Abstract
Background.
131I-metaiodobenzylguanidine (131I-MIBG) is a targeted radiopharmaceutical for patients with neuroblastoma. Despite its tumor-specific uptake, the treatment with 131I-MIBG results in whole-body radiation exposure. Our aim was to correlate whole-body radiation dose (WBD) from 131I-MIBG with tumor response, toxicities, and other clinical factors.
Methods.
This retrospective cohort analysis included 213 patients with high-risk neuroblastoma treated with 131I-MIBG at UCSF Benioff Children’s Hospital between 1996 and 2015. WBD was determined from radiation exposure rate measurements. The relationship between WBD ordered tertiles and variables were analyzed using Cochran–Mantel–Haenszel test of trend, Kruskal–Wallis test, and one-way analysis of variance. Correlation between WBD and continuous variables was analyzed using Pearson correlation and Spearman rank correlation.
Results.
WBD correlated with 131I-MIBG administered activity, particularly with 131I-MIBG per kilogram (P < 0.001). Overall response rate did not differ significantly among the three tertiles of WBD. Correlation between response by relative Curie score and WBD was of borderline significance, with patients receiving a lower WBD showing greater reduction in osteomedullary metastases by Curie score (rs = 0.16, P = 0.049). There were no significant ordered trends among tertiles in any toxicity measures (grade 4 neutropenia, thrombocytopenia < 20,000/μl, and grade > 1 hypothyroidism).
Conclusions.
This study showed that 131I-MIBG activity per kilogram correlates with WBD and suggests that activity per kilogram will predictWBD in most patients. Within the range of activities prescribed, there was no correlation between WBD and either response or toxicity. Future studies should evaluate tumor dosimetry, rather than just WBD, as a tool for predicting response following therapy with 131I-MIBG. Pediatr Blood Cancer 2016;63:436–442.
Keywords: dosimetry, 131I-metaiodobenzylguanidine (MIBG), neuroblastoma, whole-body radiation dose (WBD)
INTRODUCTION
Neuroblastoma, a malignancy arising from neural crest cells of the sympathetic nervous system, is the most common extracranial solid cancer in children. At diagnosis, approximately half of pediatric neuroblastoma patients will present with metastatic disease. Although outcomes for children with advanced disease have improved with intensive multimodal treatment, survival rates are still less than 50%.[1]
Neuroblastoma cells often express the human norepinephrine transporter that facilitates avid uptake of metaiodobenzylguanidine (MIBG), an arylalkylguanidine norepinephrine analog.[2] This proclivity of greater than 90% of neuroblastomas to accumulate MIBG allows for the use of radiolabeled MIBG for targeted imaging (123I-MIBG) and therapy (131I-MIBG).[3] The use of 131I-MIBG as targeted radiotherapy has been successful with >30% response rates in patients with refractory or relapsed neuroblastoma.[4] 131I-MIBG is now increasingly being used as a part of induction and consolidation frontline therapy.[5]
Despite its tumor-specific uptake, the treatment with 131I-MIBG results in whole-body exposure to a significant radiation doserangingfrom50to600cGy,whichcanleadtovariousacute andlatetoxicities.Phase1andotherpilotstudieshaveshownthe primary dose-limiting toxicity to be myelosuppression, though this toxicity can be alleviated through autologous hematopoietic stem cell support.[6,7] Possible nonhematological toxicities include transient nausea and vomiting, sialoadenitis, hypertensive episodes, hepatic dysfunction, hypothyroidism, infertility, and secondary cancers.[8–11]
A prior pilot study from our group showed that the whole-body radiation dose (WBD) received from 131I-MIBG therapy correlated with the activity of 131I-MIBG administered per kilogram and with hematological toxicity.[12] In the current retrospective cohort study, we aimed to analyze WBD from 131I-MIBG therapy in relation to tumor response, toxicities, and other clinical factors, including age, 131I-MIBG activity, 131I-MIBG activity per kilogram of body weight, disease extent and sites, and Curie score.
METHODS
Study Subjects
Patients of more than 1-year of age with high-risk neuroblastoma treated with 131I-MIBG at UCSF Benioff Children’s Hospital on three local and six New Approaches to Neuroblastoma Therapy (NANT) clinical trials between August 30, 1996 and May 15, 2015 were evaluated for this retrospective cohort analysis. For patients that received multiple courses of 131I-MIBG therapy, only the first treatment course was included in this analysis. Results were obtained by chart review and data abstraction. Eligibility for inclusion in this study included calculation of WBD. Of the 224 patients evaluated, 11 patients were excluded for unavailable (n=10) or incomplete (n=1) radiation exposure rate measurements. Whole-body dosimetry doses on 62 the 213 patients eligible for this study were reported in primary trial publications.[12–15]
Informed consent was obtained for all patients for each 131I-MIBG treatment protocol. This retrospective analysis was approved by the UCSF institutional review board.
Treatment
Patients were treated on the following nine clinical trials using 131I-MIBG therapy: an ongoing UCSF compassionate use study of 131I-MIBG; a Phase II study of MIBG monotherapy;[16] NANT 99–01;[17] NANT 2000–01[18], NANT 2001–02;[19] NANT 2004–06;[13] NANT 2007–01;[20] NANT 2007–03;[15] and UCSF 131I-MIBG vincristine/irinotecan [14] (Supplementary Table SI). Patients treated on NANT 2000–01, a double-infusion protocol, received 19.9–50.7 mCi/kg of 131I-MIBG over two treatments at a 2-week interval. Patients treated on the remaining clinical trials received 4.1–20.9 mCi/kg of 131I-MIBG. 131I-MIBG was given intravenously over 1–2 hr, with KI used for thyroid protection and Foley catheters were used for bladder protection. All patients were isolated for 5–7 days until radiation emissions met institutional regulations (<2 mr/hr at 1 m).
