Abstract
Background.
Around 7% of women who undergo breast-conserving surgery (BCS) or mastectomy are at risk of developing ipsilateral breast tumor recurrence (IBTR). When assessing risks that, like that of IBTR, depend on multiple clinicopathological variables, nomograms are the predictive tools of choice. In this study, we constructed two independent nomograms to estimate the individualized risk of IBTR after breast surgery.
Methods.
18,717 consecutive patients with primary invasive breast cancer were enrolled in this retrospective study. The training set used for building the nomograms comprised 15,124 patients (11,627 treated with BCS and 3,497 with mastectomy), while the validation set included 3,593 women (2,565 BCS and 1,028 mastectomy). Median follow-up time was 8 years in the training set and 6 years in the validation set. Multivariable Cox proportional Hazards regression was used to identify independent factors for IBTR. Two separated nomograms were constructed on multivariate models for BCS and mastectomy, respectively.
Results.
We identified the factors that associated with IBTR after either BCS or mastectomy. The two multivariable models were used to build nomograms for the prediction of IBTR 1 year, 5 years, and 10 years after BCS or after mastectomy. 5-year and 10-year IBTR rates in the BCS training set were equal to 3.50% and 7.00%, respectively and to 5.39% and 7.94% in the mastectomy training set. The nomograms were subsequently validated with c-index values of 0.77 and 0.69 respectively in the BCS and mastectomy validation sets.
Conclusion.
The nomograms presented in this study provide clinicians and patients with a valuable decision-making tool for choosing between different treatment options for invasive breast cancer.
Keywords: nomogram, local relapse, invasive breast cancer, breast-conserving surgery, ipsilateral breast tumor recurrence
INTRODUCTION
Ipsilateral breast tumor recurrence (IBTR) occurs in about 7% of patients who have undergone primary breast surgery with curative aim1, be it either breast-conservative surgery (BCS) or mastectomy. Risk of IBTR is estimated on the basis of margins status2, age at onset, tumor size and grade, lymphovascular invasion, tumor biology3–6, and adjuvant treatments.7–10 In particular, radiation therapy (RT), hormone therapy and chemotherapy are known to significantly decrease the risk of IBTR after breast surgery.1 Postoperative RT is recommended in selected cases, i.e., after BCS. However, while a recent study demonstrated that postmastectomy RT reduces the risk of locoregional recurrence by 12.7% in patients with stage T1–2N1 breast cancer11, clinicians are reluctant to consider all patients with invasive breast cancer “potentially” eligible for RT, especially after mastectomy.
To date, in addition to the traditional clinicopathological variables, molecular biomarkers predictive of IBTR are available in selected cases. The Oncotype DX 21-gene recurrence score (Genomic Health, Redwood City, CA), for example, is considered to be a statistically significant predictor of local recurrence for estrogen receptor (ER) positive tumors.12–13 However, due to breast cancer heterogeneity, large-scale genomic analyses of invasive cancer able to predict IBTR are not yet available.
While awaiting a multi-panel genomic predictor to be developed, physicians currently rely on the integration of multiple traditional clinicopathological variables to obtain an individualized risk estimate of IBTR that can assist them and their patients in choosing the best treatment options.
Nomograms are graphical depictions of statistically based prediction models that calculate individualized risk estimates by integrating multiple prognostic variables. Individual patients are scored based on significant risk factors, and their overall probability for a specific outcome is provided by the relevant nomogram. In the case of breast cancer, several nomograms have been formulated that predict the risk of cancer recurrence in response to chemotherapy14,15, or after ductal carcinoma in situ (DCIS) treatment.16 For example, the risk-estimation model that was developed and validated to predict the probability of IBTR in DCIS established that both the omission of RT or endocrine therapy, and close or positive margins were significantly associated with IBTR.16 The nomogram in question predicted the probability for IBTR from a final score calculated on the basis of the relevant risk factors.
The purpose of the present study was to develop nomograms able to predict the probability of IBTR in women with invasive breast cancer 5 years after they underwent either BCS or mastectomy.
PATIENTS AND METHODS
Study Design
Information regarding 18,717 consecutive patients operated for a first primary invasive breast cancer at the European Institute of Oncology (IEO) between 2000–2008 was retrieved from the IEO breast cancer database. Women who had either received neoadjuvant treatment or presented with metastatic breast disease at the time of admission, or within 3 months after surgery, were a priori excluded as well as a selected group of 87 women who received Trastuzumab for HER2 positive breast cancer.
Nomograms were built using a cohort of 15,124 patients (11,627 of which treated with BCS and 3,497 with mastectomy) diagnosed between 2000–2006 as training set, and then validated on a set of 3,593 patients (2,565 BCS and 1,028 mastectomy) treated between 2007–2008. Patients were scheduled for follow-up at 6-month intervals for almost 5 years at our outpatient clinic, and yearly thereafter. When patients were unable to attend clinical visits at our institute, follow-up information was collected by telephone. We considered as “event” any local breast tumor reappearance identified during the follow-up.
Procedures
The histological types referred to in the present study were based on the World Health Organization classification scheme.17 We classified the histology as ductal, lobular, and other minor types (mucinous, tubular, papillary, cribriform, apocrine). Tumor grade, peritumoral vascular invasion (PVI), ER and progesterone receptor (PgR) status, Ki-67 labeling index (LI), and the overexpression and/or amplification of the human epidermal growth factor 2 receptor (HER2) were evaluated as previously reported.18 Tumor grade was estimated according to Elston and Ellis.19 ER, PgR, HER2, and Ki-67 expression were evaluated by immunohistochemical staining on consecutive tissue sections from tumor-containing blocks. Detected levels of ER, PgR, and HER2 overexpression and/or amplification (FISH test), and Ki-67 LI were used to define subsets of patients on the basis of previously proposed surrogate definitions of breast cancer molecular subtypes: Luminal A–like tumors are ER-positive and HER2-negative with low Ki-67 expression (<14%) or with intermediate Ki-67 expression (14% to 19%) and high PgR levels (≥20%); Luminal B–like tumors are ER-positive with intermediate Ki-67 expression (14% to 19%) and low PgR levels (<20%) or with high Ki-67 expression (≥20%) or ER-positive and HER2-positive; Triple negative tumors are ER-, PgR- and HER2-negative and HER2 positive tumors are ER- and PgR-negative and HER2-positive.20,21 Accordingly, cases in which ER and PgR were not expressed and HER2 was neither amplified nor over-expressed were classified as triple negative tumors. IBTR cases were classified as true local recurrences (de novo carcinoma) if they occurred in the index quadrant or as ipsilateral carcinomas otherwise. Ipsilateral nodal recurrences were excluded. Adjuvant treatments were administered at the time of patient accrual following the IEO’s standard practices.
