Abstract
Sleep is undoubtedly important for human health as insufficient sleep has been associated with a plethora of diseases. Adequate sleep assessment is critical in clinical and research settings, however current sleep assessment protocols fail to account for circadian rhythms, despite the fact that sleep is a well-recognized circadian process. Purpose: The purpose of this study was to determine if circadian parameters, such as chronotype, influence sleep quality in a sleep laboratory setting. Methods: In order to investigate this, twenty participants (10 men and 10 women) aged 18–31 years old had their sleep recorded by electroencephalography in a sleep lab. Participants also complete surveys which provided data on chronotype, social jet lag and subjective sleep quality. Participants were allowed to self-select sleep time for the study, and sleep discrepancy, defined as the difference between reported and experienced mid-sleep, was determined. Results: Interestingly, results indicated a significant correlation between self-reported sleep quality and social jet lag, with those who typically experience more social jet lag being more satisfied with their sleep during the study (r = 0.549, p = 0.012). In addition, when participants were separated into groups based on chronotype, sleep discrepancy and social jet lag, sizeable differences were noted for parameters such as sleep onset latency, number of awakenings, and percent of time spent in REM sleep. Conclusion: These results suggest circadian parameters serve as predictors of both subjective and objective sleep quality, and thus illuminates a necessity for these parameters to be taken into account in the assessment and research of sleep.
Keywords: Circadian rhythms, social jet lag, electroencephalography
INTRODUCTION
Sleep, though seemingly passive, is actually a critically active stage of the day. It is a period essential for growth, differentiation and renewal of cells, and it plays an important role in immunity (12, 13). Adequate sleep is pivotal for human health, and inadequate sleep contributes to the development of disease. This is demonstrated by numerous studies on the consequences of inefficient sleep, in which impaired sleep is associated with infectious disease, increased risk of cardiovascular disease, mental illness and cancer (18). Inadequate sleep not only includes insufficient duration, poor sleep quality or the presence of sleep disturbances, but may also be due to inappropriate sleep timing (15, 31).
Sleep timing is relevant to sufficient sleep and human health because sleep is intricately linked to circadian rhythms (42); the twenty-four hour oscillations in physiological processes. Circadian rhythms are entrained to the environment and allow the body to anticipate and suitably respond to environmental changes (26). Similar to inadequate sleep, disruption of circadian rhythms is associated with a plethora of health concerns (2, 32, 35, 38). Circadian rhythms are disrupted by situations in which an individual’s endogenous rhythms do not match the exogenous environment, such as shift work or jet lag (4). Additionally, circadian disruption may occur as a result of shifts in sleep schedule from work-week to weekend, termed social jet lag (SJL) (40). SJL happens quite frequently with over two thirds of the population experiencing at least an hour of SJL, and a third experiencing two hours or more (29). Notably, just one hour of SJL increases the risk of cardiovascular disease by 11% (9). This highlights the significance of the relationship between sleep and circadian timing.
Endogenous rhythms may be measured, and an individual’s propensity to sleep at a certain time of day may be characterized by chronotype (20). Surveys, such as the Munich ChronoType Questionnaire (MCTQ), have been developed to define and measure chronotype (16, 43). The MCTQ bases chronotype on reported mid-sleep, or the halfway point between falling asleep and waking up on free days, assuming an individual’s sleep will match their endogenous cycle on free days (8). Chronotype is broken down into categories: severe early, moderate early, mild early, normal, mild late, moderate late and severe late (43). However, chronotype is often categorized more generally using the terms early (larks) or late (night owls). As one might expect, larks and night owls interact with the environment differently, and may therefore experience different degrees of circadian rhythm disruption. In fact, research has demonstrated the amount of SJL experienced is dependent on chronotype, with late chronotypes typically experiencing the most (40). Chronotype can therefore predict circadian disruption, and as such, affect sleep-wake cycles and potentially sleep quality.
