This review describes methodology used in the assessment of the manifestations of exercise-induced hypoalgesia in humans and previous findings in individuals with and without pain. Possible mechanisms and future directions are discussed.
Keywords: Exercise, Hypoalgesia, Pain sensitivity, Mechanisms
Abstract
Exercise and physical activity is recommended treatment for a wide range of chronic pain conditions. In addition to several well-documented effects on physical and mental health, 8 to 12 weeks of exercise therapy can induce clinically relevant reductions in pain. However, exercise can also induce hypoalgesia after as little as 1 session, which is commonly referred to as exercise-induced hypoalgesia (EIH). In this review, we give a brief introduction to the methodology used in the assessment of EIH in humans followed by an overview of the findings from previous experimental studies investigating the pain response after acute and regular exercise in pain-free individuals and in individuals with different chronic pain conditions. Finally, we discuss potential mechanisms underlying the change in pain after exercise in pain-free individuals and in individuals with different chronic pain conditions, and how this may have implications for clinical exercise prescription as well as for future studies on EIH.
1. Introduction
Exercise is guideline recommended treatment for a range of chronic pain conditions.49 Regular exercise and physical activity in general have well-documented positive effects on a range of physical and mental health domains including cardiovascular health, stress, mood, sleep, and sexual health.146 In addition, clinically important reductions in pain are often observed after 8 to 12 weeks of exercise therapy163; however, as little as 1 session of exercise can induce hypoalgesia. This phenomenon is known as exercise-induced hypoalgesia (EIH).92,195 The first observation of EIH was published 40 years ago by Black et al..14 During the past few decades, the number of studies investigating the effect of exercise on pain has increased dramatically, likely reflecting the increasing burden of pain as well as the recognized role of exercise in the treatment of pain.
This article will begin with a brief introduction to the methodology used in the assessment of the manifestations and mechanisms of EIH in humans. The second part of the article will present an overview of the findings from previous experimental studies investigating changes in pain perception after acute and regular exercise in pain-free individuals and in individuals with different chronic pain conditions. Possible mechanisms underlying the response to exercise in pain-free individuals and in individuals with different chronic pain conditions will also be discussed. In the last part of the article, implications for exercise prescription and future EIH studies will be addressed.
1.1. Assessment of exercise-induced hypoalgesia—methodological considerations
The effect of a single bout of exercise on pain perception in humans has primarily been investigated experimentally in laboratory settings. The methods used in these investigations are diverse, incorporating different study designs and methods of pain assessment. Most often, EIH has been investigated using a within-group pre-post design, whereby participants' pain is assessed at different exercising and nonexercising body sites before and during/after exercise.130 Controlled studies using similar methodology but different designs (eg, crossover trials and parallel trials) have also been conducted.80,162,195,204 The results of these studies, especially those where participants were randomized to exercise or control, or where the order of exercise and control were randomized and counterbalanced for crossover trials, give a less biased estimate of the effect of a single bout of exercise on pain.
1.1.1. Pain threshold, intensity, and tolerance
Pain has been quantified in a variety of ways in studies of EIH, with quantitative sensory testing used most often. Quantitative sensory testing describes a series of tests that measure the perceptual responses to systematically applied and quantifiable sensory stimuli (usually pressure, thermal, or electrical).23 These tests typically involve the assessment of a person's pain threshold or pain tolerance which are, respectively, the minimum intensity of a stimulus that is perceived as painful and the maximum intensity to a noxious stimulus that the participant is willing to tolerate.116 Ratings of pain intensity and unpleasantness during exposure to various noxious stimuli might also be measured. As an example, pressure may be applied at an increasing intensity over the lower leg using an inflated cuff, with participants asked to rate the point at which this pressure becomes painful (threshold) and then endure it for as long as possible (tolerance) while rating its intensity or unpleasantness. Using this example, EIH could manifest as an increase in pain threshold, an increase in pain tolerance, and/or a reduction in ratings of pain intensity or unpleasantness. These measures are most commonly assessed in the immediate postexercise period (eg, 0–15 minutes), but some studies have measured pain 30 to 60 minutes after exercise cessation to investigate the persistence of EIH.69,103
1.1.2. Pain modulatory mechanisms
Methods that assess an individual's ability to modulate pain have been increasingly used in recent studies of EIH. These include temporal summation, spatial summation, conditioned pain modulation, and offset analgesia. Of these paradigms, temporal summation and conditioned pain modulation have been used most often. Temporal summation refers to an increase in pain after repetitive stimulation at the same intensity137 and is considered a behavioural correlate of wind-up—the frequency-dependent increase in C-fibre-evoked responses of dorsal horn neurons after repetitive stimulation at a constant intensity.63 Temporal summation paradigms provide information mostly about facilitatory mechanisms underlying nociceptive processes.23 By contrast, conditioned pain modulation provides an index of the strength of pain inhibition. Conditioned pain modulation (ie, “pain inhibits pain”) involves the application of 2 noxious stimuli over 2 different areas of the body, with the more pronounced noxious stimulus (conditioning stimulus) subsequently inhibiting the perception of the weaker noxious stimulus (test stimulus).211,212 Using these paradigms, EIH would manifest as a reduction in temporal summation and/or an increase in conditioned pain modulation, although evidence for the latter is limited.2,36,122
1.1.3. Nociceptive processing
Although not an assessment of pain per se, techniques that assess the function of the nociceptive pathways have sometimes been used to investigate EIH.38,80 These more complex methods, which include evoked potentials and neuroimaging, may provide greater insight into the mechanisms of EIH compared to more commonly used quantitative sensory tests. Evoked potentials are cortical responses recorded at the scalp using electroencephalography in response to brief and intense stimuli. Evoked potentials are described by their polarities (negative [N] and positive [P]), latencies, and amplitudes, and consist of early, late, and ultra-late components. When analysing pain-related evoked potentials, the peak-to-peak amplitude of the N2P2 is the component most related to nociception, whereby larger N2P2 amplitude is associated with more pain.71 There is evidence that both the sensory-discriminative and affective aspects of pain are captured by this late component of the evoked potential, and studies have shown exercise to reduce the amplitude of this component.72,145 Neuroimaging is widely used in the study of pain, but to the best of our knowledge, only 2 studies have used neuroimaging to investigate acute EIH.38,165 In one study, brain responses to noxious thermal stimuli before and after rest and exercise were measured using functional magnetic resonance imaging in women with fibromyalgia and healthy pain-free controls. The results suggested that, in the women with fibromyalgia, exercise-stimulated brain regions involved in descending pain inhibition which, in turn, was associated with lower pain ratings to thermal stimuli.38 In the second study, brain responses to noxious thermal stimuli before and after walking and running exercises were measured using functional magnetic resonance imaging in 20 athletes. The results suggested that running exercise reduced the pain-induced activation in the periaqueductal gray, a key area in descending pain inhibition which, in turn, was associated with lower pain unpleasantness ratings to thermal stimuli.165 Taken together, these results provide evidence that a single bout of exercise can modulate pain-related areas of the nervous system.
In addition to the different study designs and techniques used to quantify pain in investigations of EIH, the exercise protocols have also varied considerably. Aerobic and isometric exercise have been studied most often,130 whereas dynamic resistance exercise has not commonly been used. Within each mode of exercise, the prescription has varied too. For example, aerobic exercise has consisted of cycling, running, and stepping of various durations (30 seconds–30 minutes) and intensities (low to high).69,129,195 The same is true of isometric exercise where upper-limb and lower-limb exercise of both short and long duration (<5 seconds—exhaustion) and varied intensity (10%–100% MVC) have been studied.64,195 Studies of dynamic resistance exercise have typically used whole-body training at moderate intensities.17,93 Interestingly, EIH is reproducible with each type of exercise, even when modest doses are used.129,162 This is described in more detail below.
2. Pain outcomes after acute and regular exercise in pain-free individuals
As illustrated in Table 1, a single session of exercise has repeatedly been observed to reduce pain sensitivity in pain-free individuals. Hypoalgesia after aerobic exercises (eg, bicycling or running), dynamic resistance exercises (eg, circuit training), and isometric exercises (eg, a wall squat) often produces an increase in pressure pain thresholds at exercising body areas of 15% to 20% compared with a quiet rest control condition.192,200 Increases in pain thresholds can also be observed at nonexercising body areas, although larger hypoalgesic responses are consistently observed in areas closer to the exercising muscles compared with nonexercising muscle areas. The observed EIH response is short-lasting, often with a duration lasting from 5 minutes after exercise69 to 30 minutes after exercise88 and may depend on the modality of the pain test stimulus.
Table 1.
Summary of studies investigating acute exercise-induced hypoalgesia in pain-free individuals.
| Exercise type | Exercise form | Intensity | Duration | # of partici pants | Pain test modality | Pain outcome | Local site | Remote site | Findings | Year | Author |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aerobic | Bicycling | 70% HRmax | 30 min | 10 | Chemical | Pain intensity | Thigh | — | ↑Pain intensity (hyperalgesia) | 1984 | Vecchiet et al.205 |
| Aerobic | Bicycling | 50%–70% HRmax | 20 min | 91 | Cold | CPI | — | Hand | No hypoalgesia | 1992 | Padawer and Levine143 |
|
Aerobic Aerobic |
Bicycling Bicycling |
70%–75% VO2max VO2max test |
6 min 8–12 min |
41 25 |
Cold Cold |
CPT CPTol CPI |
— — |
Hand Arm |
↑CPT ↑CPTol ↓CPI |
2013 2018 |
Pokhrel et al.154 Chretien et al.18 |
|
Aerobic Aerobic |
Bicycling Bicycling |
50 W 100 W 150 W 200 W Increasing to 300W |
Max 8 min/step 15–30 min |
6 7 |
Electrical Electrical |
EPT EPT |
— — |
Tooth Tooth |
↑EPT ↑EPT |
1984 1985 |
Pertovaara et al.149 Kemppainen et al.87 |
| Aerobic | Bicycling | HR = 150/min | 20 min | 11 | Electrical | EPT | — | Tooth | ↑EPT | 1986 | Olausson et al.140 |
| Aerobic | Bicycling | Increasing to 300 W | Unknown | 6 | Electrical | EPT | — | Tooth | ↑EPT | 1986 | Kemppainen et al.86 |
| Aerobic | Bicycling | Increasing to 200 W | Unknown | 6 | Electrical | EPT | — | Tooth | ↑EPT | 1990 | Kemppainen et al.88 |
|
Aerobic |
Bicycling |
Increasing to 250 W |
Fatigue |
10 |
Electrical |
EPT |
— |
Tooth Hand |
↑EPT tooth ↑EPT hand |
1991 |
Droste et al.30 |
|
Aerobic |
Bicycling |
Increasing to VO2max |
Unknown |
17 |
Electrical |
EPT EPTol |
— |
Hand |
↑EPT ↑EPTol |
2005 |
Drury et al.33 |
|
Aerobic Aerobic Aerobic |
Bicycling Bicycling Bicycling |
1 KP 60 W Increasing to 200 W |
5 min 10 min Unknown |
60 21 28 |
Heat Heat Heat |
HPI TSPh HPI HPI |
— — — |
Foot Lower leg Hand Forearm Hand Hand |
↓HPI lower extremity ↓TSPh (lower extremity) ↓HPI ↓HPI |
2006 2014 2019 |
George et al.50 Ellingson et al.36 St-Aubin et al.175 |
| Aerobic | Bicycling | 75% VO2max | 30 min | 16 | Pressure | PPT PPI |
— | Hand | ↑PPT ↓PPI |
1996 | Koltyn et al.95 |
|
Aerobic Aerobic |
Bicycling Bicycling |
75% VO2max 1. 75% VO2max 2. 50% VO2max |
30 min 1. 10 min 1. 20 min 2. 10 min 2. 20 min |
20 80 |
Pressure Pressure |
PPI PPT |
— Thigh |
Hand Arm Shoulder |
No hypoalgesia ↑PPTs After 75% VO2max (10 and 20 min) |
2006 2014 |
Monnier-Benoit and Groslambert128 Vaegter et al.195 |
| Aerobic | Bicycling | 75% VO2max | 15 min | 56 | Pressure | PPT | Thigh | Shoulder | ↑PPTs | 2015 | Vaegter et al.198 |
| Aerobic | Bicycling | 75% VO2max | 15 min | 56 | Pressure | PPTol TSPp |
Lower leg | Arm | ↑PPTol lower leg ↓TSPp lower leg |
2015 | Vaegter et al.196 |
|
Aerobic |
Bicycling |
1. 75% VO2max 2. 50% VO2max |
20 min |
80 |
Pressure |
PPTol TSPp |
Lower leg |
Arm |
No hypoalgesia |
2015 |
Vaegter et al.196 |