Treatment with radiotherapy was classified as following: a) no RT, b) intraoperative RT (so-called ELIOT), and c) external radiotherapy. ELIOT was performed in BCS, and in case of mastectomy in the nipple area complex (Table 1).
Table 1.
Characteristics and factors associated with IBTR after breast conserving surgery or mastectomy in patients in the training set (Patients diagnosed in 2000–2006)
| Univariate Analysis | Breast Conserving Surgery | Mastectomy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | Events | HR (95% CI) | P-value | Patients | Events | HR (95% CI) | P-value | ||
| Total | 11627 | 855 | 3497 | 273 | |||||
| Age | |||||||||
| <35 | 482 | 67 | 2.09 (1.61–2.71) | <0.0001 | 251 | 35 | 1.84 (1.25–2.70) | 0.002 | |
| 35–49 | 4007 | 341 | 1.30 (1.13–1.51) | 0.0004 | 1736 | 125 | 0.84 (0.65–1.09) | 0.19 | |
| 50–69 | 5940 | 366 | 1.00 | 1308 | 102 | 1.00 | |||
| 70+ | 1198 | 81 | 1.52 (1.20–1.94) | 0.001 | 202 | 11 | 0.86 (0.46–1.60) | 0.64 | |
| First degree family history of breast/ovarian cancer | |||||||||
| No | 7884 | 575 | 1.00 | 2443 | 172 | 1.00 | |||
| Yes | 1537 | 111 | 1.04 (0.85–1.27) | 0.72 | 386 | 34 | 1.31 (0.91–1.89) | 0.15 | |
| Histology | |||||||||
| Ductal | 9205 | 679 | 1.00 | 2733 | 225 | 1.00 | |||
| Lobular | 1101 | 106 | 1.27 (1.03–1.56) | 0.02 | 445 | 30 | 0.75 (0.51–1.10) | 0.14 | |
| Mixed | 356 | 23 | 0.93 (0.62–1.41) | 0.74 | 133 | 10 | 0.86 (0.46–1.62) | 0.64 | |
| Cribriform | 349 | 11 | 0.33 (0.18–0.59) | 0.0002 | 29 | 1 | 0.29 (0.04–2.09) | 0.22 | |
| Mucinous | 209 | 12 | 0.76 (0.43–1.35) | 0.36 | 57 | 4 | 0.73 (0.27–1.95) | 0.53 | |
| Tubular | 127 | 3 | 0.26 (0.08–0.80) | 0.02 | 18 | 2 | 1.06 (0.26–4.27) | 0.93 | |
| Apocrine | 82 | 7 | 1.42 (0.68–3.00) | 0.35 | 31 | 1 | 0.40 (0.06–2.87) | 0.36 | |
| Other | 198 | 14 | 0.87 (0.51–1.47) | 0.59 | 51 | 0 | - | ||
| pT | |||||||||
| pT1 | 8480 | 583 | 1.00 | 1137 | 76 | 1.00 | |||
| pT2 | 2979 | 254 | 1.49 (1.29–1.73) | <0.0001 | 1646 | 128 | 1.33 (1.00–1.76) | 0.05 | |
| pT3 | 80 | 6 | 1.67 (0.75–3.74) | 0.21 | 576 | 57 | 2.04 (1.44–2.87) | 0.00005 | |
| pT4 | 24 | 1 | 0.79 (0.11–5.65) | 0.82 | 102 | 10 | 2.53 (1.31–4.90) | 0.006 | |
| Positive nodes | |||||||||
| 0 | 6718 | 446 | 1.00 | 1083 | 58 | 1.00 | |||
| 1–3 | 3124 | 236 | 1.20 (1.02–1.40) | 0.03 | 1075 | 86 | 1.62 (1.16–2.25) | 0.005 | |
| 4–9 | 820 | 83 | 1.83 (1.45–2.32) | <0.0001 | 676 | 65 | 2.22 (1.56–3.17) | <0.0001 | |
| ≥10 | 515 | 42 | 1.79 (1.30–2.46) | 0.0003 | 631 | 62 | 2.76 (1.93–3.95) | <0.0001 | |
| Grade | |||||||||
| G1 | 2358 | 106 | 1.00 | 285 | 14 | 1.00 | |||
| G2 | 5017 | 357 | 1.71 (1.38–2.12) | <0.0001 | 1272 | 85 | 1.51 (0.86–2.66) | 0.15 | |
| G3 | 3285 | 302 | 2.43 (1.94–3.03) | <0.0001 | 1184 | 87 | 1.90 (1.08–3.35) | 0.03 | |
| PVI | |||||||||
| Absent | 8902 | 621 | 1.00 | 2018 | 143 | 1.00 | |||
| Present | 2714 | 233 | 1.34 (1.15–1.55) | 0.0002 | 1477 | 130 | 1.45 (1.14–1.84) | 0.002 | |
| Molecular subtype | |||||||||
| Luminal A | 4301 | 214 | 1.00 | 912 | 46 | 1.00 | |||
| Luminal B | 4652 | 366 | 1.58 (1.33–1.87) | <0.0001 | 1575 | 128 | 1.77 (1.26–2.48) | 0.001 | |
| HER-2 | 435 | 60 | 3.39 (2.55–4.52) | <0.0001 | 367 | 26 | 1.81 (1.12–2.92) | 0.02 | |
| Triple negative | 909 | 96 | 2.38 (1.87–3.03) | <0.0001 | 256 | 28 | 2.91 (1.82–4.66) | <0.0001 | |
| Hormonotherapy | |||||||||
| No | 2112 | 252 | 1.00 | 799 | 80 | 1.00 | |||
| Yes | 9314 | 600 | 0.51 (0.44–0.59) | <0.0001 | 2630 | 189 | 0.60 (0.46–0.78) | 0.0001 | |
| Chemotherapy | |||||||||
| No | 7074 | 459 | 1.00 | 1331 | 91 | 1.00 | |||
