Abstract
Aims
Emergency department (ED) visits for decompensated heart failure (HF) are frequent and associated with poor long‐term outcomes in patients with HF. Serum N‐terminal pro b‐type natriuretic peptide (NT‐proBNP) is widely used to assist diagnosis and predict clinical outcomes in HF patients. Few studies have investigated the use of urine NT‐proBNP as an HF biomarker. This study aims to assess the value of urine NT‐proBNP for predicting ED visits for decompensated HF as compared with that of serum NT‐proBNP.
Methods and results
This study included 122 HF patients with reduced left ventricular ejection fraction (<50%). Serum and urine NT‐proBNP levels were measured. Baseline data included demographics, comorbidities, and co‐medications. Medical records were used to determine the incidence of visits to the ED for decompensated HF during the 3 months following the last visit. We observed significantly higher levels of both serum and urine NT‐proBNP in patients with subsequent ED visits than in those without. Multivariate logistic regression analysis showed that urine NT‐proBNP/creatinine ratio (OR, 1.031; 95% CI, 1.001–1.061; P = 0.046) but not serum NT‐proBNP was an independent factor associated with subsequent ED visits. According to receiver‐operating characteristic‑area under the curve analysis, the optimal cut‐off value of urine NT‐proBNP/creatinine ratio for predicting subsequent heart‐failure related ED visits was 0.272 pg/μg Cr (area under the curve, 0.675; P = 0.011).
Conclusions
For HF patients with reduced left ventricular ejection fraction, a single measurement of urinary NT‐proBNP/creatinine ratio is predictive of subsequent ED visits for decompensated HF. This non‐invasive and easy measurement may be a clinically useful tool for monitoring clinical outcomes and identifying a subset of patients at higher risk of ED visits within a short time.
Keywords: Urine, N‐terminal pro b‐type natriuretic peptide, Heart failure, Emergency department
Introduction
Heart failure (HF) is a leading cause of emergency department (ED) visits and hospitalization worldwide. 1 Serum N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) is a well‐established marker used for diagnosis and to predict prognosis, including mortality and HF hospitalization. 2 , 3 , 4 However, the assessment of NT‐proBNP in serum requires a blood draw, which is not available in all clinical settings. In addition to its presence in serum, NT‐proBNP is excreted in urine, and the assessment of urine NT‐proBNP level is non‐invasive and easier than is testing for NT‐proBNP in serum. 5 Several studies have investigated the accuracy of urine NT‐proBNP concentration as an indicator of HF prognostic parameters such as 2‐month cardiac mortality 6 and acute decompensated HF. 7 One study reports that inter‐subject variability in urinary NT‐proBNP concentration can be decreased by correcting for urinary creatinine excretion (urine NT‐proBNP/creatinine), thereby optimizing its clinical use. 8
This study aims to determine whether the NT‐proBNP in fresh urine is predictive of subsequent ED visits for decompensated HF. We compare this association between serum and urine NT‐proBNP in a cohort of HF patients with impaired left ventricular ejection fraction (LVEF).
Methods
Patients
This prospective case–control study enrolled 122 consecutive patients who had chronic HF with impaired LVEF (<50%) diagnosed ≥6 months before enrolment, with New York Heart Association functional classes II and III at enrolment, and were treated with regular medications in the cardiovascular outpatient department of National Cheng Kung University Hospital from December 2018 to August 2019. Patients with end‐stage renal disease were excluded. This study was approved by the ethics committee of National Cheng Kung University Hospital and was conducted according to the guidelines of the International Conference on Harmonization for Good Clinical Practice. All patients provided written informed consent before enrolment.
