Abstract
Background:
To explore the prognostic value of diverse subsets of tumor-associated macrophages (TAMs) in prognosis in patients with nasopharyngeal carcinoma (NPC) using meta-analysis.
Methods:
Relevant studies were searched in the database of PubMed, Web of Science, Embase, Cochrane Library, Scopus, China National Knowledge Infrastructure (CNKI), and Wanfang till November 2019. The relationship between TAMs and survival outcomes was estimated by pooling hazard ratios (HRs) and 95% confidence intervals (CIs); and the correlation of TAMs and clinicopathological factors was evaluated by using odds ratios (ORs) and 95%CIs.
Results:
Six studies with 1549 patients were included in this meta-analysis. The high expression of CD68+ TAMs was associated with favorable disease-free survival (DFS) (HR = 0.66, 95%CI = 0.50–0.88, P = .005), whereas the density of M2-like TAMs (CD163+, CD68+CCL18+, and CD206+) was correlated to poor overall survival (OS) (HR = 1.77, 95%CI = 1.22–2.56, P = .003) and DFS (HR = 1.96, 95%CI = 1.00–3.85, P = .050) in patients with NPC.
Conclusions:
CD68+ TAM density is associated with superior DFS, while CD163+ M2-like TAMs predicted poor prognosis in patients with NPC.
Keywords: clinical value, meta-analysis, nasopharyngeal carcinoma, prognosis, tumor-associated macrophages
1. Introduction
Nasopharyngeal carcinoma (NPC) is originated from the lining of the nasopharynx and is a rare cancer type, accounting for 0.7% of all new cases and 0.8% of all cancer-related deaths worldwide.[1] It is estimated that about 129,079 new cases and 72,987 deaths are attributed to NPC around the world in 2018.[1] NPC is an endemic cancer in Southeast Asia (especially southern China), where the incidence could be as high as 20 per 100,000 person-years.[2] NPC has a high propensity to distant metastasis among all head and neck cancers. Due to its radiosensitive and chemosensitive behavior and the deep-seated anatomic location, radiotherapy (RT) alone and concurrent chemoradiotherapy (CCRT) are the main treatment methods for NPC.[3] Although Tumor-Node-Metastasis (TNM) staging system and plasma Epstein-Barr virus (EBV) DNA provide important prognostic implications for patients with NPC[4]; the survival outcomes in patients with stage IV disease is poor, with a 5-year survival rate being <10%.[5] Therefore, it is important to identify effective markers that could help in survival prognosis and could also be served as therapeutic targets. The recent advances[6–8] in prognostic markers provided important evidence of clinical use of those indicators. For patients with colorectal cancer (CRC), Metadherin mRNA expression is a useful non-invasive biomarker.[6] Metadherin mRNA expression is effective for screening and early diagnosis of CRC; and is correlated to advanced tumor stage and a poor prognosis.[6] Circulating high-temperature-required protein A2 (HtrA2) mRNA expression was reported as a significant diagnostic marker for breast cancer.[7] Decreased serum HtrA2 expression was associated with poor histological grade and advanced TNM stages in breast cancer patients.[7] Moreover, Octamer-binding transcription factor 4 (Oct4), which plays a pivotal role in stem cell differentiation and self-renewal, is shown to be connected with progression and prognosis of gastric carcinoma.[8] In the tumor microenvironment of NPC, substantial immune cells are infiltering and consists of the immune microenvironment.
Tumor-associated macrophages (TAMs) are significant components of the tumor microenvironment; and TAMs could also affect the tumor microenvironment, leading to tumor progression.[9] TAMs are generally identified by expressing cell surface marker CD68.[10] Macrophages can be defined into 2 polarized phenotypes: the “classically activated” M1 macrophages and “alternatively activated” M2 macrophages.[11] M1 macrophages can be induced by interferon-γ, lipopolysaccharide (LPS), and toll like receptor (TLR), and express a high level of CD86, CD40, and PD-L1.[12] M1 macrophages play pivotal roles in the elimination of pathogens and cancer.[13] In contrast, M2 macrophages are generally characterized by the expression of CD163, CD206, CD204 and production of anti-inflammatory factors (IL-10, TGFβ) and chemokines (CCL5, CCL17, CCL18, and CCL22) to facilitate tumor progression.[13] TAMs are closely resembling M2 subtype and can constitute up to 80% of tumor content.[14,15] Previous studies have explored the prognostic significance of TAMs in patients with NPC, whereas the results are inconsistent due to different TAMs markers (CD68/CD163/CD206) and distinct distribution of TAMs in tumor (intratumor [IT] or peritumor [PT] or both).[16–21] For example, some investigators reported TAMs as prognostic factors for poor prognosis,[16,18] whereas some other researchers found high density of TAMs were associated with favorable survival outcomes.[17] Therefore, we systemically searched relevant studies and performed a comprehensive meta-analysis according to different markers of TAMs in different tumor distribution. In the current meta-analysis, we investigated the difference of survival outcomes between NPC patients with high and low expression of TAMs.
