Abstract
Currently, there are no established evidence-based rating of perceived exertion (RPE) targets for physical activity (PA) in pregnant women. Yet, a set of target heart rate (HR) ranges have been recommended. Using the Borg Scale, we aimed to determine and validate the RPE target ranges for different PA intensities derived from the recommended HR ranges in the 2019 Canadian Guideline for PA throughout pregnancy. We assessed 13 pregnant women (age: 31.2 ± 3.5 years; gestational age: 20.5 ± 5.0 weeks) using the following three phases: 1) the incremental submaximal walking test to develop the linear regression equation; 2) establishment of the RPE targets for light- and moderate-intensity PA; 3) moderate-intensity exercise session aiming to cross-validate RPE targets in women whose HR ranges were within (Step 1; six participants; 36 RPE values) or outside (Step 2; seven participants; 42 RPE values) the guideline. Study Phase 1 showed a strong linear relationship between RPE x HR (RPE = −7.370 + 0.155*HR; R2 = 0.863). RPE targets for pregnant women aged ≤ 29 years are 8–12 (light-intensity) and 12–15 (moderate-intensity), respectively. For women aged ≥ 30 years, RPE targets are 8–11 (light-intensity) and 11–14 (moderate-intensity), respectively. The cross-validation suggested no differences between predicted (13.4 ± 0.7) vs. observed RPE (13.3 ± 1.4; p = 0.703) and a strong % agreement (Step 1 = 80.6%; Step 2 = 73.8%) between observed RPE and its predicted range. Thus, we have determined pregnancy-specific, evidence-based RPE targets. These RPE targets will help exercise professionals, other health care providers, and pregnant women to easily monitor exercise intensity during pregnancy to meet recommended Canadian PA Guideline.
Keywords: Pregnancy, exercise, perception, cardiovascular response, recommendation
INTRODUCTION
Recently, the “2019 Canadian Guideline for Physical Activity (PA) throughout Pregnancy” (referred throughout the text as the Canadian PA Guideline) was released by the Society of Obstetricians and Gynaecologists of Canada (SOGC) and the Canadian Society for Exercise Physiology (CSEP). It is an evidence-based guideline substantiated by extensive systematic reviews and meta-analyses. It also takes into account the user’s point of view, including obstetric care providers, exercise professionals, researchers, policy organizations, as well as pregnant and postpartum women, and is founded on feasibility, acceptability, costs, and equity aspects (19).
Based on the results of a set of systematic reviews, this current guideline states that pregnant women should aim to achieve at least 150 min of moderate-intensity PA over three or more days per week (19). Their findings suggest that meeting the recommendations is associated with reductions in the odds of developing different pregnancy complications, such as gestational diabetes mellitus and/or hypertension, as well as pre-eclampsia (12). Although there is a dose-response relationship between increasing exercise intensity and decreasing odds of developing depressive symptoms, insulin resistance, gestational diabetes, and hypertension, and pre-eclampsia (11–13), light-intensity PA has also been shown to be beneficial (19).
The recommendation of moderate-intensity PA, defined as 40–59% HR reserve (HRR) corresponding to reserve oxygen uptake (10), is comparable to the recent American College of Sports Medicine (ACSM) heart rate (HR) targets for exercise during pregnancy (2). This percentage range (i.e., 40–59%) originates from previous ACSM guidelines for prescribing exercise intensities (3) and follows the position statement on PA and exercise intensity terminologies (2, 21).
Another method to monitor PA intensity in pregnant women suggested by the Canadian PA Guideline is by using the “talk test”. For this test, women are advised to remain at a “comfortable intensity” representing the ability to hold a conversation while physically active. If they cannot maintain a conversation, the intensity should be reduced (19). The “talk test”, however, is limited in its scope as it can only measure intensity changes from moderate- to higher intensities, but fails to take into consideration changes in different exercise and PA intensity ranges (e.g., light- to moderate-intensity).
