Abstract
Several reports of second malignant neoplasm (SMN) in patients with relapsed neuroblastoma after treatment with 131I-MIBG suggest the possibility of increased risk. Incidence of and risk factors for SMN after 131I-MIBG have not been defined.
This is a multi-institutional retrospective review of patients with neuroblastoma treated with 131I-MIBG therapy. A competing risk approach was used to calculate the cumulative incidence of SMN from time of first exposure to 131I-MIBG. A competing risk regression was used to identify potential risk factors for SMN.
The analytical cohort included 644 patients treated with 131I-MIBG. The cumulative incidence of SMN was 7.6% (95% confidence interval [CI], 4.4e13.0%) and 14.3% (95% CI, 8.3−23.9%) at 5 and 10 years from first 131I-MIBG, respectively. No increase in SMN risk was found with increased number of 131I-MIBG treatments or higher cumulative activity per kilogram of 131I-MIBG received (p = 0.72 and p = 0.84, respectively). Thirteen of the 19 reported SMN were haematologic. In a multivariate analysis controlling for variables with p < 0.1 (stage, age at first 131I-MIBG, bone disease, disease status at time of first 131I-MIBG), patients with relapsed/progressive disease had significantly lower risk of SMN (subdistribution hazard ratio 0.3, 95% CI, 0.1−0.8, p = 0.023) compared to patients with persistent/refractory neuroblastoma.
The cumulative risk of SMN after 131I-MIBG therapy for patients with relapsed or refractory neuroblastoma is similar to the greatest published incidence for high-risk neuroblastoma after myeloablative therapy, with no dose-dependent increase. As the number of patients treated and length of follow-up time increase, it will be important to reassess this risk.
Keywords: Neuroblastoma, Paediatrics, Cancer, MIBG, I-metaiodobenzylguanidine, SMN, Second malignancy, Chemotherapy, Solid tumour, Survivorship
1. Introduction
Neuroblastoma, an embryonal tumour of the peripheral sympathetic nervous system, is the most common extracranial solid malignancy in children, with approximately 700 new cases diagnosed in the United States each year. Nearly half of patients have metastatic disease at presentation, and long-term survival with high-risk disease is less than 50% [1]. Treatment for neuroblastoma depends on extent of disease, age at presentation, and tumour biology. For example, surgical resection is often curative for tumours that are small and localised with favourable biology [2]. Patients with high-risk disease require multimodality therapy, including intensive chemotherapy, myeloablative therapy with autologous stem cell transplantation, local radiation, and treatment for minimal residual disease with immunotherapy and differentiation therapy. Despite these intensive measures that have improved event-free survival (EFS), outcomes remain unsatisfactory for children with high-risk disease [1,3,4].
Metaiodobenzylguanidine (MIBG) is a guanethidine derivative that, when labelled with iodine-123, has high specificity and sensitivity as an imaging tool for detection of primary and metastatic neuroblastoma [5]. Clinical trials of high-dose 131I-MIBG have utilised this agent as a radiopharmaceutical and have shown response rates of 30−40%, with apparent prolongation of survival in patients with relapsed neuroblastoma [6–8]. More recently, pilot studies are ongoing incorporating this radiopharmaceutical into up-front treatment of high-risk patients [9].
131I-MIBG therapy is generally well tolerated. The most common acute toxicity is myelosuppression, which can be abrogated with autologous haematopoietic stem cell infusion [10,11]. However, reports of second malignant neoplasm (SMN) in patients treated with 131I-MIBG suggest that this therapy may be associated with increased risk of secondary malignancy, particularly myelodysplasia and leukaemia [12,13]. It has been postulated that the increased risk for malignancy results from bystander irradiation to the bone marrow that is not high enough to be lethal to stem cells, increasing the risk for leukaemia [12]. The risk of secondary malignancy is not unique to 131I-MIBG, as the reported crude incidence of secondary malignancy in all patients with neuroblastoma is between 0.5% and 6% [14–23]. The cumulative incidence in high-risk patients has been estimated to be as great as 16.5% at 10 years [24], without evidence of plateau. High dose alkylating agents, topoisomerase II inhibitors and platinum-based drugs, have been shown to increase the risk of myelodysplastic syndrome and treatment-related acute myelogenous leukaemia [25–27]. Moreover, children with high-risk neuroblastoma typically receive external beam radiation to the primary tumour bed. Survivors of childhood cancers that were treated with radiation therapy have been shown to have an increased risk of SMN [28,29].
The incidence of and risk factors for SMN after 131I-MIBG are not well understood. The primary aim of this study was to determine the incidence, characteristics, and predisposing factors of secondary malignancy in a large, multi-institutional cohort of patients with neuroblastoma treated with 131I-MIBG. By understanding SMN risk from 131I-MIBG, we hope to guide therapeutic decisions in this vulnerable patient population.
