Abstract
Neuroblastoma, an embryonic cancer of the sympathetic nervous system, is the most common extracranial solid tumor in childhood. Dinutuximab (formerly called ch14.18), a monoclonal antibody targeting the disialoganglioside GD2, has been shown to significantly improve survival rates in patients with high-risk neuroblastoma. However, the safe and effective use of dinutuximab therapy in these high-risk patients requires medical expertise in patient selection, treatment administration, and the monitoring and management of adverse events. Findings of the randomized phase III study (ANBL0032) led to the approval of dinutuximab for the treatment of children with high-risk neuroblastoma. Multi-institutional nursing approaches to implementing standard protocols ensure the effective management of high-risk neuroblastoma patients receiving dinutuximab immunotherapy. Understanding and implementing recommendations for the management of the clinically important and most common adverse events are essential to ensuring patient continuation of therapy and improving patient outcomes.
Keywords: dinutuximab, neuroblastoma, adverse events, pediatric
Introduction
In recent years, monoclonal antibodies targeting the disialoganglioside GD2 have been added to standard therapy and have improved survival in patients with high-risk neuroblastoma (Castel, Segura, & Canete, 2010; Yu et al., 2010). However, the use of anti-GD2 antibody immunotherapy, specifically the chimeric monoclonal antibody dinutuximab (formerly called ch14.18), to treat these patients requires a high level of medical expertise in patient selection, treatment administration, and monitoring responses during therapy (Yu et al., 2010). Treatment with dinutuximab immunotherapy involves a complex regimen that includes dinutuximab, interleukin-2 (IL-2), granulocyte-macrophage colony-stimulating factor (GM-CSF), and 13-cis-retinoic acid (RA) in combination, and multiple cycles of treatment over 6 months (Yu et al., 2010). All patients experience adverse events (AEs) during dinutuximab immunotherapy. The majority of the AEs are transient and occur during treatment administration. If AEs are managed appropriately, patients may be more likely to remain on therapy. Nurses, as key members of the multidisciplinary team managing patients with high-risk neuroblastoma, play a critical role in administering therapy, managing AEs, and educating patients and families.
This review summarizes neuroblastoma treatment and provides background on the development of dinutuximab and clinical studies leading to its approval for the treatment of children with high-risk neuroblastoma. It presents multi-institutional nursing perspectives on implementing standard protocols for the safe management of high-risk neuroblastoma in patients receiving dinutuximab immunotherapy. In addition, it provides a comprehensive overview of recommendations to be used as a guide for the management of clinically important and most common AEs.
Neuroblastoma
Epidemiology
Neuroblastoma is the most common extracranial solid tumor of childhood, accounting for 6% of all cancers among children aged <15 years in the United States (American Cancer Society, 2015a), with an age-adjusted incidence of 10.1 per million (Howlader et al., 2015). The mean age at diagnosis is about 1 to 2 years, and nearly 90% of cases are diagnosed before age 5 (American Cancer Society, 2015b).
Neuroblastoma is a heterogeneous disease, with some tumors regressing or maturing while others progress despite intensive multimodality treatment (Cohn et al., 2009). According to the International Neuroblastoma Risk Group Classification System, patients are classified as very low, low, intermediate, or high risk (Cohn et al., 2009).
Nearly 50% of patients with neuroblastoma have high-risk disease (Maris, Hogarty, Bagatell, & Cohn, 2007) characterized by widespread metastasis, known genetic features, and/or unfavorable histopathologic findings. For these patients, the prognosis is poor, with 5-year event-free and overall survival rates reported as 40% to 50% (Simon et al., 2011). Despite recent advances in treatment, 50% to 60% of patients with high-risk neuroblastoma relapse after therapy, and cure remains elusive after relapse with current salvage treatments (Davidoff, 2012; Maris, 2010).
Treatment of Neuroblastoma
Treatment of neuroblastoma is driven by risk group (Pinto et al., 2015). Treatment for high-risk patients has involved dose-intensive induction chemotherapy (five to six cycles) to reduce tumor mass and surgical resection of the primary tumor (Yalcin, Kremer, Caron, & van Dalen, 2013).
This is followed by consolidation with high-dose chemotherapy to eradicate sites of minimal residual disease and autologous stem cell rescue to repopulate the bone marrow (Matthay et al., 2009; Matthay et al., 1999; Yalcin et al., 2013). Radiation therapy to the primary tumor site and to metastatic disease that remains after induction therapy is administered following the stem cell transplant (Matthay et al., 2009; Matthay et al., 1999).
Dinutuximab
A novel approach to treating high-risk neuroblastoma involves immunotherapy targeting a tumor-associated antigen, the disialoganglioside GD2 (Matthay, George, & Yu, 2012). GD2 is expressed on tumors of neuroectodermal origin and is found on the surface of neuroblastoma cells (Matthay et al., 2012; Parsons, Bernhardt, & Strickland, 2013). In normal tissue, GD2 expression is primarily limited to neurons, skin melanocytes, and peripheral nerve receptors (Svennerholm et al., 1994; Zhang et al., 1997).
Dinutuximab (Unituxin) is the first anti-glycolipid monoclonal antibody to be approved in the United States and Europe for use (in combination with GM-CSF, IL-2, and RA) in patients with high-risk neuroblastoma who achieve at least partial response to prior first-line multiagent, multimodality therapy (Dhillon, 2015; United Therapeutics Corp., 2015). Dinutuximab binds to GD2 on human neuroblastoma cells and induces antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity (Barker et al., 1991).
Trials
Preclinical and phase I clinical trials demonstrated that dinutuximab was active against neuroblastoma (Barker et al., 1991; Gilman et al., 2009; Handgretinger et al., 1995; Ozkaynak et al., 2000; Yu et al., 1998). Subsequently, a Children’s Oncology Group (COG) phase III trial, ANBL0032, was initiated to determine if adding dinutuximab and cytokines to standard treatment would result in improved event-free survival and overall survival for patients with newly diagnosed high-risk neuroblastoma who had responded to induction therapy and had received autologous stem cell transplantation (COG, 2013; Yu et al., 2010). Patients were aged <31 years at diagnosis, achieved at least a partial response to induction therapy without evidence of progressive disease, and had a life expectancy of >2 months.
A total of 226 patients were randomly assigned to receive either dinutuximab plus cytokines (GM-CSF and IL-2) in alternating cycles with standard therapy (RA) or standard therapy alone. All patients were required to receive intravenous (IV) narcotics prior to and during administration of immunotherapy.
The immunotherapy regimen was associated with significantly improved event-free survival and overall survival, compared with standard therapy (Yu et al., 2010). The estimate for event-free survival at 2 years was 66% in the immunotherapy regimen group and 46% in the standard therapy group (P = .0115). The immunotherapy regimen was also superior to standard therapy with regard to the estimated overall survival at 2 years (86% vs 75%; P = .0223).