|
Aerobic |
Bicycling |
1. 70% VO2max 2. 30% VO2max |
30 min |
10 |
Pressure |
PPT |
Thigh |
Forearm |
↑PPT thigh After 70% VO2max ↓PPT thigh and arm After 30% VO2max (hyperalgesia) |
2016 |
Micalos and Arendt-Nielsen124 |
|
Aerobic |
Bicycling |
Increasing to VO2max |
Fatigue |
50 |
Pressure |
PPT |
Knee |
Ankle Arm Chest Head |
↓PPT Chest (hyperalgesia) ↓PPT Head (hyperalgesia) |
2016 |
Kruger et al.103 |
|
Aerobic |
Bicycling |
RPE = 14–15 |
20 min |
40 |
Pressure |
PPT |
Thigh |
Shin Hand |
↑PPT thigh ↑PPT shin ↑PPT hand |
2017 |
Jones et al.81 |
|
Aerobic |
Bicycling |
RPE = 17 |
5 min |
36 |
Pressure |
PPT |
Thigh |
Hand |
↑PPT thigh ↑PPT hand |
2017 |
Jones et al.79 |
|
Aerobic |
Bicycling |
RPE = 16 |
15 min |
34 |
Pressure |
PPT |
Thigh |
Shoulder |
↑PPT thigh ↑PPT shoulder |
2018 |
Vaegter et al.193 |
|
Aerobic |
Bicycling |
1. HIIT: 90%–100% of max workload 2. MICT: 65%–75% of HR |
1. 10 × 1 min 2. 30 min |
28 |
Pressure |
PPT |
Thigh |
Shin Shoulder |
No hypoalgesia |
2018 |
Hakansson et al.58 |
|
Aerobic |
Bicycling |
75% VO2max |
15 min |
31 |
Pressure |
PPT |
Thigh |
Back Hand |
↑PPT thigh ↑PPT back ↑PPT hand |
2018 |
Gajsar et al.45 |
| Aerobic Aerobic |
Bicycling Bicycling |
50 W 75% VO2max |
12 min 15 min |
20 30 |
Pressure Pressure |
TSPp PPT |
Thigh Thigh |
Shoulder Back Hand |
↓TSPp trapezius ↑PPT thigh ↑PPT back |
2018 2019 |
Malfliet et al.118 Gomolka et al.54 |
| Aerobic | Bicycling | Lactate threshold | 15 min | 34 | Pressure | PPT | Thigh | Shoulder | ↑PPT thigh | 2019 | Vaegter et al.192 |
|
Aerobic |
Bicycling |
75%–88% HRmax |
20 min |
15 |
Pressure Electrical |
PPT EPI |
Thigh |
Shoulder Thoracic spine Hand Esophagus |
No hypoalgesia |
2017 |
van Weerdenburg et al.204 |
|
Aerobic |
Bicycling |
1. 70% HR max 2. 86% HR max |
1. 24 min 2. 4 × 4 min |
29 |
Pressure Heat |
PPT HPT HPI |
— |
Hand |
↓HPI after interval condition |
2014 |
Kodesh and Weissman-Fogel91 |
|
Aerobic |
Bicycling |
1. 70% HRR 2. 50%–55% HRR |
20 min |
27 |
Pressure Heat |
PPT PPI HPI TSPh |
— |
Forearm |
↑PPT after high intensity ↓HPI ↓TSPh |
2014 |
Naugle et al.132 |
|
Aerobic |
Bicycling |
Intensity = pain level 3/10 |
15 min |
16 |
Pressure Heat |
PPT HPT |
Thigh |
Hand |
↑PPT ↑HPT |
2016 |
Black et al.11 |
|
Aerobic |
Bicycling |
1. 75% VO2max 2. 50% VO2max |
25 min |
43 |
Pressure Heat |
PPT PPI HPI TSPh |
Forearm |
Forearm |
↑PPTs |
2016 |
Naugle et al.133 |
|
Aerobic |
Bicycling |
60–70 W |
20 min |
40 |
Pressure Heat |
PPT HPT TSPh |
Achilles |
— |
No hypoalgesia |
2016 |
Stackhouse176 |
|
Aerobic |
Bicycling |
70% HRR |
15 min |
16 |
Pressure Heat |
PPT HPT HPI |
Thigh |
Shin Foot |
↑PPT thigh ↑PPT shin ↓HPI foot |
2019 |
Jones et al.78 |
| Aerobic | Bicycling | 200 W | 20 min | 6 | Reflex | NFR | Thigh | — | ↑NFR | 1992 | Guieu et al.55 |
|
Aerobic |
Repeated back movements |
Lifting 5 kg |
7 min |
18 |
Pressure Heat Cold |
PPT HPT CPT TSPp |
Back |
Hand |
↑PPT back ↑CPT hand |
2019 |
Kuithan et al.104 |
|
Aerobic |
Running |
Near anaerobic threshold |
30 min |
27 |
Cold |
CPT CPI |
— |
Hand |
↑CPT |
2011 |
Wonders and Drury210 |
| Aerobic | Running | Unknown | 30 min | 22 | Heat | HPI | — | Forearm | No hypoalgesia | 1993 | Fuller and Robinson44 |
|
Aerobic |
Running |
Self-selected |
40 min |
1 |
Pressure |
PPT PTT |
— |
Arm |
↑PPT ↑PTT |
1979 |
Black et al.14 |
| Aerobic | Running | Self-selected | 1 mile | 15 | Pressure | PPT | — | Hand | ↑PPT hand | 1981 | Haier et al.57 |
| Aerobic | Running | VO2max test | Unknown | 29 | Pressure | PPI | — | Arm | ↓PPI | 2001 | Oktedalen et al.139 |
|
Aerobic |
Running |
1. 75% VO2max 2. 75% VO2max 3. 50% VO2max |
1. 10 min 2. 30 min 3. 10 min |
12 |
Pressure |
PPI |
— |
Hand |
↓PPI after 30 min at 75% VO2max |
2004 |
Hoffman et al.69 |
| Aerobic | Running | 65%–75% of HRR | 7 min | 12 | Pressure | PPT | — | Forearm | ↑PPT | 2004 | Drury et al.32 |
| Aerobic | Running | Unknown | 100 mile | 30 | Pressure | PPI | — | Hand | ↓PPI | 2007 | Hoffman et al.67 |
|
Aerobic |
Running |
VO2max test |
Unknown |
62 |
Pressure |
PPT |
Thigh |
Shoulder Hand |
↑PPT |
2015 |
Stolzman et al.179 |
|
Aerobic |
Running |
110% Gas exchange threshold |
30 min |
26 |
Pressure |
PPT |
Thigh |
Forearm |
↑PPT forearm ↑PPT thigh |
2019 |
Peterson et al.151 |
|
Aerobic |
Running |
85% VO2max |
44 min |
12 |
Pressure Heat Cold |
PPI HPI CPI CPT |
— |
Hand Arm |
↓HPI ↓PPI |
1984 |
Janal et al.74 |
|
Aerobic |
Running |
85% HRmax |
10 min |
63 |
Heat Cold |
HPT CPI |
— |
Hand Forearm |
↓HPT (hyperalgesia) ↓CPI |
2001 |
Sternberg et al.178 |
|
Aerobic |
Running |
75% VO2max |
30 min |
14 |
Heat Cold |
HPT CPT HPI CPI |
— |
Hand |
No hypoalgesia |
2005 |
Ruble et al.159 |
|
Aerobic |
Step |
63% VO2max |
12 min |
60 |
Pressure |
PPI PTT |
— |
Hand |
↓PPI ↑PTT |
1994 |
Gurevich et al.56 |
|
Aerobic |
Step |
50% of maximum number of steps in 1 minute |
5 min |
30 |
Pressure |
PPI TSPp |
— |
Forearm |
↓PPI ↓TSPp |
2019 |
Nasri-Heir et al.129 |
|
Aerobic Aerobic |
Walking Walking |
6.5 km/h Fast walking |
10 min 40 min 6 min |
5 35 |
Pressure Pressure |
PPT PPTol |
Thigh Calf |
Shoulder Shoulder |
No hypoalgesia ↑cPTT Calf |
2014 2019 |
Lee110 Hviid et al.73 |
|
Anaerobic |
Wingate test |
“All-out” |
30 seconds |
50 |
Pressure |
PPT |
— |
Shoulder Jaw |
↓PPTs (hyperalgesia) |
2012 |
Arroyo-Morales et al.3 |
| Anaerobic Anaerobic |
Bicycle Sprint Wingate test |
“All-out” “All-out” |
3 × 6 seconds 30 seconds |
12 50 |
Pressure Pressure Heat |
PPT PPT HPT TSPh TSPc |
Thigh Thigh |
Lower leg Hand |
↓PPTs (hyperalgesia) ↑PPT thigh ↑HPT hand ↓TSPh hand ↓TSPc hand |
2018 2018 |
Klich et al.89 Samuelly-Leichtag et al.162 |
| Dynamic resistance | Full-body circuit |
Moderate |
20 min |
17 |
Pressure |
PPT PPTol |
Shin |
— |
↑PPTol |
1996 |
Bartholomew et al.5 |
| Dynamic resistance | Full-body circuit |
75% 1RM |
4 exercises 3 × 10 repetitions (45 min) |
13 |
Pressure |
PPT PPI |
— |
Hand |
↑PPT ↓PPI |
1998 |
Koltyn and Arbogast93 |
| Dynamic resistance | Full-body circuit |
75% 1RM |
4 exercises 3 × 10 repetitions (45 min) |
21 |
Pressure |
PPT PPI |
— |
Hand |
↑PPT ↓PPI |
2009 |
Focht and Koltyn42 |
|
Dynamic resistance Dynamic resistance |
Upper-body circuit Full-body circuit |
Unknown 60% 1RM |
10 min 40 min 3 exercises 12 repetitions |
5 24 |
Pressure Pressure |
PPT PPT PPTol |
Shoulder — |
— Hand |
No hyperalgesia ↑PPTol |
2014 2017 |
Lee110 Baiamonte et al.4 |
| Dynamic resistance Dynamic resistance |
Kettlebell swings Full-body circuit |
8–12 kg 60% 1RM |
8 × 20 seconds 9 exercises 12 repetitions |
32 10 |
Pressure Pressure |
PPT PTT |
Lower back Buttock Hand |
— — |
↑PPTs ↑PTT hand |
2017 2018 |
Keilman et al.84 McKean et al.119 |
| Dynamic resistance | Handgrip | 100% MVC | 30 contractions in 1 minute | 12 | Pressure | PPT | — | Forearm | ↑PPT | 2004 | Drury et al.32 |
| Dynamic resistance | Handgrip | Medium | Maximum of 40 contractions in 1 minute | 48 | Heat | HPI | — | Hand | ↓HPI | 2008 | Weissman-Fogel et al.207 |
|
Dynamic ``Resistance |
Back extensions |
Bodyweight |
3 × 15 repetitions |
20 |
Heat |
HPI TSPh |
— |
Foot Lower leg Hand Forearm |
↓HPI (lower extremity) |
2006 |
George et al.50 |
|
Dynamic resistance |
Cervical flexions |
Head weight |
3 × 10 repetitions |
30 |
Pressure Heat |
PPT HPI TSPh |
— |
Foot Hand |
↑PPT ↓HPI |
2011 |
Bishop et al.10 |
| Eccentric Eccentric Eccentric |
Wrist extension Elbow flexion Heel-raise |
30% MVC Max Bodyweight |
5 × 10 repetitions 10 × 6 repetitions 4 × 15 contractions |
13 10 40 |
Pressure Pressure Electrical Pressure Heat |
PPT PPT EPT PPT HPT TSPh |
Forearm Arm Achilles |
— — — |
↑PPT ↓PPT ↓EPT (hyperalgesia) PPT ↓TSPh |
2010 2015 2016 |
Slater et al.171 Lau et al.108 Stackhouse et al.176 |
|
Isometric |
1. Knee extension 2. Elbow flexion |
1. 30% MVC 2. 60% MVC |
1. 90 seconds 1. 180 seconds 2. 90 seconds 2. 180 seconds |
80 |
Pressure |
PPT |
Thigh (knee extension) Arm (elbow flexion) |
Shoulder |
↑PPTs After low and high intensity exercises |
2014 |
Vaegter et al.195 |
|
Isometric |
1. Knee extension 2. Elbow flexion |
1. 30% MVC 2. 60% MVC |
3 min |
80 |
Pressure |
PPTol TSPp |
Lower leg |
Arm |
↑PPTol (after both elbow and knee exercises) ↓TSPp arm and leg (after low and high intensity exercises) |
2015 |
Vaegter et al.196 |
|
Isometric |
1. Knee extension 2. Elbow flexion |
20% of MVC |
Fatigue |
64 |
Pressure |
PPT PPI |
— |
Hand |
↑PPT after elbow flexion (women only) |
2016 |
Lemley et al.113 |
|
Isometric |
1. Knee extension 2. Shoulder rotation |
1. 1 kg 2. 0.5 kg |
Fatigue |
24 |
Pressure |
PPT |
Thigh Shoulder |
Shoulder `Thigh |
↑PPT thigh + shoulder both conditions |
2003 |
Kosek and Lundberg101 |
|
Isometric |
Back extension |
— |
2 min |
29 |
Pressure |
PPT |
Back |
Thigh Hand |
↑PPT thigh ↑PPT hand (women) |
2017 |
Gajsar et al.46 |
|
Isometric |
Elbow flexion |
1. Max contractions 2. 25% MVC 3. 25% MVC 4. 80% MVC |
1. 3 reps 2. Fatigue 3. 2 min 4. Fatigue |
40 |
Pressure |
PPT PPI |
— |
Hand |
↑PPT and ↓PPI after max and after 25% MVC until fatigue |
2008 |
Hoeger Bement et al.64 |
|
Isometric |
Elbow flexion |
25% MVC |
Fatigue |
20 |
Pressure |
PPT PPI |
Hand |
— |
↑PPT ↓PPI |
2009 |
Hoeger Bement et al.65 |
|
Isometric |
Elbow flexion |
25% MVC |
Fatigue |
26 |
Pressure |
PPT PPI |
Hand |
— |
↑PPT ↓PPI (men only) |
2014 |
Bement et al.7 |
|
Isometric |
Elbow flexion |
1. Max contractions 2. 25% MVC 3. 25% MVC |
1. 3 reps 2. Fatigue 3. 2 min |
24 |
Pressure |
PPT PPI |
Hand |
— |
↑PPT ↓PPI (women only) |
2014 |
Lemley et al.111 |
| Isometric | Elbow flexion | 25% MVC | Fatigue | 39 | Pressure | PPI | Hand | — | ↓PPI | 2014 | Lemley et al.112 |
|
Isometric |
Elbow flexion |
40% MVC |
3 min |
26 |
Pressure Heat |
PPT HPT |
Arm |
Hand |
↑PPTs |
2016 |
Jones et al.80 |
| Isometric | Arm abduction | 1 kg | Fatigue | 25 | Pressure | PPT | Shoulder | Shoulder | ↑PPTs | 2000 | Persson et al.147 |
|
Isometric |
Handgrip |
25% MVC |
2 min |
134 |
Cold |
CPT CPI |
— |
Hand |
↑CPT hand |
2017 |
Foxen-Craft and Dahlquist43 |
| Isometric | Handgrip | 25% MVC | 3 min | 34 | Electrical | EPI | — | Lower leg | ↓EPI | 2016 | Umeda et al.188 |
|
Isometric |
Handgrip |
1. 40% MVC 2. 25% MVC |
1. Fatigue 2. 3 min |
88 |
Heat |
TSPh |
Hand |
— |
↓TSPh for both conditions |
2013 |
Koltyn et al.96 |
|
Isometric |
Handgrip |
1. Maximal 2. 40%–50% MVC |
2 min |
31 |
Pressure |
PPT PPI |
Hand |
— |
↑PPT ↓PPI |
2001 |
Koltyn et al.97 |
|
Isometric |
Handgrip |
40%–50% MVC |
2 min |
40 |
Pressure |
PPT PPI |
Hand |
Hand |
↑PPT both sites ↓PPI both sites |
2007 |
Koltyn and Umeda98 |
| Isometric Isometric |
Handgrip Handgrip |
33% MVC 1. 25% MVC 2. 25% MVC |
3 min 1. 1 minute 2. 3 min |
79 23 |
Pressure Pressure |
PPTol PPT PPI |
Hand Hand |
— — |
↑PPTol No hypoalgesia |
2009 2009 |
Alghamdi and Al-Sheikh1 Umeda et al.190 |
|
Isometric |
Handgrip |
25% MVC |
1. 1 minute 2. 3 min 3. 5 min |
50 |
Pressure |
PPT PPI |
Hand |
— |
↑PPT and ↓PPI after all durations |
2010 |
Umeda et al.189 |
| Isometric | Handgrip | 50% MVC | Fatigue | 50 | Pressure | PPT | Forearm | Forearm | ↑PPT | 2017 | Black et al.12 |
| Isometric | Handgrip | 50% MVC | Fatigue | 26 | Pressure | PPT | Forearm | Thigh | ↑PPT forearm ↑PPT thigh |
2019 | Peterson et al.151 |
|
Isometric |
Handgrip |
1. 1% MVC 2. 15% MVC 3. 25% MVC |
Unknown |
2008 |
Electrical Reflex |
EPI NFR |
— |
Lower leg |
↓EPI after 15% and 25% MVC |
2008 |
Ring et al.157 |
|
Isometric |
Handgrip |
25% MVC |
3 min |
27 |
Pressure Heat |
PPT PPI HPI TSPh |
Forearm |
Forearm |
↑PPT ↓HPI (women) ↓TSPh |
2014 |
Naugle et al.131 |
|
Isometric |
Handgrip |
25% MVC |
3 min |
58 |
Pressure Heat |
PPT PPI TSPh |
Hand |
— |
↑PPT ↓PPI ↓TSPh |
2014 |
Koltyn et al.94 |
|
Isometric |
Handgrip |
25% MVC |
3 min |
43 |
Pressure Heat |
PPT PPI HPI TSPh |
Forearm |
Forearm |
↑PPT ↓TSPh |
2016 |
Naugle et al.133 |
|
Isometric |
Handgrip |
25% MVC |
3 min |
58 |
Pressure Heat |
PPT PPI TSPh |
Hand |
— |
↑PPT ↓PPI ↓TSPh |
2017 |
Brellenthin et al.16 |
|
Isometric |
Handgrip |
25% MVC |
3 min |
58 |
Pressure Heat |
PPI HPI |
Hand |
— |
↓PPI hand ↓HPI hand |
2018 |
Crombie et al.22 |
|
Isometric |
Handgrip |
25% MVC |
3 min |
52 |
Pressure Heat |
PPT HPI |
— |
Forearm |
↓PPT (hyperalgesia) |
2018 |
Ohlman et al.138 |
| Isometric | Knee extension | 21% MVC | Fatigue | 14 | Pressure | PPT | Thigh | — | ↑PPT | 1995 | Kosek and Ekholm99 |
| Isometric | Knee extension | 30% MVC | Fatigue | 134 | Pressure | PPT | — | Shoulder | ↑PPT | 2017 | Tour et al.185 |
|
Isometric |
Knee extension |
0.75 kg |
12 min |
15 |
Pressure Electrical |
PPT EPI |
Thigh |
Shoulder Thoracic spine Hand Esophagus |
No hypoalgesia |
2017 |
van Weerdenburg et al.204 |
|
Isometric |
Knee extension |
30% MVC |
3 min |
20 |
Pressure Heat |
PPT PPTol HPT |
— |
Lower leg |
↑PPTol |
2017 |
Vaegter et al.199 |
| Isometric | Knee extension | 20%–25% MVC | 5 min | Pressure Heat |
PPT PPI HPI |
Shin | Neck | ↑PPT shin | 2018 | Harris et al.61 | |
| Isometric | Pinch grip | 25% MVC | 15 seconds | 38 | Heat | HPI | Hand | Hand | No hypoalgesia | 2013 | Paris et al.144 |
|
Isometric |
Pinch grip |
1. 5% MVC 2. 25% MVC 3. 50% MVC |
15 seconds |
42 |
Heat |
HPI |
Hand |
Hand |
↓HPI with larger effects for higher intensity |
2014 |
Misra et al.127 |
| Isometric | Teeth-clenching | — | Fatigue | 33 | Pressure | PPT | Jaw | Forearm | ↑PPT jaw | 2019 | Lanefelt et al.106 |
| Isometric | Trunk flexion | — | Fatigue | 70 | Pressure | PPT | Abdomen | Nailbed | ↑PPT Abdomen | 2019 | Deering et al.27 |
|
Isometric |
Wall squat |
— |
3 min |
35 |
Pressure |
PPT |
Thigh |
Shoulder |
↑PPT thigh ↑PPT shoulder |
2019 |
Vaegter et al.200 |
The table is organized according to exercise type, exercise form, pain test modality, and year of publication.
