ABSTRACT
Aim:
Dental diseases can be prevented by reducing early bacterial colonization in biofilm, a precursor to mature dental plaque. Most studies on dental disease pathogenesis focus on mature plaque and fail to address the impact of oral cleaning on biofilm formation. Here we used next-generation metagenomics to assess the effects of a new method of regular, simple biofilm disruption on the oral metagenome.
Materials and Methods:
This was a randomized, controlled study of 45 healthy children divided into three groups. Participants avoided oral cleaning for 3 days and then performed 10 days of oral cleaning either by: (1) brushing and tongue cleaning twice a day (BT) with toothpaste; (2) Gum and tooth rubbing with Index Finger Tongue cleaning and water Swishing (GIFTS) after each meal, snack, and drink; or (3) GIFTS twice a day with nano-charcoal and tongue cleaning (CT) (n = 15 per group). Saliva, plaque, and tongue scraping samples were collected on day 0 and 10 for quantitative polymerase chain reaction (qPCR) and next-generation metagenomics sequencing to analyze microbiome taxa differences between groups.
Results:
GIFTS more significantly reduced (P < 0.004) total bacteria in saliva than BT (P < 0.02). Metagenomics revealed a significant reduction in Firmicutes in GIFTS and CT tongue samples compared to BT samples. BT and CT saliva samples showed significantly more Streptococcus species than GIFTS saliva samples. In the plaque samples, GIFTS cleaning significantly reduced early colonizers, including Streptococcus, compared to the BT and CT methods.
Conclusion:
Here, we introduce the “frequent disruption of biofilm” concept for enhanced oral hygiene. GIFTS can be used to prevent early bacterial colonization of biofilm and plaque formation in both small children and adults. Frequent biofilm disturbance more effectively disrupts early bacterial colonization than twice oral cleaning, is nonabrasive, and is, therefore, a practical and straightforward complement to regular toothbrushing for improved oral hygiene and disease prevention.
KEYWORDS: Caries, charcoal, dental hygiene, Gum and tooth rubbing with Index Finger Tongue cleaning and water Swishing (GIFTS), swishing, tongue
INTRODUCTION
Dental biofilm contains diverse microflora, including bacteria, viruses, fungi, and protozoa.[1] These organisms colonize dental surfaces within a few hours of oral cleaning and subsequently interact with each other.[2,3,4] Bacteria initially attach to salivary molecules adsorbed to the tooth surface before multiplying and secreting polymers that provide a matrix or scaffold for biofilm development. Other bacteria and fungi then attach to adherent bacteria to further increase biofilm diversity and complexity.[5,6,7]
Dental caries is now understood to be caused by dysbiosis of multiple microorganisms in the oral microbiome rather than a single organism.[8,9,10] The first and predominant initial colonizers of oral biofilm are Streptococci (yellow complex) followed by Actinomyces species (green, blue, or purple).[11]Fusobacterium species (orange) aid complex dental plaque biofilm maturation by bridging other early and late colonizing bacteria (red) in the oral cavity.[12]
Undisturbed biofilms may promote the formation of calculus, demineralization, caries, gingival inflammation, and periodontal disease.[13] Gingivitis affects 50%–90% of the adult population, and 47% of US adults have periodontitis.[14] Therefore, frequent disruption of biofilms is essential in preventing plaque formation.
Many studies have shown that daily dental biofilm disruption by mechanical means (toothbrushing and interdental cleaning) prevents biofilm development and maturation.[15] Although mechanical brushing with toothpaste removes a significant number of bacteria, tongue cleaning further enhances the cleaning effects of brushing, suggesting that tongue cleaning is critical for reducing the bacterial load.[16,17,18] Many organisms on the tongue populate the saliva and then lodge on the tooth surfaces, especially when the flushing of saliva stops during sleep, so tongue cleaning is likely to be desirable in all oral cleaning methods.
Bedtime infant feeding without oral cleansing increases the chances of dental decay.[19] Therefore, after feeding the infant, their gums should be cleaned before bedtime by gently massaging the gum tissues to aid the removal of food particles from the oral cavity.[20] For children under 6 years, toothbrushing should be supervised by parents until the child can brush independently with excellent dexterity and cognition.[21] Moreover, tooth “aches” and injuries related to toothbrush use are common in adults and especially children.[22] The stiffness of the toothbrush affects abrasion,[23] and the application of greater force causes more abrasion. The brushing frequency and brushing technique have a more significant influence on cleaning success than material-oriented toothbrushing factors such as dentifrice abrasivity or bristle stiffness.[24] Therefore, for frequent cleaning, methods that cause minimal abrasion are ideal for oral hygiene, and practices to supplement regular toothbrushing would be highly desirable.[25]
Here we describe a novel oral-cleaning technique, which we term GIFTS (Gum and tooth rubbing with Index Finger and Tongue cleaning and water Swishing). The GIFTS method was initially designed as a control group in ongoing studies, where subjects were asked to use their index finger to reach and rub all parts of their mouth, including their gums and teeth, without a toothbrush, toothpaste, or tooth powder. We found the group that used GIFTS had significantly reduced bacterial counts compared to any of the other methods tested, including in two of the most aggressive dental damaging bacteria (DDB), Streptococcus mutans and Aggregatibacter actinomycetemcomitans.