Sleep quality may be assessed in a number of ways. The gold standard for the assessment of sleep is polysomnography (23), but polysomnography is expensive and often inaccessible, therefore leading some research to rely on electroencephalography (EEG) (22, 44), which has been validated as a useful tool in the measurement of objective sleep parameters such as sleep duration, total sleep time (TST), sleep onset latency (SOL), wake after sleep onset (WASO), and time in REM and non-REM sleep. Sleep surveys, such as the Pittsburgh Sleep Quality Index (PSQI), are also often used and aid in the assessment of subjective sleep quality (5). These surveys ask for useful information about parameters such as TST, quality of sleep and WASO, however, these tools and the many of the sleep studies that utilize them, lack any mention or measure of circadian rhythms, despite the fact that chronotype may impact sleep quality. There is a growing body of evidence to support the idea that chronotype is associated with quality of sleep (1, 10, 25, 36, 41). Nielson (2010) assessed the relationship between chronotype and nightmares in 3198 subjects over a period of four and a half years and found a greater prevalence of nightmares in those with an eveningness chronotype. Barclay et. al. (2016) evaluated siblings at two time points over the span of five years to assess genetic and environmental effects on diurnal preference (chronotype) and sleep quality. Their study demonstrated a significant, but moderate correlation (r = 0.21–0.25) between diurnal preference and poor sleep quality (1). More recently, Sun et. al. (2019) evaluated the relationship between chronotype and sleep quality in medical students and observed poorer quality of sleep in those with a later chronotype (36). While these studies clearly indicate an association between chronotype and sleep quality, they do not establish how chronotype affects sleep quality within a laboratory setting. The majority of these studies evaluate sleep using self-reported data from surveys. In looking at recent studies that utilize a laboratory setting, or tools to measure qualitative data, the majority of them set the same schedule for all participants, or allow participants to choose their own start time (6, 11, 17, 45). Yet, if participants are shifted from their endogenous rhythm during the study, one might expect measures of sleep quality to be altered considering the established relationship between chronotype and sleep. As such, the current study aims to investigate the relationship between chronotype and sleep quality in a laboratory setting. In addition, past sleep studies fail to indicate if participants experience their normal mid-sleep during data collection. Alterations in mid-sleep, or circadian misalignment, during a study may serve as a confounding factor in research on sleep quality. Therefore, a second goal of this study is to determine if deviations from normal mid-sleep (hereafter referred to as sleep discrepancy) affect measures of sleep quality.
METHODS
Participants
It was determined that 18 participants were required for this study using G*POWER 3.1 (Universitat Kiel, Germany) with a power of 0.75, an effect size of 0.5 and an α = 0.05 (7). Twenty participants ages 18–31 years of age (Table 1) were recruited on a volunteer basis. Exclusion criteria included diagnosed sleep disorders or medication that would affect sleep or pose a risk of participation. All participants signed an informed consent before involvement and all study activities were approved by the Alma College Institutional Review Board. In addition, this research was carried out fully in accordance to the ethical standards of the International Journal of Exercise Science (24).
TABLE 1.
Subject characteristics for male, female and all participants.
| Variable | Male (n = 10) | Female (n = 10) | All (n = 20) |
|---|---|---|---|
| Age (years) | 20.0 ± 1.1 | 21.8 ± 4.0 | 20.9 ± 3.0 |
| Height (cm) | 182.1 ± 5.2 | 168.1 ± 5.3 | 175.1 ± 8.8 |
| Weight (kg) | 84.6 ± 7.8 | 66.4 ± 13.1 | 75.5 ± 14.1 |
| Body mass index (kg/m2) | 25.5 ± 2.1 | 23.4 ± 4.1 | 24.5 ± 3.3 |
| MCTQ reported midsleep (hour) | 5.4 ± 0.5 | 4.6 ± 0.9 | 5.0 ± 0.9 |
| Chronotype | extreme early - 0 moderate early - 0 slight early - 0 normal - 2 slight late - 8 moderate late - 0 extreme late - 0 |
extreme early - 0 moderate early - 0 slight early - 4 normal - 4 slight late - 1 moderate late - 1 extreme late - 0 |
extreme early - 0 moderate early - 0 slight early - 4 normal - 6 slight late - 9 moderate late - 1 extreme late - 0 |
| MCTQ reported social jet lag | 1.4 ± 0.6 | 1.3 ± 1.1 | 1.3 ± 0.9 |
Data is reported as mean ± standard deviation, except for chronotype which is reported as frequency.
Protocol
Objective sleep quality measures were collected using EEG. Wave activity was measured using the BIOPAC MP36 system with EEG (BIOPAC Systems Inc., Goletta, CA). Electrodes were placed at the C3 and C4 locations with a reference above the right ear and connected to a BIOPAC EEG amplifier modified from work by Jo et al. (14). Sampling frequency was set at 1000 Hz with a bandpass filter between 0.1–100 Hz. Additionally, a 60 Hz notch filter ran continuously during the experiments. EEG data were analyzed to obtain quantitative information on time spent in sleep stages, TST, SOL, and WASO, as described in past work (37). SOL was determined as the time in minutes from when participants turned the lights off and tried to fall asleep and the time the EEG recorded actual sleep onset. WASO was calculated as the time in minutes each participant spent in bed after EEG recorded sleep onset, but before wake onset. In addition to using EEG recordings, participants completed a post-sleep questionnaire (Appendix 1) to provide self-reported, subjective data on sleep quality. It yielded interval data on TST, as well as ordinal and nominal data on sleep quality and satisfaction.