2. Materials and methods
2.1. Literature search
The current meta-analysis was conducted under the guideline of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.[22] An electronic retrieval was performed in PubMed, Web of Science, Embase, Cochrane Library, Scopus, China National Knowledge Infrastructure (CNKI), and Wanfang databases. The last search was up to November 2019. The period was from inception to the last search, and the search was conducted by YLC. The search terms were: “tumor-associated macrophage,” “tumor-infiltrating macrophage,” “intratumoral macrophage,” “macrophage,” “nasopharyngeal carcinoma,” “nasopharyngeal cancer,” and “nasopharynx cancer.” The reference lists of the retrieved articles were also carefully examined to identify eligible studies. Because the current study is a meta-analysis and does not involve the collection of samples; therefore, the ethical approval is not required. The various subsets of TAMs in different tissue localization were combined to minimize the heterogeneity among studies.
2.2. Inclusion and exclusion criteria
The inclusion criteria for eligible studies were as follows: the patients were histologically diagnosed with NPC; the expression of TAMs was detected using immunochemistry (IHC) method in intratumor (IT) and/or tumor stroma (TS); a cut-off value to stratify high/low TAMs expression was determined; the relationship between TAMs expression and overall survival (OS) and disease-free survival (DFS) was investigated; the hazard ratios (HRs) and their 95% confidence intervals (95% CIs) for survival analysis were reported or sufficient data were given for HRs and 95%CIs calculation[23]; full-text studies published in English or Chinese language; there was no limitation to study design. Exclusion criteria were as follows: studies not providing sufficient data for necessary analysis; studies including overlapped patients; case reports, meeting abstracts, reviews, comments, and letters; animal studies. The inclusion and exclusion processes were performed by YLC in accordance with PRISMA guideline.
2.3. Quality assessment
Quality of the included studies was evaluated by the investigator (YLC) using Newcastle-Ottawa Scale (NOS).[24] The NOS assessed items consist of 3 parts: selection (0–4 stars), comparability (0–2 stars), and outcome (0–3 stars). Each individual study was scored between 0 and 9; and studies scored ≥6 were considered as high-quality.
2.4. Data extraction
The following information were extracted from the included studies: first author, publication year, country, sample size, patient age, sex, study duration, cut-off value, antibody used for the evaluation, treatment, tumor stage, follow-up, study design, NOS score, HRs and 95%CIs for OS and/or DFS, and clinicopathological factors.
2.5. Statistical analysis
The relationship between TAMs and survival outcomes was estimated by pooling HRs and 95%CIs; and the correlation of TAMs and clinicopathological factors was evaluated by using odds ratios (ORs) and 95%CIs. The heterogeneity among studies was evaluated by using the chi-square-based Q test and I2 statistics. In case of significant heterogeneity (I2 > 50% or P < .10), a random-effects model was used for analysis. Otherwise, a fixed-effects model was applied for calculation. Sensitivity analysis was performed to assess the stability of the pooled results. Publication bias was analyzed by using Begg funnel plot test. Stata12.0 (Stata Corporation, College Station, TX) software was used for all statistical analysis. A 2-sided P < .05 were considered statistically significant.
3. Results
3.1. Study selection
The detailed screening process was shown in Fig. 1. As shown in Fig. 1, the initial literature search yielded a total of 888 records. After duplicate records were removed, 539 studies remained. By screening title and abstract, 513 studies were eliminated. A total of 26 studies were evaluated by full-text examination. And 20 studies were discarded due to the following reasons: 19 studies did not provide HRs and 95%CIs for analysis and 1 study included overlapped patients. At last, 6 studies were included in this meta-analysis.[16–21]
Figure 1.