Many pathogenic bacteria and Candida species adhere to plastic surfaces on brush heads, even after short exposure times.[26,27] They then remain in toothbrushes for days or even weeks after brushing.[28,29] As contaminated toothbrushes can reintroduce microorganisms into the oral cavity and promote transmission of oral disease and oral infection,[30,31] the GIFTS method might be expected to help overcome these problems.
In addition to toothbrushing, swishing water around the mouth after food and drink consumption and between meals could be a safe, economical, and comfortable, but often overlooked way to improve oral hygiene, especially in resource-poor settings.[19] Mechanical disruption of biofilm through regular oral irrigation with waterjets is an effective alternative to manual toothbrushing and dental floss for reducing bleeding and gingival inflammation.[32] However, dental waterjets are expensive and inconvenient for portable use. Nevertheless, vigorous water swishing using the movement of the lips, tongue, and cheeks can be beneficial to oral hygiene.[19] We hypothesized that a simple water swishing step could be added to GIFTS to improve the technique.
The purpose of this study was to examine and compare oral hygiene practices for reducing microbiota in oral biofilm. As most existing research has been conducted on subjects with mature dental plaque,[33] which may not address the impact of dental cleaning on biofilm formation, we examined de novo biofilm on the enamel of healthy children who refrained from oral prophylaxis for several days before practicing different oral cleaning methods. Immature biofilm was then analyzed for microbiome taxa changes.
MATERIALS AND METHODS
STUDY DESIGN AND ETHICAL APPROVAL
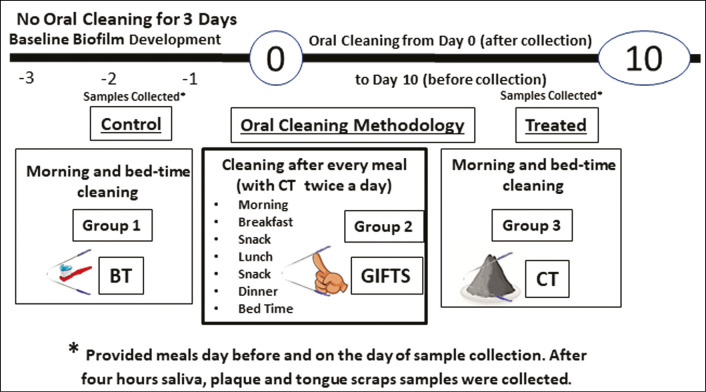
From 75 volunteers, dentists examined and selected 45 healthy subjects (10–12 years old). They were asked to randomly pick one of the three numbers in a box and were assigned into three equal groups (n = 15). Each group performed one of the three oral cleaning methods in a timed manner [Figure 1]. Each subject was provided with a kit containing sample collection tubes and cleaning materials, where appropriate, such as sodium fluoride–containing toothpaste or nano-charcoal.
Figure 1.
The study protocol. Forty-five healthy subjects (10–12 years old) were divided into three equal groups (n = 15) and avoided oral cleaning for 4 days. From the fifth day to the fifteenth day (10 days), each group performed one of the three oral cleaning methods (BT, GIFTS, CT) in a timed manner. Saliva, plaque, and tongue samples were collected from all the subjects for analysis
To build up biofilm, subjects were asked to avoid brushing for 100h (4 days). Then, on the first day (day 0) and the final day (day 10), saliva, plaque, and tongue samples were collected after breakfast [Figure 1]. Samples were collected in tubes prefilled with lysis buffer (FastID Foodchain ID, Fairfield, Iowa). On these days of oral sampling, the same meals and drinks were served to the subjects over the entire day to normalize dietary variations influencing oral microbiota.
Before beginning the study, each participant and participant’s parent signed an informed consent form for study participation, to provide saliva samples and to provide personal data as required for the study. The School SRC committee granted ethical approval, and all experiments were performed per relevant guidelines and regulations. Subjects and their parents were instructed on how to perform the institutional review board (IRB)–approved procedures, including how to use the activated charcoal.
ORAL CLEANING PROCEDURES
After breakfast and sample collection on day 1 to day 10 before the second sample collection, subjects performed one of the three oral cleaning methods:
Toothpaste brushing and tongue cleaning twice a day in the morning and at bedtime (BT): Subjects were instructed to brush with 100mg of sodium fluoride–containing toothpaste followed by tongue cleaning. Tongue cleaning was accomplished using a curved, stainless steel scraper to gently clean the tongue five times, followed by thorough rinsing of the oral cavity three times with water.
Cleaning of gums and teeth by rubbing with an index finger, tongue cleaning, and water swishing (GIFTS): Subjects were instructed to thoroughly rub their teeth and gums with their index finger, followed by tongue cleaning and water swishing. In addition to morning and bedtime GIFTS cleaning, subjects also performed GIFTS after every meal, snack, or drink (i.e., 6–8 times a day).
GIFTS method twice a day in the morning and at bedtime with nano-char cknowledgement coal (CT): Subjects were instructed to perform the GIFTS method, as aforementioned, using approximately 100mg activated charcoal powder twice a day in the morning and at bedtime.
DNA EXTRACTION AND REAL-TIME POLYMERASE CHAIN REACTION
All samples were homogenized with 0.1 mm zirconium beads (Research Products International, Mt. Prospect, Illinois) using a Mini-BeadBeater (BioSpec Products, Bartlesville, Oklahoma) for 30s at maximum speed. After homogenization, DNA extraction was performed using FastID Magnetic DNA extraction kit (Foodchain ID, Fairfield, Iowa). 200ng of purified DNA was used for quantitative real-time polymerase chain reaction (qPCR) and bacterial profile analysis.
