Supplemental Digital Content is available in the text.
Keywords: fluid balance, intensive care units, mortality, resuscitation, sepsis, septic shock
Abstract
Objectives:
Previous studies demonstrated that extensive fluid loading and consequently positive fluid balances during sepsis resuscitation are associated with adverse outcome. Yet, the association between fluid balance and mortality after reversal of shock, that is, during deresuscitation, is largely unappreciated. Our objective was to investigate the effects of fluid balance on mortality in the days after septic shock reversal.
Design:
Retrospective observational cohort study.
Setting:
ICUs of two university-affiliated hospitals in The Netherlands.
Patients:
Adult patients admitted with septic shock followed by shock reversal. Reversal of septic shock was defined based on Sepsis-3 criteria as the first day that serum lactate was less than or equal to 2 mmol/L without vasopressor requirement.
Interventions:
None.
Measurements and Main Results:
Reversal of septic shock occurred in 636 patients, of whom 20% died in the ICU. Mixed-effects logistic regression modeling, adjusted for possible confounders, showed that fluid balance in the days after reversal of septic shock (until discharge or death) was an independent predictor of ICU mortality: odds ratio 3.18 (1.90–5.32) per 10 mL/kg increase in daily fluid balance. Similar results were found for 30-day, 90-day, hospital, and 1-year mortality: odds ratios 2.09 (1.64–2.67); 1.79 (1.38–2.32); 1.70 (1.40–2.07); and 1.53 (1.17–2.01), respectively. Positive cumulative fluid balances vs. neutral or negative fluid balances on the final day in the ICU were associated with increased ICU, hospital, 30-day, and 90-day mortality: odds ratios 3.46 (2.29–5.23); 3.39 (2.35–4.9); 5.33 (3.51–8.08); and 3.57 (2.49–5.12), respectively. Using restricted cubic splines, we found a dose-response relationship between cumulative fluid balance after shock reversal and ICU mortality.
Conclusions:
A higher fluid balance in the days after septic shock reversal was associated with increased mortality. This stresses the importance of implementing restrictive and deresuscitative fluid management strategies after initial hemodynamic resuscitation. Prospective interventional studies are needed to confirm our results.
Despite major leaps in preventive measures, management modalities, and bundled care, sepsis is still one of the main causes of death in the ICU (1, 2). The foremost elements of treatment consist of early resuscitation, antibiotics, and supportive care (3). Due to capillary leakage and vasoplegia, intravascular volume is often depleted rapidly, resulting in massive fluid loading during hemodynamic resuscitation (4). As a result, cumulative fluid balances are positive, often going beyond 10 L in the first days of admission (5, 6).
Positive fluid balances are associated with adverse outcome in patients with sepsis (7, 8). This association is well established. However, most studies investigate the effects of fluid balance during the early, critical phases of septic shock. The phase after reversal of septic shock, “deresuscitation,” is generally not taken into account or specifically noted (6, 9–15). Surely, patient outcome is not solely dependent on resuscitation and the effects of fluid balance after resuscitation in this often heavily fluid loaded population are relevant. Indeed, a randomized multicenter study performed in patients after initial resuscitation of septic shock showed benefit toward fluid restriction over standard care, however, was not powered to show differences in exploratory outcomes (16). Furthermore, a systematic review and meta-analysis showed that conservative or deresuscitative fluid strategies in critically ill patients resulted in less time on mechanical ventilation and a shorter length of ICU stay compared with liberal or standard care strategies. However, the effect on mortality remained uncertain (17).
Our primary aim was to look into the effects of fluid balance on mortality in the days following reversal of septic shock. We hypothesized that there is an association between positive fluid balance and increased mortality after septic shock reversal.