| Yes | 4422 | 395 | 1.26 (1.10–1.45) | 0.001 | 2150 | 181 | 1.30 (1.01–1.67) | 0.043 | |
| Radiotherapy | |||||||||
| None | 431 | 69 | 1.00 | 2149 | 177 | 1.00 | |||
| Intraoperative | 1635 | 184 | 0.52 (0.40–0.69) | <0.0001 | 521 | 40 | 0.82 (0.58–1.15) | 0.24 | |
| External | 9360 | 598 | 0.21 (0.17–0.27) | <0.0001 | 748 | 49 | 0.97 (0.71–1.34) | 0.87 | |
HR, hazard ratio; CI, confidence interval; PVI, peritumoral vascular invasion; Bold indicates p-values < 0.05
Statistical Analysis
The cumulative incidences of IBTR from the date of surgery to the date of either first event or death were estimated using the Kalbfleisch and Prentice method, and taking competing risk events into account. Univariate Cox proportional-hazards regression models were used to calculate IBTR hazard ratios (HRs) for a series of clinicopathological factors. To identify independent factors associated with the development of IBTR, multivariable Cox proportional-hazards regression was separately applied to patients who had been treated either with BCS or with mastectomy. The following variables were considered: age, family history of breast or ovarian cancer, histologic type, pT, pN, tumor grade, PVI, surrogate molecular subtype, and primary therapies (hormone therapy, chemotherapy, radiotherapy). Backward selection was used to determine variables significantly associated with IBTR. Fitting was performed on the whole training set of patients, with dummy variables added to each model to indicate any missing values (missing indicator method).
We constructed two separate nomograms based on the results of the multivariable models in patients who underwent BCS and mastectomy. The discriminative ability of the nomograms was assessed using Harrell’s concordance statistics (c-index). We also plotted the time-dependent areas under the curve (AUCs), with 95% confidence intervals (CIs) in the training cohort for follow-up times ranging from 1–10 years, and then calculated the integrated AUC over time.
Additionally, the accuracy of each of the two multivariable models was assessed visually with calibration curves plotting the predicted 5-year IBTR probability against the 5-year IBTR cumulative incidence as observed in groups of patients defined by quantiles of predicted probabilities. Statistical analysis was performed using SAS software version 9.4 (SAS Institute, Cary, NC), and the R 3.4.4 packages rms and Hmisc.22 Statistical significance was defined by a 2-tailed p-value <0.05.
RESULTS
Development of the Nomogram
The training set included 15,124 consecutive patients who had been diagnosed with breast cancer between 2000 and 2006 and treated with either BCS (n=11,627) or mastectomy (n=3,497). The demographic and clinical characteristics of the patients in the two surgical groups are listed in Table 1.
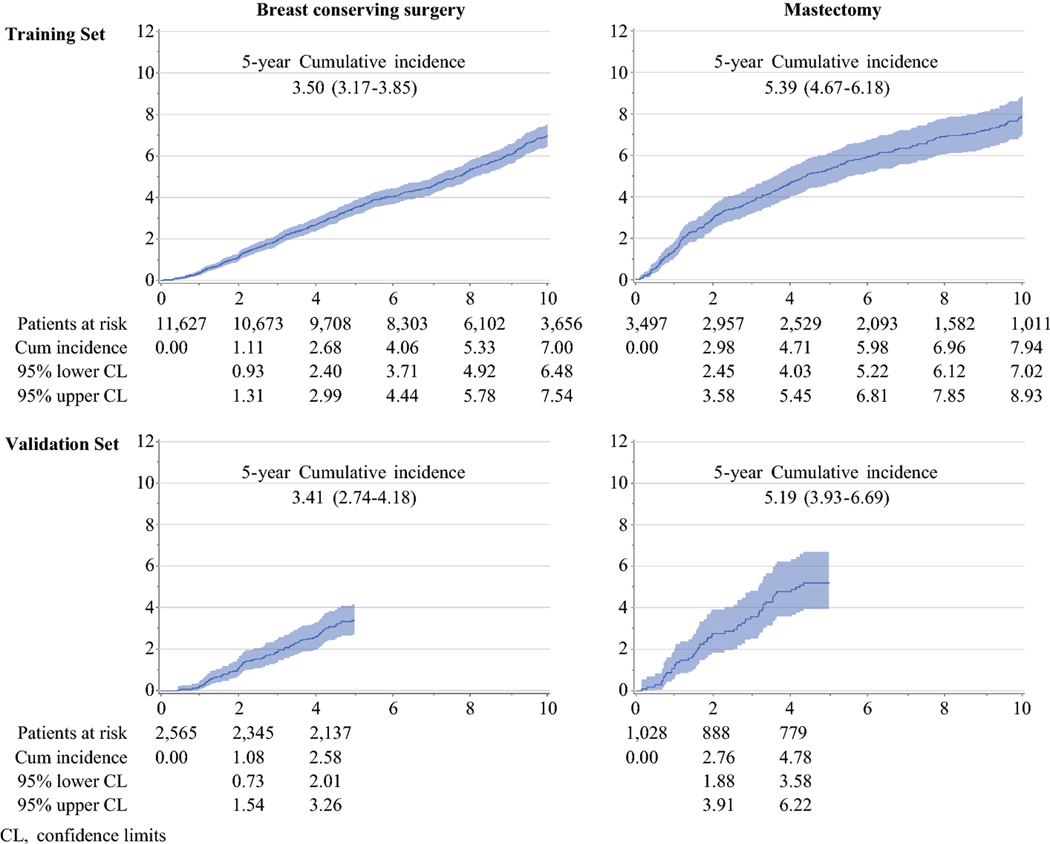
Over a median follow-up time of 8.0 years, 1,128 women developed IBTR: 855 of them after BCS and 273 following mastectomy. In the case of BCS, the annual IBTR rate stayed about constant over time, with the 5-year and 10-year IBTR rates equal to 3.50% (95% confidence interval [CI] 3.17%−3.85%) and 7.00% (95% CI 6.48%−7.54%), respectively. After mastectomy, annual IBTR rate was initially slightly higher, with 5-year and 10-year IBTR rates at 5.39% (95% CI 4.67%−6.18%) and 7.94% (95% CI 7.02%−8.93%), respectively (Fig 1). The univariate associations between each clinicopathological variable and IBTR are shown in Table 1, for the two surgical groups reported separately.