To determine the quantity of specific DDB, qPCR was performed using universal bacterial primers and TaqMan probes[34] and A. actinomycetemcomitans and S. mutans primers and probes[35] with the Taqman Universal PCR Master Mix (Applied Biosystems). qPCR was performed using a BioRad CFX 96 thermocycler (BioRad Laboratories, Hercules, California) with cycle conditions and primer–probe concentrations, as previously described.[34,35] The ratio of DDB to universal bacteria concentrations was calculated using BioRad CFX 96 software.
METAGENOMIC ANALYSIS
A metagenomic sequencing library was prepared by amplification of the 16S rRNA variable regions 3 and 4 (V3-V4). Illumina MiSeq was used to sequence the V3-V4 amplicons from both ends.[36] Ninety random samples were used for sequencing. QIIME (v 1.9.1) and Greengenes databases were used to assign bacterial taxa.[37,38]
STATISTICAL ANALYSIS
All statistical analyses, including analysis of variance (ANOVA), Wilcoxon matched-pairs signed-rank test, Holm-Sidak’s multiple comparisons test, and Student’s t test was performed using GraphPad Prism (GraphPad Software, La Jolla, California). Error bars represent the standard error of the mean (SEM).
RESULTS
ALTERATIONS IN BACTERIAL LEVELS AFTER ORAL CLEANING
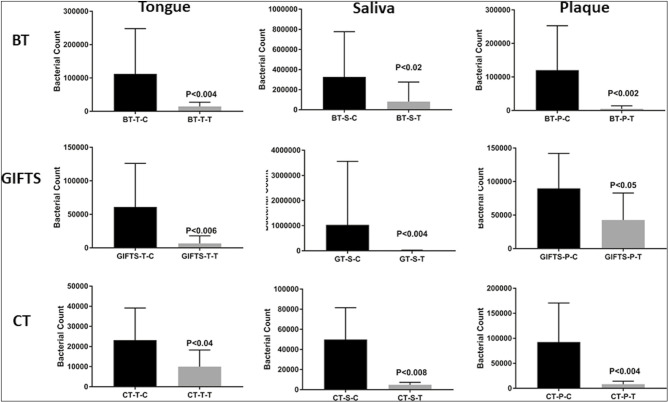
All three methods significantly reduced the total bacterial load in saliva, plaque, and tongue samples compared to no cleaning control [Figure 2]. The GIFTS method showed a more significant reduction (P < 0.004) than the BT (P < 0.02) method and the CT method (P < 0.04) in the saliva samples. In plaque, however, BT and CT reduced the total bacterial load significantly more than the GIFTS method. In the tongue samples, BT reduced the overall load more than the other two techniques. Comparing all the saliva samples from the three oral cleaning methods using Holm-Sidak’s multiple comparisons tests showed that the CT method caused a statistically significant change (P < 0.007) compared to the other two methods (P < 0.2). Multiple comparison testing of plaque samples from all three methods showed a similar significant change (P < 0.03), whereas in tongue samples, no difference was observed (P < 0.09).
Figure 2.
Reduction in bacterial levels after oral cleaning. BT = brushing followed by tongue cleaning, GIFTS = GIFTS method followed by tongue cleaning and mouth water swishing, CT = GIFT method using charcoal followed by tongue cleaning and mouth water swishing, Saliva (S), plaque (P), and tongue scraping (T) samples were collected and bacterial load was determined in comparison to no cleaning using qPCR analysis. SEM = standard error of the mean, ANOVA = analysis of variance. Total bacterial levels were significantly decreased by all of the three cleaning methods compared with that of the NC control. The error bars represent SEM values, and the P values (ANOVA) were calculated relative to the NC group
METAGENOMICS ANALYSIS OF MICROBIOME
Tongue, saliva, and plaque samples were subjected to sequence analysis to quantify and compare the relative abundance of bacteria after biofilm formation on 0 day and after 10 days of cleaning using the different methods. The bacterial species shown in Tables 1–3 are presented in the order of bacterial phyla complexes that lead from biofilm formation to maturation of the oral microbiome.[8,9,10] The first and predominant initial colonizers recognized as pathogenic are Streptococcus Firmicutes (yellow complex) followed by Actinomyces species (green, blue, or purple complex), Fusobacterium species (orange complex), and finally Bacteroides (red complex).[12]
Table 1.
Bacterial genera change after oral cleaning from day zero to ten: Statistically significant change in bacterial levels of the tongue samples of all the three oral-cleaning methods were calculated after 10 days of cleaning from day zero to ten. For each method, the level of significance is shown with an decrease in levels of bacteria (in bold), or increase in bacterial levels (not in bold). The P-values were calculated using Student t test. (BT – Brushing, followed by Tongue cleaning; GIFTS – Gum and teeth rubbing using Index Finger, followed by Tongue cleaning and water swishing; CT – GIFTS method using Charcoal, followed by Tongue cleaning). “g__” indicate OTUs only annotated to the level of Genus.