Calculation of WBD
The WBD from 131I-MIBG was calculated for every patient using radiation exposure rate measurements following the method described previously.[12] Measurements after 2001 were obtained by a ceiling-mounted ionization chamber every 3 min for 5–7 days following infusion. Measurements for patients treated before 2001 were obtained by a handheld ionization chamber, held 1 m from the patient’s body surface, taken at 4-hrtimepointsonday1aftertheinfusionandthendailyfor5–7 days following infusion. All data points within 24 hr of washout and hourly data points thereafter were used to graph a whole-body time–exposure curve, with which a three-compartment double-exponential curve was fit for most patients. Two-phase exponential decay was used with 12 patients for whom the three-compartment curve did not fit. Nonlinear regression analysis was performed using GraphPad Prism to obtain exponential time constants and coefficients, with which the whole-body cumulated activity, Ãwb, was derived. WBD was then calculated according to medical internal radiation dose methodology:
where Ãwb is the whole-body cumulated activity and Swb←wb (131I) is the weight-specific interpolated S-factor for 131I.[21,22]
Assessment of Overall Response and Relative Curie Score
For patients treated on NANT studies and the UCSF 131I-MIBG vincristine/irinotecan protocol, overall response to 131I-MIBG therapy was assessed by blinded central review according to modified International Neuroblastoma Response Criteria as utilized by NANT.[23] These criteria use Response Evaluation Criteria in Solid Tumors, Curie score, and bone marrow morphology to grade responses as complete response, partial response, mixed response, stable disease, or progressive disease.[24,25] Mixed response designates patients who achieved partial response in at least one site and stable disease in another site.[23] For patients treated on UCSF institutional studies, response was evaluated using similar criteria by UCSF radiologists and oncologists by comparison of 123I-MIBG scans, CT scans, and bone marrow biopsies obtained before and approximately 6–8 weeks following 131I-MIBG therapy.
Curie scores were determined centrally for patients on NANT protocols and for patients on UCSF institutional protocols by consensus of two nuclear medicine physicians (R.A.H. and L.N.).[26] Pretherapy extension scores were utilized to evaluate tumor burden at treatment entry. Relative extension scores, calculated by dividing posttherapy extension score by pretherapy extension score, were used to assess response to 131I-MIBG therapy, with values >1 indicating greater disease burden posttreatment and values <1 indicating lower disease burden posttreatment.
Evaluation of Hematologic and Thyroid Toxicity
Laboratory data (CBC and thyroid function) were reviewed for each patient. Myelosuppression was categorized by occurrence of grade 4 neutropenia per CTCv4.0 (ANC<500 cells/μl) or thrombocytopenia defined as platelets <20,000/μl. Pre- and posttherapy TSH and T4 values were obtained for the assessment of posttherapy thyroid dysfunction and recorded as normal or outside normal range.
Statistical Methods
This was a retrospective analysis of the impact of WBD on response and toxicity. We also tested correlation with 131I-MIBG infused activity, and clinical factors including disease sites, Curie score, age, and MYCN status. WBD was divided into tertiles for the majority of analyses, though WBD as a continuous variable was evaluated in a subset of analyses.
The Cochran–Mantel–Haenszel test of trend was used to test the relationship between WBD ordered tertiles and categorical variables. Kruskal–Wallis test and one-way analysis of variance were used to test the relationship between tertiles of WBD and patient age and 131I-MIBG activity, respectively. Mean WBD was compared between responders (partial response or better) and nonresponders (all other patients) and between patients treated with and without concomitant radiation sensitizer (irinotecan or vorinostat) using a t-test. Correlations between WBD and 131I-MIBG infused activity were assessed using Pearson correlation (r). Correlations between WBD and Curie score and relative Curie score were assessed using Spearman rank correlation (rs). Kaplan–Meier methods were used to estimate overall survival from date of 131I-MIBG administration and compared between groups with the log-rank test. All analyses were performed in Stata v12.
RESULTS
Patient Characteristics
A total of 213 patients were eligible for inclusion in this analysis, with the characteristics shown in Table I. The median WBD for the entire group was 217 cGy and for WBD Tertiles 1, 2, and 3 were 160 cGy (62–195 cGy), 217 cGy (196–253 cGy), and 314 cGy (254–659 cGy), respectively. The proportion of patients treated with 131I-MIBG alone, rather than with concomitant administration of other antitumor agents or radiosensitizers, increased with increasing WBD tertile (P = 0.002). Comparing patients who received 131I-MIBG monotherapy at 18 mCi/kg (n = 62; actual range 17–19 mCi/kg to account for rounding) with patients who received this dose of 131I-MIBG therapy with concomitant radiosensitizer (n = 26), we found no significant trend across WBD tertiles (P = 0.68). Similarly, no statistically significant difference was found between these two cohorts in evaluation of WBD as a continuous variable (mean WBD with monotherapy = 251 cGy vs. 251 cGy with concomitant radiation sensitizer; P = 0.96).
TABLE I.
