Abstract
Even though there are physiological differences between males and females, heart rate (HR), ratings of perceived exertion (RPE), power output (PO), oxygen consumption (VO2), and blood lactate (BL) levels have been used as measures of exercise intensity independently of sex. The purpose of this study was to determine differences between sexes in different exercise intensity models. Thirty (15 females) young, healthy individuals were scheduled for two testing visits 48–72 hours apart. During the first testing visit, a graded exercise test (GXT), with BL obtained at the end of each exercise step, was administered on a stationary bicycle to determine peak PO and VO2max. BL during the GXT was used to determine three 5-min steady-state workloads (low: 0–2 mmol/L; moderate: 2–4 mmol/L; and high: >4 mmol/L) for the second test. HR, %HRmax, RPE, PO, %POmax, VO2, %VO2max, and BL were obtained at the end of each steady-state workload. A two-way repeated measures ANOVA was performed to compare all exercise intensity variables obtained during the second test between males and females (α=0.05). Only RPE, %PO, and BL did not differ between sexes on all 3 exercise intensities. HR, %HR, and PO differ between sexes on at least 2 exercise intensities. Females have higher HR and %HR than males for similar %PO. VO2 and %VO2max differ between sexes on at least 1 exercise intensity. Based on the current results, traditional exercise intensity markers are different between males and females. BL and %PO appear to be markers that might be used independently of sex.
Keywords: Power output, blood lactate levels, sex
INTRODUCTION
Important components of exercise prescription include the frequency, duration, type, and intensity of physical activity (2). Among these components, the most important is the intensity of the exercise (25). In general, exercise intensity can be determined using appropriate target heart rates, ratings of perceived exertion, or blood lactate levels (23).
To achieve optimal performance, exercise prescription should be individualized for each person. However, there is not much difference when prescribing exercise intensities for males and females (1). Physiological differences under resting conditions between men and women, such as fat free mass and blood volume, are well established (7, 10, 32). The effect that these differences have on physiological responses to exercise has been tested but many controversial results have been reported (10, 16, 39). If men are larger, have more muscle mass, and less body fat than women, and women have shown greater cardiac work requirements to meet the same external workload demand as men (7), why should exercise intensity prescriptions be the same for both sexes?
Studies have shown controversial findings when comparing men and women during and after submaximal exercise tests (10, 16, 28, 39). For example, Garcin et al. (16) and Deschenes et al. (10) showed no differences between men and women in regard to heart rate (HR) during aerobic exercise. However, Wheatley et al. (39) and Laurent et al. (28) reported higher HR in females compared to males for similar workloads. Similarly, studies have shown conflicting results for oxygen uptake (VO2) with results ranging from no gender differences during resting conditions to females having higher VO2 than males for the same workload during moderate and vigorous intensities, suggesting females may be at a higher cardiopulmonary demand (28, 39).
In addition, exercise prescription following absolute or relative intensities (e.g., HR vs. %HR or VO2 vs. %VO2max) is still controversial (5, 31). For example, Berglund et al. (5) showed that peak HR during a traditional exercise test was different to real HRmax obtained in a specific HRmax test. These findings confirm that some of these exercise intensity markers have multiple factors that could affect their specificity and/or reliability.
Even though there are physiological differences between males and females, HR, rate of perceived exertion (RPE), power output (PO), VO2, and blood lactate (BL) levels have been used as measures of exercise intensity independently of the sex. Moreover, absolute or relative exercise intensities are normally prescribed also independently of sex. Therefore, the present study is novel as it seeks to determine if traditional exercise intensity markers behave similarly between males and females. The purpose of this study was to examine differences in determining exercise intensity between young, healthy males and females during steady state aerobic exercise. We hypothesized that markers of exercise intensity will be different between males and females at different exercise intensities.
METHODS
Participants
Thirty young healthy participants (15 males and 15 females) were recruited to participate in the study. A priori power and sample size analyses, using G*Power 3.1 (13, 14), showed a minimum of 8 subjects per group. Participants were eligible if they were between 18 and 35 years old and if they were considered apparently healthy based on a preliminary health questionnaire. Exclusion criteria included known history of cardiovascular, cardiac, pulmonary, or metabolic disease, taking prescription medications, any extremity injury that could affect cycling or attaching a blood pressure cuff to the arm, and pregnant females. Participants that were taking over-the-counter painkillers or nutritional supplements containing antioxidants were required to abstain from their use 12 h prior to each lab visit. Female subjects were tested within 4 days before and after menses to standardize any hormonal influence on vascular reactivity (3). Oral contraceptive female users were tested during inactive-pill phase to be consistent with other female subject’s instructions, even though the use of contraceptive has shown no differences in exercise responses (33). The study was approved by the Institutional Review Board of The University of Texas at El Paso and all individual participants included in the study signed the informed consent before scheduling the first visit to the Clinical Applied Physiology (CAPh) Lab. This research was carried out fully in accordance to the ethical standards of the International Journal of Exercise Science (36).
