Abstract
It is unclear whether the use of antibiotics is related to the efficacy of gemcitabine plus nab-paclitaxel (GnP). Therefore, we investigated the association between the use of antibiotics and efficacy of GnP.
We conducted a retrospective single center study from January 2014 to December 2018 in Hokkaido University Hospital.
Ninety-nine patients were eligible for the study. Thirty-seven used antibiotics (U) and 62 did not use antibiotics (NU) during GnP therapy. In the U group, 15 patients used β-lactam antibiotics, 21 used new quinolones, and 1 used carbapenem. The median progression-free survival was 5.8 and 2.7 months (hazards ratio [HR] .602, 95% confidence interval [CI] .391–.928, P = .022) and the median overall survival was 11.0 and 8.4 months (HR .768, 95% CI .491–1.202, P = .248) in the U and not use antibiotics groups, respectively. Antibiotic use (HR .489, 95% CI .287–.832, P = .008) and locally advanced pancreatic cancer (HR 1.808, 95% CI 1.051–3.112, P = .032) were independent prognostic factors for progression-free survival.
Antibiotic use was associated with a higher efficacy of GnP, and therefore, it may be employed as a novel treatment strategy.
Keywords: antibiotics, gemcitabine plus nab-paclitaxel, pancreatic cancer, progression-free survival, retrospective cohort
1. Introduction
Pancreatic cancer is the seventh leading cause of cancer-related death worldwide.[1] In the metastatic stage, systemic intensive chemotherapies, such as FOLFIRINOX[2] and gemcitabine plus nab-paclitaxel (GnP),[3] significantly increase the overall survival (OS) of patients compared with gemcitabine monotherapy. It is not clear whether FOLFIRINOX or GnP is a better regimen as the first-line treatment[4]; a randomized phase II trial to compare these 2 regimens for locally advanced pancreatic cancer is ongoing in Japan.[5]
Gemcitabine is a crucial agent in pancreatic cancer chemotherapy. However, in many cases, the effects of gemcitabine are limited owing to primary resistance, and even when it shows anticancer effects, most of the tumors soon acquire resistance. The mechanisms of resistance to gemcitabine include p53 function loss,[6] environment-related resistance pathways such as enriched stroma-related gene pathways,[7] micro-RNA regulation,[8] and gemcitabine metabolism pathway-associated resistance.[9] Recently, Geller et al reported that gemcitabine is inactivated by a particular isoform of cytidine deaminase (CDD) produced by microorganisms and that the intratumor microbiome contains these microorganisms; this implies that the intratumor microbiome reduces the efficacy of gemcitabine. Furthermore, antibiotics eliminate these microorganisms and restore the efficacy of gemcitabine.[10] In clinical settings, a few monocenter retrospective studies have reported the association between the use of antibiotics before or during chemotherapy and clinical outcomes of gemcitabine monotherapy[11] and combination therapy.[12] However, little is known about the association between the use of antibiotics and efficacy of GnP combination therapy, which is regarded as the standard therapy for advanced pancreatic cancer. Therefore, we conducted a retrospective study to elucidate the association between the use of antibiotics and efficacy of GnP.
2. Material and methods
2.1. Patients and study design
We retrospectively collected the clinical data of patients with advanced pancreatic cancer treated with GnP from January 2014 to December 2018 in Hokkaido University Hospital. The inclusion criteria were as follows: patients aged 20 years and older, with histologically or cytologically confirmed pancreatic cancer and a history of GnP therapy as the first-line therapy. The study design and protocol were approved by the Institutional Review Board of Hokkaido University Hospital and that of all other participating institutions (approval number: 019–0240). The need for informed consent was waived owing to the retrospective nature of the study. This research was announced on the website of Hokkaido University Hospital (http://www.huhp.hokudai.ac.jp/).
2.2. Treatment: GnP therapy
GnP was administered via intravenous infusion on days 1, 8, and 15 in a 4-week cycle of 1000 and 125 mg/m2 gemcitabine and nab-paclitaxel, respectively. The treatment was continued until the progression of disease, the occurrence of unacceptable adverse effects, or a patient expressed refusal to continue.