2. Patients and methods
2.1. Study population
We conducted a multi-institutional retrospective review of patients with neuroblastoma treated with 131I-MIBG therapy at four institutions between 1st March 1984 and 1st March 2014. Study participants were identified from neuroblastoma 131I-MIBG databases at participating institutions (University of California San Francisco, Children’s Hospital of Philadelphia, University of Michigan, and Cincinnati Children’s Hospital). Medical record abstraction was used to augment information missing from databases. To ensure most recent follow-up, we contacted referring institutions as allowed by study consents signed by families upon original 131I-MIBG trial enrolment. Institutional review board approval was obtained from all participating sites to allow transfer of deidentified patient data for study use.
2.2. Variables
Patient data obtained included age and stage at diagnosis, MYCN gene amplification status, and known history of other cancer predisposition syndrome. We classified treatment prior to 131I-MIBG by the number of prior chemotherapy regimens, prior radiation treatment, and prior myeloablative therapy with haematopoietic cell transplantation. Data collected regarding 131I-MIBG therapy were number of treatments, age at first 131I-MIBG treatment, time from neuroblastoma diagnosis to first 131I-MIBG treatment, sites of disease at 131I-MIBG therapy, disease status (refractory/persistent or relapsed) at 131I-MIBG therapy, use of stem cells or bone marrow support after 131I-MIBG, cumulative 131I-MIBG dose per kilogram, and cumulative radiation dose to the whole body.
SMN data collected included time from neuroblastoma diagnosis to SMN diagnosis, time from first 131I-MIBG therapy to SMN diagnosis, bone marrow cytogenetics or fluorescence in situ hybridisation for haematopoietic malignancy, molecular analysis for solid tumours, treatment of SMN, length of follow-up, time from SMN diagnosis to death, and cause of death, if applicable.
2.3. Statistical analysis
We used a competing risk approach to calculate the cumulative incidence of SMN from time of first exposure to 131I-MIBG. Death prior to development of SMN was considered a competing risk.
To identify potential risk factors and outcomes of secondary malignancy, we used competing risk regression by the method of Fine and Gray to model the risk of SMN according to a range of potential clinical covariates, including dose per kilogram of 131I-MIBG, age at time of treatment, and total body radiation exposure [30]. Similar to Cox survival models, the Fine−Gray approach provides estimates of the associations of risk factors with time to SMN, subhazard ratios (SHR), that accommodate the competing risk of death. Outcomes and treatment following diagnosis of SMN are also presented descriptively. All statistical analyses were performed using Stata, version 13 (Stata, College Station, TX).
3. Results
3.1. Patient characteristics
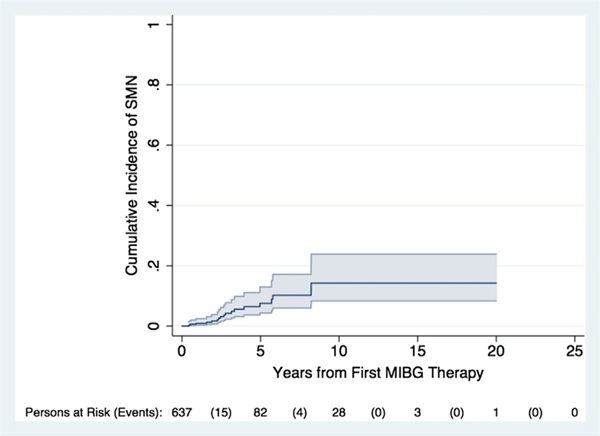
From November 1984 to March 2014, 644 patients with neuroblastoma were treated with 131I-MIBG (Table 1) at the four participating institutions. Median age at initial neuroblastoma diagnosis was 4.4 years (range, 0.5−37.8). The majority of patients (565/644, 87.7%) had stage 4 disease at diagnosis. Median follow-up time after first 131I-MIBG for surviving patients was 3.6 years (range, 0.5−20 years). Nineteen of the 644 patients were diagnosed with second malignancies after 131I-MIBG therapy. The cumulative incidence of SMN was 7.6% at 5 years (95% confidence interval [CI], 4.4−13.0%) and 14.3% at 10 years (95% CI, 8.4−23.9%) (Fig. 1).
Table 1.
Clinical and biologic characteristics of patients treated with 131I-MIBG with and without second malignant.
