Abstract
Aims
Patients undergoing percutaneous mitral valve repair (PMVR) show a substantial heterogeneity of prognostic and symptomatic benefit. Iron deficiency and anaemia are associated with worse outcomes in heart failure patients. We investigated the impact of these comorbidities on functional and clinical outcome after PMVR.
Methods and results
Iron deficiency and anaemia were prospectively assessed in 130 patients undergoing PMVR with MitraClip. Associations with functional outcomes at 6 weeks [6 min walking distance (6MWD), Short‐Form‐36 physical component score, and Minnesota Living with Heart Failure Questionnaire score, New York Heart Association class] and long‐term clinical outcome were examined. Iron deficiency and anaemia were frequent with 52% and 50%, respectively. Patients with anaemia showed significant worse baseline functional measures, whereas patients with iron deficiency showed only a trend for lower baseline 6MWD. The benefit in functional outcomes after PMVR was notable and did not differ significantly by iron deficiency or anaemia status (range of median changes in 6MWD 35 to 45 m, physical component score 5.6 to 7.2, Minnesota Living with Heart Failure Questionnaire −8.0 to −10.5; improvement of ≥1 New York Heart Association class 69% to 80%). Anaemia was associated with higher risk for the combined endpoint of mortality and heart failure hospitalization (hazard ratio: 2.51; 95% confidence interval: 1.24–5.1; P = 0.01), whereas iron deficiency showed a trend towards more heart failure hospitalizations (hazard ratio: 2.94; 95% confidence interval: 0.94–9.03; P = 0.09).
Conclusions
The prevalence of iron deficiency and anaemia is high in patients undergoing MitraClip. Clinical baseline status and long‐term outcome were worse particularly in patients with anaemia. However, the functional benefit of PMVR was equal in patients with and without iron deficiency and anaemia.
Keywords: MitraClip, Iron deficiency, Anaemia, Functional outcomes
Introduction
Percutaneous edge‐to‐edge repair [percutaneous mitral valve repair (PMVR)] of mitral regurgitation (MR) is an increasingly applied technique with more than 100 000 procedures performed worldwide. A range of observational studies showed an improvement in functionality and quality of life after PMVR. 1 , 2 However, there is a huge heterogeneity in the amount of improvement, with a substantial part of patients showing no or only minor functional benefit. 3 Furthermore, two recent randomized controlled trials in highly selected patients with functional MR also showed inconsistent results both regarding prognostic and symptomatic benefit in comparison with standard heart failure therapy. 4 , 5 The underlying causes for this effect heterogeneity are largely unclear so far and need further attention in order to adapt patient selection for optimal treatment benefit. In this context, potentially modifiable factors affecting functional and symptomatic outcome after MitraClip are of major interest.
Iron deficiency (ID) and anaemia are common in patients with cardiovascular disease, and in particular heart failure, and are associated with worse clinical outcomes. 6 , 7 Every one out of five patients with heart failure has anaemia independently of ejection fraction (EF). The presence of anaemia is an independent risk factor for all‐cause mortality or hospitalization rate. 8 , 9 , 10 A significant relationship between haemoglobin levels and health‐related quality of life through 12 months follow‐up has also been reported. 11 ID is also common in heart failure patients, with prevalence rates ranging from 37% to 61%, and is an independent predictor of cardiovascular mortality. 12 Jankowska et al. demonstrated that ID occurred frequently in the absence of anaemia (32% of non‐anaemic patients had ID). 13 The presence of ID in heart failure is associated with lower physical capacity assessed by 6 min walking distance (6MWD), more fatigue, and worse quality of life.
Given that almost all patients undergoing PMVR have severe clinical heart failure syndrome, our hypothesis was that ID and anaemia are frequent in these patients and negatively affect clinical outcomes. The aim of this study was to investigate the prevalence of ID and anaemia and the association with functional outcomes 6 weeks after the procedure and clinical long‐term outcome in consecutive patients who underwent PMVR at our centre.
Methods
We prospectively enrolled 162 consecutive patients at the Heart Centre of the University Hospital Cologne with severe MR, who planned to undergo PMVR with the MitraClip procedure between June 2016 and October 2017. Details of patient enrolment and assessments have been published recently. 3 Briefly, all patients were discussed by a Heart Team, including at least one interventional cardiologist, one non‐interventional cardiologist, and one cardiac surgeon, and a decision on interventional treatment approach using MitraClip was made based on surgical risk and MR aetiology, morphological suitability, and other relevant patient characteristics. All patients had an indication for MR treatment according to current guidelines. The patients were included in the study if written informed consent was given by the patient. The study was approved by the local ethics committee of the University of Cologne (reference 14‐116, German Registry of Clinical Trials registration DRKS00006194).
Pre‐specified baseline characteristics were extracted from records. Additionally, 6MWD, 14 New York Heart Association (NYHA) class, 15 Minnesota Living with Heart Failure Questionnaire (MLWHFQ), 16 and the generic, validated Medical Outcomes Study Short‐Form 36 (SF36, Optum insight, Life Sciences, Inc.) 17 , 18 were assessed during the hospitalization before the MitraClip procedure and at an outpatient follow‐up about 6 weeks after the procedure by a trained medical student who was blinded to procedural and echocardiographic results. Laboratory markers such as renal function and haemoglobin were part of routine pre‐procedural assessments. Additionally for this study, iron status including ferritin, transferrin, and transferrin saturation were assessed pre‐procedurally in consecutive patients.