The position statement on physical activity and exercise intensity terminology (21) suggests that an alternative subjective measure of exercise intensity could be used; rating of perceived exertion (RPE), also known as Borg RPE scales (6). Of the existing scales, the 15-point scale that ranges from 6–20, is the most widely used and is recommended for exercise testing and prescription (6). This scale was developed in an attempt to provide a user-friendly measure that increases linearly with intensity, similar to the responses of HR (6). Indeed, stress responses measured by physiological markers (e.g., blood lactate concentrations, HR, oxygen consumption) (6, 15, 21) increase positively and linearly with PA intensity. These physiological changes are cognitively perceived as a sensation of physical effort (21), which makes the Borg RPE scale useful in PA intensity monitoring. According to Borg (5), RPE complements HR responses to physical effort.
With that in mind, previous guidelines from SOGC/CSEP (14) had included Borg RPE scale target ranges for moderate-intensity PA (i.e., 12 to 14 on the 6–20 scale); however, the quality of evidence and classification of recommendations were considered poor, given that no target RPE values were derived from research in pregnant women. Due to the lack of evidence from the pregnant population, the current Canadian PA Guideline (19) does not mention RPE as a tool to monitor exercise intensity. Unlike the Canadian PA Guideline, the last American College of Obstetricians and Gynecologists (1) exercise guideline did advocate a range of 13–14 on the Borg RPE scale equivalent to moderate-intensity exercise. The ACOG (1) recommends the use of RPE in addition to HR for monitoring PA intensity due to the reported altered HR responses in pregnant women (18, 24). Nevertheless, ACOG’s suggested RPE ranges are not based on evidence in pregnant women or on other physiological responses/recommendations for this population.
The HR targets published by Davenport et al. (10) are considered the up-to-date reference for light- and moderate-intensity PA during pregnancy and are included in different guidelines for physical activity throughout pregnancy (2, 19). However, monitoring the proposed HR targets may present a real-life challenge to women without a HR measuring device. Another issue related to HR is the variable accuracy of different wearable HR monitors during aerobic exercise at light- and moderate-intensities (16).
Thus, our goal was to establish RPE target ranges for different PA intensities gleaned from the recommended HR ranges in the current Canadian PA Guideline (19) for pregnant women. To determine corresponding RPE ranges to match HR values, we developed an equation that estimates appropriate RPE target ranges for different PA intensities. We hypothesized that appropriate RPE ranges would be determined and cross-validated in a simulated bout of moderate-intensity exercise, due to the high correlation between HR and RPE (6).
METHODS
Participants
Thirteen pregnant women were recruited for participation using social media and flyers. Eligibility criteria were as follows: maternal age of 18–40 years, carrying a singleton fetus, no contraindications to exercise, and a self-reported pre-pregnancy body mass index (BMI) between 18.5 – 29.9 kg/m2, and 13–28 weeks of gestation (2nd trimester of pregnancy). Although the gestational age range we selected was wide, Pivarnik et al. (23) found no differences in HR and RPE responses during exercise across this time point (i.e., the 2nd trimester of pregnancy). Those with gestational diabetes, hypertension, or untreated thyroid disease were excluded. All experimental procedures were approved by the local Research Ethics Board (H-06-18-634). Study procedures were explained in person, after which informed written consent was obtained from all study participants. This research was carried out in accordance with the ethical standards of the International Journal of Exercise Science (20).
Protocol
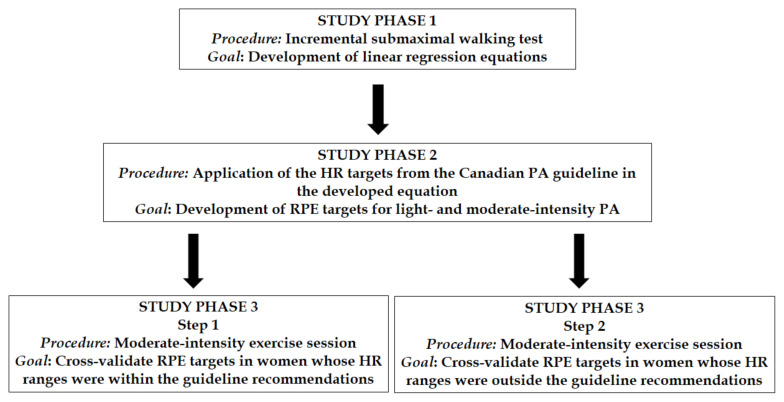
The study design is outlined in Figure 1. The procedures are detailed below.
Figure 1.