| Tongue | BT | GIFTS | CT |
|---|---|---|---|
| Firmicutes—Leuconostocaceae | 0.02 | ||
| Firmicutes—Clostridiales | |||
| Firmicutes—Clostridiales—Other | 0.03 | ||
| Firmicutes—g__ | 0.03 | ||
| Firmicutes—Blautia | 0.03 | ||
| Firmicutes—Oribacterium | 0.05 | ||
| Firmicutes—Peptococcus | 0.03 | ||
| Firmicutes—Filifactor | 0.004 | ||
| Firmicutes—Dialister | 0.02 | ||
| Firmicutes—Megasphaera | 0.01 | ||
| Firmicutes—Selenomonas | 0.03 | ||
| Firmicutes—Veillonella | 0.01 | ||
| Firmicutes—g__ | 0.04 | ||
| Firmicutes—Mogibacterium | 0.01 | 0.05 | |
| Firmicutes—Parvimonas | 0.05 | ||
| Actinobacteria—Actinomyces | 0.04 | ||
| Actinobacteria—Nesterenkonia | 0.04 | ||
| Actinobacteria—Rothia | 0.05 | ||
| Actinobacteria—Atopobium | 0.02 | ||
| Proteobacteria—Other | 0.05 | ||
| Proteobacteria—Lautropia | 0.03 | ||
| Proteobacteria—Neisseriaceae; g__ | 0.01 | ||
| Proteobacteria—Eikenella | 0.01 | ||
| Proteobacteria—Kingella | 0.03 | ||
| Proteobacteria—Cardiobacterium | |||
| Proteobacteria— Enterobacteriaceae; g__ | 0.03 | ||
| Proteobacteria—Halomonas | 0.02 | ||
| Proteobacteria—Haemophilus | 0.001 | ||
| Proteobacteria—Moraxella | 0.05 | ||
| Fusobacteria—Fusobacterium | 0.005 | 0.003 | |
| Fusobacteria—Leptotrichia | 0.01 | 0.010 | |
| Bacteroidetes—Prevotella | 0.04 | ||
| Bacteroidetes—Paraprevotella | 0.04 | 0.001 | |
| Bacteroidetes—Capnocytophaga | 0.04 | ||
| Bacteroidetes—Sediminicola | 0.05 | ||
| Synergistetes—TG5 | |||
| TM7-; g__ | 0.01 | ||
| TM7-CW040 | 0.002 | 0.01 |
Table 3.
Bacterial genera change after oral cleaning from day zero to ten: Statistically significant change in bacterial levels of the tongue samples of all the three oral-cleaning methods were calculated after 10 days of cleaning from day zero to ten. For each method, the level of significance is shown with a decrease in levels of bacteria (in bold) or an increase in bacterial levels (in unbold). The P-values were calculated using Student t test. (BT – Brushing, followed by Tongue cleaning; GIFTS – Gum and teeth rubbing using Index Finger, followed by Tongue cleaning and water swishing; CT – GIFTS method using Charcoal, followed by Tongue cleaning.). “g__” indicate OTUs only annotated to the level of Genus
| Plaque | BT | GIFTS | CT |
|---|---|---|---|
| Firmicutes—g__ | 0.003 | ||
| Firmicutes—Gemella | 0.04 | ||
| Firmicutes—Streptococcus | 0.02 | ||
| Firmicutes—g__ | 0.000005 | 0.04 | |
| Firmicutes—Mogibacterium | 0.02 | ||
| Firmicutes—Parvimonas | 0.002 | ||
| Firmicutes—g__ | 0.01 | 0.003 | |
| Firmicutes—Catonella | 0.01 | ||
| Firmicutes—Peptococcus | 0.01 | ||
| Firmicutes—Dialister | 0.02 | ||
| Firmicutes—Schwartzia | 0.02 | 0.02 | |
| Firmicutes—Selenomonas | 0.001 | 0.04 | |
| Firmicutes—Veillonella | 0.01 | ||
| Actinobacteria—Actinomyces | 0.01 | ||
| Actinobacteria—Corynebacterium | 0.02 | 0.04 | |
| Actinobacteria—Scardovia | 0.04 | ||
| Actinobacteria—Atopobium | 0.05 | ||
| Actinobacteria—Slackia | 0.03 | ||
| Proteobacteria—g__ | |||
| Proteobacteria—Rhodobacter | 0.05 | ||
| Proteobacteria—Other | 0.03 | ||
| Proteobacteria—g__ | 0.01 | ||
| Proteobacteria—Propionivibrio | 0.02 | ||
| Proteobacteria—Campylobacter | 0.01 | ||
| Proteobacteria—Cardiobacterium | 0.02 | ||
| Proteobacteria—Actinobacillus | |||
| Proteobacteria—Haemophilus | 0.04 | 0.02 | |
| Proteobacteria—Enhydrobacter | 0.03 | ||
| Proteobacteria—Moraxella | |||
| Fusobacteria—Fusobacterium | 0.00001 | 0.03 | 0.0003 |
| Fusobacteria—g__ | 0.02 | ||
| Bacteroidetes—g__ | 0.02 | ||
| Bacteroidetes—Prevotella | 0.00004 | ||
| Bacteroidetes—Paludibacter | 0.01 | ||
| Bacteroidetes—Porphyromonas | 0.004 | ||
| Bacteroidetes—Tannerella | 0.02 | ||
| Bacteroidetes—g__ | 0.04 | ||
| Bacteroidetes—Capnocytophaga | 0.001 | ||
| SR1—g__ | 0.02 | ||
| TM7—g__ | 0.05 | ||
| TM7-CW040—g__ | 0.01 | 0.04 |
As organisms on the tongue are known to populate the saliva and then adhere to the tooth surfaces, especially when salivary flushing stops during sleep, all subjects cleaned their tongues in the morning and at bedtime. In the tongue samples, the GIFTS and CT cleaning methods significantly reduced early Firmicutes colonizers. Interestingly, the BT method showed a more significant reduction in bacteria of the middle Actinobacteria and Proteobacteria colonizers. There was a substantial reduction in Fusobacterium in tongue samples, indicating that all three methods can prevent purple-complex bacteria from interacting with red-complex bacteria that would otherwise allow the biofilm to mature into pathogenic plaques. This was also evidenced by the reduction seen in red complexes of the Bacteroidetes phylum, especially Prevotella and Porphyromonas. These results show that tongue cleaning twice a day maintains a healthy microbiota in the oral cavity [Table 1].