Prior to approval by the US Food and Drug Administration, the immunotherapy regimen, with dinutuximab administered for a short duration, was associated with important treatment-related AEs, including grade 3/4 neuropathic pain (52%), hypersensitivity reactions (25%), acute capillary leak syndrome (23%), and hypotension (18%). Grade 3/4 pain was reported in 25% of the 598 cycles of immunotherapy. It was most common in cycle 1 (37%), decreasing in subsequent cycles (14% in cycle 5; P < .001). In general, pain increases during cycles containing dinutuximab in combination with IL-2. The decrease in pain in later cycles may have been due to knowledge gained from the individual patient’s experience in earlier cycles leading to improvements in pain management, such as adjustment of narcotic infusions. Hypersensitivity reactions, such as cough, bronchospasm, hives (urticaria), and rigors, occurred in 15% of cycles and were more frequently reported in the cycles including IL-2 (cycles 2 and 4; 26% and 25%) than in the three cycles involving GM-CSF therapy (cycles 1, 3, and 5; 10%, 5%, and 12%; P = .001; Yu et al., 2010). Capillary leak syndrome was reported in 23% of patients during 8% of cycles, and was reported more frequently in the two cycles involving IL-2 (11% and 13%) than in the three cycles involving GM-CSF (7%, 7%, and 3%; P = .06). As expected, withdrawals due to toxic effects were more common in patients treated with the immunotherapy regimen than in those treated with standard therapy (14% vs 4%).
Discussion
Administration of dinutuximab immunotherapy is complex, with a 10- to 20-hour infusion duration. There are multiple cycles, and cycles that differ from the one previously administered. The use of multiple therapeutic agents and supportive care measures requires a high level of attention and coordination. Because of the known antineuroblastoma activity of dinutuximab therapy, efforts to continue treatment and appropriately manage AEs should be pursued.
Points of guidance for administering components of the immunotherapy regimen have been developed by staff at institutions with experience in the phase III study (Table 1). Some institutions may allow for at-home use of a computerized ambulatory delivery device for the administration of selected medications (ie, IL-2). In addition, to minimize patient distress and discomfort, indwelling catheters may be inserted to assist in medication delivery. In all instances, nurses will review the necessary precautions and educate caregivers on appropriate procedures for troubleshooting any issues that may arise. Optimal nurse-to-patient ratios are highly recommended to ensure safe delivery of therapy and to allow optimal assessment and management of AEs. Checklists and schedules of actions, for each treatment day and for each different cycle, are recommended. Preventive and supportive care precautions need to be implemented if the anticipated AEs are to be managed.
Table 1.
Guidance on the Administration of Dinutuximab, RA, GM-CSF, and IL-2 Based on Protocols at the Comer Children’s Hospital, Chicago, IL, USA; Cook Children’s Hospital, Fort Worth, TX, USA; and the Children’s Neuroblastoma Cancer Foundation (CNCF).
| Admission | • Patient is typically admitted on Sunday evening or early Monday morning; discharge may occur on Friday or Saturday |
| Laboratory Assessment | • Labs are drawn early to allow time to administer blood products or albumin if infusion is given more than 20 hours. This helps avoid delays in starting antibody the following day • Prior to initiating dinutuximab, GM-CSF, IL-2, and RA, patients must have –Adequate liver and renal function –Platelet count: ≤20000/μL –Pulmonary: no dyspnea at rest or has resolved, and/or peripheral arterial O2 saturation >94% on room air –No systemic infection |
| Preparation | • Blood transfusions may be indicated to increase patienťs hemoglobin to minimum of 10 g/dL or hematocrit of 0.3 • Double-lumen CVC is required for drug infusion and toxicity management • Temporary PIV/PICC line may have to be placed to ensure sufficient access throughout the regimen for antibody infused through dedicated line, IL-2, and other drugs that cannot be mixed with IL-2 • Routine CVC or port dressing changes performed per institutional protocols to ensure no signs of infection prior to start of each dinutuximab cycle • ECG leads and O2 saturation probe are placed |
| Premedications | • Diphenhydramine or hydroxyzine • Acetaminophen or indomethacin • IV loading bolus pain medication followed by continuous PCA 30 minutes prior to starting dinutuximab; PCA remains throughout and often 2 to 3 hours after completion of infusion, followed by weaning |
| GM-CSFa | • 250 Mg/m2 for 14 days beginning 3 days prior to dinutuximab in cycles 1, 3, and 5 • May give as SC injection or IV as outpatient for 3 days prior to admission • On days given with dinutuximab, GM-CSF is given 1 hour before dinutuximab • Administered until day 13 and may be given at home following discharge • Monitor white blood cell counts • Local reactions at injection sites can occur; manage with diphenhydramine preinjection and postinjection • Rotate injection sites; avoid injections in areas that may be irritated • Tapping skin lightly above needle while injecting, or light massage of the area after injecting, may ease discomfort |
| IL-2b | • Should always have a stopcock and should run in lumen of line closest to central line • Check flow rate every 4 hours to ensure 24-hour infusion • Cycle is 3 MIU/m2 for 4 days in week 1 of cycles 2 and 4 (days 0–3), and 4.5 MIU/m2 for 4 days in week 2 (days 7–10) of cycles 2 and 4 • In setting pumps: 3 MIU/m2/d = 0.125 MIU/m2/h; 4.5 MIU/m2/d = 0.1875 MIU/m2/h • Flush catheter on final day; account for additional infusion volume to account for length of tubing when calculating flush • Check vitals every 15 minutes first hour, every 60 minutes thereafter day 1; every 4 hours days 2 to 4 • Allow 2 hours between starting IL-2 and starting dinutuximab • Some institutions allow first dose of IL-2 to be given as outpatient in clinic on day 0 and, if well tolerated, continued at home by CADD pump • Weigh patient twice each day • Avoid use of radiographic contrast materials during and for at least 1 week after completing IL-2 infusion (risk of anaphylactoid reaction) |
| RA | • Start 3 days after completing dinutuximab infusion • Oral dosing is done at home • Urine specimen and bloodwork to confirm electrolytes and liver function are required before starting • Patient is enrolled in iPLEDGEc program |
| Dinutuximab | • A crash cart with medications to treat anaphylaxis should be in close proximity • Begin pain medications with IV loading bolus and continuous PCA 30 minutes before beginning dinutuximab infusion • Administer normal saline bolus 1 hour immediately prior to dinutuximab • Only compatible with normal saline; no other medications should be directly infused or mixed in with dinutuximab • Infuse over 10 hours; max 20 hours • An alternative IV line/access must be readily available at all times • Check vitals every 15 minutes first hour, every 60 minutes thereafter on day 1; every 4 hours days 2 to 4 • Weigh patient twice each day |
Abbreviations: CADD, computerized ambulatory delivery device; CVC, central venous catheter; ECG, electrocardiogram; FDA, US Food and Drug Administration; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-2, interleukin 2; IV, intravenous; max, maximum; O2, oxygen; PCA, patient-controlled analgesia; PIV, peripheral intravenous; PICC, peripherally inserted central catheter; RA, 13-cis-retinoic acid; SC, subcutaneous.
Indwelling catheters may be used for delivery of GM-CSF.