MATERIALS AND METHODS
Design and Setting
This study was retrospective and observational in design. Data from the Molecular Diagnosis and Risk Stratification of Sepsis (MARS) project were used (ClinicalTrials.gov: NCT01905033). The MARS project was a prospective cohort study performed in the adult ICUs of two Dutch university-affiliated hospitals (i.e., University Medical Center Utrecht, Amsterdam University Medical Centers, Location Academic Medical Center, The Netherlands) (18–20). Both ICUs are closed-format, mixed medical-surgical units, where patients are under direct care of a team of intensive care physicians, subspecialty fellows, residents, and ICU nurses. Sepsis resuscitation was performed following the Surviving Sepsis Campaign Bundles (3).
The Institutional Review Boards (IRBs) of participating hospitals approved the study design, including an opt-out consent method (IRB: 10-056C). As this study was a substudy of the MARS project (using encrypted patient data), no separate ethics approval was required.
Definitions
Sepsis and septic shock were defined following the criteria of the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Briefly, septic shock was defined as sepsis plus persisting hypotension requiring vasopressors to maintain a mean arterial pressure greater than or equal to 65 mm Hg and with a serum lactate level greater than 2 mmol/L (21). We defined reversal of septic shock as the first day that serum lactate level was less than or equal to 2 mmol/L and the patient was weaned off vasopressors.
Patient Selection
Data from the MARS database, collected between January 2011 and December 2013, were used for the identification of patients with sepsis. The initial dataset consisted of consecutively admitted adult patients (≥ 18 yr old) diagnosed with sepsis who had at least one serum lactate level greater than 2 mmol/L during their ICU stay. Only patients with daily serum lactate levels were included in the dataset. One-thousand three-hundred twenty-five sepsis patients were identified, of whom 1,006 had one or more days of septic shock. The ICU mortality rate of the entire cohort of septic shock patients was 44% (n = 441).
Exclusively patients with septic shock at admission were included. To determine the association between fluid balance and outcome in the days after shock reversal, we only included patients who ended their ICU stay without shock. After the final reversal of shock, there was a follow-up until ICU discharge or death.
Data Collection
Demographic and bacteriologic data were collected. Furthermore, reason of ICU admission (medical/surgical), site of infection, ICU length of stay, Acute Physiology and Chronic Health Evaluation (APACHE) IV score, renal replacement therapy (RRT), vasopressor requirement, time on mechanical ventilation, lactate levels and ICU, hospital, 30-day, 90-day, and 1-year mortality were noted. Daily fluid intake and output in the ICU were noted, and daily and cumulative fluid balances were calculated (in mL/kg, based on weight at ICU admission). Data on type of fluids administered were not collected. Insensible fluid losses from transepidermal diffusion or evaporative water loss from the respiratory tract were not routinely measured and not specifically taken into account. The primary outcome was ICU mortality. Secondary outcomes were 30-day, 90-day, hospital, and 1-year mortality.
Statistical Analysis
Continuous normally distributed variables were expressed as means and sds or when not normally distributed as medians and interquartile ranges. Categorical variables were expressed as n (%). Difference testing between groups was performed using Student t tests, Mann-Whitney U tests, and chi-square tests as appropriate. As the data contained repeated measures, that is, fluid balance per day, with dependence of fluid balance within each patient, a mixed-effects logistic regression model was built with death as outcome and fluid balance as primary predictor of outcome. Covariates were included in the model when they improved the model fit. Covariates were selected from the dataset when appearing statistically and/or clinically relevant, and, after checking for multicollinearity, were: number of shock days; age; APACHE IV score; RRT requirement; gram-positive cultures; and cumulative fluid balance on the final day of shock. The model fit was further explored by adding random intercepts and/or slopes, where random intercepts were patients and random slopes fluid balance. Furthermore, we performed restricted cubic splines analyses, looking at the cumulative fluid balance on days 1, 3, 5, and 7 after shock reversal as continuous predictors of mortality. The restricted cubic spline curves had five knots, placed on the 5th, 27.5th, 50th, 72.5th, and 95th percentiles of cumulative fluid balance. Statistical significance was considered to be at p = 0.05. When appropriate, statistical uncertainty was expressed by 95% CIs. Statistical analysis was performed using R version 3.6.1 in RStudio; the mixed-effects logistic regression model was built using the glmer function of the lme4 package version 1.1.23 (22).
