fixed effect coefficient for Task Strain (i.e., the difference in SBP during Task Strain vs. no strain periods after covariate adjustment) was 0.71 mmHg, but it was distributed as a variable ranging from −3.36 to 6.52 mmHg in the random effects model. Similar analyses were run regressing DBP on momentary Task Strain, with time-varying covariates; once again, there were significant effects (b=0.61, t (22E3) = 3.52, p < .001) and significant individual differences in DBP reactivity (χ2 = 53.01, df = 2, p < 0.001). In the most reactive portion of the sample (top 10 % of each distribution, n = 47 for each), SBP was 3.4 mmHg higher during high strain periods of the day compared to low strain periods, on average, and DBP was 3.0 mmHg higher.
Task Strain Effects: Level 2 Model
Participants were identified as high in Task Strain exposure based upon their mean momentary Task Demand and Decision Latitude scores across the monitoring period (see above). Using our criteria, mean momentary scores above the median on Task Demand and below the median on Decision Latitude, 32 % of the sample was designated as high on Task Strain.
We ran Level 2 models regressing IMT on the binary mean Task Strain term, the measure of Strain Reactivity, and a cross-product interaction term. When these models were run in the context of the 4 demographic covariates, the interaction term was significant for SBP reactivity (b=.03, t (452) =2.92, p=.004) (R2 change for interaction = .011) and, in a separate model, for DBP reactivity (b= .02, t (452) = 2.24, p = .026) (R2 change for interaction= .007). Adding the risk factor covariates to these models did not alter the pattern or significance of these results (for SBP, interaction b = .02, t (445) = 2.63, p=.009; R2 change for interaction = .008; for DBP, interaction b= .02, t (445) = 2.32, p = .021; R2 change for interaction = .006).
Analyses of simple effects were run with these Strain Reactivity scores centered at 1 standard deviation (SD) above and below the sample mean (37). The effects of mean Task Strain were significant among those high in Strain Reactivity (for SBP, b=.05, t (445) =2.77, p=.006; for DBP, b=.05, t (445) =2.56, p=.011) but not among those low in Strain Reactivity (for SBP, b=−.015, t (445) =−0.84, p=.40; for DBP, b = −.01, t (445) = −.63, p = .53). (These effects are from the fully adjusted models; effects from the demographic covariate models were identical in pattern, and are available upon request). See Figures 1 and 2.
Figure 1.
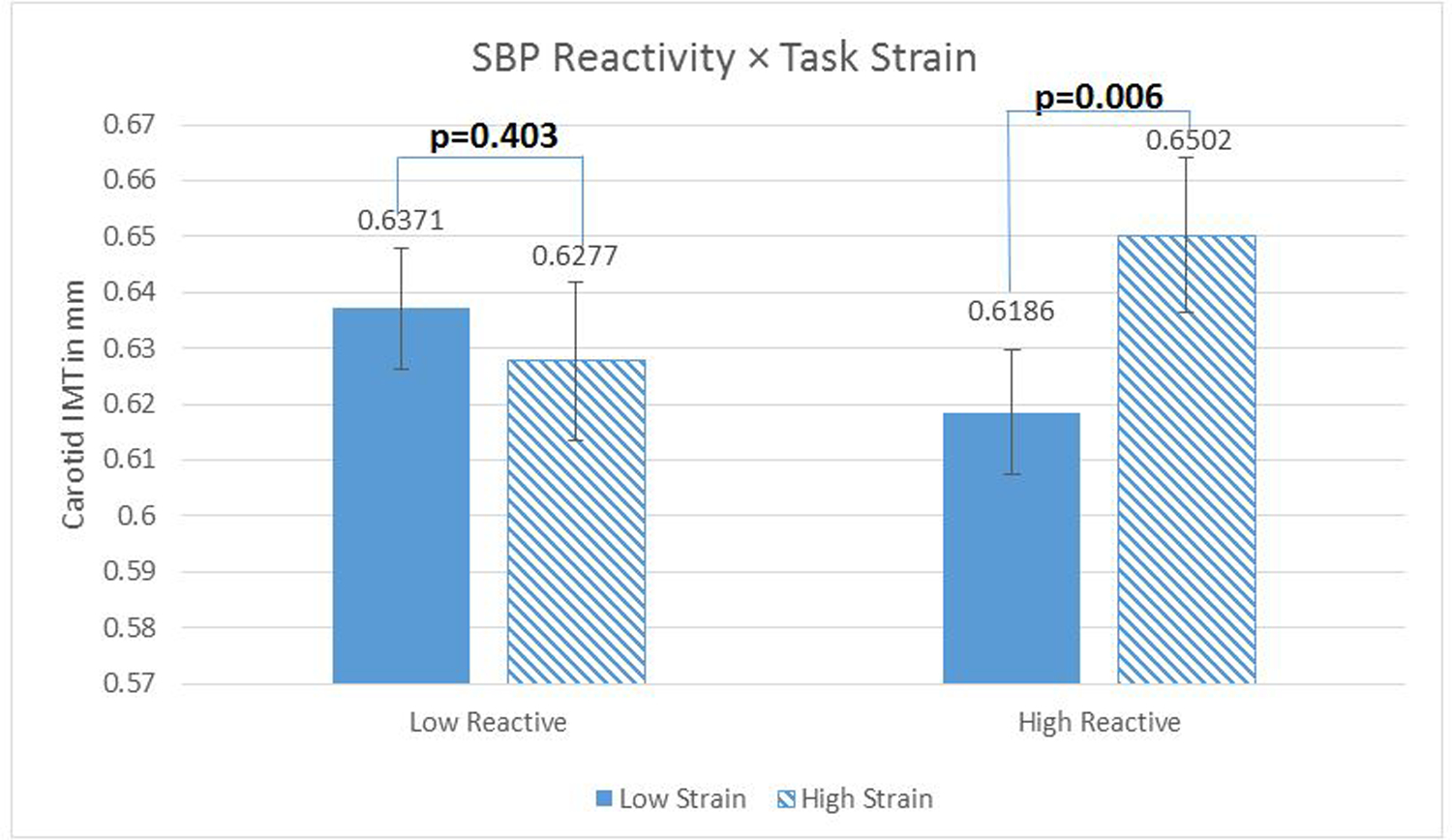
Systolic blood pressure (SBP) reactivity as a moderator of the relationship between Task Strain and carotid artery Intima-Medial Thickness (IMT). N= 460 with complete data. Regression models were fitted using log-transformed IMT scores to reduce skewness, but the figures present inverse-transformed scores and their standard errors (calculated via Taylor approximation) to enhance interpretability. Figures present the data where reactivity measures are centered 1 sd above (low reactors) and below (high reactors) the mean. Covariates in this model included age, sex, race, education, current smoking status, clinic systolic blood pressure, fasting plasma glucose, LDL and HDL cholesterol, fasting insulin, and waist circumference.