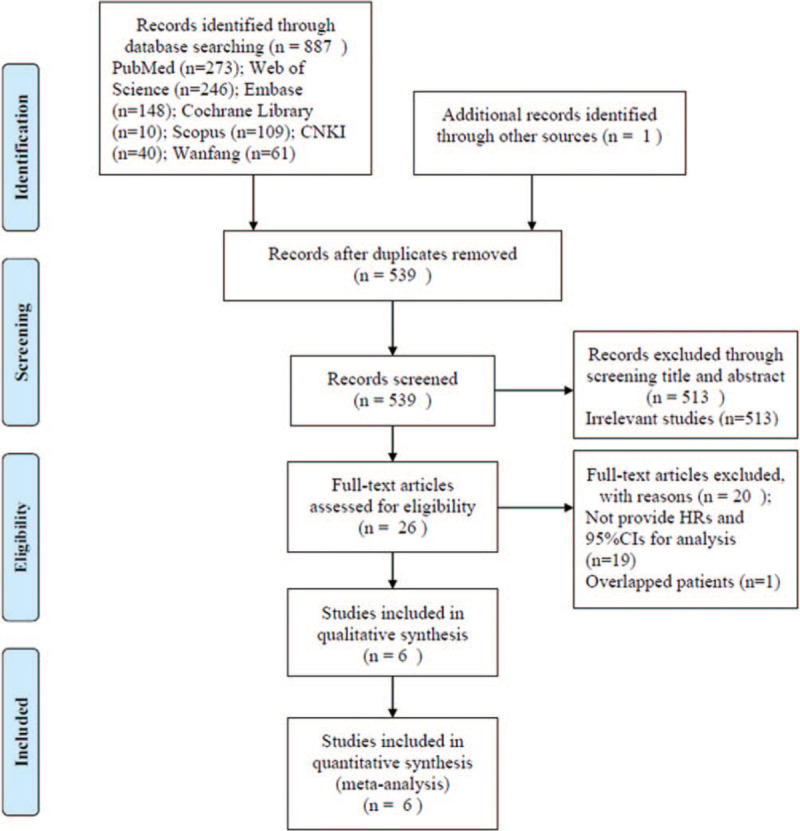
Flow diagram of study identification and selection.
3.2. The characteristics of included studies
The main characteristics of included studies are presented in Table 1. The included studies were all from China and used IHC method for TAMs detection. The included studies were published from 1999 to 2018. The total sample size was 1549, ranging from 43 to 580. Four studies marked overall TAMs as CD68(+).[16–18,21] Three studies marked M2-like TAMs as CD163+.[19–21] Two studies identified M2-like TAMs as CD68(+)CCL18(+)[18] and CD163(+).[21] All studies detected TAMs expression in intratumor (IT)[16–21] and 2 studies also measured TAMs expression in tumor stroma (TS).[17,19] All 6 studies reported the association between TAMs expression and OS,[16–21] and 5 studies presented the correlation between TAMs and DFS.[17–21] Three studies were published in the English language[18,19,21] and 3 studies were published in the Chinese language.[16,17,20] The NOS scores of the included 6 studies ranged from 6 to 8, which indicated that they were all high-quality studies.
Table 1.
Characteristics of 6 eligible studies in the meta-analysis.

3.3. CD68+ TAMs and OS and DFS
Four studies comprising 1332 cases investigated the association between density of CD68+ TAMs and prognosis in patients with NPC.[16–18,21] There were 4 studies[16–18,21] providing the data on the association of OS and density of CD68+ TAMs in IT. Because significant heterogeneity (I2 = 87.2%, P < .001) was detected, a random-effects model was used. The pooled results were HR = 1.05, 95%CI = 0.52–2.10, P = .890 (Fig. 2A; Table 2), indicating that high density of CD68+ TAMs in IT had non-significant prognostic value for OS. Two studies[17,21] provided information on the association of DFS and density of CD68+ TAMs in IT. The pooled HR and 95%CI were HR = 0.69, 95%CI = 0.54–0.88, P = .003 (I2 = 0%, P = .621; fixed-effect model; Fig. 2B, Table 2). The data suggested that high density of CD68+ TAMs in IT predicted superior DFS in patients with NPC. One included study[17] provided the data of the relationship of density of CD68+ TAMs in TS and OS and DFS. The data indicated that high expression of CD68+ TAMs in TS was associated with favorable OS (HR = 0.66, 95%CI = 0.49–0.90, P = .008) and favorable DFS (HR = 0.66, 95%CI = 0.50–0.88, P = .005) in patients with NPC (Table 2).