Figure 1.
Cumulative incidence of IBTR in patients who underwent breast conserving surgery or Mastectomy
A first set of multivariable models was constructed by fitting all categories for all variables simultaneously (Supplementary Table 1). Variables which did not retain statistical significance at this stage of the multivariable analysis, such as family history or breast or ovarian cancer, were excluded from the final model, while some categories were regrouped to propose an easier-to-use model.
In the case of patients who had undergone BCS, the factors that associated with IBTR in the final multivariable model were age <35 years (HR 2.26; 95% CI 1.72–2.97), age 35–49 years(HR 1.48; 95% CI 1.28–1.72), lobular histology (HR 1.63; 95% CI 1.31–2.03) or histology other than ductal or lobular (HR 0.61; 95% CI 0.45–0.84), pT2-pT4 (HR 1.24; 95% CI 1.06–1.45), 1–3 (HR 1.29;95% CI 1.08–1.54) or ≥4 positive nodes (HR 1.91; 95% CI 1.51–2.42), grade 2 (HR 1.29; 95% CI 1.02–1.63) or grade 3 (HR 1.36; 95% CI 1.03–1.80) tumor, peritumoral vascular invasion (PVI) (HR 1.18; 95% CI 0.99–1.40), Luminal B (HR 1.50; 95% CI 1.23–1.82), HER2 (HR 2.41; 95% CI 1.63–3.57) or triple negative (HR 1.77; 95% CI 1.23–2.54) molecular subtype, hormone therapy (HR 0.61; 95% CI 0.48–0.78), chemotherapy (HR 0.72; 95% CI 0.59–0.87), and external radiotherapy (HR 0.23; 95% CI 0.17–0.30)(Table 2). The model showed good discrimination with a Harrell’s concordance C-index of 0.73 (95% CI 0.65–0.80). A plot of the time-dependent AUC showed that the multivariable model was a good predictor of IBTR (with AUC around 0.80) in the first 5 years following BCS (Table 2).
Table 2.
Multivariable analysis of factors associated with IBTR after breast conserving surgery or mastectomy in patients in the training set (Patients diagnosed in 2000–2006) –– Models used in the construction of the nomograms.
| Breast Conserving Surgery | Mastectomy | ||||
|---|---|---|---|---|---|
| Variable | Strata | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age | <35 years | 2.26 (1.72–2.97) | <0.0001 | 1.79 (1.21–2.66) | 0.004 |
| 35–49 years | 1.48 (1.28–1.72) | <0.0001 | 0.90 (0.69–1.18) | 0.44 | |
| ≥50 years | 1.00 | 1.00 | |||
| Histology | Ductal | 1.00 | 1.00 | ||
| Lobular | 1.63 (1.31–2.03) | <0.0001 | 0.81 (0.54–1.22) | 0.32 | |
| Mixed | 1.19 (0.78–1.82) | 0.42 | 1.02 (0.54–1.95) | 0.95 | |
| Other | 0.61 (0.45–0.84) | 0.002 | 0.49 (0.24–1.00) | 0.05 | |
| pT | pT1 | 1.00 | 1.00 | ||
| pT2-pT4 | 1.24 (1.06–1.45) | 0.007 | 1.09 (0.81–1.47) | 0.57 | |
| pT3 | 1.68 (1.16–2.44) | 0.006 | |||
| pT4 | 2.05 (1.01–4.16) | 0.046 | |||
| Positive nodes | 0 | 1.00 | 1.00 | ||
| 1–3 | 1.29 (1.08–1.54) | 0.005 | 1.94 (1.36–2.77) | 0.0003 | |
| ≥4 | 1.91 (1.51–2.42) | <0.0001 | 3.32 (2.28–4.84) | <0.0001 | |
| Grade | G1 | 1.00 | |||
| G2 | 1.29 (1.02–1.63) | 0.04 | |||
| G3 | 1.36 (1.03–1.80) | 0.03 | |||
| PVI | Absent | 1.00 | |||
| Present | 1.18 (0.99–1.40) | 0.06 | |||
| Molecular | Luminal A | 1.00 | 1.00 | ||
| subtype | Luminal B | 1.50 (1.23–1.82) | <0.0001 | 1.62 (1.14–2.31) | 0.007 |
| HER-2 | 2.41 (1.63–3.57) | <0.0001 | 0.96 (0.51–1.81) | 0.89 | |
| Triple neg | 1.77 (1.23–2.54) | 0.002 | 1.71 (0.91–3.19) | 0.09 | |
| Hormone | No | 1.00 | 1.00 | ||