| Categorical variables | Alive patients with no SMN (n = 152) |
Dead patients with no SMN (n = 473) |
Patients with SMN (n = 19) |
|||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Male | 96/152 | 63.2 | 280/473 | 59.2 | 10/19 | 52.6 |
| Other than stage 4 at diagnosis | 10/152 | 6.6 | 64/473 | 13.5 | 5/19 | 26.3 |
| MYCN amplified | 25/116 | 21.6 | 140/360 | 38.9 | 3/12 | 25.0 |
| ASCT prior to MIBG | 55/151 | 36.4 | 309/470 | 65.7 | 12/19 | 63.2 |
| External beam radiation prior to MIBG | 59/145 | 40.7 | 328/440 | 74.6 | 16/19 | 84.2 |
| Bone marrow disease at initial MIBG therapy | 88/142 | 62.0 | 252/438 | 57.5 | 8/18 | 44.4 |
| Bone disease at initial MIBG therapy | 142/152 | 93.4 | 417/472 | 88.5 | 14/19 | 73.7 |
| Soft tissue disease at initial MIBG therapy rowheadDisease status at first MIBG therapy | 95/138 | 68.8 | 299/459 | 65.1 | 16/19 | 84.2 |
| Refractory/persistent | 101/145 | 70.0 | 105/385 | 27.3 | 10/16 | 62.5 |
| Relapsed/progressive rowheadNumber of MIBG treatments | 45/145 | 31.0 | 280/385 | 72.7 | 6/16 | 37.5 |
| 1 | 95/152 | 62.5 | 279/473 | 59.0 | 12/19 | 63.2 |
| 2 | 50/152 | 32.9 | 153/473 | 32.3 | 6/19 | 31.6 |
| 3 | 5/152 | 3.3 | 31/473 | 6.6 | 0/19 | 0.0 |
| ≥4 | 2/152 | 1.3 | 10/473 | 2.1 | 1/19 | 5.3 |
| Use of stem cell/bone marrow support after MIBG therapy | 98/126 | 77.8 | 234/390 | 60.0 | 8/18 | 44.4 |
| Death from progressive neuroblastoma | – | – | 443/463 | 95.7 | 3/19 | 15.8 |
| Continuous variables | N | Median (range) | N | Median (range) | N | Median (range) |
| Median age at diagnosis in years (range) | 151 | 4.4 (0.5–37.8) | 473 | 4.3 (0.2–50.8) | 19 | 5.8 (0.5–18.3) |
| Median months from ASCT to MIBG (range) | 53 | 11.5 (2.1–162.7) | 292 | 23.7 (1.3–224.7) | 19 | 23.6 (9.9–56.0) |
| Number of prior chemotherapy regimens (range) | 151 | 2.0 (1–9) | 469 | 3.0 (1–13) | 18 | 3.0 (1–5) |
| Median age in years at 1st MIBG therapy (range) | 152 | 6.1 (1.4–38.5) | 473 | 6.8 (1.3–51.2) | 19 | 8.1 (3.6–19.7) |
| Median months from diagnosis to 1st MIBG therapy (range) | 151 | 11.0 (3.4–192.9) | 473 | 26.4 (4.0–229.6) | 19 | 30.2 (6.5–87.1) |
| Median cumulative MIBG dose in mCi/kg (range) | 151 | 18.0 (8.0–96.0) | 472 | 18.2 (2.0–90.0) | 19 | 18.0 (9.6–50.5) |
| Median cumulative whole body radiation dose(range) | 64 | 271.0 (78.1–860.0) | 164 | 254.3 (79.0–681.6) | 12 | 267.9 (96.8–699.0) |
| Median months from first MIBG to last follow-up or death (range) | 150 | 40.8 (0.1–240) | 468 | 9.7 (0.5–143.8) | 19 | 39.7 (5.6–174.2) |
Neoplasm (N = 644).
131I-MIBG, iodine-131 metaiodobenzylguanidine; MYCN, V-Myc avian myelocytomatosis viral oncogene neuroblastoma derived homolog; ASCT, autologous stem cell transplant; mCi, millicurie; SMN, second malignant neoplasm.
Fig. 1.
Cumulative incidence of SMN in patients treated with 131I-MIBG therapy. SMN, second malignant neoplasm; MIBG, metaiodobenzylguanidine.
3.2. Characteristics of second malignancies
The characteristics of the 19 patients diagnosed with second malignancies are shown in Table 2. Thirteen patients were diagnosed with haematologic malignancies, including acute myelogenous leukaemia (n = 7) and acute lymphoblastic leukaemia (n = 2) (Table 3). The remaining 4 patients had myelodysplastic syndrome. Six patients were diagnosed with solid tumours, including osteosarcoma, papillary thyroid carcinoma, peritoneal mesothelioma and an inflammatory myofibroblastic tumour. One patient developed two distinct second malignancies, an undifferentiated sarcoma of the cranium and an inflammatory myofibroblastic pseudotumour of the lung (without an identifiable ALK aberration). Two patients developed benign bone tumours after 131I-MIBG, but were not included in SMN analyses. Median time from first 131I-MIBG therapy to SMN diagnosis was 32 months (range, 5.3−108.4 months) and from diagnosis of neuroblastoma was 68.5 months (range, 45.1−131.2 months). Seven of the SMN were associated with aberrations in chromosome 5 or 7, typical of myelodysplastic syndrome (MDS)/acute myelogenous leukaemia (AML) after alkylator therapy or radiation.
Table 2.