Protocol
The current protocol has been described before (8, 35). Briefly, two exercise tests were performed on a cycle ergometer (Corival, Lode, The Netherlands) in the same timeframe 24–48 h apart. Before each exercise test, height (Seca Medical, Germany), and weight (WB-110A Class III, Tanita, Japan) were obtained. Seat height was adjusted to allow a 5° to 15° of knee flexion during cycling. Participants were instructed to sit quietly on the bike for 10 min to ensure that increased sympathetic activity due to nervousness did not alter blood pressure readings (27). During the resting period, three peripheral blood pressure values were recorded using an automated brachial blood pressure cuff (BP760, Omnron Healthcare, Inc., Lake Forest, IL).
The first exercise test was an 8–12-min maximal, incremental exercise test that followed the American Heart Association and American College of Sports Medicine guidelines for exercise testing (2, 15). The first exercise test was designed to obtain individual’s VO2max, PO in watts, and lactate threshold, which were used to determine exercise intensities for the second test. Lactate levels have been employed as a biomarker for exercise intensity and training monitoring in athletes for several years (12) and currently they have been proposed as intensity markers for exercise prescription in clinical population (20, 21). Blood lactate levels, assessed via micro-sample from the earlobe and analyzed with an automated lactate analyzer (Lactate Plus, Nova Inc., Boston, MA), HR (Quinton Q-Stress Cardiac Stress System, Mortara Instrument, Milwaukee, WI), VO2 (TrueOne 2400, Parvomedics Inc., Sandy, UT), and RPE (Borg scale) were measured at rest and at the end of each 2-min stage of the exercise test. Lactate threshold curves obtained during this first exercise test were used to determine the three steady-state workloads to be used for the second exercise test.
During the second exercise test, participants were asked to exercise 5 min at each different workload, according to the following lactate training zones: low intensity (BL levels of 0–2 mmol/L), moderate intensity (BL levels of 2–4 mmol/L), and high intensity (BL levels>4 mmol/L) maintaining a constant cadence between 60 and 70 rpm (20, 29). There was no pause between 5-min workloads and all subjects performed low intensity first, then moderate intensity, and finally high intensity. HR and VO2 were continuously monitored throughout the 15-min exercise test and blood lactate and RPE were assessed at baseline and at minutes 2 and 4 of each three exercise intensities. If BL levels were over or under the target, PO was adjusted accordingly.
Statistical Analysis
Descriptive statistics, including mean, standard deviations (S.D.), standard error of the means (S.E.M), and minimum and maximum values were obtained. Normal distribution for all dependent variables was confirmed using Shapiro-Wilkins and Smirnov tests (at least one test p>0.05). Independent samples t-tests were performed for comparisons of group demographic variables. Two-way repeated measurements-ANOVAs comparing exercise intensities and sex (Intensity x Sex) were performed for all exercise intensity markers. Fisher’s Least Significant Difference was used as post-hoc analysis. Data is expressed as mean ± S.D. unless otherwise stated. All statistical analyses were performed using SPSS (version 24.0, IBM, Chicago, Il), and statistical significance was considered when p<0.05.
RESULTS
Data were confirmed normally distributed. Demographics and general characteristics of the sample are shown in Table 1. Males were taller (p=0.002) and heavier (p<0.001) than females; although no difference on BMI was observed. In addition, males had a larger VO2max (p=0.008) than females. BL levels during each workload staid within all 3 specific ranges (0–2, 2–4, and >4 mmol/L) and no PO adjustments were needed.
Table 1.
