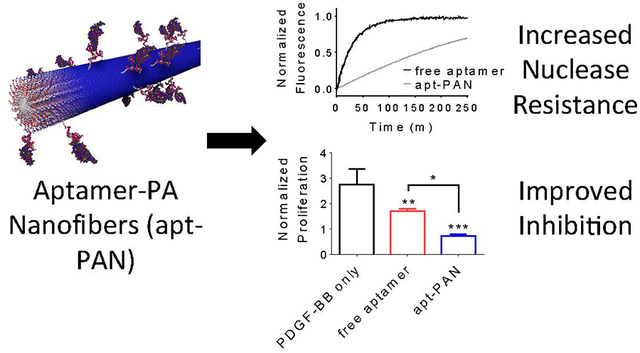
Abstract
The single stranded DNA oligonucleotides known as aptamers have the capacity to bind proteins and other molecules and offer great therapeutic potential. Further work is required to optimize their function and to diminish their susceptibility to nuclease degradation. We report here on the synthesis and supramolecular self-assembly of DNA-peptide amphiphiles that form high aspect ratio nanofibers and display aptamers for platelet-derived growth factor. The nanofibers were found to bind the growth factor with an affinity that was fivefold greater than the free aptamer. We also observed that the aptamer displayed by the supramolecular nanostructures was eight times more nuclease resistant than free aptamer. In order to highlight the therapeutic potential of these supramolecular systems, we demonstrated the improved inhibition of proliferation when the growth factor was bound to aptamers displayed by the nanofibers.
Keywords: Biomaterials, self-assembly, aptamer, peptide amphiphile, hybrid, nanotechnology
Graphical Abstract
INTRODUCTION:
Aptamers are single stranded oligonucleotides that can be selected or evolved to bind specifically a target molecule with high affinity1,2. They have shown considerable versatility and can be used as affinity reagents3, bioimaging probes4, biosensors5,6, diagnostics6,7, and therapeutics8,9. In particular, therapeutic aptamers can function as agonists for activating their target receptors or as targeted carriers for delivery of other therapeutic agents. However, their most common use is serving as an antagonist for blocking the interaction of disease-associated targets. For example, the FDA-approved pegaptanib is an RNA-based oligonucleotide that binds VEGF-165 to inhibit pathological angiogenesis in age-related macular degeneration. Generally, the development of therapeutic aptamers has been limited by their poor stability due to rapid nuclease degradation, and high renal clearance8,9. Efforts to improve the nuclease stability of aptamers include using chemical modifications that replace the 2’ hydroxyl of the ribose with a fluorine10, amine11, or methoxy group12, as these modifications render the aptamers less susceptible to nuclease degradation. Conjugation of aptamers at the 5’ end with a bulky moiety such as high molecular weight polyethylene glycol12 or cholesterol13 has extended their circulation time by increasing their size above the threshold for the renal glomerulus. Other strategies involve the formation of multimeric aptamers either through direct covalent linkage of two aptamers14, presentation on liposomes15 or dendrimers16, or attachment to cyclens17. These multimeric aptamers have demonstrated improved binding affinity, biological function, and circulation time. One strategy to improve the stability and efficacy of aptamer candidates that has scarcely been explored is the use of self-assembled nanofibrous aptamer systems. Although recent work explores the use of a supramolecular aptamer system, where hydrophobic groups drive the self-assembly into nanotubes and nanotapes, these studies were limited to only the characterization of the nanostructures and did not demonstrate improved aptamer properties18,19.
Peptide amphiphiles (PAs) are a class of self-assembling molecules consisting of a covalently linked hydrophobic tail and a peptide sequence. A specific subset of PAs containing a ß-sheet forming peptide region and a charged peptide domain is capable of forming one-dimensional nanostructures20. These PAs are useful building blocks to develop functional nanoscale assemblies for various applications in drug delivery, and regenerative medicine21–24. The self-assembly of these PAs in an aqueous environment promotes hydrophobic tail collapse and allows the display of high and tunable densities of biological signals on their surfaces25,26. This display has been shown to be useful for targeting27,28 or activating membrane receptors pathways29–32 and binding proteins33–35 or molecules of interest24,36. Our laboratory has recently investigated the use of peptide-DNA conjugates to create bioactive biomaterials37–39. One system consisted of DNA nanotubes covalently functionalized with RGDS peptides that preferentially differentiated neural stem cells into neurons37. Another system used two orthogonal peptide-DNAs to independently regulate bioactive signals over time, which enabled the discovery that neural stem cell migration out of neurospheres is reversible38. Most recently, DNA-PA conjugates were developed that can reversibly form extracellular matrix mimetic fiber bundles that can control astrocyte phenotype39. Importantly, the co-assembly of these DNA-PA molecules with diluent PA molecules does not interfere with the formation of nanofibers.
In this work we synthesized a PA conjugated at its hydrophilic terminus to a 41-nucleotide DNA aptamer that binds the B chain of platelet-derived growth factor (PDGF-BB)40. Although PDGF-BB plays an important role in developmental processes, it is also implicated in numerous proliferative disorders, such as tumor growth, fibrosis, and vascular disorders, where its inhibition is of therapeutic benefit41. We show that co-assembly of diluent PAs with aptamer-PA conjugates promotes the formation of aptamer-PA nanofibers (apt-PAN). We assess the nuclease resistance and PDGF-BB binding affinity of apt-PAN compared to the aptamer alone. In order to assess the therapeutic potential of the supramolecular structures containing the aptamer, we investigate their antagonist activity in a PDGF-BB-mediated proliferation assay.
