Abstract
Gene therapy is a powerful tool against genetic disorders and cancer, targeting the source of the disease rather than just treating the symptoms. While much of the initial success of gene delivery relied on viral vectors, non-viral vectors are emerging as promising gene delivery systems for efficacious treatment with decreased toxicity concerns. However, the delivery of genetic material is still challenging, and there is a need for vectors with enhanced targeting, reduced toxicity, and controlled release. In this article, we highlight current work in gene therapy which utilizes the cyclic oligosaccharide molecule cyclodextrin (CD). With a number of unique abilities, such as hosting small molecule drugs, acting as a linker or modular component, reducing immunogenicity, and disrupting membranes, CD is a valuable constituent in many delivery systems. These carriers also demonstrate great promise in combination therapies, due to the ease of assembling macromolecular structures and wide variety of chemical derivatives, which allow for customizable delivery systems and co-delivery of therapeutics. The use of combination and personalized therapies can result in improved patient health—modular systems, such as those which incorporate CD, are more conducive to these therapy types.
Keywords: Cyclodextrin, Drug delivery, Nanoparticle, Hydrogel, Nucleic acid delivery, Gene therapy, Cancer, Oncology, Combination therapy, Polymer
Introduction
Gene therapy
The approved therapies listed in Table 1 fall under the FDA’s “cellular and gene therapy products” category. Gene-based therapies recognized as such by the FDA can be organized into three main groups: gene replacement therapy, gene editing, and genetically engineered T cell therapies. A fourth category, gene modulation through the use of RNA interference (RNAi), is categorized separately by the FDA, but is included in this work (Fig. 1). Gene replacement therapy provides a patient’s cells with a new copy of a missing or malfunctioning gene. This area has been studied since the 1970s [1], and the first gene replacement therapy, Luxturna [2], was approved by the FDA in 2017 (Table 1). Gene editing inserts, removes, changes, or replaces a patient’s existing genetic code. The most recent and precise gene editing technique is clustered regularly interspaced short palindromic repeats (CRISPR), which is currently in clinical trials (NCT03399448, among others) [3]. However, there are no FDA-approved gene editing treatments at this time, and the CRISPR platform is used primarily to produce gene edited models to help better understand biological mechanisms and develop other therapies [4,5].
Table 1.
FDA-approved gene therapy products
| Tradename | Manufacturer | Proper name | Function | Status | |
|---|---|---|---|---|---|
| Imlygic | Amgen Inc. | Talimogene laherparepvec | Genetically modified HSV immunostimulatory injected into melanoma tumors | FDA approved | 2015 |
| Kymriah | Novartis Pharmaceuticals Co. | Tisagenlecleucel | T cells are genetically engineered to express a chimeric cell surface receptor to target cancer cells | FDA approved | 2017 |
| Luxturna | Spark Therapeutics Inc. | Voretigene rseparvovec-rzyl | AAV vector-based gene therapy for patients with inherited retinal disease and RPE65 mutations | FDA approved | 2017 |
| Yescarta | Kite Pharma Inc. | Axicabtagene ciloleucel | CAR T therapy for adults living with certain types of non-Hodgkin lymphoma. | FDA approved | 2017 |
| Zolgensma | AveXis, Inc | Onasemnogene abeparvovec-xioi | AAV delivery of SMN1 gene for treatment of spinal muscular atrophy | FDA approved | 2019 |
Fig. 1.
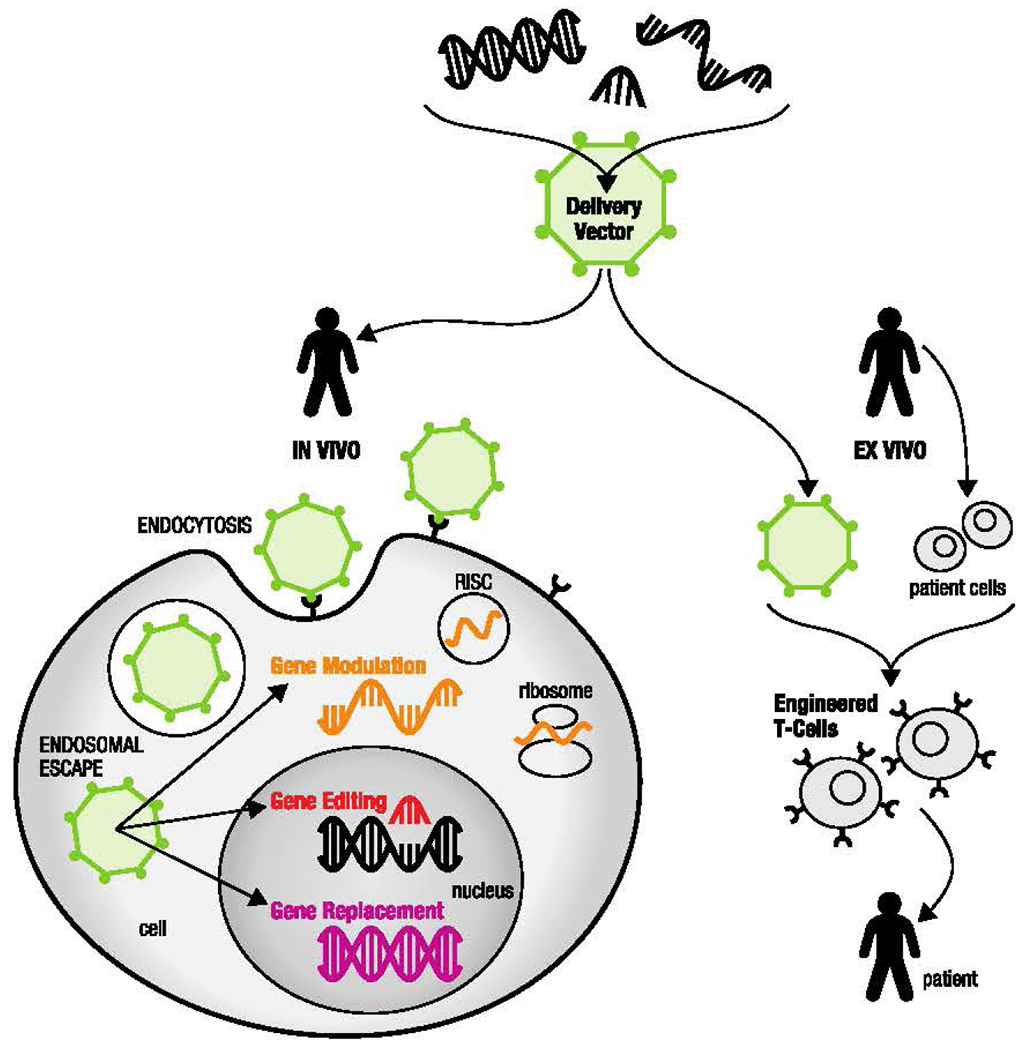
A schematic illustrating multiple types of gene-based therapy: gene modulation, gene replacement, gene editing, and engineered T cell therapy
In addition to gene replacement therapy and gene editing, there are also genetically engineered cell-based therapies, such as chimeric antigen receptor (CAR) T cell therapy, which consists of ex vivo genetic engineering of a patient’s immune cells to express CAR, enabling them to target and kill cancer cells [6–8]. There are multiple FDA-approved CAR-T therapies, the first of which is a platform called Kymriah, developed by researchers at the University of Pennsylvania [9] and approved in August 2017. However, even with a growing number of gene-based therapies entering the clinical space, the delivery of genetic material across biological barriers remains a significant challenge.
One effective means of delivering genetic material is through the use of viral vectors, which have evolved for such purposes [10]. Many viruses have been engineered to achieve therapeutic gene delivery, such as the herpes simplex virus (HSV), retro- or lenti-virus, adenovirus, and adeno-associated virus (AAV). These are very efficient methods of genetic delivery, but there are physiological barriers and safety concerns which limit their clinical implementation. Viral vectors have high immunogenicity, high cost, and can be inhibited by some drugs such as anti-coagulants [11]. They also have package size limitations, and can only deliver genetic material under a certain number of base pairs (7.5 kb for adenovirus, 4.5 kb for AAV) [12].
To overcome these challenges, non-viral vectors are being designed for gene delivery, with the long-term goal of achieving similar transfection efficiency to existing viral vectors while mitigating safety and cost concerns. This is achieved via the engineering of synthetic materials, such as lipid and polymer delivery vehicles, which use similar cell entry mechanisms to viruses but with improved safety, immunogenicity, and manufacturing [13].
Non-viral vectors have demonstrated several benefits for gene delivery, including better biocompatibility, large payload capacity, and the ability to specifically engineer their surfaces via functionalization [14, 15]. The condensation of genetic material using non-viral vectors also reduces stimulation of the innate immune response [16]. Although there are no FDA-approved non-viral gene therapies under the “cellular and gene therapy products” umbrella, there are quite a few systems which have been developed for RNA interference (RNAi) delivery [17], included here as a fourth category of gene modulation. One such system, Onpattro (patisiran, for treatment of polyneuropathy caused by hereditary ATTR amyloidosis; Alnylam Pharmaceuticals), is a lipid nanoparticle vector for RNAi delivery which was designated an orphan drug and approved by the FDA in August 2018. Patisiran is also currently in ongoing late-stage clinical trials (NCT03862807, NCT03997383) [18, 19]. Another is the spherical nucleic acid constructs being developed by Exicure [20], also currently in clinical trials (NCT03086278, NCT03684785) [21]. Multiple types of nucleic acids can decorate this nanoparticle system, including mRNA or siRNA modalities.
Non-viral vectors protect nucleic acids from nucleases and serum components [22], but often struggle to achieve delivery of sufficient genetic material. This can be caused by short retention time or insufficient endosomal release of non-viral vectors in target cells [11]. Some non-viral vectors still exhibit toxicity or immunogenicity, and can have issues with reproducibility. To fully transition to non-viral gene delivery vectors, improvements need to be made by exploring new non-viral delivery platforms and integrating new components into existing delivery vehicles.
Cyclodextrins
Cyclodextrins (CDs) have been used for years by the pharmaceutical industry to overcome drag delivery barriers such as solubility, bioavailability, and stability [23]. CDs are a family of cyclic oligosaccharides comprised of 6, 7, or 8 linked glucose units, called α-, β-, and γ-cyclodextrin respectively [24]. They are used in a variety of applications, such as foods, cosmetics, air fresheners, and pharmaceuticals [24], and there are over 50 FDA-approved formulations in which CDs are used. Their wide usage is primarily due to their ability to form molecular inclusion complexes with guest molecules [25]. The three different types of CDs can form complexes with different binding coefficients and residence times [25]. These inclusion complexes can be used to stabilize or solubilize the guest molecules, and the host/guest interactions can be 1:1 or have a great excess of CDs—these interaction properties are based on the size and charge of the guest molecule in question [26]. Finally, CDs are also attractive because of their low toxicity and immunogenicity [27].
There are also many derivatives of CD which have been developed to improve the properties of natural CDs. The many hydroxyl groups found in CD can be used as reactive sites and replaced with alternate functional groups [28] (Fig. 2). These derivatives are commonly used in pharmaceuticals and can have improved properties such as solubility or membrane penetration [29]. CDs also have two different faces—the primary, narrower face having primary hydroxyl groups, while the secondary, wider face has secondary hydroxyls. The symmetry and chemical differences of these faces allow for compatibility with multiple modification types [30].
Fig. 2.