Iron deficiency was defined according to criteria used in recent randomized trials in patients with heart failure with reduced EF (serum ferritin < 100 μg/L or ferritin between 100 and 299 μg/L and transferrin saturation < 20%). 6 , 7 Anaemia was defined according to the World Health Organization criteria (haemoglobin < 13 g/dL for male patients; haemoglobin < 12 g/dL for female patients).
Results
Patient characteristics
Of 162 patients referred for MitraClip, there were 12 who lacked iron status prior implantation; 18 did not receive MitraClip but another percutaneous device; MitraClip was not implanted in one patient due to a new vegetation shown before grasping; and a MitraClip implantation was technically not feasible in one patient due to severe biatrial dilatation. A total of 130 patients were finally included in the analysis [median age 80 (interquartile range 8) years; 51% male patients). A high surgical risk was present in all patients, and the logistic Euroscore was >20 in 29%. The MR aetiology was degenerative in 56.2% and functional in 43.9%. More than half of the patients had preserved EF (55%), whereas 34% had an EF between 30% and 50% and 11% had a severely reduced EF of <30%. Most patients (74.6%) suffered from chronic kidney disease. Around 96.2% of patients were severely symptomatic in NYHA class III or IV. Eight patients were receiving iron supplementation at study enrolment. A total of 118 patients were under anticoagulation therapy. The haemoglobin levels were significantly lower in the presence of ID.
Mitral regurgitation grade 2+ or less could be achieved in the majority of participants (90%) and was similar between groups. Blood transfusions were significantly more frequent in anaemic patients. Device‐related and procedure‐related complications were rare and did not differ between groups [patients with any of the complications reported in Table 1 : 8 (12.9%) vs. 12 (17.6%), P = 0.48 for ID groups and 9 (13.8%) vs. 11 (16.9%), P = 0.81 for anaemia groups]. Median hospital stay was 7 (5) days and was significantly prolonged in patients with anaemia (Table 1 ).
TABLE 1.
Baseline and procedural characteristics by iron deficiency and anaemia
| Total N = 130 | No iron deficiency N = 62 (48%) | Iron deficiency N = 68 (52%) | P value | No anaemia N = 65 (50%) | Anaemia N = 65 (50%) | P value | |
|---|---|---|---|---|---|---|---|
| Age (years) | 80 (8) | 78 (10) | 81 (9) | 0.07 | 80 (12) | 80 (7) | 0.44 |
| Male sex | 66 (50.8%) | 39 (62.9%) | 27 (39.7%) | 0.009 * | 35 (53.8%) | 31 (47.7%) | 0.6 |
| Functional MR | 57 (43.9%) | 32 (51.6%) | 25 (36.8%) | 0.09 | 31 (47.7%) | 26 (40.0%) | 0.38 |
| ICM | 64/129 (49.6%) | 30 (49.2%) | 34 (50%) | 0.9 | 29 (45.3%) | 35 (54.7%) | 0.27 |
| LogEuroscore > 20 | 38 (29.2%) | 15 (24.2%) | 23 (33.8%) | 0.25 | 15 (23.1%) | 23 (35.4%) | 0.18 |
| EF > 50% | 71/129 (55%) | 34 (54.8%) | 37 (55.2%) | 1 | 35 (53.8%) | 36 (56.3%) | 0.86 |
| Hypertension | 97 (74.6%) | 45 (72.6%) | 52 (76.5%) | 0.69 | 44 (67.7%) | 53 (81.5%) | 0.1 |
| Diabetes | 27 (20.8%) | 11 (17.7%) | 16 (23.5%) | 0.52 | 9 (13.8%) | 18 (27.7%) | 0.08 |
| Prior TIA or stroke | 19 (14.6%) | 8 (12.9%) | 11 (16.2%) | 0.63 | 9 (13.8%) | 10 (15.4%) | 1 |
| Prior MI | 29 (22.3%) | 12 (19.4%) | 17 (25%) | 0.53 | 14 (21.5%) | 15 (23.1%) | 1 |
| CAD | 79 (60.8%) | 36 (58.1%) | 43 (63.2%) | 0.59 | 38 (58.5%) | 41 (63.1%) | 0.72 |
| Prior cardiac surgery | 39 (30%) | 19 (30.6%) | 20 (29.4%) | 1 | 19 (29.2%) | 20 (30.8%) | 1 |
| Prior MV intervention | 4 (3.1%) | 3 (4.8%) | 1 (1.5%) | 0.35 | 2 (3.1%) | 2 (3.1%) | 1 |
| PAD | 18 (13.8%) | 8 (12.9%) | 10 (14.7%) | 0.81 | 6 (9.2%) | 12 (18.5%) | 0.2 |
| COPD | 21/129 (16.3%) | 11 (17.7%) | 10 (14.9%) | 0.81 | 12 (18.5%) | 9 (14.1%) | 0.63 |
| AF | 90 (69.2%) | 43 (69.4%) | 47 (69.1%) | 1 | 41 (63.1%) | 49 (75.4%) | 0.18 |