In salivary samples [Table 2], BT and CT but not GIFTS samples showed a significant increase in Streptococcus species, suggesting that GIFTS prevents the growth of acid-producing early colonizers. The BT method resulted in a statistically significant increase in almost all bacterial species after 10 days except for the SR1 phyla. However, the CT method significantly reduced all orange- and red-complex DDBs of the Fusobacterium and Bacteroidetes phylum, especially Prevotella and Porphyromonas. The GIFTS method showed a significant decrease in Treponema spirochetes.
Table 2.
Bacterial genera change after oral cleaning from day zero to ten: Statistically significant change in bacterial levels of the tongue samples of all the three oral-cleaning methods were calculated after 10 days of cleaning from day zero to ten. For each method, the level of significance is shown with an decrease in levels of bacteria (in bold), or increase in bacterial levels (not in bold). The P-values were calculated using Student t test. (BT – Brushing, followed by Tongue cleaning; GIFTS – Gum and teeth rubbing using Index Finger, followed by Tongue cleaning and water swishing; CT – GIFTS method using Charcoal, followed by Tongue cleaning). “g__” indicate OTUs only annotated to the level of Genus
| Saliva | BT | GIFTS | CT |
|---|---|---|---|
| Firmicutes—Planococcaceae; g__ | 0.03 | ||
| Firmicutes—Gemella | 0.03 | ||
| Firmicutes—Gemellaceae; other | 0.01 | ||
| Firmicutes—Aerococcaceae; other | |||
| Firmicutes—Streptococcus | 0.05 | 0.03 | |
| Firmicutes—Lachnospiraceae; g__ | 0.04 | ||
| Firmicutes—Peptostreptococcus | 0.02 | ||
| Actinobacteria—g__ | 0.002 | ||
| Actinobacteria—Phycicoccus | 0.03 | ||
| Actinobacteria—; g__ | 0.02 | ||
| Actinobacteria—Micrococcus | 0.01 | ||
| Proteobacteria— Hyphomicrobiaceae; g__ | 0.04 | ||
| Proteobacteria—Agrobacterium | 0.05 | ||
| Proteobacteria—Paracoccus | 0.03 | ||
| Proteobacteria— Rhodobacteraceae; other | 0.02 | ||
| Proteobacteria—Lautropia | 0.02 | ||
| Proteobacteria—Propionivibrio | |||
| Proteobacteria—Cellvibrio | 0.05 | ||
| Proteobacteria— Pseudomonadaceae; g__ | 0.03 | ||
| Fusobacteria—Fusobacterium | 0.002 | ||
| Fusobacteria—Leptotrichia | 0.05 | ||
| Bacteroidetes—Prevotella | 0.01 | ||
| Bacteroidetes—Porphyromonas | 0.03 | ||
| Bacteroidetes—Prevotella | 0.005 | ||
| Bacteroidetes—Sediminicola | 0.01 | ||
| Spirochaetes—Treponema | 0.03 | ||
| SR1-g__ | 0.03 | 0.05 | |
| SR1-f__; g__ | 0.03 | 0.05 | |
| TM7—g__ | 0.05 | ||
| TM7-CW040—g__ | 0.04 |
In the plaque samples [Table 3], the GIFTS but not the BT and CT cleaning method resulted in a significant reduction in early colonizers such as Streptococcus. However, the CT method, and to a lesser extent, the BT method caused a more substantial decrease in other early colonizers of the Firmicutes phylum.
All three methods significantly reduced Fusobacterium in the plaque samples, showing that all methods can prevent purple-complex bacteria from interacting with the red-complex bacteria that would otherwise allow the biofilm to mature into pathogenic plaques. This was also evident in the reduction of red complexes of the Bacteroidetes phylum, especially Prevotella and Porphyromonas.
DISCUSSION
Dysbiosis of microorganisms in the oral cavity can lead to biofilm and plaque formation.[39] Diet and personal oral hygiene are essential for preventing microbial plaque, the primary etiological factor for gingivitis and periodontal disease.[40] When food and drink containing sugar or starch are consumed, oral bacteria use them to produce acids, which damage the tooth and/or enamel. An undisturbed biofilm of early colonizers then allows other colonizers to promote plaque formation.[41,42,43,44] Therefore, mechanical disruption of biofilm through simple and effective methods as frequently as possible can prevent not only oral diseases but also other systemic diseases.