Characteristics of 213 Patients Treated with 131I-MIBG
| Characteristic | All patients | Tertile 1 62–195 cGy | Tertile 2 196–253 cGy | Tertile 3 254–659 cGy | P-value |
|---|---|---|---|---|---|
| No. of patients | 213 | 71 | 71 | 71 | - |
| Sex | |||||
| Male | 136 (64%) | 45 (63%) | 42 (59%) | 49 (69%) | 0.49a |
| Female | 77 (36%) | 26 (37%) | 29 (41%) | 22 (31%) | |
| Age at diagnosis (years) | |||||
| Median | 4.68 | 4.42 | 4.59 | 4.95 | 0.54b |
| Range | 0.33–50.79 | 0.33–29.22 | 0.38–26.18 | 0.83–50.79 | |
| Age at entry (years) | |||||
| Median | 7.14 | 6.19 | 7.41 | 8.14 | 0.30b |
| Range | 1.87–51.20 | 2.59–30.22 | 1.87–26.84 | 2.16–51.20 | |
| Stage at diagnosis | |||||
| 1,2,3 | 35 (16%) | 13 (18%) | 12(17%) | 10 (14%) | 0.50a |
| 4 | 178 (84%) | 58 (82%) | 59 (83%) | 61 (86%) | |
| MYCN amplificationd | |||||
| Yes | 40 (24%) | 8 (15%) | 14 (25%) | 18 (31%) | 0.05a |
| No | 127 (76%) | 45 (85%) | 42 (75%) | 40 (69%) | |
| Prior ASCT | |||||
| Yes | 120 (56%) | 35 (49%) | 43 (61%) | 42 (59%) | 0.24a |
| No | 93 (44%) | 36 (51%) | 28 (39%) | 29 (41%) | |
| Disease status prior to 131I MIBG therapy | |||||
| Relapse | 141 (66%) | 43 (61%) | 50 (70%) | 48 (68%) | 0.38a |
| Refractory | 72 (34%) | 28 (39%) | 21 (30%) | 23 (32%) | |
| Sites of disease involvement at entry | |||||
| ST + B/BM | 88 (41%) | 25 (35%) | 28 (39%) | 35 (49%) | 0.09a |
| ST only | 24(11%) | 10 (14%) | 5 (7%) | 9 (13%) | |
| B/BM only | 101 (48%) | 36 (51%) | 38 (54%) | 27 (38%) | |
| Type of 131I-MIBG protocol | |||||
| MIBG alonee | 129 (61%) | 33 (46%) | 45 (63%) | 51 (72%) | 0.002a |
| Combination | 84 (39%) | 38 (54%) | 26 (37%) | 20 (28%) | |
| Total dose of 131I-MIBG administered (mCi) | |||||
| Mean | 455.23 | 372.28 | 439.34 | 554.07 | 0.0001c |
| Standard deviation | 253.12 | 242.74 | 206.33 | 274.60 | |
| Dose of 131I-MIBG per kilogram of body weight (mCi/kg) | |||||
| Mean | 16.25 | 12.68 | 16.52 | 19.54 | <0.0001c |
| Standard deviation | 5.94 | 3.56 | 2.62 | 7.97 | |
Cochran–Mantel–Haenszel test;
Kruskal–Wallis test;
One-way analysis of variance;
MYCN amplification status unknown for 46 patients (18 Tertile 1, 15 Tertile 2, and 13 Tertile 3);
MIBG alone = any patient without concomitant administration of other antitumor agents or radiosensitizers. ASCT, autologous stem cell transplant; ST, soft tissue; B, bone; BM, bone marrow.
The proportion of patients with MYCN amplification increased with increasing WBD tertile (P = 0.051). Males predominated in all tertiles, showing no correlation between sex and WBD. Median age at diagnosis and treatment entry did not differ across tertiles. As expected, there was no significant correlation between stage at diagnosis and WBD, with stage 4 neuroblastoma patients making up greater than 80% of all tertiles. The proportion of patients with prior autologous stem cell transplant did not demonstrate a significant ordered trend across the three WBD tertiles. Only four patients in our study had received prior whole-body radiation as a part of their conditioning.
Administered 131I-MIBG
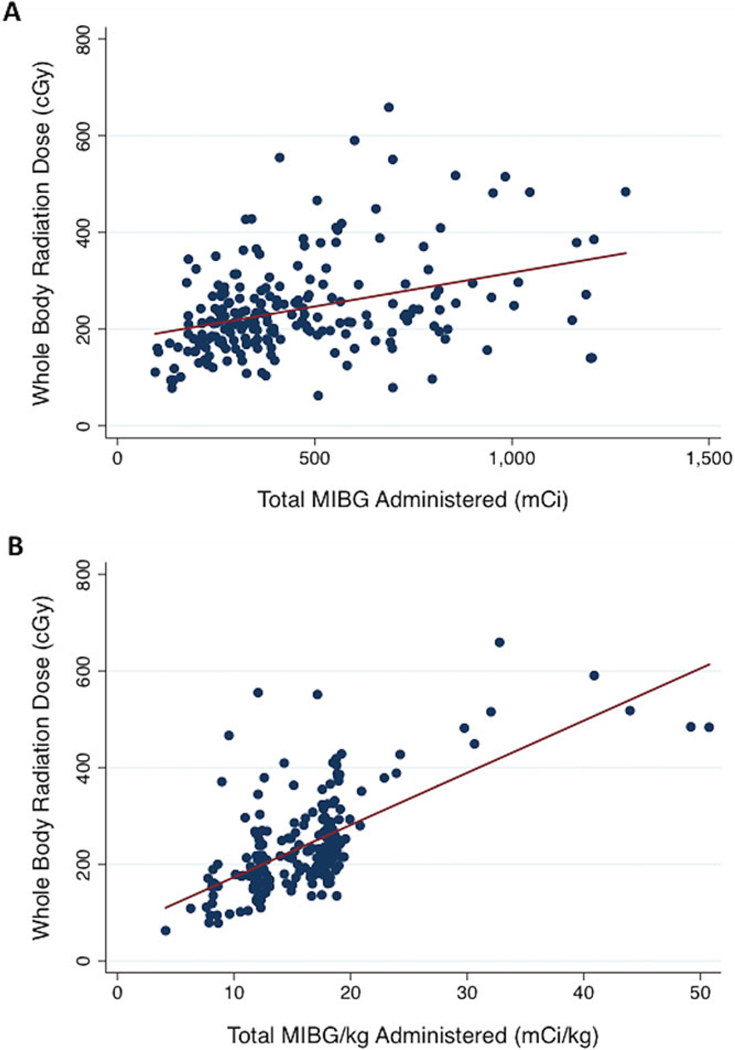
Total 131I-MIBG administered (mCi) correlated positively with WBD, as shown in Figure 1A (r = 0.3574, P < 0.001). The median WBD was 176, 213, and 250 cGy at 12, 15, and 18 mCi/kg, respectively. There was a stronger positive correlation between 131I-MIBG administered per kilogram of body weight and WBD, as shown in Figure 1B(r=0.6485, P<0.001).