| Malignancy | 23/129 (17.8%) | 12 (19.7%) | 11 (16.2%) | 0.65 | 11 (17.2%) | 12 (18.5%) | 1 |
| ICD | 13 (10%) | 7 (11.3%) | 6 (8.8%) | 0.77 | 8 (12.3%) | 5 (7.7%) | 0.56 |
| CRT | 19 (14.6%) | 8 (12.9%) | 11 (16.2%) | 0.63 | 9 (13.8%) | 10 (15.4%) | 1 |
| Oral iron supplementation | 8 (6.2%) | 2 (3.2%) | 6 (8.8%) | 0.28 | 3 (4.6%) | 5 (7.7%) | 0.72 |
| Oral anticoagulation and antiplatelet therapy | 118 (90.8%) | 57 (91.9%) | 61 (89.7%) | 0.88 | 56 (86.2%) | 62 (95.4%) | 0.16 |
| Vitamin K antagonists | 67 (51.5%) | 32 (51.6%) | 35 (51.5%) | – | 29 (44.6%) | 38 (58.5%) | – |
| DOAC | 22 (16.9%) | 12 (19.4%) | 10 (14.7%) | – | 10 (15.4%) | 12 (18.5%) | – |
| ASA | 29 (22.3%) | 13 (21%) | 16 (23.5%) | – | 17 (26.2%) | 12 (18.5%) | – |
| NYHA class III/IV | 125 (96.2%) | 58 (93.5%) | 67 (98.5%) | 0.14 | 62 (95.4%) | 63 (96.9%) | 0.64 |
| Haemoglobin (g/dL) | 12.5 (2.9) | 13.2 (2.8) | 11.6 (2.3) | 0.002 * | 13.7 (1.7) | 10.8 (1.8) | <0.001 * |
| Anaemia | 65 (50%) | 23 (37.1%) | 42 (61.8%) | 0.008 * | – | – | – |
| Iron deficiency | 68 (52.3%) | – | – | – | 26 (40%) | 42 (64.6%) | 0.008 * |
| CKD | 97 (74.6%) | 44 (71%) | 53 (78%) | 0.42 | 44 (67.7%) | 53 (81.5%) | 0.11 |
| Median NT‐proBNP (pg/mL) | 2108 (2725) (n = 95) | 1930 (2430) (n = 50) | 2184 (3199) (n = 45) | 0.59 | 1865 (2739) (n = 50) | 2421 (2242) (n = 45) | 0.053 |
| Median eGFR (mL/min/1.73 m2) | 42 (29) (n = 129) | 44 (29) (n = 61) | 37 (32) (n = 68) | 0.137 | 46.5 (29) (n = 64) | 37 (26) (n = 65) | 0.028 * |
| Technical successa | 129 (99.2%) | 62 (100%) | 67 (98.5%) | 1 | 65 (100%) | 64 (98.5%) | 1 |
| Post‐procedural MR grade < 2+ | 117 (90%) | 58 (93.5%) | 59 (86.8%) | 0.25 | 59 (90.8%) | 58 (89.2%) | 1 |
| Pericardial effusion | 0 (0%) | 0 (0%) | 0 (0%) | 1 | 0 (0%) | 0 (0%) | 1 |
| Vascular complication | 6 (4.6%) | 2 (3.2%) | 4 (5.9%) | 0.68 | 3 (4.6%) | 3 (4.6%) | 1 |
| Partial clip detachment | 6 (4.6%) | 2 (3.2%) | 4 (5.9%) | 0.68 | 2 (3.1%) | 4 (6.2%) | 0.68 |
| Periprocedural deatha | 5 (3.8%) | 3 (4.8%) | 2 (3%) | 1 | 3 (4.6%) | 2 (3.1%) | 1 |
| Periprocedural stroke a | 3 (2.3%) | 1 (1.6%) | 2 (3%) | 1 | 1 (1.5%) | 2 (3%) | 1 |
| Days of hospital stay after MitraClip | 7 (5) | 8 (4) | 7(5) | 0.6 | 6 (4) | 8 (6) | 0.01 * |
| Blood transfusion after MitraClip | 14 (10.8%) | 5 (8.1%) | 9 (13.2%) | 0.4 | 1 (1.5%) | 13 (20%) | 0.001 * |
AF, atrial fibrillation; ASA, acetylsalicylic acid; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; DOAC, direct oral Anti‐Xa inhibitor anticoagulation; EF, ejection fraction; ICD, implantable cardioverter defibrillator; ICM, ischaemic cardiomyopathy; MI, myocardial infarction; MR, mitral regurgitation; MV, mitral valve; MVARC, Mitral Valve Academic Research Consortium; NYHA, New York heart association; NT‐proBNP, N terminal pro brain natriuretic peptide; PAD, peripheral artery disease; TIA, transient ischaemic attack.
Data are presented as median with interquartile range or frequency and percentage. Comparison between groups by Mann–Whitney U test or χ2 test.
According to MVARC.
P < 0.05.
During a mean follow‐up of 14 ± 6 months, 28 patients (21.5%) died and 16 patients (12.3%) were admitted to the hospital for decompensated heart failure.
Iron deficiency
The prevalence of ID was 52% (68 patients) (Figure 1 ). The distribution of ID was equal among categories of EF; 37 patients (52.1%) with EF > 50%, 23 patients (52.3%) with EF 30–50%, and 7 patients with EF < 30% (50%) had ID. ID was also equally present in patients with functional and degenerative aetiology of MR (43.9% and 58.9%, P = 0.11). Female sex (P = 0.009) and anaemia (P = 0.008) showed a significant association with ID. In multivariate regression analysis, sex was the only significant, potentially mediating predictor of ID.