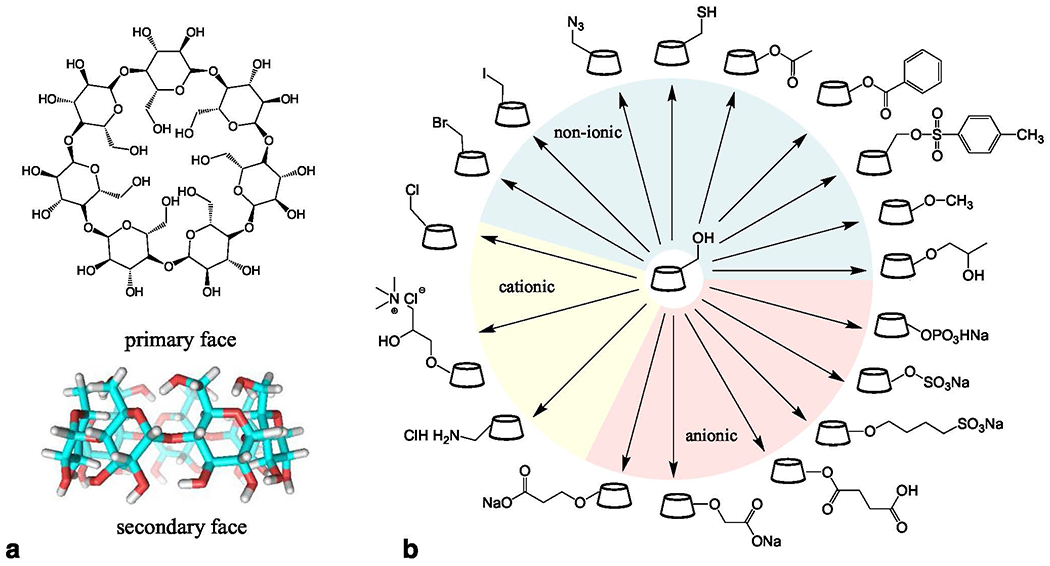
a The molecular structure of cyclodextrin (top) and a 3D conformer showing the primary and secondary faces (bottom). This structure is simplified to a truncated cone (b) where some common and commercially available derivatives of cyclodextrin are shown, broken down into cationic, non-ionic, and anionic groups
In addition to the formation of derivatives, the hydroxyl groups can also be used for bio-conjugation or polymerization. Cyclodextrin has been polymerized using multiple cross-linkers, such as epichlorohydrin [31], ethylene glycol digycetal ether [32], isocyanate [33], and polycarboxylic acid [34]. These CD polymers enable drug loading and controlled release via host/guest interactions. Other molecular edits can be done on only the head or base ring of the CD, creating different properties on each side. All of these options make the CD ring very modular, an attractive property for a delivery vector.
Based on these properties, many drug delivery systems involving CDs have been developed. These span from simple formulations, already approved for FDA use, to highly complex systems which can be engineered to specifically target disease states [35, 36]. The modularity of CDs make them useful for the delivery of genetic material, among other drug cargo types. As a result, there has been significant work in the field of CDs as delivery systems, and there are a number of excellent existing reviews on the topic [11,27, 30,36–39]. In this work, we focus on the usage of CDs in gene and combination delivery applications, as well as attempt to comment on the trajectory of the field, based on current trends and existing literature reviews.
Cyclodextrins in gene therapy applications
Cyclodextrins have been extensively researched as carriers for several types of gene therapy. They have been used in delivery systems to improve gene editing, replacement, and modulation within cells through the delivery of DNA and RNA. While there do not appear to be any current applications in CD-based ex vivo T cell engineering, this may well change if the technology successfully moves in vivo and transient expression of CAR antigens (through mRNA delivery) becomes more common [40].
Although “pure” CD vectors for gene therapy exist, CDs are more typically one of multiple components in gene delivery systems. The addition of CD can impart many of its beneficial physicochemical properties to the system as a whole, such as hosting small molecule drugs, acting as a linker or modular component, reducing immunogenicity, and disrupting membranes. In some cases, CD directly interacts with the genetic cargo. In others, genetic cargo is held by a different system component, but CD still plays an integral role such as vehicle stability or ensuring endosomal escape. Without these properties, gene delivery vectors cannot effectively deliver cargo to its desired destination. By imparting such capabilities to the larger system, the addition of CD allows for enhanced delivery in gene therapy applications.
Cationic polymers
Cationic polymers are widely utilized in gene delivery due to their positive surface charge, and are usually made of cationic monomers containing amine groups [41]. This allows for electrostatic interaction between the polymer and any nucleic acids which have a negative charge, such as plasmid DNA (pDNA), microRNA (mRNA), small interfering RNA (siRNA), messenger RNA (mRNA), and even CRISPR-associated protein-9 (cas9) RNP or S1mplex [42]. This interaction causes a condensation of polymer and nucleic acid into complexes termed polyplexes [43]. These polyplexes hold the genetic material while also protecting it during delivery.
In addition to the advantages noted above, cationic polymers are attractive non-viral delivery vectors because they are highly customizable, both in size and the type of genetic material used for delivery—this stems in part from the fact that cationic polymers can be chemically diverse. They can also be engineered to have long shelf lives, indicating potential for an easier path to clinical use [41]. Finally, cationic polymers are believed to exhibit the proton sponge effect, where protons and counter ions are drawn into the endosome by the presence of the polyplex. This causes an influx of water in an attempt to balance the osmotic gradient, which ultimately results in the rupture of the endosome, allowing the polyplex to escape and deliver its genetic cargo within the cell [43]. However, cationic polymers are currently limited in their use, in part due to low uptake and efficiency, toxicity, and immunogenicity. To help mitigate some of these concerns, many groups have worked to introduce CDs into traditional cationic polymer systems, and have shown that these CD-integrating formulations successfully deliver pDNA [44–49] and various RNAs [16, 50–52] for gene editing and gene modulation. In general, these delivery systems show greater efficacy and lower toxicity than similar formulations which do not incorporate CD.
Other groups are exploring CD-mediated polycations, which utilize CD as the base of the system, and incorporate cationic charge by modifying the hydroxyl groups of CDs [27, 53–58]. CDs modified with cationic moieties can directly form polyplexes with nucleic acids, much like existing cationic polymer systems [53, 54, 58]. These electrostatic interactions allow for self-assembly into stable structures. When these CD polycations also exhibit amphiphilic properties, they can form nanostructures where nucleic acid cargo is compacted and encapsulated within the structure [53, 59]. The complex which CD can form with host molecules enables shielding of the therapeutic cargo, up to macromolecular scale [59]. CD also naturally exhibits membrane disruption, leading to enhancement of permeability for its cargo [27]. This disruption is beyond that of the proton sponge effect alone, as it can improve delivery to the cytosol not only through enhanced uptake but through increased endosomal escape [60]. This capability is important to note, as some studies suggest that particles which include CD and their derivatives may also be able to enter cells by the endocytotic pathway [61, 62].
The cationic polymer polyethyleneimine (PEI) has been used extensively as a non-viral gene delivery vector, and is an example of the inherent cytotoxicity of cationic polymers with strong charge density. While there are both linear and branched forms of PEI, the most effective gene delivery is achieved using the branched form, as it condenses DNA to a greater extent [12]. However, as the PEI polymer network becomes larger and more branched, it also becomes more cytotoxic [63]. Regardless, PEI is considered by many to be the “gold standard” of non-viral gene delivery, due to its strong cationic charge and buffering capability, leading to high transfection efficiency. PEI, like other polycations, also exhibits the proton sponge effect, combining its ability for strong nucleic acid polyplex formation with intrinsic endosomolytic activity [64].
Overall, the use of PFI is limited by its toxicity, and reducing dosage to a non-toxic level typically results in low transfection efficiency and short duration of gene expression [64]. To mitigate these issues, several alterations to and derivatives of PEI have been explored, including the usage of CDs [16, 65–67]. By incorporating CD into PEI delivery systems, toxicity concerns can be alleviated by shortening the molecular weight of PEI used while retaining its gene transfection properties. In fact, formulations which include CD show improved DNA condensation over PEI alone [48]. An example of CD incorporation is the synthesis of PEI-β-CD, through creation of amine-reactive CD and conjugation to the amines found in branched PEI [68]. Even when only ~ 10% of the amines are conjugated with β-CD, the resulting polymer demonstrated significantly reduced cytotoxicity as compared to PEI alone [68].
Other recent studies have taken advantage of the modularity of CDs, specifically by exploiting host/guest interactions to self-assemble cationic polymers that are part of a larger macromolecular system [53, 69]. In one example, a cationic β-CD-modified PEI was synthesized, in which the branched arms of PEI were modified to terminate with a β-CD [50]. This allowed for larger self-assembly, encapsulating the polyplex within a pH responsive layer, which serves as both an added protection layer for the genetic payload and as a method for programmed endosomal escape and delivery [50]. These modifications allow for more efficient gene delivery, which can additionally reduce toxicity indirectly by reducing the dosage required to reach therapeutic transfection levels. Another example is the creation of PEI chains which terminate in a β-CD molecule. This allows for utilization of the self-assembly characteristics of CD to create a protective coating for the resulting polyplex, attaching polymers such as poly(ethylene glycol) (PEG) as an outer shielding layer [45]. By incorporating PEG, this delivery system may benefit from reduced immunogenicity and enhanced circulation times—both properties which can indirectly improve delivery efficiency. Looking forward, complex gene editing technology and the delivery of Cas9 protein could be improved using similar delivery techniques. While direct protein delivery is possible, delivery of pDNA or mRNA which encodes for Cas9 is generally preferred, as it is typically less challenging to encapsulate and deliver intracellulariy. PEI-β-CD shows promise as an effective vehicle for this approach [70], both for in vitro and in vivo applications [71].
While PEI is very commonly used in conjunction with CD, there are several other cationic polymers which have similar limitations and can take advantage of the improvements that CD can provide. One example is poly(2-dimethylaminoethyl methacrylate) (PDMAEMA) which [72], while less common, can exhibit up to 90% of the delivery efficiency of PEI and contains only tertiary amines, which are less reactive than their primary counterparts [73]. Similarly to PEI, larger molecular weight PDMAEMA shows better transfection efficiency, but increased toxicity. However, incorporating CD as a core to hold PDMAEMA [74] has proved successful for gene delivery, improving efficiency and allowing for reduced dosage and toxicity [49, 75]. The cationic lipid dioleoyl-3-trimethylammonium propane (DOTAP) has also been formulated with the addition of CD. In this case, a CD derivative was used (carboxymethyl-β-cyclodextrin), which exhibits more lipid-like (amphiphilic) properties better suited to liposome formation. When CD was introduced, increased transfection occurred in certain cell lines [76].
There are also naturally non-cationic polymers used in gene therapy which can be functionalized with polycations to provide DNA condensation properties. Poly(glycidyl methacrylate) (PGEA) can be functionalized with ethanolamine or ethylenediamine to produce a gene delivery vector [77, 78], and when combined with CD, the polymer can self-assemble on various surfaces, such as gold or iron nanoparticles [79]. This allows for gene delivery capabilities to be incorporated into many existing systems—gold nanoparticles especially have been studied extensively and their interactions within the body are well characterized. Similarly, linking ethanolamine functionalized PGEA to a β-CD backbone showed enhanced uptake, pDNA transfection, and siRNA silencing as compared to the “gold standard” 25 kDa PEI [80]. This system also took advantage of the self-assembling properties of CDs, and used the host/guest complex to create a PEG-coated and folate-targeted outer shell for additional targeting and stealth.
Overall, the incorporation of CDs into cationic polymer delivery vectors serves to improve transfection efficiency and lower cytotoxicity while also introducing modularity. Many modifications can be achieved building from the molecular structure of CDs and by taking advantage of self-assembly. This in turn allows for simple improvements to nanoparticle design to optimize delivery efficacy. CDs can also be modified directly to exhibit cationic and polyplex forming capabilities comparable to cationic polymers.
Polyrotaxanes
Polyrotaxanes are often cationic polymers themselves, but have additional characteristics which make them unique. A linear polymer backbone, such as PEG, interacts with CDs such that they thread along the PEG axis, forming a supramolecular structure [81, 82]. In a polypseudorotaxane, CDs can freely slide along and off the linear backbone. Polyrotaxanes have the ends of the backbone capped with a bulky blocking group, so that CDs are trapped in the structure until release is triggered [11], typically by changes in pH or redox state [50] to target areas such as tumor sites. Although they are trapped by the blocking group, CDs are free to slide along the length and rotate around the central polymer backbone [83].