2.3. Use of antibiotics
Antibiotic use was defined as the use of equal to or more than 1 dose of oral or intravenous antibiotics during GnP chemotherapy. Patients who used antibiotics while receiving the GnP regimen were included in the “used” group (U). Patients who did not use antibiotics during the GnP chemotherapy were included in the “not used” group (NU).
2.4. Outcome assessment
Efficacy was assessed using OS (defined as the time from the start of first GnP administration to death) and progression-free survival (PFS) (defined as the time from the start of first GnP administration to progression at the time of survival investigation). Patients whose treatment regimens were changed without evidence of progression were censored. Tumor response of patients who had an evaluable site was evaluated according to the Response Evaluation Criteria in Solid Tumors (ver. 1.1).[13] Toxicity was graded using the Common Toxicity Criteria for Adverse Events (ver. 4.0).[14]
2.5. Statistical analysis
Categorical and continuous variables were compared using the chi-squared test or Fisher exact test and the nonparametric Mann–Whitney U test, respectively. Data are presented with 95% confidence interval (CI), which was calculated using standard methods based on binomial distribution. Survival analyses were performed using the Kaplan–Meier method. A log-rank test and the Cox proportional hazards model were used to compare patient groups based on the use of antibiotics. All analyses included patients who received at least one dose of GnP. All analyses were conducted using SPSS version 25 (IBM, Armonk, NY). All tests were 2 sided, and the results with a P-value of < .05 were considered statistically significant.
3. Results
Ninety-nine patients were included in this retrospective study. The major clinicopathological characteristics of these patients are listed in Table 1. Ninety-six patients had adenocarcinoma or squamousadenocarcinoma, 2 had anaplastic carcinoma, and 1 had intraductal papillary mucinous carcinoma. Among the 99 patients, 37 were in the U group and 62 in the NU group during the GnP chemotherapy (Fig. 1). Between the U and NU groups, the location of tumor and stent implantation was significantly different (P = <.001 and .044, respectively). In the U group, 15 patients used β-lactam antibiotics, 21 used new quinolones, and 1 used carbapenem.
Table 1.
Patient characteristics.
Figure 1.
Flow diagram of the study.
At the cutoff date, October 31, 2019, 93 patients discontinued GnP treatment, with a median follow-up time of 9 months. The median treatment cycle was 6.0 and 3.0 months (P = .025) in the U and NU groups, respectively. The median relative dose intensity in the U and NU groups was .65 and .73 (P = .205) for gemcitabine and .59 and .70 for nab-paclitaxel (P = .025), respectively.
In the U and NU groups, 33 (89%) and 60 (97%) patients discontinued GnP treatment, respectively. Among them, 20 (61%) and 31 (52%) patients received subsequent chemotherapy and 1 and 3 patients received conversion surgery.
The response and disease control rates are summarized in Table 2. Of the 99 patients, 86 had measurable lesions. The objective response rate was 19.4% and 20.0% in the U and NU groups (P = 1.00), respectively. The disease control rate was 74.2% and 56.4% in the U and NU groups (P = .11), respectively. The median PFS was 5.8 and 2.7 months in the U and NU groups [hazard ratio (HR) .602, 95% CI .391–.928, P = .022], respectively (Fig. 2A). The median OS was 11.0 and 8.4 months in the U and NU groups (HR .768, 95% CI .491–1.202, P = .248), respectively (Fig. 2B). In the U group, β-lactam showed a good efficacy compared with quinolone (8.5 and 4.1 months, respectively); however, there was no significant difference (HR .592 95% CI .281–1.246, P = .167) (Fig. 3).
Table 2.
Response rate and disease control rate.

Figure 2.