Participants selected a time to arrive at a custom-built sleep laboratory at Alma College. Upon arrival, they immediately completed informed consent documents and a screening form to confirm eligibility. If eligible, participants then filled out the MCTQ (43) to determine chronotype from midsleep on free days as established by Roenneberg (2015) (28). Participants were allowed to perform activities in the room as desired until they were ready to sleep. Once ready to sleep, electrodes for EEG recording were placed on participants’ head as previously described. Research staff recorded the time the EEG was connected, the time the lights were turned off and the participant got into bed (for calculation of SOL), and the approximate time the participants fell asleep. Participants were allowed to sleep as long as they wanted (with or without an alarm clock). Upon awakening, participants signaled to the research staff by turning the lights on. The research staff recorded this time and administered the post-sleep questionnaire.
Statistical Analysis
EEG recordings were analyzed with AcqKnowledge 4.2 using visual examination and epoch-based measurement of frequency. For each 30-second epoch, sleep stage was determined according to guidelines laid out in Silber et al. (2007) (33) and the American Academy of Sleep Medicine 2004 revised scoring manual (3). One scorer analyzed all data, eliminating the possibility of low inter-rater reliability. Examination of the EEG data allowed for the following classifications: WASO, SOL, TST, number of times awake, time in Stage 1 sleep, time in Stage 2 sleep, time in deep sleep, and time in REM sleep.
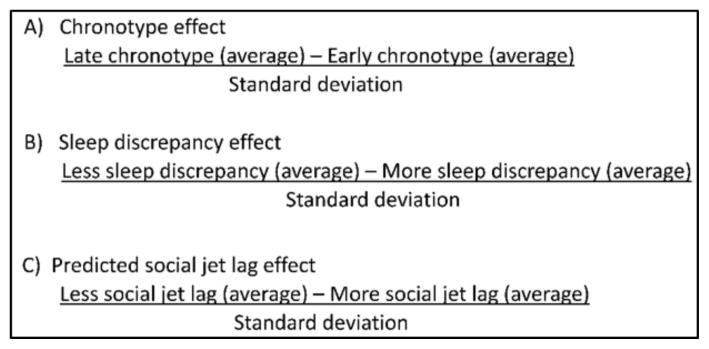
Correlation statistics were used to evaluate relationships between circadian parameters (mid-sleep, chronotype, SJL, etc.) and sleep measures. Pearson correlation analysis was done to compare interval data, Spearman rank correlation analysis was used for ordinal data, and point-biserial correlation analysis was utilized when dealing with nominal data. Student’s t-tests (interval data) and Fisher’s exact tests (nominal data) were done to investigate group differences when two groups were compared. One-way ANOVA (interval data) and Pearson’s chi-square test (nominal data) were done to investigate group differences when three groups were compared. Participants were separated into groups based on chronotype, sleep discrepancy (SD) and SJL. Chronotype was calculated by finding the time halfway between falling asleep and waking up on a non-work day. Chronotype can be classified as severe early, moderate early, mild early, normal, mild late, moderate late and severe late (43). Due to a smaller sample size in this study, participants were categorized as either early (n = 4), normal (n = 6) or late (n = 10). For reference, a mid-sleep of 5 am was considered normal, while mid-sleep before 5 am was considered early and a mid-sleep after 5 am was considered late (16). SD was determined by calculating the difference between reported mid-sleep and recorded mid-sleep. Individuals with less than 1 hour of discrepancy were considered to have low SD while participants with greater than 1 hour of difference were considered to have high SD. SJL was determined using reported data on the MCTQ (16). Those with SJL less than 1 hour were considered low while those with greater than 1 hour of SJL were considered high. Equations for these variables are shown in Figure 1.
Figure 1.
Calculations to determine effect sizes between MCTQ data and sleep quality measures.
P-values and statistical significance are highly dependent upon sample size, without necessarily indicating the practical or clinical significance of differences between groups. Therefore, effect sizes were also calculated and reported as Cohen’s d (interval data) or Cramer’s V (nominal data). Effect sizes were considered to be small = 0.2, medium = 0.5, large = 0.8 or very large = 1.3. Interval data sets were tested for normality and the significance for all correlations was set at p ≤ 0.05. All statistics were performed using Statistical Package for the Social Sciences (SPSS).
RESULTS
Results for reported chronotype from the MCTQ and EEG-measured sleep metrics are presented in Table 2. As determined by Spearman rank correlation statistics, no significant correlations were found. Pearson correlation values for SD and EEG sleep measures are also shown in Table 2. Again, no significant correlations were discovered, however non-significant trends were observed with TST (r = 0.418, p = 0.067) and percent of TST spent in deep sleep (r = −0.374, p = 0.104).