CPI, cold pain intensity; CPT, cold pain threshold; EPI, electrical pain intensity; EPT, electrical pain threshold; EPTol, electrical pain tolerance; HIIT, high-intensity interval training; HPI, heat pain intensity; HPT, heat pain threshold; HRmax, maximum heart rate; HRR, heart rate reserve; MICT, moderate-intensity continuous training; MVC, maximal voluntary contraction; NFR, nociceptive flexion reflex; PPI, pressure pain intensity; PPT, pressure pain threshold; PPTol, pressure pain tolerance; RM, repetition maximum; RPE, rating of perceived exertion; TSPc, temporal summation of cold pain; TSPh, temporal summation of heat pain; TSPp, temporal summation of pressure pain; VO2max, maximal aerobic capacity.
2.1. Exercise intensity and duration
The hypoalgesic responses seem to be similar between exercise types,133,195 although EIH differences have been observed,32 but exercise intensity and duration quite consistently affect the EIH response. Exercise intensity affects the EIH response after aerobic exercise.69,124,132,195 For example, in 80 pain-free individuals, it was observed that a moderate-to-high intensity bicycling exercise produced significantly larger EIH responses at the exercising quadriceps muscle, as well as at the nonexercising biceps and trapezius muscles, compared with a low-intensity bicycling exercise.195 Findings on the influence of aerobic exercise duration are more equivocal, with one study observing a dose-response with larger effects after bicycling for 30 minutes compared with 10 minutes,69 and one study observing no difference between bicycling for 10 minutes compared with 20 minutes.195 Moreover, the fact that very short-duration aerobic exercise can elicit EIH129,162 implies that intensity, or the combination of intensity and duration, may be more important for determining the size of EIH after aerobic exercise than either variable alone.
Exercise intensity and duration may also affect the EIH response after isometric exercises,64,127,157 although the results are more inconsistent. In 40 individuals, pressure pain thresholds at the hand were increased and pressure pain intensity was decreased after low-intensity (25% of maximal voluntary contraction [MVC]) isometric elbow flexion until exhaustion. However, no hypoalgesia was observed when the contraction was held for only 2 minutes.64 By contrast, hypoalgesia was found after 90 and 180 seconds isometric knee extensions and elbow flexion exercises at 30% MVC and 60%, respectively, in 80 healthy individuals; however, the hypoalgesic responses were not different in magnitude between low-intensity and high-intensity contractions nor between shorter or longer durations.195 The fact that very low doses of isometric exercise (eg, three maximal contractions of 5-second duration, totaling 15 seconds of exercise) can produce EIH64 lends further support to the lack of clear dose-response, which is further evidenced by a study of 50 individuals where elevations in pain threshold were not different between isometric handgrip exercises at 25% MVC for 1, 3, or 5 minutes.189
2.2. Effects on pain modulatory mechanisms
As described, robust increases in pressure pain thresholds are observed after exercise, but exercise can also affect spinal and supraspinal mechanisms of pain. Temporal summation of pressure and heat pain was reduced after submaximal isometric exercises at 25% to 40% of MVC for 3 minutes,94,96,131,196 and 20 minutes of aerobic exercise at 55% to 70% of heart rate reserve reduced temporal summation of heat pain132; however, temporal summation of pressure pain was not affected by 15 to 20 minutes of aerobic exercise at 50% to 75% of VO2max.196 However, not all studies have shown exercise to have positive effects on pain mechanisms. For example, Alsouhibani et al. observed a decrease in the CPM response after exercise.2 By contrast, other studies have found exercise to have no effect on CPM122 or offset analgesia,61 suggesting that exercise can, but does not always, influence spinal and supraspinal mechanisms of pain. Exercise can also influence the ability to cope with pain. The perceived pain intensity of a suprathreshold stimulus is consistently reduced by aerobic, isometric, and dynamic resistance exercises,42,64,98 and acute exercise can reduce ratings of pain unpleasantness even in the absence of a change in pain intensity.80 In addition, low-intensity nonpainful aerobic and isometric exercises also increase the tolerance to a painful stimulus. A 20% increase in pain tolerance was observed by Vaegter et al.199 after a 3-minute submaximal isometric knee extension exercise, and after a 6-minute walking exercise73 compared with rest in 35 pain-free individuals.
2.3. Factors influencing exercise-induced hypoalgesia
Exercise that produces acute hypoalgesia is often perceived as moderately painful with peak pain intensity ratings around 5 or 6 on a 0 to 10 numerical rating scale,193,200 and painful exercises seem to have larger hypoalgesic effects than nonpainful exercises, at least in pain-free individuals,36 but perhaps not in individuals with chronic pain.20,173
Treatment expectations are a well-recognized factor known to modulate treatment outcomes and the information about the effect of exercise given to individuals before exercise influences the magnitude of the EIH response. First, a randomized controlled trial by Jones et al.81 observed that the hypoalgesic effect after bicycling was slightly increased if positive information about EIH was given before the exercise compared to when no EIH information was given before exercise. Second, a randomized controlled trial by Vaegter et al. comparing positive vs negative pre-exercise information observed a 22% increase in pain thresholds in the positive information group, whereas the negative information group had a 4% decrease (hyperalgesia) in pain threshold at the exercising muscle (Vaegter et al., in review). Both studies observed a positive correlation between expectations and hypoalgesia after exercise.
Despite robust hypoalgesia after exercise on a group level, the response to exercise is not identical across individuals and across days. Several studies have investigated the stability of the EIH response in pain-free individuals across different days using a number of aerobic54,73,192,193 and isometric200 exercise protocols. Across protocols, some individuals consistently show hypoalgesia after exercise, some individuals consistently showed hyperalgesia after exercise, and some individuals had a change in their response from hypoalgesic to hyperalgesic or vice versa between days. Interestingly, the majority of individuals showed hypoalgesia at some point.
2.4. Regular exercise and pain
The effect of regular exercise and physical activity on pain sensitivity has been investigated, albeit less than the effect of a single session of exercise. In pain-free individuals, there have been relatively few studies investigating whether those who are more physically active experience greater EIH. The results of these studies show that EIH is usually similar between inactive and active pain-free individuals irrespective of the type of exercise they regularly perform (ie, aerobic or strength training) and the methods used to assess physical activity (ie, self-report or objectively measured using accelerometry).12,188,198 However, Ellingson et al.35 observed lower pain intensity ratings and lower pain unpleasantness ratings to suprathreshold heat pain stimulations in pain-free women who were physically active as defined by the current public health recommendations compared with women who were less physically active than recommended. There is also some evidence that individuals who are more physically fit experience greater EIH.138,166
Regarding the effect of a longer period of exercise training in pain-free individuals, Hakansson et al.58 observed changes in PPT in the legs after 6 weeks of moderate bicycling exercises (3 times/week) but not after high-intensity interval exercise. In addition, Jones et al.76 observed increases in pressure pain tolerance but not pain threshold after bicycling 30 minutes at 75% of VO2 max 3 times/week for 6 weeks compared with a control condition. These findings suggest that regular exercise in pain-free individuals specifically influences the ability to cope with pain (ie, pain perception above the pain threshold) rather than the level at which pain is first perceived (pain threshold). Similar observations have been found in athletes compared with less active individuals. A systematic review with meta-analysis by Tesarz et al.182 showed consistently higher pain tolerance across different pain modalities (ie, pressure, heat, cold, electrical, and ischemic) in athletes; however, for pain thresholds, the conclusion was less consistent.
In addition to the effect on pain tolerance, regular exercise may also affect the ability to inhibit pain as assessed by the CPM paradigm. Naugle et al. observed that pain-free individuals reporting more regular physical activity also had a larger CPM response compared with individuals reporting less regular physical activity.134,135 Although previous investigations on CPM in athletes have been equivocal because increased CPM52 as well as decreased CPM181 has been observed, the positive effect of regular exercise on CPM may be a potential mechanism underlying the preventive effect of exercise on pain because better CPM capacity has been associated with a reduced risk of chronic pain.211 The preventive effect of regular exercise is supported by a recent systematic review with meta-analysis concluding that regular exercise performed 2 to 3 times/week reduces the risk of low back pain by 33%.169 This is true even in those who are at an increased risk of developing chronic pain.115
3. Pain outcomes after acute and regular exercise in individuals with chronic pain
In individuals with different chronic pain conditions, the response to a single session of exercise is less consistent as hypoalgesia, reduced hypoalgesia, or even hyperalgesia (ie, increased sensitivity to pain) has been observed. As illustrated in Table 2, hypoalgesia after exercise has, eg, been observed in individuals with chronic musculoskeletal pain,123,197 shoulder pain,105 patella femoral pain,180 knee osteoarthritis,59,194 menstrual pain,186 and rheumatoid arthritis.117 However, reduced EIH responses or even hyperalgesia after exercise has often been demonstrated in individuals with whiplash-associated disorder,203 ME/CFS,123,202 fibromyalgia pain,100,107,177 painful diabetic neuropathy,90 chronic musculoskeletal pain,19 and also in a delayed-onset muscular soreness pain model.25 Hyperalgesia after exercise is often observed in individuals with more widespread chronic pain conditions. This was first observed by Kosek et al.100 in 5 individuals with fibromyalgia who showed a decrease in pain thresholds during and after an isometric knee extension exercise. The observation of hypoalgesia after exercise in some groups with chronic pain conditions and the observation of hyperalgesia after exercise in other groups with chronic pain may be influenced by whether the exercise is performed using a painful or nonpainful body area. Lannersten and Kosek107 observed hypoalgesia after a 5-minute submaximal (25% of MVC) isometric exercise in individuals with shoulder myalgia when the exercise was performed by a nonpainful leg muscle but when the exercise was performed by the painful shoulder muscle, no hypoalgesic response was observed. Similarly, Burrows et al.17 observed increases in pressure pain threshold after upper-body but not lower-body resistance exercise in people with knee osteoarthritis. These findings suggest that hypoalgesia can be induced by exercising nonpainful muscles in subjects with chronic pain,191 which may have important implications for exercise prescription in the clinical setting.
Table 2.
Summary of studies investigating acute exercise-induced hypoalgesia in individuals with different pain conditions.
| Exercise type | Exercise form | Intensity | Duration | # of participants | Pain condition | Pain test modality | Pain outcome | Local site | Remote site | Findings | Year | Author |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aerobic | Bicycling | Increasing to 75% HRmax | Unknown | 20 | ME/CFS | Clinical | Pain intensity | — | — | No hypoalgesia | 2017 | Oosterwijck et al.141 |
| Aerobic | Bicycling | VO2max test | 8–12 min | 25 | Chronic pain | Cold | CPI | — | Arm | No hypoalgesia | 2018 | Chretien et al.18 |
|
Aerobic |
Bicycling |
1 KPa |
5 min |
12 |
Chronic Low back pain |
Heat |
HPI TSPh |
— |
Forearm Lower leg |
↓ TSPh forearm |
2009 |
Bialosky et al.9 |
| Aerobic | Bicycling | 80% VO2max | 30 min | 23 | DOMS MODEL | Pressure | PPT | — | Arm | No hypoalgesia | 2002 | Dannecker et al.25 |
| Aerobic | Bicycling | 70% VO2max | 20 min | 8 | Chronic low back pain | Pressure | PPI | — | Hand | ↓PPI | 2005 | Hoffman et al.68 |
|
Aerobic |
Bicycling |
Increasing to 130 W |
37 min |
26 |
Chronic fatigue syndrome |
Pressure |
PPT |
Lower leg |
Hand Lower back Shoulder |
↓PPTs (hyperalgesia) |
2010 |
Meeus et al.123 |
|
Aerobic |
Bicycling |
Increasing to 130 W |
37 min |
21 |
Chronic low back pain |
Pressure |
PPT |
Lower leg |
Hand Lower back Shoulder |
↑PPTs |
2010 |
Meeus et al.123 |
|
Aerobic |
Bicycling |
1. 75% HRmax 2. Self-paced |
Unknown |
22 |
hronic fatigue syndrome |
Pressure |
PPT |
Lower leg |
Hand Lower back |
↑PPT lower back (after self-paced) ↓PPTs calf/hand (after self-paced) (hyperalgesia) ↓PPTs (after 75% HRmax) (hyperalgesia) |
2010 |
Van Oosterwijck et al.202 |
|
Aerobic |
Bicycling |
1. Increasing to 75% HRmax 2. Self-paced |
1. Unknown 2. Individual |
20 |
ME/CFS |
Pressure |
PPT |
Lower leg |
Hand Lower back |
No hypoalgesia/some hyperalgesia |
2010 |
Van Oosterwijck et al.202 |
|
Aerobic |
Bicycling |
1. 62% HRmax 2. Self-paced |
20 min |
21 |
Fibromyalgia |
Pressure |
PPT PPI PPTol |
— |
Hand |
↑PPT and PPTol (both conditions) ↓PPI (both conditions) |
2011 |
Newcomb et al.136 |
|
Aerobic |
Bicycling |
1. 75% HRmax 2. Self-paced |
Unknown |
22 |
WAD |
Pressure |
PPT |
Lower leg |
Hand Lower back |
↑PPT lower back (after self-paced) ↓PPTs calf/hand (after self-paced) (hyperalgesia) ↓PPTs (after 75% HRmax) (hyperalgesia) |
2012 |
Van Oosterwijck et al.203 |
| Aerobic | Bicycling | Increasing to 75% HRmax | Maximum of 15 min | 19 | Fibromyalgia with chronic fatigue | Pressure | TSPp | — | Shoulder Hand |