Characteristics of 19 patients with second malignancies after 131I-MIBG therapy.
| ID | Sex | Stage at NB diagnosis | Age at NB diagnosis (years) | ASCT prior to MIBG | EBRT prior to MIBG | # Chemo regimens | MIBG protocol | Total cumulative MIBG (mCi/kg) |
|---|---|---|---|---|---|---|---|---|
| Haematologic malignancies | ||||||||
| 1 | M | 4 | 3.5 | Y | N | 3 | MIBG only | 10.5 |
| 2 | F | 4 | 18.3 | Y | Y | 2 | MIBG with vincristine and irinotecan | 15 |
| 3 | M | 4 | 5.1 | Y | Y | 5 | MIBG + vorinostat, MIBG irinotecan | 36 |
| 4 | F | 4S | 0.5 | N | Y | 4 | MIBG only | 36 |
| 5 | F | 4 | 16.8 | Y | Y | 2 | MIBG only | 12.44 |
| 6 | F | 4 | 3.6 | N | Y | 2 | MIBG only | 17.7 |
| 7 | M | 4 | 1.4 | N | Y | 5 | MIBG only | 18 |
| 8 | M | 3 | 11.7 | Y | Y | 3 | MIBG only | 36 |
| 9 | M | 4 | 3.2 | Y | Y | 2 | No carrier added MIBG, MIBG only | 36 |
| 10 | M | 4 | 7.3 | Y | Y | 3 | MIBG only | 36 |
| 11 | M | 3 | 8.6 | N | Y | 5 | MIBG only | 18.8 |
| 12 | M | 4 | 13.6 | N | Y | 1 | MIBG only | 14.4 |
| 13 | F | 3 | 4.3 | Y | Y | 2 | MIBG only | 18 |
| Solid malignancies | ||||||||
| 14a | M | 4 | 5.8 | Y | Y, TBI | Unknown | MIBG only | 19 |
| 14b | M | 4 | 5.8 | Y | Y, TBI | Unknown | MIBG only | 19 |
| 15 | F | 4 | 0.7 | N | N | 2 | MIBG + CEM ASCT | 14.7 |
| 16 | M | 3 | 13.4 | Y | Y | 4 | MIBG only | 9.6 |
| 17 | F | 4 | 12.7 | N | Y | 2 | MIBG only | 50.5 |
| 18 | F | 4 | 2.8 | Y | Y | 3 | MIBG only | 36 |
| 19 | F | 4 | 1.0 | Y | Y | 4 | MIBG only | 18 |
MIBG, metaiodobenzylguanidine; NB, neuroblastoma; ASCT, autologous stem cell transplant; EBRT, external beam radiation therapy; chemo, chemotherapy; mCi, millicurie; kg, kilogram; TBI, total body irradiation; CEM, carboplatin, etoposide and melphalan.
Table 3.
SMN characteristics of 19 patients with second malignancies after 131I-MIBG therapy.
| ID | SMN diagnosis | SMN biology | SMN treatment | Time from NB diagnosis to SMN (months) | Time from MIBG to SMN (months) | Follow-up time from SMN dx (months) | Cause of death |
|---|---|---|---|---|---|---|---|
| Haematologic malignancies | |||||||
| 1 | ALL, pre-B | 46 XY | TPOG-ALL protocol | 45.1 | 22.6 | 13.9 | MDR sepsis 2/2 treatment for all recurrence |
| 2 | MDS/AML | Monosomy 7 | Umbilical cord blood transplant, 5-azacitidine (for relapse) | 44.6 | 27.0 | 8.0 | NB/AML |
| 3 | MDS/AML | Monosomy 2 | None | 64.0 | 32.0 | 3.0 | AML |
| 4 | AML | Monosomy 7; additional material in long arms of chromosomes 2 and 4 | MTX, Ara-C, HC | 97.8 | 10.7 | 4.0 | AML |
| 5 | AML | Deletion in chromosome 20; t(9; 11) | Hydroxyurea/allopurinol, Ara-C/VP-16, Mylotarg, cyclosporine, Ara-C, fludarabine, clofarabine | 65.8 | 33.1 | 6.6 | AML |
| 6 | T cell ALL | Unknown | None | 52.8 | 40.0 | 0.4 | ALL |
| 7 | AML | Unknown | None | 71.5 | 5.5 | 0.0 | AML/ARDS |
| 8 | AML | Monosomy 5q- | 5-azacytidine | 98.6 | 68.4 | 9.5 | AML |
| 9 | MDS | Trisomy 1q and 7q- t(1; 7) along with subclone containing trisomy 8 | Matched sibHSCT conditioned w/busulfan and fludarabine | 70.6 | 47.4 | 18.4+ | − |
| 10 | MDS | Monosomy 7 | 5-azacytidine | 95.9 | 37.7 | 4.4 | MDS |
| 11 | MDS | Trisomy 11 and der(12p) and monosomy 13. | Unknown | 43.1 | 6.6 | 4.6 | MDS/NB |
| 12 | MDS, AML | Monosomy 5,7q-, Y- | Allogenic HSCT from brother with melphalan, cytoxan, cyclosporin A | 25.3 | 18.8 | 4.6 | ARDS 2/2 HSCT/GVHD |
| 13 | MDS | Monosomy 7 | None | 63.0 | 28.0 | 4.0 | MDS, NB |
| Solid malignancies | |||||||
| 14a | Undifferentiated sarcoma, cranium | Not done | Resection, doxorubicin and isofosfamide, EBRT | 128.9 | 108.4 | 65.8+ | − |