Figure 2.

Forest plots showing hazard ratios (HRs) and 95% CIs for (A) density of CD68+ TAMs in IT and OS; (B) density of CD68+ TAMs in IT and DFS; (C) density of CD163+ M2-like TAMs in IT and OS; and (D) density of CD163+ M2-like TAMs in IT and DFS. CI = confidence interval; DFS = disease-free survival; IT = intratumor; OS = overall survival; TAMs = tumor-associated macrophages.
Table 2.
The pooled associations between TAMs subsets and the prognosis of patients with NPC.
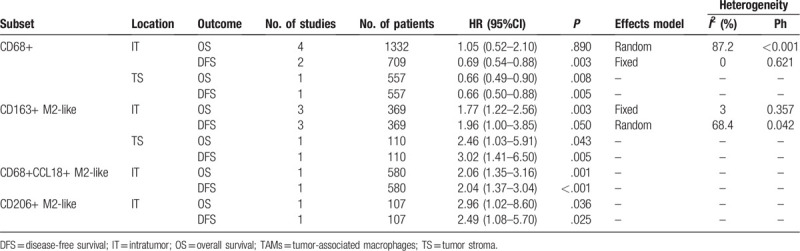
3.4. CD163+ M2-like TAMs and OS and DFS
TAMs expressing CD163 are identified as M2-like TAMs.[25] Three studies including 369 patients[19–21] offered the data on the prognostic value of CD163+ M2-like TAMs in IT on OS and DFS. The pooled data showed that high density of CD163+ M2-like TAMs in IT was correlated to inferior OS (HR = 1.77, 95%CI = 1.22–2.56, P = .003, fixed-effects model; Fig. 2C, Table 2) and inferior DFS (HR = 1.96, 95%CI = 1.00–3.85, P = .050, random-effects model; Fig. 2D, Table 2) in patients with NPC. According to data from one study with 110 patients, high expression of CD163+ M2-like TAMs in TS also predicted worse OS and poor DFS (Table 2) in patients with NPC.
3.5. CD68+CCL18+ M2-like and CD206+ M2-like TAMs and prognosis
Based on data of one study with 580 subjects,[18] elevated expression of CD68+CCL18+ M2-like TAMs in IT predicted poorer OS (HR = 2.06, 95%CI = 1.35–3.16, P = .001) and inferior DFS (HR = 2.04, 95%CI = 1.37–3.04, P < .001) (Table 2) in patients with NPC. According to a study comprising 107 cases, high density of CD206+ M2-like TAMs in IT was connected to unfavorable OS (HR = 2.96, 95%CI = 1.02–8.60, P = .036) as well as worse DFS (HR = 2.49, 95%CI = 1.08–5.70, P = .025) (Table 2) in patients with NPC.
3.6. Association between TAMs and clinicopathological characteristics of NPC
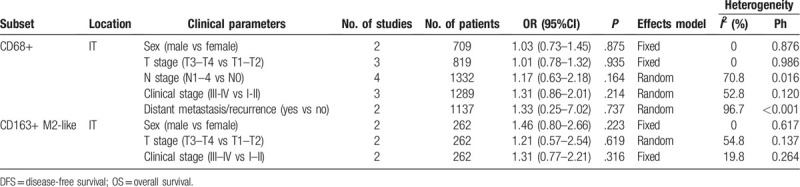
A total of 5 studies with 1442 patients[16–19,21] investigated the relationship between TAMs and clinicopathological features. The detailed pooled results were shown in Table 3. The combined data suggested that high expression of CD68+ TAMs in IT had non-significant relevance with sex (n = 2, OR = 1.03, 95%CI = 0.73–1.45, P = .875), T stage (n = 3, OR = 1.01, 95%CI = 0.78–1.32, P = .935), N stage (n = 4, OR = 1.17, 95%CI = 0.63–2.18, P = .164), clinical stage (n = 3, OR = 1.31, 95%CI = 0.86–2.01, P = .214), or distant metastasis/recurrence (n = 2, OR = 1.33, 95%CI = 0.25–7.02, P = .737) (Table 3). In addition, according to pooled data derived from 2 studies,[19,21] high density of CD163+ M2-like TAMs were not significantly correlated with sex (n = 2, OR = 1.46, 95%CI = 0.80–2.66, P = .223), T stage (n = 2, OR = 1.21, 95%CI = 0.57–2.54, P = .619), or clinical stage (n = 2, OR = 1.31, 95%CI = 0.77–2.21, P = .316) (Table 3).