No hypoalgesia | 2015 | Meeus et al.122 |
| Aerobic | Bicycling | Increasing to 75% HRmax | Maximum of 15 min | 16 | RA | Pressure | TSPp | — | Shoulder Hand |
No hypoalgesia | 2015 | Meeus et al.122 |
|
Aerobic |
Bicycling |
75% of VO2max |
15 min |
61 |
Chronic MSK pain |
Pressure |
PPT PTTol TSPp |
Thigh |
Arm Shoulder Lower leg |
↑PPTs ↑PPTol ↑TSPp (in high pain sensitive patients) |
2016 |
Vaegter et al.197 |
|
Aerobic Aerobic |
Bicycling Bicycling |
1. 70% HRmax 2. 75%–85% HRmax 75% of VO2max |
1. Continuous 20 min 2. Interval 5 × 4 min 15 min |
15 14 |
Chronic fatigue syndrome Knee OA |
Pressure Pressure |
PPT PTTol |
Thigh Thigh |
Shoulder Hand Arm Shoulder Lower leg |
↑PPT thigh after interval ↑PPTs |
2016 2017 |
Sandler et al.164 Vaegter et al.194 |
|
Aerobic |
Bicycling |
75% of HRmax |
30 min |
21 |
WAD |
Pressure |
PPT |
— |
Neck Shin |
No hypoalgesia |
2017 |
Smith et al.172 |
|
Aerobic Aerobic |
Bicycling Bicycling |
Increasing to 75% HRmax 50 W |
Unknown 12 min |
40 20 |
Knee OA Chronic fatigue syndrome |
Pressure Pressure |
PPT TSPp |
Thigh Knee Thigh |
Forearm Shoulder |
↑PPTs (if normal CPM) ↓PPTs (if abnormal CPM) No hyperalgesia |
2017 2018 |
Fingleton et al.40 Malfliet et al.118 |
|
Aerobic |
Bicycling |
70% VO2max |
30 min |
27 |
Gulf veterans |
Pressure Heat |
PPT HPI |
— |
Hand |
↑HPI (if pain) (hyperalgesia) |
2010 |
Cook et al.19 |
|
Aerobic |
Running |
Bruce test |
Fatigue |
10 |
Fibromyalgia |
Heat |
TSPh |
— |
Hands |
↑TSPh (hyperalgesia) |
2001 |
Vierck et al.206 |
|
Aerobic |
Running |
5 km/hour |
3 × 5min |
5 |
Chronic fatigue syndrome |
Pressure |
PPT |
— |
Hands |
↓PPTs (hyperalgesia) |
2004 |
Whiteside et al.208 |
|
Aerobic Aerobic |
Walking Walking |
Self-selected 1. Continuous 1.3 m/second 2. Interval 1.3 m/second |
4 min 1. 45 min 2. 3 × 15 min |
20 27 |
Plantar fasciopathy Knee OA |
Clinical pain PPT Clinical pain |
Pain intensity during test PPT Pain intensity |
Heel — |
— — |
No hypoalgesia ↑Pain intensity continuous walking (hyperalgesia) |
2018 2017 |
Riel et al.156 Farrokhi et al.39 |
|
Aerobic |
Stepping |
50% of maximum number of steps in 1 minute |
5 min |
30 |
TMD |
Pressure |
PPI TSPp |
— |
Forearm |
↓TSPp |
2019 |
Nasri-Heir et al.129 |
| Dynamic resistance | Leg exercises | 1. 60% 1RM 2. Self-selected |
2 exercises 6 × 10 repetitions |
32 |
Fibromyalgia |
Clinical |
Pain intensity |
— |
— |
Hyperalgesia |
2018 |
da Cunha Ribeiro et al.24 |
|
Dynamic resistance Dynamic resistance |
Knee extensions Knee extensions |
1RM 8RM |
6 × 10 repetitions 1 exercise 3 × 8 repetitions |
20 21 |
Knee OA Patellar tendinopathy |
Clinical Clinical pressure |
Pain intensity DOMS Pain intensity during SLS PPT |
Knee Knee shin |
— Forearm |
No change in pain intensity More DOMS than controls ↓Pain intensity ↑PPT shin |
2013 2019 |
Germanou et al.51 Holden et al.70 |
| Dynamic resistance | Arm-raises | Fast | 6 min | 24 | Knee OA | Pressure | PPT | Shoulder | Thigh | ↑PPT shoulder | 2020 | Hansen et al.59 |
|
Dynamic resistance |
1. Hip abductions 2. Knee extensions |
Load = 12RM |
3 exercises 12 repetitions |
30 |
PFP |
Pressure |
PPT PTTol TSPp |
Knee Lower leg |
Elbow (PPT) |
↑PPT (lower leg) ↑PPTol (after knee exercises) |
2019 |
Straszek et al.180 |
|
Dynamic resistance |
Lower-body circuit |
60% 1RM |
3 exercises 10 repetitions |
11 |
Knee OA |
Pressure |
PPT PPTol |
Thigh Knee Shin |
Shoulder Arm Forearm Hand |
No hypoalgesia |
2014 |
Burrows et al.17 |
|
Dynamic Resistance |
Upper-body circuit |
60% 1RM |
3 exercises 10 repetitions |
11 |
Knee OA |
Pressure |
PPT PPTol |
Shoulder Arm Forearm Hand |
Thigh Knee Shin |
↑PPTs (across sites) |
2014 |
Burrows et al.17 |
| Dynamic resistance Dynamic resistance |
Back extensions Repeated back movements |
Bodyweight Lifting 5 kg |
3 × 15 repetitions 7 min |
12 18 |
Chronic low back pain Chronic low back pain |
Heat Pressure Heat Cold |
HPI TSPh PPT HPT CPT TSPp |
— Back |
Forearm Lower Leg Hand |
↓TSPb forearm ↑CPT hand |
2009 2019 |
Bialosky et al.9 Kuithan et al.104 |
|
Dynamic resistance |
Cervical flexion |
Head weight |
10 × 10 seconds |
13 |
Chronic neck pain |
Clinical pain Pressure |
Pain intensity PPT |
Neck |
Shoulder |
↓Pain intensity ↑PPTs |
2018 |
Galindez-Ibarbengoetxea et al.47 |
|
Isometric |
Elbow flexion |
1. 25% MVC 2. 25% MVC 3. 100% MVC |
1. 2 min 2. Fatigue 3. 3 reps |
15 |
Fibromyalgia |
Pressure |
PPT PPI |
— |
Hand |
No hypoalgesia |
2011 |
Hoeger Bement et al.66 |
|
Isometric |
Handgrip |
25% MVC |
3 min |
18 |
Diabetic neuropathy |
Heat |
HPI TSPh |
Hand Forearm |
— |
↓HPI and TSPh (if no pain) No changes (if pain) |
2014 |
Knauf and Koltyn90 |
|
Isometric |
Handgrip |
25% MVC |
3 min |
64 |
Menstrual pain |
Pressure |
PPT |
Forearm Shin |
↑PPTs |
2018 |
Travers et al.186 |
|
|
Isometric |
Handgrip |
30% MVC |
90 seconds |
12 |
Fibromyalgia |
Pressure Heat |
PPT HPI |
Forearm |
Forearm |
↓PPTs ↑HPI (hyperalgesia) |
2005 |
Staud et al.177 |
| Isometric | Knee extension | 20%–25% MVC | Fatigue | 14 | Fibromyalgia | Pressure | PPT | Thigh | — | ↓PPT (hyperalgesia) | 1996 | Kosek et al.100 |
| Isometric | Knee extension | 10%–15% MVC | Fatigue | 17 | Fibromyalgia | Pressure | PPT | Thigh | Shoulder | ↑PPT (shoulder) | 2007 | Kadetoff and Kosek82 |
| Isometric | Knee extension | 50% MVC | Fatigue | 66 | Knee OA | Pressure | PPT | Thigh | Shoulder | ↑PPTs | 2013 | Kosek et al.102 |
| Isometric | Knee extension | 50% MVC | Fatigue | 47 | Hip OA | Pressure | PPT | Thigh | Shoulder | ↑PPTs | 2013 | Kosek et al.102 |
|
Isometric |
Knee extension |
30% MVC |
90 seconds |
61 |
Chronic MSK pain |
Pressure |
PPT PTTol TSPp |
Thigh |
Arm Shoulder Lower leg |
↑PPTs ↑PPTol |
2016 |
Vaegter et al.197 |
|
Isometric |
Knee extension |
30% MVC |
90 seconds |
14 |
Knee OA |
Pressure |
PPT PTTol |
Thigh |
Arm Shoulder Lower leg |
↑PPTs |
2017 |
Vaegter et al.194 |
|
Isometric |
Knee extension |
10% MVC |
5 min |
40 |
Knee OA |
Pressure |
PPT |
Thigh Knee |
Forearm |
↑PPTs (if normal CPM) ↓PPTs (if abnormal CPM) |
2017 |
Fingleton et al.40 |
|
Isometric Isometric |
Knee extension Knee extension |
30% MVC 30% MVC |
Fatigue 5 min |
130 46 |
Fibromyalgia RA |
Pressure Pressure |
PPT PPT |
— Thigh |
Shoulder Shoulder |
↑PPT ↑PPTs |
2017 2018 |
Tour et al.185 Lofgren et al.117 |
|
Isometric |
Knee extension |
70% MVC |
5 × 45 seconds |
21 |
Patellar tendinopathy |
Pressure clinical |
Pain intensity during SLS PPT |
Knee Shin |
Forearm |
↓Pain intensity ↑PPT shin |
2019 |
Holden et al.70 |
|
Isometric |
1. Knee extension 2. Shoulder rotation |
20%–25% MVC |
Fatigue |
20 |
Shoulder pain |
Pressure |
PPT |
Thigh Shoulder |
Shoulder Thigh |
↑PPTs (during knee extension) |
2010 |
Lannersten and Kosek107 |
|
Isometric |
1. Knee extension 2. Shoulder rotation |
20%–25% MVC |
Fatigue |
20 |
Fibromyalgia |
Pressure |
PPT |
Thigh Shoulder |
Shoulder Thigh |
No hypoalgesia |
2010 |
Lannersten and Kosek107 |
| Isometric | Shoulder abduction | 1 kg | Fatigue | 19 | Chronic shoulder pain | Pressure | PPT | Shoulder | — | ↑PPT | 2003 | Persson et al.148 |
| Isometric | Shoulder abduction | Weight of arms | Fatigue | 22 | Fibromyalgia | Pressure | PPT | Shoulder | Shin | ↓PPT shin (hyperalgesia) | 2012 | Ge et al.48 |
| Isometric | Shoulder abduction | 20%–25% MVC | 5 min | 24 | Shoulder pain | Pressure | PPT | Shoulder | Thigh Shin |
↑PPTs | 2016 | Kuppens et al.105 |
| Isometric | Squat | 70% MVC | 1 exercise 5 × 45 sec repetitions | 6 | Patella tendinopathy | Clinical | Pain intensity during SLS | — | — | ↓Pain intensity | 2015 | Rio et al.158 |
| Isometric | Tooth clenching | — | Fatigue | 20 | TMD | Pressure | PPT | Jaw | Forearm | ↑PPT jaw | 2019 | Lanefelt et al.106 |
|
Isometric |
Wall squat |
Bodyweight |
3 min |
21 |
WAD |
Pressure |
PPT |
— |
Neck Shin |
↑PPTs |
2017 |
Smith et al.172 |
The table is organized according to exercise type, exercise form, pain test modality, and year of publication.
DOMS, delayed-onset muscle soreness; HPI, heat pain intensity; HPT, heat pain threshold; HRmax, maximum heart rate; MVC, maximal voluntary contraction; PPI, pressure pain intensity; PPT, pressure pain threshold; PPTol, pressure pain tolerance; RM, repetition maximum; RPE, rating of perceived exertion; SLS, single-leg stand; TSPh, temporal summation of heat pain; TSPp, temporal summation of pressure pain; VO2max, maximal aerobic capacity.
3.1. Factors related to lack of exercise-induced hypoalgesia
Individuals with facilitated central pain mechanisms, which are commonly observed in several chronic musculoskeletal pain conditions,121 often report reduced hypoalgesia after exercise. Vaegter et al.197 observed reduced EIH after submaximal isometric exercise and after bicycling exercise in chronic pain patients with high widespread pain sensitivity compared with patients with low pain sensitivity. In addition, in high pain-sensitive patients, an increase in temporal summation of pain was observed after aerobic exercise177,197 possibly mimicking the pain flare-up after exercise reported in clinical practice by some individuals with widespread chronic pain.24 Also, Fingleton et al.40 observed reduced pressure pain thresholds (hyperalgesia) after both aerobic and isometric exercises in individuals with knee osteoarthritis who demonstrated an impaired CPM response. By contrast, pain thresholds increased in knee osteoarthritis individuals with a normal CPM response suggesting that patients with impaired CPM, which is also a common finding in individuals with chronic pain,114,121 may have less acute hypoalgesic effect from exercise.
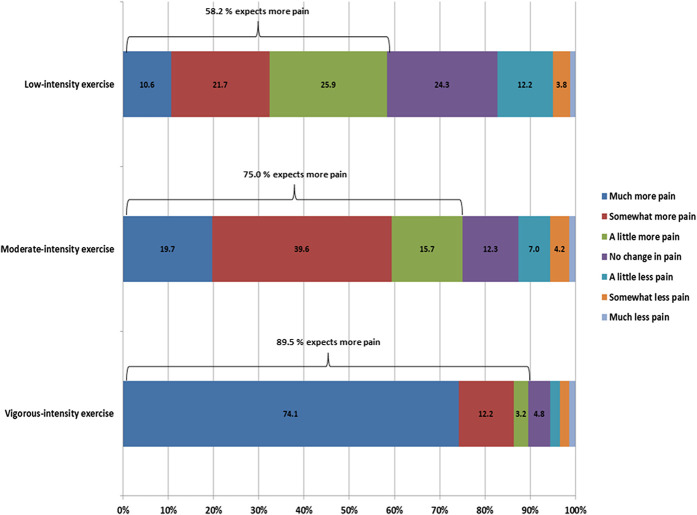
Another possible explanation for the lack of hypoalgesia after exercise often observed in individuals with chronic pain is that the exercise dose–response relationship is different in individuals with chronic pain compared with pain-free subjects. Newcomb et al.136 observed a larger EIH response in individuals with fibromyalgia after 20 minutes of aerobic exercise at a preferred intensity (45% of maximal heart rate) compared with a prescribed and higher-intensity aerobic exercise (60%–75% of maximal heart rate). Similarly, Coombes et al.20 showed that isometric exercise above but not below an individual's pain threshold increased pain responses to exercise in people with lateral epicondylalgia. These results could indicate that lower-intensity exercise creates less input to facilitated central pain mechanisms resulting in a net balance of pain inhibition and a reduction in the pain sensitivity after exercise. This may be different for chronic exercise, however, where a small benefit of painful over nonpainful exercise has been observed, albeit for clinical pain at baseline as opposed to experimental pain in the immediate post-exercise period.173 Other possible explanations for reduced EIH include use of opioids and negative expectations about the effect of exercise. Interactions between EIH mechanisms and the use of analgesics may affect the response to exercise. Individuals treated with opioids report less CPM,155 and reduced effects of opioids have been reported in animals after long-term exercise.174 As observed in pain-free individuals, negative expectations are associated with the hypoalgesic response after exercise. Interestingly, most patients with chronic pain referred to multidisciplinary pain treatment do not expect exercises to cause less pain; on the contrary, the majority expects more pain after exercise (Fig. 1).
Figure 1.
Expectations about the effects of low-intensity exercise, moderate-intensity exercise, and vigorous-intensity exercise on pain reported by patients (n = 500) referred for interdisciplinary pain treatment at a University Hospital Pain Center in Denmark (unpublished data from the clinical pain registry, PainData).
3.2. Regular exercise and pain
Regular exercise is guideline recommended treatment for a wide range of chronic pain conditions.49,146 Regular exercise is safe and generally well accepted by individuals with mild to moderate chronic pain; however, the effects on pain and pain sensitivity are somewhat conflicting, and the level of evidence for a positive effect is generally low.49 Clinically relevant reductions in pain and pain sensitivity are often observed after 8 to 12 weeks of exercise therapy in individuals with knee or hip osteoarthritis,170 but randomized controlled trials often observe smaller effects with pain reductions of less than 10 on a 100-point numerical rating scale62 or even no change in pain after exercise therapy compared with passive sham therapy.8
To the best of our knowledge, only 2 studies have investigated whether habitual physical activity levels predict pain responses to acute exercise in individuals with chronic pain. Coriolano et al.21 found that people with knee osteoarthritis who self-reported more physical activity experienced less exacerbation in pain after completing performance-based tests and a physiological test (submaximal arm ergometer test). In people with fibromyalgia, Umeda et al.187 showed that participants who were more physically active reported a smaller increase in ratings of muscle pain intensity during isometric handgrip exercise. Taken together, these results suggest that being more physically active is associated with reduced pain responses to acute exercise in individuals with chronic pain. These results are consistent with cross-sectional data showing negative associations between fitness and pain (ie, more fitness, less pain) in people with fibromyalgia77 and knee osteoarthritis (Jones et al., in review) as well as longitudinal data showing benefit of longer periods of regular exercise training on reducing pain in individuals with chronic pain.49
4. Underlying mechanisms of exercise-induced hypoalgesia in humans
There are numerous biological and cognitive factors that contribute to pain, so changes in any one or more of these by acute exercise could account for EIH. It is not clear, however, what these mechanisms are or whether the mechanisms are similar or distinct between healthy individuals and individuals with chronic pain. The contrasting magnitude of EIH between pain-free individuals and individuals with chronic pain130 suggests that the mechanisms of EIH are disrupted in individuals with chronic pain. That is, some aspect of chronic pain (eg, inflammation, sensitization, and fear of movement) interferes with the normal hypoalgesic effect of acute exercise. These potential mechanisms will be described in more detail hereafter.