Table 2.
Pearson correlation data examining relationships between EEG and MCTQ data.
| Chronotype | SD | |||
|---|---|---|---|---|
|
| ||||
| Spearman rho | p-value | Spearman rho | p-value | |
| Number of Awakenings (EEG) | 0.048 | 0.842 | 0.245 | 0.298 |
| Total Sleep Time (EEG) | 0.137 | 0.564 | 0.418 | 0.067 |
| % Deep Sleep | 0.206 | 0.383 | −0.374 | 0.104 |
| % REM Sleep | −0.020 | 0.932 | 0.075 | 0.753 |
Spearman rank and point-biserial correlation statistics between MCTQ and PSQI-determined sleep quality are shown in Table 3. There was a significant correlation between mid-sleep and sex (r = 0.486, p = 0.030), with males more likely to exhibit a late mid-sleep. In addition, significant correlations were found between amount of SJL (MCTQ) and self-reported ratings of sleep. Interestingly, those experiencing more SJL reported greater satisfaction with their stay in the lab (r = 0.549, p = 0.012), and with their sleep compared to home (r = 0.507, p = 0.023). A greater amount of SJL also correlated with affirmation that sleep was refreshing (r = 0.455, p = 0.044). However, no significant correlations were discovered between chronotype or mid-sleep and reported sleep quality.
Table 3.
Correlation coefficients examining relationships between PSQI and MCTQ data.
| Midsleep | Chronotype | Sleep debt | Sex | Social jet lag | |
|---|---|---|---|---|---|
| Satisfaction of stay | 0.119 | 0.140 | 0.397 | −0.433 | 0.549* |
| Sleep compared to home | 0.313 | 0.208 | 0.270 | −0.199 | 0.507* |
| Comfort of bed | 0.214 | 0.286 | 0.244 | −0.123 | 0.405 |
| Sleep duration | 0.038 | 0.034 | 0.092 | 0.328 | −0.005 |
| Start time of sleep | −0.140 | −0.118 | −0.113 | −0.149 | −0.088 |
| Difficulty falling asleep | −0.282 | −0.230 | −0.157 | −0.204 | 0.051 |
| Difficulty staying asleep | −0.076 | −0.105 | 0.244 | 0.105 | 0.197 |
| Refreshed after sleep | −0.156 | −0.191 | −0.287 | −0.218 | −0.436* |
| Bothered during night | 0.218 | 0.144 | 0.229 | 0.115 | 0.174 |
Note:
Spearman rank correlation analysis was done to compare interval-to-ordinal data, while point-biserial analysis was performed when one of the variables was nominal.
Correlation coefficients are considered significant if p < 0.05 (*).
Statistical tests (Student’s t-tests, Fisher’s exact tests, one-way ANOVA & Pearson’s chi-square test) revealed no differences between chronotype and the recorded EEG or the self-reported sleep data (Table 4). Despite the lack of significant differences between groups, effect size calculations demonstrated that circadian parameters were related to sleep quality (Table 5). There was a medium effect size for the relationship between chronotype and reported SOL, number of awakenings, and percent of TST spent in REM sleep. Later chronotypes reported greater difficulty falling asleep, recorded more awakenings and had a greater percent time in REM. There was also a moderate effect size for the relationship between SD and reported sleep duration, with those experiencing less SD reporting shorter sleep durations. In addition, there was a large effect size for the connection between SD and percent time spent in REM sleep as well as recorded TST and reported refreshment. Those with less SD reported less refreshment, lower percentage of TST in REM sleep and greater overall TST. Finally, there was a medium effect size for the relationship between SJL and refreshment and a very large effect size for SJL and number of awakenings, as those with lower SJL reported less refreshment and a greater number of awakenings during sleep.
Table 4.
Group differences for early/normal/late chronotype and lesser/greater sleep discrepancy (SD).