All patients received standard therapy for HF based on current guidelines. Medical histories, including co‐morbid diseases and co‐medications, were reviewed. Data on comorbidities and prescribed co‐medications were collected. The following comorbid medical conditions as well any medications prescribed for indications of the following conditions were included for analysis: diabetes mellitus (fasting plasma glucose level > 126 mg/dL on two occasions), hypertension (blood pressure >140/90 mmHg on three occasions), dyslipidaemia (≥200 mg/dL), chronic kidney disease [estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73m2 persisting for 3 months], and coronary artery disease (history of acute coronary syndrome or percutaneous coronary intervention with stents). At the start of enrolment, blood and fresh urine samples were collected, and blood pressure, heart rate, and body mass index (BMI) were measured. All patients underwent formal echocardiography, and the LVEF was evaluated using Simpson's method. The medical records were carefully reviewed for the primary endpoint: the number of ED visits for HF decompensation within 3 months after enrolment.
No patient and public involvement
This research was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop relevant patient outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Laboratory examinations
Blood and fresh urine samples were collected. Fresh, unfrozen samples were sent to the chemistry laboratory at the National Cheng Kung University. Fresh urine samples were used because previous studies suggest that the accuracy of NT‐proBNP assessment is greater for fresh than frozen urine. 8 , 9 Urine samples were collected during outpatient department visits at 9:00 am–12:00 pm or 2:00–5:00 pm. Serum NT‐proBNP levels were measured using the NT‐proBNP assay (Roche Diagnostics, Mannheim, Germany) with a Roche Modular E‐170 automated immunoanalyzer. The within‐run coefficient of variation was <2%, and the total variation was <6% at all levels measured (20–30 000 pg/mL). Urine NT‐proBNP level was measured using a miniVIDAS analyser (BioMérieux SA, Mercy I'Etoile, France). The analytical measurement range of NT‐proBNP was 7.5–100 000 pg/mL, with a coefficient of variation of 1.34–3.26%. Urine creatinine concentrations were determined using creatinine FS (DiaSys Diagnostic Systems GmbH, Holzheim, Germany) and an automated biochemical analyser (TBA‐25 FR, Toshiba Medical Systems, Tokyo, Japan). The creatinine measurement range was 0.20–15.00 mg/dL. For values exceeding the range, samples were diluted with normal saline and remeasured. The urine sample (200 μL) was pipetted onto the VIDAS NT‐proBNP2 test strip (BioMérieux SA), which then was inserted into the miniVIDAS analyser.
Statistical analysis
Data are expressed as the mean ± standard deviation. Continuous variables were compared using Student's t‐test, and categorical variables were compared using the chi‐square or Fisher's exact test. Serum NT‐proBNP levels were correlated with those in urine using Spearman's rank correlation coefficient. Multivariate logistic regression analysis was used to identify the independent factors associated with future ED visits. The receiver‐operating characteristic area under the curve from urine NT‐proBNP/creatinine and their associated 95% confidence intervals (CI) were investigated for association with future ED visits. The cut‐off values were chosen based on the results of receiver‐operating characteristic curve analysis and used to analyse the associated values of urine NT‐proBNP/creatinine (pg/μg Cr) for determining end points. All tests were two tailed. P < 0.05 was considered statistically significant. All data were analysed using SPSS statistical package version 23.0 (SPSS Inc. Chicago, IL, USA).
Results
Patient baseline characteristics
A total of 122 patients (age, 67.3 ± 10.3 years; sex, 75.4% male patients) were recruited into this study. Past admission for HF was documented in 68 patients (55.7%). Table 1 shows the characteristics of this patient cohort. The most common co‐morbidity in this study population was hypertension (55 patients; 45.1%). Atrial fibrillation was present in 41 patients (33.6%). The mean value of the LVEF was 36.2%. Serum concentrations of NT‐proBNP correlated significantly with those of fresh urine NT‐proBNP (r = 0.813; P < 0.001) and urine NT‐proBNP/creatinine (r = 0.775; P < 0.001). Most patients received guideline‐derived standard medical therapy. As such, more than 95% patients were receiving renin‐angiotensin‐aldosterone system inhibitors, and 71.3% were being treated with beta blockers.
TABLE 1.