| 14b | Inflammatory myofibroblastic pseudotumour, lung | ALK1 negative by IHC | Resection | 119.1 | 98.6 | 75.6+ | − |
| 15 | Osteosarcoma, R humerus | Stage 2A | Doxorubicin, cisplatin, high dose MTX | 68 | 57.1 | 53.3 | NB |
| 16 | Papillary thyroid carcinoma | Not done | Resection | 131.2 | 69.3 | 33.0+ | − |
| 17 | Peritoneal mesothelioma, pelvis | Translocation 13p11.2 | Unknown | 37.7 | 19.4 | 49.2 | NB |
| 18 | Inflammatory myofibroblastic tumour | ALK1+ by IHC | Unknown | 69.0 | 5.3 | 5.3 | NB |
| 19 | Papillary thyroid carcinoma | Not done | Total thyroidectomy and i131 therapy | 87.4 | 59.4 | 9.6+ | − |
SMN, second malignant neoplasm; MIBG, metaiodobenzylguanidine; NB, neuroblastoma; ALL, acute lymphoblastic leukaemia; TPOG, Turkish paediatric oncology group; MDR, multidrug resistant, MDS, myelodysplastic syndrome; AML, acute myelogenous leukaemia; MTX, methotrexate; Ara-C, cytosine arabinoside; HC, hydrocortisone; VP-16, Etoposide; ARDS, acute respiratory distress syndrome; HSCT, haematopoietic stem cell transplant; GVHD, graft versus host disease; EBRT, external beam radiation therapy; IHC, immunohistochemistry.
Twelve patients (63.0%) died of SMN or complications of SMN treatment. The remaining three died of progressive neuroblastoma. Of the 4 patients with SMN still alive at the time of data collection, median follow-up time was 34.2 months (range, 9.6−75.6).
3.3. Predictors of SMN
Few statistically significant differences in presentation were noted between patients who developed a SMN and those who did not (Table 4). There were no differences in age at diagnosis of neuroblastoma. There was no statistically significant increase in SMN risk in patients who received autologous stem cell transplant or radiotherapy prior to 131I-MIBG therapy with those who did not. Increased number of chemotherapy regimens prior to 131I-MIBG treatment was not associated with an increased risk of SMN. Increased cumulative 131I-MIBG dose per kilogram and one versus multiple 131I-MIBG treatments were not associated with an increased risk of SMN.
Table 4.
Competing risk regression results comparing patients with and without SMN.
| Subhazard ratio | 95% confidence interval | P value | |
|---|---|---|---|
| Male | 0.70 | 0.29−1.73 | 0.444 |
| Other than stage 4 at diagnosis | 0.43 | 0.15−1.17 | 0.099 |
| MYCN amplified | 0.59 | 0.16−2.17 | 0.158 |
| Age at diagnosis in years | 1.03 | 0.99−1.07 | 0.108 |
| ASCT prior to MIBG | 1.14 | 0.45−2.89 | 0.777 |
| Months from ASCT to MIBG | 1.01 | 0.99−1.02 | 0.505 |
| Number of prior chemotherapy regimens | 0.91 | 0.75−1.11 | 0.347 |
| History of external beam radiation prior to MIBG | 2.42 | 0.71−8.29 | 0.160 |
| Age in years at 1st MIBG therapy | 1.03 | 0.99−1.07 | 0.057 |
| Months from diagnosis to 1st MIBG therapy | 1.00 | 0.99−1.01 | 0.558 |
| Bone marrow disease at initial MIBG therapy | 0.60 | 0.24−1.51 | 0.236 |
| Bone disease at initial MIBG therapy | 0.36 | 0.13−0.99 | 0.049* |
| Soft tissue disease at initial MIBG therapy | 2.67 | 0.78−9.13 | 0.118 |
| Relapsed/progressive disease at first MIBG therapy (versus refractory/persistent) | 0.40 | 0.15−1.06 | 0.065 |
| One MIBG Tx (versus >1) | 0.84 | 0.33−2.14 | 0.718 |
| Cumulative MIBG dose in mCi/kg | 0.99 | 0.96−1.03 | 0.842 |
| Cumulative whole body radiation dose in cGy | 1.00 | 0.99−1.01 | 0.409 |
| Use of stem cell/bone marrow support after MIBG therapy | 0.49 | 0.19−1.24 | 0.132 |
SMN, second malignant neoplasm; 131I-MIBG, iodine-131 metaiodobenzylguanidine; MYCN, V-Myc avian myelocytomatosis viral oncogene neuroblastoma derived homolog; ASCT, autologous stem cell transplant; mCi, millicurie.