RESULTS
A flowchart of patient selection is presented in Figure 1. Final reversal of septic shock before ICU discharge or death occurred in 636 patients (63% of septic shock patients), of whom 126 (20%) died in the ICU.
Figure 1.
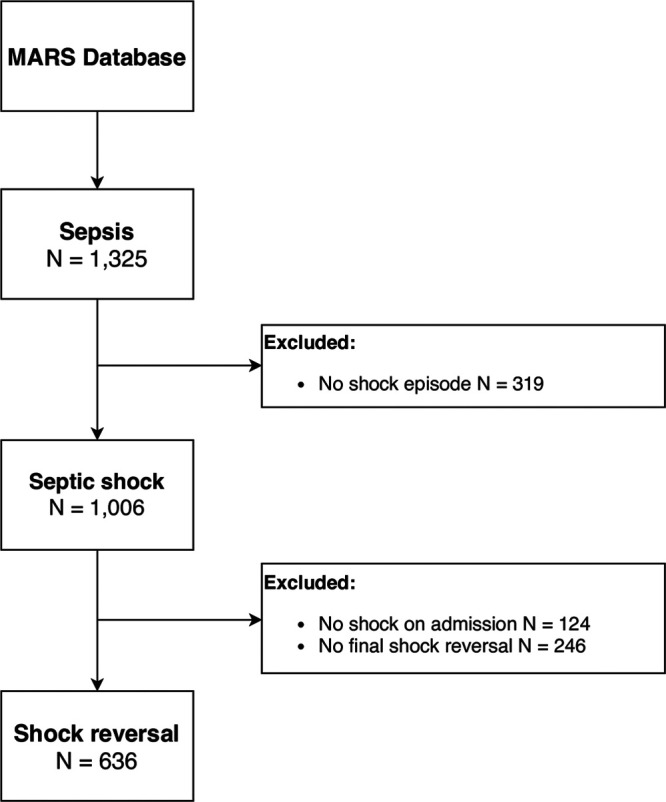
Flowchart of patient selection. MARS = Molecular Diagnosis and Risk Stratification of Sepsis.
Patient Characteristics
Characteristics of patients with septic shock reversal are presented in Table 1. See Appendix I (http://links.lww.com/CCX/A316) for an overview of the sepsis cohort. Lower respiratory tract infections were the most common cause of sepsis. Nonsurvivors were older and had higher APACHE IV scores. RRT and recurring shock were more prevalent in nonsurvivors versus survivors. Nonsurvivors had more septic shock days and shorter lengths of stays after shock reversal than survivors.
Table 1.
Demographic and Clinical Data of Patients With Septic Shock Reversal
| Data | ICU Survivors, n = 509 | ICU Nonsurvivors, n = 127 | p |
|---|---|---|---|
| Demographics | |||
| Gender | 0.655 | ||
| Male, n (%) | 295 (58.0) | 77 (60.6) | |
| Female, n (%) | 214 (42.0) | 50 (39.4) | |
| Age, yr | 62.0 (51.0–73.0) | 65.0 (58.0–75.0) | 0.009 |
| Weight, kg | 79.0 (65.0–90.0) | 75.0 (65.0–90.0) | 0.479 |
| Length, cm | 172 (165–180) | 170 (165–180) | 0.489 |
| Body mass index, kg/m2 | 24.9 (22.9–28.7) | 25.3 (22.5–28.4) | 0.678 |
| Severity of illness | |||
| Acute Physiology and Chronic Health Evaluation IV score | 84.0 (69.0–104) | 101 (78.5–125) | < 0.001 |
| Septic shock days before shock reversal | 1.00 (1.00–2.00) | 2.00 (1.00–5.00) | 0.004 |
| ICU length of stay, d | 7.00 (4.00–15.0) | 7.00 (3.00–16.0) | 0.807 |
| ICU length of stay after shock reversal, d | 5.00 (2.00–10.0) | 3.00 (1.00–7.50) | < 0.001 |
| Shock recurrence before shock reversal, n (%) | 101 (19.8) | 37 (29.1) | 0.031 |
| Mortality, n (%) | |||
| Hospital | 69 (15.1) | 114 (100.0) | < 0.001 |
| 30-d | 47 (10.3) | 99 (86.8) | < 0.001 |
| 90-d | 91 (19.9) | 112 (98.2) | < 0.001 |
| 1-yr | 155 (33.8) | 114 (100) | < 0.001 |
| Admission type, n (%) | 0.879 | ||
| Medical | 327 (64.2) | 80 (63.0) | |
| Surgical elective | 56 (11.0) | 16 (12.6) | |
| Surgical emergency | 126 (24.8) | 31 (24.4) | |
| Site of infection, n (%) | 0.063 | ||
| Bloodstream | 48 (9.66) | 17 (13.6) | |
| Central nervous system | 12 (2.4) | 2 (1.6) | |
| Lower respiratory tract | 206 (41.4) | 66 (52.8) | |
| Skin | 38 (7.7) | 5 (4.0) | |
| Urinary tract | 33 (6.6) | 8 (6.40) | |
| Other | 160 (32.2) | 27 (21.6) | |
| Infection, n (%) | |||