Once threaded onto the backbone, CDs are often modified with various conjugates, some of which are cationic and allow for the direct condensation of genetic material such as pDNA and RNA [78]. The major advantages of polyrotaxanes include their reactive release of CDs and cargo, and their biomimicry. Chemically, they appear to mimic histones. Morphologically, they have been compared to the nucleosome [46]. Together, these advantages seem to allow for innate macrophage targeting, which can be useful in applications such as immunotherapy. Threaded alpha CDs along a PEG central backbone created a polyrotaxane which was shown to be uptaken by macrophages via the macrophage mannose receptor (MMR) [83]. In fact, it appears that macrophages can even be selectively targeted in this fashion, delivering payloads specifically to macrophages exhibiting the M2 phenotype. In nanoparticle systems, CD has been found to form host/guest inclusion complexes with R848, a TLR7 and TLR8 agonist that drives the M1 phenotype in macrophages [84]. With the addition of macrophage targeting, such systems could prove useful for gene delivery.
The cationic conjugates added to CD are often large polymers themselves. PGEA [78] and PEI [46] can be functionalized to CDs and threaded along the central polymer backbone. This results in a larger architecture which is similar to that of a large molecular weight branched polycation, but without the associated toxicity [46]. Large cationic polymers can also be integrated to the polyrotaxane system by acting as the central, backbone. By threading CD onto cationic methoxy-poly(ethylene glycol)-b-poly(ε-caprolactone)-b-poly(ethylene imine) (MPEG–PCL–PEI), a complicated co-polymer with the ability to form polyplexes with DNA was formed [85]. As a block co-polymer, one section of the system holds the CDs while another is primarily responsible for holding genetic material. This allowed for hydrogel formation with sustained gene release.
As with the more general cationic polymers, the introduction of CD to polyrotaxanes allows for improved transfection and reduced cytotoxicity, with the added advantages of triggered release dependent on the bulky end group, and the potential for macrophage targeting.
CD for supramolecular assembly
CDs can be cross-linked to each other to form large polymer networks, or used as cross-linkers themselves to create supramolecular structures [46, 75, 86]. As mentioned previously, the hydroxyl groups found on CD are reactive, and can be modified in several ways to account for chemistries which require alternate functional groups (Fig. 2). Reversible, but stable, host/guest interactions with CD can serve as structural cross-linkers as well. Cross-linked systems are often advantageous for delivery. Larger particles are more likely to extravasate from the bloodstream rather than undergo renal clearance, and variations in cross-linking can affect stifiness of the polymer matrix—both examples of properties which may allow delivery systems to overcome biological barriers and improve accumulation and delivery at the target site.
By cross-linking small cationic polymers into larger cationic networks, the advantages of high molecular weights can be obtained while decreasing associated toxicity [47, 87]. In situ cross-linking can allow for injectable delivery vehicles, allowing for larger gene delivery depots as well as localized delivery without requiring invasive implantation [85]. Cross-linking via CD host/guest interactions can allow tor shear-thinning behavior during injection, as well as rapid gel healing after removal of shear, allowing for injection directly into sensitive tissue [88]. These approaches may be useful in patient cases where local gene delivery is preferred, such as when the disease state only occurs in a specific location [89, 90]. Given that there still concerns about the off-target effects of gene editing technologies, localizing therapeutics in this fashion can potentially help mitigate these concerns. Collectively, cross-linking of non-viral polymeric delivery systems can reduce both toxicity and off-target effects.
PEI has been cross-linked using CD in various fashions by several groups [45,91–93]. By activating the hydroxyl groups on the outside of β-CD or γ-CD (using reagents such as 1,1-carbonyldiimidazole), cross-linking can be achieved with the amino groups found on PEI [91]. Low molecular weight PEI can also be cross-linked via disulfide bonds with CD [47]. Both cross-linking methods show lower cytotoxicity and higher transfection efficiency for the delivery of pDNA compared with those of a PEI 25 kDa control Polycaprolactone (PCL) is another polymer which has been chemically linked to CD, to form larger networks such as amphiphilic star polymers [75]. In this system, PCL arms were linked to the hydroxyl groups on a single β-CD, creating a star polymer with 21 arms. CD serves as the core of the particle, and acts as a multifunctional initiator [75]. These cross-linked polymer systems are just as stable as their individual polymer parts, but allow for greater control over individual polymer chains. Although these examples are single polymer systems, cross-linking in this fashion would also allow for the production of co-polymer systems—which may hold interest in future combination applications.
Cross-linking via CD hydroxyl groups is very effective, but can also require harsh chemicals or temperatures depending on the desired chemistries. An attractive alternative is to exploit the innate ability of CDs to self-assemble utilizing host/guest complexes [94, 95]. Adamantane (Ad) is a non-functionalized hydrocarbon which has a number of interesting derivatives. CD forms an inclusion complex with Ad which has a high association equilibrium constant, on the scale of 104–105 M−1 (log K = 5.04) [25, 96]. CD and Ad self-assemble under physiologic conditions to create reversible but stable structures [97]. Their usage in macromolecular design allows for assembly without complicated synthesis and separation steps [48]. The host/guest complexation does not produce any heat or byproducts, and the individual components have been shown to be non-immunogenic and non-toxic [27, 98]. The utilization of the CD-Ad complex allows for construction of delivery vehicles in a stepwise fashion, taking advantage of the non-covalent hydrophobic complex to form multiple self-assembling structures such as nanoparticles and hydrogels [95, 99]. The stepwise nature of these interactions also lends itself to the engineering of systems which are layered or blocked co-polymers, further increasing modularity. A number of nanoparticles fabricated using CD-Ad interactions have been reported, and these particles successfully deliver genetic material to various cell types and in vitro models [50, 95,100, 101].
Various material types can take advantage of the host/guest interactions of CD and Ad, even inorganic materials such as gold and iron nanoparticles [79]. Often, these structures utilize cationic polymers in their systems, such as poly(N,N’-bis(acryloyl)cystamine–poly-(aminoalkyl)) (PBAP) polyplexes, which were cross-linked using CD-Ad interactions [42]. These polyplexes showed similar or enhanced transfection efficiency compared to Lipofectamine 2000 (commercially available transfection reagent) with minimal cytotoxicity, as compared to the severe toxicity associated with Lipofectamine 2000. PEI is also commonly modified using the CD-Ad assembly to enable PEG shielding [45], cell targeting [100, 101], or even cross-linking to other PEI strands by modification of PEI with an adamantyl end functional group [48]. Multiple guest molecules can be modified with adamantyl functional groups in this fashion to take advantage of this natural self-assembly (Fig. 3). These guest molecules can function to complex with genetic cargo or introduce targeting to the delivery system, and are easily modified to fit the system’s needs. An adamantine-ended linear poly(poly(ethylene glycol)ethyl ether methacrylate) polymer was formed to allow for self-assembly of gene delivering star polycations [102]. CD-Ad has also been used to link multiple star polymers to each other, allowing for pDNA delivery [95].
Fig. 3.
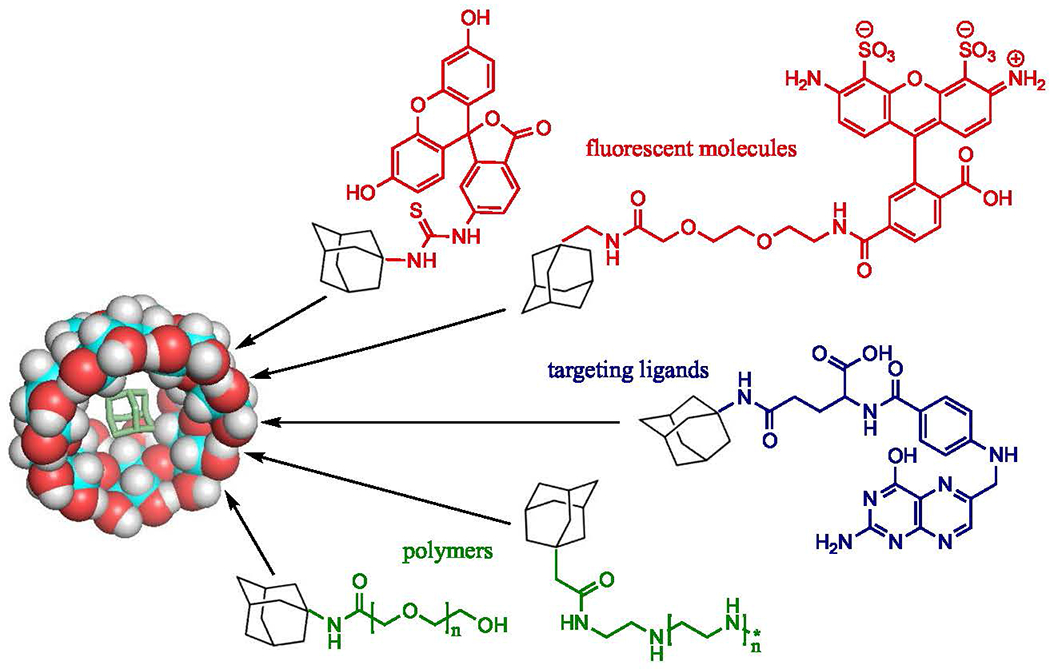
Adamantane and adamantyl functionalized molecules form host/guest inclusion complexes with cyclodextrin, allowing for self-assembly. 3D conformer of CD/Ad complex (left) and example molecule types which take advantage of this system (right) are shown: fluorescent molecules FITC and Alexa Fluor 488, targeting ligand folic acid, and polymers PEI and PEG (clockwise)
Benzimidazole can be used as a guest molecule in a similar fashion to Ad, creating a host/guest interaction that is pH mediated, and allows for triggered decomposition within the lysosome [51]. Other groups have used the benzimidazole/β-CD complex for chemotherapeutic delivery [103]. Ferrocene (Fc) has also been used in host/guest fashion. With a strong initial association (log K = 3.68) [25], oxidation of Fc leads to dissociation of the complex, creating a redox-responsive system for delivery [82]. Regardless of the molecules used to form the CD complex, all these modifications impart additional properties to existing gene delivery systems, Modification with functional groups can be used to attach many molecule types, from large polymer networks to small targeting moieties.
The addition of cell targeting creates a potential opportunity for the personalization and modification of CD-modified gene delivery vectors. The addition of PEG or folate to the surface of delivery vectors can both improve stability and reduce immunogenicity through steric hindrance and immune cell evasion. Folate specifically also allows for cell targeting, as it is overexpressed on many human cancers and can trigger cellular uptake via endocytosis [57, 80]. More specific targeting can be integrated as well via the addition of tumor-targeting peptides such as arginylglycylaspartic acid (RGD) [50]. Using CD-Ad interactions to add RGD targeting to PEI nanoparticles allows for cell-specific uptake [100,101].
The modulation of surface properties also allows for combination modifications. Multiple different types of Ad-modified molecules can be obtained: two targeting ligands (Ad-PEG-FA and Ad-PEG-LA), a cationic polymer (Ad-PEI), and a fluorescence agent (Ad-FITC) are such examples [104]. By combining these Ad-modified molecules, a single system which hosts diverse functions can be obtained and used to achieve efficient eradication of tumors [104]. Self-assembly is simple and can occur in ambient or physiological conditions with no toxic byproducts, and the integration of modularity allows for stimuli responsive, targeted gene delivery.
Pure CD vectors
Although less commonly used in gene delivery, there are other “pure” CD vectors which utilize the properties of CDs to enable gene delivery. These vectors use CD as the main constituent of the gene delivery system, as compared to the previously explored works which integrate CD to perform a secondary role. Examples include CD-based nanosponges [105], nanoparticles [84, 106, 107], vesicles [108], and hydrogels [11]. Typically, these formulations are composed of CD derivatives. Native CD does not have the innate ability to complex strongly with genetic material, and therefore has limited transfection ability. However, systems have been successfully engineered which allow for DNA condensation within the CD pocket [109]. Gene delivery can be achieved through the usage of carboxymethyl-β-cyclodextrin, a simple modification which is commercially available [110]. Some groups have had success delivering DNAzymes, which form 1:1 polyplexes with CD and can target and suppress genes of interest [107, 111]. Other groups have utilized the host/guest ability of CD to create responsive drug delivery systems, which function through binding competition or more effective isomerization, where the weaker complex that CD forms with genetic material is advantageous, as it can be undone by a molecule with a stronger affinity [112].