RESULTS AND DISCUSSION:
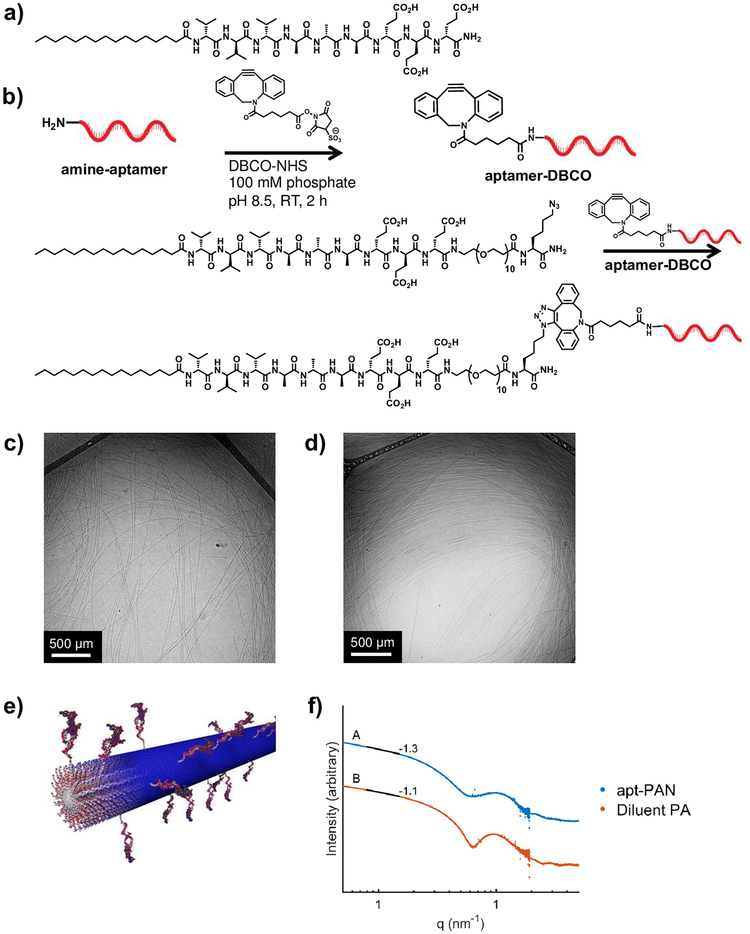
To form aptamer-PA nanofibers (apt-PAN), we co-assembled the aptamer-PA with a diluent PA (palmitoyl-V3A3E3) known to form high-aspect ratio nanofibers32,42 (Figure 1a). As the hydrophobic tails of both PAs collapse to create the core of the nanofibers, the aptamer should be displayed on their surfaces. Additionally, the negatively charged glutamic acid residues should provide charge repulsion to prevent the aptamer from folding back onto the nanostructure. Although other self-assembled morphologies can be formed, namely spherical micelles43, we chose to explore nanofibers and their potential to improve the stability and binding affinity of the aptamer. To synthesize the aptamer-PA, we adapted our previously developed methodology for peptide-DNA conjugates37,38 (see Materials and Methods and Supporting Information for details). As shown in Figure 1b, we extended the diluent PA with a spacer of ten ethylene glycol units to allow conformational flexibility for the aptamer, followed by a C-terminal azidolysine for copper-free click chemistry. We reacted the 5’-amine-modified aptamer sequence (Figure S1) with dibenzocyclooctyne-sulfo-N-hydroxysuccinimidyl ester (DBCO-sulfo-NHS) to form the aptamer-DBCO conjugate. The cyclooctyne modified aptamer was then reacted with the azido-lysine terminated PA to form the aptamer-PA conjugate (Figure 1b). The resulting material was then isolated by reverse-phase high performance liquid chromatography as a highly pure conjugate (Figure S2).
Figure 1.
Synthesis of aptamer-PA and characterization of aptamer-PA nanofibers (apt-PAN). a) The 5’-amino modified aptamer strand was reacted with a dibenzocyclooctyne-sulfo-N-hydroxysuccinimidyl ester (DBCO-NHS) to form the clickable intermediate aptamer-dibenzocyclooctyne (DBCO). This was then reacted with a C-terminal azido-lysine modified peptide amphiphile to form the resultant aptamer-PA. b) Chemical structure of the diluent PA co-assembled with the aptamer-PA. c,d) Cryogenic transmission electron microscopy micrographs of diluent PA alone and co-assembled apt-PAN (aptamer-PA:diluent-PA in a 0.5:99.5 molar ratio), respectively, show similar fibrous structures. e) Molecular representation of the co-assembly of aptamer and diluent peptide amphiphiles. f) Small angle x-ray scattering profiles of the diluent PA nanofibers and the apt-PAN fitted to a core-shell cylinder model.
We first examined whether the presence of aptamer-containing PA molecules would affect the expected self-assembly behavior in aqueous media. For this purpose we prepared a 10 mM solution containing 0.5-mol% aptamer-PA monomers and the remaining 99.5-mol% containing diluent PA monomers. We chose this co-assembly ratio since previous work demonstrated that low co-assembly ratios are important for nanofiber formation, while higher percentages of DNA-PA give rise to spherical micelles39. The solution was annealed at 95°C for 30 minutes and then cooled to room temperature at 1°C/minute. Cryogenic transmission electron microscopy (cryoTEM) micrographs of the mixture show that the co-assembled aptamer-PA, referred to as apt-PAN, forms long fibers similar to those formed by the diluent PA alone (Figure 1c,d). To confirm the results from cryoTEM, small angle x-ray scattering (SAXS) measurements were performed to determine the size and shape of the nanostructures in solution (Figure 1f). The SAXS data were fit to a core-shell cylinder model, which is expected to have a slope of −1 in the low-q region. The diluent PA has a slope of −1.1 in the low q-region, indicating good agreement with the model. The apt-PAN has a slope of −1.3, which suggests there is a distortion in the cross section of the filaments relative to that of those formed by the diluent PA, possibly an elliptical shape. However, apt-PAN are still high-aspect ratio supramolecular nanostructures. Furthermore, the radius of the apt-PAN is not appreciably different from that of the diluent PA nanofibers. It is significant that the presence of the highly charged aptamer segments does not alter the ability of the system to form fibers.