| Gram-positive | 152 (29.9) | 23 (18.1) | 0.011 |
| Gram-negative | 155 (30.5) | 36 (28.3) | 0.713 |
| Fungi | 35 (6.9) | 13 (10.2) | 0.276 |
| Organ support | |||
| Mechanical ventilation days | 5.00 (2.00–11.0) | 6.00 (3.00–14.0) | 0.013 |
| Ventilator-free daysa | 23.0 (17.0–26.0) | 0.00 (0.00–0.00) | < 0.001 |
| RRT patients, n (%) | 149 (29.3) | 54 (42.5) | 0.006 |
| RRT days | 0.00 (0.00–2.00) | 0.00 (0.00–6.00) | 0.004 |
| Cumulative fluid balances, mL/kg | |||
| Pre shock reversal | 63.3 (30.6–113) | 96.2 (48.8–163) | < 0.001 |
| Day 1 post shock reversal | 70.7 (30.9–124) | 117 (62.2–194) | < 0.001 |
| Day 3 post shock reversal | 69.6 (18.2–133) | 95.2 (30.4–208) | 0.003 |
| Day 5 post shock reversal | 67.3 (4.30–134) | 90.3 (22.4–217) | 0.071 |
| Day 7 post shock reversal | 60.9 (3.10–143) | 83.3 (31.1–173) | 0.119 |
RRT = renal replacement therapy.
aVentilator-free days at day 28, death was penalized as zero ventilator-free days.
Values indicated with n are number of patients. Medians are presented with interquartile ranges between parentheses. Statistically significant values are in italics.
Fluid Balance and Outcome After Shock Reversal
As displayed in Figures 2 and 3, negative fluid balances were less prevalent and urinary output was lower in nonsurvivors versus survivors in the days after septic shock reversal. To investigate the relationship between daily fluid balance and ICU mortality after reversal of septic shock, a mixed-effects logistic regression model was built. The modeling approach is presented in Appendix II (http://links.lww.com/CCX/A317). The best model fit was found using a model with random intercepts for patients and random slopes for fluid balance. We found that daily fluid balance after reversal of septic shock was a predictor of ICU mortality, with an odds ratio (OR) of 1.12 (95% CI, 1.07–1.18) per mL/kg increase in daily fluid balance, controlling for number of septic shock days, RRT requirement, APACHE IV score, gram-positive culture, age, and the cumulative fluid balance prior to septic shock reversal. Thus, a 10 mL/kg increase in daily fluid balance, from the moment of shock reversal to discharge or death, increases the odds of ICU mortality by 3.18 times (95% CI, 1.90–5.32). See Table 2 for the variables and corresponding ORs for mortality in our final model. Using the same model, we found similar results per 10 mL/kg increase for 30-day, 90-day, hospital, and 1-year mortality (OR, 2.09; 95% CI, 1.64–2.67); (OR, 1.79; 95% CI, 1.38–2.32); (OR, 1.70; 95% CI, 1.40–2.07); and (OR, 1.53; 95% CI, 1.17–2.01), respectively.