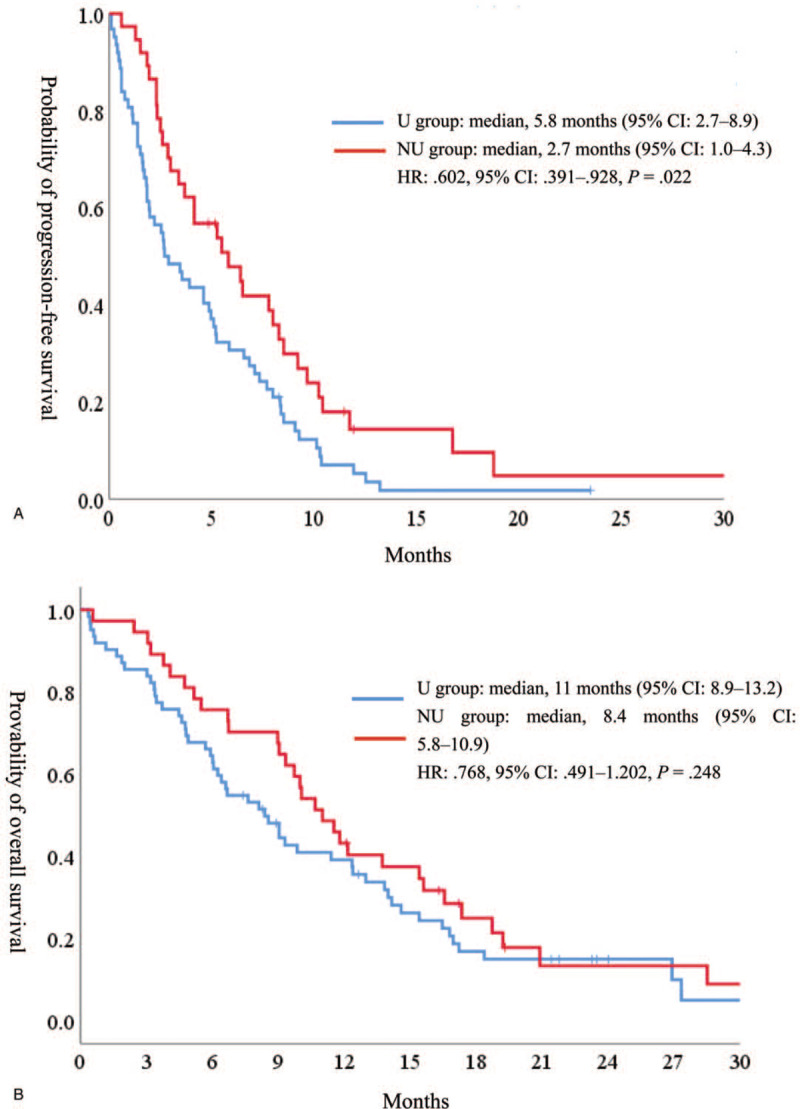
Kaplan–Meier curves for (A) progression-free survival and (B) overall survival.
Figure 3.
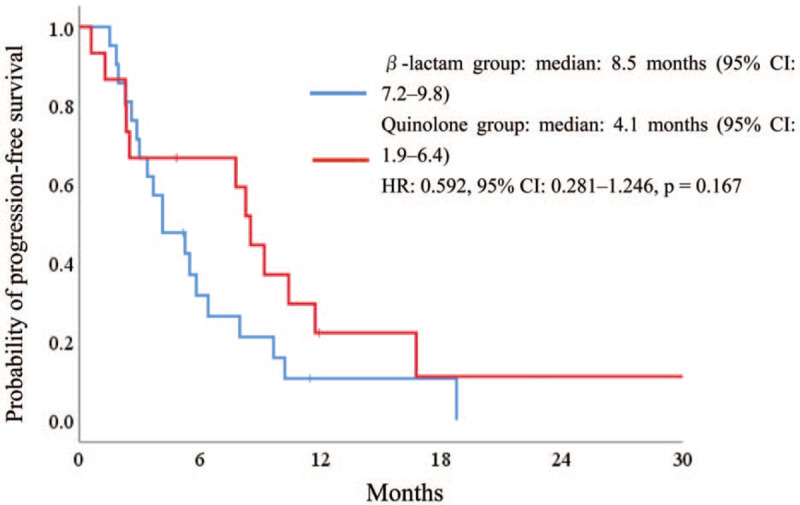
Kaplan–Meier curves for (A) progression-free survival and (B) overall survival according to the use of antibiotics.