Figure 2.
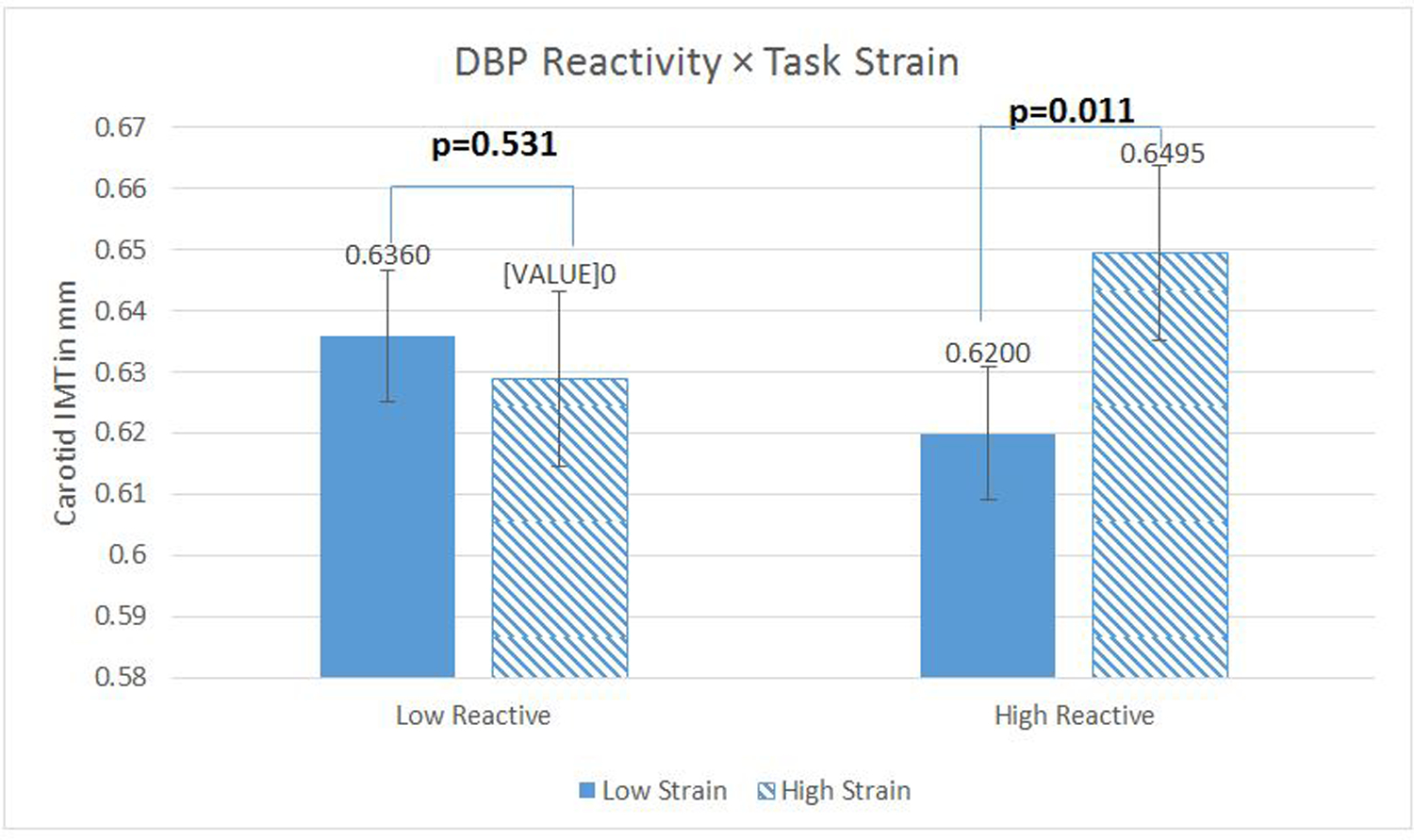
Diastolic blood pressure (DBP) reactivity as a moderator of the relationship between Task Strain and carotid artery Intima-Medial Thickness (IMT). N= 460 with complete data. Regression models were fitted using log-transformed IMT scores to reduce skewness, but the figures present inverse-transformed scores and their standard errors (calculated via Taylor approximation) to enhance interpretability. Figures present the data where reactivity measures are centered 1 sd above (low reactors) and below (high reactors) the mean. Covariates in this model included age, sex, race, education, current smoking status, clinic systolic blood pressure, fasting plasma glucose, LDL and HDL cholesterol, fasting insulin, and waist circumference.