Cationic-modified and amphiphilic CDs are also used often in gene delivery systems. Cationic functional groups and hydrophobic anchors can be added to one or both faces, creating medusa-like, skirt-shaped, or bouquet-like CDs by adding polymer chains to the primary, secondary, or both faces respectively [27,37] (Fig. 4). These amphiphilic CDs then bind to genetic material and self-assemble to form nanostructures [57, 69,113,114]. These structures vary in shape, and can be liposomal, micellar, or random in nature. Cationic-CD/genetic material complexes have been shown to deliver siRNA effectively in vitro and in vivo with favorable toxicity profiles, successfully silencing the mutant protein in Huntington’s disease [55,115]. pDNA has also been successfully delivered by cationic-modified amphiphilic CDs [53,54,116]. Adding cationic components to CD seems to be the most direct method to complex genetic material. However, some groups prefer to create polyanionic CDs instead, which can interact with traditional cationic polymer and DNA polyplexes. Sulfobutylether-β-cyclodextrin is one such polymer [117].
Fig. 4.
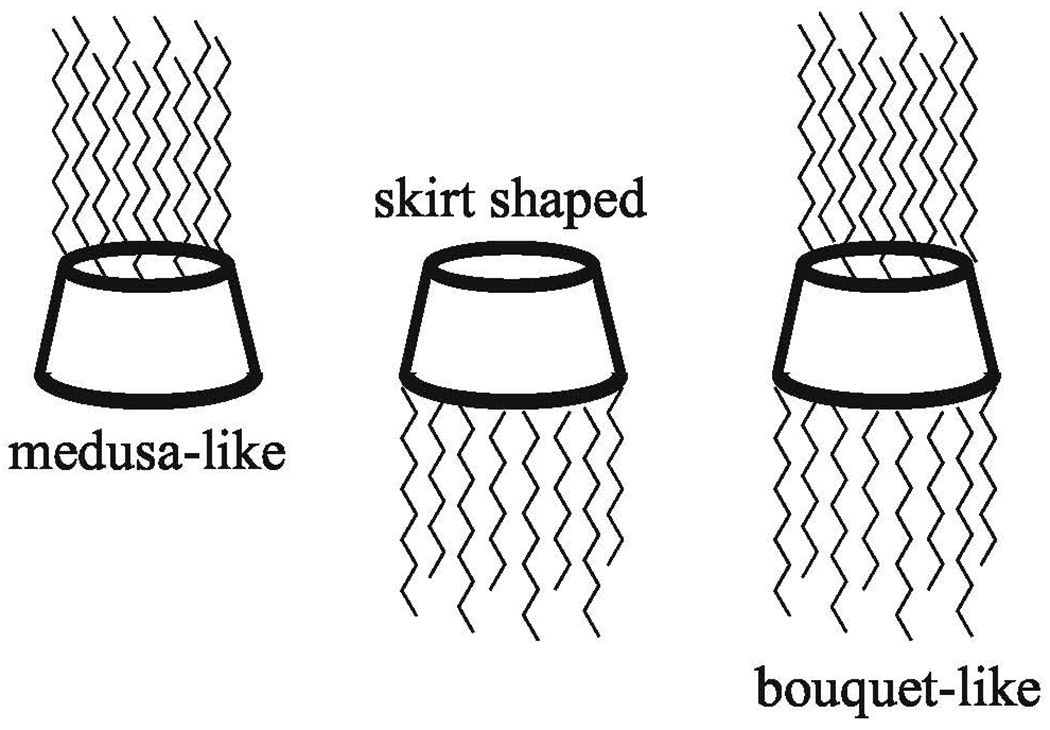
Amphiphilic cyclodextrins can be formed by adding polymer chains to the primary and/or secondary feces of CD
Other examples of functional groups being added to CD include the addition of cationic primary amines [106] and 2-hydroxylpropyl [118]. 2-hydroxylpropyl-β-cyclodextrin (HP-β-CD) is the most widely used modified CD, and is typically implemented to improve the aqueous solubility of lipophilic drugs [119]. This occurs due to the amphiphilic properties imparted by the additional polarity of the modification, It has been shown to be well tolerated in humans [120]. This CD derivative can also be further modified via click chemistry to create a wide variety of functionalized CDs, each with different properties. These ‘pure’ CD systems have a variety of advantages. They can self-assemble into a number of nanoshapes including micelles, spheres, and vesicles [109]. They can be easily modified to add targeting groups, allowing for targeted gene delivery, and can be reacted in a number of ways to create several combinations of properties at the primary and secondary faces.
CD in combination therapy: current and future directions
Combination therapy uses two or more therapeutic agents, and has been shown to be an effective treatment strategy in oncology. Gene, immuno-, and chemotherapies have been used in various combinations to improve patient outcome [121–123]. While combination therapy is widespread in cancer treatment it also holds promise for treating other disease states such as Alzheimer’s [124], cystic fibrosis [125], and infections [126]. The usage of multiple therapeutics simultaneously comes with a number of positive effects. Combination therapies allow for use of reduced individual dosages, and potentially achieving therapeutic effects without hitting dose-limiting toxicity levels. The use of multiple drug types can also result in synergistic effects, where the outcome is better than the sum of the individual drag effects [51]. Combination therapy also helps to reduce the likelihood of acquired resistance, by introducing alternate mechanisms of action [127]. In cancer especially, acquired resistance remains a large obstacle to many otherwise efficacious therapeutics.
Due to the number of advantages that combination therapy offers, it is a current and promising field of study. There are thousands of clinical studies investigating combination therapies, which results from the fact that the possible drug combinations are vast In the current climate of clinical treatment, which is moving away from a “one-size-fits-all” approach and towards the more widespread implementation of precision medicine, combination therapy is well suited to overcome limitations of single therapies by introducing secondary agents. One limitation to gene delivery is the inability to deliver enough cargo within target cells, due to the numerous biological barriers that delivery systems face. Secondary therapeutics can potentially enhance the delivery of genes, by down-regulating the immune cells that capture circulating delivery systems, or by beginning to break up the tumor microenvironment to improve distribution within solid tumors, for example.
However, in order to effectively deliver multiple therapeutic cargo types, delivery systems must be optimized for combination therapy. Therapeutics typically have alternate mechanisms of action and, resultingly, different final destinations within the body. The usage of CD is advantageous in these cases due to its ability to fulfill multiple functions: hosting small molecule drugs, acting as a linker or modular component, reducing immunogenicity, and disrupting membranes. CD forms inclusion complexes with many different drug types, including chemotherapeutics [59, 104, 128–130], immunomodulators [105, 118, 131], cytokines [132], and even proteins [82, 133]. When the modularity of CD hydroxyl groups is used to create gene delivery systems, the CD pocket often goes unused, and could be easily loaded with a small-molecule drug to integrate a secondary therapy type such as chemo- or immunotherapy. Similarly, delivery vehicles which use the CD pocket can often be modified at either of the CD faces to add an additional delivery component to the system. This concept makes it fairly easy to create CD delivery systems which can hold two or more payloads with variable properties and applications.
Existing CD delivery systems for combination therapy
CD delivery systems for combination therapy are generally very similar to those used in gene mono-therapy, and are covered in the previous sections of this work. While genetic material is delivered via cationic polymers, supramolecular assemblies, or pure CD vectors, alternate therapies are loaded into secondary parts of the system [65, 130]. These therapies are commonly chemotherapeutics or immunotherapies, and these complex systems can allow for the incorporation of tracking agents, active targeting, and responsive properties, among others. The ability to choose from multiple methods of therapy incorporation allows for modularity and the ability to personalize a drug delivery system, both advantages which should allow systems to be engineered to produce the best therapeutic results.
Chemotherapeutic drugs are one of the most commonly administered cancer treatments, and there are over a hundred used clinically in both mono- and co-therapies. Due to their promiscuous toxicity, effective delivery of chemotherapeutics aims to maximize exposure to tumor cells while minimizing exposure to healthy tissues. While these drugs are heterogeneous, they are typically small molecules with limited water solubility. Resultingly, they often have high affinities to complex with CDs, and can easily co-loaded when the CD pocket is not in use for gene delivery applications. Doxorubicin (DOX) is a very common chemotherapeutic which has been co-delivered via host/guest interactions with CD [51, 59, 60, 128, 134]. As an example, polycationic brushes have been used as multifunctional carriers, delivering both the p53 gene (which functions as a tumor suppressor), held by the cationic comb block, and DOX held in the CD pocket via host/guest interactions [59,134]. Another gene co-delivery system used PEI and CD for the loading of nucleic acids and DOX respectively [51,128]. DOX interacts directly with DNA, breaking it down to cause cell death. As it has the same final destination (intracellular, nucleus) as genetic cargo, the addition of DOX to a gene delivery system is fairly simple and typically does not require additional optimization. However, for nucleic acids which illicit effects within the cytosol (e.g. RNAi), additional design considerations may improve intracellular trafficking to the nucleus.
Paclitaxel (PTX) is another small molecule chemotherapeutic which can undergo host/guest interactions with CD, and has been successfully co-delivered with genetic cargo [117]. Researchers have seen successful co-delivery of the B cell lymphoma-2 conversion gene Nur77 and PTX using an injectable hydrogel system, which incorporated PEI, PCL, and CD [85,129]. Using this system, PTX delivery was measured over the course of a week. This time scale is important to note as PTX targets microtubules, disrupting mitosis. If PTX is not administered during a mitotic cycle, effects are limited. Although it is not a traditional chemotherapeutic, there has also been success with the co-delivery of genetic material and TPP, a photosensitizer which creates reactive oxygen species [135]. When complexed with CD as a guest molecule, TPP can impart additional tumor-killing effects as well as increased endosomal escape.
Co-delivery of these small molecules is not limited to the utilization of host/guest interactions alone. There are other CD systems in which DOX is encapsulated within the complex. In one such study, DOX was encapsulated in the center of a nanoparticle decorated with targeting ligands, PEI, and a fluorescent agent [104]. Another nanoparticle system loaded DOX into a PAMAM dendrimer core [60]. In these applications, these systems both reduced drug leakage and showed significant antitumor activity after intravenous administration. In the case of chemotherapy drugs, enhanced stability and encapsulation may be preferred to reduce off-target effects.
In addition to chemotherapeutics, gene therapies are also co-delivered with immunotherapies. Immunotherapeutics can be small molecules (immunomodulators, cytokines), proteins (antibodies, checkpoint inhibitors), or even cell-based therapies. Many of these molecule types have stability or solubility concerns, and are limited in their efficacy when administered in free drug form, Due to the wide range of sizes and properties of these therapies, immunotherapy delivery systems are varied. The modularity of CD, as well as its ability to complex with guest molecules of multiple types, shows promise for use in immunotherapy applications. Additionally, delivery vectors formulated with CD have been shown to have high macrophage affinity [136], which could be advantageous for the introduction of immunotherapies.
Combination therapies also can incorporate tracking agents, active targeting, and responsive properties, further improving modularity. There are existing nanoparticle formulations for combination therapy which demonstrate this modular nature. CD coatings can be used to allow multiple molecules to dock to the surface via Ad/CD complexes. In one such system targeting ligands, PEI, and a fluorescent agent were attached to the surface via Ad/CD complexes [104]. Ad/CD complexes self-assemble, making the fabrication of this particle facile. This allowed for targeted delivery of genetic cargo, as well as tracking of the delivery system.
Although this review has focused mainly on non-viral vectors which incorporate CD, there are a few usages of CD in viral gene delivery as well, some of which are combinatorial. By linking CD to the surface of an adenovirus, reduced immunogenicity can be achieved [86], as well as increased modularity via delivery of a secondary therapeutic, such as TGF-beta inhibitors [65]. While these examples focus specifically on single delivery systems which hold multiple therapeutic cargo types, it is also important to note that there is a large body of work in which co-delivery does not utilize a single delivery vector. In many of these applications, delivery systems are used to improve efficacy of one therapeutic, and another is administered as free drug. In many cases, these studies serve to check the interactions of two drug types before time is spent optimizing a delivery system for both. However, in some cases, the synergy of drugs may only be seen when delivery efficiency is above a certain level. To that point, there are a number of existing mono-therapies which could show significant improvement if coordinated delivery of multiple therapeutics could be attained.