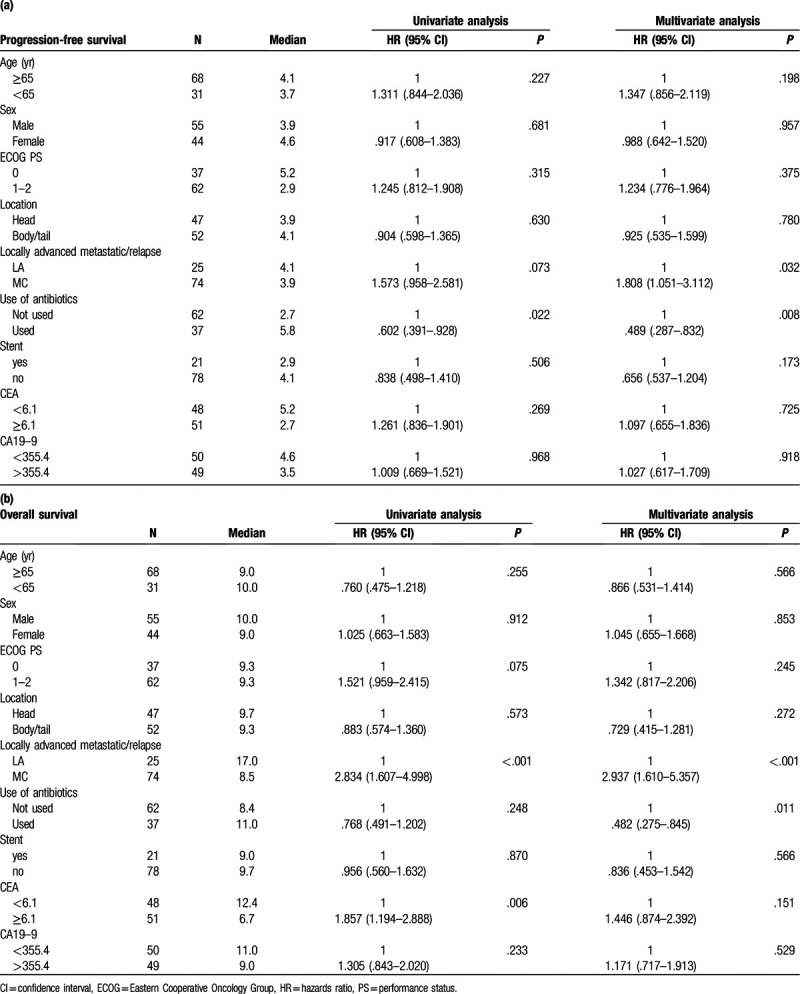
The univariate and multivariate analyses of PFS and OS are shown in Table 3. Locally advanced pancreatic cancer and antibiotic use were significantly associated with PFS and OS. Locally advanced pancreatic cancer and antibiotic use were independent prognostic factors for PFS and OS.
Table 3.
Univariate and multivariate analyses of (a) progression-free survival and (b) overall survival.
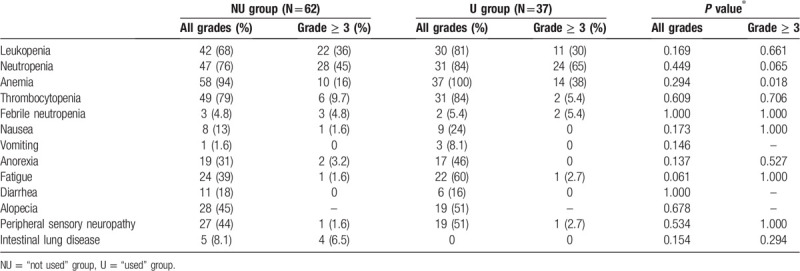
The treatment-related adverse events (AEs) are shown in Table 4. No patient died of a treatment-related cause. Severe anemia was significantly more frequently observed in the U group (P = .018) than in the NU group. The common (5% or higher) severe AEs were decreased white blood cell count (30% and 36%), neutropenia (65% and 45%), anemia (38% and 16%), thrombocytopenia (5.4% and 9.7%), febrile neutropenia (5.4% and 5.0%), and intestinal lung disease (0% and 6.5%) in the U and NU groups.