| Nominal Data (Y/N) | Interval Data (mean ± std. dev.) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Diff. falling asleep? | Diff. staying asleep? | Refresh. sleep? | Bothered during night? | Rep. sleep duration | Total sleep time | % Deep sleep | % REM sleep | WASO | ||
| Chronotype | Early | 1/3 | 3/1 | 3/1 | 1/1 | 1.78 ± 0.15 | 419.10 ± 30.81 | 1.78 ± 0.15 | 22.85 ± 3.43 | 1.78 ± 0.15 |
| Normal | 1/2 | 1/1 | 1/1 | 1/5 | 6.33 ± 0.36 | 396.50 ± 21.74 | 19.66 ± 3.98 | 28.53 ± 3.84 | 2.00 ± 0.55 | |
| Late | 1/1 | 7/3 | 4/1 | 1/4 | 6.70 ± 0.34 | 1.30 ± 0.15 | 19.10 ± 1.94 | 1.30 ± 0.15 | 3.00 ± 0.62 | |
|
| ||||||||||
| p-value | 0.72 | 0.70 | 0.47 | 0.52 | 0.62 | 0.79 | 0.96 | 0.60 | 0.47 | |
|
| ||||||||||
| SD | Lesser | 4/8 | 7/5 | 6/6 | 3/9 | 6.75 ± 0.99 | 430.00 ± 76.19 | 17.41 ± 7.23 | 23.35 ± 8.70 | 2.25 ± 1.48 |
| Greater | 4/4 | 6/2 | 8/0 | 2/6 | 6.19 ± 0.97 | 375.50 ± 60.99 | 21.9 ± 9.80 | 29.88 ± 12.35 | 2.75 ± 1.88 | |
|
| ||||||||||
| p-value | 0.65 | 0.64 | 0.042* | 1.00 | 0.24 | 0.13 | 0.28 | 0.21 | 0.56 | |
Note:
Nominal (Y/N) data is reported as frequencies while interval data is reported as mean (standard deviation).
For comparing early/normal/late chronotype groups, nominal data was analyzed using a Chi-square test for association and interval data was analyzed using one-way ANOVA. When assessing lesser/greater SD groups, nominal data was analyzed using Fisher’s exact test and interval data was analyzed using Student’s t-test. (*p < 0.05). Abbreviations are as follows: diff. = difficulty, refresh. = refreshing.
Table 5.
Effect sizes examining the magnitude of difference in sleep measures between chronotype, sleep discrepancy and social jet lag groups.
| Group Comparison | ||||
|---|---|---|---|---|
|
| ||||
| Early/late chronotype | Lesser/greater SD | Little/more reported SJL | ||
| Self-reported | Reported sleep duration | 0.36 | 0.69 | 0.02 |
| Difficulty falling asleep | 0.60 | 0.17 | 0.12 | |
| Difficulty staying asleep | 0.29 | 0.17 | 0.18 | |
| Refreshing nature of sleep | 0.21 | 0.54 | 0.37 | |
| Bothered during night | 0.29 | 0.00 | 0.06 | |
| Recorded | # of awakenings | 0.57 | 0.48 | 1.36 |
| Total sleep time | −0.30 | 0.90 | −0.03 | |
| % Deep sleep | −0.06 | −0.36 | −0.04 | |
| % REM sleep | 0.53 | −0.88 | −0.20 | |
Cramer’s V values are reported for “Self-reported” data and Cohen’s d values are reported for “Recorded” data.
DISCUSSION
Our data indicate a significant correlation between MCTQ-determined SJL and self-reported sleep quality parameters such as satisfaction of stay, refreshing nature of sleep and sleep quality compared to home. Notably, this was not the amount of SJL experienced during the study, but rather the amount of SJL experienced based on preferred mid-sleep and normal weekday schedule. This suggests an effect of regularly experienced SJL on sleep quality, even during a preferred night’s sleep. In addition, our results identified a number of moderate to very large effect sizes. When participants were separated into groups based on chronotype, SD and SJL, there was a sizable difference between groups for sleep parameters such as SOL, number of awakenings, percent REM sleep and refreshment. Those effect sizes indicate that significance may be obtained with a greater sample size. This would implicate chronotype and discrepancies in mid-sleep as predictors of both objective and subjective measures of sleep.
As stated, SJL was significantly correlated to self-reported sleep quality parameters. In general, those individuals experiencing more SJL reported greater satisfaction with their stay in the lab and a more refreshing night of sleep. Past studies have demonstrated a sex difference in self-reported sleep quality, with females reporting poorer sleep (21). However, the sex split for those with lesser SJL (5 males, 6 females) and those with greater SJL (5 males, 4 females) was quite even. This indicates the difference found in this study is due to something other than biological sex. A study by Vitale and colleagues, used actigraphy to determine sleep efficiency in college students and found that evening-types were more likely to have lower sleep efficiency (39). Yet this contradicts the self-reported data in this study, as evening types ordinarily experience greater amounts of SJL, but those with greater SJL rated their sleep quality higher. Individuals who experience greater SJL accumulate more sleep debt, and may therefore report greater satisfaction because they have been sleep-deprived. This is speculation however, and more research is necessary to better elucidate the relationship between self-reported sleep quality and SJL.
