Abstract
Aims
Data on the early course and use of systemic thrombolysis in pregnant women with pulmonary embolism associated or not with haemodynamic failure are scarce. We investigated these aspects using the information from the German Nationwide Inpatient Registry (years 2005–2016).
Methods and results
In Germany, all diagnoses referring to hospitalized patients are coded according to the International Classification of Diseases and Related Health Problems, 10th Revision with German Modification. We analysed data of pregnant women aged 18–50 years for whom the following diagnoses were recorded during hospitalization: (i) pulmonary embolism (I26) during pregnancy or peripartum (O09) or (ii) obstetric thromboembolism (O88.2). Haemodynamic failure at any time during the in‐hospital stay was defined as need for cardiopulmonary resuscitation (OPS code 8‐77) or the presence of shock (International Classification of Diseases and Related Health Problems, 10th Revision with German Modification code R57). The primary study outcome was in‐hospital death. A total of 8 271 327 births were registered in Germany from 2005 to 2016. During this 12 year time period, there were 1846 hospitalizations for pregnancy‐associated pulmonary embolism in patients aged 18–50, corresponding to 2.2 [95% confidence interval (CI): 2.1–2.3] cases every 10 000 births and 0.2% of all hospitalizations for pulmonary embolism in Germany. The median age was 31 years, and the median length of hospitalization was 8 days. A total of 63 deaths were reported, corresponding to an overall in‐hospital fatality rate of 3.4% (95% CI: 2.7–4.4) and a pulmonary embolism‐related mortality rate of 0.8 (95% CI: 0.6–1.0) per 100 000 (live) births per year. Pulmonary embolism‐related deaths in hospitalized pregnant women represented 14% of all maternal deaths recorded in Germany between 2005 and 2016. A total of 135 (7.3%) women had haemodynamic failure, of whom 51 (37.8%) received systemic thrombolysis and 50 (37.0%) died.
Conclusions
Pulmonary embolism‐related fatality remains substantial in pregnant women with pulmonary embolism and represents a frequent cause of maternal mortality. The use of systemic thrombolysis was reported in one third of pregnant women with pulmonary embolism and haemodynamic failure. Better preventive and management strategies should be urgently implemented in this vulnerable patient group.
Keywords: Pulmonary embolism, Pregnancy, Mortality, Systemic thrombolysis, Epidemiology
Introduction
Acute venous thromboembolism complicates one in 500–3000 pregnancies 1 , 2 , 3 and represents a leading cause of death among younger women in Western countries. 4 , 5 , 6 , 7 A recent analysis of medically certified vital registration data in Europe demonstrated that, despite an overall decreasing trend in age‐standardized pulmonary embolism‐related mortality, more than 1% of all deaths in younger women are due to pulmonary embolism. 8 This figure is almost two times higher than that observed in men of the same age. 8
While the mainstay of therapy for acute pulmonary embolism is anticoagulation, systemic thrombolysis is recommended to rapidly relieve the right ventricle and reduce fatality in patients who present with haemodynamic instability, which comprise up to 10% of all cases and have a fatality of up to 68% in the first 5 days of hospitalization. 9 , 10 Other reperfusion options, including surgical embolectomy or catheter‐directed treatment, are less frequently used therapeutic alternatives, depending on the patient's profile and the expertise available at a given centre. However, systemic thrombolysis is probably underused. It has been recently reported that it is used in only 26% of haemodynamically unstable patients with acute pulmonary embolism in Germany. 11
The management of acute pulmonary embolism during pregnancy is challenging. First, pregnancy has been associated with improper diagnostic management of suspected pulmonary embolism because validated diagnostic algorithms were unavailable until recently, 12 , 13 and concerns on the potential harm of radiation or iodine contrast exposure fuel an ongoing debate on the best imaging modality. 14 , 15 Interventional studies for treatment of pregnancy associated pulmonary embolism are not available. 10 , 16 The evidence on the role of reperfusion strategies during pregnancy is limited too: its use for high‐risk pulmonary embolism during pregnancy is therefore only supported by a low level of recommendation. 9 , 10 A recent systematic review of the literature identified a limited number of 83 published cases and showed that pregnant women with high‐risk or intermediate‐risk pulmonary embolism had a high survival rate (94%) after systemic thrombolysis, although major maternal bleeding was observed in 28.4% of women. 17
In the present study, we investigated the case fatality and the use of systemic thrombolysis for acute pulmonary embolism in pregnant women with and without haemodynamic failure included in the German Nationwide Inpatient Registry from 2005 to 2016.