Some institutions approve the use of CADD pumps at home, with adequate home care support.
iPLEDGE is an FDA-approved program that uses verifiable, trackable links between prescriber, patient, pharmacy, and wholesaler in order to prevent fetal exposure to RA and to inform prescribers, pharmacists, and patients about RA serious risks and safe-use conditions.
Most patients receiving the dinutuximab immunotherapy regimen experience pain, fluid retention, and tachycardia. Allergic or hypersensitivity reactions, hypotension, and other AEs, varying in seriousness and severity, may occur (Yu et al., 2010). Pretreatment with analgesics for pain management and with antihistamines for hypersensitivity reaction management is recommended. However, most AEs are acute and limited to days of actual treatment. Patients can usually resume a normal schedule between dinutuximab administration days, and some patients have attended school and other activities while receiving monotherapy with RA or continued GM-CSF.
Toxicities associated with dinutuximab infusion may be minimized by anticipation and preventive measures and could be resolved by either slowing the rate of or temporarily stopping the infusion. If needed, the dinutuximab infusion may be prolonged up to 20 hours. Once the infusion rate has been reduced, it is prudent and recommended to initiate treatment with the slower rate in subsequent infusions (with same cytokine), then slowly increase the rate if tolerated.
Pain Management
Pain, particularly neuropathic pain, is the most common AE experienced by patients receiving dinutuximab therapy, despite premedication steps to reduce or attenuate events (United Therapeutics Corp., 2015; Yu et al., 2010). Pain is often described as abdominal (United Therapeutics Corp., 2015; Yu et al., 2010), but can also be tingling, numbness, burning, or general all-over body pain.
Required premedication is administered prior to initiation of each dinutuximab infusion (Table 2). IV loading bolus pain medication followed by a continuous patient-controlled analgesia (PCA) infusion of morphine, hydromorphone, or fentanyl is initiated 30 minutes prior to administration of dinutuximab. Pain medication infusions are routinely administered and are increased as clinically indicated (Gorges et al., 2015). Drugs such as lidocaine and gabapentin combined with opioids may be considered for severe or persistent neuropathic pain. Dinutuximab therapy is rarely discontinued beacause of pain, as most pain can be effectively managed.
Table 2.
Preventive and Supportive Care for Management of Anticipated Toxicities Associated With Administration of Dinutuximab in Combination With GM-CSF, IL-2, and RA.a
| Drug and Indication | Dosing and Administration | Patient Monitoring |
|---|---|---|
| Morphine sulfate Pain management |
Loading dose of 50 μg/kg immediately prior to dinutuximab infusion; continuous/PCA morphine during infusion | Substitute with fentanyl or hydromorphone if patient is unable to tolerate morphine |
| Lidocaineb Pain management |
IV bolus at 2 mg/kg in 50 mL normal saline over 30 minutes prior to start of dinutuximab infusion Once dinutuximab started, IV push at 1 mg/kg/h and continue until 2 hours after completion |
Discontinue if patient becomes dizzy or develops perioral numbness, or tinnitus attributable to lidocaine |
| Gabapentinc Pain management |
If needed, 10 mg/kg PO may be given when starting morphine premedication; dose may be subsequently increased (to max 60 mg/kg/d, max daily dose 3600 mg/d) as needed | Titrate as ordered and recommended per institution pharmacy guidelines |
| Hydroxyzine and diphenhydramined Allergic reactions |
0.5 to 1.0 mg/kg, max 50 mg, or diphenhydramine (0.5–1.0 mg/kg, max 50 mg) over 10 to 15 minutes, starting 20 minutes prior to dinutuximab infusion and continuing, as tolerated, every 4 to 6 hours until end of dinutuximab infusion |
Careful patient monitoring is required, as this drug combination has the potential for increased side effects, such as drowsiness, hypotension, difficulty urinating, and tachycardia |
| Acetaminophen Fever or pain |
10 mg/kg, max 650 mg, IV or PO 20 minutes prior to dinutuximab infusion and every 4 hours as needed for fever; administer every 4 to 6 hours during IL-2 plus dinutuximab cycles | |
| Ibuprofene Fever or pain |
5 to 10 mg/kg/dose; not to exceed every 6 hours; administered between acetaminophen doses for control of persistent fever | Discontinue if bleeding occurs, platelet count drops below 50 000/μL, or evidence of renal dysfunction occurs |
| Antibiotics | Broad-spectrum antibiotics are recommended for febrile patients; dinutuximab immunotherapy should be discontinued until infection has resolved; some centers recommend using prophylactic antibiotics for patients with bacteremia during previous cycle | |
Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-2, interleukin 2; IV, intravenous; max, maximum; PCA, patient-controlled analgesia; PO (per os), by mouth; RA, 13-cis-retinoic acid.
Modified from the ANBL0032 Protocol (Children’s Oncology Group, 2013).
Rarely used and should be given under direction of pediatric pain specialist.
In general, institutions report starting gabapentin 3 days prior to start of dinutuximab infusion, although some may start 1 to 2 weeks prior depending on institutional and prescriber preferences.
May be used in combination.
Not preferred.
On the first cycle of dinutuximab, an increase in analgesic titration is often required until pain is adequately managed. With subsequent cycles, the individualized patient opioid infusion can begin at the rate found to be adequate during the previous cycle in which the same cytokine was used (ie, IL-2: cycles 2 and 4; GM-CSF: cycles 1, 3, and 5).
Careful review is recommended to ensure that all pain medications are accurately included in the patient’s care plan. Pain should be addressed prophylactically, with ongoing assessments and adjustments to maintain adequate control. Individualized patient experiences during the first infusion should be used to guide the regimen in subsequent infusions.
The prescribing information advises that pain medications continue for 2 hours after completion of the dinutuximab infusion. Pain generally resolves, but some patients may have short-term residual pain after completion of the infusion, necessitating the administration of pain medications at home. Hence, it is important to confirm in advance that all patients have pain medications at home, along with prescriptions and refills. Patients and caregivers should be aware that high doses of opiates are associated with their own AEs, such as respiratory depression, constipation, itching, and urinary retention (Benyamin et al., 2008). Patients prone to constipation may benefit from proactive intervention in advance of treatment. Although there are effective treatment options (naloxone hydrochloride [NarcanTM] and nalbuphine hydrochloride [NubainTM]) to alleviate opiate-induced itching, they may diminish the effect of narcotics. Therefore, patients should also be monitored for signs of withdrawal if significant narcotic support is required.
Peripheral Neuropathy
Severe grades 3 and 4 peripheral neuropathy (loss of muscle function, disabling numbness, tingling, burning sensation) is rare with dinutuximab immunotherapy. It is recommended to discontinue dinutuximab therapy for severe unresponsive peripheral neuropathy.