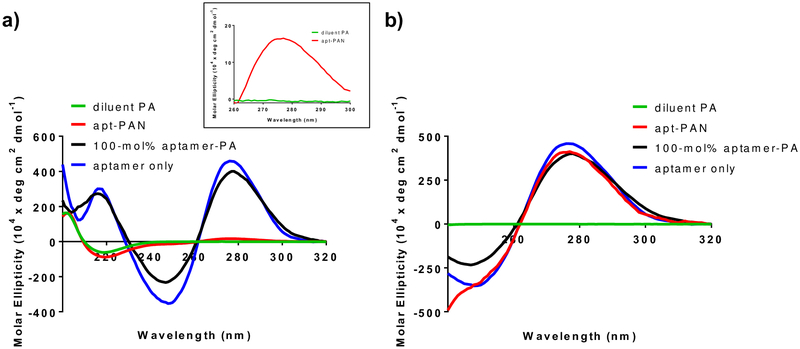
Circular dichroism (CD) spectroscopy was used to determine both the conformation of the aptamer on the apt-PAN and the secondary structure of the peptide. The secondary structure of the aptamer is important for binding its target since various folds allow the aptamer to access diverse binding mechanisms such as hydrophobicity, molecular shape complementarity, or in the case of small molecules binding through intercalation in double-stranded regions of an aptamer44,45. Aptamers can adopt a variety of self-associative conformations including loops attached to stems formed through Watson–Crick base pairing (stem-loop structures), which give rise to a maximum peak at ~277 nm and a minimum at ~245 nm18,46. We observed that the PDGF-BB aptamer alone has peaks at 275 nm and 247 nm, characteristic of the stem-loop structures (Figure 2). The apt-PAN in a pH 7.4 adjusted aqueous solution containing 1 mM sodium chloride reveals a peak at 275 nm and a shoulder at 247 nm, which we interpret as an indication that the aptamer covalently conjugated to the PA segment is likely forming the same secondary structure as the aptamer on its own (Figure 2b). Similarly, the aptamer-PA at 100-mol% shows a CD spectrum that closely resembles that of the aptamer alone. This is an important observation since the secondary structures of aptamers play an important role in binding of targets.44,45. The secondary structure of the peptide is important since formation of a β-sheet structure contributes to nanofiber formation42. The CD spectra of the diluent PA reveal the characteristic negative peak for β-sheet formation at 219 nm and a maximum at 202 nm47 (Figure 2a). The co-assembled apt-PAN has a nearly identical CD spectrum, indicating that the presence of the aptamer does not affect β-sheet formation at this co-assembly ratio (Figure 2a). The aptamer-PA at 100-mol%, however, does not show β-sheet formation, as this likely forms micelles39. The β-sheet signal is expected for the apt-PAN because the aptamer is displayed on the surfaces of the nanofibers given its hydrophilic nature (Figure 1e).
Figure 2.
Circular dichroism (CD) spectra of solutions of diluent PA nanofibers and apt-PAN at 100 μM total PA and 100-mol% aptamer-PA and aptamer alone at 100 μM total DNA concentration. All samples are at pH 7.4. a) CD spectra of apt-PAN plotted with regard to peptide molar ellipticity show the formation of a β-sheet structure evidenced by peaks at 219 nm and 202 nm in both the diluent PA and apt-PAN. The presence of a peak at 275 nm indicates the presence of the DNA on the apt-PAN (see inset). b) Plotting the molar ellipticity in terms of the DNA concentration shows that the apt-PAN display a similar maximum peak as the free aptamer at 275 and a shoulder at 247 nm, indicating that the aptamer still adopts the same stem-loop conformation on the nanostructure.
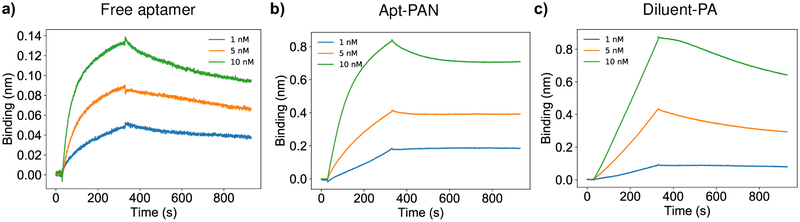
After observing that the secondary structure of the aptamer on the apt-PAN was not altered compared to the free aptamer, we explored how the binding properties of the aptamer were affected by its presentation on PA nanofibers. Using biolayer interferometry, a label-free method for measuring bimolecular interactions, we modified amine reactive probes with PDGF-BB using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide and N-hydroxysuccinimide coupling. Solutions of free aptamer, diluent PA nanofibers, or apt-PAN at various concentrations were allowed to associate with a freshly prepared PDGF-BB functionalized probe for 5 minutes followed by a 10-minute dissociation step. Kinetic constants were derived using the Pall Forte Bio BLItz software. For the free aptamer, an equilibrium dissociation constant (KD) of 780 pM was calculated which is in good agreement with the value reported previously40. We found that the apt-PAN had a KD of 150 pM, a more than five-fold decrease relative to the aptamer alone (Figure 3, Figure S3 and Table 1). The diluent PA, in contrast, had less affinity than both the free aptamer and the apt-PAN with a KD of 460 nM (Figure 3 and Table 1). The diluent PA appears to have some nonspecific electrostatic affinity for the positively charged PDGF-BB (isoelectric point = 9.8). Zeta (ζ) potential measurements show that the surface charge of the diluent PA is strongly negative due to the presence of the glutamic acid residues (Figure S4). The ζ-potential of the various nanofibers is identical, indicating that the binding of the PDGF-BB by the apt-PAN is primarily driven by specific interactions between the aptamer and the protein. Electrostatics, therefore, are not primarily responsible for the difference in binding affinity between the apt-PAN and the diluent PA.
Figure 3.
Binding affinities of free aptamer and PA systems to PDGF-BB. Biolayer interferometry sensorgrams of several dilutions of a) free aptamer, b) apt-PAN, and c) diluent PA. Kinetic constants calculated from the curves are shown in Table 1.
Table 1.