Methods
Diagnoses, procedural codes, and definitions
In Germany, all diagnoses referring to hospitalized patients are coded according to the International Classification of Diseases and Related Health Problems, 10th Revision with German Modification (ICD‐10‐GM) and included in a Nationwide Inpatient Registry. Diagnostic, general medical, and surgical invasive procedures are classified according to the German Procedure Classification [Operationen‐ und Prozedurenschlüssel (OPS), surgery and procedures codes]. The data of hospitalized patients with their Diagnosis‐Related Group (DRG) are collected and managed by the Federal Statistical Office of Germany. 11 This DRG system, which had been introduced in 2003–2004, applies to all hospitals, departments, and patients with the exclusion of rehabilitation and psychiatric institutions. The coding quality of German DRG data is routinely monitored by the regional medical review boards of the sickness funds, which verify the assignment of individual cases and corresponding resource utilization across randomly selected institutions (12% of all in‐hospital cases were monitored in 2009). Hospitals that intentionally up‐coded cases to increase their DRG‐based reimbursement would receive notification for a penalty payment. Further details concerning these procedures have been summarized by the European Observatory on Health Systems and Policies. 18 , 19
Study population and definition of pulmonary embolism and clinical variables
The ICD‐10‐GM code O09 for pregnancy and peripartum served for the identification of pregnant women up to 41 gestational weeks hospitalized with a primary diagnosis of pulmonary embolism (code I26) from 2005 to 2016. Alternatively, the code O88.2 for obstetric thromboembolism was used to identify women with pregnancy‐associated pulmonary embolism. The analysis was restricted to women aged 18–50 years at delivery who were treated with anticoagulant therapy or with systemic thrombolysis (source: RDC of the Federal Statistical Office and the Statistical Offices of the federal states, DRG Statistics 2005‐2016, and own calculations).
Haemodynamic failure at any time during the in‐hospital stay was defined as need for cardiopulmonary resuscitation (OPS code 8‐77) or the presence of shock (ICD‐10‐GM code R57). For subgroup analysis, patients with haemodynamic failure were further stratified into (i) patients who required cardiopulmonary resuscitation and (ii) patients with haemodynamic failure but requiring neither cardiopulmonary resuscitation nor mechanical ventilation (OPS codes 8‐70 and 8‐71).
The use of systemic thrombolysis was based on the OPS code 8‐020.8. We excluded patients for whom the following OPS codes were reported: 8‐838.d0 (percutaneous rotational pulmonary embolectomy), 8‐838.70 (percutaneous pulmonary embolus defragmentation), 8‐838.50 (or percutaneous pulmonary embolus aspiration), and catheter‐directed treatment (OPS 8‐83860 and OPS 8‐83bj). The following variables were also captured: pre‐eclampsia (O14), eclampsia (O15), cancer not including pregnancy‐related malignant neoplasms (ICD‐10‐GM codes C00‐C97), heart failure (ICD‐10‐GM code I50), obesity (ICD‐10‐GM code E66), deep vein thrombosis or thrombophlebitis of the leg veins (ICD‐10‐GM codes I80) transfusion of erythrocyte concentrates (OPS code 8‐800) and surgery during in‐hospital stay (OPS code 5). Information on pulmonary embolism occurring during post‐partum could not be extracted because no specific code allows unique identification of this period.
Because this study did not involve direct access to data of individual patients by the investigators, approval by an Ethics Committee and informed consent were not required, in accordance with German regulations. We could not obtain information on specific conditions and outcomes observed in five or fewer patients for each subgroup, which were censored by the Federal Statistical Office and the Statistical Offices of the federal states to prevent potential re‐identification of patients.
Study outcomes
The primary outcome is all‐cause in‐hospital death.
Statistical methods
Continuous variables are presented as median and interquartile range or mean and standard deviation, depending on their distribution. Categorical variables are provided as counts and percentages. Total numbers of pulmonary embolism cases, incidence, in‐hospital death rate, and length of in‐hospital stay were calculated for each calendar year. Crude fatality rates by dividing the number of deaths by pulmonary embolism by the number of pregnancy‐associated diagnoses of pulmonary embolism recorded in the Nationwide Inpatient Registry; their confidence interval (CI) was calculated using the Wilson score formula. We used smoothed lines generated by locally estimated scatterplot smoothing with least squares fitting to depict the trends in the incidence and crude fatality rate of pulmonary embolism over the study period. Univariable and multivariable (age‐adjusted) logistic regression models explored the association between systemic thrombolysis and fatality in patients who developed haemodynamic failure during hospitalization.
Crude incidence rates were estimated by dividing the number of pregnancy‐associated diagnoses of pulmonary embolism by the number of live births. For calculation of the annual incidence rates of pulmonary embolism among pregnant women and death rates related to pregnancy‐associated pulmonary embolism, the number of all births in Germany in the same year was used as denominator, as provided by the Federal Statistical Office of Germany. Because we did not anticipate major differences in the distribution of pregnancies across the 5year age groups during the period 2005–2016, no age‐standardization of rates was performed.
