Abstract
Background
Exposure to adverse childhood experiences (ACEs) is associated with many childhood diseases and poor health outcomes in adulthood. However, the association with childhood obesity is inconsistent. We investigated the association between reported cumulative ACE score and body mass index (BMI) in a large sample of patients at a single institution.
Methods
This cross-sectional study included children aged 2–20 years that were screened in a general pediatrics clinic for ACEs utilizing the Center for Youth Wellness ACEs questionnaire between July 2017 and July 2018. Overall ACE score was categorized as ‘no exposure’ (score = 0), ‘low exposure’ (score = 1), and ‘high exposure’ (score≥ 2). BMI was categorized as overweight/obese (BMI percentile ≥ 85) or non-obese (BMI percentile < 85). The association between ACEs score and obesity was determined using univariate and multivariable logistic regression.
Results
Of the 948 patients included in the study, 30% (n = 314) were overweight/obese and 53% (n = 504) had no ACE exposure, 19% (n = 179) had low ACE exposure, and 28% (n = 265) had high ACE exposure. High ACE exposure was associated with increased odds of obesity (OR = 1.47, 95%CI = 1.07–2.03, p = 0.026). However, after adjusting for age, race/ethnicity, insurance type, and birth weight, the association attenuated and was null (OR = 1.01, 95%CI = 0.70–1.46, p = 0.97).
Conclusion
The study findings may suggest an association between ACE and childhood obesity. However, the association attenuated after adjusting for age, race/ethnicity, insurance type, and birth weight. Larger prospective studies are warranted to better understand the association.
Introduction
Adverse Childhood Experiences (ACEs), a term coined by Felitti and colleagues in 1998, refers to psychological, physical, or sexual abuse and household dysfunction experienced by youth under the age of eighteen [1]. In their landmark study, “The Adverse Childhood Experiences Study”, they described a dose-response relationship between exposure to traumatic or emotionally distressing experiences during childhood and the development of chronic health conditions in adulthood, such as heart disease, chronic respiratory disease, cancer, and diabetes [1]. Since then, the presence of multiple childhood exposures have also been associated with adult health risk behaviors, such as alcohol and substance use disorders, as well as mental disorders and obesity in adults and well-being throughout the life span [2, 3].
The association between ACE and obesity is plausible. Parental incarceration and child maltreatment are associated with poor dietary and sleep practices including sweets, soda and salty snack consumption and short sleep duration [4, 5]. These poor dietary and sleep practices are associated with obesity [6]. Similarly, ACE affects telomere length [7, 8], which is associated with obesity [9, 10]. In addition, food insecurity is associated with obesity in adults [11] and a recent study showed that a high cumulative adverse childhood experiences is associated with food insecurity [12].
Emerging evidence in the last decade also suggests that childhood adversity negatively affects pediatric health, including obesity. Children with separated or divorced parents, mothers exposed to intimate partner violence or children who lived in a dysfunctional household were more likely to be overweight [13]. In addition, adolescent females exposed to maltreatment or had been sexually abused were more likely to be obese [14, 15].
These previous studies are informative, however the majority of them examined individual aspects or a limited number of ACEs. It is known that the risk of negative outcomes associated with adverse experiences is more pronounced in individuals who experience multiple ACEs [3, 16, 17]. Presently, there is a paucity of knowledge on the cumulative effects of ACEs on childhood obesity. Understanding this association, could ultimately guide future interventions that might have a positive, life-long influence on a child’s overall health and well-being.
In July 2017, as a result of the increasing awareness of the effects of ACEs on our pediatric population, the Johns Hopkins All Children’s Hospital General Pediatric and Adolescent Medicine (GPAM) clinic initiated screening of all children for ACEs at annual well child visits utilizing a questionnaire published by the Center for Youth Wellness [18]. In this analysis, we examined the association between cumulative ACE scores and body mass index (BMI) using data on 1,200 children screened between July 2017 and July 2018.