Allergic and Hypersensitivity Reactions
Symptoms of allergic and hypersensitivity reactions include cough, bronchospasm, hives (urticaria), rigors, itching, and secondary hypotension. Cough is reported in many patients. Although an occasional cough may be the early sign of developing bronchospasm, most patients can be managed with a combination of slowing the infusion rate, increasing antihistamines, and giving regularly scheduled respiratory therapies (eg, albuterol, racemic epinephrine, ipratropium [Atrovent™]). Treatment should be delayed in patients with viral infections, as cough may be aggravated by immunotherapy.
Dose modification guidelines, from the current prescribing information for managing infusion-related reactions during administration of dinutuximab in combination with GM-CSF, IL-2, and RA, are shown in Table 3. For transient rash, the recommendations are to temporarily reduce the rate of dinutuximab infusion with use of antihistamines. Diphenhydramine is usually given prior to the initiation of dinutuximab and as tolerated every 4 to 6 hours during the infusion. Hydroxyzine and the histamine 2 blocker ranitidine may also be effective. Pramoxine hydrochloride lotion (Sarna™), colloidal oats and/or oat extracts (Aveeno™), and other non–alcohol-based products may relieve skin itch. If symptoms are mild to moderate, the dinutuximab infusion rate should be gradually increased to a maximum of 1.75 mg/m2/h.
Table 3.
Dose Modification Guidance for Management of Infusion-Related Reactions During Administration of Dinutuximab in Combination With GM-CSF, IL-2, and RA.
| Grade 1 or 2a: Transient rash; fever <38°C; and localized urticaria that responds promptly to symptomatic treatment | |
| Onset of symptoms | • Reduce rate of dinutuximab infusion to 0.875 mg/m2/h • Administer supportive measuresb |
| Symptoms resolve | • Resume dinutuximab infusion at 1.75 mg/m2/h • If not tolerated, reduce to 0.875 mg/m2/h |
| Grade 3 or 4: Prolonged symptoms, with life-threatening consequences | |
| Onset of symptoms | • Immediately discontinue dinutuximab and GM-CSF or IL-2 • Administer supportive measuresc |
| Symptoms resolve | • If signs and symptoms resolve rapidly, dinutuximab infusion may resume at reduced rate of 0.875 mg/m2/h • Do not resume GM-CSF or IL-2 until following day • For GM-CSF cycles, administer GM-CSF starting next day at 50% of starting dose (125 μg/m2) and, if tolerated, at full dose (250 Mg/m2) after completion of dinutuximab infusion for that cycle • For IL-2 cycles, administer IL-2 starting next day at 50% of starting dose (1.5 × MIU/m2) and continue for remainder of cycle • If symptoms recur with addition of GM-CSF or IL-2, discontinue it. If symptoms resolve the following day, resume dinutuximab at tolerated rate without GM-CSF or IL-2 |
| First recurrence | • Discontinue dinutuximab and GM-CSF or IL-2 for that day • If symptoms resolve that day, resume next day with premedication in intensive care setting |
| Subsequent cycles | • Maintain tolerated dinutuximab infusion rate for all subsequent cycles with GM-CSF or IL-2 |
Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-2, interleukin 2; RA, 13-cis-retinoic acid.
Graded per Common Terminology Criteria for Adverse Events version 3.0.
Supportive measures for grade 1 or 2 symptoms refers to sections of the prescribing instructions describing use of premedication and containing the warning regarding risk of infusion-related reactions with dinutuximab therapy. In these sections, recommendations include administration of an antihistamine every 4 to 6 hours, as required; administration of acetaminophen (10–15 mg/kg) every 4 to 6 hours, as needed; and use of ibuprofen (5–10 mg/kg/dose) no more than every 6 hours between acetaminophen doses for persistent fever.
Supportive measures for grade 3 or 4 symptoms refers to the section of prescribing instructions describing use of premedications for patients receiving dinutuximab therapy. In this section, recommendations include administration of an antihistamine every 4 to 6 hours, as required; administration of acetaminophen (10–15 mg/kg) every 4 to 6 hours, as needed; and use of ibuprofen (5–10 mg/kg/dose) no more than every 6 hours between acetaminophen doses for persistent fever.
Skin should be closely monitored during dinutuximab infusion for any change in appearance, hives, or other symptoms. If the patient has a mild hypersensitivity reaction and responds well to hydroxyzine, scheduled hydroxyzine is added every 6 hours, alternating with diphenhydramine, through the remainder of the cycle. In subsequent cycles, the patient may be started on both hydroxyzine and diphenhydramine.
For grade 3 or 4 allergic reactions (eg, persistent, distressing cough, angioedema, severe hypersensitivity), the infusion of dinutuximab and of GM-CSF or IL-2 should be immediately discontinued and supportive care measures started, including the administration of diphenhydramine and steroids. Corticosteroid therapy should be used only for life-threatening conditions, since steroids counter the dinutuximab mechanism of action. As symptoms improve, dinutuximab may resume at the 50% rate (0.875 mg/m2/h), with GM-CSF and IL-2 resuming the next day at a 50% reduced dose if the symptoms do not recur. The full GM-CSF dose may resume for the remainder of the cycle, if tolerated. The rate of dinutuximab infusion should not be decreased by more than 50% in order for the infusion to be completed in 20 hours. Following completion of infusion, any dinutuximab remaining after 20 hours from the start of that day’s infusion must be discarded.
Anaphylactoid reactions (acute grade >3 hypersensitivity) rarely occur with dinutuximab immunotherapy, but can be life-threatening. If these reactions occur, then dinutuximab and IL-2 or GM-CSF treatment should be immediately discontinued. Epinephrine (adrenaline) and hydrocortisone for IV bolus administration should be immediately available at the bedside during dinutuximab administration to manage life-threatening allergic reactions. Treatment for such reactions is given once every 3 to 5 minutes as necessary according to clinical response. Most patients respond to epinephrine.
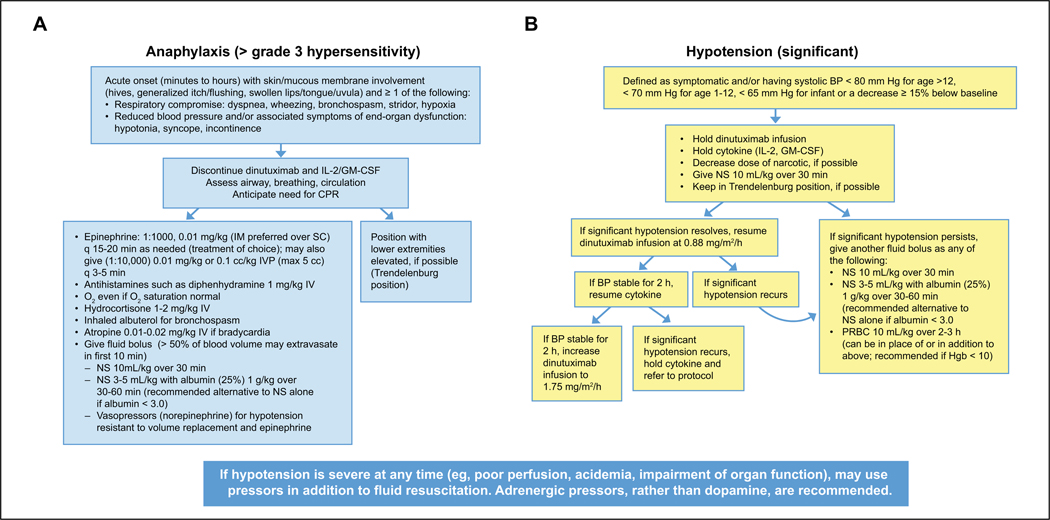
The COG recommends that management guidelines and algorithms for anaphylaxis be reviewed and made available on the inpatient unit to facilitate treatment decisions. The COG algorithm for managing anaphylaxis is shown in Figure 1A.