We estimated the proportionate mortality (95% CI) for pulmonary embolism, calculated as the number of pulmonary embolism‐related deaths registered in the Nationwide Inpatient Registry database of the total number of maternal deaths reported in the Global Health Observatory data repository of the World Health Organization (available at apps.who.int and data.worldbank.org).
The statistical analyses were run on behalf of the investigators by the Research Data Center of the Federal Statistical Office and the Statistical Offices of the federal states in Wiesbaden, Germany, who provided the results as aggregated data. SPSS® (version 20.0, Chicago, Illinois, USA) served for data analysis. The figures and the CIs of incidence, mortality, and case fatality rates were obtained by the investigators using R (version 3.5.3, package epitools).
Role of the funding source
The funder of the study (German Federal Ministry of Education and Research) had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
A total of 8 362 030 births were registered in Germany from 2005 to 2016, including 30 131 stillbirths. During this 12 year time period, there were 1846 hospitalizations for pregnancy‐associated pulmonary embolism in patients aged 18–50 years, corresponding to 2.2 (95% CI: 2.1–2.3) cases every 10 000 births/live births in women of the same age and 0.2% of all hospitalizations for pulmonary embolism in Germany. The annual rate of pulmonary embolism among pregnant women over time is depicted in Figure 1 . A total of 1839 patients were included in the present analysis after exclusion of six women who underwent pulmonary endarterectomy and one treated with catheter‐directed reperfusion therapy.
FIGURE 1.
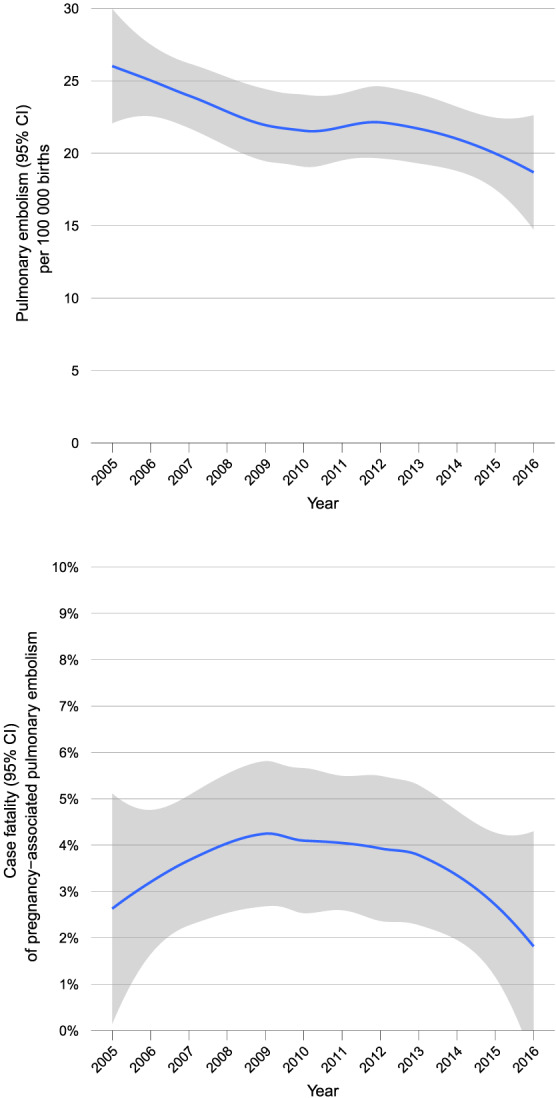
Incidence and case fatality of pregnancy‐associated pulmonary embolism in Germany, 2005–2016. CI, confidence interval.
Patient risk profile and clinical characteristics
Table 1 summarizes the baseline characteristics of pregnant women hospitalized for acute pulmonary embolism. The median age was 31 years, and the median length of hospitalization was 8 days. The majority of the cases of pulmonary embolism (n = 946; 51.4%) were diagnosed between the 34th and the 41th week of gestation. A code for haemodynamic failure or cardiopulmonary resuscitation was assigned to a total of 135 (7.3%) women, who were classified as haemodynamically unstable for the purposes of the present analysis. Differences in demographic and baseline characteristics between patients with versus without haemodynamic instability are summarized in Table 1 .
TABLE 1.