4.1. Opioid and cannabinoid systems
The most commonly proposed mechanism of EIH is enhanced descending inhibition by activation of the opioid and cannabinoid systems. The contraction of skeletal muscle increases the discharge of mechanosensitive afferents (ie, A-delta and C-fibres) which, in turn, activates central descending opioid pain pathways.29,184 Exercise also increases the release of endogenous cannabinoids. These opioid and cannabinoid pathways have receptors throughout the peripheral and central nervous systems that can produce analgesia when stimulated.29,184
Human studies investigating the role of opioids and cannabinoids in EIH have yielded equivocal findings. For example, opioid antagonists such as naloxone and naltrexone have been shown to increase, decrease, or have no effect on EIH.30,31,74,94,140 Moreover, correlations between EIH and exercise-induced changes in plasma concentrations of beta-endorphins and endocannabinoids are not always observed.94,139,165 A limitation of these human investigations is that they are more constrained than rodent studies in their ability to investigate whether opioids and cannabinoids are acting through peripheral and/or central actions to influence pain after exercise; however, there is some evidence that blocking blood flow to a limb during exercise attenuates EIH in pain-free individuals, suggesting that peripheral factors are important.79
4.2. Stress-induced hypoalgesia
Exercise-induced hypoalgesia might also be a form of stress-induced analgesia, related to the release of various stress hormones during exercise. However, evidence to support this in humans is mixed. For example, EIH is related to increases in growth hormone during exercise,149 but another study found that the suppression of exercise-induced growth hormone release by cyproheptadine had no effect on EIH.86 Dexamethasone, a steroid medication, has been shown to attenuate EIH by reducing secretion of adrenocorticotropin88; however, other studies have found no effect of dexamethasone on pain in healthy individuals.209 A small pilot study of 7 healthy individuals showed that exercise-induced changes in neuropeptide Y, allopregnanolone, pregnenolone, and dehydroepiandrosterone were related to EIH.167 However, because concentrations of these substances were only measured in the plasma, it is not clear whether they were acting through peripheral or central mechanisms to influence pain. Moreover, because this was only a small pilot study, more studies are needed to confirm the findings.
4.3. Cardiovascular systems
Exercise-induced changes in the cardiovascular system have also been proposed as a mechanism of EIH. That is, elevations in blood pressure by exercise are thought to attenuate pain through baroreceptor-related mechanisms (ie, the activation of arterial baroreceptors by exercise subsequently activates pain-related brain areas involved in pain modulation). Although it is true that people with high blood pressure are less sensitive to pain (ie, hypertension-associated hypoalgesia),161 there is currently little evidence that acute changes in blood pressure by exercise are related to EIH.28,157,189,190 Moreover, acute increases in blood pressure by exercise could not account for the persistence of EIH after exercise (eg, 15 minutes after exercise cessation210 because blood pressure would have presumably returned to baseline, or indeed be lower, by this time).
4.4. Central pain modulatory systems
The influence of exercise on reducing the sensitivity of the central nervous system has also been explored as a mechanism of EIH. These studies show that acute exercise can reduce temporal summation96,131,196,206 and increase thresholds to elicit the nociceptive withdrawal reflex,55 although there is some evidence contrary to the latter observation.125 These results imply that exercise can reduce pain through reductions in central nervous system sensitivity at spinal and supraspinal levels, but exactly where in the nociceptive pathway these changes occur is not known. Improved efficacy of descending inhibitory pathways by exercise has been studied as a mechanism of EIH as well, but there is little direct evidence to support this. For example, Alsouhibani et al.2 observed a decrease in the CPM response after exercise, Meeus et al. found no effect of aerobic exercise on CPM in healthy individuals,122 and Ellingson et al.36 showed that EIH was comparable for nonpainful and painful exercise, although the latter should have evoked a larger “pain inhibits pain” effect. A few studies have found small positive correlations between conditioned pain modulation and EIH13,112,198 suggesting that the 2 may share similar mechanisms; however, EIH is usually somewhat smaller in magnitude but more enduring than conditioned pain modulation so the 2 are likely distinct.112,195
4.5. Psychological contributing factors
Changes in pain cognition might also account for some of the effect of acute exercise on pain. It has been shown that exercise can reduce ratings of pain unpleasantness in the absence of a change in ratings of pain intensity,80 suggesting that alterations in the appraisal of noxious stimuli contribute to EIH. Cognitive and psychosocial factors including pain self-efficacy, coping strategies, fear of pain, and stress are known to underlie some of the difference in pain between athletes and nonathletes,53,75,142 but their relation to EIH is less clear. For example, several studies have shown that individuals with higher levels of catastrophizing experience less EIH,16,131,207 although this is not always observed and correlations between EIH and other psychosocial factors (eg, fear of pain, pain attitudes, and anxiety) seem negligible.112,201 Therefore, the contribution of cognitive factors to EIH remains poorly understood but seems limited. More studies are needed to investigate whether these cognitive factors are related to EIH and, more importantly, whether they can be manipulated to augment it.81
4.6. Impaired EIH: disrupted or distinct mechanisms
The mechanisms of EIH in individuals with chronic pain are equally if not more unclear. Because exercise has such varying effects on pain within and between individuals with chronic pain, it is difficult to determine whether there is a consistent mechanism that contributes to changes in pain with acute exercise. Moreover, it is not clear if the mechanisms of EIH in individuals with chronic pain are the same as pain-free individuals and are just disrupted, or whether separate mechanisms related to the presence of chronic pain are involved as well.
The fact that EIH can occur at exercised and remote sites in individuals with chronic pain shows that EIH is not always disrupted in these individuals.38,136,197 However, there are also several demonstrations that exercise with a painful joint or muscle can either diminish EIH compared to when a nonpainful body part is exercised (ie, exercise of the upper limb in people with knee osteoarthritis, but pain measurement in the lower limb)17 or, worse, can increase pain.19,20,107,177 These results are both opposite to what is normally seen in pain-free individuals where EIH is usually greatest for the exercised body part. Therefore, the results of the above studies provide some evidence that compared to healthy individuals, the mechanisms of EIH in individuals with chronic pain are both similar and distinct. However, because the mechanisms of EIH are still poorly understood in both groups, there is little direct evidence to support this.
Regarding mechanisms of EIH that may be similar, but disrupted, in individuals with chronic pain compared to pain-free individuals, altered excitability of the central nervous system after exercise is perhaps the most obvious. In pain-free individuals, acute exercise reliably reduces temporal summation,96,196,206 whereas the opposite effect has been observed in individuals with chronic pain.197,206 By contrast, one of the few studies to combine acute exercise with analgesic medication showed that paracetamol and placebo had comparable effects on temporal summation and conditioned pain modulation after exercise in pain-free individuals and individuals with chronic pain.122 Because paracetamol is a predominantly central acting agent that can affect opioids, cannabinoid and serotonergic pathways,168 this finding provides little support to the notion that exercise reduces pain through central changes in these pathways or that differences in the sensitivity of these pathways through exercise accounts for the greater EIH in pain-free individuals compared to individuals with chronic pain. More studies using drugs with less ubiquitous effects would be useful to further investigate how different substances are involved in EIH in humans and whether these differ between pain-free individuals and individuals with chronic pain.
As for mechanisms of EIH that might be distinct between pain-free individuals and individuals with chronic pain, reductions in inflammation by acute exercise are one such possibility. Inflammation plays a key role in the pathogenesis of several chronic pain states, so it is possible that reductions in inflammation by exercise may reduce pain in these individuals. However, the results of studies examining the effect of acute exercise on inflammation in individuals with chronic pain are mixed and the relation between the changes in inflammatory markers and pain has seldom been explored. Moreover, differences in the exercise-induced changes in inflammatory markers between individuals with chronic pain and pain-free individuals were only sometimes, but not always, observed. Therefore, it remains unclear to what extent EIH is related to acute changes in inflammation by exercise in individuals with chronic pain or whether this is a distinct mechanism of EIH in these populations. Another possibility is opioid-induced hyperalgesia. As already mentioned, interactions between EIH mechanisms and the use of analgesics may affect the response to exercise. Individuals treated with opioids report less CPM,155 and reduced effects of opioids have been reported in animals after long-term exercise.174 This may be explained by opioid-induced hyperalgesia which, paradoxically, leads to a reduction in central opioid receptor availability60 and hence less potential to modulate pain through opioidergic mechanisms (as shown in pain-free individuals.152
Psychosocial and cognitive factors are heavily implicated in the development and persistence of chronic pain.34 These same cognitive factors influence responses to experimental noxious stimuli in pain-free individuals as well,150 but their relation to EIH has seldom been examined, particularly in individuals with chronic pain. Accordingly, it is still not known whether cognitive factors are directly involved in EIH, or, perhaps more importantly, whether they can be manipulated to influence pain responses to exercise. Although there is some evidence to support this in pain-free individuals,81 it remains to be determined whether preceding exercise with education can also influence EIH in individuals with chronic pain in whom negative expectations about pain and exercise are more prevalent and therefore likely harder to change. It may be that, because of their more entrenched negative beliefs about pain and exercise, more intensive education is required in individuals with chronic pain to produce the same effect. Some combination of pain neuroscience education and EIH education might also be required. Nonetheless, if the effect can be replicated in individuals with chronic pain, it could have important applications for exercise prescription in clinical practice.
Regarding regular exercise, despite the large number of studies that have shown exercise training to reduce pain in people with chronic pain,49 the mechanisms by which it does this is poorly understood. This is largely because many of the studies did not analyze which changes occurring with exercise (biological and/or psychological changes) were associated with the observed improvements in pain. Moreover, few of the studies investigated where in the nociceptive pathways (ie, peripheral, spinal, and/or supraspinal pathways) changes might be occurring due to exercise, which could account for the observed reductions in pain. As a result, the precise mechanisms of pain attenuation by exercise training are not known, but several possibilities exist that are likely common to individuals with chronic pain.
Improved structure and function of the musculoskeletal system is one such possibility. In people with knee osteoarthritis, chronic exercise can improve several musculoskeletal factors important in the development and progression of the disease including body mass, joint alignment, proprioception, cartilage structure and function, inflammation, and muscle strength.6,160 Of these possible mediators, improvements in muscle strength are the strongest contributor to the positive effect of physical exercise on improved osteoarthritis symptoms.160
Desensitization of the nervous system is another possibility. In humans, exercise-induced changes in biomarkers associated with nociceptive pathways have been reported (eg, inflammatory factors and neurotransmitters),83 but again it is not clear whether these changes reduce pain due to the peripheral or central actions of these factors. Preliminary evidence shows that exercise can normalise aberrant brain activity in people with fibromyalgia.41 This finding is in agreement with the results of a few cross-sectional studies showing that people with fibromyalgia who are more physically active have more typical brain responses to pain compared to less active individuals.37,120 However, not all studies have shown chronic exercise to attenuate aberrant brain responses in individuals with chronic pain,126 so the role of changes in brain activity as a mechanism of pain relief by regular exercise remains unclear.
Finally, exercise-induced improvements in mood could be another shared mediator of the positive effect of exercise on pain in individuals with chronic pain. The role of both general (eg, depression and anxiety) and pain-specific (eg, catastrophizing and self-efficacy) psychosocial processes in the development and maintenance of chronic pain is clear.34 Many of these psychosocial factors are positively influenced by exercise,85,183 so it is plausible that this could result in improvements in pain either directly or indirectly through changes in both the sensory and emotional aspects of pain.
5. Implications and future perspectives
5.1. Clinical implications
Most types of exercise can reduce pain sensitivity at exercising and nonexercising muscles in pain-free individuals, with a larger hypoalgesic response at the exercising muscles. In individuals with chronic pain, the hypoalgesic response after exercise is less consistent; however, in addition to other well-documented physical and mental health benefits related to exercise, exercise can sometimes induce hypoalgesia in individuals with chronic pain. Regarding exercise prescription in clinical settings, it may be worth considering: (1) that exercising nonpainful body areas if possible as well as using low-intensity exercises such as walking may be useful as a first step, (2) that individuals' beliefs, expectations, and exercise preference should be assessed before exercise prescription to minimize the risk of a poor outcome, and (3) that these beliefs and expectations could be modified through education or other interventions to improve pain responses to exercise in people with chronic pain. There is some evidence that combining exercise training and education has superior effects compared to exercise alone in individuals with chronic pain,15,153 but this is yet to be properly explored in the context of pain responses to a single bout of acute exercise in individuals with chronic pain.
5.2. Implications for future exercise-induced hypoalgesia studies
In addition to the above-mentioned implications, we also propose several methodological recommendations for future studies of EIH. First, studies should use a randomized controlled design (parallel or crossover), or at the very least include a control group/condition. This is because the causal effects of exercise on pain are best inferred from randomized controlled trials. As shown in Tables 1 and 2, there have been well over 150 studies of EIH in pain-free individuals and individuals with chronic pain. However, the minority of these used a randomized controlled design or a nonrandomized controlled design. Instead, EIH was often investigated using a single-arm pre-post design. A major limitation of this type of study design is that the effects of habituation to noxious stimuli, as well as statistical phenomena such as regression to the mean, are not accounted for. To truly determine whether a single bout of exercise causes a reduction in pain, randomized controlled trials are needed. Second, it is important that these randomized controlled trials use large(r) sample sizes. The majority of EIH studies are small (n ≤ 50), and it is well documented that small studies are inherently biased to find larger effects.26 Hence, most studies of EIH probably overestimate the effect of exercise on pain. Consequently, despite the enormous amount of EIH studies to date, the true effect of a single bout of exercise on pain is still unknown. Larger randomized controlled trials, of which there are currently very few, are clearly needed to determine this.
As evident in Tables 1 and 2, there is substantial heterogeneity in methodology used in EIH studies, making it difficult to synthesise the results of this vast literature. Therefore, we also recommend that future EIH studies share a somewhat common methodology so that the results between studies can be more easily compared. To this end, it may be useful for future studies to share a common method of pain assessment. Pressure pain thresholds at local and remote sites may be the most appropriate because these have been studied most often and do not require expensive equipment (although they may be more prone to experimenter bias if using handheld algometry). It would also be of benefit to include assessment of both experimental and clinical pain in individuals with chronic pain to better understand the effects of exercise on “real life” pain. Moreover, it may be useful to prescribe and report exercise using a common index so that the amount of work performed can be quantified. This would help clarify the dose–response effect of exercise on pain, a result that may have important clinical implications such as determining the minimal effective dose with respect to hypoalgesia for each mode of exercise as well as identifying volumes and/or intensities of exercise that may be more likely to exacerbate pain in individuals with chronic pain. Finally, Lee et al.109 recently outlined several issues in clinical pain research including transparency, underpowered studies, and researcher degrees of freedom. The use of preregistration and registered reports, data sharing, and greater adherence to reporting guidelines were suggested as areas for improvement and we believe that EIH studies would benefit from adopting these recommendations.