Fig. 1.
(A) Total 131I-MIBG administered (mCi) correlates positively with whole-body radiation dose (cGy) (r = 0.36, P < 0.001). (B) 131I-MIBG administered per kilogram body weight (mCi/kg) correlates positively with whole-body radiation dose (cGy) (r=0.65, P < 0.001).
Lack of Effect of Disease Burden on WBD
Sites of disease involvement at treatment entry did not correlate significantly with tertiles of WBD (Table I). For Tertiles 1 and 2, the majority of patients (51% and 54%, respectively) had bone or bone marrow involvement only. For Tertile 3, the patients predominantly had disease involvement in both soft tissue and bone/bone marrow compartments (49%). We tested Curie score at entry as a measure of disease burden and observed no significant correlation with WBD (rs = 0.07, P = 0.35; Fig. 2A).
Fig. 2.
(A) Correlation of whole-body radiation dose with baseline Curie score (n =179, rs = 0.07, P = 0.35). (B) Correlation of relative Curie score with whole-body radiation dose (n =160, rs = 0.16, P = 0.049).
Overall Response
Overall response rate did not differ significantly among the three tertiles of WBD (Table II). By examination of WBD as a continuous variable, the mean WBD for patients with an objective response (partial response or better) was 227 cGy (95% CI 206–249 cGy), while the mean WBD for patients without an objective response was 246 cGy (95% CI 229–262 cGy), which was not significantly different (P = 0.23). In sensitivity analyses restricted to patients who received 131I-MIBG at 18 mCi/kg (actual range 17–19 mCi/kg to account for rounding) and no associated myeloablative chemotherapy, we also did not see statistically significant difference analyzing the data across WBD tertiles (P = 0.14) or as a continuous variable (mean WBD for responders = 231 vs. 258 cGy for nonresponders; P = 0.10).
TABLE II.
Response to 131I-MIBG by Tertiles of Whole-Body Radiation Dose
| Alla | Tertile 1 | Tertile 2 | Tertile 3 | P-valueb | |
|---|---|---|---|---|---|
| Response | 57 (27%) | 21 (30%) | 19 (27%) | 17 (24%) | 0.45 |
| No response | 156 (73%) | 50 (70%) | 52 (73%) | 54 (76%) | |
| CR | 16 (8%) | 9 (13%) | 4 (6%) | 3 (4%) | |
| PR | 42 (20%) | 13 (18%) | 15 (22%) | 14 (20%) | |
| SD | 96 (46%) | 33 (47%) | 32 (46%) | 31 (44%) | 0.08 |
| PD | 38 (18%) | 9 (13%) | 14 (20%) | 15 (21%) | |
| MR | 18 (8%) | 6 (9%) | 4 (6%) | 8 (11%) | |
Response for three patients was not evaluable;
Cochran–Mantel–Haenszel test. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; MR, mixed response; ASCT, autologous stem cell transplant.
In evaluation of the specific response categories (complete response, partial response, mixed response, stable disease, and progressive disease), there was no correlation among the three tertiles of WBD (P = 0.08). However, correlation between response, as assessed by relative Curie score, and WBD was of borderline significance, with patients receiving a lower WBD paradoxically showing a greater reduction in osteomedullary metastases by Curie score (rs = 0.16, P = 0.049; Fig. 2B).
Toxicities
Of the patients with available CBC values, 115 of 160 (72%) and 115 of 157 (73%) experienced grade 4 neutropenia and thrombocytopenia (< 20,000/μl), respectively. Of the patients with available baseline and posttherapy TSH and/or T4 lab values (n = 134), 17% experienced posttreatment grade ≥ 1 hypothyroidism. Comparable results were found in the subanalysis of patients treated with 131I-MIBG monotherapy. Details of toxicities by tertiles of WBD are presented in Table III. There were no significant ordered trends among tertiles in any of the measures of toxicity.
TABLE III.