Baseline characteristics and clinical presentation of women with pregnancy‐associated acute pulmonary embolism
| Parameters | Total women (N = 1839) | Women with haemodynamic failure a (n = 135) | Women without haemodynamic failure a (n = 1704) | Absolute risk difference (95% CI) |
|---|---|---|---|---|
| Age (years) | 31 (27–35) | 33 (30–37) | 31 (26–35) | — |
| In‐hospital stay (days) | 8 (4–14) | 10 (2–19) | 8 (5–14) | — |
| Duration of pregnancy | ||||
| <5 weeks | 11 (0.6%) | 0 (0.0%) | 11 (0.6%) | +0.0% (−4.5%; +6.6%) |
| 5–13 weeks | 193 (10.5%) | 15 (11.1%) | 178 (10.4%) | |
| 14–19 weeks | 60 (3.3%) | 3 (2.2%) | 57 (3.3%) | +0.2% (−4.5%; +6.9%) |
| 20–25 weeks | 155 (8.4%) | 13 (9.6%) | 142 (8.3%) | |
| 26–33 weeks | 407 (22.1%) | 18 (13.3%) | 389 (22.8%) | −13.8% (−20.4%; −5.9%) |
| 34–36 weeks | 224 (12.2%) | 11 (8.1%) | 213 (12.5%) | |
| 37–41 weeks b | 722 (39.3%) | 67 (49.6%) | 655 (38.4%) | +11.2% (+2.6%; +19.8%) |
| Clinical presentation, co‐morbidities, and provoking risk factors | ||||
| Surgery during the in‐hospital stay | 1251 (68.0%) | 119 (88.1%) | 1132 (66.4%) | +21.7% (+14.8%; +26.7%) |
| Obesity | 104 (5.7%) | 10 (7.4%) | 94 (5.5%) | +1.9% (−1.6%; +7.7%) |
| Heart failure | 80 (4.4%) | 19 (14.1%) | 61 (3.6%) | +10.5% (+5.5%; +17.4%) |
| Deep venous thrombosis or thrombophlebitis | 109 (5.9%) | 10 (9.2%) | 99 (5.8%) | +1.6% (−2.0%; +7.4%) |
| Preeclampsia | 139 (7.6%) | 10 (7.4%) | 129 (7.6%) | −0.16% (−3.8%; +5.6%) |
| Eclampsia | 17 (0.8%) | 3 (2.2%) | 14 (0.8%) | +1.4% (−0.2%; +5.5%) |
| HELLP syndrome | 30 (1.6%) | 3 (2.2%) | 27 (1.6%) | +0.6% (−1.0%; +4.8%) |
| In‐hospital stay (days) | 8 (4–14) | 10 (2–19) | 8 (5–14) | — |
| Systemic thrombolysis | 67 (3.6%) | 51 (37.8%) | 16 (0.9%) | +36.8% (+29.1%; +45.3%) |
| In‐hospital cumulative death rate | 63 (3.4%) | 50 (37.0%) | 13 (0.8%) | +36.3% (+28.6%; +44.7%) |
Pulmonary embolism‐related fatality and death rate
A total of 63 deaths were reported in pregnant women hospitalized for acute pulmonary embolism from 2005 to 2016, corresponding to an in‐hospital case fatality rate of 3.4% (95% CI: 2.7–4.4). Using as denominator the number of (live) births reported by the Federal Statistical Office of Germany during the same time period, we estimated an average pulmonary embolism‐related mortality rate of 0.8 (95% CI: 0.6–1.0) per 100 000 (live) births per year. The estimated proportionate mortality for pregnancy‐associated pulmonary embolism was 13.7% (95% CI: 10.9–17.1), corresponding to the number of deaths in women hospitalized for pulmonary embolism out of all maternal deaths (N = 460) reported in Germany from 2005 to 2016.
Of the 63 deaths, 50 occurred among patients with haemodynamic failure (case fatality rate 37.0% in this subgroup) and 13 among patients without haemodynamic failure (case fatality rate 0.8%) for an absolute risk difference of +36.3% (95% CI: +28.6% to +44.7%).
Use of systemic thrombolysis
Systemic thrombolysis was administered to 67 (3.6%) of 1839 pregnant women with pulmonary embolism and to 51 (37.8%) of the 135 patients with haemodynamic failure. Systemic thrombolysis was more frequent applied during the first 13 weeks of pregnancy and between weeks 37 and 41 (Supporting Information, Table S1 ). The proportions of patients with haemodynamic failure among all cases of pregnancy‐related pulmonary embolism and that of patients receiving systemic thrombolysis are depicted in Figure 2 . Among women with pulmonary embolism and haemodynamic failure, the odds of death was higher among patients receiving systemic thrombolysis [crude odds ratio 3.43 (95% CI: 1.64–7.16); age‐adjusted odds ratio 3.48 (95% CI: 1.66–7.27)].
FIGURE 2.