Task Strain Effects: Multilevel Structural Equation Model
Within the MSEM framework, the association between momentary Task Strain and SBP were modeled at Level 1, and associations with IMT were modeled at Level 2. Random slopes (Strain Reactivity measures) were represented as latent or unobserved variables in models that regressed IMT on the binary mean Strain term, Strain Reactivity, and a cross-product interaction term.
Adjusting for 4 demographic covariates, the interaction terms were significant for both SBP and DBP (for SBP, interaction b=.02, t (28) =2.97, p=.003; for DBP, b = .02, t (28)= 2.25, p = .025). Adding the risk factor covariates to this model did not alter the pattern or significance of these effects (for SBP, interaction b = .01, t (35) = 2.67, p=.008; for DBP, b = .02, t (35) = 2.36, p = .018). Thus, the one-step modeling in Mplus yielded results very similar to the two-step modeling approach performed in SAS.
Social Conflict Effects: Level 1 Model
Once again, we were concerned that CVR may not be reliably estimated among individuals for whom variation in stress exposure is not adequately sampled, so we deleted participants from the analytic models who endorsed no Social Conflict (60 participants with no variance in social conflict throughout were deleted, yielding a sample size of 414). Within this sample, an average of 34 paired observations (ABP and diary entry during recent social interactions) per person were available for analysis.
We ran a Level 1 model regressing momentary SBP on the continuous momentary Social Conflict term, with 14 time-varying covariates. We specified 2 random effects in this fully adjusted model (intercept and Social conflict). Results revealed a significant independent association between momentary Social Conflict and SBP (b = .48, t (14E3) = 2.97, p=0.003). The associations between momentary Social Conflict and DBP were close to but did not cross the threshold for significance (b = 0.21, t (14E3) =1.80, p = 0.071).
Constraining the Social Conflict term to be a fixed effect (Random intercept only) significantly reduced model fit (for SBP, χ2 = 8.10, df = 2, p < 0.017 for DBP, χ2 = 7.00, df = 2, p < 0.030). The fixed effect coefficient for Social Conflict (e.g., the increase in SBP associated with 1 unit change in Social Conflict after covariate adjustment) was .48 mmHg, but it ranged from −1.02 to 2.59 mmHg in the random effects model. Coefficients for DBP reactivity (fixed effect= .21) similarly ranged from −0.65 to 1.37 mmHg. Given that the most reactive portion of the sample (top 10 % of each distribution, n = 43 for each) showed an increase of 1.3 mmHg in SBP and an increase of .81 mmHg DBP for each point increase in Social Conflict, and that the average within-person range in Social Conflict scores was 2.5 points, we can infer that the most reactive subjects showed increases of about 3.3 mmHg SBP and 2.0 mmHg DBP during and shortly after social interactions marked by Social Conflict, compared to low conflict social interaction periods. Random effects coefficients associated with Task Strain and Social Conflict were modestly correlated (r=.10, n = 406, p= .04 for SBP; r = .04, n = 406, p =0.37 for DBP). The partial regression coefficients linking momentary Social conflict with SBP or DBP (measures of Social Conflict reactivity) were used as part of a Level 2 model with IMT as the outcome variable (see below).
Social Conflict Effects: Level 2 Model
The average score on the mean Social Conflict scale across all subjects in this sample (n= 414) was 1.7 (sd= .65) (Theoretical range=1–6, Empirical range=1.01–3.41). We ran Level 2 models regressing IMT on the continuous mean Social Conflict term, the measures of “Social Conflict reactivity,” and a cross-product interaction term. When these models were run in the context of the 4 demographic covariates, the interaction term was significant for SBP (b=.05, t (406) =2.03, p=.043; R2 change for interaction = .006) and it approached the significance threshold for DBP (b = .06, t (406) =1.92, p = .055; R2 change for interaction = .005). In the model with the additional risk factor covariates, this effect was no longer significant for either parameter (for SBP, interaction b = .04, t (399) = 1.66, p=.10; R2 change for interaction = .004; for DBP, b = .05, t (399)= 1.52, p = .13; R2 change for interaction = .003).
Analyses of simple effects were run with the Social Conflict Reactivity score centered at 1 SD above and below the sample mean (37). The effects of mean Social Conflict were significant among those high in Social Conflict reactivity (b=0.05, t (399) = 3.65, p < .001 for SBP; b= .05, t (399) = 3.58, p <.001 for DBP) but not among low Social Conflict reactors (for SBP, b =0.02, t (399) =1.11, p=0.27, for DBP, b = .02, t (399) =1.43, p= .155). (fully adjusted models are depicted here, but the patterns for demographic covariate models are identical; results available upon request). See Figures 3 and 4.
Figure 3.