Table 4.
Safety profile.
4. Discussion
In this single center retrospective study, we showed that the use of antibiotics may be associated with a higher efficacy of GnP treatment as the first-line chemotherapy. Gemcitabine is a cytidine analog and an important drug for the treatment of various cancers, such as pancreatic,[2,3] biliary,[15,16] ovarian,[17,18] breast,[19] and lung cancers.[20,21] Gemcitabine is inactivated by CDD, which is abundant in the liver and muscle tissue.[22] In preclinical studies, CDD-related mechanisms, such as the upregulation of CDD,[23,24] have been attributed to gemcitabine resistance. nab-Paclitaxel, used with gemcitabine as the standard chemotherapy for pancreatic cancer, increased the activity of gemcitabine by reducing the CDD level in a mouse cancer model.[25] Therefore, we speculate that CDD is related to the efficacy and toxicity of gemcitabine. In clinical settings, neutrophil count and malnutrition are associated with serum CDD activity,[26] and serum CDD is a prognostic marker for gemcitabine/platinum-treated advanced nonsmall cell lung cancer.[27]
Recently, Geller et al reported that some bacteria, mainly Gammaproteobacteria members, possess a long isoform of CDD, CDDL, which inactivates gemcitabine and reduces its anticancer efficacy.[10] A few studies have revealed that patients with pancreatic cancer have more microorganisms in the pancreatic tissue than patients without pancreatic cancer, and the intratumor microbiome predominantly consists of Proteobacteria members.[10,28] This implies that these intratumor microorganisms inactivate gemcitabine and impair its anticancer effects. In clinical settings, a few studies have reported the association between the use of antibiotics and efficacy of gemcitabine. Sunakawa et al showed that the use of antibiotics before gemcitabine monotherapy in patients with advanced pancreatic cancer is associated with a higher efficacy of gemcitabine monotherapy.[11] Imai et al reported that the use of antibiotics during a gemcitabine-containing regimen for various cancers is associated with increased survival.[12] However, little is known about whether the use of antibiotics leads to a better clinical outcome of GnP.
Our study showed that the use of antibiotics during GnP was associated with increased survival. It also supports the possibility that the microbiome influences the efficacy of gemcitabine combination therapy. In addition, GnP may be a better target for antibiotic therapy among gemcitabine-containing therapies. Several studies have reported that both nab-paclitaxel[25,29,30] and gemcitabine[31] enhance the concentration of each other via a transport-based mechanism. Therefore, the use of antibiotics enhances the efficacy of not only gemcitabine, but also nab-paclitaxel. Furthermore, combination chemotherapy and intratumor microorganism manipulation by antibiotics can be potential strategies for the treatment of pancreatic cancer. The most effective type of antibiotics to modify the microbiome is still unclear; however, our results imply the β-lactam antibiotics are associated with a better outcome than quinolone antibiotics. Nevertheless, the use of antibiotics may prove to be a double-edged sword. A recent study reported that a high diversity of the intratumor microbiome in resected pancreatic tumor tissue is associated with a longer survival than a low diversity microbiome. It is not clear how the intratumor microbiome affects the prognosis of patients with pancreatic cancer, but this study showed that the intratumor microbiome may affect the clinical outcome by influencing immunogenicity.[32] Antibiotics generally disturb the diversity of the microbiome; thus, the use of antibiotics may lead to a lower survival rate. For example, several studies have revealed that the efficacy of immune checkpoint inhibitor therapy is associated with the gut microbiome and that a highly diverse microbiome is related to an improved response and increased survival.[33–35] Furthermore, the use of antibiotics is associated with a poor efficacy of immune checkpoint inhibitors.[36,37] Therefore, modifying the microbiome using antibiotics may lead to a better outcome in some cases, such as during gemcitabine-containing therapy, but it may also lead to a lower survival rate by disrupting microbiome diversity as a whole. An appropriate choice of antibiotics is needed to ensure better outcomes for patients.