Baseline characteristics of the patient cohort
| Parameter | Mean value |
|---|---|
| Age (years) | 67.3 ± 10.3 |
| Male sex (n) | 92 (75.4%) |
| Body height (cm) | 162.5 ± 7.6 |
| Body weight (kg) | 65.8 ± 12.1 |
| Body mass index (kg/m2) | 24.8 ± 4.1 |
| Systolic blood pressure (mmHg) | 121 ± 17 |
| Diastolic blood pressure (mmHg) | 71 ± 11 |
| Heart rate (bpm) | 80 ± 15 |
| Heart failure duration (years) | 4.5 ± 3.2 |
| Diabetes mellitus (n) | 54 (44.3%) |
| Hypertension (n) | 55 (45.1%) |
| Dyslipidemia (n) | 46 (37.7%) |
| Coronary artery disease (n) | 45 (36.9%) |
| Chronic kidney disease (n) | 52 (42.6%) |
| Atrial fibrillation (n) | 41 (33.6%) |
| Creatinine (mg/dL) | 1.38 ± 1.24 |
| eGFR (mL/min/1.73m2) | 62.0 ± 22.5 |
| Sodium (meq/L) | 144 ± 34 |
| Potassium (meq/L) | 4.2 ± 0.6 |
| Blood NT‐proBNP (pg/mL) | 2856.7 ± 3999.8 |
| Urine creatinine (mg/dL) | 73.9 ± 51.4 |
| Urine NT‐proBNP (pg/mL) | 1210.7 ± 6122.8 |
| Urine NT‐proBNP/creatinine ratio (pg/μgCr) | 1.89 ± 8.71 |
| Left atrial diameter (cm) | 4.3 ± 0.9 |
| Average mitral E/e′ | 12.6 ± 5.5 |
| Ejection fraction (%) | 36.2 ± 9.2 |
| Medications taken (n, patients; %) | |
| ACEI | 7 (5.7%) |
| ARB | 84 (68.9%) |
| ARNI | 25 (20.5%) |
| MRA | 48 (39.3%) |
| Beta blockers | 87 (71.3%) |
| Diuretics | 66 (54.1%) |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; eGFR, estimated glomerular filtration rate; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro b‐type natriuretic peptide.
Total N, 122. Data are presented as the mean ± standard deviation.
Parameter comparison between patients with and without future emergency department visits for heart failure decompensation within 3 months
No mortality was reported during the follow‐up period. The average BMI of patients with primary endpoint (ED visit for HF decompensation within 3 months) was lower than that of patient without primary endpoint (Table 2 ). No significant differences between patients with and without primary endpoint were observed with respect to HF duration, blood pressure, heart rate, co‐morbidities, and co‐medications; however, both coronary artery disease and chronic kidney disease were more common in patients with primary endpoint (Table 2 ). Significantly higher levels of serum NT‐proBNP, urine NT‐proBNP levels, and urine NT‐proBNP/creatinine were observed in patients with primary endpoint (Table 2 ). Only LVEF was significantly lower in patients with a subsequent HF‐related ED visit than in those without (28.7 ± 8.5% vs. 37.8 ± 8.6%; P < 0.001). After adjusting for confounders (age and sex) and covariates (BMI, eGFR, coronary artery disease, and LVEF), multivariate logistic regression analysis revealed that urine NT‐proBNP/creatinine (pg/μg Cr) was an independent factor (OR, 1.031; 95% CI, 1.001–1.061; P = 0.046), while blood NT‐proBNP and urine NT‐proBNP were not (Table 3 ).
TABLE 2.