This study was done to help define the role of chronotype on sleep quality in a laboratory setting. This relationship is critical to understand in order to accurately set up sleep studies and collect sleep data. While there are some studies that have begun to explore to relationship between chronotype and sleep quality, many of these evaluate sleep through survey only, and thus do not require the use of a laboratory setting (10, 34, 41, 45). Recent research that has used the laboratory setting, or collected quantitative measures of sleep via EEG or polysomnography allow the participants to self-report to the lab, or provide a set arrival/sleep time that is the same for every participant (6, 11, 17). If arrival time or sleep time are set, SD is almost guaranteed, as no group of participants could be expected to share the same chronotype. Therefore, it would seem the better protocol to follow would be to allow participants to self-report to the lab and self-select sleep time. Interestingly, when participants were given the freedom to select their own sleep times in this study, 40% selected sleep times that shifted their mid-sleep at least one hour from the expected time. Our data shows that SD influences sleep measures and suggests that chronotype should be assessed and controlled for in sleep studies. Researchers should set sleep times such that participants are as close to mid-sleep as possible in the laboratory setting, or at the very least take chronotype into account during data analysis when sleep times cannot be adjusted to individual rhythms. This could prevent circadian parameters from becoming confounding variables in the interpretation of results.
The results of this study include a significant correlation between chronotype and biological sex, with men exhibiting later chronotypes, which is in agreement with past literature (8). Many studies have demonstrated the effect of sex on chronotype, but the mechanism responsible for this difference is largely undiscovered. Sex hormones likely play a key role in determining diurnal preference. In fact, several studies implicate estrogen and testosterone as modulators of the circadian clock (19, 27, 30). Sex hormone receptors have been shown to be present in the suprachiasmatic nucleus (SCN), which is thought of as the master circadian clock within the body. Every cell of the body has a molecular clock, which generates circadian rhythms, but the ability of the SCN to coordinate and synchronize the body clocks has been well described (26). The presence of these receptors in the SCN suggests a capacity for sex hormones to regulate the molecular clock, and therefore circadian timing. Furthermore, both estrogen and testosterone levels are correlated with chronotype. Randler and colleagues evaluated the level of salivary testosterone in college aged men and self-reported chronotype. They found a positive relationship between testosterone level and eveningness in men (27). While men typically have later chronotypes than women, the sex difference in chronotype disappears around the age of 50, which coincides with the timing of menopause (30). This implies the presence of estrogen affects chronotype as well. Further research is necessary to discern the underlying reasons for the sex effect on chronotype.
Limitations to this study include the sample size and narrow range of participants. Only twenty participants were included, all of which were young, apparently healthy individuals. Many sleep studies include a broader participant pool, and future research is necessary to determine if our findings hold true across populations. This study only evaluated sleep for one night. Moreover, no familiarization with the sleep lab prior to the study night was done. It would be beneficial to further evaluate the effects of circadian parameters on sleep quality over a number of nights. Finally, EEG was used according to previous work (14, 33, 37) to obtain objective sleep measures, but polysomnography is the gold standard. Polysomnography includes EEG, but provides additional data on measures of heart rhythms, eye movement, and muscle movement in addition to the electrical activity of the brain. Subsequent work should include polysomnography in addition to a longer timeline and a larger, more diverse sample to better elucidate the influence chronotype has on sleep within the lab. Though limitations in the study exist, our data are some of the first to indicate circadian parameters influence both objective and subjective sleep quality. This has potential implications that are crucial in the consideration of experimental design for future sleep studies.
Supplementary Information
ACKNOWLEDGEMENTS
This research was supported by the Alma College e-STEM CORE program.