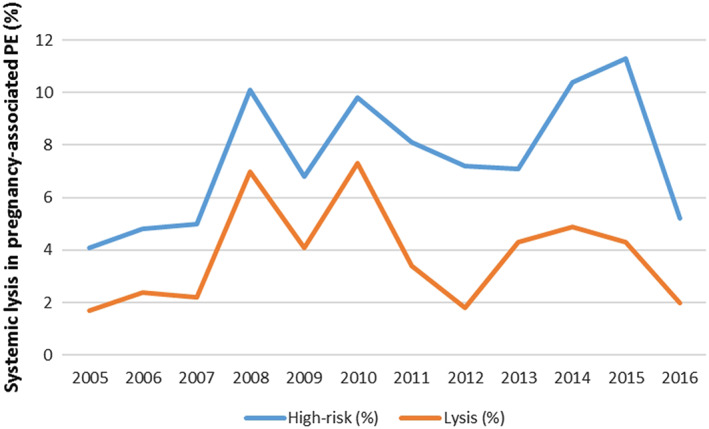
Proportion of pregnant women with pulmonary embolism and haemodynamic failure (high risk) and use of systemic thrombolysis in Germany from 2005 to 2016.
The distribution of co‐morbidities between patients who underwent (versus those who did not undergo) systemic thrombolysis tended to parallel that presented in Table 1 for patients stratified by the presence of haemodynamic failure. A total of five intracerebral bleeding events were recorded, but we were unable to determine whether they occurred in women who received systemic thrombolysis to prevent potential re‐identification of patients.
Discussion
Comprehensive analyses of the rate of occurrence and early outcome of acute pregnancy‐associated pulmonary embolism are not available to this date, particularly with regard the use of systemic thrombolysis. Our nationwide analysis, covering a 12 year period and based on administrative data of more than 1800 women with pregnancy‐associated pulmonary embolism in Germany, provides nationwide estimates of case fatality and the use of systemic thrombolysis in this population (Table 2 ).
TABLE 2.
Baseline characteristics of 1839 pregnant women with pulmonary embolism stratified by systemic lysis treatment
| Parameters | All pregnant women with pulmonary embolism (n = 1839) | Thrombolysis (n = 67; 3.6%) | No thrombolysis (n = 1771; 96.3%) | Absolute risk difference (95% CI) |
|---|---|---|---|---|
| Age (years) | 31 (27–35) | 32 (29–36) | 31 (27–35) | — |
| Obesity | 104 (5.7%) | 6 (8.8%) | 98 (5.5%) | +3.3% (−1.6%; +12.5%) |
| In‐hospital stay (days) | 8 (4–14) | 9 (2–20) | 8 (4–14) | — |
| Pregnancy <5 weeks | 11 (0.6%) | 0 (0.0%) | 11 (0.6%) | +8.3% (+0.6%; +19.3%) |
| Pregnancy 5–13 weeks | 193 (10.5%) | 13 (19.1%) | 180 (10.2%) | |
| Pregnancy 14–19 weeks | 60 (3.3%) | Censored | Censored | −1.1% (−5.5%; +7.7%) |
| Pregnancy 20–25 weeks | 155 (8.4%) | 5 (7.4%) | 150 (8.5%) | |
| Pregnancy 26–33 weeks | 407 (22.1%) | 9 (13.2%) | 398 (22.5%) | −17.3% (−24.9%; −6.4%) |
| Pregnancy 34–36 weeks | 224 (12.2%) | 3 (4.4%) | 221 (12.5%) | |
| Pregnancy 37–41 weeks | 722 (39.3%) | 34 (50.0%) | 688 (38.8%) | +11.2% (−0.6%; +22.9%) |
| Co‐morbidities | ||||
| Surgery during the in‐hospital stay | 1251 (68.0%) | 53 (77.9%) | 1198 (67.6%) | +10.3% (−1.10; +18.8%) |
| Heart failure | 80 (4.4%) | 9 (13.2%) | 71 (4.0%) | +9.2% (+3.0%; +19.3%) |
| Deep venous thrombosis or thrombophlebitis | 109 (5.9%) | 6 (8.8%) | 103 (5.8%) | +3.0% (−1.9%; +12.2%) |
| Haemodynamic failure | 135 (7.3%) | 52 (76.5%) | 83 (4.7%) | +71.8% (+60.4%; +80.3%) |
| Mechanical ventilation | 175 (9.5%) | 40 (58.8%) | 135 (7.6%) | +51.2% (+39.3%; +62.2%) |
| Outcome | ||||
| In‐hospital mortality | 63 (3.4%) | 29 (42.6%) | 34 (1.9%) | +40.7% (+29.7%; +52.6%) |
We found that the in‐hospital fatality rate in women hospitalized for pulmonary embolism is substantial (3.4 deaths per 100 pregnant women with a diagnosis of pulmonary embolism) and approximately 500 times higher than the maternal mortality in Germany during the same time period (five to six deaths per 100 000 live births) excluding pulmonary embolism. 20 Deaths during hospitalizations for pulmonary embolism in pregnant women accounted for almost 14% of all maternal deaths. In particular, pregnant women with pulmonary embolism and haemodynamic failure were characterized by very high death rates, with more than one third of these patients dying during hospitalization as compared with less than 1% in women without haemodynamic failure. This observation could partly explain why pulmonary embolism represented a much more frequent cause of death among younger women aged 15–55 years than in men of the same age. 8 While these estimates concerning the overall fatality rates are similar to what described in prior studies, 21 the in‐hospital fatality rate among women with pulmonary embolism and haemodynamic failure (37%) depicts a much more pessimistic picture than that derived from small case series and reports (7.6% in high‐risk or intermediate‐risk patients). 17 Fatality estimates obtained from case reports were even lower (approximately 5%) if only antenatal pulmonary embolism were considered. 17