Presence of bone disease at time of first 131I-MIBG therapy was associated with a decreased risk of SMN (SHR 0.36, p = 0.049, 95% CI, 0.13−0.99). No association between presence of bone marrow disease or soft tissue disease at time of first 131I-MIBG therapy was found (p = 0.236 and p = 0.118, respectively). Four variables had p < 0.1 (stage, age at first 131I-MIBG, bone disease, disease status at time of first 131I-MIBG) on univariate analysis. When controlling for these variables in a multivariate analysis, only disease status remained statistically significant, with relapsed/progressive disease associated with decreased risk of SMN (SHR 0.3, 95% CI, 0.1−0.8, p = 0.023) compared to persistent/refractory.
4. Discussion
This retrospective study examined the risk of second malignancy in 644 neuroblastoma patients treated with 131I-MIBG therapy and found a cumulative 10-year incidence of 14.3%. Nineteen patients in our cohort were diagnosed with second malignancies, with the most common being MDS and AML. No dose-dependent increases in risk was found in patients who received more treatments or higher doses per kilogram of 131I-MIBG. The only risk factor in multivariate analysis for SMN was having primary refractory rather than relapsed disease.
Increased SMN risk for high-risk neuroblastoma patients has been well described in the literature. Appelbaum et al. found in a SEER analysis the cumulative incidence of SMN at 30 years for high-risk patients (n = 933) to be 10.5%, with an estimate of only <5% at 10 years, lower than our incidence in relapsed/refractory neuroblastoma patients [31]. Another study of long-term survivors found the crude incidence of SMN to be 6% in high-risk patients [22]. It is likely that in these prior studies, the intensity of treatment and risk stratification may have differed, partially accounting for the difference in incidence. Additionally, all of our population reported here consisted of heavily pretreated patients with multiple regimens, which may have further contributed to the SMN risk. In a study looking specifically at SMN after autologous stem cell transplant for high-risk neuroblastoma, the 5- and 10-year cumulative incidence rates of 7.2% and 16.5%, respectively, were similar to our observations [24]. Six of the 10 patients with SMN developed haematologic malignancies, similar to our population. Unfortunately, some patients in these study populations may have received 131I-MIBG therapy, making its contribution to the results difficult to tease out. However, it is striking that in our heavily pretreated relapsed/refractory population, this cohort treated with 131I-MIBG, and this cohort does not appear to have a higher cumulative risk of SMN than the high-risk post-transplant population overall.
When controlling for patient variables with an association with second malignancy (defined as p < 0.1), refractory disease status after completing induction chemotherapy was significantly associated with SMN, even when controlling for longer survival in this patient population. This finding warrants further exploration. It is possible that refractory/persistent disease status is a surrogate for an underlying genetic predisposition to SMN or that these patients received more aggressive treatment regimens, which increased their risk of SMN. Unlike known increases in SMN risk from treatments such as total body radiation and alkylating agents, the number of 131I-MIBG treatments and cumulative dose per kilogram were not associated with increasing risk of SMN in our patient population [24,32]. It is unexpected that bone disease was associated with a decreased risk of SMN, and this may reflect different biological characteristics.
Our study has many strengths. This is one of the largest sample sizes in a study of this kind, with highly annotated combined data from four large neuroblastoma referral centres in the United States with treatments dating back to 1984. Over 85% of living patients have updated follow-up within the last year, with the longest follow-up after 131I-MIBG therapy being 20 years.
There were a few limitations to this study. Despite being the largest study of its kind, the small number SMN cases (only 19 overall) limited our ability to identify variables with only a modest effect on risk of SMN. Second, although the study encompassed three decades, the number of patients surviving more than 15 years was very small, and the risk of SMN may continue to increase. Furthermore, the inclusion of patients from 131I-MIBG institutions who were often referred from other centres made it impossible to quantify the doses of cytotoxic chemotherapy received, nor to be certain that all medical records obtained detailed family history of cancer. This study was also limited by missing whole body dosimetry information on 62.7% of patients. It is reassuring that available surrogates for this information such as 131I-MIBG dose per kilogram and number of 131I-MIBG treatments did not show an association with 131I-MIBG therapy and risk of SMN [33].
5. Conclusion
The risk of SMN after 131I-MIBG therapy for patients with relapsed or refractory neuroblastoma is similar to the greatest published incidence in high-risk neuroblastoma. We found no dose-dependent increase in SMN risk in patients who received more 131I-MIBG treatments or had larger cumulative doses of 131I-MIBG. In the context of the current study, we were not able to detect an increased risk of SMN above and beyond the known risk associated with other therapies used in the treatment of patients with high-risk neuroblastoma. As the number of patients treated with 131I-MIBG earlier after diagnosis and length of follow-up time from 131I-MIBG therapy increase, it will be important to reassess this risk.