REFERENCES
- 1.Barclay NL, Rowe R, O’Leary R, Bream D, Gregory AM. Longitudinal stability of genetic and environmental influences on the association between diurnal preference and sleep quality in young adult twins and siblings. J Biol Rhythms. 2016;31(4):375–386. doi: 10.1177/0748730416653533. [DOI] [PubMed] [Google Scholar]
- 2.Becker RC, Corrao JM. Circadian variations in cardiovascular disease. Cleve Clin J Med. 1989;56(7):676–680. doi: 10.3949/ccjm.56.7.676. [DOI] [PubMed] [Google Scholar]
- 3.Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, Troester MT, Vaughn BV. Aasm scoring manual updates for 2017 (version 2.4) J Clin Sleep Med. 2017;13(5):665–666. doi: 10.5664/jcsm.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boivin DB, Boudreau P. Impacts of shift work on sleep and circadian rhythms. Pathol Biol. 2014;62(5):292–301. doi: 10.1016/j.patbio.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 6.Della Monica C, Johnsen S, Atzori G, Groeger JA, Dijk DJ. Rapid eye movement sleep, sleep continuity and slow wave sleep as predictors of cognition, mood, and subjective sleep quality in healthy men and women, aged 20–84 years. Front Psychiatry. 2018;9:255. doi: 10.3389/fpsyt.2018.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using g*power 3.1: Tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 8.Fischer D, Lombardi DA, Marucci-Wellman H, Roenneberg T. Chronotypes in the us - influence of age and sex. PloS One. 2017;12(6):e0178782. doi: 10.1371/journal.pone.0178782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forbush S, Fisseha E, Gallagher R, Hale L, Malone S, Patterson F, Branas C, Barrett M, Killgore W, Gehrels J, Alfonso-Miller P, Grandner M. 1067 sociodemographics, poor overall health, cardiovascular disease, depression, fatigue, and daytime sleepiness associated with social jetlag independent of sleep duration and insomnia. Sleep. 2017;40(suppl_1):A396–A397. [Google Scholar]
- 10.Gangwar A, Tiwari S, Rawat A, Verma A, Singh K, Kant S, Garg RK, Singh PK. Circadian preference, sleep quality, and health-impairing lifestyles among undergraduates of medical university. Cureus. 2018;10(6):e2856. doi: 10.7759/cureus.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamanishi S, Eguchi E, Ito T, Nagaoka K, Ogino K. Head cooling during sleep improves sleep quality in the luteal phase in female university students: A randomized crossover-controlled pilot study. PloS One. 2019;14(3):e0213706. doi: 10.1371/journal.pone.0213706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin JC. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996;10(5):643–653. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- 13.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nature Rev Immunol. 2011;11(9):625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jo HG, Park JY, Lee CK, An SK, Yoo SK. Genetic fuzzy classifier for sleep stage identification. Comput Biol Med. 2010;40(7):629–634. doi: 10.1016/j.compbiomed.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Juda M, Vetter C, Roenneberg T. Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers. J Biol Rhythms. 2013;28(2):141–151. doi: 10.1177/0748730412475042. [DOI] [PubMed] [Google Scholar]
- 16.Juda M, Vetter C, Roenneberg T. The munich chronotype questionnaire for shift-workers (mctqshift) J Biol Rhythms. 2013;28(2):130–140. doi: 10.1177/0748730412475041. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan KA, Hardas PP, Redline S, Zeitzer JM, Sleep Heart Health, Study Research G. Correlates of sleep quality in midlife and beyond: A machine learning analysis. Sleep Med. 2017;34:162–167. doi: 10.1016/j.sleep.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kecklund G, Axelsson J. Health consequences of shift work and insufficient sleep. BMJ. 2016;355:i5210. doi: 10.1136/bmj.i5210. [DOI] [PubMed] [Google Scholar]
- 19.Kruijver FP, Swaab DF. Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology. 2002;75(5):296–305. doi: 10.1159/000057339. [DOI] [PubMed] [Google Scholar]
- 20.Lehnkering H, Siegmund R. Influence of chronotype, season, and sex of subject on sleep behavior of young adults. Chronobiol Int. 2007;24(5):875–888. doi: 10.1080/07420520701648259. [DOI] [PubMed] [Google Scholar]
- 21.Lindberg E, Janson C, Gislason T, Bjornsson E, Hetta J, Boman G. Sleep disturbances in a young adult population: Can gender differences be explained by differences in psychological status? Sleep. 1997;20(6):381–387. doi: 10.1093/sleep/20.6.381. [DOI] [PubMed] [Google Scholar]
- 22.Lucey BP, McLeland JS, Toedebusch CD, Boyd J, Morris JC, Landsness EC, Yamada K, Holtzman DM. Comparison of a single-channel eeg sleep study to polysomnography. J Sleep Res. 2016;25(6):625–635. doi: 10.1111/jsr.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcus CL, Chapman D, Ward SD, McColley SA, Herrerias CT, Sitllwell PC, Laskosz LN. Clinical practice guideline: Diagnosis and management of childhood obstructive sleep apnea. Pediatrics. 2002;109(4):704–712. doi: 10.1542/peds.109.4.704. [DOI] [PubMed] [Google Scholar]