Toothbrushing is effective in reducing dental plaque levels and is considered the reference technique for mechanical control of plaque. It is, therefore, recommended by the World Health Organization (WHO).[44,45] However, toothbrushing does not remove over 40% of plaque, even by well-trained individuals.[20] To prevent dental caries, other oral cleaning methods should be used to supplement toothbrushing, such as tongue cleaning and oral irrigation, to remove food particles, and therefore, bacterial flora in the oral cavity.[46,47] Swishing 20–30 mL of water for a couple of minutes after eating or drinking and also between meals can help to remove food particles from the oral cavity.[19]
Furthermore, cleaning frequency is essential, and brushing teeth more than once a day reduces the occurrence of caries.[46,48] Individuals who state that they brush their teeth infrequently are at higher risk of developing or worsening carious lesions than those brushing more frequently.[49,50] However, too frequent brushing causes dental damage through corrosion and can be a risk factor for periodontitis.[51,52]
An optimal approach is to strike a balance between the frequency of cleaning and reducing abrasion. Here we show that all three methods of oral cleaning tested significantly reduced the bacterial load, including damaging dental bacteria, in plaque. However, early colonizers were notably reduced by the GIFTS method, probably due to the frequency of cleaning (average every 4h), thereby preventing early colonizers from establishing a stable biofilm.
Frequent snacking or sipping of sugary soft drinks and sedentary and food-abundant lifestyles have increased in the postindustrial society, and this is reflected in the high incidence of oral and systemic diseases. It is usually impractical to use toothbrushing with toothpaste away from home after every consumption of snacks and drinks. However, the GIFTS method can easily be carried out anywhere with only water required for swishing after gentle massaging of the gums and inner cavity. This process reduces the number of retained food particles in the oral cavity that could promote the growth of oral bacteria. This is particularly important when sugars remain in the mouth and are subsequently fermented by Streptococcus to produce the acids that damage enamel. Furthermore, the flexibility afforded by fingers with the GIFTS method allows the individual to reach all areas of the teeth, gums, and inner cheeks that are not easily accessible by a toothbrush.
This study shows that simply mechanically disturbing biofilm formation without the need for a cleaning agent every time food or drink is consumed reduces early colonizers. This frequent disturbance was more effective than once or twice daily oral cleaning. GIFTS significantly decreased several biofilm genera, presumably because frequent water swishing in the GIFTS method removed food particles that would otherwise be metabolized by bacteria to support their growth. Furthermore, tongue cleaning twice a day maintained a healthy microbiota in the oral cavity, which was supported by the observed decrease in bacterial load in all samples.
The GIFTS method was also tested with a cleaning agent in the CT group, albeit only twice a day and not after each meal and drink. However, the CT method still effectively decreased DDB in all three oral cavity samples, most likely due to its adsorptive properties. It has been shown that frequent toothbrushing causes abrasion of dental enamel.[51,52] Still, we have observed that finely powdered charcoal is minimally abrasive and safe for enamel, similar to toothbrushing. Dental abrasion is very unlikely with finger rubbing. Therefore, the GIFTS method is a safe way to remove food particles in the oral cavity that might cause acid damage and plaque formation.
CONCLUSION
Here we studied oral hygiene practices, including a novel, minimally abrasive, economic, eco-friendly, frequently useable, and convenient method. This is the first description of “frequent disruption of biofilm” by GIFTS. This method can be used by small children and adults alike to frequently clean the oral cavity without causing abrasion to the enamel. The GIFTS method is not an alternative to toothbrushing but can be regarded as a complementary method for use after any snack, meal, or drink, to prevent biofilm formation before it matures to plaque.
FINANCIAL SUPPORT AND SPONSORSHIP
This research received no specific grant from any funding agency.
CONFLICTS OF INTEREST
There are no conflicts of interest.
AUTHOR CONTRIBUTIONS
P. Chhaliyil, is principal investigator who designed the study, collected subject’s data, samples and analysed and also contributed in manuscript writing. K. F. Fischer, helped in 16s DNA sequencing part of the study. B. Schoel, is Guide and and critically revised the manuscript; P. Chhalliyil, is co-guide for the research work, drafted and critically revised the manuscript. Finally, all the authors approved the final version of the manuscript for publication.
ETHICAL POLICY AND INSTITUTIONAL REVIEW BOARD STATEMENT
The study and treatment protocol were explained in detail to the parents and informed consent was obtained. Institutional ethics committee approval was also obtained.
PATIENT DECLARATION OF CONSENT
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
ACKNOWLEDGEMENT
We thank the Principal Mr. Venkatesan, the students and teachers of Andavar Porayar School, Tamil Nadu, India, for their support in conducting the research study. We thank Seohee Park (uBiota LLC) for editing the manuscript. We thank Randal Hatfield (Pacific Surface Science Inc) for analyzing the charcoal size.