Demographic characteristics of the sample.
| Overall n=30 |
Females n=15 |
Males n=15 |
|
|---|---|---|---|
| Age (years) | 21.9 ± 3.2 | 21.3 ± 2.3 | 22.7 ± 4.0 |
| Height (cm) | 172.1 ± 9.1 | 166.5 ± 8.4 | 177.6 ± 5.9* |
| Weight (kg) | 72.9 ± 18.4 | 62.1 ± 9.4 | 83.7 ± 19.0* |
| BMI (kg/m2) | 25.0 ±4.9 | 23.4 ± 3.5 | 27.0 ± 5.3 |
| SBP (mmHg) | 114.5 ± 7.2 | 113.1 ± 6.9 | 116.1 ± 7.5 |
| DBP (mmHg) | 77.6 ± 5.6 | 77.1 ± 5.8 | 78.2 ± 5.6 |
| VO2max (ml/kg/min) | 31.8 ± 7.4 | 28.3 ± 6.3 | 35.2 ± 6.9* |
| Resting BL (mmol/L) | 0.9 ± 0.3 | 0.8 ± 0.3 | 0.9 ± 0.3 |
BMI= Body mass index; SBP=Systolic blood pressure; DBP=Diastolic blood pressure; VO2max= Maximal oxygen uptake; BL= Blood lactate;
=p<0.05 males vs. females
Two-way repeated measurements ANOVA statistics are presented in Table 2. All exercise intensity markers showed a significant exercise intensity effect. Only PO, HR, and %HR showed a significant sex effect. Significant interaction was only observed in PO, %PO, VO2, and %VO2max.
Table 2.
Two-way repeated measurements ANOVA data.
| Variable | Intensity Effect | Sex Effect | Interaction |
|---|---|---|---|
| PO (Watts) | F(2, 28)=470.4 | F(1, 14)=7.868 | F(2, 28)=30.88 |
| p<0.001 | p=0.014 | p<0.001 | |
| %PO (%) | F(2, 28)=2496 | F(1, 14)=3.148 | F(2, 28)=4.447 |
| p<0.001 | p=0.098 | p=0.021 | |
| VO2 (mL/kg/min) | F(2, 28)=203.3 | F(1, 14)=2.977 | F(2, 28)=8.890 |
| p<0.001 | p=0.106 | p=0.001 | |
| %VO2max (%) | F(2, 28)=292.7 | F(1, 14)=4.458 | F(2, 28)=3.931 |
| p<0.001 | p=0.053 | p=0.031 | |
| BL (mmol/L) | F(2, 28)=321.1 | F(1, 14)=0.148 | F(2, 28)=0.812 |
| p<0.001 | p=0.706 | p=0.454 | |
| RPE | F(2, 28)=253.3 | F(1, 14)=0.073 | F(2, 28)=0.558 |
| p<0.001 | p=0.791 | p=0.578 | |
| HR (bpm) | F(2, 28)=152.0 | F(1, 14)=9.310 | F(2, 28)=9.310 |
| p<0.001 | p=0.009 | p=0.705 | |
| %HR (%) | F(2, 28)=412.6 | F(1, 14)=24.04 | F(2, 28)=1.443 |
| p<0.001 | p<0.001 | p=0.253 |
PO= Power Output; VO2= Oxygen uptake; BL= Blood Lactate; RPE= Ratings of Perceived Exertion, HR= Heart Rate.
Different exercise intensity markers for all 3 physiological workloads are presented in Table 3. Females worked at a higher percentage of the VO2max during the lower physiological workload (p=0.010). However, at higher intensities males worked out at a higher VO2 and PO than females (p=0,034 and p=0.001, respectively), but no differences were observed between males and females for percentage of the VO2max and relative PO (%VO2max and %PO, respectively). Throughout the stages of Low (0–2mmol/L), Moderate (2–4mmol/L), and High (>4mmol/L) workloads RPE, and BL did not differ between sexes.
Table 3.
Different exercise intensity markers at different physiological workloads.