Future combination therapy applications
The previous examples were created with the intent of co-delivering therapeutics, but there are other delivery vehicles which have been crested for monotherapies and may be easily translatable for use in combination therapy. These typically fall into two categories: delivery systems which were co-administered with a free drug, and delivery systems which have a currently unused component
In the first category, a pure β-CD nanoparticle was fabricated to hold DNAzyme, which demonstrated minimal cancer cell killing actvity (10–20% cell death) on its own, but was found to work synergistically with DOX to improve effects [107]. In this work, DOX was not loaded into the nanoparticle itself but was instead administered in a soluble form which mimics systemic administration. However, DOX is known to form inclusion complexes with CD, and could likely be delivered more consistently and for longer periods of time if co-delivery was explored. Similarly, the immunotherapeutic NLG919 was found to form host/guest complexes with a CD derivative, improving solubilization and anti-tumor efficacy when delivered in concert with free PTX [137]. By co-delivering these two therapeutics, even greater efficacy may be possible. Another study showed that the delivery of R848 (Toll-like receptor agonist) from a CD-based delivery system could promote the M1 macrophage phenotype and enhance the efficacy of the checkpoint inhibitor anti-PD-1 [84]. These, and other immunotherapy applications, could be improved through the usage of smart delivery system design for co-therapeutic delivery.
An example of a delivery system with an unused component is a co-delivering cationic nanocapsule [138]. A cationic poly(CD) and alginate shell was formulated for the delivery of 4-hydroxy-tamoxifen, a selective estrogen receptor (ER) modulator used for the treatment of breast cancer. A small molecule drug, tamoxifen, was loaded into the nanocapsule via inclusion with the CD pocket. The cationic nature of the CD polymer in this system brings forward the idea of integrating gene delivery as well, through the formation of polyplexes. In this system specifically, the cationic charge is used to create layering, via interactions with the negatively charged alginate, but the idea still holds as inspiration for similar systems which already utilize cationic polymers. These are only a few examples of such systems, but the numerous methods which have been used to create delivery systems for chemo-, immune-, and gene therapy support the theory that as more complex systems are developed, it will become easier to incorporate multiple therapeutic types and optimize for both.
Conclusions
The utilization of CDs in gene delivery vectors can improve overall efficacy of the system. Formulations incorporating CD show more efficient gene condensation, delivery, and transfection, compared to the common standards tested: 25 kDa PEI and Lipofectamine 2000. These improvements can come from direct action of CDs, as is the case when improved membrane disruption occurs, or can be indirect applications—improvements to stability and size control can enhance passive targeting and uptake. Additionally, CDs have been shown to reduce related toxicities. Many efficient gene delivery vectors currently come with significant side effects, and these can be mitigated through smart engineering of the delivery system—including the introduction of CD.
CDs are generally biocompatible, and have already been approved for clinical applications. As of January 2020, 18 clinical trials in the USA with the term “cyclodextrin” in the study title are listed as completed (based on a ClinicalTrials.gov search). Another 9 trials are listed as either active or recruiting. This makes the path to market marginally easier, which is not often the case when adding components to a delivery system. There are several current studies which utilize CDs in gene delivery, and as more uses are discovered, the utility of the CD system increases. There are also a fair number of systems which have shown efficacy through in vivo testing, on both small animals and large non-human primates [139], further supporting the idea that this toolbox of gene delivery vectors, including those which incorporate CD, is growing (Fig. 5).
Fig. 5.
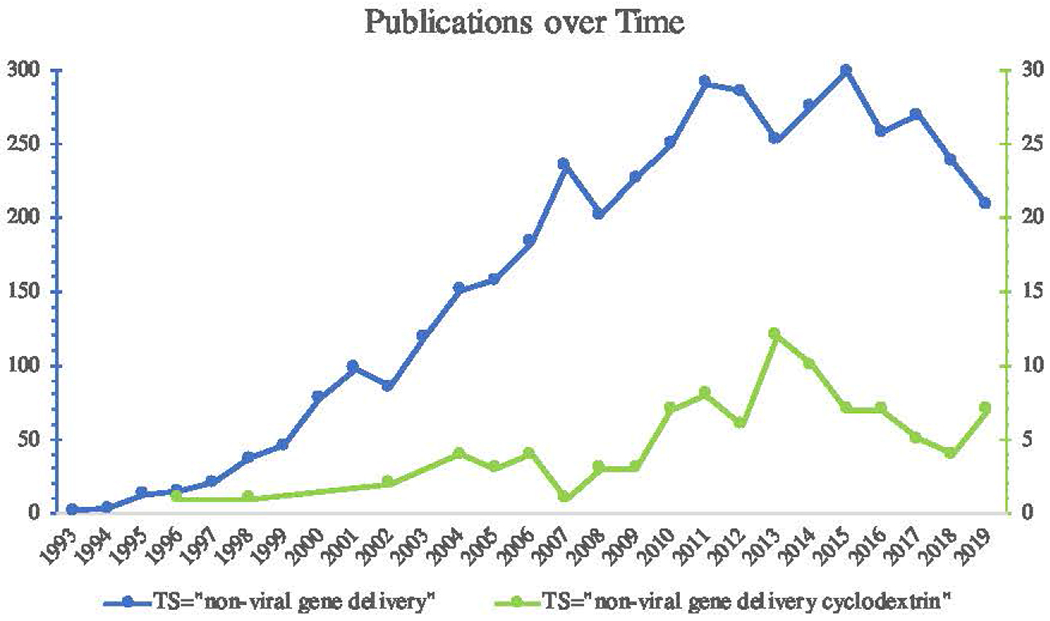
Publications on non-viral gene delivery over time. Search terms (on pubmed.gov) used ware TOPIC = “non-viral gene delivery” or TOPIC = “non-viral gene delivery cyclodextrin” Far fewer gens delivery papers are published on CD each year, hut both searches seem to follow the same trend
Over the past 10 years, the body of work which explores CDs for gene delivery has been growing, with a fairly consistent number of new publications each year. The content of this work has also been consistent. CDs are still cross-linked using epichlorahydrin and isocyanate cross-linkers, and CDs and cationic polymers continue to be incorporated to balance polyplex stability and toxicity concerns. Adamantane remains the chemical of choice for many CD-based self-assembling nanoparticles, and CD polyrotaxanes continue to be developed and modified. In 2011, CDs were called “novel nanoobjects of undeveloped potential” [27]. In 2020, new subclasses of CDs in gene delivery systems have begun to explore this potential and optimize the initial discoveries in the field. Of the literature cited in this work, almost 40% is from the last 2 years (2019, 2018). The achievements of these groups are complex engineering designs which are more tightly controlled and allow for more specificity of outcome.
Systems incorporating CD allow for modularity in the design. Self-assembly allows for the simple fabrication of complex systems, making delivery of multiple therapeutics facile. Of course, there are still challenges that remain. Guest recognition has been well studied, but delivery from the inner CD pocket can be affected by the larger polymer structure. It will likely not be so easy that any small molecule drug can be switched out for another—differing structures and charges will likely result in differing release profiles. More research needs to be done to fully understand the mechanisms behind these controlled release systems, so that we can better predict which functionalities can be achieved. As we continue to move towards combinatorial and personalized treatments, it is important that the treatment systems we design make allowances for this direction, CD can fulfill many roles in delivery systems, making it an important addition to platforms that require modularity.
Acknowledgments
Funding information M.J.M. acknowledges support from a Burroughs Wellcome Fund Career Award at the Scientific Interface (CASI), a US National Institutes of Health (NIH) Director’s New Innovator Award (DP2 TR002776), a grant from the American Cancer Society (129784-IRG-16-188-38-IRG), the National Institutes of Health (NCI R01 CA241661, NCI R37 CA244911, and NIDDK R01 DK123049), an Abramson Cancer Center (ACC)-School of Engineering and Applied Sciences (SEAS) Discovery Grant (P30 CA016520), and a 2018 AACR-Bayer Innovation and Discovery Grant, Grant Number 18-80-44-MITC (to M.J.M.).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Declaration of informed consent or animal studies No animal or human studies were carried out by the authors for this article.
References
- 1.Terheggen HG, Lowenthal A, Lavinha F, Colombo JP, Rogers S. Unsuccessful trial of gene replacement in arginase deficiency. Zeitschrift für Kinderheilkunde. 1975;119:1–3. [DOI] [PubMed] [Google Scholar]
- 2.Maguire AM, Russell S, Wellman JA, Chung DC, Yu Z-F, Tillman A, et al. Efficacy, safety, and durability of voretigene neparvovecrzyl in RPE65 mutation-associated inherited retinal dystrophy: results of phase 1 and 3 trials. Ophthalmology. 2019; 126:1273–85. 10.1016/j.ophtha.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 3.ClinicalTrials.gov [Internet] Identifier NCT03399448, NY-ESO-1-redirected CRISPR (TCRendo and PD1) Edited T Cells (NYCE T Cells), 2018. January 16 Available from: https://clinicaltrials.gov/ct2/show/NCT03399448?term=03399448&draw=2&rank=1.
- 4.Reardon S CRISPR gene-editing creates wave of exotic model organisms. Nature. 2019;568:441–2. [DOI] [PubMed] [Google Scholar]
- 5.Reardon S Welcome to the CRISPR zoo. Nature. 2016;531:160–3. 10.1038/531160a. [DOI] [PubMed] [Google Scholar]
- 6.Qasim W, Zhan H, Samarasinghe S, Adams S, Amrolia P, Stafford S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017;9:1–9. 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]
- 7.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RPT, Carpenter RO, Maryalice SS, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–9. 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalos M, Levine BL, Porter DL, Katz S, Stephan A, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects. Sci Transl Med. 2011;3:1–21, 10.1126/scitranslmed.3002842.T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cope S, Ayers D, Zhang J, Batt K, Jansen JP. Integrating expert opinion with clinical trial data to extrapolate long-term survival: a case study of CAR-T therapy for children and young adults with relapsed or refractory acute lymphoblastic leukemia. BMC Med Res Methodol. 2019;19:1–11. 10.1186/s12874-019-0823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walther W, Stein U. Viral vectors for gene transfer. Drugs. 2000;60:249–71. 10.2165/00003495-200060020-00002. [DOI] [PubMed] [Google Scholar]
- 11.Rey-Rico A, Cucchiarini M, Supramolecular cyclodextrin-based hydrogels for controlled gene delivery. Polymers (Basel). 2019;11: 1–9. 10.3390/polym11030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine) and its role in gene delivery. J Control Release. 1999;60:149–60. 10.1016/S0168-3659(99)00090-5. [DOI] [PubMed] [Google Scholar]
- 13.Nyamay’Antu A, Dumont M, Kedinger V, Erbacher P. Non-viral vector mediated gene delivery: the outsider to watch out for in gene therapy. Cell Gene Ther Insights. 2019;5:51–7. 10.18609/cgti.2019.007. [DOI] [Google Scholar]
- 14.De Laporte L, Cruz Rea J, Shea LD. Design of modular non-viral gene therapy vectors. Biomaterials. 2006;27:947–54. 10.1016/j.biomaterials.2005.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodik RV, Klymchenko AS, Mely Y, Kalchenko VI. Calixarenes and related macrocycles as gene delivery vehicles, J Incl Phenom Macrocycl Chem. 2014;80:189–200. 10.1007/s10847-014-0412-8. [DOI] [Google Scholar]
- 16.Li M, Zhao M, Fu Y, Li Y, Gong T, Zhang Z, et al. Enhanced intranasal delivery of mRNA vaccine by overcoming the nasal epithelial barrier via intra- and paracellular pathways. J Control Release. 2016;228:9–19. 10.1016/j.jconrel.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 17.Nikam RR, Gore KR, Journey of siRNA: clinical developments and targeted delivery. Nucleic Acid Ther. 2018:28:209–24. 10.1089/nat.2017.0715. [DOI] [PubMed] [Google Scholar]
- 18.ClinicalTrials.gov [Internet], Identifier NCT03997383, APOLLO-B: A Study to Evaluate Patisiran in Participants With Transthyretin Amyloidosis With Cardiomyopathy (ATTR Amyloidosis With Cardiomyopathy) - Full Text View - ClinicalTrials.gov 2019. June 25 Av.