Figure 1.
(A) Anaphylaxis algorithm for patients receiving dinutuximab. (B) Hypotension algorithm for patients receiving dinutuximab.
Abbreviations: BP, blood pressure; CPR, cardiopulmonary resuscitation; GM-CSF, granulocyte-macrophage colony-stimulating factor; Hgb, hemoglobin; IL-2, interleukin 2; IM, intramuscular; IV, intravenous; IVP, intravenous push; NS, normal saline; O2, oxygen; PRBC, packed red blood cells; SC, subcutaneous.
Specific precautions are necessary for patients with a history of asymptomatic bronchospasm. In consultation with institutional clinical care teams, patients may start on scheduled ipratropium bromide HFA/albuterol every 6 hours and racemic epinephrine every 2 to 4 hours in subsequent cycles. For patients with rapidly resolving symptoms of either angioedema that does not affect the airway or mild bronchospasm without any other symptoms, dinutuximab may be resumed on resolution of symptoms at the 50% rate with close observation. If patients have had a grade 3 or 4 reaction requiring the infusion rate to be slowed, subsequent cycles should be infused at the slower rate. The rate of subsequent infusions should not be increased.
Capillary Leak Syndrome
Capillary leak syndrome is a common AE, defined as a decreased intravascular volume, decreased urine output, fluid retention, pulmonary interstitial edema ascites, peripheral edema, and decreased albumin (Baluna & Vitetta, 1997). Experience has shown that many patients receiving dinutuximab immunotherapy will experience some grade of capillary leak syndrome, which occursmore commonly when the agent is given with IL-2 in cycles 2 and 4. Therefore, careful monitoring is particularly important during these cycles. Patients should be weighed twice daily, and fluid intake and excretion closely monitored to calculate fluid balance. The patient’s history should be assessed to determine previous experience, and a management plan should be established before dinutuximab infusion begins. Albumin levels should be monitored, and albumin with furosemide should be started when the levels are within low normal or are 3.0 g/dL or lower. Close monitoring of fluid balance and blood pressure is important, with implementation of diuretics so as to not further contribute to hemodynamic instability.
If capillary leak is not well controlled, it can progress to symptomatic pulmonary edema or symptomatic ascites. Managing fluid balance during dinutuximab infusion can be challenging. Hypotension can result from fluid depletion. Assessing hydration status and measuring fluid balance can ensure optimal hydration.
It is important to differentiate capillary leak syndrome from urinary retention, and a bladder scan, in addition to albumin and hemoglobin measurements, may assist in the diagnosis. Foley catheter placement and medications to counter urinary retention will help relieve retention symptoms. Patients with capillary leak syndrome generally respond quickly to furosemide, especially if administered following albumin or blood products to increase oncotic pressure.
Dose modifications outlined in the prescribing information for managing capillary leak syndrome during dinutuximab immunotherapy in combination with GM-CSF, IL-2, and RA are shown in Table 4.
Table 4.
Dose Modification Guidance for Management of Capillary Leak Syndrome During Administration of Dinutuximab in Combination With GM-CSF, IL-2, and RA.a
| Grade 3b: Severe symptoms | |
| Onset of symptoms | • Discontinue dinutuximab infusion and IL-2 or GM-CSF • Administer supportive measuresc |
| Symptoms resolve | • Resume dinutuximab infusion at 0.875 mg/m2/h • Resume IL-2 or GM-CSF the following day at 50% of starting dose until last dose of dinutuximab for that cycle |
| Subsequent cycles | • If 50% dose of IL-2 or GM-CSF is tolerated, start at this dose and infuse dinutuximab at 0.875 mg/m2/h. If tolerated, increase IL-2 or GM-CSF to full dose the next day • If 50% dose of IL-2 is not tolerated, substitute GM-CSF for IL-2 in remainder of cycles • If 50% dose of GM-CSF is not tolerated, administer dinutuximab alone for remainder of GM-CSF cycles quences |
| Grade 4: Life-threatening conse | |
| Onset of symptoms | • Discontinue dinutuximab infusion and IL-2 or GM-CSF for that cycle • Administer supportive measuresc |
| Subsequent cycles | • If capillary leak syndrome occurred during an IL-2 cycle, substitute GM-CSF for IL-2 in remainder of cycles • If capillary leak syndrome occurred during a GM-CSF cycle, administer dinutuximab alone for remainder of GM-CSF cycles |
Data from Unituxin (dinutuximab) injection, for intravenous use [prescribing information] (United Therapeutics Corp., 2015).
Graded per Common Terminology Criteria for Adverse Events version 3.0.
Supportive measures for grade 3 or 4 symptoms refers to sections of the prescribing instructions describing use of premedication and containing the warning regarding risk of capillary leak syndrome with dinutuximab therapy. In these sections, recommendations include administration of oral metolazone or IV furosemide every 6 to 12 hours, supplemental O2, respiratory support, and albumin replacement as required.
Grade 4 capillary leak syndrome is rare and, with proper patient management, can be avoided. In rare cases where capillary leak is life threatening and requires pressor support or mechanical ventilation, dinutuximab and either IL-2 or GM-CSF should be halted for the remainder of the cycle and the patient given supportive care. If the syndrome begins in an IL-2 cycle, then GM-CSF should be substituted in subsequent cycles. If the syndrome begins in a GM-CSF cycle, then dinutuximab alone should be administered in subsequent cycles, with no IL-2 and no GM-CSF.
Hypotension
It should be noted that IL-2 has been associated with hypotension in studies where it was used at high doses (Kammula, White, & Rosenberg, 1998). Experience with dinutuximab immunotherapy has shown that increases in infusion rate may frequently cause transient and sometimes severe hypotension, and patients who receive immunotherapy over 10 to 20 hours are less likely to need intensive care unit (ICU) intervention.
Dose modifications outlined in the prescribing information for managing hypotension during dinutuximab immunotherapy in combination with GM-CSF, IL-2, and RA are shown in Table 5. The COG algorithm for the definition and management of hypotension is presented in Figure 1B.
Table 5.