Comparison of clinical characteristics between patients with and without subsequent emergency department visits for heart failure decompensation
| Without ED visits (n = 100) | With ED visits (n = 22) | P | |
|---|---|---|---|
| Age (years) | 67.1 ± 10.1 | 68.3 ± 11.1 | 0.601 |
| Male gender | 76 (76.0%) | 16 (72.7%) | 0.607 |
| Body mass index (kg/m2) | 25.2 ± 3.9 | 23.2 ± 4.8 | 0.048 |
| Heart failure duration (years) | 4.6 ± 3.1 | 4.0 ± 3.6 | 0.434 |
| Systolic blood pressure (mmHg) | 122 ± 17 | 115 ± 19 | 0.197 |
| Diastolic blood pressure (mmHg) | 72 ± 10 | 69 ± 11 | 0.442 |
| Heart rate (bpm) | 81 ± 16 | 78 ± 10 | 0.679 |
| Diabetes mellitus | 46 (46.0%) | 8 (36.4%) | 0.410 |
| Hypertension | 45 (45.0%) | 10 (45.5%) | 0.969 |
| Dyslipidemia | 39 (39.0%) | 7 (31.8%) | 0.529 |
| Coronary artery disease | 32 (32.0%) | 13 (59.1%) | 0.017 |
| Chronic kidney disease | 36 (36.0%) | 16 (72.7%) | 0.002 |
| Atrial fibrillation | 31 (31.0%) | 10 (45.5%) | 0.194 |
| ACEI | 6 (6.0%) | 1 (4.5%) | 0.791 |
| ARB | 70 (70.0%) | 14 (63.6%) | 0.580 |
| ARNI | 20 (20.0%) | 5 (22.7%) | 0.880 |
| MRA | 37 (37.0%) | 11 (50.0%) | 0.258 |
| Beta blockers | 69 (69.0%) | 18 (81.8%) | 0.229 |
| Diuretics | 51 (51.0%) | 15 (68.2%) | 0.143 |
| Creatinine (mg/dL) | 1.17 ± 0.50 | 2.31 ± 2.56 | 0.002 |
| Potassium (Meq/L) | 4.2 ± 0.5 | 4.1 ± 0.7 | 0.516 |
| eGFR (mL/min/1.73m2) | 65.3 ± 20.6 | 46.9 ± 25.0 | 0.015 |
| Blood NT‐proBNP (pg/mL) | 2271.5 ± 3075.5 | 5504.0 ± 6208.7 | 0.001 |
| Urine creatinine (mg/dL) | 72.1 ± 51.7 | 82.2 ± 50.0 | 0.403 |
| Urine NT‐proBNP (pg/mL) | 321.3 ± 613.2 | 5253.1 ± 13904.9 | <0.001 |
| Urine NT‐proBNP/creatinine ratio (pg/μg Cr) | 0.59 ± 1.11 | 7.81 ± 19.65 | <0.001 |
| Left atrial diameter (cm) | 4.3 ± 0.9 | 4.4 ± 0.9 | 0.768 |
| Average mitral E/e′ | 12.4 ± 5.7 | 13.4 ± 4.7 | 0.467 |
| Left ventricular ejection fraction (%) | 37.8 ± 8.6 | 28.7 ± 8.5 | <0.001 |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; eGFR, estimated glomerular filtration rate; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro b‐type natriuretic peptide.
TABLE 3.