Acknowledgements
The authors would like to acknowledge Megan Trieu, Scarlett Czarnecki, and Elizabeth Pons for their assistance in data collection for this manuscript.
Funding
This work was supported by The Thrasher Foundation Early Career Award (KH, provided salary support, statistical analysis consultation, travel money, and funded data collection), The American Academy of Pediatrics Resident Research Award (KH, provided salary support, travel money, and funded data collection), University of San Francisco Clinical & Translational Science Institute (KH, provided salary support, travel money, and funded data collection), Alex Lemonade MIBG Infrastructure grant (KKM, provided salary support, funded data collection), Dougherty fund (KKM, funded data collection), Mildred V. Strouss Chair (KKM, funded data collection), National Institutes of Health (Grant PO1 81403 to KKM, funded data collection), Connor’s Heroes Grant (KKM, funded data collection).
Footnotes
Conflict of interest statement
None declared.
References
- [1].Park JR, Bagatell R, London WB, Maris JM, Cohn SL, Mattay KM, et al. Children’s Oncology Group’s 2013 blueprint for research: neuroblastoma. Pediatr Blood Cancer 2013;60(6):985–393. [DOI] [PubMed] [Google Scholar]
- [2].Strother DR, London WB, Schmidt ML, Brodeur GM, Shimada H, Thorner P, et al. Outcome after surgery alone or with restricted use of chemotherapy for patients with low-risk neuroblastoma: results of Children’s Oncology Group study P9641. J Clin Oncol 2012;30(15):1842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010;363(14):1324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kreissman SG, Seeger RC, Matthay KK, London WB, Sposto R, Grupp SA, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol 2013;14(10):999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Matthay KK, Shulkin B, Ladenstein R, Michon J, Giammarile F, Lewington V, et al. Criteria for evaluation of disease extent by (123)I-metaiodobenzylguanidine scans in neuroblastoma: a report for the International Neuroblastoma Risk Group (INRG) Task Force. Br J Cancer 2010;102(9):1319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Matthay KK, Yanik G, Messina J, Quach A, Huberty J, Cheng SC, et al. Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol 2007;25(9):1054–60. [DOI] [PubMed] [Google Scholar]
- [7].DuBois SG, Matthay KK. 131I-Metaiodobenzylguanidine therapy in children with advanced neuroblastoma. Q J Nucl Med Mol Imaging 2013;57(1):53–65. [PubMed] [Google Scholar]
- [8].Wilson JS, Gains JE, Moroz V, Wheatley K, Gaze MN. A systematic review of 131I-meta iodobenzylguanidine molecular radiotherapy for neuroblastoma. Eur J Cancer 2014;50(4):801–15. [DOI] [PubMed] [Google Scholar]
- [9].de Kraker J, Hoefnagel KA, Verschuur AC, van Eck B, van Santen HM, Caron HN. Iodine-131-metaiodobenzylguanidine as initial induction therapy in stage 4 neuroblastoma patients over 1 year of age. Eur J Cancer 2008;44:551–6. [DOI] [PubMed] [Google Scholar]
- [10].Dubois SG, Messina J, Maris JM, Huberty J, Glidden DV, Veatch J, et al. Hematologic toxicity of high-dose iodine-131-metaiodobenzylguanidine therapy for advanced neuroblastoma. J Clin Oncol 2004;22(12):2452–60. [DOI] [PubMed] [Google Scholar]
- [11].Matthay KK, DeSantes K, Hasegawa B, Huberty J, Hattner RS, Ablin A, et al. Phase I dose escalation of 131I-metaiodobenzylguanidine with autologous bone marrow support in refractory neuroblastoma. J Clin Oncol 1998;16:229–36. [DOI] [PubMed] [Google Scholar]
- [12].Garaventa A, Gambini C, Villavecchia G, Di Cataldo A, Bertolazzi L, Pizzitola MR, et al. Second malignancies in children with neuroblastoma after combined treatment with 131I-metaiodobenzylguanidine. Cancer 2003;97(5):1332–8. [DOI] [PubMed] [Google Scholar]
- [13].Weiss B, Vora A, Huberty J, Hawkins RA, Matthay KK. Secondary myelodysplastic syndrome and leukemia following 131I-metaiodobenzylguanidine therapy for relapsed neuroblastoma. J Pediatr Hematol Oncol 2003;25(7):543–7. [DOI] [PubMed] [Google Scholar]
- [14].Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis- retinoic acid. Children’s Cancer Group. N Engl J Med 1999;341(16):1165–73. [DOI] [PubMed] [Google Scholar]
- [15].Baker DL, Schmidt ML, Cohn SL, Maris JM, London WB, Buxton A, et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma.NEngl J Med 2010;363(14):1313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol 2005;6:649–58. [DOI] [PubMed] [Google Scholar]