We showed that use of systemic thrombolysis was rarely recorded among pregnant women, even in the presence of haemodynamic failure; in fact, the code for the use of lytic therapy was reported in only one third of women with pulmonary embolism and haemodynamic failure. The fact that the death rate was higher among those women who received systemic thrombolysis most likely suggests that this treatment was used as a last resort option in the most severely compromised patients. Of note, the observational nature of our data prevents us from drawing any conclusions concerning the efficacy and safety of systemic thrombolysis during pregnancy. A prospective registry endorsed by the International Society on Thrombosis and Haemostasis will set out to investigate the course of high‐risk pulmonary embolism among pregnant women receiving systemic or catheter‐directed thrombolysis. 22
Our results are consistent with prior epidemiological studies estimating the incidence of pulmonary embolism during pregnancy. 4 , 5 , 7 , 23 In particular, we found that pulmonary embolism was recorded as a complication in an average of one every 4000 pregnancies in Germany between 2005 and 2007, decreasing to approximately one per 5000 between 2014 and 2016. The observed incidence of 25 cases of pulmonary embolism per 100 000 pregnancies in Germany in 2005 is consistent with the estimates obtained in Canada (26 per 100 000 pregnancies in 2004–2005), 24 United Kingdom (13 per 100 000 in 2005–2006), 21 and Scotland (30 per 100 000 pregnancies in 2001–2005). 25 In our study, the majority of events occurred after week 34 of gestation and a number of co‐morbidities were more prevalent in women with pulmonary embolism associated with haemodynamic failure or requiring cardiopulmonary resuscitation, including caesarean section, obesity, and heart failure. Caesarean section and the latter co‐morbidities have been previously identified as key risk factors for the development of pulmonary embolism among pregnant women 26 but not for the severity of pulmonary embolism. Of note, one in nine hospitalizations for pulmonary embolism were recorded in women before 13 weeks of gestation, a time when clinicians may not be thinking of pulmonary embolism as the most likely diagnosis in a symptomatic woman.
Our study has limitations. First, because our results are based on administrative data and the original charts could not be directly verified, we cannot exclude code misclassification or missed diagnoses of other complications that can manifest during pregnancy or delivery, including amniotic fluid embolism. Fatal pulmonary embolism events occurring out of hospital or diagnosed post‐mortem are not captured in the German Inpatient Registry, possibly leading to an underestimation of real estimates. Second, we estimated the incidence of pulmonary embolism events during pregnancy based on the number of (live) births in Germany in the same year; however, this figure did not include pregnancies interrupted by miscarriages until the 20th week. Third, we were able to study the association between variables or events registered during hospitalization, but we had no information on their temporal/causal relationship. In light of this issue and the relatively small number of deaths recorded, we could not further investigate the potential influence of specific patient characteristics or factors on the outcomes, including caesarean section. With this respect, it would be impossible to distinguish whether it represented an underlying cause of pulmonary embolism or, conversely, was attempted as a last resort option after its diagnosis. Finally, due to German legal regulations, we were not allowed to show the results of analyses for complications or procedures involving fewer than five patients: therefore, we could not analyse the rate of major bleeding complications in women undergoing systemic thrombolysis. For the same reason, we were not allowed to identify individual patients, and we cannot exclude that some had repeated hospitalizations.
The case fatality rate of acute pulmonary embolism remains substantial among pregnant women in a high‐income country like Germany. Systemic thrombolysis was used in a minority of pregnant women with pulmonary embolism and haemodynamic failure. Our findings may partly explain the discrepancy between women and men in age‐specific mortality rate and proportionate mortality related to pulmonary embolism previously described in Western European countries. Better preventive and management strategies should be urgently implemented in this vulnerable patient group.