| Workload | Variable | Females (n=15) | Males (n=15) |
|---|---|---|---|
| Low (0–2 mmol/L) | PO (Watts) | 61.3 ± 20.3 | 67.3 ± 15.3 |
| %PO (%) | 44.1 ± 11.2 | 36.4 ± 9.7 | |
| VO2 (mL/kg/min) | 15.1 ± 3.7 | 15.7 ± 3.6 | |
| %VO2max (%) | 54.0 ± 9.8 | 45.6 ± 8.4* | |
| BL (mmol/L) | 1.5 ± 0.4 | 1.3 ± 0.4 | |
| RPE | 7.7 ± 2.0 | 7.5 ± 1.6 | |
| Moderate (2–4 mmol/L) | PO (Watts) | 93.3 ± 22.0 | 116.7 ± 19.5* |
| %PO (%) | 67.6 ± 10.3 | 62.1 ± 8.3 | |
| VO2 (mL/kg/min) | 20.1 ± 4.2 | 24.7 ± 5.1* | |
| %VO2max (%) | 71.9 ± 10.0 | 71.3 ± 6.7 | |
| BL (mmol/L) | 2.6 ± 0.6 | 2.8 ± 0.7 | |
| RPE | 10.5 ± 2.4 | 10.8 ± 2.4 | |
| High (>4 mmol/L) | PO (Watts) | 123.7 ± 23.0 | 166.0 ± 29.2* |
| %PO (%) | 89.9 ± 8.6 | 87.8 ± 8.2 | |
| VO2 (mL/kg/min) | 26.3 ± 5.5 | 31.0 ± 5.6 | |
| %VO2max (%) | 93.3 ± 7.4 | 89.6 ± 5.5 | |
| BL (mmol/L) | 4.7 ± 0.7 | 4.8 ± 1.1 | |
| RPE | 13.7 ± 2.4 | 14.1 ± 2.4 |
PO= Power Output; VO2= Oxygen uptake; BL=Blood Lactate; RPE= Ratings of Perceived Exertion;
=p<0.05 males vs. females.
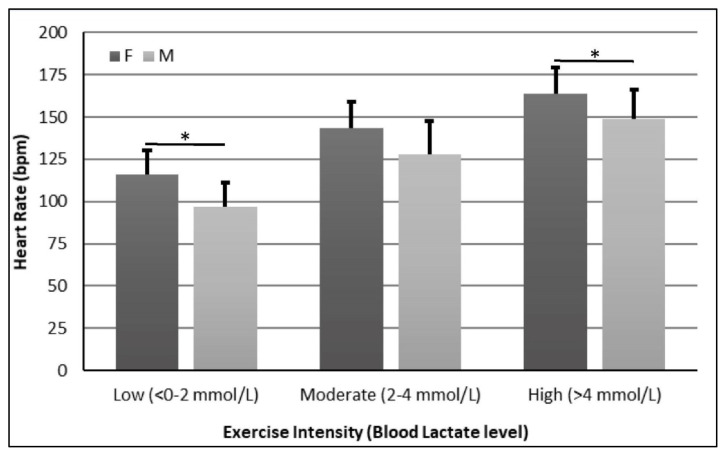
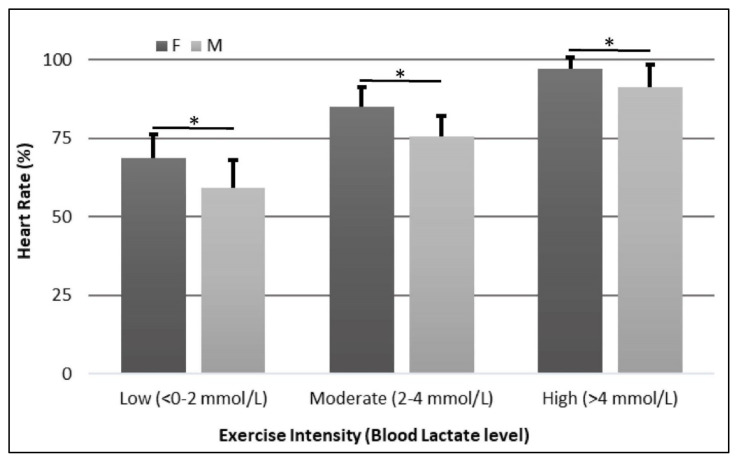
Females had higher HR than males at Low (116 ± 14 bpm vs. 97 ± 14 bpm, p=0.004) and High (164 ± 16 bpm vs. 149 ± 17 bpm, p=0.016) workloads (Figure 1). In addition, females had higher %HR than males in all three workloads (Low: 69 ± 8% vs. 59 ± 9%, p=0.002; Moderate: 85 ± 6% vs. 75 ± 7%, p<0.001; High: 97 ± 4% vs. 91 ± 7%, p=0.016) (Figure 2).
Figure 1.
Heart Rate Comparison between Females and Males. * = p<0.05
Figure 2.
Percent Heart Rate Comparison between Females and Males. * = p<0.05
DISCUSSION
The present study intended to find differences in determining exercise intensity between young, healthy males and females during steady state aerobic exercise. The present study found that some exercise intensity markers were different between males and females. The key findings of the present study were 3-fold; 1) VO2 and %VO2max differed between sexes only at Moderate and Low intensities, respectively; 2) variables which differed on at least 2 exercise intensities were HR, %HR, and PO; and 3) only RPE, %PO, and BL did not differ between sexes on all 3 exercise intensities.