Study design. HR = heart rate; PA = physical activity; RPE = rating of perceived exertion.
Participants were asked to visit the lab following a minimal 8 h fast and to abstain from exercise for 12 h before the session. Height and body weight were measured using a Tanita HR-200 wall-mounted stadiometer (accuracy: 0.1cm; Lachine, QC) and Tanita BWB-800 scale, respectively. The study protocol required participants to arrive fasted for a baseline blood draw, followed by a standardized (~400 kcal) snack before the exercise session. The participants were fasted overnight as the current study was part of a larger study assessing blood biomarkers in pregnancy.
Resting HR was determined by obtaining the average HR recorded at 1 min intervals during the last 5 min of a 10 min seated resting phase.
The first part of the exercise session consisted of an incremental submaximal walking test conducted using a Woodway Pro XL 27 treadmill (Woodway USA, Waukesha, WI) described as follows: 3 min warm-up at a speed of 2.0 miles per hours (mph) and an incline of 2.0%. Then, the incline increased to 6.0%, with speed increasing by 0.2 mph at 1-min intervals. HR was continuously measured using a Polar V800 watch (Polar Electro Canada, Lachine, QC) until the target calculated moderate-intensity HR range was reached (i.e., the upper limit of the 40–59% of HRR range). This target is consistent with the Canadian PA Guideline (19). The following equation was employed to determine the moderate-intensity target HR range using the following calculation: %HRR = [(HRmax – resting HR)* %intensity] + resting HR; where HRmax = 220 – age.
Along with HR monitoring throughout the incremental submaximal walking test, RPE values were recorded using the 6–20 Borg Scale (6) 15 seconds before the end of each stage of the test. From the HR data, HR values were pooled at the same time RPE was collected in order to create the linear regression equations (Study Phase 1) and subsequently develop the RPE targets for light- and moderate-intensity PA (Study Phase 2).
Moderate-intensity exercise session (Study Phase 3): Once the upper limit of the 40–59% HRR range was achieved in the incremental submaximal walking test, the participants continued to exercise at moderate-intensity for 30 min as close as possible to the upper limit of the 40–59% HRR range using the same treadmill and with continuous measurement of HR as described above. The speed was adjusted accordingly to ensure that the target moderate-intensity HR range was maintained. RPE and HR were recorded every 5 min (i.e., 5th, 10th, 15th, 20th, 25th, and 30th minutes) until the completion of the exercise session. A 30-min moderate-intensity exercise session was chosen to replicate a suggested bout of prenatal PA (19).
To cross-validate RPE targets, Study Phase 3 was further divided into two steps (Steps 1 and 2). Separation of study processes was necessary because some of our participants were found to be exercising outside of the recommended HR targets based on findings from Davenport et al. (10). This identification of exercise intensities outside the recommended range is due to differences in observed resting HR (used to calculate 40–59% HRR) in our study population compared to those in Davenport et al. (10).
Thus, in Step 1 the observed RPE values obtained from the 5th–30th minutes of the moderate-intensity exercise session were compared with the predicted RPE values from the developed equation (Study Phase 1) for women whose HR ranges were within the guideline recommendations (n = 6 participants; total of 36 RPE values) according to their individualized 40–59% HRR target ranges (10). In Step 2 of the cross-validation of the RPE targets, we used the observed RPE values from seven participants whose suggested moderate-intensity HR ranges fell outside the guideline recommendations. Assuming that the exercise session for this group was also considered a moderate-intensity exercise bout, we used observed RPE values in the seven women captured from the 5th–30th minutes (a total of 42 RPE values) of the exercise session separately.
Statistical Analysis
A power analysis conducted using G*POWER 3.1 (Universitat Kiel, Germany) determined that a minimum of 11 participants were required in the present study for a power of 80%, an effect size of 0.7, and an α = 0.05. The effect size of 0.7 was determined based on the results of a large (n = 2560) (non-pregnant) cohort study (25) examining the association between HR and RPE during an incremental exercise test.
Data are presented as mean ± standard deviation, and the Shapiro-Wilk normality test was used to determine if parametric (paired t-test) or non-parametric (Wilcoxon test) tests would be used in the comparison analysis.