The multivariate logistic regression analysis for independent predictors for future emergency department visits for heart failure decompensation
| OR | 95% CI for B | P | |
|---|---|---|---|
| Age (years) | 0.937 | 0.868–1.011 | 0.095 |
| Sex (male) | 0.196 | 0.039–0.992 | 0.049 |
| Body mass index (kg/m2) | 0.691 | 0.533–0.897 | 0.005 |
| eGFR (mL/min/1.73m2) | 0.933 | 0.892–0.976 | 0.002 |
| LVEF (%) | 0.867 | 0.780–0.964 | 0.008 |
| CAD (yes) | 10.035 | 2.080–48.420 | 0.004 |
| Blood NT‐proBNP (pg/mL) | 1.000 | 1.000–1.000 | 0.132 |
| Age (years) | 0.928 | 0.856–1.007 | 0.073 |
| Sex (male) | 0.245 | 0.045–1.335 | 0.104 |
| Body mass index (kg/m2) | 0.684 | 0.522–0.897 | 0.006 |
| eGFR (mL/min/1.73m2) | 0.935 | 0.892–0.979 | 0.004 |
| LVEF (%) | 0.854 | 0.766–0.952 | 0.004 |
| CAD (yes) | 8.682 | 1.679–44.897 | 0.010 |
| Urine NT‐proBNP (pg/mL) | 1.001 | 1.000–1.002 | 0.084 |
| Age (years) | 0.936 | 0.866–1.013 | 0.102 |
| Sex (male) | 0.283 | 0.055–1.454 | 0.131 |
| Body mass index (kg/m2) | 0.670 | 0.512–0.876 | 0.003 |
| eGFR (mL/min/1.73m2) | 0.935 | 0.893–0.979 | 0.004 |
| LVEF (%) | 0.851 | 0.762–0.949 | 0.004 |
| CAD (yes) | 8.205 | 1.676–40.161 | 0.009 |
| Urine NT‐proBNP/creatinine (pg/μg Cr) | 1.031 | 1.001–1.061 | 0.046 |
CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro b‐type natriuretic peptide
We further divided the study population into four quartiles based on the urine NT‐proBNP/creatinine (pg/μg Cr) (quartile 1, 0–0.05595; quartile 2, 0.05596–0.26305; quartile 3, 0.26306–0.82646; and quartile 4, >0.82647) and found that the number of patients with a subsequent HF‐related ED visit significantly increased with increasing quartile (10%, 12.9%, 12.9%, and 36.7%, respectively; P = 0.024) (Figure 1 ).
FIGURE 1.
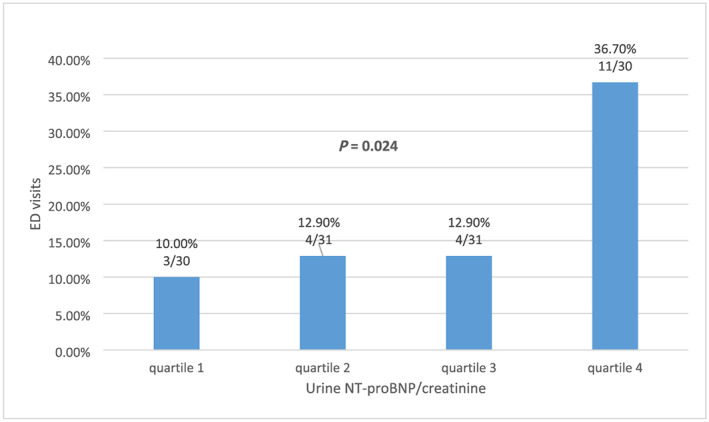
Subsequent emergency department visits significantly increased with quartiles of urine NT‐proBNP/creatinine ratio.
Receiver‐operating characteristic‑area under the curve determination of serum and urine NT‐proBNP cut‐off values for association with subsequent heart failure‐related emergency department visit
We found that fresh urine NT‐proBNP/creatinine but not serum NT‐proBNP was independently associated with subsequent HF‐related ED visits (Table 3 ). The optimal urine NT‐proBNP/creatinine cut‐off value for association with ED visits was determined to be 0.272 pg/μg Cr (sensitivity, 68.2%; specificity, 55.0%; positive predictive value, 25%; negative predictive value, 88.7%; area under the curve, 0.675; 95% CI, 0.543–0.806; P = 0.011) (Figure 2 ). The positive predictive value was higher in patients with eGFR < 60 mL/min/1.73 m2 than in those with eGFR > 60 mL/min/1.73 m2 (32.3% vs. 17.2%).
FIGURE 2.