Kinetic constants calculated from curve fitting of biolayer interferometry data shown in Figure 3.
| System | KD (M) | KD Error | ka (1/Ms) | ka Error | kd (1/s) | kd Error |
|---|---|---|---|---|---|---|
| Free aptamer | 7.82 × 10−10 | 3.64 × 10−11 | 1.59 × 106 | 7.41 × 104 | 1.24 × 10−3 | 5.18 × 10−5 |
| Apt-PAN | 1.51 × 10−10 | 4.18 × 10−12 | 1.25 × 106 | 1.68 × 104 | 1.89 × 10−4 | 5.22 × 10−6 |
| Diluent PA | 4.61 × 10−7 | 3.73 × 10−7 | 1.08 × 103 | 6.96 × 103 | 4.98 × 10−4 | 7.09 × 10−6 |
The supramolecular nature of the nanofiber itself also influences the difference in KD between the free aptamer and the nanofiber-bound aptamer. Compared to free aptamer, the apt-PAN dissociates much more slowly from PDGF-BB (Table 1). We hypothesize this is due to the fact that the aptamer chosen for this study binds specifically to the B chain of PDGF40. PDGF-BB has two B-chains and therefore two potential binding sites for the DNA aptamer. In solution, two different free aptamers might bind independently; however, because the apt-PAN presents copies of the aptamer in high density, the nanofiber-bound aptamers may cooperatively bind the PDGF-BB. Other multivalent aptamer systems have previously shown enhanced affinity for targets with multiple ligands or multidentate ligands48,49. For example, circular bivalent aptamers have been shown to reduce the KD by more than twofold48. Furthermore, the internal dynamics of molecules within the nanofibers allow the rearrangement of the individual aptamer-PA monomers for optimal binding to PDGF-BB50,51. The presence of many aptamers on the surface of the apt-PAN also allows for rapid rebinding after a dissociation event occurs. Thus, we suggest the supramolecular design of the apt-PAN contributes to the observed enhanced binding properties.
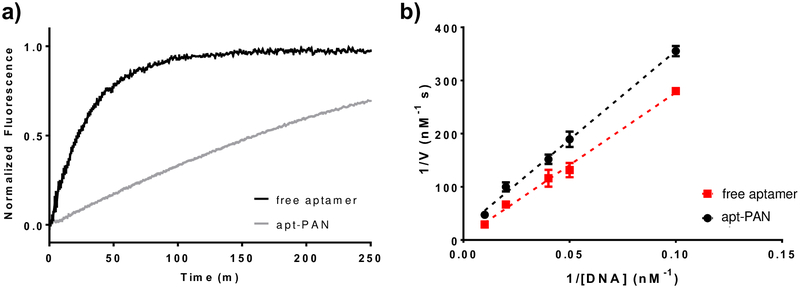
Nanoparticles with a highly negative surface charge have been shown to increase the stability of DNA on their surface by creating a high local salt concentration52. For this reason, we tested whether presentation on the surface of a nanofiber conferred greater nuclease resistance to the hybrid aptamer-PA compared to the free aptamer. To do so, we designed an oligonucleotide that was complementary to the aptamer sequence and modified it at the 5’-end with a carboxyfluorescein and at the 3’-end with a dabcyl quencher (Figure S1). We hybridized the free aptamer and apt-PAN with the modified complementary oligomer at 1 μM in PBS by annealing at 70°C followed by slow cooling to room temperature. The hybridized free aptamer and apt-PAN were diluted to various concentrations and mixed with DNAse I. The degradation reactions were monitored using fluorescence over several hours, and the half-lives of the free and bound aptamers were calculated (Figure 4, Table 2, and Figure S5). Under these conditions, apt-PAN has a half-life that is 8.1 times longer than the free aptamer. Double-reciprocal analysis was performed to determine the enzyme–substrate association (1/KM) and maximum reaction velocity (vmax) for both the free aptamer and apt-PAN. This analysis shows that the enzyme association is more favorable in the case of apt-PAN, perhaps due to a greater local concentration of DNA along the surface of the fiber. However, vmax is much slower than the free aptamer, suggesting that nanofiber association slows the rate of DNA hydrolysis and improves nuclease resistance of the apt-PAN, despite the higher observed enzyme association. Thus, the supramolecular assembly of the apt-PAN provides greater nuclease resistance compared to the free aptamer.
Figure 4.
Comparison of the degradation rates of apt-PAN and free aptamer systems. a) Free aptamer and apt-PAN samples labeled with quenched fluorophores are mixed with DNAse I to initiate hydrolysis of the DNA. As the hydrolysis proceeds, the fluorescence increases as a function of time indicating the nuclease-mediated degradation rates of the two systems. b) Double-reciprocal plot of the initial degradation velocity calculated from the progress curves for various DNA concentrations for the free aptamer and apt-PAN systems. Data points represent the reciprocal initial degradation velocity plotted against the reciprocal DNA concentration. Dashed lines represent the linear best fit for each system. Kinetic parameters calculated from the double-reciprocal plot are shown in Table 2.
Table 2.
Kinetic parameters derived from the double reciprocal plot shown in Figure 4.