Dose Modification Guidance for Management of Hypotension During Administration of Dinutuximab in Combination With GM-CSF, IL-2, and RA.a,b
| Symptomatic and/or systolic BP less than 70 mm Hg or a decrease >15% below baseline | |
| Onset of symptoms | •Discontinue dinutuximab infusion and IL-2 or GM-CSF • Administer supportive measuresc |
| Symptoms resolve | •Resume dinutuximab infusion at 0.875 mg/m2/h • If BP remains stable for >2 hours, resume IL-2 or G-CSF • If BP remains stable for >2 hours after resuming IL-2 or GM-CSF, increase dinutuximab to 1.75 mg/m2/h |
| First recurrence | •Discontinue dinutuximab infusion and IL-2 or GM-CSF • Resume dinutuximab infusion at 0.875 mg/m2/h once BP is stable |
| Symptoms resolve | •Resume IL-2 or GM-CSF following day at 50% dose if BP remains stable • Start IL-2 or GM-CSF at 50% dose when given with dinutuximab. Increase to full dose if tolerated for remainder of cycle • If IL-2 or GM-CSF is not tolerated at 50% dose, give dinutuximab alone for remainder of cycle |
| Second recurrence | •Discontinue IL-2 or GM-CSF for remainder of cycle |
| Subsequent cycles | • Start IL-2 or GM-CSF at 50% dose, if tolerated increase to full dose next day • If IL-2 is not tolerated at 50% dose, substitute with GM-CSF for remainder of IL-2 cycle • If GM-CSF is not tolerated at 50% dose, administer chl4.l8 alone for remainder of GM-CSF cycles |
Abbreviations: BP, blood pressure; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-2, interleukin 2; IV, intravenous; RA, 13-cis-retinoic acid.
Variation in these guidelines may be necessary according to institutional protocols and clinical judgment of individual patients.
Data from Unituxin (dinutuximab) injection, for intravenous use [prescribing information]. (United Therapeutics Corp., 2015).
Supportive measures for patients with symptomatic hypotension and/or systolic BP less than 70 mm Hg or a decrease >15% below baseline refers to sections of the prescribing instructions containing the warning regarding risk of hypotension with dinutuximab therapy. In this section, recommendations include administration of normal saline as clinically indicated. If hypotension persists, repeat normal saline infusion, or administer IV albumin or packed red cells as clinically indicated. Vasopressor therapy may be necessary to restore adequate perfusion pressure.
For symptomatic hypotensive patients (systolic blood pressure [SBP] less than the lower limit of normal for age, or SBP decreased by >15% compared with baseline), dinutuximab infusion and cytokine administration should be stopped. The patient should be started as soon as possible on an infusion of normal saline at 10 mL/kg over 30 minutes; a more rapid infusion (20 mL/kg) has also been recommended according to the Pediatric Advanced Life Support Standards (Fleegler & Kleinman, 2016). The dose of narcotics and sedating histamine 1 blockers, if used, should be decreased.
If hypotension resolves, then dinutuximab should be resumed at the 50% rate. If hypotension recurs at the reduced dinutuximab infusion rate, then measures to stabilize blood pressure should be repeated and dinutuximab discontinued for that day. If blood pressure remains stable for at least 2 hours, the IL-2 or GM-CSF can be resumed and the dinutuximab infusion increased to 1.75 mg/m2/h. If hypotension recurs, the cytokine administration should be halted. Dinutuximab may be started the next day at the 50% rate if hypotension has resolved and at the full rate if blood pressure is stable for 2 hours, if tolerated.
If hypotension persists with the initial infusion of normal saline or recurs, then an additional bolus of normal saline may be administered. Alternatively, either normal saline and albumin (25%) or packed red blood cells may be administered. Saline with albumin is recommended if the albumin level is ≤3 g/dL. Packed red blood cells can be given in place of saline or saline with albumin, and are recommended if hemoglobin is <10 g/dL.
Vasopressor therapy may be necessary in addition to fluid resuscitation to restore adequate perfusion pressure. ICU consultation and/or transfer to an ICU for pressor therapy (eg, epinephrine or dopamine) should be considered for all patients with persistent hypotension after initial treatment. If the blood pressure is stable when the patient is off pressors for more than 6 hours, then dinutuximab infusion may be resumed at the 50% rate on the following day. In rare instances where patients continue to require pressor support, dinutuximab should be discontinued.
Fever and Association With Systemic Infection
Although an increased risk of fever is associated with both IL-2 and GM-CSF cycles with or without dinutuximab, it is more commonly related to IL-2 infusion (United Therapeutics Corp., 2015). Fever and chills can be a sign of infection, particularly if they persist for more than 24 hours after completion of IL-2 infusion. Fevers are not common during the first week of IL-2 therapy. Acetaminophen is the preferred medication for pain or fever, as the use of ibuprofen, especially during cycles with IL-2, may increase risk of thrombocytopenia and renal issues as per the US Food and Drug Administration. Acetaminophen should be given as premedication and every 4 to 6 hours during dinutuximab and IL-2 therapy. It may also be given for fever every 4 to 6 hours as needed and continued throughout treatment. Ice packs should be available and cool washcloths on hand to treat patients with fevers.
For fever >101°F (38.5°C), blood cultures should be obtained and broad-spectrum antibiotics given until the patient is afebrile for >24 hours and cultures are negative for 48 hours. If a patient has a confirmed infection, the prescribing information indicates that dinutuximab immunotherapy should be discontinued until the systemic infection has resolved.
Patients who had an autologous stem cell transplant and are receiving dinutuximab immunotherapy are likely to be immunocompromised. In addition, IL-2 may cause a reversible defect in neutrophil function, contributing to increased risk of gram-positive systemic bacterial infections (Klempner, Noring, Mier, & Atkins, 1990; Snydman et al., 1990). Therefore, administration of prophylactic broad-spectrum antibiotics may be recommended. Some centers may recommend using prophylactic antibiotics for patients with bacteremia during the previous cycle.
Other Toxicities
Diarrhea following therapy has been reported and may lead to dehydration, causing necessary treatment interventions, including fluid therapy and careful monitoring of fluid and electrolyte balance. Treatment with loperamide (Imodium™) is recommended and generally helps resolve symptoms. Rare side effects, including grade 3/4 central nervous system symptoms (encephalopathy, confusion, and psychosis), seizures, and serum sickness have been noted. Medical staff should be aware of these unusual events to ensure their appropriate management, should they occur. Patients should also be carefully monitored for laboratory abnormalities, including hypokalemia, hyponatremia, and transaminitis, which occurs in many patients and is usually transient and mild. To maintain fluid and electrolyte balance, it is recommended that serum electrolytes be monitored, and hypotonic fluids be avoided. Although rare, dilated pupils and persistent light sensitivity have been reported. In addition, neurological disorders of the eye, such as blurred vision, photophobia, and fixed or unequal pupil sizes, have been reported.
Nursing Implications
Treatment of high-risk disease through induction chemotherapy, surgery, and consolidation therapy is rigorous and difficult and raises many issues for nurses and other health care professionals. In addition, managing toxicities and the patient/parent experience is complex. Therefore, a multidisciplinary team approach and optimization of the use of resources available at each center are integral to the effective management and care of patients receiving the dinutuximab immunotherapy regimen. Collaboration among critical care, pain, respiratory, and hematology/oncology teams is often reinforced and can improve the quality of patient care.