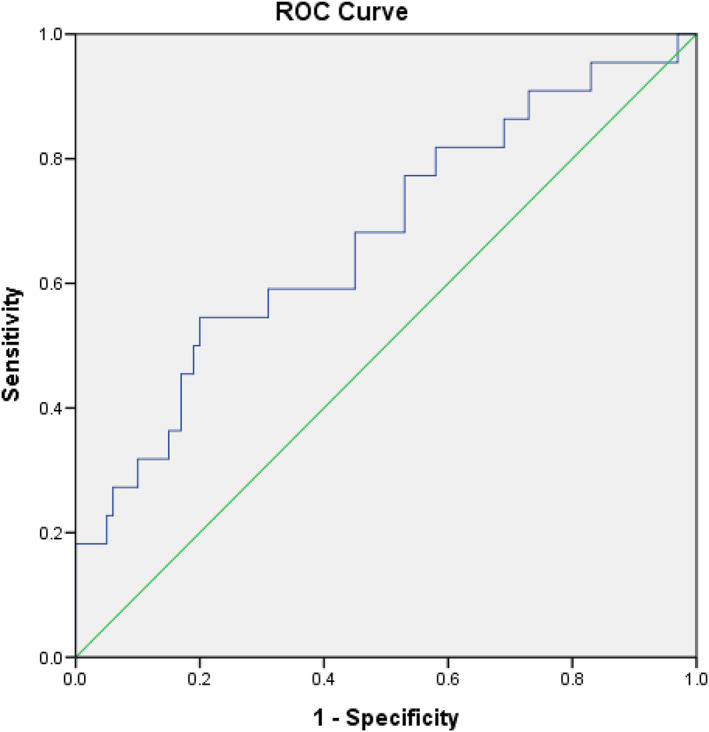
Receiver‐operating characteristic curve analysis of fresh urine NT‐proBNP/creatinine (pg/μg Cr) in heart failure patients with subsequent emergency department visits for decompensated heart failure. Urine NT‐proBNP/creatinine: cut‐off value, 0.272 pg/μgCr; sensitivity, 68.2%; specificity, 55.0%; AUC, 0.675; 95% CI, 0.543–0.806; P = 0.011.
Discussion
The results of this study show that the ratio of NT‐proBNP/creatinine in fresh urine but not serum NT‐proBNP level or urine NT‐proBNP level was independently associated with subsequent ED visits for HF decompensation within 3 months. We also observed a strong correlation between NT‐proBNP concentrations in serum and fresh urine. Thus, a single, non‐invasive test of fresh urine provides an accurate measure of NT‐proBNP/creatinine level and has great value for predicting short‐term future ED visits.
Few studies have investigated the effectiveness of urine NT‐proBNP as a biomarker to assess the status of HF patients during ED visits or hospitalization. The diagnostic value of serum NT‐proBNP concentration is greater for acute 10 than chronic 11 HF. Based on the results of several trials and current guidelines, a serum NT‐proBNP level of 300 pg/mL indicates a diagnosis of HF, 4 and 1000 pg/mL indicates that HF treatment should be started. 11 Our data indicate that regardless of future ED visits, the mean values of serum NT‐proBNP were significantly higher than 1000 pg/mL. Although all of our patients received guideline‐derived medical treatment including RAAS inhibitors and beta‐blockers, more aggressive drug therapy may be needed to lower the serum NT‐proBNP concentration.