Conflict of interest
L. Hobohm reports lecture/consultant fees from MSD and Actelion. F. A. Klok reports research grants from Bayer, Bristol‐Myers Squibb, Boehringer‐Ingelheim, Daiichi‐Sankyo, MSD, and Actelion, the Dutch Heart foundation and the Dutch Thrombosis association. F. Ni Ainle reports unrestricted research grants (paid to the PI's institution) from Actelion, Bayer and Leo Pharma. M. Lankeit reports having received consultancy and lecture honoraria from Actelion, Bayer, Daiichi‐Sankyo, MSD, Pfizer – Bristol‐Myers Squibb and research funding from BRAHMS – Thermo Fisher scientific. S. V. Konstantinides reports grants and personal fees from Bayer AG; grants from Boehringer Ingelheim, grants and personal fees from Actelion, grants and personal fees from Daiichi‐Sankyo, grants and personal fees from Biocompatibles Group UK, personal fees from Pfizer‐Bristol‐Myers Squibb, grants and personal fees from MSD, all outside the submitted work. S. Barco received lecture/consultant fees from Bayer HealthCare, BTG Pharmaceuticals, and LeoPharma; and economical support for travel/congress costs from Daiichi Sankyo and Bayer HealthCare, outside the submitted work. The remaining authors declare no competing financial interests.
Funding
This study was supported by the German Federal Ministry of Education and Research (BMBF 01EO1503). The authors are responsible for the contents of this publication.
Supporting information
Table S1. Baseline characteristics of 1,839 pregnant women with pulmonary embolism stratified by systemic lysis treatment.
Hobohm, L. , Keller, K. , Valerio, L. , Ni Ainle, F. , Klok, F. A. , Münzel, T. , Kucher, N. , Lankeit, M. , Konstantinides, S. V. , and Barco, S. (2020) Fatality rates and use of systemic thrombolysis in pregnant women with pulmonary embolism. ESC Heart Failure, 7: 2365–2372. 10.1002/ehf2.12775.
References
- 1. Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ 3rd. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30‐year population‐based study. Ann Intern Med 2005; 143: 697–706. [DOI] [PubMed] [Google Scholar]
- 2. Meng K, Hu X, Peng X, Zhang Z. Incidence of venous thromboembolism during pregnancy and the puerperium: a systematic review and meta‐analysis. J Matern Fetal Neonatal Med 2015; 28: 245–253. [DOI] [PubMed] [Google Scholar]
- 3. Danwang C, Temgoua MN, Agbor VN, Tankeu AT, Noubiap JJ. Epidemiology of venous thromboembolism in Africa: a systematic review. J Thromb Haemost 2017; 15: 1770–1781. [DOI] [PubMed] [Google Scholar]
- 4. Chang J, Elam‐Evans LD, Berg CJ, Herndon J, Flowers L, Seed KA, Syverson CJ. Pregnancy‐related mortality surveillance—United States, 1991–1999. MMWR Surveill Summ 2003; 52: 1–8. [PubMed] [Google Scholar]
- 5. Sanisoğlu S, Uygur D, Keskinkılıç B, Engin‐Üstün Y, Keskin HL, Karaahmetoğlu S, Özcan A, Esen M, Ongun V, Özkan S. Maternal mortality cases from pulmonary embolism: a nation‐wide study in Turkey. J Obstet Gynaecol 2017; 37: 151–156. [DOI] [PubMed] [Google Scholar]
- 6. Heyl PS, Sappenfield WM, Burch D, Hernandez LE, Kavanaugh VM, Hill WC. Pregnancy‐related deaths due to pulmonary embolism: findings from two state‐based mortality reviews. Matern Child Health J 2013; 17: 1230–1235. [DOI] [PubMed] [Google Scholar]
- 7. Bødker B, Hvidman L, Weber TO, Møller M, Aarre A, Nielsen KM, Sørensen JL. Maternal deaths in Denmark 2002‐2006. Acta Obstet Gynecol Scand 2009; 88: 556–562. [DOI] [PubMed] [Google Scholar]
- 8. Barco S, Mahmoudpour SH, Valerio L, Klok FA, Münzel T, Middeldorp S, Ageno W, Cohen AT, Hunt BJ, Konstantinides SV. Trends in mortality related to pulmonary embolism in the European Region from 2000 to 2015: analysis of vital registration data from the World Health Organization mortality database. Lancet Respir Med 2020; 8: 277–287. [DOI] [PubMed] [Google Scholar]
- 9. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G‐J, Harjola V‐P, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Áinle FN, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2019; 54: 1901647. [DOI] [PubMed] [Google Scholar]
- 10. Bates SM, Rajasekhar A, Middeldorp S, McLintock C, Rodger MA, James AH, Vazquez SR, Greer IA, Riva JJ, Bhatt M, Schwab N, Barrett D, LaHaye A, Rochwerg B. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv 2018; 2: 3317–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keller K, Hobohm L, Ebner M, Kresoja KP, Münzel T, Konstantinides SV, Lankeit M. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur Heart J 2019. 41: 522–529. [DOI] [PubMed] [Google Scholar]