Toxicities by Tertiles of Whole-Body Radiation Dose
| AH 131I-MIBG Protocolsa | |||||
|---|---|---|---|---|---|
| Toxicity | All | Tertile 1 | Tertile 2 | Tertile 3 | P-valuec |
| Grade 4 neutropenia | |||||
| Yes | 115 (72%) | 32 (67%) | 37 (67%) | 46 (81%) | 0.10 |
| No | 45 (28%) | 16(33%) | 18 (33%) | 11 (19%) | |
| Thrombocytopeniab | |||||
| Yes | 115 (73%) | 29 (64%) | 44 (83%) | 42 (71%) | 0.54 |
| No | 42 (27%) | 16(36%) | 9 (17%) | 17 (29%) | |
| Posttreatment hypothyroidism | |||||
| Yes | 23 (17%) | 8 (18%) | 10 (23%) | 5(11%) | 0.35 |
| No | 111 (83%) | 36 (82%) | 34 (77%) | 41 (89%) | |
| 131I-MIBG Monotherapy Protocolsd | |||||
| Grade 4 neutropenia | |||||
| Yes | 80 (71%) | 19 (63%) | 27 (71%) | 34 (77%) | 0.19 |
| No | 32 (29%) | 11 (37%) | 11 (29%) | 10 (23%) | |
| Thrombocytopeniab | |||||
| Yes | 91 (76%) | 22 (71%) | 35 (88%) | 34 (71%) | 0.79 |
| No | 28 (24%) | 9 (29%) | 5 (12%) | 14 (29%) | |
| Posttreatment hypothyroidism | |||||
| Yes | 16(16%) | 4(16%) | 7 (21%) | 5 (12%) | 0.62 |
| No | 82 (84%) | 21 (84%) | 26 (79%) | 35 (88%) | |
Lab values unavailable for 53 patients (ANC), 56 patients (platelet count), and 79 patients (TSH/T4);
Assessed using a platelet count threshold of 20,000/μl;
Cochran–Mantel–Haenszel test;
Lab values unavailable for 17 patients (ANC), 10 patients (platelet count), and 31 patients (TSH/T4).
Overall Survival
The 24-month overall survival was lowest for Tertile 3 patients at 37.8% (95% CI 26.0–49.5%), compared to 54.5% (95% CI 41.6–65.7%) for Tertile 1 patients and 57.4% (95% CI 44.1– 68.1%) for Tertile 2 patients, although there was not a significant difference (P = 0.22; Fig. 3).
Fig. 3.
Estimated overall survival according to tertiles of whole-body radiation dose (P = 0.22).
DISCUSSION
In this study, we found that WBD correlated with 131I-MIBG activity, particularly with 131I-MIBG administered per kilogram. This finding serves to validate the results of our previous study, suggesting that activity administered per kilogram can be used as a measure of expected whole-body radiation exposure.[12] As expected, 131I-MIBG protocol correlated significantly with WBD, because the MIBG monotherapy cohort included patients treated on the double-infusion protocol (NANT 2000–01) and administered 131I-MIBG activity was decreased in myeloablative protocols for toxicity. Furthermore, we found trends to suggest correlations between both MYCN amplification and relative Curie score. In contrast to our hypothesis and results of previous studies, we did not find a significant correlation between WBD and response or toxicity.[6,12,13,27–32]
Our findings regarding the correlation between WBD and 131I-MIBG activity support the results of previous studies.[12, 17,20,31,33] The strong positive correlation of WBD to 131I-MIBG activity per kilogram suggests that activity prescriptions for131I-MIBGtherapiesshouldbemadebasedonpatientweight as opposed to a predetermined total activity dose in order to achieve a targeted WBD. A recent study by Minguez et al. proposed an equation, which describes whole-body absorbed dose per unit of administered activity as a function of patient mass, as an alternative for prescriptions of activity on first administration when dosimetry data for the individual patient are unknown.[31]
The lack of correlation of disease sites and baseline Curie score to WBD suggests that tumor burden does not impact WBD. Although this association has not previously been investigated in 131I-MIBG therapy, prior studies have assessed the effect of tumor burden on the biodistribution of different radiopharmaceuticals and its subsequent impact on WBD. These studies on non-Hodgkin lymphoma patients treated with 131I-rituximab (anti-CD20 antibody) and 131I-tositumomab (anti-B1 antibody) and patients with gastrointestinal malignancies treated with 131I-COL-1 suggest the dependence of clearance kinetics on tumor burden.[34–36] The studies independently proposed that patients with larger tumor burden might bind a greater fraction of the administered radiopharmaceutical, yielding a decreased concentration in serum and, therefore, an increased clearance of the serum radioactivity. We expected that increased neuroblastoma burden would similarly increase uptake and retention of 131I-MIBG, effectively increasing WBD. While Tertile 3 had the greatest proportion of patients with disease involvement in all compartments, we found no significant correlation between tumor burden and WBD, perhaps due to the more rapid plasma clearance of 131I-MIBG compared to radiolabeled antibodies.
Our previous pilot study found that tumor self-absorbed radiation dose (TSARD) predicted tumor volume decrease and also correlated with both WBD and overall tumor response.[12] Therefore, we hypothesized that WBD might correlate with overall response. However, similar to other previous studies, we found no relationship between WBD and overall response.[12, 30] Although not statistically significant, our results suggest that responders received a lower mean WBD (227 cGy) compared to nonresponders (246 cGy), similar to the results of DuBois et al.[13] These findings were also reflected in our comparison of relative Curie score and WBD, in which patients, who received lower WBD, showed the greatest reduction in Curie score. This unexpected finding could be explained if overall response is described as a function of a therapeutic ratio (TSARD/WBD), wherein response is not so much dependent on the value of WBD, but rather the fraction of WBD localized in the tumor. Because TSARD was not calculated in our study, this relationship could not be evaluated. Future studies including more accurate tumor dosimetry such as 124I-MIBG with PET-CT technology will improve our assessment of this ratio.[37]
Many past studies have found a significant association between WBD and hematologic toxicity.[6,12,13,27–30,32] However, in our study, we were unable to establish any relationship between hematologic or thyroid toxicity and WBD. Inclusion of patients treated on myeloablative protocols may have confounded our analysis by increasing the hematologic toxicity at lower WBD. To account for this, we conducted a subanalysis of MIBG monotherapy protocols and again no significant association was observed. A recent study also found no correlation between grade of hematologic toxicity and WBD, but they suggested that the lack of correlation might be attributed to small sample size, wide age variance, or prior hematotoxic treatments.[31] One possible explanation for the low correlation in our study is the narrow range of WBD. In a study by Lashford et al., 31% of patients developed grade 3 or 4 thrombocytopenia at WBD of 2.0 Gy and 40% of patients developed grade 3 or 4 neutropenia at WBD of 2.5 Gy.[30] In our Phase I dose escalation study, grade 4 hematologic toxicity occurred in 80% of patients receiving >12 mCi/kg. Because it has been previously established that 18 mCi/kg is the maximal dose of 131I-MIBG, the majority of the patients in our study received WBD levels above the toxicity threshold, so no significant association could be assessed. Variable receipt of hematopoietic stem cell support after 131I-MIBG therapy may have also impacted our ability to detect a difference in hematologic toxicity according to WBD. Whether WBD may correlate with other late toxicities, such as second malignancy or reduced fertility, will require further study.