Blood sampling is invasive and cannot be carried out in every clinical setting. Thus, fresh and frozen urine have been investigated as alternative sources of NT‐proBNP concentration assessment. Results show that fresh and frozen urine concentrations of NT‐proBNP are acceptably accurate biomarkers for diagnosing HF, with cut‐off values of 94.2 8 and 74.2 pg/mL, 6 respectively. By comparison, our patient cohort had higher NT‐proBNP levels (1210.7 ± 6122.8 pg/mL). This difference may result from our use of fresh urine, which is reported to be more accurate than frozen urine for predicting HF from NT‐proBNP levels. 8 Investigating the prognostic power of fresh urine NT‐proBNP, one study showed that the 12‐month combined event rate was 21% in patients with low urine NT‐proBNP compared with 52% in those with NT‐proBNP levels > 92.61 pg/mL. 6 For comparison, our study showed that the 3‐month ED visit rate was 11% in patients with low urine NT‐proBNP (less than median, 128.0 pg/mL) and 25% in those with NT‐proBNP levels > 128.0 pg/mL. Another study showed that fresh urine NT‐proBNP had significant and independent predictive value, especially regarding all‐cause mortality, during a 5‐year‐follow‐up, a relatively long‐term outcome. 12 Our study, however, did not indicate NT‐proBNP as an independent predictor for short‐term ED visits. We found that the serum NT‐proBNP concentration correlated more significantly with LVEF than with urine NT‐proBNP/Cr (r = −0.435 vs. −0.210, data not shown). Using LVEF as a covariate factor in multivariate regression analysis, only urine NT‐proBNP/Cr remained an independent predictor of short‐term ED visits.
One study reported that urine NT‐proBNP concentrations needed correction for creatinine excretion to reduce inter‐subject variability. 1 Although no study has shown that urine NT‐proBNP/creatinine is a better predictor than urine NT‐proBNP for cardiovascular outcomes in HF patients, we observed the power for independently predicting future ED visits was significantly higher for the urine NT‐proBNP/creatinine ratio than for urine NT‐proBNP alone. Together, these observations indicate that fresh urine NT‐proBNP/creatinine may be a promising marker for HF prognostication in short‐term follow‐up.
This study has several limitations. The sample size was small. In addition, our primary endpoint was short term; thus, these results might not be applicable to long‐term outcomes. Third, urine samples were obtained during two different time periods, which may have affected the results. The average difference in measurements between the two time periods was less than 5% of the value. Although a urine sample collected after waking up in the morning may be less error prone, this timing is not clinically practical, limiting its usefulness in outpatient settings. Fourth, hypertension can potentially decrease the specificity of NT‐proBNP. Finally, suboptimal beta blocker dosage or poor drug compliance may lead to a mildly elevated heart rate in this study population, possibly resulting in more ED visits. Despite these limitations, our results indicate that high NT‐proBNP concentration in urine but not serum in HF patients predicts a risk for decompensation within 3 months. These patients might need more aggressive treatment for HF.
Conclusions
A single measurement of the NT‐proBNP/creatinine ratio (pg/μg Cr) in fresh urine may be an alternative biomarker to serum or urine NT‐proBNP for predicting subsequent ED visits within 3 months in HF patients with impaired LVEF (<50%). This non‐invasive and easy measurement may be a clinically useful tool in outpatient settings for monitoring future outcomes.
Conflict of interest
None declared.
Funding
The authors would like to thank the Ministry of Science and Technology of the Republic of China, Taiwan, for financially supporting this research under contract MOST 107‐2218‐E‐006‐033 and 108‐2218‐E‐006‐019‐.
Contributors
Conception and design: J‐YC; data acquisition: J‐YC, D‐SC, S‐YL; data analysis and interpretation: J‐YC, D‐SC, W‐CT; statistical analysis: J‐YC, W‐CT; drafting and finalizing the article: J‐YC, S‐YL, W‐CT, C‐YL, M‐DS; critical revision of the article for important intellectual content: J‐YC, W‐CT.
Acknowledgements
The authors would like to thank Convergence CT for assistance with English editing of the manuscript.
Chen, J.‐Y. , Lee, S.‐Y. , Tsai, W.‐C. , Lin, C.‐Y. , Shieh, M.‐D. , and Ciou, D.‐S. (2020) Urine N‐terminal pro b‐type natriuretic peptide is predictive of heart failure‐related emergency department visits. ESC Heart Failure, 7: 2672–2678. 10.1002/ehf2.12856.