| System | vmax (nM/s) | 1/KM (uM-1) | Half-life (min) |
|---|---|---|---|
| Free aptamer | 0.21 ± 0.12 | 1.7 ± 0.5 | 21 ± 2 |
| Apt-PAN | 0.046 ± 0.014 | 6.5 ± 1.8 | 170 ± 2 |
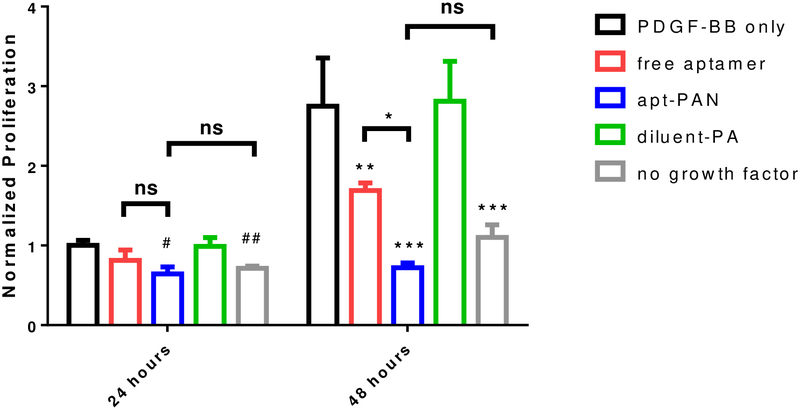
After demonstrating that the apt-PAN confers greater nuclease resistance to the aptamer, we investigated how this would impact the ability of the aptamer to block the activity of PDGF-BB in a proliferation assay. To determine whether our apt-PAN has enhanced antagonist activity, we plated human lung fibroblasts in a 96-well plate and treated them with different concentrations of PDGF-BB ranging from 0 to 100 ng/mL. Except for control samples without growth factor, the cells were all cultured in the presence of PDGF-BB and then a portion of the cells were further treated with free aptamer, apt-PAN, or diluent PA before being allowed to proliferate for 48 hours (time points were taken at 24 and 48 hours). After 24 hours, both free aptamer and apt-PAN reduced proliferation compared to PDGF-BB alone or the diluent PA without aptamer (Figure 5 and Figure S6). After 48 hours, there was a more than two-fold increase in total DNA in the wells of PDGF-BB only samples. The condition with free aptamer did show significant an increase in the total amount of DNA between 24h and 48h, but this increase was much less than without aptamer. The apt-PAN inhibited the PDGF-BB mediated proliferation of the fibroblasts at both time points and displayed levels of proliferation comparable to those observed without the use of growth factor (Figure 5). Based on these data, it is clear that the apt-PAN is superior to free aptamer at inhibiting PDGF-BB mediated proliferation. The improved antagonist activity of the apt-PAN is likely due to enhanced nuclease stability and continued function after 24 hours. Additionally, the slower dissociation constant of apt-PAN (Table 1) may also contribute to the enhanced efficacy by increasing the amount of time PDGF-BB remains associated to nanofibers thus remaining inactive during those periods. Thus, our study suggests that apt-PAN is an effective structure to improve the activity of an aptamer.
Figure 5.
Inhibition of PDGF-BB induced proliferation of fibroblasts by free aptamer and apt-PAN systems measured by total DNA content in a fluorescent assay. Data are mean ± SD; # is significance relative to samples after 24 hour containing only PDGF-BB; #P<0.05, ##P<0.01; * is significance with respect to samples kept for 48 hour in the presence of PDGF-BB only; *P<0.05, **P<0.01, ***P<0.001.
CONCLUSION:
We have reported on the design and biological activity of supramolecular PA nanofibers that display aptamer molecules on their surfaces. We conclude that compared to free aptamer, aptamer-PA nanofibers have improved binding to their target protein due to cooperative binding effects. They also display enhanced resistance to nuclease-mediated degradation, and improved capacity to block PDGF-BB activity compared to free aptamer. This study highlights the potential for using peptide amphiphile nanotechnology as a strategy to enhance biological activity of aptamers.
MATERIALS AND METHODS:
Materials
All reagents were purchased from Sigma-Aldrich and used without any further purification unless otherwise indicated. Normal human lung fibroblasts (NHLF) were purchased from Lonza (CC-2512). Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), penicillin/streptomycin (P/S), Dulbecco’s phosphate buffered saline (DPBS), and trypsin/EDTA were ordered from Gibco® (Life Technologies). Dibenzocyclooctyne-sulfo-N-hydroxysuccinimidyl ester (DBCO-sulfo-NHS) was purchased from Sigma-Aldrich and dissolved in DMSO at 50 mM and stored at −20°C prior to use. All DNA strands were purchased from IDT. Both the unmodified aptamer and 5’-amino modified aptamer were ordered without additional purification from and dissolved at 1 mM in either in water or pH 8.3 sodium phosphate buffer, respectively, and stored at −20°C prior to use. The stability strand was ordered from IDT with HPLC purification and was dissolved in water at 1 mM and stored at −20°C prior to use.
Peptide synthesis and purification
The C16VVVAAAEEE and C16VVVAAAEEE(PEG)10Kaz (Kaz denotes the unnatural amino acid azidolysine) peptide amphiphiles were synthesized at the Peptide Synthesis Core at the Simpson Querrey Institute using standard fluoren-9-ylmethoxycarbonyl (Fmoc) solid-phase peptide synthesis on rink amide 4-methylbenzhydrylamine (MBHA) resin (100–200 mesh, 0.55 mmol/g). Following synthesis, each peptide was cleaved from the resin in a mixture of 95% trifluoroacetic acid (TFA), 2.5% triisopropylsilane (TIS), and 2.5% water. The solvent was removed under reduced pressure by rotary evaporation, and the peptide was precipitated using cold diethyl ether. The precipitate was filtered and washed with ether, then dissolved in water with 0.1% ammonium hydroxide. The solution was purified using reverse phase HPLC (Varian Prostar 363, Jupiter 10u Proteo 90A column) using a water/acetonitrile gradient (2–100% acetonitrile over 45 minutes) with 0.1% ammonium hydroxide. Purified peptides were lyophilized and stored at −20°C. The purity of the peptides was confirmed by liquid chromatography mass spectrometry (LC-MS, Agilent 6510 Q-TOF).
Aptamer-peptide amphiphile conjugation and purification
To a solution of 5’-amine terminated aptamers (1 mM in 100 mM phosphate buffer, pH 8.3), 5 equivalents of dibenzocylooctyne-sulfo-N-hydroxysuccinimidyl ester (DBCO-sulfo-NHS, 50 mM) was added. The mixture was incubated for 2 hours at room temperature with vigorous shaking. Afterwards, an equivalent volume of 100 mM sodium phosphate buffer, pH 9.5, was added along with a fresh addition of 5 equivalents of DBCO-sulfo-NHS and again the mixture was allowed to react for 2 hours with vigorous agitation. Excess DBCO was removed using a size exclusion column (Illustra Nap-5, GE Healthcare) pre-equilibrated with water with 0.1% sodium hydroxide. To this solution was added two equivalents of the azide-modified peptide amphiphile and 100 mM sodium chloride. The solution was gently agitated at room temperature for at least 24 hours. Afterwards, excess peptide was removed by spin-concentration (Amicon Ultra spin concentrators, 3,000 DA molecular weight cutoff, Millipore) and desalted using a Nap-5 column pre-equilibrated with 50 mM triethylammonium acetate (TEAA) buffer, pH 7, prior to reverse phase HPLC purification.