FIGURE 1.
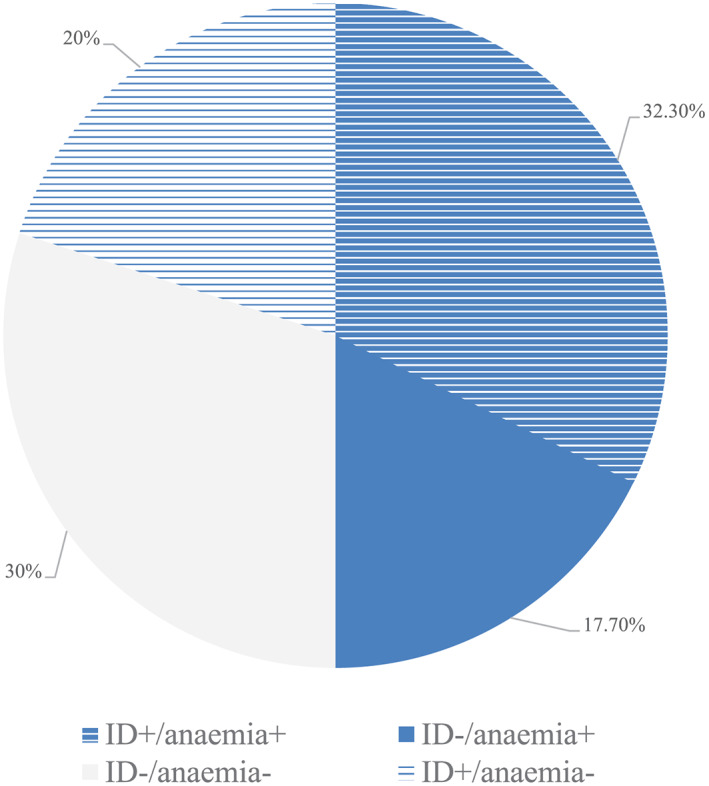
Prevalence of iron deficiency and anaemia in the study population. ID, iron deficiency.
At baseline, PCS, MCS, MLWHFQ, and NYHA class were similar by ID status, whereas 6MWD was lower in patients with ID, which was of borderline significance (Figure 2 A–E ). Absolute changes of functional measures did not differ significantly by ID status, with improvements in both patients with and without ID (Figure 2 A–D ). The 6MWD improved by 33 (0 to 137) vs. 39 (0 to 109) m, the PCS improved by 5.63 (1.64 to 9.88) vs. 7.24 (2.68 to 15.01), the MCS improved by 1.94 (−3.16 to 6.83) vs. 1.27 (−5.03 to 4.63), and the MLWHFQ improved by −8 (−14 to −3) vs. −10.5 (−17 to −3) (Figure 2 A–D ). ID was not associated with changes of functional parameters in linear regression analysis with adjustment for baseline values and in repeated‐measures ANOVA (all P values > 0.05). The NYHA class improved by ≥1 class in 71% iron‐deficient vs. 78% non‐iron‐deficient patients (Figure 2 E ). There was no evidence for an interaction of ID with MR aetiology on functional outcomes (all tests for interaction with P > 0.05, Supporting Information, Table S1 ).
FIGURE 2.
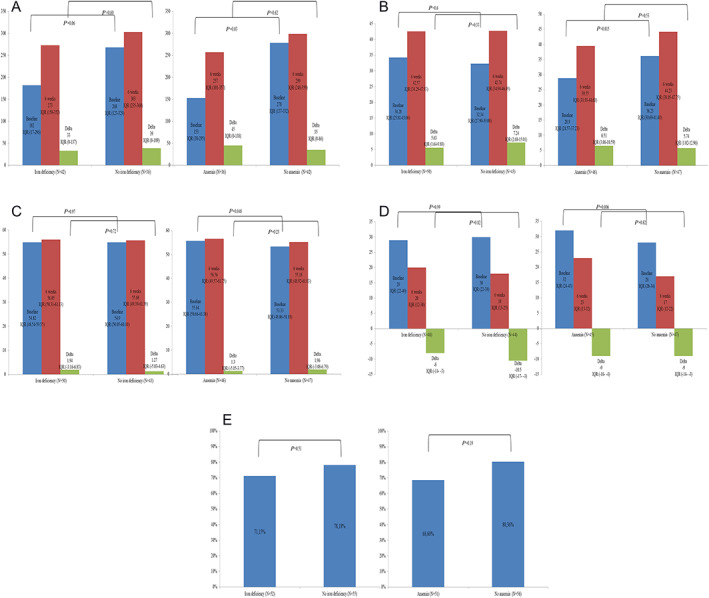
(A) Changes in 6MWD from baseline to 6 weeks by iron deficiency and anaemia, (B) changes in SF36 PCS from baseline to 6 weeks by iron deficiency and anaemia, (C) changes in SF36 MCS from baseline to 6 weeks by iron deficiency and anaemia, (D) changes in MLWHFQ from baseline to 6 weeks by iron deficiency and anaemia, and (E) changes in NYHA functional class from baseline to 6 weeks by iron deficiency and anaemia. IQR, interquartile range.