Table 3.
The relationship between TAMs and clinicopathological characteristics.
3.7. Sensitivity analysis
To test the stability of the pooled results, sensitivity analysis was carried out by omitting each eligible study. As shown in Fig. 3A–D, the combined results were not substantially altered by any individual study. Therefore, the results of meta-analysis were reliable.
Figure 3.

Sensitivity analysis for the (A) density of CD68+ TAMs in IT and OS; (B) density of CD68+ TAMs in IT and DFS; (C) density of CD163+ M2-like TAMs in IT and OS; and (D) density of CD163+ M2-like TAMs in IT and DFS. CI = confidence interval; DFS = disease-free survival; IT = intratumor; OS = overall survival; TAMs = tumor-associated macrophages.
3.8. Publication Bias
Begg funnel plot was conducted to test potential publication bias. The funnel plots were shown in Fig. 4A–D. The results indicated that there was no significant publication in the current meta-analysis.
Figure 4.
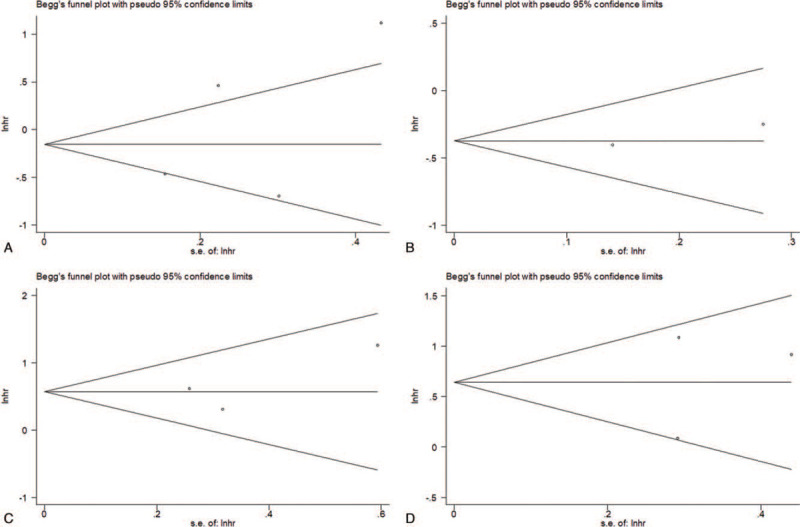
Publication bias examined by Begg plot test for (A) density of CD68+ TAMs in IT and OS (P = .734); (B) density of CD68+ TAMs in IT and DFS (P = 1); (C) density of CD163+ M2-like TAMs in IT and OS (P = .602); and density of CD163+ M2-like TAMs in IT and DFS (P = 1). CI = confidence interval; DFS = disease-free survival; IT = intratumor; OS = overall survival; TAMs = tumor-associated macrophages.
4. Discussion
TAMs are an extremely heterogeneous cell population regarding morphology, functions, and tissue location. TAMs also showed different even conflicting prognostic significance for patients with NPC based on relevant studies.[16–21] The results demonstrated that high density of CD68+ TAMs in IT predicted favorable DFS, whereas high density of CD68+ TAMs in TS was a predictor of superior prognosis. Moreover, high density of CD163+ M2-like TAMs, CD68+CCL18+ M2-like TAMs, and CD206+ M2-like TAMs were all connected with poor survival outcomes in patients with NPC. However, significant correlation between TAMs and clinicopathological features was not found based on pooled results. Collectively, the present meta-analysis indicated that CD68+ TAMs in IT predicted poor DFS, and high density of various subsets of M2-like TAMs was predictive of inferior prognosis in patients with NPC. To our knowledge, the current study is the first meta-analysis exploring the prognostic significance of TAMs in patients with NPC.