Disclosures
The authors have no conflicts of interest to declare.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Alghamdi KS, Al-Sheikh MH. Effect of stress on pain perception in young women. Saudi Med J 2009;30:478–84. [PubMed] [Google Scholar]
- [2].Alsouhibani A, Vaegter HB, Hoeger Bement M. Systemic exercise-induced hypoalgesia following isometric exercise reduces conditioned pain modulation. Pain Med 2019;20:180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Arroyo-Morales M, Rodriguez LD, Rubio-Ruiz B, Olea N. Influence of gender in the psychoneuroimmunological response to therapeutic interval exercise. Biol Res Nurs 2012;14:357–63. [DOI] [PubMed] [Google Scholar]
- [4].Baiamonte BA, Kraemer RR, Chabreck CN, Reynolds ML, McCaleb KM, Shaheen GL, Hollander DB. Exercise-induced hypoalgesia: pain tolerance, preference and tolerance for exercise intensity, and physiological correlates following dynamic circuit resistance exercise. J Sports Sci 2017;35:1–7. [DOI] [PubMed] [Google Scholar]
- [5].Bartholomew JB, Lewis BP, Linder DE, Cook DB. Post-exercise analgesia: replication and extension. J Sports Sci 1996;14:329–34. [DOI] [PubMed] [Google Scholar]
- [6].Beckwee D, Vaes P, Cnudde M, Swinnen E, Bautmans I. Osteoarthritis of the knee: why does exercise work? A qualitative study of the literature. Ageing Res Rev 2013;12:226–36. [DOI] [PubMed] [Google Scholar]
- [7].Bement MH, Drewek B, Hunter SK. Men report greater pain relief following sustained static contractions than women when matched for baseline pain. J Mot Behav 2014;46:107–13. [DOI] [PubMed] [Google Scholar]
- [8].Bennell KL, Egerton T, Martin J, Abbott JH, Metcalf B, McManus F, Sims K, Pua YH, Wrigley TV, Forbes A, Smith C, Harris A, Buchbinder R. Effect of physical therapy on pain and function in patients with hip osteoarthritis: a randomized clinical trial. Jama 2014;311:1987–97. [DOI] [PubMed] [Google Scholar]
- [9].Bialosky JE, Bishop MD, Robinson ME, Zeppieri G, Jr, George SZ. Spinal manipulative therapy has an immediate effect on thermal pain sensitivity in people with low back pain: a randomized controlled trial. Phys Ther 2009;89:1292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bishop MD, Beneciuk JM, George SZ. Immediate reduction in temporal sensory summation after thoracic spinal manipulation. Spine J 2011;11:440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Black CD, Gonglach AR, Renfroe JB, Hight RE. The effects of caffeine ingestion on exercise-induced hypoalgesia: a pilot study. Physiol Behav 2016;161:1–6. [DOI] [PubMed] [Google Scholar]
- [12].Black CD, Huber JK, Ellingson LD, Ade CJ, Taylor EL, Griffeth EM, Janzen NR, Sutterfield SL. Exercise-induced hypoalgesia is not influenced by physical activity type and amount. Med Sci Sports Exerc 2017;49:975–82. [DOI] [PubMed] [Google Scholar]
- [13].Black CD, Tynes BK, Gonglach AR, Waddell DE. Local and generalized endogenous pain modulation in healthy men: effects of exercise and exercise-induced muscle damage. Pain Med 2016;17:2422–33. [DOI] [PubMed] [Google Scholar]
- [14].Black J, Chesher GB, Starmer GA, Egger G. The painlessness of the long distance runner. Med.J.Aust. 1979;1:522–3. [PubMed] [Google Scholar]
- [15].Bodes Pardo G, Lluch Girbes E, Roussel NA, Gallego Izquierdo T, Jimenez Penick V, Pecos Martin D. Pain neurophysiology education and therapeutic exercise for patients with chronic low back pain: a single-blind randomized controlled trial. Arch Phys Med Rehabil 2018;99:338–47. [DOI] [PubMed] [Google Scholar]
- [16].Brellenthin AG, Crombie KM, Cook DB, Sehgal N, Koltyn KF. Psychosocial influences on exercise-induced hypoalgesia. Pain Med 2017;18:538–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Burrows NJ, Booth J, Sturnieks DL, Barry BK. Acute resistance exercise and pressure pain sensitivity in knee osteoarthritis: a randomised crossover trial. Osteoarthritis Cartilage 2014;22:407–14. [DOI] [PubMed] [Google Scholar]
- [18].Chretien R, Lavoie S, Chalaye P, de Vette E, Counil FP, Dallaire F, Lafrenaye S. Reduced endogenous pain inhibition in adolescent girls with chronic pain. Scand J Pain 2018;18:711–17. [DOI] [PubMed] [Google Scholar]
- [19].Cook DB, Stegner AJ, Ellingson LD. Exercise alters pain sensitivity in Gulf War veterans with chronic musculoskeletal pain. J Pain 2010;11:764–72. [DOI] [PubMed] [Google Scholar]
- [20].Coombes BK, Wiebusch M, Heales L, Stephenson A, Vicenzino B. Isometric exercise above but not below an individual's pain threshold influences pain perception in people with lateral epicondylalgia. Clin J Pain 2016;32:1069–75. [DOI] [PubMed] [Google Scholar]
- [21].Coriolano K, Aiken A, Pukall C, Harrison M. Changes in self-reported disability after performance-based tests in obese and non-obese individuals diagnosed with osteoarthritis of the knee. Disabil Rehabil 2015;37:1152–61. [DOI] [PubMed] [Google Scholar]
- [22].Crombie KM, Brellenthin AG, Hillard CJ, Koltyn KF. Endocannabinoid and opioid system interactions in exercise-induced hypoalgesia. Pain Med 2018;19:118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cruz-Almeida Y, Fillingim RB. Can quantitative sensory testing move us closer to mechanism-based pain management? Pain Med 2014;15:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].da Cunha Ribeiro RP, Franco TC, Pinto AJ, Pontes Filho MAG, Domiciano DS, de Sa Pinto AL, Lima FR, Roschel H, Gualano B. Prescribed versus preferred intensity resistance exercise in fibromyalgia pain . Front Physiol 2018;9:1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dannecker EA, Koltyn KF, Riley JL, III, Robinson ME. The influence of endurance exercise on delayed onset muscle soreness. J.Sports Med Phys Fitness 2002;42:458–65. [PubMed] [Google Scholar]
- [26].Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ 2013;346:f2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Deering R, Pashibin T, Cruz M, Hunter SK, Hoeger Bement M. Fatiguing trunk flexor exercise decreases pain sensitivity in postpartum women. Front Physiol 2019;10:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Devoize L, Chalaye P, Lafrenaye S, Marchand S, Dallel R. Relationship between adaptation and cardiovascular response to tonic cold and heat pain adaptability to tonic pain and cardiovascular responses. Eur J Pain 2016;20:731–41. [DOI] [PubMed] [Google Scholar]
- [29].Dietrich A, McDaniel WF. Endocannabinoids and exercise. Br J Sports Med 2004;38:536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Droste C, Greenlee MW, Schreck M, Roskamm H. Experimental pain thresholds and plasma beta-endorphin levels during exercise. Med Sci Sports Exerc 1991;23:334–42. [PubMed] [Google Scholar]
- [31].Droste C, Meyer-Blankenburg H, Greenlee MW, Roskamm H. Effect of physical exercise on pain thresholds and plasma beta-endorphins in patients with silent and symptomatic myocardial ischaemia. Eur Heart J 1988;9(suppl N):25–33. [DOI] [PubMed] [Google Scholar]
- [32].Drury D, Stuempfle K, Shannon R, Miller J. An investigation of exercise-induced hypoalgesia after isometric and cardiovascular exercise. J Exerc Physiol 2004;7:1–5. [Google Scholar]
- [33].Drury DG, Greenwood K, Stuempfle KJ, Koltyn KF. Changes in pain perception in women during and following an exhaustive incremental cycling exercise. J Sports Sci Med 2005;4:215–22. [PMC free article] [PubMed] [Google Scholar]
- [34].Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain 2016;17(9 suppl):T70–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ellingson LD, Colbert LH, Cook DB. Physical activity is related to pain sensitivity in healthy women. Med Sci Sports Exerc 2012;44:1401–6. [DOI] [PubMed] [Google Scholar]
- [36].Ellingson LD, Koltyn KF, Kim JS, Cook DB. Does exercise induce hypoalgesia through conditioned pain modulation? Psychophysiology 2014;51:267–76. [DOI] [PubMed] [Google Scholar]
- [37].Ellingson LD, Shields MR, Stegner AJ, Cook DB. Physical activity, sustained sedentary behavior, and pain modulation in women with fibromyalgia. J Pain 2012;13:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ellingson LD, Stegner AJ, Schwabacher IJ, Koltyn KF, Cook DB. Exercise strengthens central nervous system modulation of pain in fibromyalgia. Brain Sci 2016;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Farrokhi S, Jayabalan P, Gustafson JA, Klatt BA, Sowa GA, Piva SR. The influence of continuous versus interval walking exercise on knee joint loading and pain in patients with knee osteoarthritis. Gait Posture 2017;56:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fingleton C, Smart KM, Doody CM. Exercise-induced hypoalgesia in people with knee osteoarthritis with normal and abnormal conditioned pain modulation. Clin J Pain 2017;33:395–404. [DOI] [PubMed] [Google Scholar]
- [41].Flodin P, Martinsen S, Altawil R, Waldheim E, Lampa J, Kosek E, Fransson P. Intrinsic brain connectivity in chronic pain: a resting-state fMRI study in patients with rheumatoid arthritis. Front Hum Neurosci 2016;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Focht BC, Koltyn KF. Alterations in pain perception after resistance exercise performed in the morning and evening. J Strength Cond Res 2009;23:891–7. [DOI] [PubMed] [Google Scholar]
- [43].Foxen-Craft E, Dahlquist LM. Brief submaximal isometric exercise improves cold pressor pain tolerance. J Behav Med 2017;40:760–71. [DOI] [PubMed] [Google Scholar]
- [44].Fuller AK, Robinson ME. A test of exercise analgesia using signal detection theory and a within-subjects design. Percept Mot Skills 1993;76(3 pt 2):1299–310. [DOI] [PubMed] [Google Scholar]
- [45].Gajsar H, Nahrwold K, Titze C, Hasenbring MI, Vaegter HB. Exercise does not produce hypoalgesia when performed immediately after a painful stimulus. Scand J Pain 2018;18:311–20. [DOI] [PubMed] [Google Scholar]
- [46].Gajsar H, Titze C, Hasenbring MI, Vaegter HB. Isometric back exercise has different effect on pressure pain thresholds in healthy men and women. Pain Med 2017;18:917–23. [DOI] [PubMed] [Google Scholar]
- [47].Galindez-Ibarbengoetxea X, Setuain I, Ramirez-Velez R, Andersen LL, Gonzalez-Izal M, Jauregi A, Izquierdo M. Immediate effects of osteopathic treatment versus therapeutic exercise on patients with chronic cervical pain. Altern Ther Health Med 2018;24:24–32. [PubMed] [Google Scholar]
- [48].Ge HY, Nie H, Graven-Nielsen T, Danneskiold-Samsoe B, Arendt-Nielsen L. Descending pain modulation and its interaction with peripheral sensitization following sustained isometric muscle contraction in fibromyalgia. Eur J Pain 2012;16:196–203. [DOI] [PubMed] [Google Scholar]
- [49].Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev 2017;4:Cd011279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].George SZ, Bishop MD, Bialosky JE, Zeppieri G, Jr, Robinson ME. Immediate effects of spinal manipulation on thermal pain sensitivity: an experimental study. BMC Musculoskelet Disord 2006;7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Germanou EI, Chatzinikolaou A, Malliou P, Beneka A, Jamurtas AZ, Bikos C, Tsoukas D, Theodorou A, Katrabasas I, Margonis K, Douroudos I, Gioftsidou A, Fatouros IG. Oxidative stress and inflammatory responses following an acute bout of isokinetic exercise in obese women with knee osteoarthritis. Knee 2013;20:581–90. [DOI] [PubMed] [Google Scholar]
- [52].Geva N, Defrin R. Enhanced pain modulation among triathletes: a possible explanation for their exceptional capabilities. PAIN 2013;154:2317–23. [DOI] [PubMed] [Google Scholar]
- [53].Geva N, Pruessner J, Defrin R. Acute psychosocial stress reduces pain modulation capabilities in healthy men. PAIN 2014;155:2418–25. [DOI] [PubMed] [Google Scholar]
- [54].Gomolka S, Vaegter HB, Nijs J, Meeus M, Gajsar H, Hasenbring MI, Titze C. Assessing endogenous pain inhibition: test-retest reliability of exercise-induced hypoalgesia in local and remote body parts after aerobic cycling. Pain Med 2019;20:2272–82. [DOI] [PubMed] [Google Scholar]
- [55].Guieu R, Blin O, Pouget J, Serratrice G. Nociceptive threshold and physical activity. Can J Neurol Sci 1992;19:69–71. [PubMed] [Google Scholar]
- [56].Gurevich M, Kohn PM, Davis C. Exercise-induced analgesia and the role of reactivity in pain sensitivity. J.Sports Sci 1994;12:549–59. [DOI] [PubMed] [Google Scholar]
- [57].Haier RJ, Quaid K, Mills JC. Naloxone alters pain perception after jogging. Psychiatry Res 1981;5:231–2. [DOI] [PubMed] [Google Scholar]
- [58].Hakansson S, Jones MD, Ristov M, Marcos L, Clark T, Ram A, Morey R, Franklin A, McCarthy C, Carli LD, Ward R. Keech. Intensity-dependent effects of aerobic training on pressure pain threshold in overweight men: a randomized trial. Eur J Pain 2018;22:1813–23. [DOI] [PubMed] [Google Scholar]
- [59].Hansen S, Vaegter HB, Petersen KK. Pre-treatment exercise-induced hypoalgesia is associated with change in pain and function after standardized exercise therapy in painful knee osteoarthritis. Clin J Pain 2019;36:16–24. [DOI] [PubMed] [Google Scholar]
- [60].Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci 2007;27:10000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Harris S, Sterling M, Farrell SF, Pedler A, Smith AD. The influence of isometric exercise on endogenous pain modulation: comparing exercise-induced hypoalgesia and offset analgesia in young, active adults. Scand J Pain 2018;18:513–23. [DOI] [PubMed] [Google Scholar]
- [62].Henriksen M, Klokker L, Graven-Nielsen T, Bartholdy C, Jorgensen TS, Bandak E, Danneskiold-Samsoe B, Christensen R, Bliddal H. Exercise therapy reduces pain sensitivity in patients with knee osteoarthritis: a randomized controlled trial. Arthritis Care Res (Hoboken) 2014;66:1836–43. [DOI] [PubMed] [Google Scholar]
- [63].Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol 2000;61:169–203. [DOI] [PubMed] [Google Scholar]
- [64].Hoeger Bement MK, Dicapo J, Rasiarmos R, Hunter SK. Dose response of isometric contractions on pain perception in healthy adults. Med Sci Sports Exerc 2008;40:1880–9. [DOI] [PubMed] [Google Scholar]
- [65].Hoeger Bement MK, Rasiarmos RL, DiCapo JM, Lewis A, Keller ML, Harkins AL, Hunter SK. The role of the menstrual cycle phase in pain perception before and after an isometric fatiguing contraction. Eur J Appl Physiol 2009;106:105–12. [DOI] [PubMed] [Google Scholar]
- [66].Hoeger Bement MK, Weyer A, Hartley S, Drewek B, Harkins AL, Hunter SK. Pain perception after isometric exercise in women with fibromyalgia. Arch Phys Med Rehabil 2011;92:89–95. [DOI] [PubMed] [Google Scholar]
- [67].Hoffman MD, Lee J, Zhao H, Tsodikov A. Pain perception after running a 100-mile ultramarathon. Arch Phys Med Rehabil 2007;88:1042–8. [DOI] [PubMed] [Google Scholar]
- [68].Hoffman MD, Shepanski MA, Mackenzie SP, Clifford PS. Experimentally induced pain perception is acutely reduced by aerobic exercise in people with chronic low back pain. J Rehabil Res Dev 2005;42:183–90. [DOI] [PubMed] [Google Scholar]
- [69].Hoffman MD, Shepanski MA, Ruble SB, Valic Z, Buckwalter JB, Clifford PS. Intensity and duration threshold for aerobic exercise-induced analgesia to pressure pain. Arch Phys Med Rehabil 2004;85:1183–7. [DOI] [PubMed] [Google Scholar]
- [70].Holden S, Lyng K, Graven-Nielsen T, Riel H, Olesen JL, Larsen LH, Rathleff MS. Isometric exercise and pain in patellar tendinopathy: a randomized crossover trial. J Sci Med Sport 2020;23:208–14. [DOI] [PubMed] [Google Scholar]
- [71].Hu L, Cai MM, Xiao P, Luo F, Iannetti GD. Human brain responses to concomitant stimulation of a-delta and C nociceptors. J Neurosci 2014;34:11439–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hu L, Valentini E, Zhang ZG, Liang M, Iannetti GD. The primary somatosensory cortex contributes to the latest part of the cortical response elicited by nociceptive somatosensory stimuli in humans. Neuroimage 2014;84:383–93. [DOI] [PubMed] [Google Scholar]