The immunotherapy regimen lasts for 6 months, involves infusions lasting 10 to 20 hours, and requires frequent hospital or clinic visits. All components of the immunotherapy regimen are associated with significant toxicities. The expert assessment and management of AEs, as well as preparation of and collaboration with patients and families, will facilitate patients’ remaining on therapy and receiving the full cycle of treatment, thereby optimizing the possibility of long-term control. Physicians and nurses, particularly those less experienced with immunotherapy, often need support in managing AEs, especially those that are unexpected and uncommon. In the view of many health care providers, a critical unmet need is the lack of appropriate tools and standardized procedures for managing AEs associated with the immunotherapy regimen.
It is critical that management of these patients be based on clinical laboratory data, practice guidelines, and interprofessional collaboration of practitioners with expert clinical judgment. A sample case study for a patient receiving courses 1 and 2 of therapy is presented in the appendix.
Summary
Dinutuximab has demonstrated significant clinical efficacy in children with high-risk neuroblastoma, with treatment-related AEs that are manageable if recognized early and treated with appropriate care. The critical and diverse roles of the nurse should not be underestimated and include responsibilities of patient education, pretreatment planning, monitoring during therapy, early AE identification, and safe treatment when AEs occur. As the use of dinutuximab in the treatment of high-risk neuroblastoma expands, astute assessment and management of treatment-related AEs will be fundamental to improving patient adherence to therapy and outcomes.
Acknowledgments
The authors would like to acknowledge all the interprofessional team members who continue to provide outstanding quality care for patients with neuroblastoma and their families. The authors also wish to acknowledge Julia D’Ambrosio, PhD, for support in preparation of this article.
Funding
The authors received no financial support for the authorship, preparation, and/or publication of this article.
Author Biographies
Rita Secola, PhD, RN, CPON, FAAN, is the Clinical Services Director for the Hematology Oncology Service Line at Children’s Hospital Los Angeles and Assistant Adjunct Professor, UCLA School of Nursing, Los Angeles, CA. She received her MSN from the University of Pittsburgh, PA and PhD from University of California Los Angeles.
Araz Marachelian, MD, is a pediatric hematologist/oncologist, Medical Director of NANT Operations Center and Clinical Director of the Neuroblastoma Program at Children’s Hospital Los Angeles, Los Angeles, CA.
Susan L. Cohn, MD, is a Pediatric Oncologist and Acting Section Chief of Hematology/Oncology at Comer Children’s Hospital, the University of Chicago, Chicago, IL.
Bonnie Toy, RN, MSN, CPNP, CPON, is a pediatric hematology/oncology nurse practitioner on the solid tumor program at Comer Children’s Hospital, the University of Chicago, Chicago, IL.
Kathleen Neville, MD, MS, is the Director of Experimental Therapeutics Program and Professor of Pediatrics at the University of Arkansas for Medical Sciences, Little Rock, AR.
Meaghan Granger, MD, is a Pediatric Hematologist/Oncologist at Cook Children’s Hospital in Fort Worth, TX.
Angela Brentlinger, MSN, RN, CPNP, is a pediatric hematology/oncology nurse practitioner on the Neuroblastoma and Stem Cell Transplantation Program at Cook Children’s Medical Center, Fort Worth, TX.
Gina Martin, BSN, CCRP, is a clinical research specialist in the Pediatric Hematology/Oncology Department at Washington University School of Medicine, St. Louis, MO.
Appendix
Case Study for a Patient Receiving Courses 1 and 2 of Immunotherapy
The patient is a 5-year-old male with high-risk stage IV neuroblastoma post 6 cycles of chemotherapy and peripheral stem cell collection, with surgical resection and high-dose chemotherapy followed by autologous stem cell transplantation. He received and tolerated nasogastric tube feedings to assist in weight recovery after significant weight loss and poor appetite throughout chemotherapy and stem cell transplantation.
Course 1, days 0–3 receiving granulocyte-macrophage colony-stimulating factor (GM-CSF) and dinutuximab (day 3):
Premedication and continuous hydromorphone (Dilaudid™) infusion were administered. The patient tolerated dinutuximab infusions well and did not require a decrease or pause of infusions. Intermittent decrease in urine output was noted, with no weight gain or signs of pulmonary edema or capillary leak syndrome. A minor complaint of abdominal pain was managed with intermittent use of hydromorphone by patient-controlled analgesia (PCA).
Course 2, days 0–3 receiving interleukin 2 (IL-2) only:
Premedications were administered. The patient remained afebrile. A moderate complaint of nausea was managed by administration of ondansetron hydrochloride (Zofran™) and continued nasogastric tube feedings.
Course 2, days 7–10 receiving IL-2 and dinutuximab:
Premedications were administered. Intermittent hives developed and were managed with an additional dose of diphenhydramine (Benadryl™) and the addition of hydroxyzine (Atarax™). Following further complaints of nausea and vomiting, nasogastric tube feedings were held. Frequent febrile episodes required collection of blood cultures, acetaminophen (Tylenol™), empiric use of cefotaxime (Claforan™), and close monitoring of blood pressure. No positive blood cultures were found. Persistent coughing episodes required administration of three albuterol treatments during the course. Decreased urine output and highly concentrated urine required a 10 mL/kg normal saline bolus with no signs of pulmonary edema or capillary leak. Additionally, the patient received two infusions of albumin and furosemide (Lasix™) and one transfusion of packed red blood cells. Tachycardia and tachypnea occurred during dinutuximab therapy and resolved on completion of the infusion. Complaints of abdominal pain post infusion required use of PCA and one dose of polyethylene glycol (Miralax™) for constipation.
The patient remained stable in hematology oncology unit with 1:2 nurse patient ratio and was discharged to home 1 day postcompletion of course 2.
Footnotes
Declaration of Conflicting Interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Susan Cohn reports equity in United Therapeutics. Meaghan Granger reports personal fees from United Therapeutics for a scientific advisory board and serving as Unituxin Training Speaker. Araz Marachelian reports personal fees from United Therapeutics for facilitating scientific/regional advisory board. Kathleen Neville reports personal fees from United Therapeutics for reimbursement for study costs. All other authors declared no potential conflicts of interests.