TAMs are a diverse collection of cell types and exert a wide range of biological and pathological roles in tumor environment.[25] CD68 is the most extensively used marker of macrophages and is used to identify overall TAMs.[26,27] Whereas M1 and M2 subsets of TAMs are identified according to polarization.[28] M1 TAMs are known to induce inflammation and play a crucial role in anti-tumor activity. Whereas, M2 TAMs are related to tumor growth, angiogenic, and immunosuppressive functions.[29] M2 TAMs can also promote tumor cell trans endothelial migration through interaction with tumor cells.[30] Previous meta-analyses also explored the prognostic role of different subsets of TAMs in various cancer types.[31] A recent meta-analysis including 17 studies with 3547 patients suggested that a high density of M2 TAMs in IT was significantly correlated with OS in patients with hepatocellular carcinoma (HCC).[32] Whereas CD68+ TAMs in the IT or TS have no prognostic effects on OS in HCC. Another meta-analysis focusing on TAMs and Non-Hodgkin's lymphoma (NHL) showed that high-density CD68+ TAMs was associated with poor OS and poor PFS, which suggested that TAMs was a robust predictor of outcomes in NHL.[33] In addition, a recent meta-analysis demonstrated that high stromal expression of CD163+ TAMs correlated with both poor OS and poor DFS in patients with squamous cell carcinoma of the head and neck (SCCHN). However, abundance of CD68+ TAMs was not associated with OS or DFS in SCCHN.[34]
In the present meta-analysis, the results showed the prognostic of CD68+ TAMs in IT was also not significant for OS in patients with NPC, which was in accordance with the results of SCCHN.[34] Referring relevant meta-analyses on TAMs,[10,27,35,36] I defined TAMs as CD68+ as the overall cell population and CD163+, CD68+CCL18+, and CD206+ as M2-like TAMs. The tissue distribution (IT and/or TS) was used to further stratify the location of diverse subsets of TAMs. The CD68+ TAMs or different subsets of M2-like TAMs in IT or TS are incorporated for meta-analysis, which guarantees the homogeneity of the TAMs subpopulation. In the present meta-analysis, we found the high density of CD68+ TAMs in IT were associated with favorable DFS in NPC, which was in line with the results of TAMs in non-small cell lung cancer (NSCLC).[37] In addition, we identified M2-like TAMs as 3 different cell subpopulations (CD163+, CD68+CCL18+, and CD206+). The pooled data indicated that abundance of all 3 subsets of M2-like TAMs were correlated to poor OS and DFS in patients with NPC. These findings validated the prognostic role of CD163+ M2-like and CD206+ M2-like TAMs in NPC, as the results derived from other cancer types including pancreatic cancer,[38] HCC,[32] esophageal cancer,[39] and bladder cancer.[40] More importantly, for the first time, we reported the significant impact of CD68+CCL18+ M2-like TAMs in meta-analysis. Those results highlighted the potential prognostic and therapeutic value of CD68+CCL18+ M2-like TAMs in patients with NPC. The results of this meta-analysis should be validated in non-Asian patients in clinical trials and open datasets.
Although this is the first meta-analysis focusing on TAMs and prognosis in patients with NPC, there are still several limitations should be acknowledged. First, the sample size was relatively small. Only 6 studies were included, and the data of CD68+CCL18+ M2-like and CD206+ M2-like TAMs on prognosis were extracted from an individual study. Second, all included studies were from China, which may compromise the generalizability of the results to patients in other countries or with other ethnicity. Third, the antibodies to PD-L1 and cut-off values varied in included studies; and the treatment methods were not uniform. Those elements could introduce inherent heterogeneity to this meta-analysis and affect the reliability of results. Fourth, all eligible studies were of retrospective study design, which may increase heterogeneity to this meta-analysis.
5. Conclusions
The presented meta-analysis demonstrated that the high expression of CD68+ TAMs was associated with favorable DFS, whereas the density of M2-like TAMs (CD163+, CD68+CCL18+, and CD206+) was correlated to poor prognosis in patients with NPC. Nevertheless, due to several limitations, further randomized controlled trials recruiting patients from various countries are needed to warrant our results.