References
- 1. Hahn RG, Jaarsma T, Waldreus N, Linssen GC. Urine measurement indicates the plasma brain natriuretic peptide concentration during optimization of heart failure treatment. Scand J Clin Lab Invest 2016; 76: 112–117. [DOI] [PubMed] [Google Scholar]
- 2. Cowie MR, Jourdain P, Maisel A, Dahlstrom U, Follath F, Isnard R, Luchner A, McDonagh T, Mair J, Nieminen M, Francis G. Clinical applications of B‐type natriuretic peptide (BNP) testing. Eur Heart J 2003; 24: 1710–1718. [DOI] [PubMed] [Google Scholar]
- 3. Jourdain P, Jondeau G, Funck F, Gueffet P, Le Helloco A, Donal E, Aupetit JF, Aumont MC, Galinier M, Eicher JC, Cohen‐Solal A, Juillière Y. Plasma brain natriuretic peptide‐guided therapy to improve outcome in heart failure: the STARS‐BNP Multicenter Study. J Am Coll Cardiol 2007; 49: 1733–1739. [DOI] [PubMed] [Google Scholar]
- 4. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2016; 68: 1476–1488. [DOI] [PubMed] [Google Scholar]
- 5. Ng LL, Geeranavar S, Jennings SC, Loke I, O'Brien RJ. Diagnosis of heart failure using urinary natriuretic peptides. Clin Sci (Lond) 2004; 106: 129–133. [DOI] [PubMed] [Google Scholar]
- 6. Cortes R, Portoles M, Salvador A, Bertomeu V, García de Burgos F, Martínez‐Dolz L, Lletí ER, Climent V, Jordán A, Payá R, Sogorb F, Rivera M. Diagnostic and prognostic value of urine NT‐proBNP levels in heart failure patients. Eur J Heart Fail 2006; 8: 621–627. [DOI] [PubMed] [Google Scholar]
- 7. Manzano‐Fernandez S, Januzzi JL, Boronat Garcia M, Bonaque‐González JC, Muñoz‐Esparza C, Albaladejo‐Otón MD, Pastor‐Pérez FJ, Pastor P, Valdés M, Pascual‐Figal DA. Comparative prognostic value of plasma and urinary N‐terminal pro‐B‐type natriuretic peptide in patients with acute destabilized heart failure. Rev Esp Cardiol 2011; 64: 365–372. [DOI] [PubMed] [Google Scholar]
- 8. Toufan M, Namdar H, Abbasnezhad M, Habibzadeh A, Esmaeili H, Yaraghi S, Samani Z. Diagnostic values of plasma, fresh and frozen urine NT‐proBNP in heart failure patients. J Cardiovasc Thorac Res 2014; 6: 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jungbauer CG, Buchner S, Birner C, Resch M, Heinicke N, Debl K, Buesing M, Biermeier D, Schmitz G, Riegger G, Luchner A. N‐terminal pro‐brain natriuretic peptide from fresh urine for the biochemical detection of heart failure and left ventricular dysfunction. Eur J Heart Fail 2010; 12: 331–337. [DOI] [PubMed] [Google Scholar]
- 10. Kagiyama N, Kitai T, Hayashida A, Yamaguchi T, Okumura T, Kida K, Mizuno A, Oishi S, Inuzuka Y, Akiyama E, Suzuki S, Yamamoto M, Shimizu A, Urakami Y, Toki M, Aritaka S, Matsumoto K, Nagano N, Yamamoto K, Matsue Y. Prognostic value of BNP reduction during hospitalization in patients with acute heart failure. J Card Fail 2019; 25: 712–721. [DOI] [PubMed] [Google Scholar]
- 11. Brunner‐La Rocca HP, Sanders‐van Wijk S. Natriuretic peptides in chronic heart failure. Card Fail Rev 2019; 5: 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jungbauer CG, Stadler S, Birner C, Resch M, Ücer E, Fredersdorf S, Maier Lars S, Luchner A. Urinary NT‐proBNP is independently associated with long‐term prognosis of mortality in chronic heart failure. J Dis Markers 2015; 2: 1027. [Google Scholar]