- 12. van der Pol LM, Tromeur C, Bistervels IM, Ni Ainle F, van Bemmel T, Bertoletti L, Couturaud F, van Dooren Y, Elias A, Faber LM, Hofstee HMA, van der Hulle T, Kruip MJHA, Maignan M, Mairuhu ATA, Middeldorp S, Nijkeuter M, Roy PM, Sanchez O, Schmidt J, ten Wolde M, Klok FA, Huisman MV, Artemis Study Investigators . Pregnancy‐adapted years algorithm for diagnosis of suspected pulmonary embolism. N Engl J Med 2019; 380: 1139–1149. [DOI] [PubMed] [Google Scholar]
- 13. Righini M, Robert‐Ebadi H, Elias A, Sanchez O, le Moigne E, Schmidt J, le Gall C, Cornuz J, Aujesky D, Roy PM, Chauleur C, Rutschmann OT, Poletti PA, le Gal G, for the CT‐PE‐Pregnancy Group . Diagnosis of pulmonary embolism during pregnancy: a multicenter prospective management outcome study. Ann Intern Med 2018; 169: 766–773. [DOI] [PubMed] [Google Scholar]
- 14. Barco S, Nijkeuter M, Middeldorp S. Pregnancy and venous thromboembolism. Semin Thromb Hemost 2013; 39: 549–558. [DOI] [PubMed] [Google Scholar]
- 15. Roy PM, Meyer G, Vielle B, le Gall C, Verschuren F, Carpentier F, Leveau P, Furber A, for the EMDEPU Study Group* . Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med 2006; 144: 157–164. [DOI] [PubMed] [Google Scholar]
- 16. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs JSR, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Noordegraaf AV, Zamorano JL, Zompatori M, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol Ç, Fagard R, Ferrari R, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Erol Ç, Jimenez D, Ageno W, Agewall S, Asteggiano R, Bauersachs R, Becattini C, Bounameaux H, Büller HR, Davos CH, Deaton C, Geersing G‐J, Sanchez MAG, Hendriks J, Hoes A, Kilickap M, Mareev V, Monreal M, Morais J, Nihoyannopoulos P, Popescu BA, Sanchez O, Spyropoulos AC. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 2014; 35: 3033–3080.25173341 [Google Scholar]
- 17. Martillotti G, Boehlen F, Robert‐Ebadi H, Jastrow N, Righini M, Blondon M. Treatment options for severe pulmonary embolism during pregnancy and the postpartum period: a systematic review. J Thromb Haemost 2017; 15: 1942–1950. [DOI] [PubMed] [Google Scholar]
- 18. Busse R, Geissler A, Aaviksoo A, Cots F, Hakkinen U, Kobel C, Mateus C, Or Z, O'Reilly J, Serden L, Street A, Tan SS, Quentin W. Diagnosis related groups in Europe: moving towards transparency, efficiency, and quality in hospitals? BMJ 2013; 346: f3197. [DOI] [PubMed] [Google Scholar]
- 19. Busse R, Geissler A, Quentin W, Wiley M. Diagnosis‐related groups in Europe. Moving towards transparency, efficiency and quality in hospitals. Maidenhead, Berkshire, England: Open University Press; 2011. [DOI] [PubMed] [Google Scholar]
- 20. WHO U , UNFPA , World Bank Group , and the United Nations Population Division . Trends in maternal mortality: 2000 to 2017. 2019. https://data.worldbank.org/indicator/SH.STA.MMRT?locations=DE (28 October 2019).
- 21. Knight M. Ukoss. Antenatal pulmonary embolism: risk factors, management and outcomes. BJOG 2008; 115: 453–461. [DOI] [PubMed] [Google Scholar]
- 22. McIntyre KM, Sasahara AA. The hemodynamic response to pulmonary embolism in patients without prior cardiopulmonary disease. Am J Cardiol 1971; 28: 288–294. [DOI] [PubMed] [Google Scholar]
- 23. James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol 2006; 194: 1311–1315. [DOI] [PubMed] [Google Scholar]
- 24. Liu S, Rouleau J, Joseph KS, Sauve R, Liston RM, Young D, Kramer MS. Epidemiology of pregnancy‐associated venous thromboembolism: a population‐based study in Canada. J Obstet Gynaecol Can 2009; 31: 611–620. [DOI] [PubMed] [Google Scholar]
- 25. Kane EV, Calderwood C, Dobbie R, Morris C, Roman E, Greer IA. A population‐based study of venous thrombosis in pregnancy in Scotland 1980‐2005. Eur J Obstet Gynecol Reprod Biol 2013; 169: 223–229. [DOI] [PubMed] [Google Scholar]
- 26. Larsen TB, Sorensen HT, Gislum M, Johnsen SP. Maternal smoking, obesity, and risk of venous thromboembolism during pregnancy and the puerperium: a population‐based nested case‐control study. Thromb Res 2007; 120: 505–509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of 1,839 pregnant women with pulmonary embolism stratified by systemic lysis treatment.