Even though VO2 and %VO2max did not show a main sex effect (Table 2), the observed interaction can be attributed to differences between sexes on at least one intensity (Table 3). Males exercised at a larger VO2 than females during Moderate intensity (BL: 2–4 mmol/L), while females exercised at a larger %VO2 than males during Low intensity (BL: 0–2 mmol/L). Previous reports have shown that women have lower maximal oxygen uptake in comparison to men of similar age and fitness level (9, 10). These differences can be attributed to a decrease in cardiac output and stroke volume in females while exercising at equivalent relative submaximal intensities (%VO2max) compared to males (41) and/or to a lower hemoglobin concentration observed in females (40–42). On the other hand, other studies have shown consistent findings expressing no significant differences between genders when accounting for a relative VO2 (10, 37). The results on the present study show that VO2 might be associated with PO in both males and females (Table 2 and 3); as PO increases, VO2 increases. In addition, %VO2max might also be associated with %PO and there were not significant differences between males and females, except at the lower exercise intensity, where females had to exercise at a higher percentage of the VO2max (54.0%±9.8% vs. 45.6%±8.4%, p<0.05) to maintain the same BL levels (<2.0 mmol/L). This finding could be associated to a lower relative muscle mass observed in females who might need to recruit more muscle fibers at lower intensities, increasing peripheral oxygen extraction and %VO2max (6, 39).
The present study showed that HR and %HR were higher on females than males during all 3 workloads (Figures 1 and 2). These results are consistent with previous reports (28, 34, 38, 39, 41). These differences can be attributed to females having smaller hearts and levels of blood volume than males, even when adjusted for body mass, which will elicit a higher HR to keep blood supply to exercising muscles (34). Considering that our sample was obtained from young, apparently healthy, non-trained individuals, we could attribute the observed differences to normal baseline differences such as heart size and lower blood volume reported by others (41). Deschenes et al. (10) performed a similar study conducted to compare the physiological responses of males and females to similar relative submaximal intensity where they found that HR and %HR behaved similarly between males and females during both exercise and recovery periods. However, they did not compare males and females during individual workloads what make difficult to compare with the present study (10). The present study also showed similar HR and %HR behaviors in both males and females. HR and %HR in both males and females increased with increased exercise intensity (Table 2 and Figures 1 and 2). However, in the present study females’ HR and %HR increased to a greater extent than males in at least 2 exercise intensity levels, in all 3 levels for %HR, showing that %HR and HR may not be good markers for exercise intensity to use interchangeably in males and females.
The present study showed no differences in RPE between males and females during all 3 exercise workloads. These results are consistent to other reports where RPE did not differ between males and females using different exercise intensity markers (10, 37, 39). Wheatley et al. (39) studied 31 males and 33 females during 2 exercise intensities (i.e. 40% and 75% PO) and showed no differences in RPE between males and females. Robertson et al. (37) studied 9 males and 10 females during different exercise intensities performing 3 types of weight bearing exercise. Even though the authors found differences on RPE between males and females at absolute VO2, these differences disappeared when using %VO2max. Consistently, Deschenes et al. (10) studied 10 males and 10 females during one 60–65 %VO2max bout of exercise and found that there were no differences in RPE values reported by males and females throughout a 30-minute exercise session. Even though RPE might not represent the best marker for exercise intensity (11, 18, 19, 21, 26), the present and previous studies show that RPE works independently of biological sex, which could make it a consistent tool for prescribing exercise intensity in both males and females.