Although there was no significant difference in the 24-month OS among the three tertiles of WBD, 24-month OS was lowest for Tertile 3 patients at 37.8%. As shown in Table I, lack of difference in 24-month OS among the three tertiles does not appear to be due to a higher proportion of relapsed patients compared to refractory patients.[38] Instead, it may be due to the increased proportion of Tertile 3 patients with disease involvement in all three compartments, previously shown to be a higher risk group.[16] The low 24-month OS may also be due to decreased response rate, as assessed by relative Curie score.
Limitations of our study may include (i) inter patient variability, such as differing rates of renal excretion, (ii) comparison of different 131I-MIBG protocols, some of which include concomitant administration of different agents and radiosensitizers that may have confounded response or toxicity results, (iii) lack of tumor dosimetry for most patients because of the requirement for pretreatment serial imaging, and (iv) a narrow range of WBD, which may have limited the evaluation of toxicity.
CONCLUSIONS
This study has shown that 131I-MIBG activity per kilogram correlates with WBD and suggests that 131I-MIBG activity per kilogram will predict WBD in most patients. Despite lack of correlation to response and toxicity, WBD correlated inversely with relative Curie score. This unexpected finding prompts future studies to evaluate tumor dosimetry, rather than just WBD, as a tool for predicting response following therapy with 131I-MIBG.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by Alex’s Lemonade Stand Foundation POST Award (M.T.),Alex’s Lemonade Stand Foundation Infrastructure Award (K.K.M.), Mildred V. Strouss Chair, Conner Fund, Dougherty Foundation, PO1CA81403, and Frank A. Campini Foundation. We thank Shih-Ying Huang and John Huberty for advice on dosimetry calculations.
Abbreviations:
- MIBG
metaiodobenzylguanidine
- NANT
New Approaches to Neuroblastoma Therapy
- TSARD
tumor self-absorbed radiation dose
- WBD
whole-body radiation dose
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of interest: Nothing to declare.
REFERENCES
- 1.Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, Gerbing RB, London WB, Villablanca JG. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A children’s oncology group study. J Clin Oncol 2009;27:1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howman-Giles R, Shaw PJ, Uren RF, Chung DK. Neuroblastoma and other neuroendocrine tumors. Semin Nucl Med 2007;37:286–302. [DOI] [PubMed] [Google Scholar]
- 3.Taggart D, Dubois S, Matthay KK. Radiolabeled metaiodobenzylguanidine for imaging and therapy of neuroblastoma. Q J Nucl Med Mol Imaging 2008;52:403–418. [PubMed] [Google Scholar]
- 4.Wilson JS, Gains JE, Moroz V, Wheatley K, Gaze MN. A systematic review of 131I-meta iodobenzylguanidine molecular radiotherapy for neuroblastoma. Eur J Cancer 2014;50:801–815. [DOI] [PubMed] [Google Scholar]
- 5.Gaze MN, Gains JE, Walker C, Bomanji JB. Optimization of molecular radiotherapy with [131I]-meta iodobenzylguanidine for high-risk neuroblastoma. Q J Nucl Med Mol Imaging 2013:57: 66–78. [PubMed] [Google Scholar]
- 6.DuBois SG, Messina J, Maris JM, Huberty J, Glidden DV, Veatch J, Charron M, Hawkins R, Matthay KK. Hematologic toxicity of high-dose iodine-131-metaiodobenzylguanidine therapy for advanced neuroblastoma. J Clin Oncol 2004;22:2452–2460. [DOI] [PubMed] [Google Scholar]
- 7.Matthay KK, DeSantes K, Hasegawa B, Huberty J, Hattner RS, Ablin A, Reynolds CP, Seeger RC, Weinberg VK, Price D. Phase I dose escalation of 131I-metaiodobenzylguanidine with autologous bone marrow support in refractory neuroblastoma. J Clin Oncol 1998;16:229–236. [DOI] [PubMed] [Google Scholar]
- 8.Kosmin MA, Bomanji JB, Cork NJ, Shankar A, Gaze MN. Hypertension complicating 131I-metaiodobenzylguanidine therapy for neuroblastoma. Eur J Nucl Med Mol Imaging 2012;39:597–601. [DOI] [PubMed] [Google Scholar]
- 9.Modak S, Pandit-Taskar N, Kushner BH, Kramer K, Smith-Jones P, Larson S, Cheung NK. Transient sialoadenitis: A complication of 131I-metaiodobenzylguanidinetherapy.PediatrBloodCancer 2008;50:1271–1273. [DOI] [PubMed] [Google Scholar]