Statistical analysis followed similar procedures described previously by Davenport et al. (8) to establish the cut-off points for HR described in the Canadian PA Guideline. In Study Phase 1, linear regression equations were developed for each of the 13 participants. The regression equations and other linear regression variables were pooled to determine mean values for the final equation. A one-sample t-test was used to compare mean intercept and slope with the line of identity (Intercept = 0; Slope = 1) to verify whether HR and RPE were equivalent.
Subsequently, in Study Phase 2, HR targets were pooled from the Canadian PA Guideline (17) and applied to the equation to determine the RPE targets for light- and moderate-intensity PA, considering that the incremental submaximal walking test only included these intensity zones. RPE targets were developed for women aged ≤ 29 years and ≥ 30 years separately, as also presented in the Canadian PA Guideline for HR targets during PA.
The final phase of the analysis (Study Phase 3) was the cross-validation of the RPE targets, separated in two steps. In Step 1, the observed RPE values obtained at 5th, 10th, 15th, 20th, 25th, and 30th minutes of the moderate-intensity exercise session were compared with the predicted RPE values from the developed equation (HR x RPE) for women whose HR ranges were within the guideline recommendations (n = 6 participants; total of 36 RPE values; Wilcoxon test) according to their individualized 40–59% HRR target ranges (10). We also calculated the percentage of agreement (% agreement = [n of agreements/total sample size] * 100) between the observed RPE values and the predicted RPE targets derived from the HR ranges for the moderate-intensity exercise session (total of 36 RPE values). This percentage value was also classified according to McHugh (17) to determine the level of agreement (0–4% None; 4–15% Minimal; 15–35% Weak; 35–63% Moderate; 64–81% Strong; 82–100% Almost Perfect).
In Step 2 of the cross-validation of the RPE targets, we used the observed RPE values from seven participants whose suggested moderate-intensity HR ranges were outside the guideline recommendations. Assuming that the exercise session for this group was also considered a moderate-intensity exercise bout, we used observed RPE values in the seven women captured at the 5th, 10th, 15th, 20th, 25th, and 30th minutes (total of 42 RPE values) of the exercise session and analyzed the percentage of agreement described above with the RPE targets derived from the HR cut-offs.
Participants in Step 1 and 2 of Study Phase 3 were compared with regards their descriptive characteristics using the Mann-Whitney test or unpaired t-test, according to the normality test results. For all the inferential analyses performed, significance was set at p < 0.05.
RESULTS
The characteristics of pregnant women participating in the present study, as well as the subgroup analyzed in the cross-validation procedure, are described in Table 1. The incremental submaximal walking test lasted, on average, 10 ± 2 min.
Table 1.
Study population characteristics.
| Demographics | Equation development (n = 13) | Cross-validation 1 (n = 6) | Cross-validation 2 (n = 7) |
|---|---|---|---|
| Age (years) | 31.2 ± 3.5 | 33.5 ± 3.3 | 29.3 ± 2.6* |
| Gestational age (weeks) | 20.5 ± 5.0 | 18.8 ± 5.5 | 22.0 ± 4.4 |
| Height (cm) | 166.7 ± 5.4 | 167.7 ± 4.0 | 165.8 ± 6.5 |
| Weight at visit (kg) | 69.6 ± 10.3 | 70.2 ± 11.0 | 69.0 ± 10.5 |
| Gestational weight gain (kg) | 5.8 ± 3.9 | 4.8 ± 4.3 | 6.7 ± 4.3 |
| Pre-pregnant body weight (kg) | 63.7 ± 9.5 | 65.4 ± 9.5 | 62.3 ± 10.0 |
| Pre-pregnant BMI (kg·m−2) | 23.7 ± 3.6 | 23.4 ± 3.9 | 24.0 ± 3.5 |
| Resting HR (bpm) | 81 ± 14.5 | 73 ± 11 | 89 ± 14* |
| Incremental submaximal walking test | |||
| Speed to reach the upper limit of 40–59% HRR (mph) | 3.4 ± 0.4 | 3.7 ± 0.5 | 3.4 ± 0.3 |
| Moderate-intensity exercise session | |||
| 40% HRR (bpm) | 125 ± 9.5 | 119 ± 6.1 | 130 ± 9.2* |
| 60% HRR (bpm) | 146 ± 7.1 | 141 ± 4.2 | 150 ± 6.8* |
Note.
p < 0.05.