Methods
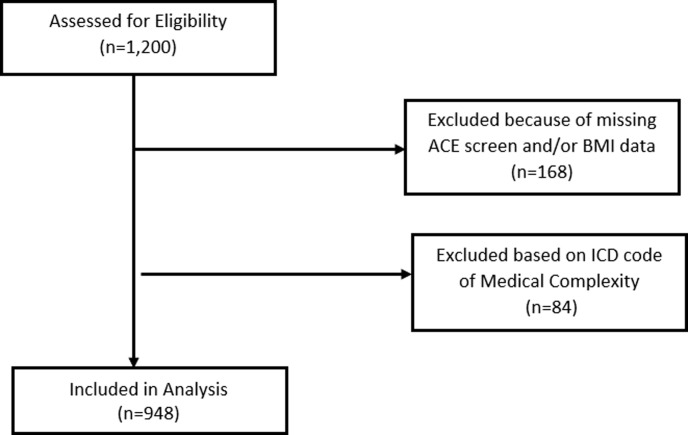
This is a cross-sectional study that utilized existing data from the electronic health record-derived Data Warehouse (EHR-DW) at Johns Hopkins All Children’s Hospital between July 2017 and July 2018. The study included children and adolescents, aged 2–20 years, presenting for well child visits at the Johns Hopkins All Children’s Hospital General Pediatric and Adolescent Medicine (GPAM) who were screened for ACEs (n = 1,200) (Fig 1). Patients were excluded if they did not have a completed ACEs screener or BMI obtained during the same visit (n = 168). Additionally, patients with a medically complex diagnosis code (ICD Z78.9) were excluded (n = 84), because this diagnosis code is usually used to identify patients who typically have chronic and/or severe conditions, functional limitations, and may be technology dependent for activities of daily living [19]. The final analytic dataset had 948 participants.
Fig 1. Participant flow diagram.
Diagram visually representing the number of participants excluded due to history of medical complexity and incomplete ACE screen and/or BMI data.
The primary outcome variable was obesity status based on BMI. BMI obtained from the data warehouse were used to determine age-adjusted BMI percentiles using the 2000 Centers for Disease Control and Prevention BMI for age growth charts for children between 2 and 20 years of age (www.cdc.gov/growthcharts). Due to small numbers in some groups, participants were classified as obese by combining overweight/obese (≥85th percentile) and as non-obese (<85th percentile). ACEs score was the main exposure variable. A child’s ACE score was obtained in the clinic using an existing questionnaire developed and published by Center for Youth Wellness [18]. This questionnaire includes 10 ACEs described in the Adverse Childhood Experiences Study as well as other additional early life stressors identified by childhood adversity experts, including living in a foster care, being bullied, death of a caregiver, deportation or migration, discrimination, experiencing a life-threatening illness or invasive medical procedure, and, exposure to community or school violence [1, 20].
As a tool designed to calculate the cumulative exposure to adverse experiences, parents are asked to count the number of ACEs their child has been exposed to and to write the total number on the form. The total ACE score was categorized as ‘no ACE exposure’ (ACE score = 0), ‘low ACE exposure’ (ACE = 1), and ‘high ACE exposure’ (ACE≥ 2) as previously reported [21]. We also obtained, from the data warehouse, other demographic data, including age, gender, race/ethnicity, insurance status, birthweight and history of prematurity, which may increase a child’s risk for obesity. The study was approved by the Johns Hopkins All Children's Hospital Institutional Review Board, with a waiver of informed consent.
Statistical analysis
Demographic characteristics of the study participants were summarized using counts and percentages for categorical variables and mean (standard deviation) or median (range) for continuous variables. The association between ACEs score, as continuous or categorical variable, and obesity was determined using univariate and multivariable logistic regression. Variables that were screened as potential confounders included age, gender, race/ethnicity, insurance status, birthweight and history of prematurity. A variable was included in the multivariable model if it had a p-value<0.1 in a univariate model. All statistical analyses were performed using SAS v 9.4 and a p-value <0.05 was considered statistically significant.