REFERENCES
- 1.Avila M, Ojcius DM, Yilmaz O. The oral microbiota: Living with a permanent guest. DNA Cell Biol. 2009;28:405–11. doi: 10.1089/dna.2009.0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 1994;8:263–71. doi: 10.1177/08959374940080022001. [DOI] [PubMed] [Google Scholar]
- 3.Ramberg P, Sekino S, Uzel NG, Socransky S, Lindhe J. Bacterial colonization during de novo plaque formation. J Clin Periodontol. 2003;30:990–5. doi: 10.1034/j.1600-051x.2003.00419.x. [DOI] [PubMed] [Google Scholar]
- 4.Sekino S, Ramberg P, Uzel NG, Socransky S, Lindhe J. The effect of a chlorhexidine regimen on de novo plaque formation. J Clin Periodontol. 2004;31:609–14. doi: 10.1111/j.1600-051X.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson L, Lif Holgerson P, Esberg A, Johansson I. Microbial complexes and caries in 17-year-olds with and without Streptococcus mutans. J Dent Res. 2018;97:275–82. doi: 10.1177/0022034517731758. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson L, Lif Holgerson P, Johansson I. Saliva and tooth biofilm bacterial microbiota in adolescents in a low caries community. Sci Rep. 2017;7:5861. doi: 10.1038/s41598-017-06221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer RJ, Jr., Shah N, Valm A, Paster B, Dewhirst F, Inui T, et al. Interbacterial adhesion networks within early oral biofilms of single human hosts. Appl Environ Microbiol. 2017;83:1–16. doi: 10.1128/AEM.00407-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nath SG, Raveendran R. Microbial dysbiosis in periodontitis. J Indian Soc Periodontol. 2013;17:543–5. doi: 10.4103/0972-124X.118334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 10.Tanner ACR, Kressirer CA, Rothmiller S, Johansson I, Chalmers NI. The caries microbiome: Implications for reversing dysbiosis. Adv Dent Res. 2018;29:78–85. doi: 10.1177/0022034517736496. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Helmerhorst EJ, Leone CW, Troxler RF, Yaskell T, Haffajee AD, et al. Identification of early microbial colonizers in human dental biofilm. J Appl Microbiol. 2004;97:1311–8. doi: 10.1111/j.1365-2672.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- 12.Larsen T, Fiehn NE. Dental biofilm infections—An update. APMIS. 2017;125:376–84. doi: 10.1111/apm.12688. [DOI] [PubMed] [Google Scholar]
- 13.Kolenbrander PE, Palmer RJ, Jr, Rickard AH, Jakubovics NS, Chalmers NI, Diaz PI. Bacterial interactions and successions during plaque development. Periodontol 2000. 2006;42:47–79. doi: 10.1111/j.1600-0757.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 14.Eke PI, Thornton-Evans G, Dye B, Genco R. Advances in surveillance of periodontitis: The Centers for Disease Control and Prevention Periodontal Disease Surveillance Project. J Periodontol. 2012;83:1337–42. doi: 10.1902/jop.2012.110676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahni K, Khashai F, Forghany A, Krasieva T, Wilder-Smith P. Exploring mechanisms of biofilm removal. Dentistry (Sunnyvale) 2016;6:1–9. doi: 10.4172/2161-1122.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandekerckhove B, Van den Velde S, De Smit M, Dadamio J, Teughels W, Van Tornout M, et al. Clinical reliability of non-organoleptic oral malodour measurements. J Clin Periodontol. 2009;36:964–9. doi: 10.1111/j.1600-051X.2009.01473.x. [DOI] [PubMed] [Google Scholar]
- 17.Bordas A, McNab R, Staples AM, Bowman J, Kanapka J, Bosma MP. Impact of different tongue cleaning methods on the bacterial load of the tongue dorsum. Arch Oral Biol. 2008;53:S13–8. doi: 10.1016/S0003-9969(08)70004-9. [DOI] [PubMed] [Google Scholar]
- 18.Matsui M, Chosa N, Shimoyama Y, Minami K, Kimura S, Kishi M. Effects of tongue cleaning on bacterial flora in tongue coating and dental plaque: A crossover study. BMC Oral Health. 2014;14:4. doi: 10.1186/1472-6831-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Math MV, Balasubramaniam P. Water swishing. Br Dent J. 2009;207:304. doi: 10.1038/sj.bdj.2009.859. [DOI] [PubMed] [Google Scholar]
- 20.Thornton J. More health warnings on food are needed to reduce tooth decay, says BMA. BMJ. 2018;361:k2177. doi: 10.1136/bmj.k2177. [DOI] [PubMed] [Google Scholar]
- 21.Bakdash B. Current patterns of oral hygiene product use and practices. Periodontol 2000. 1995;8:11–4. doi: 10.1111/j.1600-0757.1995.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 22.Breitenmoser J, Mörmann W, Mühlemann HR. [Gingival injuries from toothbrush bristles] SSO Schweiz Monatsschr Zahnheilkd. 1978;88:79–89. [PubMed] [Google Scholar]
- 23.Joshi CP, Patil AG, Karde PA, Mahale SA, Dani NH. Comparative evaluation of cemental abrasion caused by soft and medium bristle hardness toothbrushes at three predetermined toothbrushing forces: An in vitro study. J Indian Soc Periodontol. 2017;21:10–5. doi: 10.4103/jisp.jisp_118_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergström J, Lavstedt S. An epidemiologic approach to toothbrushing and dental abrasion. Community Dent Oral Epidemiol. 1979;7:57–64. doi: 10.1111/j.1600-0528.1979.tb01186.x. [DOI] [PubMed] [Google Scholar]
- 25.Chhaliyil P, Fischer KF, Schoel B, Chhalliyil P. Impact of Different Bedtime Oral Cleaning Methods on Dental Damaging Microbiota Levels. Dent Hypotheses. 2020;11:40–6. [Google Scholar]