- [73].Hviid JT, Thorlund JB, Vaegter HB. Walking increases pain tolerance in humans an experimental cross-over study. Scand J Pain 2019. doi: 10.1515/sjpain-2019-0070 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [74].Janal MN, Colt EW, Clark WC, Glusman M. Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: effects of naloxone. PAIN 1984;19:13–25. [DOI] [PubMed] [Google Scholar]
- [75].Johnson MH, Stewart J, Humphries SA, Chamove AS. Marathon runners' reaction to potassium iontophoretic experimental pain: pain tolerance, pain threshold, coping and self-efficacy. Eur J Pain 2012;16:767–74. [DOI] [PubMed] [Google Scholar]
- [76].Jones MD, Booth J, Taylor JL, Barry BK. Aerobic training increases pain tolerance in healthy individuals. Med Sci Sports Exerc 2014;46:1640–7. [DOI] [PubMed] [Google Scholar]
- [77].Jones MD, Booth J, Taylor JL, Barry BK. Limited association between aerobic fitness and pain in healthy individuals: a cross-sectional study. Pain Med 2016;17:1799–808. [DOI] [PubMed] [Google Scholar]
- [78].Jones MD, Nuzzo JL, Taylor JL, Barry BK. Aerobic exercise reduces pressure more than heat pain sensitivity in healthy adults. Pain Med 2019;20:1534–46. [DOI] [PubMed] [Google Scholar]
- [79].Jones MD, Taylor JL, Barry BK. Occlusion of blood flow attenuates exercise-induced hypoalgesia in the occluded limb of healthy adults. J Appl Physiol (1985) 2017;122:1284–91. [DOI] [PubMed] [Google Scholar]
- [80].Jones MD, Taylor JL, Booth J, Barry BK. Exploring the mechanisms of exercise-induced hypoalgesia using somatosensory and laser evoked potentials. Front Physiol 2016;7:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Jones MD, Valenzuela T, Booth J, Taylor JL, Barry BK. Explicit education about exercise-induced hypoalgesia influences pain responses to acute exercise in healthy adults: a randomized controlled trial. J Pain 2017;18:1409–16. [DOI] [PubMed] [Google Scholar]
- [82].Kadetoff D, Kosek E. The effects of static muscular contraction on blood pressure, heart rate, pain ratings and pressure pain thresholds in healthy individuals and patients with fibromyalgia. Eur J Pain 2007;11:39–47. [DOI] [PubMed] [Google Scholar]
- [83].Kawi J, Lukkahatai N, Inouye J, Thomason D, Connelly K. Effects of exercise on select biomarkers and associated outcomes in chronic pain conditions: systematic review. Biol Res Nurs 2016;18:147–59. [DOI] [PubMed] [Google Scholar]
- [84].Keilman BM, Hanney WJ, Kolber MJ, Pabian PS, Salamh PA, Rothschild CE, Liu X. The short-term effect of kettlebell swings on lumbopelvic pressure pain thresholds: a randomized controlled trial. J Strength Cond Res 2017;31:3001–9. [DOI] [PubMed] [Google Scholar]
- [85].Kelley GA, Kelley KS, Hootman JM. Effects of exercise on depression in adults with arthritis: a systematic review with meta-analysis of randomized controlled trials. Arthritis Res Ther 2015;17:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kemppainen P, Pertovaara A, Huopaniemi T, Johansson G. Elevation of dental pain threshold induced in man by physical exercise is not reversed by cyproheptadine-mediated suppression of growth hormone release. Neurosci Lett 1986;70:388–92. [DOI] [PubMed] [Google Scholar]
- [87].Kemppainen P, Pertovaara A, Huopaniemi T, Johansson G, Karonen SL. Modification of dental pain and cutaneous thermal sensitivity by physical exercise in man. Brain Res 1985;360:33–40. [DOI] [PubMed] [Google Scholar]
- [88].Kemppainen P, Paalasmaa P, Pertovaara A, Alila A, Johansson G. Dexamethasone attenuates exercise-induced dental analgesia in man. Brain Res 1990;519:329–32. [DOI] [PubMed] [Google Scholar]
- [89].Klich S, Krymski I, Michalik K, Kawczynski A. Effect of short-term cold-water immersion on muscle pain sensitivity in elite track cyclists. Phys Ther Sport 2018;32:42–7. [DOI] [PubMed] [Google Scholar]
- [90].Knauf MT, Koltyn KF. Exercise-induced modulation of pain in adults with and without painful diabetic neuropathy. J Pain 2014;15:656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kodesh E, Weissman-Fogel I. Exercise-induced hypoalgesia—interval versus continuous mode. Appl Physiol Nutr Metab 2014;39:829–34. [DOI] [PubMed] [Google Scholar]
- [92].Koltyn KF. Analgesia following exercise: a review. Sports Med 2000;29:85–98. [DOI] [PubMed] [Google Scholar]
- [93].Koltyn KF, Arbogast RW. Perception of pain after resistance exercise. Br.J.Sports Med 1998;32:20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Koltyn KF, Brellenthin AG, Cook DB, Sehgal N, Hillard C. Mechanisms of exercise-induced hypoalgesia. J Pain 2014;15:1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Koltyn KF, Garvin AW, Gardiner RL, Nelson TF. Perception of pain following aerobic exercise. Med Sci Sports Exerc 1996;28:1418–21. [DOI] [PubMed] [Google Scholar]
- [96].Koltyn KF, Knauf MT, Brellenthin AG. Temporal summation of heat pain modulated by isometric exercise. Eur J Pain 2013;17:1005–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Koltyn KF, Trine MR, Stegner AJ, Tobar DA. Effect of isometric exercise on pain perception and blood pressure in men and women. Med Sci Sports Exerc 2001;33:282–90. [DOI] [PubMed] [Google Scholar]
- [98].Koltyn KF, Umeda M. Contralateral attenuation of pain after short-duration submaximal isometric exercise. J Pain 2007;8:887–92. [DOI] [PubMed] [Google Scholar]
- [99].Kosek E, Ekholm J. Modulation of pressure pain thresholds during and following isometric contraction. PAIN 1995;61:481–6. [DOI] [PubMed] [Google Scholar]
- [100].Kosek E, Ekholm J, Hansson P. Modulation of pressure pain thresholds during and following isometric contraction in patients with fibromyalgia and in healthy controls. PAIN 1996;64:415–23. [DOI] [PubMed] [Google Scholar]
- [101].Kosek E, Lundberg L. Segmental and plurisegmental modulation of pressure pain thresholds during static muscle contractions in healthy individuals. Eur J Pain 2003;7:251–8. [DOI] [PubMed] [Google Scholar]
- [102].Kosek E, Roos EM, Ageberg E, Nilsdotter A. Increased pain sensitivity but normal function of exercise induced analgesia in hip and knee osteoarthritis—treatment effects of neuromuscular exercise and total joint replacement. Osteoarthritis Cartilage 2013;21:1299–307. [DOI] [PubMed] [Google Scholar]
- [103].Kruger S, Khayat D, Hoffmeister M, Hilberg T. Pain thresholds following maximal endurance exercise. Eur J Appl Physiol 2016;116:535–40. [DOI] [PubMed] [Google Scholar]
- [104].Kuithan P, Heneghan NR, Rushton A, Sanderson A, Falla D. Lack of exercise-induced hypoalgesia to repetitive back movement in people with chronic low back pain. Pain Pract 2019;19:740–50. [DOI] [PubMed] [Google Scholar]
- [105].Kuppens K, Struyf F, Nijs J, Cras P, Fransen E, Hermans L, Meeus M, Roussel N. Exercise- and stress-induced hypoalgesia in musicians with and without shoulder pain: a randomized controlled crossover study. Pain Physician 2016;19:59–68. [PubMed] [Google Scholar]
- [106].Lanefelt SV, Melo-Gomez M, Chizari M, Krsek M, Christidis N, Kosek E, Ernberg M. Tooth clenching until exhaustion evokes exercise-induced hypoalgesia in healthy persons and in patients with temporomandibular disorders. J Oral Facial Pain Headache 2019;33:14–24. [DOI] [PubMed] [Google Scholar]
- [107].Lannersten L, Kosek E. Dysfunction of endogenous pain inhibition during exercise with painful muscles in patients with shoulder myalgia and fibromyalgia. PAIN 2010;151:77–86. [DOI] [PubMed] [Google Scholar]
- [108].Lau WY, Blazevich AJ, Newton MJ, Wu SS, Nosaka K. Changes in electrical pain threshold of fascia and muscle after initial and secondary bouts of elbow flexor eccentric exercise. Eur J Appl Physiol 2015;115:959–68. [DOI] [PubMed] [Google Scholar]
- [109].Lee H, Lamb SE, Bagg MK, Toomey E, Cashin AG, Moseley GL. Reproducible and replicable pain research: a critical review. PAIN 2018;159:1683–9. [DOI] [PubMed] [Google Scholar]
- [110].Lee HS. The effects of aerobic exercise and strengthening exercise on pain pressure thresholds. J Phys Ther Sci 2014;26:1107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Lemley KJ, Drewek B, Hunter SK, Hoeger Bement MK. Pain relief after isometric exercise is not task-dependent in older men and women. Med Sci Sports Exerc 2014;46:185–91. [DOI] [PubMed] [Google Scholar]
- [112].Lemley KJ, Hunter SK, Hoeger Bement MK. Conditioned pain modulation predicts exercise-induced hypoalgesia in healthy adults. Med Sci Sports Exerc 2015;47:176–84. [DOI] [PubMed] [Google Scholar]
- [113].Lemley KJ, Senefeld J, Hunter SK, Hoeger Bement M. Only women report increase in pain threshold following fatiguing contractions of the upper extremity. Eur J Appl Physiol 2016;116:1379–85. [DOI] [PubMed] [Google Scholar]
- [114].Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain 2012;13:936–44. [DOI] [PubMed] [Google Scholar]
- [115].Lier R, Mork PJ, Holtermann A, Nilsen TI. Familial risk of chronic musculoskeletal pain and the importance of physical activity and body mass index: prospective Data from the HUNT Study, Norway. PLoS One 2016;11:e0153828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Loeser JD, Treede R. The Kyoto protocol of IASP basic pain terminology. PAIN 2008;137:473–7. [DOI] [PubMed] [Google Scholar]
- [117].Lofgren M, Opava CH, Demmelmaier I, Friden C, Lundberg IE, Nordgren B, Kosek E. Pain sensitivity at rest and during muscle contraction in persons with rheumatoid arthritis: a substudy within the Physical Activity in Rheumatoid Arthritis 2010 study. Arthritis Res Ther 2018;20:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Malfliet A, Pas R, Brouns R, De Win J, Hatem SM, Meeus M, Ickmans K, van Hooff RJ, Nijs J. Cerebral blood flow and heart rate variability in chronic fatigue syndrome: a randomized cross-over study. Pain Physician 2018;21:E13–e24. [PubMed] [Google Scholar]
- [119].McKean CC, Baiamonte BA, Kraemer RR, Hollander DB. Attention-deficit/hyperactivity disorder medication does not alter exercise-induced hypoalgesia following an acute bout of dynamic circuit resistance exercise. Biol Sport 2018;35:321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].McLoughlin MJ, Stegner AJ, Cook DB. The relationship between physical activity and brain responses to pain in fibromyalgia. J Pain 2011;12:640–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].McPhee ME, Vaegter HB, Graven-Nielsen T. Alterations in pro-nociceptive and anti-nociceptive mechanisms in patients with low back pain: a systematic review with meta-analysis. PAIN 2020;161:464–75. [DOI] [PubMed] [Google Scholar]
- [122].Meeus M, Hermans L, Ickmans K, Struyf F, Van Cauwenbergh D, Bronckaerts L, De Clerck LS, Moorken G, Hans G, Grosemans S, Nijs J. Endogenous pain modulation in response to exercise in patients with rheumatoid arthritis, patients with chronic fatigue syndrome and comorbid fibromyalgia, and healthy controls: a double-blind randomized controlled trial. Pain Pract 2015;15:98–106. [DOI] [PubMed] [Google Scholar]
- [123].Meeus M, Roussel NA, Truijen S, Nijs J. Reduced pressure pain thresholds in response to exercise in chronic fatigue syndrome but not in chronic low back pain: an experimental study. J Rehabil Med 2010;42:884–90. [DOI] [PubMed] [Google Scholar]
- [124].Micalos PS, Arendt-Nielsen L. Differential pain response at local and remote muscle sites following aerobic cycling exercise at mild and moderate intensity. Springerplus 2016;5:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Micalos PS, Harris J, Drinkwater EJ, Cannon J, Marino FE. Perceptual and cerebro-spinal responses to graded innocuous and noxious stimuli following aerobic exercise. J Sports Med Phys Fitness 2015;55:1407–15. [PubMed] [Google Scholar]
- [126].Micalos PS, Korgaonkar MS, Drinkwater EJ, Cannon J, Marino FE. Cerebral responses to innocuous somatic pressure stimulation following aerobic exercise rehabilitation in chronic pain patients: a functional magnetic resonance imaging study. Int J Gen Med 2014;7:425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Misra G, Paris TA, Archer DB, Coombes SA. Dose-response effect of isometric force production on the perception of pain. PLoS One 2014;9:e88105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Monnier-Benoit P, Groslambert A, Rouillon JD. Effects of steady-state exercise on perceived pain: comparison of sedentary students and cyclists. Percept Mot Skills 2006;103:659–66. [DOI] [PubMed] [Google Scholar]
- [129].Nasri-Heir C, Patil AG, Korczeniewska OA, Zusman T, Khan J, Heir G, Benoliel R, Eliav E. The effect of nonstrenuous aerobic exercise in patients with chronic masticatory myalgia. J Oral Facial Pain Headache 2019;33:143–52. [DOI] [PubMed] [Google Scholar]
- [130].Naugle KM, Fillingim RB, Riley JL., III A meta-analytic review of the hypoalgesic effects of exercise. J Pain 2012;13:1139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Naugle KM, Naugle KE, Fillingim RB, Riley JL., III Isometric exercise as a test of pain modulation: effects of experimental pain test, psychological variables, and sex. Pain Med 2014;15:692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Naugle KM, Naugle KE, Fillingim RB, Samuels B, Riley JL., III Intensity thresholds for aerobic exercise-induced hypoalgesia. Med Sci Sports Exerc 2014;46:817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Naugle KM, Naugle KE, Riley JL., III Reduced modulation of pain in older adults after isometric and aerobic exercise. J Pain 2016;17:719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Naugle KM, Ohlman T, Naugle KE, Riley ZA, Keith NR. Physical activity behavior predicts endogenous pain modulation in older adults. PAIN 2017;158:383–90. [DOI] [PubMed] [Google Scholar]
- [135].Naugle KM, Riley JL., III Self-reported physical activity predicts pain inhibitory and facilitatory function. Med Sci Sports Exerc 2014;46:622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Newcomb LW, Koltyn KF, Morgan WP, Cook DB. Influence of preferred versus prescribed exercise on pain in fibromyalgia. Med Sci Sports Exerc 2011;43:1106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Nie H, Graven-Nielsen T, Arendt-Nielsen L. Spatial and temporal summation of pain evoked by mechanical pressure stimulation. Eur J Pain 2009;13:592–9. [DOI] [PubMed] [Google Scholar]
- [138].Ohlman T, Miller L, Naugle KE, Naugle KM. Physical activity levels predict exercise-induced hypoalgesia in older adults. Med Sci Sports Exerc 2018;50:2101–9. [DOI] [PubMed] [Google Scholar]
- [139].Oktedalen O, Solberg EE, Haugen AH, Opstad PK. The influence of physical and mental training on plasma beta-endorphin level and pain perception after intensive physical exercise. Stress Health 2001;17:121–7. [Google Scholar]
- [140].Olausson B, Eriksson E, Ellmarker L, Rydenhag B, Shyu BC, Andersson SA. Effects of naloxone on dental pain threshold following muscle exercise and low frequency transcutaneous nerve stimulation: a comparative study in man. Acta Physiol Scand 1986;126:299–305. [DOI] [PubMed] [Google Scholar]
- [141].Oosterwijck JV, Marusic U, De Wandele I, Paul L, Meeus M, Moorkens G, Lambrecht L, Danneels L, Nijs J. The role of autonomic function in exercise-induced endogenous analgesia: a case-control study in myalgic encephalomyelitis/chronic fatigue syndrome and healthy people. Pain Physician 2017;20:E389–e399. [PubMed] [Google Scholar]