Figure 2.

Fluid balance after reversal of septic shock. Boxplots for fluid balance per day after final septic shock reversal until ICU discharge or death for survivors and nonsurvivors. The number of survivors and nonsurvivors are presented below the boxplots. Survival is based on ICU mortality. Fluid balance is in mL/kg.
Figure 3.

Urinary output after reversal of septic shock. Boxplots for urinary output per day after final septic shock reversal until ICU discharge or death for survivors and nonsurvivors. The number of survivors and nonsurvivors are presented below the boxplots. Survival is based on ICU mortality. Fluid balance is in mL/kg.
Table 2.
Variables and Corresponding Odds Ratios for ICU Mortality of the Final Mixed-Effects Logistic Regression Model
| Variables | OR (95% CI) |
|---|---|
| Daily fluid balance (mL/kg) | 1.12 (1.07–1.18) |
| Acute Physiology and Chronic Health Evaluation IV score | 1.08 (1.03–1.14) |
| Age at ICU admission | 0.99 (0.92–1.07) |
| Renal replacement therapy | 0.11 (0.01–2.02) |
| Gram-positive infection | 1.03 (0.08–12.81) |
| Septic shock days before shock reversal | 2.37 (1.12–5.03) |
| Cumulative fluid balance pre shock reversal (mL/kg) | 0.99 (0.98–1.00) |
OR = odds ratio.
Patients who ended their ICU stay with a positive cumulative fluid balance had greater odds of dying in the ICU (OR, 3.46; 95% CI, 2.29–5.23); hospital (OR, 3.39; 95% CI, 2.35–4.9); at 30 days (OR, 5.33; 95% CI, 3.51–8.08); or at 90 days (OR, 3.57; 95% CI, 2.49–5.12) versus patients with a neutral or negative fluid balance on their final day of ICU stay.
Using restricted cubic splines, we explored the relationship between cumulative fluid balance and ICU mortality after septic shock reversal. As presented in Figure 4, we found a J-shaped dose-response relationship between probability of death and cumulative fluid balance on the first day after shock reversal: both a more negative fluid balance, as a more positive fluid balance were associated with increased mortality, with a flattening of the curve for the highest fluid balances. This J-shape was not observed on days 3, 5, and 7 after shock reversal. For all days, a gradual increase in mortality was found for cumulative fluid balances higher than approximately 50 mL/kg.
Figure 4.

The relationship between probability of death and cumulative fluid balance after shock reversal. Restricted cubic splines models. 95% CIs are displayed in gray. D1 denotes day 1 after septic shock reversal; D3, day 3; D5, day 5; and D7, day 7. Probability of death is based on ICU mortality. Cumulative fluid balance is in mL/kg.
DISCUSSION
In a context of sepsis care bundles, our study demonstrated that a higher fluid balance in the days after reversal of septic shock was independently associated with ICU mortality. This suggests that late fluid management may improve patient outcome, supporting the implementation of restrictive and deresuscitative fluid management after initial resuscitation.
Fluid bolusing is a necessary, unavoidable supportive measure in hypotensive, hypoperfused, septic patients in the critical phase of their disease (3). Given the major part of fluids administered extravasates and has rather short-lived hemodynamic effects, fluids accumulate (23). This results in positive fluid balances during resuscitation (24). We demonstrated that these positive fluid balances persist until after shock reversal, which was associated with increased mortality. Of course, not only resuscitative fluid administration adds to a positive fluid balance, but also other fluids contribute. A multicenter retrospective cohort study performed in 400 mechanically ventilated patients in 10 ICUs across Canada and the United Kingdom showed that 60% of fluid input during the first 3 days was from drugs and maintenance fluids, whereas only 24.4% of input was accounted for by fluid boluses (25). Unfortunately, data on fluid source were not collected in the MARS database.