BMI = body mass index; HR = heart rate; HRR = heart rate reserve; bpm = beats per minute.
The regression equation for HR vs. RPE was significantly different from the line of identity (slope = 1; intercept = 0), as presented in Table 2. The proportion of variability from one variable explained by the other was determined according to the coefficient of determination of the model, which was R2 = 0.863 for the raw analysis and R2 = 0.836 for the adjusted analysis. Other parameters of the linear regression are described in Table 3 (Study Phase 1).
Table 2.
Means and standard deviation for the intercept and slope of linear regression analysis for RPE predicted from HR (n = 13).
| RPE vs. HR | Mean ± SD | p-value for the line of identity |
|---|---|---|
| Intercept | −7.370 ± 5.849 | 0.002a |
| Slope | 0.155 ± 0.050 | < 0.001b |
Note.
RPE = rating of perceived exertion; HR = heart rate; SD = standard deviation.
Significantly different than 0.
Significantly different than 1.
Table 3.
R-value, p-value from the correlation, coefficient of determination, adjusted coefficient of determination, and standard error of estimation derived from the linear regression to predict RPE based on HR.
| R-value (correlation) | p-value (correlation) | R2 | Adjusted R2 | SEE | |
|---|---|---|---|---|---|
| RPE vs. HR | 0.933 | 0.005 | 0.863 | 0.836 | 0.801 |
Note. RPE = rating of perceived exertion; HR = heart rate; SEE = standard error of estimation.
The RPE targets were calculated by applying the HR values described in the Canadian PA Guideline in the regression equation developed in the Study Phase 1. The derived RPE targets for light- and moderate-intensity PA are described in Table 4 (Study Phase 2).
Table 4.
RPE targets predicted according to the HR targetsa described in the 2019 Canadian Guideline for PA throughout pregnancy.
| Maternal age (years) | Intensity | HR targets (bpm) | RPE targetsb (Borg scale 6–20) |
|---|---|---|---|
| ≤ 29 | Light | 102–124 | 8–12 |
| Moderate | 125–146 | 12–15 | |
|
| |||
| ≥ 30 | Light | 101–120 | 8–11 |
| Moderate | 121–141 | 11–14 | |
Note.
HR targets were pooled and adapted from Mottola et al.1.
RPE targets were determined based on the equation RPE = −7.370 + 0.155*HR.
bpm = beats per minute; HR = heart rate; RPE = rating of perceived exertion.
When our equation was used in the first step of cross-validation analyses (36 RPE observations), predicted RPE (13.4 ± 0.7) based on the HR obtained in the 30-min moderate-intensity exercise session was not different from the observed RPE (13.3 ± 1.4) obtained throughout the exercise session (p = 0.703). We also compared the predicted and observed RPE values only in the first 15-min (1st half) as well as in the last 15-min (2nd half of the exercise session). For both first (18 RPE observations) and last half (18 RPE observations) of the exercise session, there was no difference between predicted and observed RPE values (Predicted RPE = 13.4 ± 0.7; Observed RPE = 13.6 ± 1.2; p = 0.624 for 1st half and predicted RPE = 13.4 ± 0.7; Observed RPE = 13.0 ± 1.6; p = 0.372 for 2nd half).
The percentage agreement between the observed RPE and RPE targets for moderate-intensity exercise (RPE of 12–15 for ≤ 29 years old pregnant women and RPE of 11–14 for ≥ 30 years old pregnant women; Table 4) was calculated for the six women engaged in Step 1 of cross-validation (36 RPE observations) and revealed 80.6% agreement (classified as a ‘strong’ level of agreement). The percentage agreement was also determined for the seven women in Step 2 (42 RPE observations) of cross-validation with 73.8% agreement, classified as a ‘strong’ level of agreement.
DISCUSSION
An important outcome of the study is the development and cross-validation of RPE ranges for light- and moderate-intensity PA for pregnant women. For those aged ≤ 29 years, light-intensity PA RPE targets are 8–12 on the 6–20 Borg RPE scale, whereas moderate-intensity corresponds to 12–15. Women aged ≥ 30 years should associate light-intensity PA to RPE scores of 8–11 and moderate-intensity to 11–14, using the same scale.