Anaemia
The prevalence of anaemia was 50% (n = 65) (Figure 1 ). Anaemia was significantly associated with ID and renal function. The association with N terminal pro brain natriuretic peptide levels was of borderline significance. The prevalence of ID and anaemia combined was 32.3% (N = 42). Multivariate regression revealed ID and diabetes as independent predictors of anaemia.
Patients with anaemia had significantly worse baseline 6MWD, PCS, MCS, and MLWHFQ, whereas NYHA class did not differ significantly (Figure 2 A–E ). The absolute change of functional measures between baseline and 6 weeks was not significantly different by anaemia status. The 6MWD improved by 45 (0 to 138) vs. 35 (0 to 86) m, the PCS improved by 6.51 (3.06 to 10.59) vs. 5.74 (1.02 to 12.96), the MCS improved by 1.3 (−5.05 to 3.77) vs. 1.96 (−3.08 to 6.79), and the MLWHFQ improved by −9 (−16 to −4) vs. −9 (−14 to −3) (Figure 2 A–D ). Anaemia was not associated with changes of functional parameters in linear regression analysis with adjustment for baseline values and in repeated‐measures ANOVA (all P values > 0.05). The NYHA class improved by ≥1 class in 69% anaemic vs. 80% non‐anaemic patients (Figure 2 E ). There was no evidence for an interaction of anaemia with MR aetiology on functional outcomes (all tests for interaction with P > 0.05, Supporting Information, Table S2 ).
Results for the association of ID and anaemia with functional parameters were virtually unchanged after exclusion of patients without technical success of MitraClip, that is, not achieving a reduction of MR severity of grade 2 or lower, and after exclusion of patients taking oral iron supplementation or receiving blood transfusions (N = 109). Associations were also comparable for patients with both anaemia and ID present.
Clinical outcomes
Iron deficiency was not associated with mortality [hazard ratio (HR): 0.91; 95% confidence interval (CI): 0.42–1.97; P = 0.81] but showed a trend towards more heart failure hospitalizations (HR: 2.94; 95% CI: 0.94–9.03; P = 0.09). Anaemia showed a borderline significant association with mortality (HR: 2.2; 95% CI: 0.96–5.08; P = 0.06) and a significantly increased risk of heart failure hospitalization (HR: 3.28; 95% CI: 1.06–10.19; P = 0.04). ID was not associated with higher risk for the combined endpoint of mortality and heart failure hospitalization (HR: 1.38; 95% CI: 0.72–2.66; P = 0.33), whereas anaemia was significantly associated with higher risk for the combined endpoint of mortality and heart failure hospitalization (HR: 2.51; 95% CI: 1.24–5.09; P = 0.01) and remained significant (HR: 2.29; 95% CI: 1.08–4.85; P = 0.03) after multivariate adjustment for age, sex, EF, estimated glomerular filtration rate, and ID. The association of anaemia due to ID with the combined endpoint of mortality and heart failure hospitalization was slightly weaker (HR: 1.46, 95% CI: 0.76–2.82; P = 0.26). Figure 3 shows a Kaplan–Meier analysis for the combined endpoint of mortality and heart failure hospitalization by iron deficiency and anaemia.
FIGURE 3.
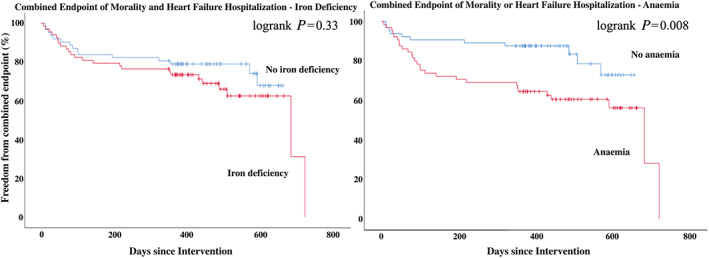
FIGUREKaplan–Meier analysis for the combined endpoint of mortality and heart failure hospitalization by iron deficiency and anaemia.
Discussion
There are four key findings arising from this study: the high prevalence of anaemia and ID in patients undergoing PMVR; the worse baseline functional status in patients particularly with anaemia; the same absolute benefit in functional outcomes after PMVR regardless of anaemia or ID; and a worse clinical long‐term outcome in patients with ID and particularly anaemia.
Both ID and anaemia are of particular clinical relevance because diagnosis is easy through laboratory tests and well‐established treatments are available. In patients with heart failure and reduced EF, intravenous iron supplementation can improve symptoms, functional capacity, and quality of life, and there is preliminary evidence for beneficial effects on renal function and disease progression. 19 Although randomized controlled trials on anaemia treatment using erythropoietin derivates in heart failure patients were neutral, much experience exists for this treatment in patients with chronic kidney disease.
In our study, one out of two patients had anaemia and one out of two patients had ID. These results on ID are almost identical to data reported for patients with heart failure with reduced EF. 20 The rate of ID in patients with heart failure with preserved EF in a recent multicentre study is also comparable with a prevalence of 55%. 21 Similarly, a large study with more than 13 000 heart failure patients showed a prevalence of anaemia (42%) across different EF categories. 8 Important to note, half of our patients did have degenerative MR without primary myocardial disease. Accordingly, mechanisms driving development of ID and anaemia might be associated with heart failure independently of underlying causes and independently whether myocardial disease is causative. In support of the latter, in patients with aortic stenosis, 64% and 53% had pre‐procedural anaemia and ID, respectively, suggesting a similar impact of valvular heart failure. 22
We observed a weak association of ID and a strong association of anaemia with baseline functional status in patients undergoing MitraClip therapy. This extends existing data on patients with myocardial heart failure and patients undergoing transcatheter aortic valve replacement. 22 The very weak association of ID might be due to the severe cardiac and extra‐cardiac morbidity in our patients, which might overlay ID effects. Additionally, heart failure resulting from severe MR might dominate functional status of patients. In this context, it would be interesting to examine the association between ID and functional status after successful MitraClip procedure, which was not the objective of this study and we do not have data on this.