- 10.Quach A, Ji L, Mishra V, Sznewajs A, Veatch J, Huberty J, Franc B, Sposto R, Groshen S, Wei D, Fitzgerald P, Maris JM, Yanik G, Hawkins RA, Villablanca JG, Matthay KK. Thyroid and hepatic function after high-dose 131 I-metaiodobenzylguanidine (131 I-MIBG) therapy for neuroblastoma. Pediatr Blood Cancer 2011;56:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clement SC, Kraal KC, van Eck-Smit BL, van den Bos C, Kremer LC, Tytgat GA, van Santen HM. Primary ovarian insufficiency in children after treatment with 131I-metaiodobenzylguanidine for neuroblastoma: Report of the first two cases. J Clin Endocrinol Metab 2014;99: E112–E116. [DOI] [PubMed] [Google Scholar]
- 12.Matthay KK, Panina C, Huberty J, Price D, Glidden DV, Tang HR, Hawkins RA, Veatch J, Hasegawa B. Correlation of tumor and whole-body dosimetry with tumor response and toxicity in refractory neuroblastoma treated with (131)I-MIBG. J Nucl Med 2001;42:1713–1721. [PubMed] [Google Scholar]
- 13.DuBois SG, Chesler L, Groshen S, Hawkins R, Goodarzian F, Shimada H, Yanik G, Tagen M, Stewart C, Mosse YP, Maris JM, Tsao-Wei D, Marachelian A, Villablanca JG, Matthay KK. Phase I study of vincristine, irinotecan, and (1)(3)(1)I-metaiodobenzylguanidine for patients with relapsed or refractory neuroblastoma: A new approaches to neuroblastoma therapy trial. Clin Cancer Res 2012:18:2679–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DuBois SG, Allen S, Bent M, Hilton JF, Hollinger F, Hawkins R, Courtier J, Mosse YP, Matthay KK. Phase I/II study of (131)I-MIBG with vincristine and 5 days of irinotecan for advanced neuroblastoma. Br J Cancer 2015;112:644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DuBois SG, Groshen S, Park JR, Haas-Kogan DA, Yang X, Geier E, Chen E, Giacomini K, Weiss B, Cohn SL, Granger MM, Yanik GA, Hawkins R, Courtier J, Jackson H, Goodarzian F, Shimada H, Czarnecki S, Tsao-Wei D, Villablanca JG, Marachelian A, Matthay KK. Phase I study of vorinostat asaradiationsensitizerwith131I-metaiodobenzylguanidine(131I-MIBG)forpatientswithrelapsed or refractory neuroblastoma. Clin Cancer Res 2015;21:2715–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthay KK, Yanik G, Messina J, Quach A, Huberty J, Cheng SC, Veatch J, Goldsby R, Brophy P, Kersun LS, Hawkins RA, Maris JM. Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol 2007;25:1054–1060. [DOI] [PubMed] [Google Scholar]
- 17.Matthay KK, Tan JC, Villablanca JG, Yanik GA, Veatch J, Franc B, Twomey E, Horn B, Reynolds CP, Groshen S, Seeger RC, Maris JM. Phase I dose escalation of iodine-131-metaiodobenzylguanidine with myeloablative chemotherapy and autologous stem-cell transplantation in refractory neuroblastoma: A new approaches to neuroblastoma therapy consortium study. J Clin Oncol 2006;24:500–506. [DOI] [PubMed] [Google Scholar]
- 18.Matthay KK, Quach A, Huberty J, Franc BL, Hawkins RA, Jackson H, Groshen S, Shusterman S, Yanik G, Veatch J, Brophy P, Villablanca JG, Maris JM. Iodine-131-metaiodobenzylguanidine double infusion with autologous stem-cell rescue for neuroblastoma: A new approaches to neuroblastoma therapy phase I study. J Clin Oncol 2009;27:1020–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanik GA, Villablanca JG, Maris JM, Weiss B, Groshen S, Marachelian A, Park JR, Tsao-Wei D, Hawkins R, Shulkin BL, Jackson H, Goodarzian F, Shimada H, Courtier J, Hutchinson R, Haas-Koga D, Hasenauer CB, Czarnecki S, Katzenstein HM, Matthay KK. 131I-metaiodobenzylguanidine with intensive chemotherapy and autologous stem cell transplantation for high-risk neuroblastoma. A new approaches to neuroblastoma therapy (NANT) phase II study. Biol Blood Marrow Transplant 2015;21:673–681. [DOI] [PubMed] [Google Scholar]
- 20.Matthay KK, Weiss B, Villablanca JG, Maris JM, Yanik GA, Dubois SG, Stubbs J, Groshen S, Tsao-Wei D, Hawkins R, Jackson H, Goodarzian F, Daldrup-Link H, Panigrahy A, Towbin A, Shimada H, Barrett J, Lafrance N, Babich J. Dose escalation study of no-carrier-added 131I-metaiodobenzylguanidine for relapsed or refractory neuroblastoma: New approaches to neuroblastoma therapy consortium trial. J Nucl Med 2012;53:1155–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loevinger R, Budinger TF, Watson EE, Committee SoNMMIRD MIRD primer for absorbed dose calculations. New York, NY: Society of Nuclear Medicine; 1988. [Google Scholar]
- 22.Cristy M, Eckerman K. Specific absorbed fractions of energy at various ages from internal photon sources: Ornl/tm-8381 v1-v7. Oak Ridge, TN: Oak Ridge National Laboratory; 1987. [Google Scholar]
- 23.Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, De Bernardi B, Evans AE, Favrot M, Hedborg F, Kaneko M, Kemshead J, Lampert F, Lee REJ, Look T, Pearson ADJ, Philip T, Roald B, Sawada T, Seeger RC, Tsuchida Y, Voute PA. Revisions of the international criteriaforneuroblastomadiagnosis,staging,andresponsetotreatment. J ClinOncol 1993;11:1466–1477. [DOI] [PubMed] [Google Scholar]