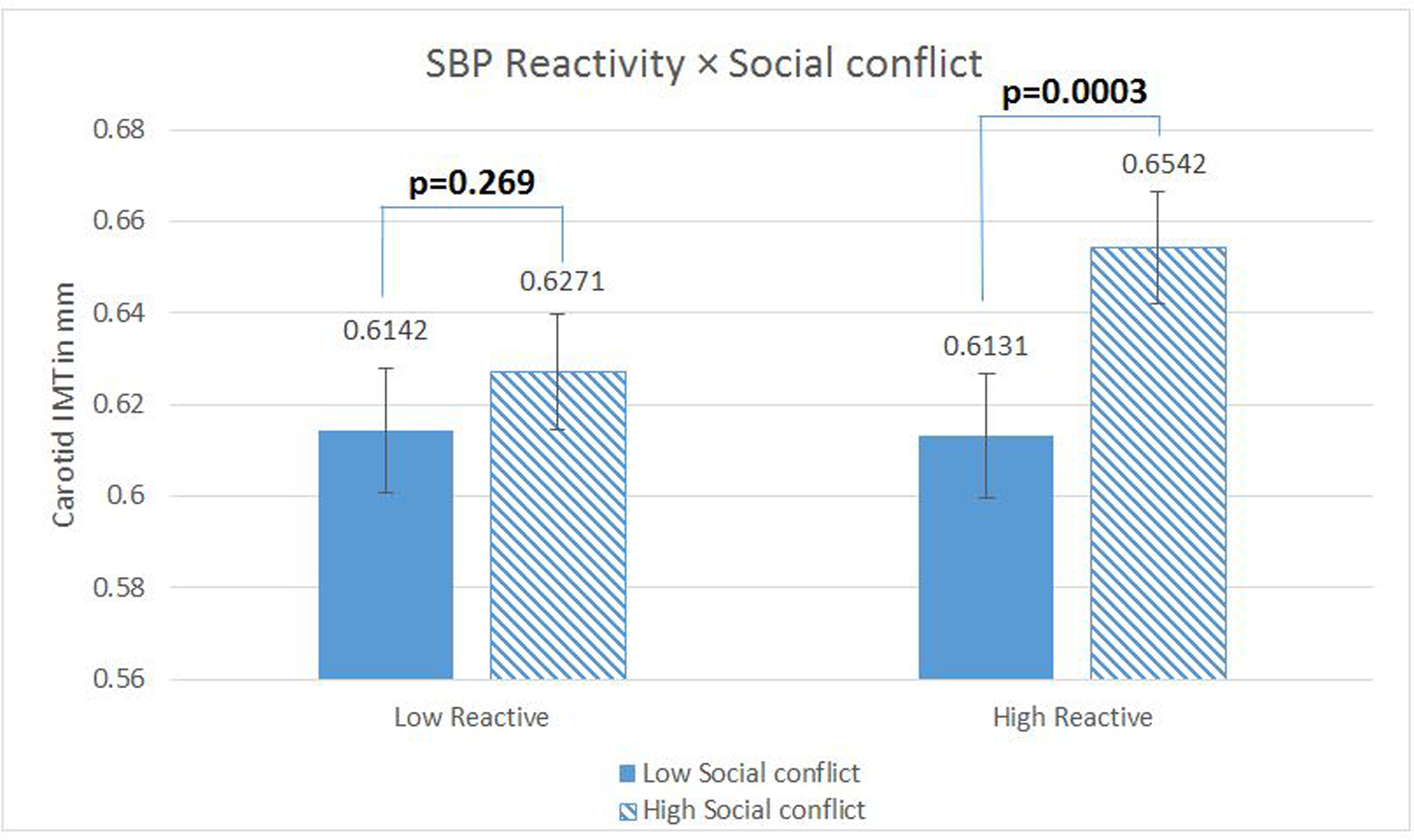
Systolic blood pressure (SBP) reactivity as a moderator of the relationship between Social Conflict and carotid artery Intima-Medial Thickness (IMT). N= 414 with complete data. Regression models were fitted using log-transformed IMT scores to reduce skewness, but the figures present inverse-transformed scores and their standard errors (calculated via Taylor approximation) to enhance interpretability. Figures present the data where reactivity measures are centered 1 sd above (low reactors) and below (high reactors) the mean. Covariates in this model included age, sex, race, education, current smoking status, clinic systolic blood pressure, fasting plasma glucose, LDL and HDL cholesterol, fasting insulin, and waist circumference.
Figure 4.
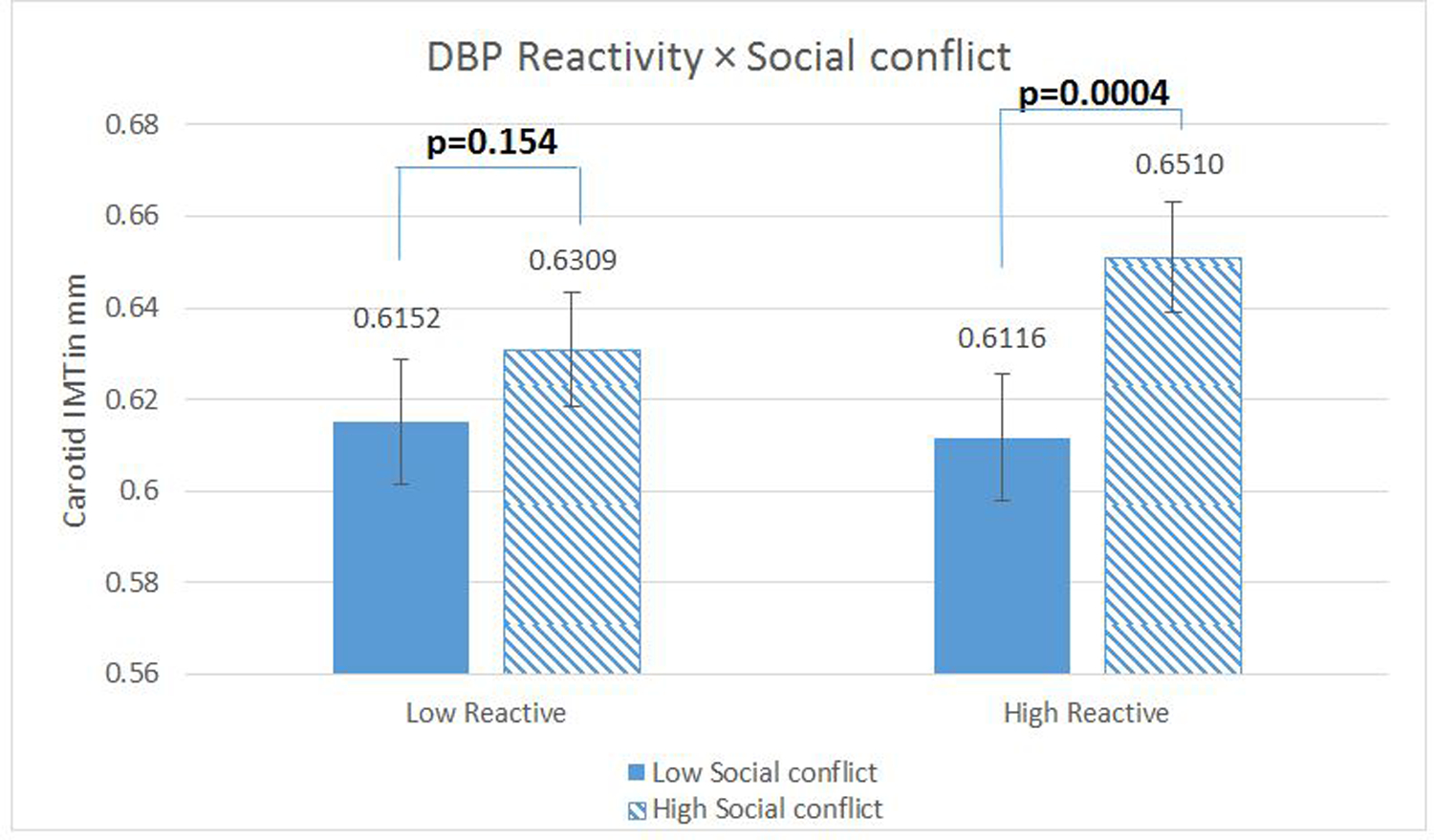
Diastolic Blood Pressure (SBP) Reactivity as a Moderator of the relationship between Social Conflict and Carotid Artery Intima-Medial Thickness (IMT). N= 414 with complete data. Regression models were fitted using log-transformed IMT scores to reduce skewness, but the figures present inverse-transformed scores and their standard errors (calculated via Taylor approximation) to enhance interpretability. Figures present the data where reactivity measures are centered 1 sd above (low reactors) and below (high reactors) the mean. Covariates in this model included age, sex, race, education, current smoking status, clinic systolic blood pressure, fasting plasma glucose, LDL and HDL cholesterol, fasting insulin, and waist circumference.
Social Conflict Effects: Multilevel Structural Equation Model
When models were run adjusting for the 4 demographic covariates, the interaction term of interest approached the significance threshold for SBP (b=.02, t (28) =1.91, p=.056) and was significant for DBP, (b=.04, t (28) = 2.20, p = .028). After the risk factor covariates were added to this model, interaction terms were not significant for either parameter (for SBP, interaction b = .02, t (35) =1.44, p=.149; for DBP, interaction b = .03, t (35) = 1.58, p = .114). For analyses examining SBP reactivity to Social Conflict, the MSEM models produced results that were similar to those using the two-step approach, in that one of the parameters yielded a significant interaction for the demographic covariate models whereas neither was significant in the fully adjusted models.
Exploratory Analyses
We examined whether stress reactivity parameters derived from the Task Strain measure might moderate the effects of Social Conflict exposures on IMT, and whether the stress reactivity parameters associated with Social Conflict might moderate the effects of Task Strain. Neither of these interaction effects were significant (for example, for the full models, Strain with Social Conflict SBP reactivity interaction b = −.02, t (391) = −.67, p =.502; Social Conflict with Strain SBP reactivity interaction b = .003, t (391) = .40, p =.69).
Discussion