Author contributions
Ya-Lian Chen designed the project and performed data extraction and analysis. Ya-Lian Chen performed the quality assessment and drafted the article. Ya-Lian Chen revised the manuscript critically and supervised the project. Author read and approved the final manuscript.
Footnotes
Abbreviations: CCRT = concurrent chemoradiotherapy, CI = confidence interval, CNKI = China National Knowledge Infrastructure, CRC = colorectal cancer, DFS = disease-free survival, EBV = Epstein-Barr virus, HR = hazard ratio, HtrA2 = high-temperature-required protein A2, IHC = immunochemistry, IT = intratumor, LPS = lipopolysaccharide, NOS = Newcastle-Ottawa Scale, NPC = nasopharyngeal carcinoma, Oct4 = Octamer-binding transcription factor 4, OS = overall survival, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, PT = peritumor, RT = radiotherapy, TAMs = tumor-associated macrophages, TLR = toll like receptor, TNM = tumor-node-metastasis, TS = tumor stroma.
How to cite this article: Chen YL. Prognostic significance of tumor-associated macrophages in patients with nasopharyngeal carcinoma: A meta-analysis. Medicine. 2020;99:39(e21999).
The author declares that there are no sources of funding to be acknowledged.
The author declares that he has no competing interests.
The author has no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 2006;15:1765–77. [DOI] [PubMed] [Google Scholar]
- [3].Sun XS, Li XY, Chen QY, et al. Future of radiotherapy in nasopharyngeal carcinoma. Br J Radiol 2019;92:20190209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet 2019;394:64–80. [DOI] [PubMed] [Google Scholar]
- [5].Jain A, Chia WK, Toh HC. Immunotherapy for nasopharyngeal cancer-a review. Chin Clin Oncol 2016;5:22. [DOI] [PubMed] [Google Scholar]
- [6].Abdel Ghafar MT, Gharib F, Abdel-Salam S, et al. Role of serum Metadherin mRNA expression in the diagnosis and prediction of survival in patients with colorectal cancer. Mol Biol Rep 2020;47:2509–19. [DOI] [PubMed] [Google Scholar]
- [7].Ghafar MTA, Gharib F, Al-Ashmawy GM, et al. Serum high-temperature-required protein A2: a potential biomarker for the diagnosis of breast cancer. Gene Rep 2020;20:100706. [Google Scholar]
- [8].El-Guindy DM, Wasfy RE, Abdel Ghafar MT, et al. Oct4 expression in gastric carcinoma: association with tumor proliferation, angiogenesis and survival. J Egypt Natl Canc Inst 2019;31:3. [DOI] [PubMed] [Google Scholar]
- [9].Allavena P, Sica A, Garlanda C, et al. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev 2008;222:155–61. [DOI] [PubMed] [Google Scholar]
- [10].Zhao X, Qu J, Sun Y, et al. Prognostic significance of tumor-associated macrophages in breast cancer: a meta-analysis of the literature. Oncotarget 2017;8:30576–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012;122:787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mills CD, Ley K. M1 and M2 macrophages: the chicken and the egg of immunity. J Innate Immun 2014;6:716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tamura R, Tanaka T, Yamamoto Y, et al. Dual role of macrophage in tumor immunity. Immunotherapy 2018;10:899–909. [DOI] [PubMed] [Google Scholar]
- [14].Zhong XM, Chen B, Yang ZW. The role of tumor-associated macrophages in colorectal carcinoma progression. Cell Physiol Biochem 2018;45:356–65. [DOI] [PubMed] [Google Scholar]
- [15].Mantovani A, Marchesi F, Malesci A, et al. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017;14:399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].He C, Zhuang HG, Zhang WY. Immunocyte reaction in stage III nasopharyngeal carcinoma in relation to biological findings and prognosis. Chin J Pathol 1999;34–7. [PubMed] [Google Scholar]
- [17].Cai MB, Shao JY, Zeng YX, et al. Influence of CD68+ macrophages in different parts in the tumor microenvironment on progression and prognosis of nasopharyngeal carcinoma. Med Sci J Central South China 2016;44:258–62. [Google Scholar]