- 24.Navalta J, Stone W, Lyons T. Ethical issues relating to scientific discovery in exercise science. Int J Exerc Sci. 2019;12(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen T. Nightmares associated with the eveningness chronotype. J Biol Rhythms. 2010;25(1):53–62. doi: 10.1177/0748730409351677. [DOI] [PubMed] [Google Scholar]
- 26.Panda S, Hogenesch JB, Kay SA. Circadian rhythms from flies to human. Nature. 2002;417(6886):329–335. doi: 10.1038/417329a. [DOI] [PubMed] [Google Scholar]
- 27.Randler C, Ebenhoh N, Fischer A, Hochel S, Schroff C, Stoll JC, Vollmer C. Chronotype but not sleep length is related to salivary testosterone in young adult men. Psychoneuroendocrinology. 2012;37(10):1740–1744. doi: 10.1016/j.psyneuen.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Roenneberg T. Having trouble typing? What on earth is chronotype? J Biol Rhythms. 2015;30(6):487–491. doi: 10.1177/0748730415603835. [DOI] [PubMed] [Google Scholar]
- 29.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22(10):939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 30.Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, Merrow M. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11(6):429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP, Jr, Vitiello MV, Zhdanova IV American Academy of Sleep M. Circadian rhythm sleep disorders: Part i, basic principles, shift work and jet lag disorders An American academy of sleep medicine review. Sleep. 2007;30(11):1460–1483. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sigurdardottir LG, Valdimarsdottir UA, Mucci LA, Fall K, Rider JR, Schernhammer E, Czeisler CA, Launer L, Harris T, Stampfer MJ, Gudnason V, Lockley SW. Sleep disruption among older men and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(5):872–879. doi: 10.1158/1055-9965.EPI-12-1227-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silber MH, Ancoli-Israel S, Bonnet MH, Chokroverty S, Grigg-Damberger MM, Hirshkowitz M, Kapen S, Keenan SA, Kryger MH, Penzel T, Pressman MR, Iber C. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3(2):121–131. [PubMed] [Google Scholar]
- 34.Simor P, Zavecz Z, Palosi V, Torok C, Koteles F. The influence of sleep complaints on the association between chronotype and negative emotionality in young adults. Chronobiol Int. 2015;32(1):1–10. doi: 10.3109/07420528.2014.935786. [DOI] [PubMed] [Google Scholar]
- 35.Souetre E, Salvati E, Belugou JL, Pringuey D, Candito M, Krebs B, Ardisson JL, Darcourt G. Circadian rhythms in depression and recovery: Evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Res. 1989;28(3):263–278. doi: 10.1016/0165-1781(89)90207-2. [DOI] [PubMed] [Google Scholar]
- 36.Sun J, Chen M, Cai W, Wang Z, Wu S, Sun X, Liu H. Chronotype: Implications for sleep quality in medical students. Chronobiol Int. 2019;36(8):1115–1123. doi: 10.1080/07420528.2019.1619181. [DOI] [PubMed] [Google Scholar]
- 37.Swalve N, Harfmann BD, Mitrzyk J, Montoye AHK. Utility of activity monitors and thermometry in assessing sleep stages and sleep quality. J Meas Phys Behav. 2018;1(3):108–121. [Google Scholar]
- 38.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian clock mutant mice. Science. 2005;308(5724):1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vitale JA, Roveda E, Montaruli A, Galasso L, Weydahl A, Caumo A, Carandente F. Chronotype influences activity circadian rhythm and sleep: Differences in sleep quality between weekdays and weekend. Chronobiol Int. 2015;32(3):405–415. doi: 10.3109/07420528.2014.986273. [DOI] [PubMed] [Google Scholar]
- 40.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: Misalignment of biological and social time. Chronobiol Int. 2006;23(1–2):497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 41.Yun JA, Ahn YS, Jeong KS, Joo EJ, Choi KS. The relationship between chronotype and sleep quality in korean firefighters. Clinical Psychopharmacol Neurosci. 2015;13(2):201–208. doi: 10.9758/cpn.2015.13.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaki NFW, Spence DW, BaHammam AS, Pandi-Perumal SR, Cardinali DP, Brown GM. Sleep and circadian rhythms in health and disease: A complex interplay. Eur Arch Psychiatry Clin Neurosci. 2018;269(3):365–366. doi: 10.1007/s00406-018-0866-6. [DOI] [PubMed] [Google Scholar]
- 43.Zavada A, Gordijn MC, Beersma DG, Daan S, Roenneberg T. Comparison of the munich chronotype questionnaire with the horne-ostberg’s morningness-eveningness score. Chronobiol Int. 2005;22(2):267–278. doi: 10.1081/cbi-200053536. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Wang B, Jing J, Zhang J, Zou J, Nakamura M. A comparison study on multidomain eeg features for sleep stage classifcation. Comput Intell Neurosci. 2017;2017 doi: 10.1155/2017/4574079. 4574079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zwart TC, Smits MG, Egberts TCG, Rademaker CMA, van Geijlswijk IM. Long-term melatonin therapy for adolescents and young adults with chronic sleep onset insomnia and late melatonin onset: Evaluation of sleep quality, chronotype, and lifestyle factors compared to age-related randomly selected population cohorts. Healthcare. 2018;6(1):23. doi: 10.3390/healthcare6010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.