As for safety, our study revealed that the use of antibiotics was not associated with an increase in antibiotic-related adverse events such as diarrhea. Although hematological adverse events showed a higher incidence, they probably reflected the infectious events in the U group. At least a short course of antibiotic therapy seems safe for patients who received chemotherapy.
Our study had some limitations. First, this was a single center retrospective study and had some biases. Second, the antibiotics used were different among patients; thus, it is not clear which antibiotic is better in enhancing the efficacy of gemcitabine. Finally, we did not evaluate the intratumor microorganisms; thus, we do not know whether they truly interfere with antibiotics. Therefore, a large cohort prospective study should be carried out to support the results.
In conclusion, our retrospective study showed that antibiotic use during GnP combination therapy was associated with increased survival. Although further research is needed in both basic and clinical settings, our results may lead to the use of antibiotics with GnP to treat advanced, gemcitabine-resistant pancreatic cancer in the future.
Acknowledgments
We thank the patients and their families for participating in the study. We would like to thank Editage (www.editage.com) for English language editing.
Author contributions
XXXX.
Footnotes
Abbreviations: CDD = cytidine deaminase, CI = confidence interval, GnP = gemcitabine plus nab-paclitaxel, HR = hazards ratio, NU = “not used” group, OS = overall survival, PFS = progression-free survival, U = “used” group.
How to cite this article: Nakano S, Komatsu Y, Kawamoto Y, Saito R, Ito K, Nakatsumi H, Yuki S, Sakamoto N. Association between the use of antibiotics and efficacy of gemcitabine plus nab-paclitaxel in advanced pancreatic cancer. Medicine. 2020;99:39(e22250).
The authors report no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].International Agency for Research on Cancer WHO. Global Cancer Observatory. Available at: https://gco.iarc.fr/ [access date November 21, 2019]. [Google Scholar]
- [2].Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25. [DOI] [PubMed] [Google Scholar]
- [3].Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Society JP. Clinical Practice Guidelines for Pancreatic Cancer 2019. Tokyo: Kanehara-shuppan; 2019. [Google Scholar]
- [5].Mizusawa J, Fukutomi A, Katayama H, et al. Protocol digest of randomized phase II study of modified FOLFIRINOX versus gemcitabine plus nab-paclitaxel combination therapy for locally advanced pancreatic cancer: Japan clinical oncology group study (JCOG1407). Pancreatology 2018;18:841–5. [DOI] [PubMed] [Google Scholar]
- [6].Wormann SM, Song L, Ai J, et al. Loss of P53 function activates JAK2-STAT3 signaling to promote pancreatic tumor growth, stroma modification, and gemcitabine resistance in mice and is associated with patient survival. Gastroenterology 2016;151:180–93.e112. [DOI] [PubMed] [Google Scholar]
- [7].Garrido-Laguna I, Uson M, Rajeshkumar NV, et al. Tumor engraftment in nude mice and enrichment in stroma-related gene pathways predict poor survival and resistance to gemcitabine in patients with pancreatic cancer. Clin Cancer Res 2011;17:5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Drakaki A, Iliopoulos D. MicroRNA-gene signaling pathways in pancreatic cancer. Biomedical J 2013;36:200–8. [DOI] [PubMed] [Google Scholar]
- [9].Amrutkar M, Gladhaug IP. Pancreatic cancer chemoresistance to gemcitabine. Cancers 2017;9:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Geller LT, Barzily-Rokni M, Danino T, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017;357:1156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sunakawa Y. Antibiotics may enhance the efficacy of gemcitabine treatment for advanced pancreatic cancer. Ann Oncol 2018;29: suppl_8: viii205–70. [Google Scholar]