The main objective of our study was to examine whether patients with ID or anaemia show benefit in functional outcomes and quality of life after percutaneous treatment of MR. Both patients with ID or anaemia showed the same magnitude of benefit regarding NYHA class, 6MWD, MLWHFQ, and SF36 as patients without anaemia or ID. This extends findings from an earlier small study by Hellhammer et al. showing that patients with and without anaemia experience equal improvement in NYHA class and MLWHFQ after MitraClip. 23 The magnitude of improvement in functional outcomes is comparable with that of cardiac resynchronization device therapy in appropriate patients, where it showed an improvement of NYHA class in 58–75% of patients, 39–50 m in 6MWD, and −16 to −21 in MLWHFQ score. 24 Interestingly, ID was strongly associated with symptomatic response, reverse remodelling, and outcome in patients undergoing cardiac resynchronization therapy implantation, 25 which somehow contrasts our findings for MitraClip treatment. However, besides differences between both patient populations in general, different mechanisms of respective therapies might explain these findings. MitraClip directly improves haemodynamics with reduction of pulmonary capillary pressure. 26 This might be the primary cause of the short‐term improvement in functional parameters, which is independent of ID and anaemia. Although MitraClip treatment has also the potential to reverse remodelling in selected patients, 27 we do not have data on follow‐up echocardiography in our patients and hence cannot estimate the contribution of remodelling for clinical outcome.
The presence of ID in patients with heart failure with reduced EF is significantly associated with mortality irrespective of other risk factors. 13 , 28 In our study population, mortality was not associated with ID. However, the former data were obtained from chronic stable heart failure with reduced EF patients under optimized therapy. In our cohort, patients underwent a structural procedure with the potential to improve prognosis including mortality, which might overlay the association of ID with outcome. Accordingly, the association with heart failure hospitalization was only of borderline significance.
Existing data on anaemia in the setting of MitraClip are inconsistent. Hellhammer et al. showed similar survival in patients with and without anaemia. 23 However, the study sample was small und follow‐up was short. Kaneko et al. showed a significant association of pre‐procedural anaemia with higher mortality after MitraClip after adjusting the data of 392 patients for covariates such as NYHA class, renal function, age, and gender. 29 We observed a similar risk of about factor 2 associated with anaemia. However, we extend previous data by showing a highly significant association of anaemia with heart failure hospitalization by factor 3, indicating a particular role of anaemia with progression of the cardiac disease.
Study limitations
There are several limitations to mention. The definition of ID is complex, and the usual approach of serum ferritin measurement as a correlate of body iron store is not recommended in heart failure and other inflammatory conditions. We used the criteria of the substitution trials in heart failure patients (e.g. CONFIRM‐HF) to define ID, which is a pragmatic method because it is directly linked to therapeutic benefit. The sample size of 130 patients was limited and the patients were heterogeneous as discussed above. This might be one reason why association of ID with baseline functional measures was weak. In addition, the duration of follow‐up of 6 weeks might be too short to achieve full functional recovery after the procedure and gain maximum functional benefit. However, we have shown earlier that there was no heterogeneity of functional benefit across short‐term and long‐term follow‐up. 3 Finally, differences in volume and congestion status across patients might impact functional parameters, and we did not quantitatively assess this in our patients. However, premise for undergoing MitraClip in our institution is clinical euvolaemia because overload might attenuate the procedural success of grasping leaflets. Hence, we regard this potential bias as minor.
Conclusions
The prevalence of ID and anaemia is high in patients undergoing PMVR with MitraClip and is similar for patients with myocardial heart failure, that is, secondary MR and patients with primary MR. There was a worse clinical baseline status and a worse clinical long‐term outcome particularly in patients with anaemia. However, the benefit in functional outcomes after MitraClip was regardless of iron status and anaemia.
Statistical analysis
Baseline characteristics were presented as median (interquartile range) and frequency (percentage) by status of ID and anaemia and compared using Mann–Whitney U test and χ2 test, as appropriate. Independent predictors of ID and anaemia were identified by multivariate regression analysis including baseline variables with a P < 0.1 in univariate association tests. Associations of ID and anaemia were examined with baseline measures of 6MWD, NYHA class, physical component score (PCS) and MCS (mental component score), and MLWHFQ, as well as with the absolute change of these measures between 6 weeks follow‐up and baseline. To account for differences in baseline values impacting the absolute change at follow‐up, we used linear regression with absolute changes as dependent variable and ID and anaemia as independent variables adjusting for baseline values of functional parameters and repeated‐measures ANOVA. Association of ID and anaemia with long‐term mortality and heart failure hospitalization was analysed using Kaplan–Meier survival and Cox regression analysis. Multivariate analyses were conducted adjusting for age and sex as well as potentially effect‐mediating variables such as EF, estimated glomerular filtration rate, and ID. Analyses were performed using SPSS© Version 25 (IBM© Corp. Armonk, NY, USA) and STATA/SE 12.1 (STATACorp LP, TX, USA).