- 24.Ady N, Zucker JM, Asselain B, Edeline V, Bonnin F, Michon J, Gongora R, Manil L. A new 123I-MIBG whole body scan scoring method—Application to the prediction of the response of metastases to induction chemotherapy in stage IV neuroblastoma. Eur J Cancer 1995;31A:256–261. [DOI] [PubMed] [Google Scholar]
- 25.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–216. [DOI] [PubMed] [Google Scholar]
- 26.Matthay KK, Shulkin B, Ladenstein R, Michon J, Giammarile F, Lewington V, Pearson AD, Cohn SL. Criteria for evaluation of disease extent by (123)I-metaiodobenzylguanidine scans in neuroblastoma: A report for the international neuroblastoma risk group (inrg) task force. Br J Cancer 2010:102:1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beierwaltes WH. Treatment of neuroblastoma with 131I-MIBG: Dosimetric problems and perspectives. Med Pediatr Oncol 1987;15:188–191. [DOI] [PubMed] [Google Scholar]
- 28.Buckley SE, Chittenden SJ, Saran FH, Meller ST, Flux GD. Whole-body dosimetry for individualized treatment planning of 131I-MIBG radionuclide therapy for neuroblastoma. J Nucl Med 2009;50:1518–1524. [DOI] [PubMed] [Google Scholar]
- 29.Fielding SL, Flower MA, Ackery D, Kemshead JT, Lashford LS, Lewis I. Dosimetry of iodine 131 metaiodobenzylguanidine for treatment of resistant neuroblastoma: Results of a UK study. Eur J Nucl Med 1991;18:308–316. [DOI] [PubMed] [Google Scholar]
- 30.Lashford LS, Lewis IJ, Fielding SL, Flower MA, Meller S, Kemshead JT, Ackery D. Phase I/II study of iodine 131 metaiodobenzylguanidine in chemoresistant neuroblastoma: A United Kingdom Children’s Cancer Study Group investigation. J Clin Oncol 1992;10:1889–1896. [DOI] [PubMed] [Google Scholar]
- 31.Minguez P, Flux G, Genolla J, Guayambuco S, Delgado A, Fombellida JC, Sjogreen Gleisner K. Dosimetric results in treatments of neuroblastoma and neuroendocrine tumors with (131)I-metaiodobenzylguanidine with implications for the activity to administer. Med Phys 2015;42:3969–3978. [DOI] [PubMed] [Google Scholar]
- 32.Sisson JC, Shapiro B, Hutchinson RJ, Carey JE, Zasadny KR, Zempel SA, Normolle DP. Predictors of toxicity in treating patients with neuroblastoma by radiolabeled metaiodobenzylguanidine. Eur J Nucl Med 1994;21:46–52. [DOI] [PubMed] [Google Scholar]
- 33.Gaze MN, Chang YC, Flux GD, Mairs RJ, Saran FH, Meller ST. Feasibility of dosimetrybased high-dose 131I-meta-iodobenzylguanidine with topotecan as a radiosensitizer in children with metastatic neuroblastoma. Cancer Biother Radiopharm 2005;20:195–199. [DOI] [PubMed] [Google Scholar]
- 34.Illidge TM, Bayne M, Brown NS, Chilton S, Cragg MS, Glennie MJ, Du Y, Lewington V, Smart J, Thom J, Zivanovic M, Johnson PW. Phase 1/2 study of fractionated (131)I-rituximab in low-grade b-cell lymphoma: The effect of prior rituximab dosing and tumor burden on subsequent radioimmunotherapy. Blood 2009;113:1412–1421. [DOI] [PubMed] [Google Scholar]
- 35.Sgouros G, Squeri S, Ballangrud AM, Kolbert KS, Teitcher JB, Panageas KS, Finn RD, Divgi CR, Larson SM, Zelenetz AD. Patient-specific, 3-dimensional dosimetry in non-Hodgkin’s lymphoma patients treated with 131i-anti-b1 antibody: Assessment of tumor dose-response. J Nucl Med 2003;44:260–268. [PubMed] [Google Scholar]
- 36.Yu B, Carrasquillo J, Milenic D, Chung Y, Perentesis P, Feuerestein I, Eggensperger D, Qi CF, Paik C, Reynolds J, Grem J, Curt G, Siler K, Schlom J, Allegra C. Phase I trial of iodine 131-labeled col1 in patients with gastrointestinal malignancies: Influence of serum carcinoembryonic antigen and tumor bulk on pharmacokinetics. J Clin Oncol 1996;14:1798–1809. [DOI] [PubMed] [Google Scholar]
- 37.Huang SY, Bolch WE, Lee C, Van Brocklin HF, Pampaloni MH, Hawkins RA, Sznewajs A, DuBois SG, Matthay KK, Seo Y. Patient-specific dosimetry using pretherapy [(1)(2)(4)I]miodobenzylguanidine ([(1)(2)(4)I]mIBG) dynamic PET/CT imaging before [(1)(3)(1)I]mIBG targeted radionuclide therapy for neuroblastoma. Mol Imaging Biol 2015;17:284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou MJ, Doral MY, DuBois SG, Villablanca JG, Yanik GA, Mattay KK. Impact of response to prior therapy on outcome for refractory vs. relapsed neuroblastoma patients treated with 131i-metaiodobenzylguanidine (131i-MIBG). Eur J Cancer 2015;51(16):2465–2472. doi: 10.1016/j.ejca.2015.07.023. Epub 2015 Aug 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.