Results
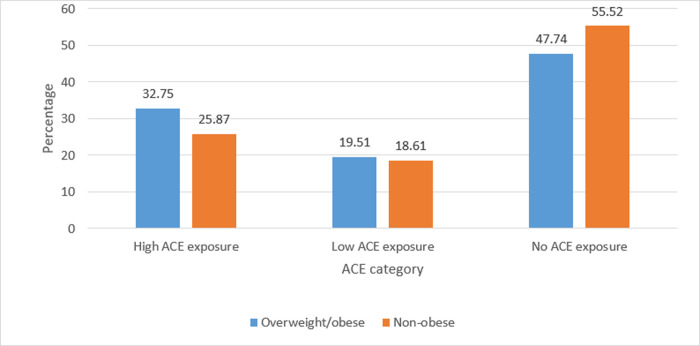
Characteristics of the study population are presented in Table 1. Of the 948 patients included in the study, 48% (n = 499) were females. The median (range) age was 7 (2–19) years. The proportion of Non-Hispanic Blacks (37%, n = 353) or Non-Hispanic Whites (37%, n = 351) was higher than Hispanics (12%, n = 112). The majority of the study participants did not have a history of prematurity (93%) or low birthweight (94%). Approximately 30% (n = 314) of the participants were overweight/obese and 52% (n = 540) had no ACE exposure, 19% (n = 201) had low ACE exposure, and 291 (28%) had high ACE exposure. The prevalence of high ACE exposure among overweight/obese participants (33%) was higher than that of non-obese participants (26%) (Fig 2).
Table 1. Characteristics of study participants by obesity status.
| Covariate | Level | Overweight/Obese N = 287 | Non-Obese N = 661 | |
|---|---|---|---|---|
| Age (Years) | N | 287 | 661 | |
| Mean (SD) | 9.08 (4.78) | 7.2 (4.59) | ||
| Median (IQR) | 9 (5–13) | 6 (3–10) | ||
| Age category (years) | N (Col %) | 0–5 | 88 (30.66) | 323 (48.87) |
| N (Col %) | 6–12 | 120 (41.81) | 220 (33.28) | |
| N (Col %) | 13+ | 79 (27.53) | 118 (17.85) | |
| Gender | N (Col %) | Female | 141 (49.13) | 327 (49.47) |
| N (Col %) | Male | 146 (50.87) | 334 (50.53) | |
| Race/ethnicity | N (Col %) | Hispanic | 41 (16.08) | 61 (10) |
| N (Col %) | Non-Hispanic Black | 101 (39.61) | 225 (36.89) | |
| N (Col %) | Non-Hispanic White | 78 (30.59) | 239 (39.18) | |
| N (Col %) | Other | 35 (13.73) | 85 (13.93) | |
| Insurance type | N (Col %) | Other | 6 (2.09) | 12 (1.82) |
| N (Col %) | Private | 76 (26.48) | 227 (34.34) | |
| N (Col %) | Public | 205 (71.43) | 422 (63.84) | |
| premature | N (Col %) | No | 275 (95.82) | 620 (93.8) |
| N (Col %) | Yes | 12 (4.18) | 41 (6.2) | |
| Low Birth Weight | N (Col %) | No | 279 (97.21) | 624 (94.4) |
| N (Col %) | Yes | 8 (2.79) | 37 (5.6) | |
| ACE | Median (range) | 1 (0–12) | 0 (0–15) | |
| ACE category | N (Col %) | High ACE exposure | 94 (32.75) | 171 (25.87) |
| N (Col %) | Low ACE exposure | 56 (19.51) | 123 (18.61) | |
| N (Col %) | No ACE exposure | 137 (47.74) | 367 (55.52) |
Fig 2. Prevalence of ACE by obesity status: Figure demonstrates percentage of individuals who were overweight and obese by category of ACE exposure.