- 26.Svanberg M. Contamination of toothpaste and toothbrush by Streptococcus mutans. Scand J Dent Res. 1978;86:412–4. doi: 10.1111/j.1600-0722.1978.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 27.Ratson T, Greenstein RB, Mazor Y, Peretz B. Salivary candida, caries and candida in toothbrushes. J Clin Pediatr Dent. 2012;37:167–70. [PubMed] [Google Scholar]
- 28.Assed Bezerra Da Silva L, Nelson-Filho P, Saravia ME, De Rossi A, Lucisano MP, Assed Bezerra Da Silva R. Mutans streptococci remained viable on toothbrush bristles, in vivo, for 44h. Int J Paediatr Dent. 2014;24:367–72. doi: 10.1111/ipd.12079. [DOI] [PubMed] [Google Scholar]
- 29.Celepkolu T, Toptancı IR, Bucaktepe PG, Sen V, Dogan MS, Kars V, et al. A microbiological assessment of the oral hygiene of 24-72-month-old kindergarten children and disinfection of their toothbrushes. BMC Oral Health. 2014;14:94. doi: 10.1186/1472-6831-14-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richards D. How clean is your toothbrush? Evid Based Dent. 2012;13:111. doi: 10.1038/sj.ebd.6400895. [DOI] [PubMed] [Google Scholar]
- 31.van Palenstein Helderman WH, Soe W, van ‘t Hof MA. Risk factors of early childhood caries in a southeast Asian population. J Dent Res. 2006;85:85–8. doi: 10.1177/154405910608500115. [DOI] [PubMed] [Google Scholar]
- 32.Fabbri S, Johnston DA, Rmaile A, Gottenbos B, De Jager M, Aspiras M, et al. Streptococcus mutans biofilm transient viscoelastic fluid behaviour during high-velocity microsprays. J Mech Behav Biomed Mater. 2016;59:197–206. doi: 10.1016/j.jmbbm.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Uzel NG, Teles FR, Teles RP, Song XQ, Torresyap G, Socransky SS, et al. Microbial shifts during dental biofilm re-development in the absence of oral hygiene in periodontal health and disease. J Clin Periodontol. 2011;38:612–20. doi: 10.1111/j.1600-051X.2011.01730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology. 2002;148:257–66. doi: 10.1099/00221287-148-1-257. [DOI] [PubMed] [Google Scholar]
- 35.Masunaga H, Tsutae W, Oh H, Shinozuka N, Kishimoto N, Ogata Y. Use of quantitative PCR to evaluate methods of bacteria sampling in periodontal patients. J Oral Sci. 2010;52:615–21. doi: 10.2334/josnusd.52.615. [DOI] [PubMed] [Google Scholar]
- 36.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–4. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6:610–8. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kilian M, Chapple IL, Hannig M, Marsh PD, Meuric V, Pedersen AM, et al. The oral microbiome—An update for oral healthcare professionals. Br Dent J. 2016;221:657–66. doi: 10.1038/sj.bdj.2016.865. [DOI] [PubMed] [Google Scholar]
- 40.Greenstein G. Periodontal response to mechanical non-surgical therapy: A review. J Periodontol. 1992;63:118–30. doi: 10.1902/jop.1992.63.2.118. [DOI] [PubMed] [Google Scholar]
- 41.Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, et al. Dental caries. Nat Rev Dis Primers. 2017;3:17030. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 42.Sbordone L, Bortolaia C. Oral microbial biofilms and plaque-related diseases: Microbial communities and their role in the shift from oral health to disease. Clin Oral Investig. 2003;7:181–8. doi: 10.1007/s00784-003-0236-1. [DOI] [PubMed] [Google Scholar]
- 43.Bortolaia C, Sbordone L. [Biofilms of the oral cavity. Formation, development and involvement in the onset of diseases related to bacterial plaque increase] Minerva Stomatol. 2002;51:187–92. [PubMed] [Google Scholar]
- 44.Harvey JD. Periodontal microbiology. Dent Clin North Am. 2017;61:253–69. doi: 10.1016/j.cden.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Petersen PE. The World Oral Health Report 2003: Continuous improvement of oral health in the 21st century—The approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2003;31:3–23. doi: 10.1046/j..2003.com122.x. [DOI] [PubMed] [Google Scholar]
- 46.Frazelle MR, Munro CL. Toothbrush contamination: A review of the literature. Nurs Res Pract. 2012;2012:420630. doi: 10.1155/2012/420630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winnier JJ, Rupesh S, Nayak UA, Reddy V, Prasad Rao A. The comparative evaluation of the effects of tongue cleaning on existing plaque levels in children. Int J Clin Pediatr Dent. 2013;6:188–92. doi: 10.5005/jp-journals-10005-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melo P, Fine C, Malone S, Frencken JE, Horn V. The effectiveness of the brush day and night programme in improving children’s toothbrushing knowledge and behaviour. Int Dent J. 2018;68:7–16. doi: 10.1111/idj.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holmes RD. Tooth brushing frequency and risk of new carious lesions. Evid Based Dent. 2016;17:98–9. doi: 10.1038/sj.ebd.6401196. [DOI] [PubMed] [Google Scholar]
- 50.Borgnakke WS, Brignardello-Petersen R. Insufficient evidence to claim that more frequent toothbrushing reduces the risk of developing new caries. J Am Dent Assoc. 2017;148:e1. doi: 10.1016/j.adaj.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 51.Kumar S, Tadakamadla J, Johnson NW. Effect of toothbrushing frequency on incidence and increment of dental caries: A systematic review and meta-analysis. J Dent Res. 2016;95:1230–6. doi: 10.1177/0022034516655315. [DOI] [PubMed] [Google Scholar]
- 52.Zimmermann H, Zimmermann N, Hagenfeld D, Veile A, Kim TS, Becher H. Is frequency of tooth brushing a risk factor for periodontitis? A systematic review and meta-analysis. Community Dent Oral Epidemiol. 2015;43:116–27. doi: 10.1111/cdoe.12126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.