Results suggest that individual differences in daily life stress-related BP reactivity may moderate the association between daily psychosocial stress and carotid artery intima medial thickness (IMT), a marker of subclinical atherosclerosis, among healthy, community adults. We found the same pattern of effects across two different types of stressor exposures--to daily life “task strains” and to daily life “social conflicts.” For both types of exposures, for two different cardiovascular parameters (SBP and DBP), and for two types of models (with demographic covariates, and with both demographic and risk factor covariates) a common pattern of results emerged: daily life psychosocial stress was associated with increased IMT, but this was significant only among those showing larger BP responses to stressor exposures during daily life, and not among low BP reactors.
Although the same pattern of results was found across stressor types, parameters, and models, the statistical significance of the interaction effect (the difference in effect between high and low reactors) emerged more consistently for Task Strain than for Social Conflict: Interaction effects involving Social Conflict were reduced to non-significance after adjustment for risk factor covariates, suggesting that sensitivity to social conflict may be correlated with standard CVD risk factors. Overt social conflict likely occurs less frequently than task strain during daily life as well (the average score on the 6-point Social Conflict scale was only 1.7 in this sample) which may reduce our power to detect significant moderation.
We used two different approaches to model these interactions: a two-step approach (in which ABP reactivity scores were extracted for each individual) and a one-step approach (in which ABP reactivity was treated as a latent variable). Both approaches yielded largely the same results. Any measurement error introduced by extracting ABP reactivity scores from partial regression coefficient estimates was apparently not sufficient to change the overall pattern of the findings.
These data represent a conceptual replication of our previous work, from the Pittsburgh Healthy Heart Study (PHHP), showing that daily experiences of demanding and uncontrollable environments may be associated with subclinical IMT (13–15). Unlike in the PHHP sample, there were no main effects of Task Demand or Decision Latitude on IMT in the current cohort; instead, associations with IMT only emerged for those who showed the “strain” pattern (high in Demand and low in Latitude) and among those who were most strongly stress reactive. The reason for these discrepant patterns across samples is unknown, but may be attributed to differences in population characteristics (the PHHP involved older adults, who have been shown to demonstrate larger CVR (38, 39)) or methods used in the two studies (e.g., we sampled both working and nonworking days in the present sample, perhaps enhancing our metrics of stress-related CVR as a result). The current sample was considerably larger as well, with additional contributions to statistical power.