Interestingly, the current results showed an intensity x sex interaction for %PO, without any differences between sexes (Tables 2 and 3). This interaction might be associated with a smaller difference between sexes at high intensity (Δ=2.1%) when compared to low intensity (Δ=7.7%), which reflects a rather dynamic workload. Nevertheless, BL and %PO appear to be the exercise intensity models that showed more consistency and might be used independently of biological sex. These results are consistent with previous studies (9, 22, 39). On their study, Wheatley at al. (39) also showed no differences in BL between males and females at resting, moderate (40% PO), vigorous (75% PO), and after exercise. Demello et al. (9) studied 10 untrained males and females during several bouts of aerobic exercise at different exercise intensities determined by %VO2max. Their findings showed that there were no differences in BL between males and females in any of the 5 exercise intensities (9). Blood lactate levels have also shown to be dependable across multiple studies finding that males and females achieve maximal lactate steady state at similar relative intensities (4, 6, 22, 24). In addition, Green at al. (17) did not find any difference in BL levels between males and females during 2 bouts of exercise, constant load cycling and interval cycling. Similarly, Zhang and Li (43) showed no differences in BL at resting, peak (after a VO2max test), and at any time of 30-min recovery between untrained males and females. Finally, Hafen and Vehrs (22) and Hoffmann et al. (24) also observed similar results that the current study in well-trained males and females. Interestingly, Deschenes et al. (10) showed a significant difference in BL levels between males and females during a submaximal (i.e. 60–65 %VO2max) exercise bout. These contrasting results might have several reasons. First, even though Deschenes et al. (10) stated that their subjects were untrained, their VO2max (45.1 and 38.2 mL/kg/min, males and females respectively) are considered average (2, 7) and were higher than the observed in the current study (35.2 and 28.3 mL/kg/min, males and females respectively), which are considered ‘way below average’. Secondly, it is not clear the %PO their subjects performed to stay on target as they altered the workload (in watts) as needed. Finally, there is a notorious difference in the BL levels between both studies during a similar %VO2max (~2.5 mmol/L @~71.5% in the current study vs. ~6.5 mmol/L in males and ~4.3 mmol/L in females @~63.0%), which might be due to plasma processing time, which did not include any glycolysis inhibitor such as Glyceraldehyde (30). Nevertheless, it appears that subjects in the current study workout at lower absolute PO than the previous study what could have driven the differences in BL levels between studies.
The present study was not without limitations. First, our sample size was composed of only young healthy individuals that may not allow us to generalize our findings. In addition, our subjects were categorized as ‘way below average’ by their VO2max (2, 7), which could have affected some of the results at the High intensity workload, especially after 15 minutes of continuous exercise. Moreover, measurements of body composition, to characterize fat mass and fat free mass, could help explaining some of the current results. A larger sample size, including subjects of different age groups and fitness levels, would serve as a better representation of the population. For further studies, we may include well-trained individuals and a randomization of the workloads in different days to see if these results can be replicated. Finally, the present study used cycle ergometer testing and results might be different when using other exercise modalities such as running, swimming, or rowing.
Although VO2, %VO2max, HR, %HR, PO, %PO, RPE, and BL have continuously been used interchangeable in males and females, the present study found different behaviors of exercise intensity markers in males and females. Based on the present and other studies, it appear that the use of %PO, supported by BL and RPE, might be a more consistent way to determine exercise intensities, regardless of biological sex. Traditional exercise intensity markers, such as HR, %HR, PO, VO2, and %VO2max, showed more variability when comparing males and females; therefore, should not be used interchangeably between males and females.
REFERENCES
- 1.ACSM. American college of sports medicine position stand: The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 2.ACSM. American college of sports medicine's guidelines for exercise testing and prescription. Philadelphia, PA: Lippincott, Williams & Wilkins; 2000. [Google Scholar]