Conflict of interest
Relationship with industry and other entities: S.B. has received lecture honoraria from Edwards Lifesciences, Bayer Vital, CVRx, MSD Sharp&Dome GmbH, JenaValve Technology, Abbott, and research grant from IcoVifor, Symetis SA, Pfizer, JenaValve Technology, Valtech, OptumInsight, Biotronik and Abbott, ‘modest’, outside the submitted work. R.P. has received travel support by Abbott, ‘modest’, outside the submitted work. C.I., C.M., and M.K. have no relationship with industry and other entities.
Funding
No funding sources to report.
Supporting information
Table S1: Changes of functional parameters by iron deficiency and aetiology of mitral regurgitation.
Table S2: Changes of functional parameters by anaemia and aetiology of mitral regurgitation.
Acknowledgements
We thank all the study participants, medical students, and administrative staff for their invaluable contribution.
Iliadis, C. , Metze, C. , Körber, M. I. , Baldus, S. , and Pfister, R. (2020) Association of iron deficiency, anaemia, and functional outcomes in patients undergoing edge‐to‐edge mitral valve repair. ESC Heart Failure, 7: 2379–2387. 10.1002/ehf2.12778.
These authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
This manuscript has not been published and is not under consideration for publication elsewhere. All authors have read and approved the manuscript.
References
- 1. Kalbacher D, Schafer U, Bardeleben RS, Eggebrecht H, Sievert H, Nickenig G, Butter C, May AE, Bekeredjian R, Ouarrak T, Kuck KH, Plicht B, Zahn R, Baldus S, Ince H, Schillinger W, Boekstegers P, Senges J, Lubos E. Long‐term outcome, survival and predictors of mortality after MitraClip therapy: results from the German Transcatheter Mitral Valve Interventions (TRAMI) registry. Int J Cardiol 2019; 277: 35–41. [DOI] [PubMed] [Google Scholar]
- 2. Arnold SV, Li Z, Vemulapalli S, Baron SJ, Mack MJ, Kosinski AS, Reynolds MR, Hermiller JB, Rumsfeld JS, Cohen DJ. Association of transcatheter mitral valve repair with quality of life outcomes at 30 days and 1 year: analysis of the Transcatheter Valve Therapy Registry. JAMA Cardiol 2018; 3: 1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iliadis C, Lee S, Kuhr K, Metze C, Matzik AS, Michels G, Rudolph V, Baldus S, Pfister R. Functional status and quality of life after transcatheter mitral valve repair: a prospective cohort study and systematic review. Clin Res Cardiol 2017; 106: 1005–1017. [DOI] [PubMed] [Google Scholar]
- 4. Obadia JF, Messika‐Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, Lefevre T, Piot C, Rouleau F, Carrie D, Nejjari M, Ohlmann P, Leclercq F, Saint Etienne C, Teiger E, Leroux L, Karam N, Michel N, Gilard M, Donal E, Trochu JN, Cormier B, Armoiry X, Boutitie F, Maucort‐Boulch D, Barnel C, Samson G, Guerin P, Vahanian A, Mewton N. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018; 379: 2297–2306. [DOI] [PubMed] [Google Scholar]
- 5. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ. Transcatheter mitral‐valve repair in patients with heart failure. N Engl J Med 2018; 379: 2307–2318. [DOI] [PubMed] [Google Scholar]
- 6. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole‐Wilson PA, Ponikowski P. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448. [DOI] [PubMed] [Google Scholar]
- 7. Ponikowski P, van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD. Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur Heart J 2015; 36: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berry C, Poppe KK, Gamble GD, Earle NJ, Ezekowitz JA, Squire IB, McMurray JJV, McAlister FA, Komajda M, Swedberg K, Maggioni AP, Ahmed A, Whalley GA, Doughty RN, Tarantini L. Prognostic significance of anaemia in patients with heart failure with preserved and reduced ejection fraction: results from the MAGGIC individual patient data meta‐analysis. QJM 2016; 109: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaiafa G, Kanellos I, Savopoulos C, Kakaletsis N, Giannakoulas G, Hatzitolios AI. Is anemia a new cardiovascular risk factor? Int J Cardiol 2015; 186: 117–124. [DOI] [PubMed] [Google Scholar]
- 10. Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CS, Cowie MR, Kjeldsen K, Jankowska EA, Atar D, Butler J, Fiuzat M, Zannad F, Pitt B, O'Connor CM. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2014; 64: 2281–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adams KF Jr, Pina IL, Ghali JK, Wagoner LE, Dunlap SH, Schwartz TA, Stough WG, Mehra MR, Felker GM, Chiong JR, Patterson JH, Kim J, Butler J, Oren RM. Prospective evaluation of the association between hemoglobin concentration and quality of life in patients with heart failure. Am Heart J 2009; 158: 965–971. [DOI] [PubMed] [Google Scholar]