These data are not only a partial replication of our previous findings, but they are also an extension of our recently published work, showing a significant association between social conflict exposure and IMT in the AHAB-2 cohort (20, 35). In our previous publications from AHAB-2, we did not examine CVR as a moderator of Social Conflict on IMT, as here.
The present findings are consistent with a diathesis-stress model, the notion that those showing more exaggerated cardiovascular responses to stress may be more vulnerable to the effects of life stressors on CVD. The few previous studies examining this hypothesis have used measures of stress and stress reactivity drawn from incomparable assessment contexts. EMA methods, in contrast, can be used to sample stressor exposure and stress responding using comparable metrics, and in the course of daily living, thus they may be optimally suited for examining how stress and CVR may interact in association with disease risk.
Interpreting the clinical significance of our results requires us to understand the utility of small differences in carotid IMT for predicting the probability of clinical events. A recent meta-analysis of prospective studies in this literature has shown that a carotid IMT difference of just 0.1 mm is associated with a 17 % increased risk for myocardial infarction and stroke over a the next 4–7 year period (40). Our own findings involve mean IMT differences ranging from .03–.04 mm (high vs. low Task Strain or high vs. low Conflict among high SBP or DBP reactors; see Figures 1–4). Assuming some consistency of effects across samples, our results would translate into about a 5–6 % increased risk of clinical events in the near term among the high risk (high stress-high reactive) subgroups here.
Our results are consistent with the possibility that individual differences in stress susceptibility may have stressor-specific effects. Indeed, our measures of Strain reactivity were only modestly correlated with reactivity to Social Conflict, and they were stronger moderators of the effects of Task Strain exposures than they were of exposures to Social Conflict (and vice versa). Previous laboratory studies of CVR have found much stronger correlations across stressor types (41, 42). We speculate that there may be a common evaluative performance context which drives individual differences in CVR in the laboratory; such a context may not be as strongly shared across stressor domains in the natural environment. Importantly, we have previously shown that laboratory CVR measures are moderately associated with BP responses to experiences of Demand and Control in daily life (42, 43).
The pattern of results shown here may have implications not only for understanding individual differences in stress susceptibility, but for characterizing some of the potential mechanisms by which daily stressor exposures may contribute to CVD risk as well. If daily psychosocial stress is linked with the development of carotid atherosclerosis only for those for whom daily stressors are triggers of exaggerated BP fluctuations, this is consistent with the possibility that the recurrent BP fluctuations characteristic of exaggerated stress reactors may constitute a causal mechanism by which stressor exposure may contribute to atherosclerosis (8). Downstream effects of this type of recurrent activation could include pathogenic processes-- such as endothelial dysfunction, inflammation, and platelet activation— previously shown to be associated with sympathetic nervous system and HPA axis activation as well as atherogenesis (44–46).
The use of EMA-based assessments and the internal replication across stressors, measures, and analytic models constitute important contributions of this study. Additionally, the use of subclinical CVD measures among healthy adults reduces potential confounding effects of clinical symptoms or medication treatment which could otherwise confound the measurement of stress or stress responding. An important limitation of the study, at the same time, involves its use of a cross-sectional correlational design, precluding our ability to rule out third factor or reverse causality processes. Use of samples with a wider educational background in the future would allow us to generalize to more representative groups as well.
This is the first study, to our knowledge, that has employed an integrated EMA assessment approach for evaluating the association between daily life stressor exposure, stress-related CVR and CVD risk. Future research will need to demonstrate that these results can be replicated in a prospective cohort sample, examining the stability of daily life reactivity, the temporal processes that may moderate the effects of daily life stressor exposure, and the prediction of disease progression or clinical events. Future research could potentially make use of EMA methods, as well, to target psychosocial exposures with real-time interventions, using ambulatory methods to identify stress-susceptible individuals as candidates for these types of treatments. Such efforts would build upon emerging evidence linking the effects of chronic psychosocial stress with daily life behavioral and biological processes, effects that may turn out to have important implications for cardiovascular health.
Glossary
- CVD
cardiovascular disease
- CVR
cardiovascular reactivity
- IMT
intima medial thickness
- ABP
ambulatory blood pressure
- EMA
ecological momentary assessment
- BP
blood pressure
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- JCQ
Job Content Questionnaire
- MSEM
multilevel structural equation modeling
- SD
standard deviation
- AHAB-II
Adult Health and Behavior Project II
- PHHP
Pittsburgh Healthy Heart Project
Footnotes
Conflicts of Interest and Source of Funding: This research was supported by the National Heart, Lung, and Blood Institute (HL040962). No conflicts of interest were declared.