- 3.Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: Fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood) 2010;235(1):111–118. doi: 10.1258/ebm.2009.009186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beneke R, Hutler M, Leithauser RM. Maximal lactate-steady-state independent of performance. Med Sci Sports Exerc. 2000;32(6):1135–1139. doi: 10.1097/00005768-200006000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Berglund IJ, Sørås SE, Relling BE, Lundgren KM, Kiel IA, Moholdt T. The relationship between maximum heart rate in a cardiorespiratory fitness test and in a maximum heart rate test. J Sci Med Sport. 2019;22(5):607–610. doi: 10.1016/j.jsams.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Castagna C, Bizzini M, D'Ottavio S, Araújo Póvoas SC. Sex differences in aerobic fitness in top-class soccer referees. J Strength Cond Res. 2018;32(11):3216–3221. doi: 10.1519/JSC.0000000000002292. [DOI] [PubMed] [Google Scholar]
- 7.Charkoudian N, Joyner MJ. Physiologic considerations for exercise performance in women. Clin Chest Med. 2004;25(2):247–255. doi: 10.1016/j.ccm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Coovert D, Evans LD, Jarrett S, Lima C, Lima N, Gurovich AN. Blood flow patterns during incremental and steady-state aerobic exercise. J Sports Med Phys Fitness. 2018;58(10):1537–1543. doi: 10.23736/S0022-4707.17.07142-0. [DOI] [PubMed] [Google Scholar]
- 9.Demello JJ, Cureton KJ, Boineau RE, Singh MM. Ratings of perceived exertion at the lactate threshold in trained and untrained men and women. Med Sci Sports Exerc. 1987;19(4):354–362. [PubMed] [Google Scholar]
- 10.Deschenes MR, Hillard MN, Wilson JA, Dubina MI, Eason MK. Effects of gender on physiological responses during submaximal exercise and recovery. Med Sci Sports Exerc. 2006;38(7):1304–1310. doi: 10.1249/01.mss.0000227316.93351.56. [DOI] [PubMed] [Google Scholar]
- 11.Duke JW, Lane AR, Behr MB, Ondrak KS, Hackney AC. Exercise training biomarkers: Influence of short-term diet modification on the blood lactate to rating of perceived exertion (la:Rpe) ratio. Acta Physiol Hung. 2011;98(2):128–136. doi: 10.1556/APhysiol.98.2011.2.4. [DOI] [PubMed] [Google Scholar]
- 12.Faude O, Kindermann W, Meyer T. Lactate threshold concepts: How valid are they? Sports Med. 2009;39(6):469–490. doi: 10.2165/00007256-200939060-00003. [DOI] [PubMed] [Google Scholar]
- 13.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using g*power 3.1: Tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 14.Faul F, Erdfelder E, Lang AG, Buchner A. G*power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. Exercise standards for testing and training: A scientific statement from the American Heart Association. Circulation. 2013;128(8):873–934. doi: 10.1161/CIR.0b013e31829b5b44. [DOI] [PubMed] [Google Scholar]
- 16.Garcin M, Fleury A, Mille-Hamard L, Billat V. Sex-related differences in ratings of perceived exertion and estimated time limit. Int J Sports Med. 2005;26(8):675–681. doi: 10.1055/s-2004-830440. [DOI] [PubMed] [Google Scholar]
- 17.Green JM, Bishop PA, Muir IH, McLester JR, Jr, Heath HE. Effects of high and low blood lactate concentrations on sweat lactate response. Int J Sports Med. 2000;21(8):556–560. doi: 10.1055/s-2000-8483. [DOI] [PubMed] [Google Scholar]
- 18.Green JM, McLester JR, Crews TR, Wickwire PJ, Pritchett RC, Lomax RG. Rpe association with lactate and heart rate during high-intensity interval cycling. Med Sci Sports Exerc. 2006;38(1):167–172. doi: 10.1249/01.mss.0000180359.98241.a2. [DOI] [PubMed] [Google Scholar]
- 19.Green JM, McLester JR, Crews TR, Wickwire PJ, Pritchett RC, Redden A. Rpe-lactate dissociation during extended cycling. Eur J Appl Physiol. 2005;94(1–2):145–150. doi: 10.1007/s00421-004-1311-2. [DOI] [PubMed] [Google Scholar]
- 20.Gurovich AN, Flores OE, Diaz RA. Comparison of the observed heart rate during blood lactate-based exercise intensity vs. three heart rate-based methods in cardiovascular rehabilitation. Cardiopulm Phys Ther J. 2014;25(2):50–54. [Google Scholar]
- 21.Gurovich AN, Heiser B, Hayes C, Marshall E, Roath S, Kabous NG. Clinical markers of exercise intensity as a surrogate for blood lactate levels only during low-intensity exercise in patients with coronary artery disease. Cardiopulm Phys Ther J. 2018;29(4):144–151. [Google Scholar]