Shock reversal was defined as the first day that the patient had a lactate less than or equal to 2 mmol/L and was weaned off vasopressors. This was based on the latest sepsis definitions. By including both vasopressor use and hyperlactatemia in our definition, we take into account cellular dysfunction and cardiovascular compromise, both characteristics of septic shock (21). Previous studies used similar definitions to define reversal of septic shock explicitly, however, to our knowledge, only one relatively small study investigated the impact of fluids on outcome after shock reversal (5, 26, 27). This was a prospective, observational study performed in 40 septic shock patients, showing that after shock reversal, a higher cumulative fluid balance was associated with prolonged lengths of stay (5).
Negative daily fluid balances were more prevalent in survivors, whereas nonsurvivors had more positive fluid balances. Maybe nonsurvivors were the group of patients in whom it was not feasible to implement restrictive or deresuscitative fluid therapy approaches. Indeed, RRT, indicative of acute kidney injury, was more prevalent in nonsurvivors. Also, we found that nonsurvivors had lower daily urinary output than survivors, but it should be noted that urinary output does not include ultrafiltrate from RRT, which was more prevalent in nonsurvivors. We adjusted for RRT in our model. It is plausible that nonsurvivors received more fluids or less deresuscitative interventions, due to different, clinician-dependent practice methods, resulting in, perhaps preventable, fluid overload.
Our study has several limitations. First, the retrospective and observational study design limits the possibility to determine a cause-effect relationship between fluid balance and mortality. Therefore, no firm conclusions can be drawn about active fluid management in the deresuscitation phase. However, we have a relatively large cohort of patients in which we used mixed-effects logistic regression models adjusting for possible confounding variables. Furthermore, we used random effects to take dependence of fluid balance within each patient into account. Second, we purposely excluded patients who did not end their ICU stay with shock reversal. One could argue that fluid balance may play a role in shock recurrence, or that patients with worse prognoses are less responsive to supportive therapy, presenting more fluctuations in their hemodynamic state. Indeed, the mortality rate in patients with shock reversal was relatively low compared with the entire septic shock cohort (20% vs 44%), which might suggest selection bias. However, by investigating the effects of fluid balance on mortality in a patient cohort with no shock on the final day(s) of their ICU stay, the impact of fluid balance on mortality becomes less obscure and prone to confounding by severity of illness or insufficient resuscitation. Still, even in this population with better a priori survival chances, higher fluid balances were associated with mortality. Third, it would be interesting to look into long-term (functional) effects of fluid balance. Previous studies demonstrated that fluid overload may not only have its effects on outcome during (or shortly after) critical illness, it is also associated with decreased renal recovery rates after hospital discharge (28, 29). Furthermore, a previous study showed that a conservative fluid-management strategy in patients with acute lung injury, often due to sepsis, is associated with long-term cognitive impairment (30). Unfortunately, we did not collect long-term functional outcomes. Fourth, our data were collected between 2011 and 2013. With increasing attention to the potential harms of excess fluids, fluid practice may have changed since then. Nevertheless, fluid overload is still a frequently encountered problem in the ICU (and on the wards) and protocols initiating active deresuscitation are uncommon and not yet widely implemented. Last, data were collected in the ICUs of two academic hospitals in The Netherlands. The external validity of our results to places with less resources (e.g., smaller hospitals, developing countries) may be limited.