In univariate analysis, high ACE exposure was associated with increased odds of obesity (OR = 1.47, 95%CI = 1.07–2.03, p = 0.017) (Table 2). Low ACE exposure was also associated with a slight increase in the odds of obesity, but the association was not statistically significant (OR = 1.22, 95%CI = 0.84–1.77, p = 0.30). Age, race, insurance type and low birth weight met the criterion to be included in the multivariable model. However, after controlling for these potential confounding variables both associations of ACE (High exposure: OR = 1.01, 95%CI = 0.70–1.46, p = 0.97; Low exposure: OR = 1.03, 95%CI = 0.68–1.55, p = 0.89) were null (Table 3).
Table 2. Univariate association between obesity and ACE.
| Covariate | Level | N | Odds Ratio | OR P-value |
|---|---|---|---|---|
| (95% CI) | ||||
| ACE continuous | 948 | 1.07 (1.01–1.15) | 0.029 | |
| ACE category | High ACE exposure | 265 | 1.47 (1.07–2.03) | 0.017 |
| Low ACE exposure | 179 | 1.22 (0.84–1.77) | 0.3 | |
| No ACE exposure | 504 | - | - | |
| Age (Years) | 948 | 1.09 (1.06–1.12) | < .001 | |
| Gender | Female | 468 | 0.99 (0.75–1.30) | 0.92 |
| Male | 480 | - | - | |
| Race/ethnicity | Hispanic | 102 | 2.06 (1.29–3.30) | 0.003 |
| Non-Hispanic Black | 326 | 1.38 (0.97–1.95) | 0.07 | |
| Other | 120 | 1.26 (0.79–2.02) | 0.33 | |
| Non-Hispanic White | 317 | - | - | |
| Insurance type | Other | 18 | 1.49 (0.54–4.12) | 0.44 |
| Public | 627 | 1.45 (1.07–1.98) | 0.018 | |
| Private | 303 | - | - | |
| Premature | Yes | 53 | 0.66 (0.34–1.28) | 0.22 |
| No | 895 | - | - | |
| Low Birth Weight | Yes | 45 | 0.48 (0.22–1.05) | 0.07 |
| No | 903 | - | - |
Table 3. Multivariable association between obesity and ACE.
| Covariate | Level | Odds Ratio | OR P-value |
|---|---|---|---|
| (95% CI) | |||
| ACE category | High ACE exposure | 1.01 (0.70–1.46) | 0.97 |
| Low ACE exposure | 1.03 (0.68–1.55) | 0.89 | |
| No ACE exposure | - | - | |
| Age (Years) | 1.08 (1.05–1.12) | < .001 | |
| Race/ethnicity | Hispanic | 2.04 (1.26–3.31) | 0.0038 |
| Non-Hispanic Black | 1.26 (0.88–1.82) | 0.21 | |
| Other | 1.49 (0.92–2.41) | 0.11 | |
| Non-Hispanic White | - | - | |
| Insurance type | Other | 1.90 (0.64–5.64) | 0.25 |
| Public | 1.35 (0.95–1.91) | 0.09 | |
| Private | - | - | |
| Low Birth Weight | Yes | 0.44 (0.18–1.08) | 0.07 |
| No | - | - |
Discussion
In this cross-sectional study consisting of 948 patients ages 2–19 years of age, the prevalence of high ACE exposure among overweight/obese participants (33%) was higher than that of non-obese participants (26%). Increased odds of obesity with high ACE exposure was also found among pediatric participants. However, this association attenuated and was no longer statistically significant after controlling for potential confounding variables (age, gender, race/ethnicity, insurance status, and birth weight).
The overall prevalence of ACE observed in this study is similar to other previous studies [22, 23]. However, it was higher than that reported by Caballero and colleagues and the difference could be due to the higher proportion of children immigrant families in their study, who have lower ACE than children in US native families [21].