- 22.Hafen PS, Vehrs PR. Sex-related differences in the maximal lactate steady state. Sports (Basel) 2018;6(4) doi: 10.3390/sports6040154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hills AP, Byrne NM, Ramage AJ. Submaximal markers of exercise intensity. J Sports Sci. 1998;16(Suppl):S71–S76. doi: 10.1080/026404198366696. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann SM, Skinner TL, van Rosendal SP, Osborne MA, Emmerton LM, Jenkins DG. The efficacy of the lactate threshold: A sex-based comparison. J Strength Cond Res Publish Ahead of Print. 2019 doi: 10.1519/JSC.0000000000002654. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann P, Tschakert G. Special needs to prescribe exercise intensity for scientific studies. Cardiol Res Pract. 2011;2011 doi: 10.4061/2011/209302. 209302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irving BA, Rutkowski J, Brock DW, Davis CK, Barrett EJ, Gaesser GA, Weltman A. Comparison of Borg- and Omni-rpe as markers of the blood lactate response to exercise. Med Sci Sports Exerc. 2006;38(7):1348–1352. doi: 10.1249/01.mss.0000227322.61964.d2. [DOI] [PubMed] [Google Scholar]
- 27.Joyner MJ, Charkoudian N, Wallin BG. Sympathetic nervous system and blood pressure in humans: Individualized patterns of regulation and their implications. Hypertension. 2010;56(1):10–16. doi: 10.1161/HYPERTENSIONAHA.109.140186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laurent CM, Vervaecke LS, Kutz MR, Green JM. Sex-specific responses to self-paced, high-intensity interval training with variable recovery periods. J Strength Cond Res. 2014;28(4):920–927. doi: 10.1519/JSC.0b013e3182a1f574. [DOI] [PubMed] [Google Scholar]
- 29.Maglischo EW. Training zones revisited. J Swim Res. 2012;19(2):18. [Google Scholar]
- 30.Mangukiya KK, Saxena P, Patel NR, Shaherawala J, Rajput A, Sodavadiya K. Comparison of alternate glycolysis inhibitors with fluoride for preservation of blood for glucose and other common clinical chemistry examinations. Natl J Integr Res Med. 2013;4(3):97–102. [Google Scholar]
- 31.Mann T, Lamberts RP, Lambert MI. Methods of prescribing relative exercise intensity: Physiological and practical considerations. Sports Med. 2013;43(7):613–625. doi: 10.1007/s40279-013-0045-x. [DOI] [PubMed] [Google Scholar]
- 32.Maruf FA, Ogochukwu UN, Dim PA, Alada AR. Absence of sex differences in systolic blood pressure and heart rate responses to exercise in healthy young adults. Niger J Physiol Sci. 2012;27(1):95–100. [PubMed] [Google Scholar]
- 33.Mattu AT, Iannetta D, MacInnis MJ, Doyle-Baker PK, Murias JM. Menstrual and oral contraceptive cycle phases do not affect submaximal and maximal exercise responses. Scand J Med Sci Sports. 2020;30(3):472–484. doi: 10.1111/sms.13590. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell JH, Tate C, Raven P, Cobb F, Kraus W, Moreadith R, O'Toole M, Saltin B, Wenger N. Acute response and chronic adaptation to exercise in women. Med Sci Sports Exerc. 1992;24(6 Suppl):S258–S265. [PubMed] [Google Scholar]
- 35.Morales-Acuna F, Ratcliffe B, Harrison C, Crowe S, Bockover E, Pawlak R, Gurovich AN. Comparison between cuff-based and radial tonometry exercise-induced central blood pressure. Eur J Appl Physiol. 2019;119(4):901–911. doi: 10.1007/s00421-019-04079-9. [DOI] [PubMed] [Google Scholar]
- 36.Navalta JW, Stone WJ, Lyons S. Ethical issues relating to scientific discovery in exercise science. Int J Exer Sci. 2019;12(1):1–8. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robertson RJ, Moyna NM, Sward KL, Millich NB, Goss FL, Thompson PD. Gender comparison of rpe at absolute and relative physiological criteria. Med Sci Sports Exerc. 2000;32(12):2120–2129. doi: 10.1097/00005768-200012000-00024. [DOI] [PubMed] [Google Scholar]
- 38.Seebauer M, Sidler M-A, Kohl J. Gender differences in workload effect on coordination between breathing and cycling. Med Sci Sports Exerc. 2003;35(3):495–499. doi: 10.1249/01.MSS.0000053657.42138.3F. [DOI] [PubMed] [Google Scholar]
- 39.Wheatley CM, Snyder EM, Johnson BD, Olson TP. Sex differences in cardiovascular function during submaximal exercise in humans. Springerplus. 2014;3:445. doi: 10.1186/2193-1801-3-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiebe CG, Gledhill N, Warburton DE, Jamnik VK, Ferguson S. Exercise cardiac function in endurance-trained males versus females. Clin J Sport Med. 1998;8(4):272–279. doi: 10.1097/00042752-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Wilmore JH, Stanforth PR, Gagnon J, Rice T, Mandel S, Leon AS, Rao DC, Skinner JS, Bouchard C. Cardiac output and stroke volume changes with endurance training: The heritage family study. Med Sci Sports Exerc. 2001;33(1):99–106. doi: 10.1097/00005768-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 42.Woodson RD. Hemoglobin concentration and exercise capacity. Am Rev Respir Dis. 1984;129(2 Pt 2):S72–S75. doi: 10.1164/arrd.1984.129.2P2.S72. [DOI] [PubMed] [Google Scholar]
- 43.Zhang JQ, Ji LL. Gender differences in peak blood lactate concentration and lactate removal. Ann Sports Med Res. 2016;3(7):1088. [Google Scholar]