Contributor Information
Thomas W. Kamarck, Department of Psychology and Psychiatry, University of Pittsburgh
Xingyuan Li, Department of Biostatistics, University of Pittsburgh
Aidan G.C. Wright, Department of Psychology, University of Pittsburgh
Matthew F. Muldoon, Department of Medicine, University of Pittsburgh
Stephen B. Manuck, Department of Psychology, University of Pittsburgh
References
- 1.Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nature Reviews Cardiology. 2012;9:360–70. [DOI] [PubMed] [Google Scholar]
- 2.Kivimaki M, Batty GD, Ferrie JE, Kawachi I. Cumulative meta-analysis of job strain and CHD. Epidemiology. 2014;25:464–5. [DOI] [PubMed] [Google Scholar]
- 3.Orth-Gomer K, Wamala S, Horsten M, Schenck-Gustafsson K, Schneiderman N, Mittleman MA. Marital stress worsens prognosis in women with coronary heart diseas: The Stockholm female coronary risk study. Journal of the American Medical Association. 2000;284:3008–14. [DOI] [PubMed] [Google Scholar]
- 4.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental streess are associated with poor subsequent cardiovascular risk status: A meta-analysis of prospective evidence. Hypertension. 2010;55(1026–1032). [DOI] [PubMed] [Google Scholar]
- 5.Everson S, Lynch J, Kaplan G, Lakka T, Sivenius J, Salonen J. Stress-induced blood pressure reactivity and incident stroke in middle-aged men. Stroke. 2001;32:1263–70. [DOI] [PubMed] [Google Scholar]
- 6.Carroll D, Ginty A, Der G, Hunt K, Benzeval M, Phillips A. Increased blood pressure reactions to acute mental stress are associated with 16-year cardivascular disease mortality. Psychophysiology. 2012;49:1444–8. [DOI] [PubMed] [Google Scholar]
- 7.Krantz D, Manuck S. Acute psychophysiologic reactivity and risk of cardiovascular disease: A review and methodologic critique. Psychological Bulletin. 1984;96:435–64. [PubMed] [Google Scholar]
- 8.Schwartz A, Gerin W, Davidson K, Pickering T, Brosschot J, Thayer JF CN, Linden W. Toward a causal model of cardiovascular responses to stress and the development of cardiovascular disease. Psychosomatic Medicine. 2003;65:22–35. [DOI] [PubMed] [Google Scholar]
- 9.Everson S, Lynch J, Chesney M, Kaplan G, Goldberg D, Shade SB CR, Salonen R, Salonen JT. Interactions of workplace demands and cardiovascular reactivity in progression of carotid atherosclerosis. British Medical Journal. 1997;314:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch JW, Everson SA, Kaplan GA, Salonen R, Salonen JT. Does low socioeconomic status potentiate the effects of heightened cardiovascular responses to stress on the progression of carotid atherosclerosis? Am J Pub Health. 1998;88:389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamarck T, Shiffman S, Wethington E. Measuring psychosocial stress using ecological momentary assessment methods In: Contrada R, Baum A, editors. Handbook of stress science: Biology, psychology, and health. New York: Springer; 2011. p. 597–617. [Google Scholar]
- 12.Karasek RA, Theorell T. Healthy work: stress, productivity and the reconstruction of working life. New York: Basic Books; 1990. [Google Scholar]
- 13.Kamarck T, Muldoon M, Shiffman S, Sutton-Tyrrell K. Experiences of demand and control during daily life are predictors of carotid artery atherosclerotic progression among healthy men. Health Psychology. 2007;26:324–32. [DOI] [PubMed] [Google Scholar]
- 14.Kamarck TW, Muldoon M, Shiffman S, Sutton-Tyrrell K, Gwaltney CJ. Experiences of demand and control in daily life as correlates of subclinical carotid atherosclerosis in a healthy older sample: the Pittsburgh Healthy Heart Project. Health Psychology. 2004;23:24–32. [DOI] [PubMed] [Google Scholar]
- 15.Kamarck T, Shiffman S, Sutton-Tyrrell K, Muldoon M, Tepper P. Daily psychological demands are associated with six-year progression of carotid artery atherosclerosis: The Pittsburgh Healthy Heart Project. Psychosomatic Medicine. 2012;74:432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Vogli R, Chandola T, Marmot MG. Negative Aspects of Close Relationships and Heart Disease. Archive of Internal Medicine. 2007;167:1951–7. [DOI] [PubMed] [Google Scholar]
- 17.Gallo LC, Bogart LM, Vranceanu A, Walt LC. Job characteristics, occupational status, and ambulatory cardiovascular activity in women. Annals of Behavioral Medicine. 2004;28:62–73. [DOI] [PubMed] [Google Scholar]
- 18.Smith TW, Uchino BN, Berg CA, Forsheim P. Marital discord and coronary artery disease: A comparison of behaviorally defined discrete groups. Journal of Consulting and Clinical Psychology. 2012;80:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan J, Manuck S, Clarkson T, Lusso F, Taub D. Social status, environment, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 1982;2:359–68. [DOI] [PubMed] [Google Scholar]
- 20.Joseph NT, Kamarck T, Muldoon MF, Manuck SB. Daily marital interaction quality and carotid intima-medial thickness in healthy middle-aged adults. Psychosomatic Medicine. 2014;76:347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson L, Miller KG, Wong PM, Anderson BA, Kamarck TW, Matthews KA KC, Manuck SB. Sleep duration partially accounts for race differences in diurnal cortisol dynamics. Health Psychology. 2017;36:502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodwin J, Bilous M, Winship S, Finn P, Jones SC. Validation of the Oscar 2 oscillometric 24-h ambulatory blood pressure monitor according to the British Hypertension Society protocol. Blood Pressure Monitoring. 2007;12:113–7. [DOI] [PubMed] [Google Scholar]
- 23.Johannsen DL, Calabro MA, Stewart J, Franke W, Rood JC, Welk GJ. Accuracy of armband monitors for measuring daily energy expenditure in healthy adults. Medicine and Science in Sports and Exercise. 2010;42:2134–40. [DOI] [PubMed] [Google Scholar]