| therapy | Yes | 0.61 (0.48–0.78) | <0.0001 | 0.44 (0.29–0.67) | 0.0002 |
| Chemotherapy | No | 1.00 | 1.00 | ||
| Yes | 0.72 (0.59–0.87) | 0.0006 | 0.66 (0.49–0.89) | 0.007 | |
| Radiotherapy | None | 1.00 | 1.00 | ||
| intraoperative | 0.76 (0.56–1.04) | 0.08 | 0.98 (0.68–1.40) | 0.90 | |
| External | 0.23 (0.17–0.30) | <0.0001 | 0.57 (0.40–0.82) | 0.002 | |
| Time-dependent area under the curve (AUC)* | 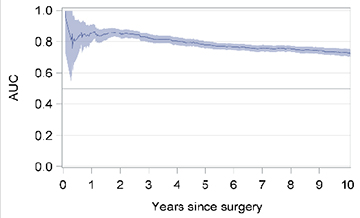 |
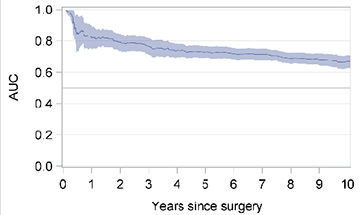 |
|||
| Harrell’s concordance | 0.73 (0.65–0.80) | 0.70 (0.56–0.82) | |||
| C-index (95% CI) | |||||
CI, confidence interval; PVI, peritumoral vascular invasion; AUC, area under curve
The shaded area represents the 95% confidence intervals (CIs)
Similarly, for patients treated with mastectomy, the factors that correlated with IBTR in the final multivariable model included age <35 years (HR 1.79; 95% CI 1.21–2.66), histology other than ductal or lobular (HR 0.49; 95% CI 0.24–1.00), pT3 (HR 1.68, 95% CI 1.16–2.44), pT4 (HR 2.05; 95% CI 1.01–4.16), 1–3 (HR 1.94; 95% CI 1.36–2.77) or ≥4 positive nodes (HR 3.32; 95% CI 2.28–4.84), Luminal B (HR 1.62; 95% CI 1.14–2.31) or triple negative (HR 1.71; 95% CI 0.91–3.19) molecular subtype, hormone therapy (HR 0.44; 95% CI 0.29–0.67), chemotherapy (HR0.66; 95% CI 0.49–0.89), and external radiotherapy (HR 0.57; 95% CI 0.40–0.82) (Table 2). The model showed good Harrell’s concordance with a C-index of 0.70 (95% CI 0.56–0.82), and was a good predictor of IBTR in the first 5 years following mastectomy (with AUC above 0.75) (Table 2).
Each of the 2 multivariable models was subsequently used to build a corresponding nomogram for the prediction of IBTR at 1, 5 and 10 years after surgery (Fig 2a–2b). The formulae from which the nomograms were derived and that allowed to calculate the predicted individualized risk 5 and 10 years after surgery are provided in Supplementary Table 2.
Figure 2a.
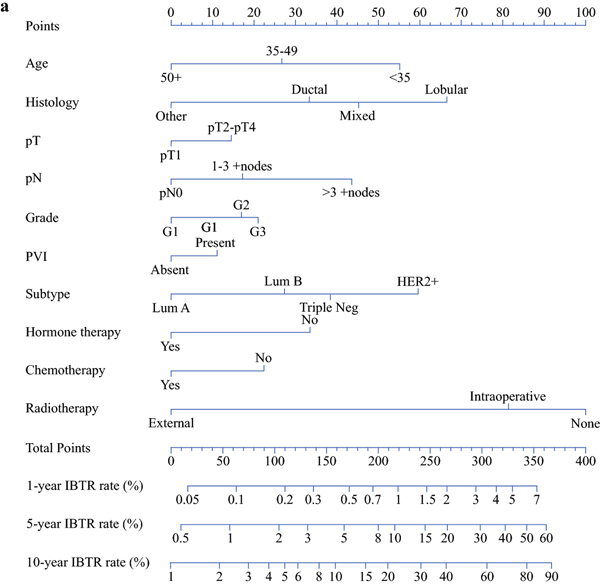
Nomograms for the prediction of IBTR 1, 5 and 10 years after breast conserving surgery
Points
Age: <35=55 points; 35–49=27 points; 50+= 0 points
Histology: Lobular=67 points; Mixed=45 points; Ductal=33 points; Other= 0 points
pT: pT1=0 points; pT2-pT4=15 points
pN: pN0=0 points; 1–3 +nodes=17 points; >3 +nodes=44 points
Grade: G1= 0 points; G2=17 points; G3= 21 points
PVI: Absent=0 points; Present=11 points
Subtype: Lum A=0 points; Lum B=27 points; Triple neg=38 points; HER2+=60 points
Hormone therapy: No=34 points; Yes=0 points
Chemotherapy: No=22 points; Yes=0 points
Radiotherapy: None=100 points; Intraoperative=81 points; External=0 points
Validation of the Nomogram
The validation set included 3,593 consecutive patients who had been diagnosed with breast cancer between 2007–2008 and who had received either BCS (n=2,565) or mastectomy (n=1,028). They presented characteristics that were similar to those of the women in the training set (Supplementary Table 3). After a median follow-up of 6.2 years, 212 of the women from the validation set developed IBTR; 146 after BCS and 66 after mastectomy. The cumulative incidence of IBTR was similar to that observed in the training set, with 5-year rates equal to 3.41% (95% CI 2.74%−4.18%) after BCS, and to 5.19% (95% CI 3.93%−6.69%) after mastectomy (Fig 1).