- [18].Huang D, Song SJ, Wu ZZ, et al. Epstein-Barr Virus-induced VEGF and GM-CSF drive nasopharyngeal carcinoma metastasis via recruitment and activation of macrophages. Cancer Res 2017;77:3591–604. [DOI] [PubMed] [Google Scholar]
- [19].Huang HR, Liu X, Zhao FP, et al. M2-polarized tumour-associated macrophages in stroma correlate with poor prognosis and Epstein-Barr viral infection in nasopharyngeal carcinoma. Acta Otolaryngol 2017;137:888–94. [DOI] [PubMed] [Google Scholar]
- [20].Xia TL, Song SJ, Hu ZY, et al. Tumor-associated macrophages promote invasion and metastasis of nasopharyngeal carcinoma. J Trop Med 2017;17:333–6. 342, 413. [Google Scholar]
- [21].Yu YH, Ke LR, Lv X, et al. The prognostic significance of carcinoma-associated fibroblasts and tumor-associated macrophages in nasopharyngeal carcinoma. Cancer Manag Res 2018;10:1935–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [23].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [25].DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol 2019;19:369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lissbrant IF, Stattin P, Wikstrom P, et al. Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol 2000;17:445–51. [DOI] [PubMed] [Google Scholar]
- [27].Ding W, Tan Y, Qian Y, et al. Clinicopathologic and prognostic significance of tumor-associated macrophages in patients with hepatocellular carcinoma: a meta-analysis. PLoS One 2019;14:e0223971. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [28].Najafi M, Goradel NH, Farhood B, et al. Macrophage polarity in cancer: a review. J Cell Biochem 2019;120:2756–65. [DOI] [PubMed] [Google Scholar]
- [29].Krishnan V, Schaar B, Tallapragada S, et al. Tumor associated macrophages in gynecologic cancers. Gynecol Oncol 2018;149:205–13. [DOI] [PubMed] [Google Scholar]
- [30].Pignatelli J, Bravo-Cordero JJ, Roh-Johnson M, et al. Macrophage-dependent tumor cell transendothelial migration is mediated by Notch1/Mena(INV)-initiated invadopodium formation. Sci Rep 2016;6:37874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang QW, Liu L, Gong CY, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One 2012;7:e50946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang J, Chang L, Zhang X, et al. Meta-analysis of the prognostic and clinical value of tumor-associated macrophages in hepatocellular carcinoma. J Investig Surg 2019;1–0. [DOI] [PubMed] [Google Scholar]
- [33].Xu X, Li Z, Liu J, et al. The prognostic value of tumor-associated macrophages in Non-Hodgkin's Lymphoma: a systematic review and meta-analysis. Scand J Immunol 2020;91:e12814. [DOI] [PubMed] [Google Scholar]
- [34].Troiano G, Caponio VCA, Adipietro I, et al. Prognostic significance of CD68(+) and CD163(+) tumor associated macrophages in head and neck squamous cell carcinoma: a systematic review and meta-analysis. Oral Oncol 2019;93:66–75. [DOI] [PubMed] [Google Scholar]
- [35].Yin SC, Huang JY, Li Z, et al. The prognostic and clinicopathological significance of tumor-associated macrophages in patients with gastric cancer: a meta-analysis. PLoS One 2017;12:e0170042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Alves AM, Diel LF, Lamers ML. Macrophages and prognosis of oral squamous cell carcinoma: a systematic review. J Oral Pathol Med 2018;47:460–7. [DOI] [PubMed] [Google Scholar]
- [37].Mei JD, Xiao ZL, Guo CL, et al. Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: a systemic review and meta-analysis. Oncotarget 2016;7:34217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yu M, Guan RG, Hong WF, et al. Prognostic value of tumor-associated macrophages in pancreatic cancer: a meta-analysis. Cancer Manag Res 2019;11:4141–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li JF, Xie YF, Wang XL, et al. Prognostic impact of tumor-associated macrophage infiltration in esophageal cancer: a meta-analysis. Future Oncol 2019;15:2303–17. [DOI] [PubMed] [Google Scholar]
- [40].Wu SQ, Xu R, Li XF, et al. Prognostic roles of tumor associated macrophages in bladder cancer: a system review and meta-analysis. Oncotarget 2018;9:25294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]