Evidence for the negative impact of ACEs on weight status in children is inconsistent in the literature with most studies supporting an increased risk of obesity with ACE, albeit different experiences exert an influence at various timeframes during childhood and adolescence. In the Fragile Families and Child Wellbeing Study, a longitudinal study consisting of a cohort of 1,538 children born between 1998 and 2000 in urban hospitals, Schmeer found that children whose mothers dissolved a union or those with stable single mothers had an 80% higher risk of becoming overweight or obese between ages 3 and 5 years, as compared with children of stable married mothers [24]. Lynch et al. examined data from the 2011–2012 National Survey of Children’s Health and found that exposure to two or more adverse family experiences (AFEs) was associated with higher odds of overweight and obese status [25]. Heerman et al reported a similar result utilizing the 2011–2012 National Survey of Children’s Health. They found that children who had more AFEs were also at higher risk for overweight or obesity status [26]. Furthermore, Lynch et al found that exposure to certain childhood experiences, death of a parent and hardship due to family income, were stronger predictors of childhood obesity than other adverse experiences [25]. Isohookana et al observed that female adolescents who had experienced adverse childhood events were more likely to be obese and engage in unhealthy weight control behaviors [27]. However, all these previous studies did not control for birth weight, which is an important potential confounder [28, 29]. Boynton-Jarrett et al found that childhood exposure to maternal chronic intimate partner violence (IPV) correlated with an increased risk of obesity at age 5 years after adjusting for potential confounders including birth weight and socioeconomic status [23]. However, this association disappeared with early childhood exposure (less than 12 months of age) or late childhood exposure (at age 3 and/or 5 years) to maternal IPV suggesting that the timing of exposure might also be an important confounder. Future studies should consider these potential confounders and the timing of exposure.
The association between ACE and childhood health outcomes, including obesity, is biologically plausible and emerging evidence suggests that toxic stress, the accumulation or chronic activation of the body’s physiological stress response, is a potential mechanism [30]. Toxic stress exposure may manifest in the release of inflammatory cytokines, increased cortisol levels, and epigenetic modifications [25]. Kaufman et al. observed that ten methylation sites interact with ACEs to predict cross-sectional measures of BMI and an additional six sites were found to exert a main effect in predicting BMI in youth [25]. In addition to influencing biological pathways, early life stressors may affect child behavioral patterns, such as eating, sedentary behavior, and sleep, which could potentially result in increased childhood obesity [4, 5, 7–12, 31].
Frohlich et al found that a history of family adversity, maternal depression, and maternal attachment styles were important factors in predicting long-term success for a cohort of overweight and obese children ages 7 to 15 enrolled in a weight reduction program [32]. Lynch et al found that the probability of developing childhood obesity decreased when positive contextual factors were present, such as supportive neighborhoods and school safety [33]. Therefore, improving intervention programs and public policies focused on optimizing early environments may modify obesity risk in childhood.
Although several previous studies report an increased risk of obesity with ACEs, many of them relied on variable proxy measures, such as free/reduced lunch status, parental education level or place of residence to assess socioeconomic status, which could be a potential confounder or effect modifier [34, 35]. Min et al found that children living in poverty were more likely to engage in sedentary behaviors and have a poor BMI growth trajectory than children of higher socioeconomic status [36]. Other recent studies have also reported a higher ACEs prevalence among children of lower socioeconomic status [37–39]. In addition, previous studies show that birth weight is an important predictor of childhood obesity and therefore could be a potential confounder [28, 29]. Taken together, socioeconomic status and birth weight may be potential confounders or effect modifiers for the association between ACEs and childhood obesity and needs to be controlled in future studies. In addition, the timing of exposure could also play an important role in the association and warrants further investigation.
The present study has several limitations. It is a cross-sectional design and thus limits determination of causality. Additionally, our ACE measure does not ascertain important items, such as incarceration of a parent. Our study population included children and adolescence from a single institution and the findings may not be generalizable. Furthermore, it is difficult to discern the impact of ACE exposure over time on development of obesity in childhood. While multiple factors influencing development of obesity may be controlled for in a cross-sectional study design, longitudinal studies are better equipped to capture the effect of duration of ACE exposure on weight status during childhood [33]. Therefore, further prospective studies are warranted to explore the relationship between ACEs and obesity development in youth.