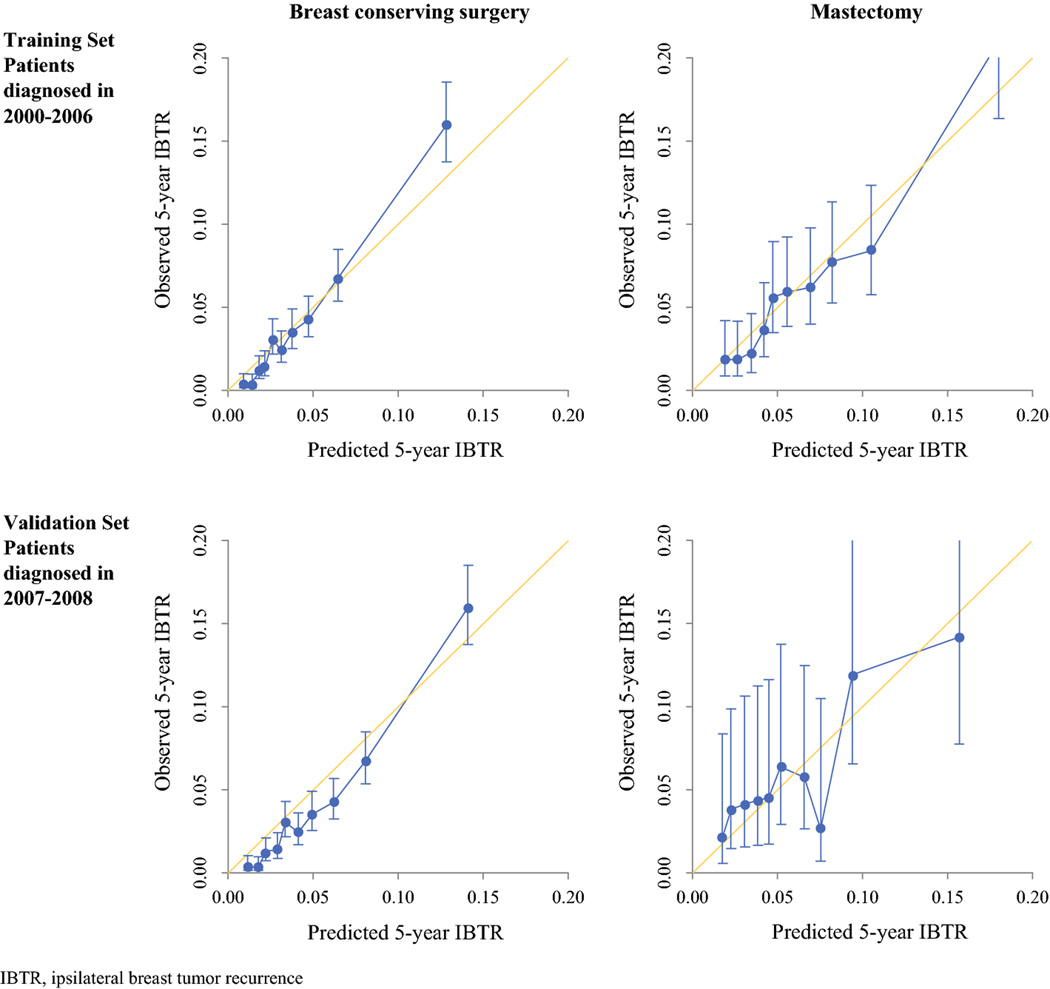
The nomograms constructed with the data from the training set were used to predict the probability of IBTR for patients in the validation cohort. For each nomogram, the predicted 5-year cumulative incidence of IBTR was plotted against the observed 5-year cumulative incidence of IBTR, showing good calibration (Fig 3). The number of observed versus predicted 5-year IBTRs across deciles of risk are presented in Table 3, again showing good discriminative ability on the part of the models. The predictive performance of the nomograms was also assessed by concordance index (C-index) with respective values of 0.77 (95% CI 0.58–0.92) in the BCS validation set and 0.69 (95% CI 0.41–0.92) in the mastectomy validation set.
Figure 3.
Calibration curves of the nomograms predicting the 5-year cumulative incidence of IBTR in patients who had undergone breast conserving surgery or Mastectomy
IBTR, ipsilateral breast tumor recurrence
Table 3.
Observed versus predicted 5-year IBTR after breast conserving surgery or mastectomy in patients in the validation set
| Breast Conserving Surgery | Mastectomy | |||||
|---|---|---|---|---|---|---|
| Decile of risk | Patients | Observed | Predicted* | Patients | Observed | Predicted* |
| number | IBTR | IBTR | number | IBTR | IBTR | |
| D1 | 279 | 1 | 3.2 | 97 | 2 | 1.7 |
| D2 | 226 | 3 | 4.0 | 106 | 4 | 2.4 |
| D3 | 267 | 5 | 5.9 | 108 | 4 | 3.3 |
| D4 | 256 | 2 | 7.4 | 99 | 4 | 3.8 |
| D5 | 253 | 4 | 8.5 | 105 | 4 | 4.7 |
| D6 | 258 | 6 | 10.6 | 100 | 6 | 5.2 |
| D7 | 256 | 7 | 12.6 | 111 | 6 | 7.3 |
| D8 | 269 | 11 | 16.7 | 97 | 2 | 7.3 |
| D9 | 245 | 14 | 19.8 | 103 | 10 | 9.7 |
| D10 | 256 | 31 | 36.1 | 102 | 10 | 16.0 |
| Hosmer & Lemeshow | Chi-square =3.67 with 9 DF | Chi-square =4.56 with 9 DF | ||||
| Goodness of fit | P=0.93 | P=0.87 | ||||
IBTR, ipsilateral breast tumor recurrence; DF, degrees of freedom
based on the nomogram constructed with data from the training set
DISCUSSION
Around 7% of women who undergo breast surgery are at risk of developing ipsilateral breast tumor recurrence (IBTR). Indeed, adjuvant treatments, such as RT, hormone therapy, and chemotherapy are still unable to completely prevent the development of IBTR. However, the risk of IBTR could be predicted at the time of surgery from the value of some clinicopathological variables. For example, metastatic axillary lymph nodes, >pT1, high tumor grade G2/G3, luminal B, HER2 positivity, PVI, and lobular and triple negative subtypes have been shown to significantly associate with an increased risk of IBTR. Conversely, hormone therapy, chemotherapy, and external RT have been proven to act as significant protective factors from IBTR.1
Nomograms estimate individualized risks based on a combination of multiple variables, thus suggesting the best available treatment and making the most reliable prediction of outcome. So far, they have been successfully used in several malignancies23–26 including breast cancer, where validated nomograms are routinely applied in the case of DCIS16 and sentinel node metastasis.27,28
In this study, we constructed and validated novel nomograms able to predict the risk of IBTR in patients with invasive breast cancer treated either with BCS or mastectomy. Using a large number of patients diagnosed and treated according to standard protocols, we were able to identify risk factors for the development of IBTR. In particular, we found that, in the case of BCS, high risk of IBTR associated with younger age at onset (age<35 years), pT2-pT4, metastatic lymph nodes (≥4 positive nodes), G2-G3 tumor grade, PVI positive, HER2 positive, and luminal B subtypes, and lobular histology. Similarly, following mastectomy, high risk for developing IBTR was observed in patients with pT3-pT4, metastatic lymph nodes (≥4 positive nodes), and luminal B and triple negative subtypes. Interestingly, on multivariable analysis, external RT was found to act as a protective factor in both surgical groups, confirming results from previous studies. Indeed, postmastectomy RT was shown to decrease both local recurrence and breast cancer-related mortality in women with node-positive diseases; results from the EBCTCG study demonstrated that radiotherapy reduced both recurrence and breast cancer mortality in women with 1–3 positive lymph nodes.29 Analysis of the BIG 02–98 trial showed excellent outcomes in women with pT1-pT2 tumors and 1–3 positive lymph nodes found in axillary dissection. Postmastectomy RT improved locoregional recurrence in this cohort at 10 years.30