References
- American Cancer Society. (2015a). Cancer facts and figures, 2015. Retrieved from http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf
- American Cancer Society. (2015b). Neuroblastoma. Retrieved from http://www.cancer.org/cancer/neuroblastoma
- Baluna R, & Vitetta ES (1997). Vascular leak syndrome: A side effect of immunotherapy. Immunopharmacology, 37, 117–132. [DOI] [PubMed] [Google Scholar]
- Barker E, Mueller BM, Handgretinger R, Herter M, Yu AL, & Reisfeld RA (1991). Effect of a chimeric antiganglioside GD2 antibody on cell-mediated lysis of human neuroblastoma cells. Cancer Research, 51, 144–149. [PubMed] [Google Scholar]
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, … Vallejo R. (2008). Opioid complications and side effects. Pain Physician, 11(2 Suppl.), S105–S120. [PubMed] [Google Scholar]
- Castel V, Segura V, & Canete A. (2010). Treatment of high-risk neuroblastoma with anti-GD2 antibodies. Clinical & Translational Oncology, 12, 788–793. doi: 10.1007/s12094-010-0600-y [DOI] [PubMed] [Google Scholar]
- Children’s Oncology Group. (2013). ANBL0032 protocol: Phase III randomized study of chimeric antibody 14.18 (Ch14.18) in high risk neuroblastoma following myeloablative therapy and autologous stem cell rescue. Version 11/25/13. Amendment 16A. [Google Scholar]
- Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, …Matthay KK (2009). The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. Journal of Clinical Oncology, 27, 289–297. doi: 10.1200/jco.2008.16.6785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff AM (2012). Neuroblastoma. Seminars in Pediatric Surgery, 21, 2–14. doi: 10.1053/j.sempedsurg.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon S. (2015). Dinutuximab: First global approval. Drugs, 75, 923–927. doi: 10.1007/s40265-015-0399-5 [DOI] [PubMed] [Google Scholar]
- Fleegler E, & Kleinman M. (2016). Pediatric Advanced Life Support (PALS). UpToDate. Retrieved from http://www.uptodate.com/contents/pediatric-advanced-life-supportpals [Google Scholar]
- Gilman AL, Ozkaynak MF, Matthay KK, Krailo M, Yu AL, Gan J, … Sondel PM (2009). Phase I study of ch14.18 with granulocyte-macrophage colony-stimulating factor and interleukin-2 in children with neuroblastoma after autologous bone marrow transplantation or stem-cell rescue: A report from the Children’s Oncology Group. Journal of Clinical Oncology, 27, 85–91. doi: 10.1200/jco.2006.10.3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorges M, West N, Deyell R, Winton P, Cheung W, & Lauder G. (2015). Dexmedetomidine and hydromorphone: A novel pain management strategy for the oncology ward setting during anti-GD2 immunotherapy for high-risk neuroblastoma in children. Pediatric Blood & Cancer, 62, 29–34. doi: 10.1002/pbc.25197 [DOI] [PubMed] [Google Scholar]
- Handgretinger R, Anderson K, Lang P, Dopfer R, Klingebiel T, Schrappe M, … Neithammer D. (1995). A phase I study of human/mouse chimeric antiganglioside GD2 antibody ch14.18 in patients with neuroblastoma. European Journal of Cancer, 31, 261–267. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, … Cronin KA. (2015). SEER Cancer Statistics Review, 1975–2012. Retrieved from http://seer.cancer.gov/csr/1975_2012/
- Kammula US, White DE, & Rosenberg SA (1998). Trends in the safety of high dose bolus interleukin-2 administration in patients with metastatic cancer. Cancer, 83, 797–805. [PubMed] [Google Scholar]
- Klempner MS, Noring R, Mier JW, & Atkins MB (1990). An acquired chemotactic defect in neutrophils from patients receiving interleukin-2 immunotherapy. New England Journal of Medicine, 322, 959–965. doi: 10.1056/nejm199004053221404 [DOI] [PubMed] [Google Scholar]
- Maris JM (2010). Recent advances in neuroblastoma. New England Journal of Medicine, 362, 2202–2211. doi: 10.1056/NEJMra0804577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris JM, Hogarty MD, Bagatell R, & Cohn SL (2007). Neuroblastoma. Lancet, 369, 2106–2120. doi: 10.1016/s0140-6736(07)60983-0 [DOI] [PubMed] [Google Scholar]
- Matthay KK, George RE, & Yu AL (2012). Promising therapeutic targets in neuroblastoma. Clinical Cancer Research, 18, 2740–2753. doi: 10.1158/1078-0432.ccr-11-1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, … Villablanca JG (2009). Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A Children’s Oncology Group study. Journal of Clinical Oncology, 27, 1007–1013. doi: 10.1200/jco.2007.13.8925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, … Reynolds CP (1999). Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. New England Journal of Medicine, 341, 1165–1173. doi: 10.1056/nejm199910143411601 [DOI] [PubMed] [Google Scholar]
- Ozkaynak MF, Sondel PM, Krailo MD, Gan J, Javorsky B, Reisfeld RA, …Seeger RC (2000). Phase I study of chimeric human/murine anti-ganglioside G(D2) monoclonal antibody (ch14.18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after hematopoietic stem-cell transplantation: A Children’s Cancer Group Study. Journal of Clinical Oncology, 18, 4077–4085. [DOI] [PubMed] [Google Scholar]
- Parsons K, Bernhardt B, & Strickland B. (2013). Targeted immunotherapy for high-risk neuroblastoma: The role of monoclonal antibodies. Annals of Pharmacotherapy, 47, 210–218. doi: 10.1345/aph.1R353 [DOI] [PubMed] [Google Scholar]
- Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF, … Cohn SL (2015). Advances in risk classification and treatment strategies for neuroblastoma. Journal of Clinical Oncology, 33,3008–3317. doi: 10.1200/jco.2014.59.4648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Klingebiel T, & Berthold F. (2011). Long term outcome of high-risk neuroblastoma patients after immunotherapy with antibody ch14.18 or oral metronomic chemotherapy. BMC Cancer, 11, 21. doi: 10.1186/1471-2407-11-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snydman DR, Sullivan B, Gill M, Gould JA, Parkinson DR, & Atkins MB (1990). Nosocomial sepsis associated with interleukin-2. Annals of Internal Medicine, 112, 102–107. [DOI] [PubMed] [Google Scholar]
- Svennerholm L, Bostrom K, Fredman P, Jungbjer B, Lekman A, Mansson JE, & Rynmark BM (1994). Gangliosides and allied glycosphingolipids in human peripheral nerve and spinal cord. Biochimica et Biophysica Acta, 1214, 115–123. [DOI] [PubMed] [Google Scholar]
- United Therapeutics Corp. (2015). Unituxin™ (dinutuximab) injection, for intravenous use [prescribing information]. Retrieved from https://www.unituxin.com/downloads/fullprescribing-information.pdf
- Yalcin B, Kremer LC, Caron HN, & van Dalen EC (2013). High-dose chemotherapy and autologous haematopoietic stem cell rescue for children with high-risk neuroblastoma. Cochrane Database of Systematic Reviews, 8, CD006301. doi: 10.1002/14651858.CD006301.pub3 [DOI] [PubMed] [Google Scholar]
- Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, … Sondel PM (2010). Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. New England Journal of Medicine, 363, 1324–1334. doi: 10.1056/NEJMoa0911123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AL, Uttenreuther-Fischer MM, Huang CS, Tsui CC, Gillies SD, Reisfeld RA, & Kung FH (1998). Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. Journal of Clinical Oncology, 16, 2169–2180. [DOI] [PubMed] [Google Scholar]
- Zhang S, Cordon-Cardo C, Zhang HS, Reuter VE, Adluri S, Hamilton WB, … Livingston PO (1997). Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. International Journal of Cancer, 73, 42–49. [DOI] [PubMed] [Google Scholar]