Conclusion
The findings of this cross-sectional analysis may suggest an association between ACE and childhood obesity. However, the association attenuated after adjusting for age, race/ethnicity, insurance type, and birth weight. Larger prospective studies are warranted to better understand the association.
If an association actually exists, it could guide future interventions that may have a positive, life-long influence on a child’s overall health and well-being.
Data Availability
The dataset from our hospital cannot be publicly shared because it contains sensitive patient information. Data request may be directed to Sue Ellie (sue.ellie@jhmi.edu) the Johns Hopkins All Children's Hospital Institutional Review Board, 501 6th Avenue South, St. Petersburg, FL 33701
Funding Statement
The author(s) received no specific funding for this work
References
- 1.Felitti VJ Anda RF., Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med, 1998;14(4):245–258. 10.1016/s0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- 2.Merrick MT, Ford DC, Ports KA, Guinn AS, Chen J, Klevens J, Metzler M, Jones CM, Simon TR, Daniel VM, Ottley P, Mercy JA. Vital Signs: Estimated Proportion of Adult Health Problems Attributable to Adverse Childhood Experiences and Implications for Prevention—25 States, 2015–2017. MMWR Morb Mortal Wkly Rep. 2019;68(44):999–1005. 10.15585/mmwr.mm6844e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, Jones L, Dunne MP. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. 2018;2(8): e356–e366. [DOI] [PubMed] [Google Scholar]
- 4.Jackson DB & Vaughn MG. Parental incarceration and child sleep and eating behaviors. Journal of Pediatrics. 2017; (185): 211–217. [DOI] [PubMed] [Google Scholar]
- 5.Jackson DB & Vaughn MG. Obesogenic food consumption among young children: the role of maltreatment. Public Health Nutrition. 2019; 22(10): 1840–1849. 10.1017/S1368980019000065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown CL, Halvorson EE, Cohen GM, Lazorick S, Skelton JA. Addressing Childhood Obesity: Opportunities for Prevention. Pediatr Clin North Am. 2015;62(5):1241–61. 10.1016/j.pcl.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drury SS, Theall K, Gleason MM, Smyke AT, De Vivo I, Wong JYY, … & Nelson CA. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Molecular Psychiatry; 2012; 17(7): 719–727. 10.1038/mp.2011.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rentscher KE, Carroll JE, Mitchell C. Psychosocial Stressors and Telomere Length: A Current Review of the Science. Annual Rev Public Health. 2020;(41):223–245. [DOI] [PubMed] [Google Scholar]
- 9.Davis SK, Xu R, Khan RJ, Gaye A. Adiposity and Leukocyte Telomere Length in US Adults by Sex-Specific Race/Ethnicity: National Health and Nutrition Examination Survey. Ethn Dis. 2020; 30(3):441–450 10.18865/ed.30.3.441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamprokostopoulou A, Moschonis G, Manios Y, Critselis E et al. Childhood obesity and leucocyte telomere length. Eur J Clin Invest. 2019;49(12):e13178 10.1111/eci.13178 [DOI] [PubMed] [Google Scholar]
- 11.Dinour LM, Bergen D, & Yeh MC. The food insecurity–obesity paradox: a review of the literature and the role food stamps may play. Journal of the American Dietetic Association. 2007; 107(11), 1952–1961. 10.1016/j.jada.2007.08.006 [DOI] [PubMed] [Google Scholar]
- 12.Jackson DB, Chilton M, Johnson KR, & Vaughn MG. Adverse Childhood Experiences and Household Food Insecurity: Findings From the 2016 National Survey of Children's Health. American Journal of Preventive Medicine. 2019; 57(5), 667–674. 10.1016/j.amepre.2019.06.004 [DOI] [PubMed] [Google Scholar]