We used the results of our multivariable analysis to construct easy-to-interpret nomograms able to assess individualized IBTR risks (Figg 2a–2b). As an example, the nomogram predicts that a BC patient aged 60 years (9 points), with ductal histotype (59 points), pT3 (43 points), pN0 (0 points), triple negative subtype (48 points), who undergoes neither hormone therapy (68 points) nor RT (46 points), and who is treated with adjuvant chemotherapy (0 points) has a risk of about 10% of developing a recurrence 5 years after mastectomy (total points: 273). If the same patient is instead treated with RT (0 points), she would have a lower than 6% risk of IBTR (total points: 227) 5 years from the date of definitive surgery. Keeping all variables the same except for changing the cancer pathological state to pT2 (7 points), 5 years after mastectomy, IBTR is predicted to develop in 4% of patients treated with RT (191 points) and in 7% of those who do not undergo RT (237 points). 10 years after mastectomy, the IBTR risk for the same patients is predicted to increase to 7% and 12%, in the presence and absence of RT, respectively.
We validated the nomograms on an independent cohort of patients who had characteristics similar to those of the women in the training set, and who were treated in the same institution and used the same protocols as them. However, they had been diagnosed and had undergone surgery at a later point in time with respect to the women in the training set. The cumulative incidence of IBTR was similar in the training and validation sets (Fig 1), providing further evidence of the validity of the 2 cohorts. The follow-up time was shorter for the patients in the validation set, but it was still long enough to allow validation of the prediction of IBTR at 5 years. The number of IBTR cases observed in the validation set 5 years after surgery was close to that predicted by the nomogram, for both BCS and mastectomy patients, and across different levels of risk, demonstrating good calibration of our instrument.
The main limitations of this study are its use of retrospective data and its monocentric setting. Retrospective data imply that treatment was not assigned in a randomized fashion; patients believed to be at lower risk of IBTR, for example, might have been less likely to receive RT or chemotherapy. A monocentric setting means that datasets might have been biased by institutional practice patterns. Moreover, as the training and validation cohorts comprised patients treated in the same institution, internal validation might have led to over-optimistic results. Consequently, it would be important to further validate the two proposed nomograms on sets of patients treated in other institutions and, particularly, in prospective studies. Another important limitation of this study is the heterogeneity of the enrolled population. Patients with HER2 positive BC today are eligible to receive targeted inhibitor. The issue HER2 positive is challenging and important for BC patients care and prognosis. However, in our population this issue is a great limitation as patients were enrolled in the pre targeted therapy era with HER2 inhibitor. The very few and highly selected patients who received anti-HER2 drugs were a-priori excluded and the resulting nomogram apply to untreated patients. Further studies will consider specific risks for IBTR in accord with these targeted therapies.
Lastly, as with any other statistical model, our nomograms are not flawless. Although statistically validated, results should be considered carefully and translated into clinical practice only after multidisciplinary counseling.
In this study, we proposed a new approach for risk quantification and patient stratification using the prognostic nomogram and decision tree analysis in this setting. We have described a predictive model for the personalized estimate of the risk of developing IBTR after BCS or mastectomy, and we have built two novel nomograms that can help clinicians and patients, in accord with a multidisciplinary team evaluation.
Supplementary Material
Figure 2b.
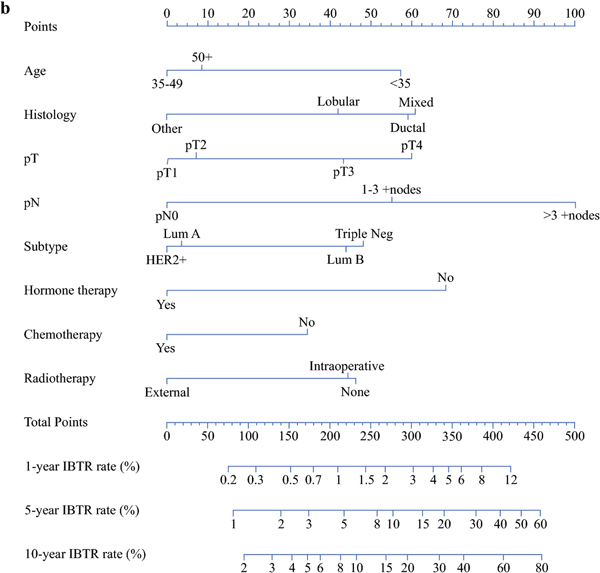
Nomograms for the prediction of IBTR 1, 5 and 10 years after mastectomy
Points
Age: <35=57 points; 35–49=0 points; 50+= 9 points
Histology: Lobular=42 points; Mixed=61 points; Ductal=59 points; Other= 0 points
pT: pT1=0 points; pT2=7 points; pT3=43 points; pT4=60 points
pN: pN0=0 points; 1–3 +nodes=55 points; >3 +nodes=100 points
Subtype: Lum A=4 points; Lum B=44 points; Triple neg=48 points; HER2+=0 points
Hormone therapy: No=68 points; Yes=0 points
Chemotherapy: No=35 points; Yes=0 points
Radiotherapy: None=46 points; Intraoperative=44 points; External=0 points
Acknowledgments
This study was approved by the review board/ethics committee of the European Institute of Oncology. The preparation of this study was supported in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center. The authors have no conflict of interest disclosures to report.