The HR targets published by Davenport et al. (10), considered the up-to-date reference for light- and moderate-intensity PA during pregnancy, are those associated with the exercise guidelines in this population (2, 19). While HR targets are an excellent tool for those with access to a HR monitor, this strategy is not realistic for monitoring PA intensity in all women. Additionally, there are concerns regarding the accuracy and reliability of different commercially-available HR monitors, specifically during aerobic exercise conducted at light- or moderate-intensities (16).
Although RPE, a relatively simple perceptual tool with high practical application, has previously been included in pregnancy-related PA guidelines as a surrogate measure for moderate-intensity PA monitoring, the quality of evidence for its inclusion lacked rigor (14). As such, our study is the first to address this shortcoming and provide RPE targets for light- and moderate-intensity exercise during pregnancy. We do not include RPE targets corresponding to vigorous-intensity PA (i.e., 60–79% HRR) for two reasons. First, the incremental submaximal walking test used to determine the predictive equation was designed to have women exercise at the recommended moderate-intensity range. Thus, vigorous-intensity HR and RPE values were not captured. During incremental exercise tests, including the vigorous-intensity range, participant data illustrate a change from the linear HR-exercise intensity relationship, referred to as the HR deflection point (8). The departure from linearity is generally reflected as a concave trend in the HR curve (9). However, this curve has a wide range of inter-subject variability, allowing the observation of not only concave but also convex and linear trends (5). Consequently, extrapolating RPE values for vigorous-intensity HR targets from light- and moderate-intensities could be premature.
The second reason as to why vigorous-intensity RPE targets were omitted from our study is that the Canadian PA Guideline recommends vigorous-intensity PA only in a monitored environment (19). Additionally, there is still a lack of information regarding the benefits of exercising within this higher intensity range, especially with-respect-to the upper limit, which prompted the Canadian PA Guideline to recommend that pregnant women consult their obstetric care provider before starting a vigorous-intensity exercise regimen (19).
Despite the optimistic results from our cross-validation phase, they do not agree with those found by O’Neill et al. (22), which showed that HR prediction based on RPE underestimated the observed HR values in pregnant women. O’Neill’s results can be explained by the predictive approach taken to calculate HR based on RPE that these authors used. Essentially, they added a “0” value to the 6–20 RPE Borg Scale, representing participants’ HR. Although Borg (6) suggested that the 6–20 values corresponded to HR ranges from 60–200 beats·min−1, it was also mentioned that a given exercise session that evokes a HR of 150 beats·min−1 may be assigned an RPE of either 13 or 17 as a result of a multitude of physical and emotional factors. Ideally, both HR and RPE should be used together to monitor exercise intensity (6), which was acknowledged by O’Neill et al. (22). In lieu of adding a “0” value to the RPE scale to predict HR values, we followed the same approach (i.e., simple linear regression) as the one used in pregnant women by Davenport et al. (10) to determine the HR ranges.
The RPE target ranges found in the current study showed an overlap between the upper limit for light-intensity and the lower limit for moderate-intensity RPE values in both younger (i.e., ≤ 29 years; RPE = 12) and older pregnant women (i.e., ≥ 30 years; RPE = 11). These differing results were observed because the RPE values obtained from the prediction equation were rounded as a necessity. As 6–20 RPE values are not commonly reported using decimals, we deemed that rounding was acceptable.