- 19.Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang C-C, Ueda M, Kristen AV, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:11–21. 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Qin L, Yamankurt G, Skakuj K, Huang Z, Chen PC, et al. Rational vaccinology with spherical nucleic acids. Proc Natl Acad Sci U S A. 2019;116:10473–81. 10.1073/pnas.1902805116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ClinicalTrials.gov [Internet] Identifier NCT03684785, Intratumoral AST-008 Combined With Pembrolizumab in Patients With Advanced Solid Tumors - Full Text View - ClinicalTrials.gov 2018. September 26 Available from: https://clinicaltrials.gov/ct2/show/NCT03684.
- 22.Pengnam S, Leksantikul L. Patrojanasophon P, Opanasopit P, Niyomtham N, Yingyongnarongkul B, et al. Influence of serum on DNA protection ability and transfection efficiency of cationic lipid-based nanoparticles for gene delivery. MATEC Web Conf. 2018;192:1025 10.1051/matecconf/201819201025. [DOI] [Google Scholar]
- 23.Loftsson T, Jarho P, Másson M, Järvinen T, Cyclodextrins in drug delivery. Expert Opin Drug Deliv. 2005;2:335–51. 10.1517/17425247.2.1335. [DOI] [PubMed] [Google Scholar]
- 24.Loftsson T, Duchêne D, Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007:329:1–11. 10.1016/j.ijpharm.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 25.Rekharsky MV, Inoue Y. Complexation thermodynamics of cyclodextrins, Chem Rev. 1998;98:1875–918. 10.1021/cr970015o. [DOI] [PubMed] [Google Scholar]
- 26.Balabai N, Linton B, Napper A, Priyadarshy S, Sukharevsky AP, Waldeck DH. Orientational dynamics of β-cyclodextrin inclusion complexes. J Phys Chem B. 1998;102:9617–24. 10.1021/jp982756e. [DOI] [Google Scholar]
- 27.Mellet CO, Fernández JMG, Benito JM Cyclodextrin-based gene delivery systems. Chem Soc Rev. 2011;40:1586–608. 10.1039/C0CS00019A. [DOI] [PubMed] [Google Scholar]
- 28.Muankaew C, Loftsson T. Cyclodextrin-based formulations: a non-invasive platform for targeted drug delivery. Basic Clin Pharmacol Toxicol. 2018;122:46–55. 10.1111/bcpt.12917. [DOI] [PubMed] [Google Scholar]
- 29.Klein K, Mann JFS, Rogers P, Shattock RJ. Polymeric penetration enhancers promote humoral immune responses to mucosal vaccines. J Control Release. 2014;183:43–50. 10.1016/j.jconrel.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Jiménez Blanco JL, Benito JM, Ortiz Mallet C, Garcîa Fernández JM. Molecular nanoparticle-based gene delivery systems. J Drug Deliv Sci Technol. 2017;42:18–37. 10.1016/j.jddst.2017.03.012. [DOI] [Google Scholar]
- 31.Renard E, Deratani A, Volet G, Sebille B. Preparation and characterization of water soluble high molecular weight α-cyclodextrin-epichlorohydrin polymers. Eur Polym J. 1997;33:49–57. 10.1016/S0014-3057(96)00123-1. [DOI] [Google Scholar]
- 32.Rodriguez-Tenreiro C, Alvarez-Lorenzo C, Rodriguez-Perez A, Concheiro A, Torres-Labandeira JJ. New cyclodextrin hydrogels cross-linked with diglycidylethers with a high drug loading and controlled release ability. Pharm Res. 2006;23:121–30. 10.1007/s11095-005-8924-y. [DOI] [PubMed] [Google Scholar]
- 33.Thatiparti TR, von Recum HA. Cyclodextrin complexation for affinity-based antibiotic delivery. Macromol Biosci. 2010; 10:82–90. 10.1002/mabi.200900204. [DOI] [PubMed] [Google Scholar]
- 34.Euvrard É, Morin-Crini N, Druart C, Bugnet J, Martel B, Cosentino C, et al. Cross-linked cyclodextrin-based material for treatment of metals and organic substances present in industrial discharge waters. Beilstein J Org Chem. 2016;12:1826–38. https://doi.org/103762/bjoc.12.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shelley H, Babu RJ. Role of cyclodextrins in nanoparticle-based drug delivery systems. J Pharm Sci. 2018;107:1741–53. 10.1016/j.xphs.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 36.Bai H, Wang J, Li Z, Tang G. Macrocyclic compounds for drug and gene delivery in immune-modulating therapy. Int J Mol Sci. 2019. 20:2097 10.3390/ijms20092097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geng W-C, Huang Q, Xu Z, Wang R, Guo D-S. Gene delivery based on macrocyclic amphiphiles. Theranostics. 2019;9:3094–106. 10.7150/thno.31914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai W-F. Cyclodextrins in non-viral gene delivery. Biomaterials. 2014;35:401–11. 10.1016/j.biomaterials.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li JJ, Zhao F, Li J. Supramolecular polymers based on cyclodextrins for drug and gene delivery BT - biofunctionalization of polymers and their applications. In: Nyanhongo GS, Steiner W, Gübitz G, editors. Berlin: Springer Berlin Heidelberg; 2011, p. 207–49. [Google Scholar]
- 40.Billingsley M, Singh N, Ravikumar P, Zhang R, June CH, Mitchell MJ, Ionizable lipid nanoparticle mediated mRNA delivery for human CAR T cell engineering. Nano Lett 2020. 10.1021/acs.nanolett.9b04246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olden B, Cheng Y, Yu J, Pun S. Cationic polymers for non-viral gene delivery to human T cells. J Control Release, 2018;282 10.1016/j.jconrel.2018.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Ma B, Abdeen AA, Chen G, Xie R, Saha K, et al. Versatile redox-responsive polyplexes for the delivery of plasmid DNA, messenger RNA, and CRISPR-Cas9 genome-editing machinery. ACS Appl Mater Interfaces. 2018;10:31915–27. 10.1021/acsami.8b09642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wojnilowicz M, Glab A, Bertucci A, Caruso F, Cavalieri F. Super-resolution imaging of proton sponge-triggered rupture of endosomes and cytosolic release of smalt interfering RNA. ACS Nano. 2019;13:187–202. 10.1021/acsnano.8b05151. [DOI] [PubMed] [Google Scholar]
- 44.Yao H, Chen S-C, Shen Z, Huang Y-C, Zhu X, Wang X, et al. Functional characterization of a PEI-CyD-FA-coated adenovirus as delivery vector for gene therapy. Curr Med Chem. 2013;20: 2601–8. [DOI] [PubMed] [Google Scholar]
- 45.Wright KJ, Badwaik VD, Samaddar S, Hyun S-H, Glauninger K, Eom T, et al. Organocaialytic synthesis and evaluation of polycarbonate pendant polymer: β-cydodextrin-based nucleic acid delivery vectors. Macromolecules. 2018;51:670–8. 10.1021/acs.macromol.7b02293. [DOI] [Google Scholar]
- 46.Ardeleanu R, Dascalu AI, Neamtu A, Peptanariu D, Uritu CM, Maier SS, et al. Multivalent polyrotaxane vectors as additive cargo complexes for gene therapy, Polym Chem. 2018;9:845–59. 10.1039/C7PY01256J. [DOI] [Google Scholar]
- 47.Xu F, Zhong H, Chang Y, Li D, Jin H, Zhang M, et al. Targeting death receptors for drug-resistant cancer therapy: codelivery of pTRAIL and monensin using dual-targeting and stimuli-responsive self-assembling nanocomposites. Biomaterials. 2018;158:56–73. 10.1016/j.biomaterials.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 48.Yan F, Wu JS, Liu ZL, Yu HL, Wang YH, Zhang WF, et al. Ruthenium-containing supramolecular nanoparticles based on bipyridine-modified cyclodextrin and adamaniyl PEI with DNA condensation properties. Nanoscale Res Lett 2018;13 10.1186/s11671-018-2820-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loh XJ, Wu Y-L. Cationic star copolymers based on β-cyclodextrins for efficient gene delivery to mouse embryonic stem cell colonies. Chem Commun, 2015;51:10815–8. 10.1039/C5CC03686K. [DOI] [PubMed] [Google Scholar]
- 50.Wong L-Y, Xia B, Wolvetang E, Cooper-White J. Targeted, stimuli-responsive delivery of plasmid DNA and miRNAs using a facile self-assembled supramolecular nanoparticle system, Biomacromolecules. 2018;19:353–63. 10.1021/acs.biomac.7b01462. [DOI] [PubMed] [Google Scholar]
- 51.Zhou X, Xu L, Xu J, Wu J, Kirk TB, Ma D, et al. Construction of a High-efficiency drug and gene co-delivery system for cancer ther apy from a pH-sensitive supramolecular inclusion between oligoethylenimine-graft-β-cyclodextrin and hyperbranched polyglycerol derivative. ACS Appl Mater Interfaces. 2018;10: 35812–29. 10.1021/acsami.8b14517. [DOI] [PubMed] [Google Scholar]
- 52.Xu C, Wu Y-L, Li Z, Loh XJ. Cyclodestrin-based sustained gene release systems; a supramolecular solution towards clinical applications. Mater Chem Front. 2019;3:181–92, 10.1039/C8QM00570B. [DOI] [Google Scholar]
- 53.Mández-Ardoy A, Guilloteau N, Di Giorgio C, Vierling P, Santoyo-González F, Ortiz Mellet C, et al. β-Gyriocknctrin-based polycaticmis amphiphilic “click’ clusters: effect of structural modifications in their DNA complexing and delivery properties. J Organomet Chem. 2011;76:5882–94, 10.1021/jo2007785. [DOI] [PubMed] [Google Scholar]
- 54.Ortega-Caballero F, Mdlet CO, Le Gourriérec L, Guilloteau N, Di Giorgio C, Vierling P, et al. Tailoring β-cyclodextrin for DNA complexation and delivery by homogeneous functionalization at the secondary face. Org Lett. 2008;10:5143–6. 10.1021/o1802081z. [DOI] [PubMed] [Google Scholar]
- 55.Godinho BMDC, Ogier JR, Darcy R, O’Driscoll CM, Cryan JF. Self-assembling modified β-cyclodextrin nanoparticies as neuronal siSNA delivery vectors: focus on Huntington’s disease. Mol Pharm. 2013;10:640–9. 10.1021/mp3003946. [DOI] [PubMed] [Google Scholar]
- 56.Cryan SA, Donohue R, Ravoo BJ, Darcy R, O’Driscoll CM. Cationic cyclodextrin amphiphiles as gene delivery vectors. J Drug Deliv Sci Technol. 2004;14:57–62. 10.1016/S1773-2247(04)50006-0. [DOI] [Google Scholar]
- 57.Yao H, Ng SS, Tucker WO, Tsang Y-K-T, Man K, Wang X, et al. The gene transfection efficiency of a folate-PEI600-cyclodextrin nanopolymer. Biomaterials. 2009;30:5793–803. 10.1016/j.biomaterials.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 58.Pun SH, Bellocq NC, Liu A, Jensen G, Machemer T, Quijano E, et al. Cyclodextrin-modified polyethylenimine polymers for gene delivery, Bioconjug Chem. 2004;15:831–40. 10.1021/bc049891g. [DOI] [PubMed] [Google Scholar]
- 59.Chen W, Zhang M, Shen W, Du B, Yang J, Zhang Q, A polycationic brush mediated co-delivery of doxorubicin and gene for combination therapy. Polymers (Basel). 2019;11:60, https://doi.org/103390/polym11010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohammed AFA, Higashi T, Motoyama K, Ohyama A, Onodera R, Khaled KA, et al. In vitro and in vivo co-delivery of siRNA and doxorubicin by folate-PEG-appended dendrimer/glucuronylglucosyl-β-cyclodextrin conjugate. AAPS J. 2019;21:54 10.1208/s1248-019-0327-9. [DOI] [PubMed] [Google Scholar]