- 13.Oh DL, Jerman P, Silvério Marques S, Koita K, Purewal Boparai SK, Burke Harris N, Bucci M. Systematic review of pediatric health outcomes associated with childhood adversity. BMC Pediatr. 2018;18(1):83 10.1186/s12887-018-1037-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noll JG, Zeller MH, Trickett PK, Putnam FW. Obesity risk for female victims of childhood sexual abuse: a prospective study. Pediatrics. 2007;120(1), e61–67. 10.1542/peds.2006-3058 [DOI] [PubMed] [Google Scholar]
- 15.Shenk CE, Noll JG Peugh JL, Griffin AM, Bensman HE. Contamination in the Prospective Study of Child Maltreatment and Female Adolescent Health. J Pediatr Psychol. 2016;41(1): 37–45. 10.1093/jpepsy/jsv017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellis MA, Hughes K, Ford K, Ramos Rodriguez G, Sethi D, Passmore J. Life course health consequences and associated annual costs of adverse childhood experiences across Europe and North America: a systematic review and meta-analysis. Lancet Public Health. 2019;4(10): e517–e528. 10.1016/S2468-2667(19)30145-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones CM, Merrick MT, Houry DE. Identifying and Preventing Adverse Childhood Experiences: Implications for Clinical Practice. JAMA. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Center for Youth Wellness Adverse Childhood Experiences Questionnaire (ACE-Q) Child. (2015). Center for Youth Wellness. Retrieved March 26 from centerforyouthwellness.org/aceq-pdf/
- 19.Cohen E, Kuo DZ, Agrawal R, Berry JG, Bhagat SK, Simon TD, Srivastava R. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538 10.1542/peds.2010-0910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bucci MGW, Koita K, Purewal SK, Silverio Marques S, Burke Harris N. Center for Youth Wellness ACE-Questionnaire User Guide. Center for Youth Wellness. Retrieved March 26th from https://centerforyouthwellness.org/wp-content/uploads/2018/06/CYW-ACE-Q-USer-Guide-copy.pdf
- 21.Caballero TM, Johnson SB, Buchanan CRM, DeCamp LR. Adverse childhood experiences among Hispanic children in immegrant families versus US-native families. Pediatrics, 2017:140(5),e20170297 10.1542/peds.2017-0297 [DOI] [PubMed] [Google Scholar]
- 22.Merrick MT, Ford DC, Ports KA, Guinn AS. Prevalence of Adverse Childhood Experiences From the 2011–2014 Behavioral Risk Factor Surveillance System in 23 States. JAMA Pediatr. 2018;172(11):1038–1044. 10.1001/jamapediatrics.2018.2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boynton-Jarrett R, Fargnoli J, Suglia SF, Zuckerman B, & Wright RJ. Association between maternal intimate partner violence and incident obesity in preschool-aged children: results from the Fragile Families and Child Well-being Study. Arch Pediatr Adolesc Med. 2010;164(6):540–546. 10.1001/archpediatrics.2010.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmeer KK. Family structure and obesity in early childhood. Soc Sci Res. 2012;41(4):820–832. 10.1016/j.ssresearch.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 25.Kaufman J, Montalvo-Ortiz JL, Holbrook H, O'Loughlin K, Orr C, Kearney C, Yang BZ, Wang T, Zhao H, Althoff R, Garavan H, Gelernter J, Hudziak J. Adverse Childhood Experiences, Epigenetic Measures, and Obesity in Youth. J Pediatr. 2011;202:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heerman WJ, Krishnaswami S, Barkin SL, McPheeters M. (2016, March). Adverse family experiences during childhood and adolescent obesity. Obesity (Silver Spring), 24(3), 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isohookana R, Marttunen M, Hakko H, Ripinen P, Riala K. The impact of adverse childhood experiences on obesity and unhealthy weight control behaviors among adolescents. Compr Psychiatry. 2016;11(71):17–24. [DOI] [PubMed] [Google Scholar]