Our results add to previous literature demonstrating that iatrogenic fluid overload is associated with adverse outcome in sepsis patients. Unlike most previously performed studies, we specifically investigated the post-shock phase by defining reversal of septic shock explicitly. The Goldilocks principle seems to apply for fluid administration in the critically ill septic patient: neither too little nor too much fluid administration is beneficial (31). Our results suggest that, if possible, neutral or negative daily fluid balances should be pursued in patients after septic shock reversal. Furthermore, patients with a positive cumulative fluid balance on their final day of ICU stay had greater odds of mortality than patients ending their ICU stay with a neutral or negative cumulative fluid balance. Furthermore, we found a possible cutoff point around 50–75 mL/kg for cumulative fluid balance in the days after septic shock reversal, but more data are needed to draw firm conclusions. These results could aid in the development of strategies to prevent positive fluid balances in the deresuscitation phase of shock. It would be interesting to conduct interventional studies implementing restrictive and/or deresuscitative fluid management strategies in septic patients.
CONCLUSIONS
In patients with septic shock reversal, a higher fluid balance was associated with increased mortality in the days after shock reversal. This supports the implementation of restrictive and deresuscitative fluid management strategies after initial hemodynamic resuscitation. Prospective interventional studies are needed to confirm our results.
ACKNOWLEDGMENTS
Molecular Diagnosis and Risk Stratification of Sepsis (MARS) consortium collaborators were Laura R. A. Schouten, Lonneke A. van Vught, Maryse A. Wiewel, David S. Y. Ong, Jos F. Frencken, Marc Bonten, Peter M. C. Klein Klouwenberg, Roosmarijn T. M. van Hooijdonk, Mischa A. Huson, Marleen Straat, Esther Witteveen, Gerie J. Glas, Luuk Wieske, Brendon P. Scicluna, Hakima Belkasim-Bohoudi, and Arie J. Hoogendijk.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
The authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
Collaborators: Laura R. A. Schouten, Lonneke A. van Vught, Maryse A. Wiewel, David S. Y. Ong, Jos F. Frencken, Marc Bonten, Peter M. C. Klein Klouwenberg, Roosmarijn T. M. van Hooijdonk, Mischa A. Huson, Marleen Straat, Esther Witteveen, Gerie J. Glas, Luuk Wieske, Brendon P. Scicluna, Hakima Belkasim-Bohoudi, and Arie J. Hoogendijk
REFERENCES
- 1.Fleischmann C, Scherag A, Adhikari NK, et al. ; International Forum of Acute Care Trialists. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016; 193:259–272 [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Marshall JC, Namendys-Silva SA, et al. ; ICON investigators. Assessment of the worldwide burden of critical illness: The intensive care over nations (ICON) audit. Lancet Respir Med. 2014; 2:380–386 [DOI] [PubMed] [Google Scholar]
- 3.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017; 43:304–377 [DOI] [PubMed] [Google Scholar]
- 4.Marik P, Bellomo R. A rational approach to fluid therapy in sepsis. Br J Anaesth. 2016; 116:339–349 [DOI] [PubMed] [Google Scholar]
- 5.Cunha AR, Lobo SM. What happens to the fluid balance during and after recovering from septic shock? Rev Bras Ter Intensiva. 2015; 27:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd JH, Forbes J, Nakada TA, et al. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011; 39:259–265 [DOI] [PubMed] [Google Scholar]
- 7.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006; 354:2564–2575 [DOI] [PubMed] [Google Scholar]
- 8.Malbrain ML, Marik PE, Witters I, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: A systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014; 46:361–380 [DOI] [PubMed] [Google Scholar]
- 9.Acheampong A, Vincent JL. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care. 2015; 19:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Oliveira FS, Freitas FG, Ferreira EM, et al. Positive fluid balance as a prognostic factor for mortality and acute kidney injury in severe sepsis and septic shock. J Crit Care. 2015; 30:97–101 [DOI] [PubMed] [Google Scholar]