Overall, this study has important strengths. The statistical procedures used to determine appropriate RPE targets were based on similar methods employed by Davenport et al. (8). Additionally, the cross-validation (Study Phase 3) was based on a moderate-intensity PA session with both women who were exercising within the HR guideline targets (i.e., Step 1) and those exercising outside the HR guideline targets according to their resting HR (i.e., Step 2). The study also has limitations. Only women in their 2nd trimester were studied, similar to the HR target ranges described in the Canadian PA Guideline (19), and we cannot assure this will be applicable in other pregnancy time points (i.e., earlier or later in pregnancy). Also, we included pregnant women who were classified as normal and overweight, while Davenport et al. (10) included women categorized as overweight or obese in their study to develop the HR target ranges. The reason for analyzing both women categorized as normal-weight and overweight in the present study is that the current Canadian PA Guideline (19) extrapolated the HR target ranges for those in all BMI categories. Due to the small sample size (n=9 classified as normal weight and n=4 considered overweight), we could not perform our analysis separately between BMI categories. On a similar note, the impact of gestational weight gain on these RPE ranges was not assessed in the present study. Based on self-reported PA questionnaire assessment, we found that many of our participants reported being quite active (unpublished data), but none of them were actively engaged in exercise training (i.e., systematic and prescribed training). As this study included only 13 women, we opted not to include this self-reported data in the analysis since subjective data have been found to overestimate PA levels in pregnancy (7). One more shortcoming is that the same set of pregnant women were used in the cross-validation, although we have changed the exercise modality (i.e., submaximal incremental test to continuous exercise session). Lastly, vigorous-intensity PA was not examined in our study. More studies are required to fully understand the benefits of exercising at higher intensities during pregnancy, although there is evidence showing positive maternal and fetal outcomes (4). Given these limitations, future studies should examine the influence of pre-pregnancy BMI categories, gestational weight gain, PA levels, and other exercise modes and intensities on the relationship between HR and RPE during exercise.
We concluded that RPE ranges for light- and moderate-intensity PA for pregnant women aged ≤ 29 years old should be 8–12 on the 6–20 Borg RPE scale, whereas Borg 12–15 reflects moderate-intensity. Women aged ≥ 30 years old should perform light-intensity PA within the range of 8–11 and moderate-intensity in the range of 11–14 using the same scale. The cross-validation phase suggested no differences between predicted vs. observed RPE values and a strong percentage of agreement between the observed RPE and its predicted range. The availability of appropriate RPE ranges will ultimately guide the monitoring of exercise-intensity by exercise professionals, researchers, and pregnant women.
ACKNOWLEDGEMENTS
We would like to thank all of the study participants for their efforts. S.M. holds the Faculty of Health Sciences uOttawa/Children’s Hospital of Eastern Ontario (CHEO) Doctoral Fellowship for Advancement of Biological Perspectives for Exercise Interventions Across Lifespan. K.A.H. is the recipient of the Canada Graduate Scholarships-Masters from the Natural Sciences and Engineering Research Council of Canada (NSERC). We acknowledge the support of NSERC (RGPIN-2017-05457) and the Canadian Institutes of Health Research (CIHR) (MOP 142298) grants awarded to K.B.A.
REFERENCES
- 1.American College of Obstetricians and Gynecologists. Committee Opinion. Physical activity and exercise during pregnancy and the postpartum period. Obstetr Gynecol. 2015;126(6):e135–e142. doi: 10.1097/AOG.0000000000001214. [DOI] [PubMed] [Google Scholar]
- 2.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 10th ed. Amsterdam: Wolters Kluwer; 2017. [DOI] [PubMed] [Google Scholar]
- 3.American College of Sports Medicine. Guidelines for exercise testing and exercise prescription. 7th ed. Lippincott: Williams & Wilkins, Philadelphia, Penn; 2005. [Google Scholar]