- 28.Rito A, Buoncristiano M, Spinelli A, Salanave B, Kunesova M, Jejgaard T, Garcia SM, Fijalkowska A, Sturua L, Hyska J, Kelleher C, Duleva V, Music Milanovic S, Farrugia Sant'Angelo V, Abdrakhmanova S, Kujundzic E, Peterkova V, Gualtieri A, Pudule J, Petrauskiene A, Tanrygulyyeva M, Sherali R, Huidumac-Petrescu C, Williams J, Ahrens W, Breda J. Association between characteristics at birth, breasfeeding and obesity in 22 countries: The WHO European Childhood Obesity Surveillance Initiative-COSI 2015/2017. Obes Facts. 2019;12(2):226–243. 10.1159/000500425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapral N, Miller SE, Scharf RJ, Gurka MJ, DeBoer MD. Associations between birthweight and overweight and obesity in school-age children. Pediatric Obesity. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bucci M, Marques SS, Oh D, & Harris NB. Toxic Stress in Children and Adolescents. Adv Pediatr. 2018;63(1):403–428. [DOI] [PubMed] [Google Scholar]
- 31.Miller AL, Lumeng JC. Pathways of Association from Stress to Obesity in Early Childhood. Obesity (Silver Spring). 2018;26(7):1117–1124. [DOI] [PubMed] [Google Scholar]
- 32.Fröhlich G, Pott W, Albayrak Ö, Hebebrand J, Pauli-Pott U. Conditions of long-term success in a lifestyle intervention for overweight and obese youths. Pediatrics. 2011;128(4):e779–785. 10.1542/peds.2010-3395 [DOI] [PubMed] [Google Scholar]
- 33.Lynch BA, Finney Rutten LJ, Wilson PM, Kumar S, Phelan S, Jacobson RM, Fan C, Agunwamba A. The impact of positive contextual factors on the association between adverse family experiences and obesity in a National Survey of Children. Prev Med. 2018;116(11):81–86. [DOI] [PubMed] [Google Scholar]
- 34.Davis L, Barnes AJ, Gross AC, Ryder JR, Shlafer RJ. Adverse Childhood Experiences and Weight Status among Adolescents. J Pediatr. 2019;204(1):71–76. [DOI] [PubMed] [Google Scholar]
- 35.Fuemmeler BF, Dedert E, McClernon FJ, Beckham JC. Adverse childhood events are associated with obesity and disordered eating: results from a U.S. population-based survey of young adults. J Trauma Stress. 2009;22(4):329–333. 10.1002/jts.20421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Min J, Xue H, Wang Y. Association between household poverty dynamics and childhood overweight risk and health behaviours in the United States: a 8-year nationally representative longitudinal study of 16 800 children. Pediatr Obes. 2018;13(10):590–597. 10.1111/ijpo.12292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardner R, Feely A, Layte R, Williams J, McGavock J. Adverse childhood experiences are associated with an increased risk of obesity in early adolescence: a population-based prospective cohort study. Pediatr Res, 2019;86(4):522–528. 10.1038/s41390-019-0414-8 [DOI] [PubMed] [Google Scholar]
- 38.Manyema M, Richter LM. (2019, Dec). Adverse childhood experiences: prevalence and associated factors among South African young adults. Heliyon. 2019;5(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh D, McCartney G, Smith M, Armour G. (2019, Dec). Relationship between childhood socioeconomic position and adverse childhood experiences (ACEs): a systematic review. J Epidemiol Community Health. 2019;73(12):1087–1093. 10.1136/jech-2019-212738 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset from our hospital cannot be publicly shared because it contains sensitive patient information. Data request may be directed to Sue Ellie (sue.ellie@jhmi.edu) the Johns Hopkins All Children's Hospital Institutional Review Board, 501 6th Avenue South, St. Petersburg, FL 33701