Footnotes
DISCLOSURES
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Corso G, Maisonneuve P, Santomauro GI, et al. Ipsilateral Breast Tumor Reappearance and Contralateral Breast Cancer after Primary Breast Cancer Treatment: A Comprehensive Retrospective Study of 15,168 Patients. Oncology. 95:147–55,2018 [DOI] [PubMed] [Google Scholar]
- 2.Morrow M, Van Zee KJ, Solin LJ, et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation in Ductal Carcinoma In Situ. J Clin Oncol. 34:4040–6,2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houssami N, Macaskill P, Marinovich ML, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer. 46:3219–32,2010 [DOI] [PubMed] [Google Scholar]
- 4.Abdulkarim BS, Cuartero J, Hanson J, et al. Increased risk of locoregional recurrence for women with T1–2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol. 29:2852–8,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman GM, Fowble BL. Local recurrence after mastectomy or breast-conserving surgery and radiation. Oncology (Williston Park). 14:1561–81; discussion 81–2, 82–4,2000 [PubMed] [Google Scholar]
- 6.Millar EK, Graham PH, O’Toole SA, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 27:4701–8,2009 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 26:2373–8,2008 [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Anderson S, Redmond CK. Surgery for early breast cancer. N Engl J Med. 334:987–8,1996 [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Dignam J, Mamounas EP, et al. Sequential methotrexate and fluorouracil for the treatment of node-negative breast cancer patients with estrogen receptor-negative tumors: eight-year results from National Surgical Adjuvant Breast and Bowel Project (NSABP) B-13 and first report of findings from NSABP B-19 comparing methotrexate and fluorouracil with conventional cyclophosphamide, methotrexate, and fluorouracil. J Clin Oncol. 14:1982–92,1996 [DOI] [PubMed] [Google Scholar]
- 10.Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 24:2028–37,2006 [DOI] [PubMed] [Google Scholar]
- 11.Luo C, Zhong X, Deng L, et al. Nomogram Predicting Locoregional Recurrence to Assist Decision-Making of Postmastectomy Radiation Therapy in Patients With T1–2N1 Breast Cancer. Int J Radiat Oncol Biol Phys. 103:905–12,2019 [DOI] [PubMed] [Google Scholar]
- 12.Mamounas EP, Liu Q, Paik S, et al. 21-Gene Recurrence Score and Locoregional Recurrence in Node-Positive/ER-Positive Breast Cancer Treated With Chemo-Endocrine Therapy. J Natl Cancer Inst. 109,2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 28:1677–83,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouzier R, Pusztai L, Delaloge S, et al. Nomograms to predict pathologic complete response and metastasis-free survival after preoperative chemotherapy for breast cancer. J Clin Oncol. 23:8331–9,2005 [DOI] [PubMed] [Google Scholar]
- 15.Fujii T, Kogawa T, Wu J, et al. Nomogram to predict pathologic complete response in HER2-positive breast cancer treated with neoadjuvant systemic therapy. Br J Cancer. 116:509–14,2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudloff U, Jacks LM, Goldberg JI, et al. Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ. J Clin Oncol. 28:3762–9,2010 [DOI] [PubMed] [Google Scholar]
- 17.Tavassoli FA, Devilee P. Pathology and genetics of tumours of the breast and female genital organs: WHO Classification of Tumour Series. Lyon, France: IARC; 2003: 13–48. [Google Scholar]
- 18.Colleoni M, Bagnardi V, Rotmensz N, et al. A risk score to predict disease-free survival in patients not achieving a pathological complete remission after preoperative chemotherapy for breast cancer. Ann Oncol. 20:1178–84,2009 [DOI] [PubMed] [Google Scholar]
- 19.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. C. W. Elston & I. O. Ellis. Histopathology 1991; 19; 403–410. Histopathology. 41:151–2, discussion 2–3,2002 [DOI] [PubMed] [Google Scholar]
- 20.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 22:1736–47,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hugh J, Hanson J, Cheang MC, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 27:1168–76,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The R Project for Statistical Computing. http://www.r-project.org/ (Accessed June 14, 2019).
- 23.Brennan MF, Kattan MW, Klimstra D, et al. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg 240:293–8,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kattan MW, Scardino PT. Evidence for the usefulness of nomograms. Nat Clin Pract Urol. 4:638–9,2007 [DOI] [PubMed] [Google Scholar]
- 25.Voelkel V, Draeger T, Groothuis-Oudshoorn CGM, et al. Predicting the risk of locoregional recurrence after early breast cancer: an external validation of the Dutch INFLUENCE-nomogram with clinical cancer registry data from Germany. J Cancer Res Clin Oncol. 2019. in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiser MR, Landmann RG, Kattan MW, et al. Individualized prediction of colon cancer recurrence using a nomogram. J Clin Oncol. 26:380–5,2008 [DOI] [PubMed] [Google Scholar]
- 27.Bevilacqua JL, Kattan MW, Fey JV, et al. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. J Clin Oncol. 25:3670–9,2007 [DOI] [PubMed] [Google Scholar]
- 28.Van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 10:1140–51,2003 [DOI] [PubMed] [Google Scholar]
- 29.Early Breast Cancer Trialists’ Collaboration Group (EBCTCG), McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 383:2127–35,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeidan YH, Habib JG, Ameye L, et al. Postmastectomy Radiation Therapy in Women with T1-T2 Tumors and 1 to 3 Positive Lymph Nodes: Analysis of the Breast International Group 02–98 Trial. Int J Radiat Oncol Biol Phys. 101:316–24,2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.