- 11.Koonrangsesomboon W, Khwannimit B. Impact of positive fluid balance on mortality and length of stay in septic shock patients. Indian J Crit Care Med. 2015; 19:708–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Micek ST, McEvoy C, McKenzie M, et al. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care. 2013; 17:R246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadaka F, Juarez M, Naydenov S, et al. Fluid resuscitation in septic shock: The effect of increasing fluid balance on mortality. J Intensive Care Med. 2014; 29:213–217 [DOI] [PubMed] [Google Scholar]
- 14.Sirvent JM, Ferri C, Baró A, et al. Fluid balance in sepsis and septic shock as a determining factor of mortality. Am J Emerg Med. 2015; 33:186–189 [DOI] [PubMed] [Google Scholar]
- 15.Vincent JL, Sakr Y, Sprung CL, et al. ; Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med. 2006; 34:344–353 [DOI] [PubMed] [Google Scholar]
- 16.Hjortrup PB, Haase N, Bundgaard H, et al. ; CLASSIC Trial Group; Scandinavian Critical Care Trials Group. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: The CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensive Care Med. 2016; 42:1695–1705 [DOI] [PubMed] [Google Scholar]
- 17.Silversides JA, Major E, Ferguson AJ, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: A systematic review and meta-analysis. Intensive Care Med. 2017; 43:155–170 [DOI] [PubMed] [Google Scholar]
- 18.Engele LJ, Straat M, van Rooijen IHM, et al. ; MARS Consortium. Transfusion of platelets, but not of red blood cells, is independently associated with nosocomial infections in the critically ill. Ann Intensive Care. 2016; 6:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frencken JF, van Vught LA, Peelen LM, et al. ; MARS Consortium. An unbalanced inflammatory cytokine response is not associated with mortality following sepsis: A prospective cohort study. Crit Care Med. 2017; 45:e493–e499 [DOI] [PubMed] [Google Scholar]
- 20.Bos LD, Schouten LR, van Vught LA, et al. ; MARS consortium. Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax. 2017; 72:876–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R Core Team. R: A Language and Environment for Statistical Computing. 2015. Available at: https://www.R-project.org/. Accessed November 26, 2019
- 23.Jacob M, Chappell D, Hofmann-Kiefer K, et al. The intravascular volume effect of Ringer’s lactate is below 20%: A prospective study in humans. Crit Care. 2012; 16:R86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malbrain MLNG, Van Regenmortel N, Saugel B, et al. Principles of fluid management and stewardship in septic shock: It is time to consider the four D’s and the four phases of fluid therapy. Ann Intensive Care. 2018; 8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silversides JA, Fitzgerald E, Manickavasagam US, et al. ; Role of Active Deresuscitation After Resuscitation (RADAR) Investigators. Deresuscitation of patients with iatrogenic fluid overload is associated with reduced mortality in critical illness. Crit Care Med. 2018; 46:1600–1607 [DOI] [PubMed] [Google Scholar]
- 26.Oppert M, Schindler R, Husung C, et al. Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Crit Care Med. 2005; 33:2457–2464 [DOI] [PubMed] [Google Scholar]
- 27.Bayer O, Reinhart K, Kohl M, et al. Effects of fluid resuscitation with synthetic colloids or crystalloids alone on shock reversal, fluid balance, and patient outcomes in patients with severe sepsis: A prospective sequential analysis. Crit Care Med. 2012; 40:2543–2551 [DOI] [PubMed] [Google Scholar]
- 28.Heung M, Wolfgram DF, Kommareddi M, et al. Fluid overload at initiation of renal replacement therapy is associated with lack of renal recovery in patients with acute kidney injury. Nephrol Dial Transplant. 2012; 27:956–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodward CW, Lambert J, Ortiz-Soriano V, et al. Fluid overload associates with major adverse kidney events in critically ill patients with acute kidney injury requiring continuous renal replacement therapy. Crit Care Med. 2019; 47:e753–e760 [DOI] [PubMed] [Google Scholar]
- 30.Mikkelsen ME, Christie JD, Lanken PN, et al. The adult respiratory distress syndrome cognitive outcomes study: Long-term neuropsychological function in survivors of acute lung injury. Am J Resp Crit Care Med. 2012; 185:1307–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Genga KR, Russell JA. How much excess fluid impairs outcome of sepsis? Intensive Care Med. 2017; 43:680–682 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