- [17].Cohen LE, Gordon JH, Popovsky EY, Gunawardene S, Duffey-Lind E, Lehmann LE, et al. Late effects in children treated with intensive multimodal therapy for high-risk neuroblastoma: high incidence of endocrine and growth problems. Bone Marrow Transplant 2014;49(4):502–8. [DOI] [PubMed] [Google Scholar]
- [18].Laverdiere C, Cheung NK, Kushner BH, Kramer K, Modak S, LaQuaglia MP, et al. Long-term complications in survivors of advanced stage neuroblastoma. Pediatr Blood Cancer 2005;45(3):324–32. [DOI] [PubMed] [Google Scholar]
- [19].Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a Children’s Oncology Group study. J Clin Oncol 2009;27(7):1007–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Moreno L, Vaidya SJ, Pinkerton CR, Lewis IJ, Imeson J, Machin D, et al. Long-term follow-up of children with high-risk neuroblastoma: the ENSG5 trial experience. Pediatr Blood Cancer 2013;60(7):1135–40. [DOI] [PubMed] [Google Scholar]
- [21].Rubino C, Adjadj E, Guerin S, Guibout C, Shamsaldin A, Dondon MG, et al. Long-term risk of second malignant neoplasms after neuroblastoma in childhood: role of treatment. Int J Cancer 2003;107(5):791–6. [DOI] [PubMed] [Google Scholar]
- [22].Laverdiere C, Liu Q, Yasui Y, Nathan PC, Gurney JG, Stovall M, et al. Long-term outcomes in survivors of neuroblastoma: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst 2009;101(16):1131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Federico SM, Allewelt HB, Spunt SL, Hudson MM, Wu J, Billups CA, et al. Subsequent malignant neoplasms in pediatric patients initially diagnosed with neuroblastoma. J Pediatr Hematol Oncol 2015;37(1):e6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Martin A, Schneiderman J, Helenowski IB, Morgan E, Dilley K, Danner-Koptik K, et al. Secondary malignant neoplasms after high-dose chemotherapy and autologous stem cell rescue for high-risk neuroblastoma. Pediatr Blood Cancer 2014;61(8):1350–6. [DOI] [PubMed] [Google Scholar]
- [25].Sandoval C, Pui C, Bowman LC, Heaton D, Hurwitz CA, Raimondi SC, et al. Secondary acute myeloid leukemia in children previously treated with alkylating agents, intercalating topoisomerase II inhibitors, and irradiation. J Clin Oncol 1993;11(6):1039–45. [DOI] [PubMed] [Google Scholar]
- [26].Le Deley MC, Leblanc T, Shamsaldin A, Raquin MA, Lacour B, Sommelet D, et al. Risk of secondary leukemia after a solid tumor in childhood according to the dose of epipodophyllotoxins and anthracyclines: a case-control study by the Societe Francaise d’Oncologie Pediatrique. J Clin Oncol 2003;21(6):1074–81. [DOI] [PubMed] [Google Scholar]
- [27].Smith MA, Rubinstein L, Cazenave L, Ungerleider RS, Maurer HM, Heyn R, et al. Report of the Cancer Therapy Evaluation Program monitoring plan for secondary acute myeloid leukemia following treatment with epipodophyllotoxins. J Natl Cancer Inst 1993;85(7):554–8. [DOI] [PubMed] [Google Scholar]
- [28].Inskip PD, Robison LL, Stovall M, Smith SA, Hammond S, Mertens AC, et al. Radiation dose and breast cancer risk in the childhood cancer survivor study. J Clin Oncol 2009;27(24):3901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Henderson TO, Rajaraman P, Stovall M, Constine LS, Olive A, Smith SA, et al. Risk factors associated with secondary sarcomas in childhood cancer survivors: a report from the childhood cancer survivor study. Int J Radiat Oncol Biol Phys 2012;84(1):224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fine J, Gray R. A proportional hazards maodel for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- [31].Applebaum MA, Henderson TO, Lee SM, Pinto N, Volchenboum SL, Cohn SL. Second malignancies in patients with neuroblastoma: the effects of risk-based therapy. Pediatr Blood Cancer 2015;62(1):128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Flandin I, Hartmann O, Michon J, Pinkerton R, Coze C, Stephan JL, et al. Impact of TBI on late effects in children treated by megatherapy for Stage IV neuroblastoma. A study of the French Society of Pediatric oncology. Int J Radiat Oncol Biol Phys 2006;64(5):1424–31. [DOI] [PubMed] [Google Scholar]
- [33].Trieu M, DuBois SG, Pon E, Nardo L, Hawkins RA, Marachelian A, et al. Impact of whole-body radiation dose on response and toxicity in patients with neuroblastoma after therapy with (131) I-Metaiodobenzylguanidine (MIBG). Pediatr Blood Cancer 2016;63(3):436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]