- 61.Pipemo A, Mazzaglia A, Scala A, Pennisi R, Zagami R, Neri G, et al. Casting light on intracellular tracking of a new functional graphene-based Micro RNA delivery system by FLIM and Raman imaging. ACS Appl Mater Interfaces. 2019;11:46101–11. 10.1021/acsami.9b15826. [DOI] [PubMed] [Google Scholar]
- 62.Fenyvesi F, Réti-Nagy K, Bacsó Z, Gutay-Tóth Z, Malanga M, Fenyvesi É, et al. Fluorescently labeled metiryl-beta-cyclodextrin enters intestinal epithelial Caco-2 cells by fluid-phase eridocytosis. PLoS One. 2014;9:1–11. 10.1371/journal.pone.0084856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barua S, Ramos J, Potta T, Taylor D, Huang H-C, Montanez G, et al. Discovery of cationic polymers for non-viral gene delivery using combinatorial approaches. Comb Chem High Throughput Screen. 2011;14:908–24. 10.2174/138620711797537076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lungwitz U, Breunig M, Blunk T, Göpferich A. Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm. 2005;60:247–66. 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 65.Jiang J, Zhang Y, Peng K, Wang Q, Hong X, Li H, et al. Combined delivery of a TGF-β inhibitor and an adenoviral vector expressing interleukin-12 potentiates cancer immunotherapy, Acta Biomater, 2017;61:114–23. 10.1016/j.actbio.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 66.Yao H, Ng S, Huo L-F, Chow B, Shen Z, Yang M, et al. Effective melanoma immunotherapy with interleukin-2 delivered by a novel polymeric nanoparticle. Mol Cancer Ther. 2011;10:1082–92, https://dri.org/10.1158/1535-7163.MCT-10-0717. [DOI] [PubMed] [Google Scholar]
- 67.Li J-M, Wang Y-Y, Zhang W, Su H, Ji L-N, Mao Z-W. Low-weight polyethylenimine cross-linked 2-hydroxypopyl-β-eyebdextrin and folic and as an efficient and nontoxic siRNA carrier for gene silencing and tumor inhibition by VEGF siRNA. Int J Nanomedicine, 2013;8:2101–17. https://doi.oig/10.2147/IJN.S42440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Forrest M, Gabrielson N, Pack D. Cyclodextrin - polyethylenimine conjugates for targeted in vitro gene delivery. Biotodhnol Bioeng. 2005;89:416–23. 10.1002/bit.20356. [DOI] [PubMed] [Google Scholar]
- 69.Villari V, Mazzaglia A, Darcy R, O’Driscoll CM, Micali N. Nanostructures of cationic amphiphilic cyclodextrin complexes with DNA. Biomacromolecules. 2013;14:811–7. 10.1021/bm3018609. [DOI] [PubMed] [Google Scholar]
- 70.Ping Y, Hu Q, Tang G, Li J, FGFR-targeted gene delivery mediated by supramolecular assembly between β-cyclodextrin-crosslinked PEI and redox-sensitive PEG. Biomaterials. 2013:34:6482–94. 10.1016/j.biomaterials.2013.03.071. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Z, Wan T, Chen Y, Chen Y, Sun H, Cao T, et al. Cationic polymer-mediated CRISPR/Cas9 plasmid delivery for genome editing. Macromol Rapid Common, 2019;40:1800068 10.1002/marc.201800068. [DOI] [PubMed] [Google Scholar]
- 72.Chemg J-Y, van de Watering P, Talsma H, Crommelin DJA, Hennink WE. Effect of size and serum proteins on transfection efficiency of poly ((2-dimeffiylamino)eibyl methacrylate)-plasmid nanoparticles. Pharm Res. 1996;13:1038–42. https://dri.org/10.1023/A:1016054623543. [DOI] [PubMed] [Google Scholar]
- 73.Agarwal S, Zhang Y, Maji S, Greiner A. PDMAEMA based gene delivery materials. Mater Today. 2012;15:388–93. 10.1016/S1369-7021(12)70165-7. [DOI] [Google Scholar]
- 74.Zhou Z, Guo F, Wang N, Msng M, Li G. Dual pH-sensitive supramolecular micelles from star-shaped PDMAEMA based on β-cyclodextrin for drug release. Int J Biol Macromol. 2018;116:911–9. 10.1016/j.ijbiomac.2018.05.092. [DOI] [PubMed] [Google Scholar]
- 75.Fan X, Cheng H, Wu Y, Loh XJ, Wu Y-L, Li Z. Incorporation of polycaprolactone to cyclodextrin-based nanocarrier for potent gens delivery, Macromol Mater Eng. 2018;303:1800255 10.1002/mamc.201800255. [DOI] [Google Scholar]
- 76.Elsana H, Olusanya TOB, Carr-wilkinson J, Darby S, Faheem A, Elkordy AA. Evaluation of novel cationic gene based liposomes with cyclodextrin prepared by thin film hydration and microfluidic systems. Sci Rep. 2019;9:1–17. 10.1038/s41598-019-51065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu FJ, Zhu Y, Chai MY, Liu FS. Comparison of etbsnolamine/ethylenedamine-functionalized poly(glycidyl methacrylate) for efficient gene delivery. Acta Biomater. 2011. ;73131–40. 10.1016/j.actbio.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 78.Song H-Q, Qi Y, Li R-Q, Cheng G, Zhao N, Xu F-J. High-performance cationic polyrotaxanes terminated with polypeptides as promising nucleic acid delivery systems. Polym Chem. 2018;9: 2281–9. 10.1039/C8PY00333E. [DOI] [Google Scholar]
- 79.Duan S, Li J, Zhao N, Xu F-J. Multifunctional hybrids with versatile types of nanoparticles via self-assembly for complementary tumor therapy. Nanoscale. 2018;10:7649–57. 10.1039/C8NR00767E. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y, Jiang Q, Wojnilowicz M, Pan S, Ju Y, Zhang W, et al. Acid-sensitive poly(β-cyclodextrin)-based multifunctional supramolecular gene vector. Polym Chem. 2018;9:450–62. 10.1039/C7PY01847A. [DOI] [Google Scholar]
- 81.Loethen S, Kim J, Thompson DH. Biomedical applications of cyclodextrin based polyrotaxanes. Polym Rev. 2007;47:383–418. https://doi.org/l0.1080/15583720701455145. [Google Scholar]
- 82.Dong Z, Kang Y, Yuan Q, Luo M, Gu Z. H(2)O(2)-responsive nanqpaitide based on the supramoleeular selt-assemble of cyclodextrin, Front Pharmacol. 2018;9:552 10.3389/fphar.2018.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shibaguchi K, Tamura A, Terauchi M, Matsumura M, Miura H, Yui N. Mannosylated polyrotaxanes for increasing cellular uptake efficiency in macrophages through receptor-mediated endocytosis. Molecules. 2019;24:439 10.3390/molecules24030439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, et al. TLR7/8-agonist-loaded nanopartides promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng. 2018;2:578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu X, Chen X, Chua MX, Li Z, Loh XJ, Wu Y-L. Injectable supramolecular hydrogels as delivery agents of Bcl-2 conversion gene for the effective shrinkage of therapeutic resistance tumors. Adv Healthc Mater. 2017;6:1700159 10.1002/adhm.201700159. [DOI] [PubMed] [Google Scholar]
- 86.Fan G, Fan M, Wang Q, Jiang J, Wan Y, Gong T, et al. Bio-inspired polymer envelopes around adenoviral vectors to reduce immunogenicity and improve in vivo kinetics. Acta Biomater. 2016;30:94–105. 10.1016/j.actbio.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 87.Wen Y, Pan S, Luo X, Zhang X, Zhang W, Feng M, A biodegradable low molecular weight polyethylenimine derivative as low toxicity and efficient gene vector. Bieconjug Chem. 2009;20: 322–32. 10.1021/bc800428y. [DOI] [PubMed] [Google Scholar]
- 88.Wang LL, Liu Y, Chung JJ, Wang T, Gaffey AC, Lu M, et al. Local and sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischemic injury. Nat Biomed Eng. 2017;1:983–92. 10.1038/s41551-017-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Badea I, Virtanen C, Verrall RE, Rosenberg A, Foldvari M. Effect of topical interferon-γ gene therapy using gemini nanoparticles on pathophysiological markers of cutaneous sclerodema in Tsk/+ mice. Gene Iher. 2012;19:978–87. 10.1038/gt.2011.159. [DOI] [PubMed] [Google Scholar]
- 90.Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sd U S A. 2008;105:15112–7. 10.1073/pna.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang H, Tang G, Wang Q, Li D, Shen F, Zhou J, et al. Two novel non-viral gene delivery vectors: low molecular weight polyethylenimine cross-linked by (2-hydroxypropyl)-β-cyclodextrin or (2-hydroxypropyl)-γ-cyctodestria Chem Commun. 2006:2382–4. https://doi.oig/10.1039/B601130F. [DOI] [PubMed] [Google Scholar]
- 92.Lai W-F, Green D, Jung H-S. Linear poly(ethyleramine) cross-linked by methyl-β-cyclodextrin for gene delivery. Curr Gene Ther. 2014;14 10.2174/1566523214666140612160042. [DOI] [PubMed] [Google Scholar]
- 93.Hu Q, Wang K, Sun X, Li Y, Fu Q, Liang T, et al. A redox-sensitive, oligppsptide-guided, self-assembling, and efficiency-enhanced (ROSE) system for functional delivery of microRNA therapeutics for treatment of hepatocellular carcinoma. Biomaterials. 2016;104:192–200. 10.1016/j.bromaterials.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 94.Schmidt BVKJ, Bamer-Kowollik C. Dynamic macromolecular material design—foe versatility of cyclodextrin-based host-guest chemistry. Angew Chem Int Ed. 2017;56:8350–69, 10.1002/ame.201612150. [DOI] [PubMed] [Google Scholar]
- 95.Chen F, Kong L, Wang L, Fan Y, Shen M, Shi X. Construction of core–shell tecto dendrimers based on supramoleeular host–guest assembly for enhanced gene delivery. J Mater Chem B. 2017;5:8459–66. 10.1039/C7TB02585H. [DOI] [PubMed] [Google Scholar]
- 96.Granadero D, Bordello J, Pérez-Alvite MJ, Novo M, Al-Soufi W. Host-guest complexation studied by fluorescence correlation spectroscopy: adamantane-cydodextrin inclusion. Int J Mol Sci. 2010;11:173–88. 10.3390/ijms11010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Connors KA, The stability of cyclodextrin complexes in solution, Chem Rev. 1997;97:1325–58. 10.1021/cr960371r. [DOI] [PubMed] [Google Scholar]
- 98.Štimac A, Šekutor M, Mlinarić-Majerski K, Frkanec L, Frkanec R. Adamantane in drug delivery systems and surface recognition. Molecules. 2017;22:297 10.3390/molecules22020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee HJ, Le PT, Kwon HJ, Park KD. Supramoleeular assembly of tetronic–edamantaae and poly (β-cyclodextrin) as injectable shear-thinning hydrogels. J Mater Chem B, 2019;7:3374–82, 10.1039/C9TBG0072K. [DOI] [Google Scholar]
- 100.Wang H, Chen K-J, Wang S, Ohashi M, Kamei K, Sun J, et al. A small, library of DNA-encapsulated supramoleeular nanoparticles for targeted gene delivery, Chem Commun (Camb), 2010;46: 1851–3. https://doi.org/l0.1039/b923711a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang H, Liu K, Chen K-J, Lu Y, Wang S, Lin W-Y, et al. A rapid pathway toward a superb gene delivery system: programming structural and functional diversity into a supramoleeular nanoparticle library. ACS Nano. 2010;4:6235–43. 10.1021/nn101908e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu Y, Yuan W, Zhao N-N, Ma J, Yang W-T, Xu F-J. Supramoleeular pseudo-block gene carriers based on hioredudble star polycations. Biomaterials, 2013;34:5411–22. 10.1016/j.biomaterials.2013.03.092. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Z, Ding J, Chen X, Xiao C, He C, Zhuang X, et al. Intracellular pH-sensitive supramolecular amphiphiles based on host–guest recognition between benzimidazole and β-cyclodextrin as potential drug delivery vehicles. Polym Chem. 2013;4:3265–71. 10.1039/C3PY00141E. [DOI] [Google Scholar]