- 4.Beetham KS, Giles C, Noetel M, Clifton V, Jones JC, Naughton G. The effects of vigorous intensity exercise in the third trimester of pregnancy: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19:281. doi: 10.1186/s12884-019-2441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodner ME, Rhodes EC. A review of the concept of the heart rate deflection point. Sports Med. 2000;30:31–46. doi: 10.2165/00007256-200030010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 7.Brett KE, Wilson S, Ferraro ZM, Adamo KB. Self-report Pregnancy Physical activity questionnaire overestimates physical activity. Can J Public Health. 2015;106(5):e297–302. doi: 10.17269/cjph.106.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conconi F, Ferrari M, Ziglio PG, Droghetti P, Codeca L. Determination of the anaerobic threshold by a noninvasive field test in runners. J Appl Physiol Respir Environ Exerc Physiol. 1982;52:869–873. doi: 10.1152/jappl.1982.52.4.869. [DOI] [PubMed] [Google Scholar]
- 9.Da Silva DF, Peserico CS, Machado FA. Relationship between heart rate deflection point determined by Dmax method and 10-km running performance in endurance recreationally-trained female runners. J Sports Med Phys Fitness. 2015;55:1064–1071. [PubMed] [Google Scholar]
- 10.Davenport MH, Charlesworth S, Vanderspank D, et al. Development and validation of exercise target heart rate zones for overweight and obese pregnant women. Appl Physiol Nutr Metab. 2008;33:984–989. doi: 10.1139/H08-086. [DOI] [PubMed] [Google Scholar]
- 11.Davenport MH, McCurdy AP, Mottola MF, et al. Impact of prenatal exercise on both prenatal and postnatal anxiety and depressive symptoms: a systematic review and meta-analysis. Br J Sports Med. 2018a;52:1376–1385. doi: 10.1136/bjsports-2018-099697. [DOI] [PubMed] [Google Scholar]
- 12.Davenport MH, Ruchat SM, Poitras VJ, et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Br J Sports Med. 2018b;52:1367–1375. doi: 10.1136/bjsports-2018-099355. [DOI] [PubMed] [Google Scholar]
- 13.Davenport MH, Sobierajski F, Mottola MF, et al. Glucose responses to acute and chronic exercise during pregnancy: a systematic review and meta-analysis. Br J Sports Med. 2018c;52:1357–1366. doi: 10.1136/bjsports-2018-099829. [DOI] [PubMed] [Google Scholar]
- 14.Davies GAL, Wolfe LA, Mottola ML, et al. Joint SOGC/SCEP Clinical practice guideline: exercise in pregnancy and the postpartum period. Can J Appl Physiol. 2003;28(3):329–341. [PubMed] [Google Scholar]
- 15.Faude O, Kindermann W, Meyer T. Lactate threshold concept: how valid are they? Sports Med. 2009;39:469–490. doi: 10.2165/00007256-200939060-00003. [DOI] [PubMed] [Google Scholar]
- 16.Gillinov S, Etiwy M, Wang R, et al. Variable accuracy of wearable heart rate monitors during aerobic exercise. Med Sci Sports Exerc. 2017;49(8):1697–1703. doi: 10.1249/MSS.0000000000001284. [DOI] [PubMed] [Google Scholar]
- 17.McHugh ML. Interrater reliability: the Kappa statistic. Biochemia Med. 2012;22(3):276–282. [PMC free article] [PubMed] [Google Scholar]
- 18.McMurray RG, Mottola MF, Wolfe LA. Recent advances in understanding maternal and fetal responses to exercise. Med Sci Sports Exerc. 1993;25(12):1305–1321. [PubMed] [Google Scholar]
- 19.Mottola MF, Davenport MH, Ruchat SM, et al. 2019 Canadian guideline for physical activity throughout pregnancy. Br J Sports Med. 2018;52:1339–1346. doi: 10.1136/bjsports-2018-100056. [DOI] [PubMed] [Google Scholar]
- 20.Navalta JW, Stone WJ, Lyons TS. Ethical issues relating to scientific discovery in exercise science. Int J Exerc Sci. 2019;12(1):1–8. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norton K, Norton L, Sadgrove D. Position statement on physical activity and exercise intensity terminology. J Sci Med Sport. 2010;13:496–502. doi: 10.1016/j.jsams.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 22.O’Neill ME, Cooper KA, Mills CM, et al. Accuracy of Borg’s ratings of perceived exertion in the prediction of heart rates during pregnancy. Br J Sp Med. 1992;26(2):121–124. doi: 10.1136/bjsm.26.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pivarnik JM, Lee W, Miller JF. Physiological and perceptual responses to cycle and treadmill exercise during pregnancy. Med Sci Sports Exerc. 1991;23(4):470–475. [PubMed] [Google Scholar]
- 24.Purdy GM, James MA, Wakefield PK, et al. Maternal cardioautonomic responses during and following exercise throughout pregnancy. Appl Physiol Nutr Metab. 2019;44(3):263–270. doi: 10.1139/apnm-2018-0397. [DOI] [PubMed] [Google Scholar]
- 25.Scherr J, Wolfarth B, Christle JW, Pressler A, Wagenpfeil S, Halle M. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur J Appl Physiol. 2013;113:147–155. doi: 10.1007/s00421-012-2421-x. [DOI] [PubMed] [Google Scholar]