The aptamer-PA solutions were purified using reverse phase HPLC (Agilent 1260 Infinity, DIKMA Inspire C18 column (5 μm, 250 × 4.6 mm)) with a gradient of organic buffer B (90% acetonitrile in water, + 50 mM TEAA, pH 7) in water + 50 mM TEAA, pH 7 (buffer A). The gradient used was 5–100% B over 45 min. Absorbance at 260 nm was monitored, with unmodified DNA eluting at ~7–10 min, and aptamer-PA conjugates ~20–24 min. The aptamer-PA peak was collected and lyophilized and then dissolved in water to determine the concentration of aptamer-PA by UV-Vis absorbance measured at 260 nm. The aptamer-PA was then aliquoted, lyophilized and stored at −20°C until use. The purity of the aptamer-PA conjugates was confirmed by matrix-assisted laser desorption ionization (MALDI). The matrix was prepared as follows: 75 mg/mL of 2,6-dihydroxyacetophenone in methanol was mixed in a 3:1 ratio with saturated ammonium citrate in water. 1 uL of the matrix solution was used to reconstitute lyophilized DNA PA, which was then dispensed onto a MALDI plate. Data was collected on a Bruker AutoFlex-III MALDI-ToF (laser = 355 nm).
Aptamer-peptide amphiphile preparation
Apt-PANs were prepared by first dissolving the diluent PA solution in 150 mM NaCl and 10 mM NaOH. The pH of the diluent PA was checked with pH paper and adjusted if needed so that the pH was approximately 7.4. Aptamer-PA was then dissolved with the diluent PA solution, such that the total PA concentration was 10 mM with 0.5-mol% being aptamer-PA and 99.5-mol% being diluent PA. The diluent PA samples were prepared at 10 mM with the same solvent. All PA solutions were horn sonicated three times for 10 seconds with 30-second rests at 10% amplitude. All PA solutions were then annealed using a thermal cycler (Mastercycler® pro, Eppendorf) set to incubate at 95°C for 30 minutes before cooling the samples to 20°C over the course of 75 minutes (1°C/min).
Cryogenic transmission electron microscopy
CryoTEM was performed using a JEOL 1230 TEM fitted with a LaB6 filament working at an accelerating voltage of 100 kV. Images were acquired with a Gatan 832 CCD camera. After annealing, samples were deposited on a 300-mesh copper grid with lacey carbon support (Electron Microscopy Sciences), blotted, and plunged into liquid ethane using a Vitrobot Mark IV (FEI) vitrification robot operating at 22°C with 100% humidity. After vitrification, the sample was transferred under liquid nitrogen to a Gatan 626 cryo-holder.
Circular Dichroism (CD)
Circular dichroism spectra were recorded using a Jasco Circular Dichroism Spectrophotometer (model J-815). PA samples were dissolved in water at 10 mM, with the apt-PAN sample having 0.5-mol% aptamer-PA (50 μM), and pH adjusted to 7.4 with 1 M sodium hydroxide. Before each measurement, the PA or DNA samples were diluted to final PA concentration of 100 μM with 1 mM sodium chloride. A quartz cuvette of 2 mm path length was used for the measurements. Each trace represents an average of five scans.
Zeta potential measurements
The ζ-potential of the PAs was measured using Zetasizer Nano ZSP (Malvern) using disposable folded capillary cells (#DTS1070, Malvern). The different PA solutions were prepared at 1-wt% in 150 mM NaCl in milli-Q water and were diluted 1:10 in PBS. For ζ-potential measurements, the sample was considered to be protein with an RI of 1.45, the dispersant was considered to be water with a RI of 1.33, the temperature was set at 25°C, the model used was Smoluchowski, the measurement duration was set on automatic, each sample was measured 5 times with a delay of 30 seconds between measurements, and both automatic attenuation and automatic voltage were selected.
Biolayer Interferometry
Biolayer interferometry was performed on a FortéBio BLItz® system using second generation amine reactive (AR2G) biosensors. PDGF-BB was immobilized to the surface of the biosensor by first activating the surface with 40 mM EDC and 20 mM sulfo-NHS for 5 minutes followed by incubation with the protein for 10 minutes at 10 μg/mL in 1:20 diluted PBS pH 7.4 solution. The reaction was quenched for 5 minutes with 1 M ethanolamine and then washed twice with TBS and water. For the standard association and dissociation experiments, the analyte was allowed to associate with the biosensor at a given concentration for 5 minutes and then dissociate for 10 minutes. A new biosensor was used for each concentration and the spectra represent an average of three independent runs. The data was fit using the FortéBio software to calculate the kinetic parameters. For the kinetic titration experiments, the biosensors were prepared in the same way as before and the analyte was allowed to associate for 5 minutes followed by 1 minute dissociation for five consecutive concentrations from low to high. Data for the kinetic titration were fit using CLAMP software.
Nuclease Stability Assay
The nuclease stability experiments were performed as described elsewhere with some modifications52. Briefly, fluorophore/quencher-labeled complementary sequences and either the apt-PAN or the free aptamer were hybridized at 1 μM in PBS. The solutions were heated to 80°C and allowed to cool to room temperature at 1°C/min. Samples were diluted further into PBS at various concentrations from 250 nM to 10 nM and then 90 μL of the diluted solutions was added to a 96-well fluorescence microplate and allowed to equilibrate for 10 minutes before adding 10 μL of DNAse I (2 units/L). To prevent evaporation, the plate was covered with a plate sealer. The fluorescence of the samples (excitation = 490 nm, emission =530 nM) was measured every minute for 8 hours using a Cytation3 plate reader. All samples were measured with n = 4. For the Lineweaver–Burk analysis, the initial reaction velocity was determined from the slope of the progress curve from the first 15 data points after nuclease addition.
Cell culture
Normal human lung fibroblasts (NHLF) (Lonza CC-2512) were used for experiments between passage 3 or 4 and cultured in 10% fetal bovine serum (FBS) (GIBCO) in Dulbecco’s Modified Eagle’s Medium (DMEM) (GIBCO) with 1% penicillin/streptomycin (Life Technologies).