- 104.Liu J, Liu X, Yuan Y, Li Q, Chang B, Xu L, et al. Supramolecular modular approach toward conveniently constructing and multifunctioning a pH/redox dual-responsive drug delivery nanoplatform for improved Cancer chemotherapy, ACS Appl Mater interfaces. 2018;10:26473–84. 10.1021/acsami.8b05232. [DOI] [PubMed] [Google Scholar]
- 105.Argenziano M, Haimhoffer A, Bastiandch C, Jicsinszky L, Caldera F, Trotta F, et al. In vitro enhanced skin permeation and retention of imiquimod loaded in β-cydodestrin nanosponge hydrogel. Pharmaceutics. 2019;11:138 https://doi.erg/10.3390/pharmaceuticsl1030138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Evans JC, Malhotra M, Sweeney K, Darcy R, Nelson CC, Hollier BG, et al. Folate-targeted amphiphilic cyclodextrin nanoparticles incorporating a fusogenic peptide deliver therapeutic siRNA and inhibit tie invasive capacity of 3D prostate cancer tumours. Int J Pharm. 2017;532:511–8. 10.1016/j.ijpharm.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 107.Farrokhi F, Karami Z, Esmaeili-Mahani S, Heydari A. Delivery of DNAzyme targeting c-Myc gene using β-cydodextrin polymer nanocarrier for therapeutic application in human breast cancer cell line, J Drug Deliv Sci Technol. 2018;47:477–84. 10.1016/j.jddst2018.08.015. [DOI] [Google Scholar]
- 108.Falvey P, Lim C, Darcy R, Revermann T, Karst U, Giesbers M, et al. Bilayer vesicles of amphiphilic cyclodextrins: host membranes that recognize guest molecules. Chemistry. 2005;11:1171–80. 10.1002/chem.200400905. [DOI] [PubMed] [Google Scholar]
- 109.Zerkoune L, Angelova A, Lesieur S. Nano-assemblies of modified cyclodextrins and their complexes with guest molecules: incorporation in nano structured membranes and Amphiphile Nanoarchitectonics design. Nanomaterials. 2014;4:741–65. 10.3390/nano4030741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Elsana H, Mysina S, Elkordy E, Carr-Wilkinson J, Elkordy A. Insights into the influences of carboxymethyl-β-Cyclodextrin on DNA formulations characteristics and gene transfection efficiency. Curr Drug Deliv. 2018;15 10.2174/1567201815666180226115503. [DOI] [PubMed] [Google Scholar]
- 111.Zokaei E, Badoei-dalfrad A, Ansari M, Karami Z, Eslaminejad T, Nematollahi-Mahani SN, Therapeutic potential of DNAzyme loaded on chitosan/cyclodextrin nanoparticle to recovery of chemosensitivity in the MCF-7 cell line. Appl Biochem Biotechnol, 2019;187:708–23. 10.1007/s12010-018-2836-x. [DOI] [PubMed] [Google Scholar]
- 112.Moratz J, Stricker L, Engel S, Ravoo BJ. Controlling complex stability in photoresponsive macromolecular host–guest systems: toward reversible capture of DNA by cyclodextrin vesicles. Macromol Rapid Commun. 2018;39:1700256 10.1002/raarc.201700256. [DOI] [PubMed] [Google Scholar]
- 113.Gooding M, Malhotra M, McCarthy DJ, Godinho BMDC, Cryan JF, Darcy R, et al. Synthesis and characterization of rabies virus glycoprotein-tagged amphiphilic cyclodextrins for siRNA delivery in human glioblastoma cells: in vitro analysis. Eur J Pharm Sci. 2015;71:80–92, 10.1016/j.ejps.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 114.Štimac A, Tokić M, Ljubetič A, Vuletić T, Šekutor M, Požar J, et al. Functional, self-assembled narsovesicles based on β-cyclodextrin, liposomes and adamantyl guanidines as potential non viral gene delivery vectors. Org Biomol Chem. 2019;17:4640–51. 10.1039/C90B00488B. [DOI] [PubMed] [Google Scholar]
- 115.O’Mahony AM, Ogier J, Desgranges S, Cryan JF, Darcy R, O’Driscoll CM, A slick chemistry route to 2-functionalised PEGylatod and cationic β-cyclodextrins: co-formulation opportunities for siRNA delivery. Org Biomol Chem. 2012;10:4954–60. 10.1039/C2OB25490E. [DOI] [PubMed] [Google Scholar]
- 116.Li Y, Qian Y, Liu T, Zhang G, Hi J, Liu S. Asymmetrically functionalized β-cydodextrirs-based star copolymers for integrated gene delivery and magnetic resonance imaging contrast enhancement Polym Chem. 2014;5:1743–50. 10.1039/C3PY01278F. [DOI] [Google Scholar]
- 117.Xu J, Xu B, Tan J, Yang Y, Hu Y, Huang Y. Micronesdle-assisted, DC-targeted codslivery of pTRP-2 and adjuvant of paclitaxel for transcutaneous immunotherapy. Small. 2017;13:1700666 10.1002/smll.201700666. [DOI] [PubMed] [Google Scholar]
- 118.Ramineni SK, Cunningham LL Jr, Dziubla TD, Puleo DA. Development of imiquimod-loaded mucoadhssive films for oral dysplasia. J Pharm Sci. 2013;102:593–603. 10.1002/jps.23386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Onishi M, Ozasa K, Kobiyama K, Ohata K, Kitano M, Taniguchi K, et al. Hydroxypropyl-β-cyclodextrin spikes local, inflammation that induces Th2 cell and T follicular helper cell responses to the coadministered antigen. J Immunol. 2015;194:2673–82, 10.4049/jimmunol.1402027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li RQ, Niu YL, Zhao NN, Yu BR, Mao C, Xu FJ. Series of new β-cyclodextrin-cored starlike carries for gene delivery, ACS Appl Mater Interfaces, 2014;6:3969–78. 10.1021/am5005255. [DOI] [PubMed] [Google Scholar]
- 121.Sterman DH, Alley E, Stevenson JP. Friedberg J, Metzger S, Recio A, et al. a Clin Cancer Res. 2016. 522:3791–800. 10.1158/1078-0432.CCR-15-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tamura RE, Lana MG, Costanzi-Strauss E, Strauss BE. Combination of cabazitaxel and p53 gene therapy abolishes prostate carcinoma tumor growth. Gene Ther. 2019:1–12. 10.1038/s1434-019-0071-x. [DOI] [PubMed] [Google Scholar]
- 123.Kamran N, Kadiyala P, Saxena M, Candolfi M, Li Y, Moreno-Ayala MA, et al. Immunosuppressive myeloid cells’ blockade in the glioma microenvironment enhances the efficacy of immunestimulatory gene therapy. Mol Ther, 2017;25:232–48, 10.1016/j.ymthe.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Patel L, Grositeig GT. Combination therapy for Alzheimer’s disease. Drugs Aging. 2011;28:539–46. 10.2165/11591860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 125.Schneider-Futschik EK. Beyond cystic fibrosis transmembrane conductance regulator therapy: a perspective on gene therapy and small molecule treatment for cystic fibrosis. Gene Ther, 2019;26:354–62, 10.1038/s41434-019-0092-5. [DOI] [PubMed] [Google Scholar]
- 126.Grassi L, Maisetta G, Esin S, Batoni G. Combination strategies to enhance the efficacy of antimicrobial peptides against bacterial biofilms. Front Microbiol. 2017;8:2409 https://doi.org/103389/fmicb.2017.02409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hill JA, Cower LE, Using combination therapy to thwart drug resistance. Future Microbiol. 2015;10:1719–26. 10.2217/fmb.15.68. [DOI] [PubMed] [Google Scholar]
- 128.Li D, Li Y, Xing H, Guo J, Ping Y, Tang G. Synergistic enhancement of lung cancer therapy through nanocarrier-mediated sequential delivery of superantigen and tyrosin kinase inhibitor, Adv Funct Mater. 2014;24:5482–92. 10.1002/adfm/201400456. [DOI] [Google Scholar]
- 129.Liu X, Li Z, Loh XI, Chen K, Li Z, Wu Y-L. Targeted and sustained corelease of chemotherapeutics and gene by injectable supramolecular hydrogel for drug-resistant cancer therapy. Macromol Rapid Commun. 2019;40:1800117 https://doi.oig/10.1002/marc.201800117. [DOI] [PubMed] [Google Scholar]
- 130.Kejík Z, Břǭza T, Králová J, Poučková P, Král A, Martásek P, et al. Coordination conjugates of therapeutic proteins with drag carriers: a new approach for versatile advanced drug delivery. Bioorg Med Chem Lett. 2011;21:5514–20. https://doi.org/10.I016/j.bmcl.2011.06.101. [DOI] [PubMed] [Google Scholar]
- 131.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11:895–905. 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Song Q, Yin Y, Shang L, Wu T, Zhang D, Kong M, et al. Tumor microenvironment responsive naaogel for the combinatorial antitumor effect of chemotherapy and immunotherapy. Nano Lett, 2017;17:6366–75. 10.1021/acs.nanclett.7b03186. [DOI] [PubMed] [Google Scholar]
- 133.He M, Zhong C, Hu H, Jin Y, Chen Y, Lou K, et al. Cydodextrin/chitosan nanoparticles for oral ovalbumin delivery: preparation, characterization and intestinal mucosal immunity in mice. Asian J Pharm Sci, 2019;14:193–203. 10.1016/j.ajps.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang M, Xiong Q, Wang Y, Zhang Z, Shen W, Liu L, et al. A well-defined coil–comb polyeationic brush with “star polymers” as side chains for gene delivery, Polym Chem, 2014;5:4670–8. 10.1039/C4PY00311J. [DOI] [Google Scholar]
- 135.Wang J, Liu L, Chen J, Deng M, Feng X, Chen L. Supramolecular nanoplatforms via cyclodextrin host-guest recognition for synergistic gene-photodynarnic therapy, Eur Polym J. 2019;118:222–30. https://doi.org/l0.1016/j.empolymj.2019.04.051. [Google Scholar]
- 136.Ahmed MS, Rodell CB, Hulsmans M, Kohler RH, Aguirre AD, Nahrendorf M, et al. A supramolecular nanocarrier for delivery of amiodarone anti-arrhythmic therapy to the heart. Bioconjug Chem. 2019;30:733–40. 10.1021/acs.bioconjchem.gb00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu J, Rea X, Guo T, Sun X, Chen X, Patterson LH, et al. NLG919/cyclodextrin complexation and anti-cancer therapeutic benefit as a potential immunotherapy in combination with paditaxel Eur J Pharm Sci. 2019;138:10S034 10.1016/j.ejps.2019.105034. [DOI] [PubMed] [Google Scholar]
- 138.Bdbskhouche S, Oniszczuk J, Pawlak A, El Joukhar I, Goffin A, Varrault G, et al. Cationic poly (cyclodextrin)/alginate nanocapsules; from design to application as efficient delivery vehicle of 4-hydroxy tamoxifen to podocyte in vitro. Colloids Surf B: Biointerfaces. 2019;179:128–35. 10.1016/j.colsurfb.2019.03.060. [DOI] [PubMed] [Google Scholar]
- 139.Heidel JD, Yu Z, Liu JY-C, Reis SM, Liang Y, Zeidan RK, et al. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc Nad Acad Sd U S A. 2007;104: 5715–21. 10.1073/pnas.0701458104. [DOI] [PMC free article] [PubMed] [Google Scholar]