- [142].Ord P, Gijsbers K. Pain thresholds and tolerances of competitive rowers and their use of spontaneous self-generated pain-coping strategies. Percept Mot Skills 2003;97(3 pt 2):1219–22. [DOI] [PubMed] [Google Scholar]
- [143].Padawer WJ, Levine FM. Exercise-induced analgesia: fact or artifact? PAIN 1992;48:131–5. [DOI] [PubMed] [Google Scholar]
- [144].Paris TA, Misra G, Archer DB, Coombes SA. Effects of a force production task and a working memory task on pain perception. J Pain 2013;14:1492–501. [DOI] [PubMed] [Google Scholar]
- [145].Pazzaglia C, Testani E, Giordano R, Padua L, Valeriani M. Expectation to feel more pain disrupts the habituation of laser-pain rating and laser-evoked potential amplitudes. Neuroscience 2016;333:244–51. [DOI] [PubMed] [Google Scholar]
- [146].Pedersen BK, Saltin B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 2015;25(suppl 3):1–72. [DOI] [PubMed] [Google Scholar]
- [147].Persson AL, Hansson GA, Kalliomaki A, Moritz U, Sjolund BH. Pressure pain thresholds and electromyographically defined muscular fatigue induced by a muscular endurance test in normal women. Clin J Pain 2000;16:155–63. [DOI] [PubMed] [Google Scholar]
- [148].Persson AL, Hansson GA, Kalliomaki J, Sjolund BH. Increases in local pressure pain thresholds after muscle exertion in women with chronic shoulder pain. Arch Phys Med Rehabil 2003;84:1515–22. [DOI] [PubMed] [Google Scholar]
- [149].Pertovaara A, Huopaniemi T, Virtanen A, Johansson G. The influence of exercise on dental pain thresholds and the release of stress hormones. Physiol Behav 1984;33:923–6. [DOI] [PubMed] [Google Scholar]
- [150].Peters ML. Emotional and cognitive influences on pain experience. Mod Trends Pharmacopsychiatri 2015;30:138–52. [DOI] [PubMed] [Google Scholar]
- [151].Peterson JA, Schubert DJ, Campbell J, Bemben MG, Black CD. Endogenous pain inhibitory function: endurance-trained athletes vs active controls. Pain Med 2019;20:1822–30. [DOI] [PubMed] [Google Scholar]
- [152].Piche M, Watanabe N, Sakata M, Oda K, Toyohara J, Ishii K, Ishiwata K, Hotta H. Basal mu-opioid receptor availability in the amygdala predicts the inhibition of pain-related brain activity during heterotopic noxious counter-stimulation. Neurosci Res 2014;81-82:78–84. [DOI] [PubMed] [Google Scholar]
- [153].Pires D, Cruz EB, Caeiro C. Aquatic exercise and pain neurophysiology education versus aquatic exercise alone for patients with chronic low back pain: a randomized controlled trial. Clin Rehabil 2015;29:538–47. [DOI] [PubMed] [Google Scholar]
- [154].Pokhrel BR, Malik SL, Ansari AH, Paudel BH, Sinha R, Sinha M. Effect of sub-maximal exercise stress on cold pressor pain: a gender based study. Kathmandu Univ Med J (KUMJ) 2013;11:54–9. [DOI] [PubMed] [Google Scholar]
- [155].Ram KC, Eisenberg E, Haddad M, Pud D. Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain—new perspective of opioid-induced hyperalgesia. PAIN 2008;139:431–8. [DOI] [PubMed] [Google Scholar]
- [156].Riel H, Vicenzino B, Jensen MB, Olesen JL, Holden S, Rathleff MS. The effect of isometric exercise on pain in individuals with plantar fasciopathy: a randomized crossover trial. Scand J Med Sci Sports 2018;28:2643–50. [DOI] [PubMed] [Google Scholar]
- [157].Ring C, Edwards L, Kavussanu M. Effects of isometric exercise on pain are mediated by blood pressure. Biol Psychol 2008;78:123–8. [DOI] [PubMed] [Google Scholar]
- [158].Rio E, Kidgell D, Purdam C, Gaida J, Moseley GL, Pearce AJ, Cook J. Isometric exercise induces analgesia and reduces inhibition in patellar tendinopathy. Br J Sports Med 2015;49:1277–83. [DOI] [PubMed] [Google Scholar]
- [159].Ruble SB, Hoffman MD, Shepanski MA, Valic Z, Buckwalter JB, Clifford PS. Thermal pain perception after aerobic exercise. Arch Phys Med Rehabil 2005;86:1019–23. [DOI] [PubMed] [Google Scholar]
- [160].Runhaar J, Luijsterburg P, Dekker J, Bierma-Zeinstra SM. Identifying potential working mechanisms behind the positive effects of exercise therapy on pain and function in osteoarthritis; a systematic review. Osteoarthritis Cartilage 2015;23:1071–82. [DOI] [PubMed] [Google Scholar]
- [161].Sacco M, Meschi M, Regolisti G, Detrenis S, Bianchi L, Bertorelli M, Pioli S, Magnano A, Spagnoli F, Giuri PG, Fiaccadori E, Caiazza A. The relationship between blood pressure and pain. J Clin Hypertens 2013;15:600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Samuelly-Leichtag G, Kodesh E, Meckel Y, Weissman-Fogel I. A fast track to hypoalgesia—the anaerobic exercise effect on pain sensitivity. Int J Sports Med 2018;39:473–81. [DOI] [PubMed] [Google Scholar]
- [163].Sandal LF, Roos EM, Bøgesvand SJ, Thorlund JB. Pain trajectory and exercise-induced pain flares during 8 weeks of neuromuscular exercise in individuals with knee and hip pain. Osteoarthritis Cartilage 2016;24:589–92. [DOI] [PubMed] [Google Scholar]
- [164].Sandler CX, Lloyd AR, Barry BK. Fatigue exacerbation by interval or continuous exercise in chronic fatigue syndrome. Med Sci Sports Exerc 2016;48:1875–85. [DOI] [PubMed] [Google Scholar]
- [165].Scheef L, Jankowski J, Daamen M, Weyer G, Klingenberg M, Renner J, Mueckter S, Schürmann B, Musshoff F, Wagner M, Schild HH, Zimmer A, Boecker H. An fMRI study on the acute effects of exercise on pain processing in trained athletes. PAIN 2012;153:1702–14. [DOI] [PubMed] [Google Scholar]
- [166].Schmitt A, Wallat D, Stangier C, Martin JA, Schlesinger-Irsch U, Boecker H. Effects of fitness level and exercise intensity on pain and mood responses. Eur J Pain 2019;24:568–79. [DOI] [PubMed] [Google Scholar]
- [167].Scioli-Salter E, Forman DE, Otis JD, Tun C, Allsup K, Marx CE, Hauger RL, Shipherd JC, Higgins D, Tyzik A, Rasmusson AM. Potential neurobiological benefits of exercise in chronic pain and posttraumatic stress disorder: pilot study. J Rehabil Res Dev 2016;53:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Sharma CV, Mehta V. Paracetamol: mechanisms and updates. Contin Educ Anaesth Crit Care Pain 2014;14:153–58. [Google Scholar]
- [169].Shiri R, Coggon D, Falah-Hassani K. Exercise for the prevention of low back pain: systematic review and meta-analysis of controlled trials. Am J Epidemiol 2018;187:1093–101. [DOI] [PubMed] [Google Scholar]
- [170].Skou ST, Roos EM. Physical therapy for patients with knee and hip osteoarthritis: supervised, active treatment is current best practice. Clin Exp Rheumatol 2019;37(suppl 120):112–17. [PubMed] [Google Scholar]
- [171].Slater H, Theriault E, Ronningen BO, Clark R, Nosaka K. Exercise-induced mechanical hypoalgesia in musculotendinous tissues of the lateral elbow. Man Ther 2010;15:66–73. [DOI] [PubMed] [Google Scholar]
- [172].Smith A, Ritchie C, Pedler A, McCamley K, Roberts K, Sterling M. Exercise induced hypoalgesia is elicited by isometric, but not aerobic exercise in individuals with chronic whiplash associated disorders. Scand J Pain 2017;15:14–21. [DOI] [PubMed] [Google Scholar]
- [173].Smith BE, Hendrick P, Smith TO, Bateman M, Moffatt F, Rathleff MS, Selfe J, Logan P. Should exercises be painful in the management of chronic musculoskeletal pain? A systematic review and meta-analysis. Br J Sports Med 2017;51:1679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Smith MA, Yancey DL. Sensitivity to the effects of opioids in rats with free access to exercise wheels: mu-opioid tolerance and physical dependence. Psychopharmacology (Berl) 2003;168:426–34. [DOI] [PubMed] [Google Scholar]
- [175].St-Aubin MO, Chalaye P, Counil FP, Lafrenaye S. Beneficial effects of regular physical activity on exercise-induced analgesia in adolescent males. Pediatr Exerc Sci 2019;31:425–31. [DOI] [PubMed] [Google Scholar]
- [176].Stackhouse SK, Taylor CM, Eckenrode BJ, Stuck E, Davey H. Effects of noxious electrical stimulation and eccentric exercise on pain sensitivity in asymptomatic individuals. PM R 2016;8:415–24. [DOI] [PubMed] [Google Scholar]
- [177].Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. PAIN 2005;118:176–84. [DOI] [PubMed] [Google Scholar]
- [178].Sternberg WF, Bokat C, Kass L, Alboyadjian A, Gracely RH. Sex-dependent components of the analgesia produced by athletic competition. J Pain 2001;2:65–74. [DOI] [PubMed] [Google Scholar]
- [179].Stolzman S, Danduran M, Hunter SK, Bement MH. Pain response after maximal aerobic exercise in adolescents across weight status. Med Sci Sports Exerc 2015;47:2431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Straszek CL, Rathleff MS, Graven-Nielsen T, Petersen KK, Roos EM, Holden S. Exercise-induced hypoalgesia in young adult females with long-standing patellofemoral pain—a randomized crossover study. Eur J Pain 2019;23:1780–9. [DOI] [PubMed] [Google Scholar]
- [181].Tesarz J, Gerhardt A, Schommer K, Treede RD, Eich W. Alterations in endogenous pain modulation in endurance athletes: an experimental study using quantitative sensory testing and the cold-pressor task. PAIN 2013;154:1022–9. [DOI] [PubMed] [Google Scholar]
- [182].Tesarz J, Schuster AK, Hartmann M, Gerhardt A, Eich W. Pain perception in athletes compared to normally active controls: a systematic review with meta-analysis. PAIN 2012;153:1253–62. [DOI] [PubMed] [Google Scholar]
- [183].Thomas EN, Blotman F. Aerobic exercise in fibromyalgia: a practical review. Rheumatol Int 2010;30:1143–50. [DOI] [PubMed] [Google Scholar]
- [184].Thoren P, Floras JS, Hoffmann P, Seals DR. Endorphins and exercise: physiological mechanisms and clinical implications. Med Sci Sports Exerc 1990;22:417–28. [PubMed] [Google Scholar]
- [185].Tour J, Löfgren M, Mannerkorpi K, Gerdle B, Larsson A, Palstam A, Bileviciute-Ljungar I, Bjersing J, Martin I, Ernberg M, Schalling M, Kosek E. Gene-to-gene interactions regulate endogenous pain modulation in fibromyalgia patients and healthy controls-antagonistic effects between opioid and serotonin-related genes. PAIN 2017;158:1194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Travers M, Moss P, Gibson W, Hince D, Yorke S, Chung C, Langford R, Tan EEW, Ng J, Palsson TA. Exercise-induced hypoalgesia in women with varying levels of menstrual pain. Scand J Pain 2018;18:303–10. [DOI] [PubMed] [Google Scholar]
- [187].Umeda M, Corbin LW, Maluf KS. Examination of contraction-induced muscle pain as a behavioral correlate of physical activity in women with and without fibromyalgia. Disabil Rehabil 2015;37:1864–9. [DOI] [PubMed] [Google Scholar]
- [188].Umeda M, Kempka LE, Greenlee BT, Weatherby AC. A smaller magnitude of exercise-induced hypoalgesia in African Americans compared to non-Hispanic Whites: a potential influence of physical activity. Biol Psychol 2016;113:46–51. [DOI] [PubMed] [Google Scholar]
- [189].Umeda M, Newcomb LW, Ellingson LD, Koltyn KF. Examination of the dose-response relationship between pain perception and blood pressure elevations induced by isometric exercise in men and women. Biol Psychol 2010;85:90–6. [DOI] [PubMed] [Google Scholar]
- [190].Umeda M, Newcomb LW, Koltyn KF. Influence of blood pressure elevations by isometric exercise on pain perception in women. Int J Psychophysiol 2009;74:45–52. [DOI] [PubMed] [Google Scholar]
- [191].Vaegter HB. Exercising non-painful muscles can induce hypoalgesia in individuals with chronic pain. Scand J Pain 2017;15:60–1. [DOI] [PubMed] [Google Scholar]
- [192].Vaegter HB, Bjerregaard LK, Redin MM, Rasmussen SH, Graven-Nielsen T. Hypoalgesia after bicycling at lactate threshold is reliable between sessions. Eur J Appl Physiol 2019;119:91–102. [DOI] [PubMed] [Google Scholar]
- [193].Vaegter HB, Dorge DB, Schmidt KS, Jensen AH, Graven-Nielsen T. Test-retest reliabilty of exercise-induced hypoalgesia after aerobic exercise. Pain Med 2018;19:2212–22. [DOI] [PubMed] [Google Scholar]
- [194].Vaegter HB, Handberg G, Emmeluth C, Graven-Nielsen T. Preoperative hypoalgesia after cold pressor test and aerobic exercise is associated with pain relief 6 Months after total knee replacement. Clin J Pain 2017;33:475–84. [DOI] [PubMed] [Google Scholar]
- [195].Vaegter HB, Handberg G, Graven-Nielsen T. Similarities between exercise-induced hypoalgesia and conditioned pain modulation in humans. PAIN 2014;155:158–67. [DOI] [PubMed] [Google Scholar]
- [196].Vaegter HB, Handberg G, Graven-Nielsen T. Isometric exercises reduce temporal summation of pressure pain in humans. Eur J Pain 2015;19:973–83. [DOI] [PubMed] [Google Scholar]
- [197].Vaegter HB, Handberg G, Graven-Nielsen T. Hypoalgesia after exercise and the cold pressor test is reduced in chronic musculoskeletal pain patients with high pain sensitivity. Clin J Pain 2016;32:58–69. [DOI] [PubMed] [Google Scholar]
- [198].Vaegter HB, Handberg G, Jorgensen MN, Kinly A, Graven-Nielsen T. Aerobic exercise and cold pressor test induce hypoalgesia in active and inactive men and women. Pain Med 2015;16:923–33. [DOI] [PubMed] [Google Scholar]
- [199].Vaegter HB, Hoeger Bement M, Madsen AB, Fridriksson J, Dasa M, Graven-Nielsen T. Exercise increases pressure pain tolerance but not pressure and heat pain thresholds in healthy young men. Eur J Pain 2017;21:73–81. [DOI] [PubMed] [Google Scholar]
- [200].Vaegter HB, Lyng KD, Yttereng FW, Christensen MH, Sorensen MB, Graven-Nielsen T. Exercise-induced hypoalgesia after isometric wall squat exercise: a test-retest reliabilty study. Pain Med 2019;20:129–37. [DOI] [PubMed] [Google Scholar]
- [201].Vaegter HB, Madsen AB, Handberg G, Graven-Nielsen T. Kinesiophobia is associated with pain intensity but not pain sensitivity before and after exercise: an explorative analysis. Physiotherapy 2018;104:187–93. [DOI] [PubMed] [Google Scholar]
- [202].Van Oosterwijck J, Nijs J, Meeus M, Lefever I, Huybrechts L, Lambrecht L, Paul L. Pain inhibition and postexertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: an experimental study. J Intern Med 2010;268:265–78. [DOI] [PubMed] [Google Scholar]
- [203].Van Oosterwijck J, Nijs J, Meeus M, Van Loo M, Paul L. Lack of endogenous pain inhibition during exercise in people with chronic whiplash associated disorders: an experimental study. J Pain 2012;13:242–54. [DOI] [PubMed] [Google Scholar]
- [204].van Weerdenburg LJ, Brock C, Drewes AM, van Goor H, de Vries M, Wilder-Smith OH. Influence of exercise on visceral pain: an explorative study in healthy volunteers. J Pain Res 2017;10:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Vecchiet L, Marini I, Colozzi A, Feroldi P. Effects of aerobic exercise on muscular pain sensitivity. Clin Ther 1984;6:354–63. [PubMed] [Google Scholar]
- [206].Vierck CJ, Jr, Staud R, Price DD, Cannon RL, Mauderli AP, Martin AD. The effect of maximal exercise on temporal summation of second pain (windup) in patients with fibromyalgia syndrome. J Pain 2001;2:334–44. [DOI] [PubMed] [Google Scholar]
- [207].Weissman-Fogel I, Sprecher E, Pud D. Effects of catastrophizing on pain perception and pain modulation. Exp Brain Res 2008;186:79–85. [DOI] [PubMed] [Google Scholar]
- [208].Whiteside A, Hansen S, Chaudhuri A. Exercise lowers pain threshold in chronic fatigue syndrome. PAIN 2004;109:497–9. [DOI] [PubMed] [Google Scholar]
- [209].Wingenfeld K, Wolf S, Kunz M, Krieg JC, Lautenbacher S. No effects of hydrocortisone and dexamethasone on pain sensitivity in healthy individuals. Eur J Pain 2015;19:834–41. [DOI] [PubMed] [Google Scholar]
- [210].Wonders KY, Drury DG. Exercise intensity as a determinant of exercise induced hypoalgesia. J Exerc Physiol (Online) 2011;14:134–44. [Google Scholar]
- [211].Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 2010;23:611–15. [DOI] [PubMed] [Google Scholar]
- [212].Yarnitsky D, Arendt-Nielsen L, Bouhassira D, Edwards RR, Fillingim RB, Granot M, Hansson P, Lautenbacher S, Marchand S, Wilder-Smith O. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain 2010;14:339. [DOI] [PubMed] [Google Scholar]