Inhibition of proliferation assay
In a 96-well plate, 10,000 NHLF were plated per well in complete media and allowed to recover overnight. The cells were then starved for 6 hours in 0.5% FBS in DMEM and then treated with 1:2 serial dilutions of PDGF-BB from 100 ng/ml to 1.56 ng/mL. Cells were then allowed to grow for 48 hours with no further treatment, with addition of 100 μM diluent PA, 0.5-mol% aptamer-PA with 99.5-mol% diluent-PA (500 nM aptamer-PA, 100 μM total PA), or 500 nM free aptamer. The cells were assayed with CyQuant Direct Cell Proliferation Assay (Thermo Fisher) at 24 and 48 hours according to the manufacturer’s instructions. The level of proliferation was normalized to the growth factor only condition and each bar represents an average of three independent experiments.
Statistical analysis
The data are shown as a mean ± standard deviation. Statistical comparisons were performed using Student’s t test. P-values of <0.05 were considered to be statistically significant. Statistical analysis was performed using Graphpad Prism v.6 software. Analysis of variance (ANOVA) with the post hoc test was used for all multiple group experiments. P values<0.05 were deemed significant. Values in graphs are shown are Mean ± S.E.M.
Supplementary Material
ACKNOWLEDGMENTS
This project was supported by the National Institutes of Health NIDCR (Grant No. 2R01DE015920–11) and the Northwestern University Center for Regenerative Nanomedicine (CRN) through a Catalyst Award. C.M.S. acknowledges support from the NSF Graduate Research Fellowship Program. R.F. acknowledges support from an EMBO Long-Term Postdoctoral Fellowship (ALTF 233–2012). J.G. acknowledges support from Northwestern University through a Ryan Fellowship. J.A.L. acknowledges support from a National Science Foundation Graduate Research Fellowship.
We are grateful to the Peptide Synthesis Core and the Analytical bioNanoTechnology Core at the Simpson Querrey Institute at Northwestern University. The U.S. Army Research Office, the U.S. Army Medical Research and Materiel Command, and Northwestern University provided funding to develop these facilities and ongoing support is being received from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205). The authors acknowledge also the use of the following Northwestern University facilities: the Biological Imaging Facility (supported by the Northwestern University Office for Research), the Keck Biophysics Facility (supported by the NCI CCSG P30 CA060553 grant awarded to the Robert H. Lurie Comprehensive Cancer Center), and the Electron Probe Instrumentation Center facility of Northwestern University’s NUANCE Center, which has received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205). This work made use of the IMSERC at Northwestern University, which has received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205), the State of Illinois, and the International Institute for Nanotechnology (IIN). This research used resources of the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02–06CH11357. This work also made use of the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) located at Sector 5 of the APS. DND-CAT is supported by Northwestern University, E.I. DuPont de Nemours & Co., and The Dow Chemical Company. The authors also thank Mark Seniw for designing the schematic drawing in the manuscript.
ABBREVIATIONS
- PDGF-BB
Platelet derived growth factor-BB
- KD
Equilibrium binding constant
- ka
Association rate constant
- kd
Dissociation rate constant
- KM
Michaelis constant
- vmax
Maximum reaction rate
- PA
Peptide amphiphile
Footnotes
Supporting Information
Synthesis, purification, characterization, and preparation details for the aptamer-PA conjugate, details of transmission electron microscopy, circular dichroism, zeta potential, nuclease stability, binding affinity, cell culture, and proliferation experiments are available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest
References
- 1.Tuerk C & Gold L Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249, 505–510 (1990). [DOI] [PubMed] [Google Scholar]
- 2.Ellington AD & Szostak JW In vitro selection of RNA molecules that bind specific ligands. Nature 346, 818–822 (1990). [DOI] [PubMed] [Google Scholar]
- 3.Groff K, Brown J & Clippinger AJ Modern affinity reagents: Recombinant antibodies and aptamers. Biotechnol. Adv 33, 1787–1798 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Bagalkot V et al. Quantum Dot−Aptamer Conjugates for Synchronous Cancer Imaging, Therapy, and Sensing of Drug Delivery Based on Bi-Fluorescence Resonance Energy Transfer. Nano Lett 7, 3065–3070 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Song S, Wang L, Li J, Fan C & Zhao J Aptamer-based biosensors. TrAC Trends Anal. Chem 27, 108–117 (2008). [Google Scholar]
- 6.Zhou W, Jimmy Huang P-J, Ding J & Liu J Aptamer-based biosensors for biomedical diagnostics. Analyst 139, 2627–2640 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Dirkzwager RM, Liang S & Tanner JA Development of Aptamer-Based Point-of-Care Diagnostic Devices for Malaria Using Three-Dimensional Printing Rapid Prototyping. ACS Sensors 1, 420–426 (2016). [Google Scholar]
- 8.Zhou J & Rossi J Aptamers as targeted therapeutics: current potential and challenges. Nat. Rev. Drug Discov 16, 181–202 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keefe AD, Pai S & Ellington A Aptamers as therapeutics. Nat. Rev. Drug Discov 9, 537–550 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruckman J et al. 2’-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J. Biol. Chem 273, 20556–20567 (1998). [DOI] [PubMed] [Google Scholar]
- 11.Lin Y, Qiu Q, Gill SC & Jayasena SD Modified RNA sequence pools for in vitro selection. Nucleic Acids Res 22, 5229–5234 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burmeister PE et al. Direct In Vitro Selection of a 2′-O-Methyl Aptamer to VEGF. Chem. Biol 12, 25–33 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Rusconi CP et al. Antidote-mediated control of an anticoagulant aptamer in vivo. Nat. Biotechnol 22, 1423–1428 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Soule EE, Bompiani KM, Woodruff RS & Sullenger BA Targeting Two Coagulation Cascade Proteases with a Bivalent Aptamer Yields a Potent and Antidote-Controllable Anticoagulant. Nucleic Acid Ther 26, 1–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willis MC et al. Liposome-Anchored Vascular Endothelial Growth Factor Aptamers. Bioconjug. Chem 9, 573–582 (1998). [DOI] [PubMed] [Google Scholar]