- 24.Perloff D, Grim C, Glack J, Frohlich E, Hill M, McDonald M, Morgenstern BZ. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–7. [DOI] [PubMed] [Google Scholar]
- 25.Wendelhag I, Liang Q, Gustavsson T, Wikstrand J. A new automated computerized analyzing system simplifies readings and reduces the variability in ultrasound measurement of intima-media thickness. Stroke. 1997;28:2195–200. [DOI] [PubMed] [Google Scholar]
- 26.Verdecchia P, Porcellati C, Schillaci G. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. [DOI] [PubMed] [Google Scholar]
- 27.Kamarck T, Everson S, Kaplan G, Manuck S, Jennings JR SR, Salonen JT. Exaggerated blood pressure responses during mental stress are associated with enhanced carotid atherosclerosis in middle-aged Finnish men: Findings from the Kuopio Ischemic Heart Disease Study. Circulation. 1997;96:3842–8. [DOI] [PubMed] [Google Scholar]
- 28.Jennings J, Kamarck T, Everson-Rose S, Kaplan G, Manuck S, Salonen J. Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation. 2004;110:2198–203. [DOI] [PubMed] [Google Scholar]
- 29.Karasek RA, Pieper C, Schwartz J, Fry L, D. S. Job content instrument questionnaire and user’s guide. New York: Columbia Job/Heart Project; 1985. [Google Scholar]
- 30.Bobko P A solution to some dilemmas when testing hypotheses about ordinal interactions. J Appl Psychol. 1986:323. [Google Scholar]
- 31.Kivimaki M, Nyberg ST, Batty GD, Fransson EJ, Heikkila K, Alfredsson LBJ, Borritz M, Burr H, Casini A, Clays E, De Bacquer D, Dragano N, Ferrie JE, Geuskens GA, Goldberg M, Hamer M, Hooftman WE, Houtman IL, Joensuu M, Jokela M, Kittel F, Knutsson A, Koskenvuo M, Koskinen A, Kouvonen A, Kumari M, Madsen IE, Marmot MG, Nielsen ML, Nordin M, Oksanen T, Pentti J, Rugulies R, Salo P, Siegrist J, Singh-Manoux A, Suominen SB, Väänänen A, Vahtera J, Virtanen M, Westerholm PJ, Westerlund H, Zins M, Steptoe A, Theorell T, IPD-Work Consortium. Job strain as a risk factor for coronary heart disease: A collaborative meta-analysis of individual participant data. Lancet. 2012;380:1491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landsbergis PA, Schnall PL, Warren K, Pickering T, Schwartz J. Association between ambulatory blood pressure and alternative formulations of job strain. Scandinavian Journal of Work and Environmental Health. 1994;20:349–63. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert-Ouimet M, Brisson C, Millot A, Vezina M. Double exposure to adverse psychosocial work factors and high family responsibilities as related to ambulatory blood pressure at work: A 5-year prospective study in women with white-collar jobs. Psychosomatic Medicine. 2017;79:593–602. [DOI] [PubMed] [Google Scholar]
- 34.Janicki DL, Kamarck TW, Shiffman S, Gwaltney CJ. Application of ecological momentary assessment to the study of marital adjustment and social interactions during daily life. Journal of Family Psychology. 2006;20:168–72. [DOI] [PubMed] [Google Scholar]
- 35.John-Henderson N, Kamarck T, Muldoon M, Manuck S. Early life family conflict, social interactions, and carotid artery intima-media thickness in adulthood. Psychosomatic Medicine. 2016;78:319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamarck TW, Shiffman SM, Smithline LGJ, Paty JA, Gnys M, Jong JY. Effects of task strain, social conflict, and emotional activation on ambulatory cardiovascular activity: Daily life consequences of recurring stress in a multiethnic adult sample. Health Psychology. 1998;17:17–29. [DOI] [PubMed] [Google Scholar]
- 37.Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park: Sage; 1991. [Google Scholar]
- 38.Jennings J, Kamarck T, Manuck S, Everson S, Kaplan G, Salonen J. Aging or disease: Cardiovascular reactivity in Finnish men over the middle years. Psychology and Aging. 1997;12:225–38. [DOI] [PubMed] [Google Scholar]
- 39.Uchino B, Berg C, Smith T, Pearce G, Skinner M. Age-related dfifferences in ambulatory blood pressure during daily stress: Evidence for greater blood pressure reactivity with age. Psychology and Aging. 2006;21:231–9. [DOI] [PubMed] [Google Scholar]
- 40.van den Oord SCH, Sijbrands EJG, ten Kate GL, van Klaveren D, van Domburg RT, van der Steen AF, Schinkel AF. Carotid intima-media thickness for cardiovascular risk assessment: Systematic review and meta-analysis. Atherosclerosis. 2013;228:1–11. [DOI] [PubMed] [Google Scholar]
- 41.Kamarck TW, Debski TT, Manuck SB. Enhancing the laboratory-to-life generalizability of cardiovascular reactivity using multiple occasions of measurement. Psychophysiology. 2000;37:533–42. [PubMed] [Google Scholar]
- 42.Kamarck T, Lovallo WR. Cardiovascular reactivity to psychological challenge: Conceptual and measurement considerations. Psychosomatic Medicine. 2003;65:9–21. [DOI] [PubMed] [Google Scholar]
- 43.Kamarck T, Schwartz J, Janicki DL, Shiffman S, Raynor D. Correspondence between laboratory and ambulatory measures of cardiovascular reactivity: A multilevel modeling approach. Psychosomatic Medicine. 2003;40:675–83. [DOI] [PubMed] [Google Scholar]
- 44.Mausbach BT, Roepke SK, Ziegler MG, Milic M, von Kanel R, Dimsdale JE, Mills PJ, Patterson TL, Allison MA, Ancoli-Israel S, Grant I. Association between chronic caregiving stress and impaired endothelial function in the elderly. Journal of the American College of Cardiology. 2010;55:2599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansel A, Hong S, Camara R, von Kanel R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neuroscience and Biobehavioral Reviews. 2010;35:115–21. [DOI] [PubMed] [Google Scholar]
- 46.Brydon L, Magid K, Steptoe A. Platelets, coronary heart disease, and stress. Brain, Behavior, and Immunity. 2006;20:113–9. [DOI] [PubMed] [Google Scholar]