- 12. Parikh A, Natarajan S, Lipsitz SR, Katz SD. Iron deficiency in community‐dwelling US adults with self‐reported heart failure in the National Health and Nutrition Examination Survey III: prevalence and associations with anemia and inflammation. Circ Heart Fail 2011; 4: 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin‐Nadzieja L, Banasiak W, Polonski L, Filippatos G, McMurray JJ, Anker SD, Ponikowski P. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J 2010; 31: 1872–1880. [DOI] [PubMed] [Google Scholar]
- 14. Rasekaba T, Lee AL, Naughton MT, Williams TJ, Holland AE. The six‐minute walk test: a useful metric for the cardiopulmonary patient. Intern Med J 2009; 39: 495–501. [DOI] [PubMed] [Google Scholar]
- 15. Association TCCotNYH . Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels, 9th ed. Boston: Little, Brown & Co; 1994. p 253–256. [Google Scholar]
- 16. Rector TS, Kubo SH, Cohn JN. Patients' self‐assessment of their congestive heart failure: content, reliability, and validity of a new measure, the Minnesota Living with Heart Failure questionnaire. Heart Fail 1987; 3: 198–209. [Google Scholar]
- 17. Muller‐Nordhorn J, Roll S, Willich SN. Comparison of the short form (SF)‐12 health status instrument with the SF‐36 in patients with coronary heart disease. Heart 2004; 90: 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ware J Jr, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34: 220–233. [DOI] [PubMed] [Google Scholar]
- 19. Jankowska EA, Tkaczyszyn M, Suchocki T, Drozd M, von Haehling S, Doehner W, Banasiak W, Filippatos G, Anker SD, Ponikowski P. Effects of intravenous iron therapy in iron‐deficient patients with systolic heart failure: a meta‐analysis of randomized controlled trials. Eur J Heart Fail 2016; 18: 786–795. [DOI] [PubMed] [Google Scholar]
- 20. Klip IT, Comin‐Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165: 575–582 e3. [DOI] [PubMed] [Google Scholar]
- 21. Bekfani T, Pellicori P, Morris D, Ebner N, Valentova M, Sandek A, Doehner W, Cleland JG, Lainscak M, Schulze PC, Anker SD, von Haehling S. Iron deficiency in patients with heart failure with preserved ejection fraction and its association with reduced exercise capacity, muscle strength and quality of life. Clin Res Cardiol 2019; 108: 203–211. [DOI] [PubMed] [Google Scholar]
- 22. DeLarochelliere H, Urena M, Amat‐Santos IJ, Ribeiro HB, Allende R, Laflamme L, Laflamme J, Paradis JM, Dumont E, Doyle D, Mohammadi S, DeLarochelliere R, Cote M, Laroche V, Rodes‐Cabau J. Effect on outcomes and exercise performance of anemia in patients with aortic stenosis who underwent transcatheter aortic valve replacement. Am J Cardiol 2015; 115: 472–479. [DOI] [PubMed] [Google Scholar]
- 23. Hellhammer K, Balzer J, Zeus T, Rammos C, Niebel S, Kubatz L, Wagstaff R, Kelm M, Rassaf T. Percutaneous mitral valve repair using the MitraClip system in patients with anemia. Int J Cardiol 2015; 184: 399–404. [DOI] [PubMed] [Google Scholar]
- 24. Abraham WT, Leon AR, St John Sutton MG, Keteyian SJ, Fieberg AM, Chinchoy E, Haas G. Randomized controlled trial comparing simultaneous versus optimized sequential interventricular stimulation during cardiac resynchronization therapy. Am Heart J 2012; 164: 735–741. [DOI] [PubMed] [Google Scholar]
- 25. Martens P, Verbrugge F, Nijst P, Dupont M, Tang WH, Mullens W. Impact of iron deficiency on response to and remodeling after cardiac resynchronization therapy. Am J Cardiol 2017; 119: 65–70. [DOI] [PubMed] [Google Scholar]
- 26. Gaemperli O, Moccetti M, Surder D, Biaggi P, Hurlimann D, Kretschmar O, Buehler I, Bettex D, Felix C, Luscher TF, Falk V, Grunenfelder J, Corti R. Acute haemodynamic changes after percutaneous mitral valve repair: relation to mid‐term outcomes. Heart 2012; 98: 126–132. [DOI] [PubMed] [Google Scholar]
- 27. Asch FM, Grayburn PA, Siegel RJ, Kar S, Lim DS, Zaroff JG, Mishell JM, Whisenant B, Mack MJ, Lindenfeld J, Abraham WT, Stone GW, Weissman NJ. Echocardiographic outcomes after transcatheter leaflet approximation in patients with secondary mitral regurgitation: the COAPT trial. J Am Coll Cardiol 2019; 74: 2969–2979. [DOI] [PubMed] [Google Scholar]
- 28. Yeo TJ, Yeo PS, Ching‐Chiew Wong R, Ong HY, Leong KT, Jaufeerally F, Sim D, Santhanakrishnan R, Lim SL. M MYC, Chai P, Low AF, Ling LH, Ng TP, Richards AM, Lam CS. Iron deficiency in a multi‐ethnic Asian population with and without heart failure: prevalence, clinical correlates, functional significance and prognosis. Eur J Heart Fail 2014; 16: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 29. Kaneko H, Neuss M, Okamoto M, Weissenborn J, Butter C. Impact of preprocedural anemia on outcomes of patients with mitral regurgitation who underwent MitraClip implantation. Am J Cardiol 2018; 122: 859–865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Changes of functional parameters by iron deficiency and aetiology of mitral regurgitation.
Table S2: Changes of functional parameters by anaemia and aetiology of mitral regurgitation.