- [12].Imai H, Saijo K, Komine K, et al. Antibiotic therapy augments the efficacy of gemcitabine-containing regimens for advanced cancer: a retrospective study. Cancer Manag Res 2019;11:7953–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- [14].Common terminology criteria for adverse events v4.0 (ctcae). Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcae_4_with_lay_terms.pdf [access date November 21, 2019]. [Google Scholar]
- [15].Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273–81. [DOI] [PubMed] [Google Scholar]
- [16].Morizane C, Okusaka T, Mizusawa J, et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol 2019;30:1950–8. [DOI] [PubMed] [Google Scholar]
- [17].Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 2012;30:2039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pfisterer J, Plante M, Vergote I, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol 2006;24:4699–707. [DOI] [PubMed] [Google Scholar]
- [19].Albain KS, Nag SM, Calderillo-Ruiz G, et al. Gemcitabine plus Paclitaxel versus Paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol 2008;26:3950–7. [DOI] [PubMed] [Google Scholar]
- [20].Treat JA, Gonin R, Socinski MA, et al. A randomized, phase III multicenter trial of gemcitabine in combination with carboplatin or paclitaxel versus paclitaxel plus carboplatin in patients with advanced or metastatic non-small-cell lung cancer. Ann Oncol 2010;21:540–7. [DOI] [PubMed] [Google Scholar]
- [21].Ohe Y, Ohashi Y, Kubota K, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol 2007;18:317–23. [DOI] [PubMed] [Google Scholar]
- [22].Ciccolini J, Serdjebi C, Peters GJ, et al. Pharmacokinetics and pharmacogenetics of Gemcitabine as a mainstay in adult and pediatric oncology: an EORTC-PAMM perspective. Cancer Chemother Pharmacol 2016;78:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Weizman N, Krelin Y, Shabtay-Orbach A, et al. Macrophages mediate gemcitabine resistance of pancreatic adenocarcinoma by upregulating cytidine deaminase. Oncogene 2014;33:3812–9. [DOI] [PubMed] [Google Scholar]
- [24].Funamizu N, Okamoto A, Kamata Y, et al. Is the resistance of gemcitabine for pancreatic cancer settled only by overexpression of deoxycytidine kinase? Oncol Rep 2010;23:471–5. [PubMed] [Google Scholar]
- [25].Frese KK, Neesse A, Cook N, et al. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov 2012;2:260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cohen R, Preta LH, Joste V, et al. Determinants of the interindividual variability in serum cytidine deaminase activity of patients with solid tumours. Br J Clin Pharmacol 2019;85:1227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tibaldi C, Camerini A, Tiseo M, et al. Cytidine deaminase enzymatic activity is a prognostic biomarker in gemcitabine/platinum-treated advanced non-small-cell lung cancer: a prospective validation study. Br J Cancer 2018;119:1326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pushalkar S, Hundeyin M, Daley D, et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov 2018;8:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 2011;29:4548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Awasthi N, Zhang C, Schwarz AM, et al. Comparative benefits of Nab-paclitaxel over gemcitabine or polysorbate-based docetaxel in experimental pancreatic cancer. Carcinogenesis 2013;34:2361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Borsoi C, Leonard F, Lee Y, et al. Gemcitabine enhances the transport of nanovector-albumin-bound paclitaxel in gemcitabine-resistant pancreatic ductal adenocarcinoma. Cancer Lett 2017;403:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Riquelme E, Zhang Y, Zhang L, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 2019;178:795–806.e712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018;359:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359:91–7. [DOI] [PubMed] [Google Scholar]
- [35].Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018;359:104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Khan U, Peña C, Brouwer J, et al. Impact of antibiotic use on response to treatment with immune checkpoint inhibitors. J Clinical Oncol 2019;37: 4_suppl: 143. [Google Scholar]
- [37].Pinato DJ, Howlett S, Ottaviani D, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