- 16.Kim Y, Dennis DM, Morey T, Yang L & Tan W Engineering Dendritic Aptamer Assemblies as Superior Inhibitors of Protein Function. Chem. – An Asian J 5, 56–59 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Borbas KE, Ferreira CSM, Perkins A, Bruce JI & Missailidis S Design and Synthesis of Mono- and Multimeric Targeted Radiopharmaceuticals Based on Novel Cyclen Ligands Coupled to Anti-MUC1 Aptamers for the Diagnostic Imaging and Targeted Radiotherapy of Cancer. Bioconjug. Chem 18, 1205–1212 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Pearce TR, Waybrant B & Kokkoli E The role of spacers on the self-assembly of DNA aptamer-amphiphiles into micelles and nanotapes. Chem. Commun 50, 210–212 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Harris MA et al. Aptamer micelles targeting fractalkine-expressing cancer cells in vitro and in vivo. Nanomedicine Nanotechnology, Biol. Med 14, 85–96 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Hartgerink JD, Beniash E & Stupp SI Self-Assembly and Mineralization of Peptide-Amphiphile Nanofibers. Science 294, 1684–1688 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Matson JB & Stupp SI Self-assembling peptide scaffolds for regenerative medicine. Chem. Commun 48, 26–33 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui H, Webber MJ & Stupp SI Self-assembly of peptide amphiphiles: From molecules to nanostructures to biomaterials. Pept. Sci 94, 1–18 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato K, Hendricks MP, Palmer LC & Stupp SI Peptide supramolecular materials for therapeutics. Chem. Soc. Rev 47, 7539–7551 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chow LW et al. A bioactive self-assembled membrane to promote angiogenesis. Biomaterials 32, 1574–1582 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Storrie H et al. Supramolecular crafting of cell adhesion. Biomaterials 28, 4608–4618 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Webber MJ et al. Development of bioactive peptide amphiphiles for therapeutic cell delivery. Acta Biomater 6, 3–11 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moyer TJ et al. Shape-Dependent Targeting of Injured Blood Vessels by Peptide Amphiphile Supramolecular Nanostructures. Small 11, 2750–2755 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.So MM et al. Peptide Amphiphile Nanostructures for Targeting of Atherosclerotic Plaque and Drug Delivery. Adv. Biosyst 2, 1700123 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webber MJ et al. Supramolecular nanostructures that mimic VEGF as a strategy for ischemic tissue repair. Proc. Natl. Acad. Sci 108, 13438–13443 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edelbrock AN et al. Supramolecular Nanostructure Activates TrkB Receptor Signaling of Neuronal Cells by Mimicking Brain-Derived Neurotrophic Factor. Nano Lett 18, 6237–6247 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubert Pérez CM, Álvarez Z, Chen F, Aytun T & Stupp SI Mimicking the Bioactivity of Fibroblast Growth Factor-2 Using Supramolecular Nanoribbons. ACS Biomater. Sci. Eng 3, 2166–2175 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva GA et al. Selective Differentiation of Neural Progenitor Cells by High-Epitope Density Nanofibers. Science 303, 1352–1355 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Lee SS et al. Sulfated glycopeptide nanostructures for multipotent protein activation. Nat. Nanotechnol 12, 821–829 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SS et al. Gel Scaffolds of BMP-2-Binding Peptide Amphiphile Nanofibers for Spinal Arthrodesis. Adv. Healthc. Mater 4, 131–141 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah RN et al. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc. Natl. Acad. Sci 107, 3293–3298 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webber MJ, Newcomb CJ, Bitton R & Stupp SI Switching of self-assembly in a peptide nanostructure with a specific enzyme. Soft Matter 7, 9665–9672 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stephanopoulos N et al. Bioactive DNA-Peptide Nanotubes Enhance the Differentiation of Neural Stem Cells Into Neurons. Nano Lett 15, 603–609 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freeman R et al. Instructing cells with programmable peptide DNA hybrids. Nat. Commun 8, 15982 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freeman R et al. Reversible self-assembly of superstructured networks. Science (2018). at <http://science.sciencemag.org/content/early/2018/10/03/science.aat6141.abstract> [DOI] [PMC free article] [PubMed]
- 40.Green LS et al. Inhibitory DNA Ligands to Platelet-Derived Growth Factor B-Chain. Biochemistry 35, 14413–14424 (1996). [DOI] [PubMed] [Google Scholar]
- 41.Andrae J, Gallini R & Betsholtz C Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22, 1276–1312 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pashuck ET, Cui H & Stupp SI Tuning Supramolecular Rigidity of Peptide Fibers through Molecular Structure. J. Am. Chem. Soc 132, 6041–6046 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith JD et al. Aptamer-displaying peptide amphiphile micelles as a cell-targeted delivery vehicle of peptide cargoes. Phys. Biol 15, 65006 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Günter M The Chemical Biology of Aptamers. Angew. Chemie Int. Ed 48, 2672–2689 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Gelinas AD, Davies DR & Janjic N Embracing proteins: structural themes in aptamer–protein complexes. Curr. Opin. Struct. Biol 36, 122–132 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Kypr J, Kejnovská I, Renčiuk D & Vorlíčková M Circular dichroism and conformational polymorphism of DNA. Nucleic Acids Res 37, 1713–1725 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greenfield NJ Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc 1, 2876–2890 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuai H et al. Circular Bivalent Aptamers Enable in Vivo Stability and Recognition. J. Am. Chem. Soc 139, 9128–9131 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasegawa H, Taira K, Sode K & Ikebukuro K Improvement of Aptamer Affinity by Dimerization. Sensors 8, 1090–1098 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ortony JH et al. Internal dynamics of a supramolecular nanofibre. Nat. Mater 13, 812–816 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.da Silva RMP et al. Super-resolution microscopy reveals structural diversity in molecular exchange among peptide amphiphile nanofibres. Nat. Commun 7, 11561 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seferos DS, Prigodich AE, Giljohann DA, Patel PC & Mirkin CA Polyvalent DNA Nanoparticle Conjugates Stabilize Nucleic Acids. Nano Lett 9, 308–311 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.