Abstract
Aims
Cardiac resynchronization therapy (CRT) has become an important therapy in patients with heart failure with reduced left ventricular ejection fraction (LVEF). The effect of diabetes on long‐term outcome in these patients is controversial. We assessed the effect of diabetes on long‐term outcome in CRT patients and investigated the role of diabetes in ischaemic and non‐ischaemic cardiomyopathy.
Methods and results
All patients undergoing CRT implantation at our institution between November 2000 and January 2015 were enrolled. The study endpoints were (i) a composite of ventricular assist device (VAD) implantation, heart transplantation, or all‐cause mortality; and (ii) reverse remodelling (improvement of LVEF ≥ 10% or reduction of left ventricular end‐systolic volume ≥ 15%). Median follow‐up of the 418 patients (age 64.6 ± 11.6 years, 22.5% female, 25.1% diabetes) was 4.8 years [inter‐quartile range: 2.8;7.4]. Diabetic patients had an increased risk to reach the composite endpoint [adjusted hazard ratio (aHR) 1.48 [95% CI 1.12–2.16], P = 0.041]. Other factors associated with an increased risk to reach the composite endpoint were a lower body mass index or baseline LVEF (aHR 0.95 [0.91; 0.98] and 0.97 [0.95; 0.99], P < 0.01 each), and a higher New York Heart Association functional class or creatinine level (aHR 2.14 [1.38; 3.30] and 1.04 [1.01; 1.05], P < 0.05 each). Early response to CRT, defined as LVEF improvement ≥ 10%, was associated with a lower risk to reach the composite endpoint (aHR 0.60 [0.40; 0.89], P = 0.011). Reverse remodelling did not differ between diabetic and non‐diabetic patients with respect to LVEF improvement ≥ 10% (aHR 0.60 [0.32; 1.14], P = 0.118). However, diabetes was associated with decreased reverse remodelling with respect to a reduction of left ventricular end‐systolic volume ≥ 15% (aHR 0.45 [0.21; 0.97], P = 0.043). In patients with ischaemic cardiomyopathy, survival rates were not significantly different between diabetic and non‐diabetic patients (HR 1.28 [0.83–1.97], P = 0.101), whereas in patients with non‐ischaemic cardiomyopathy, diabetic patients had a higher risk of reaching the composite endpoint (HR 1.65 [1.06–2.58], P = 0.027). The latter effect was dependent on other risk factors (aHR 1.47 [0.83–2.61], P = 0.451). The risk of insulin‐dependent patients was not significantly higher than in patients under oral antidiabetic drugs (HR 1.55 [95% CI 0.92–2.61], P = 0.102).
Conclusions
Long‐term follow‐up revealed diabetes mellitus as independent risk factor for all‐cause mortality, heart transplantation, or VAD in heart failure patients undergoing CRT. The detrimental effect of diabetes appeared to weigh heavier in patients with non‐ischaemic compared with ischaemic cardiomyopathy.
Keywords: Cardiac resynchronization therapy, Diabetes mellitus, All‐cause mortality, Ischaemic cardiomyopathy, Non‐ischaemic cardiomyopathy
Introduction
The beneficial effects of cardiac resynchronization therapy (CRT) in selected patients with heart failure with reduced ejection fraction have been well established. 1 , 2 , 3 However, a relevant proportion of patients do not derive the expected benefit from CRT. Whereas this may partially be due to a lack of uptitration of medical therapy, 4 a suboptimal position of the coronary sinus lead or undeliberated device programming, 5 patient selection remains a key issue. 6 Therefore, thorough investigation of all available data is warranted to identify potential predictors of CRT response in order to further ameliorate outcome in daily clinical practice.
Diabetes mellitus (DM) is a strong cardiovascular risk factor of increasing significance with a growing prevalence among elderly patients owing to demographic changes worldwide, and it is associated with increased mortality in patients with heart failure. 7 , 8 , 9 , 10 However, the effect of DM on outcome, especially long‐term outcome, in patients receiving CRT remains controversial. While some cohort studies have demonstrated a less favourable outcome of CRT in diabetic compared with non‐diabetic patients, other cohort studies as well as post hoc analyses from the MADIT‐CRT, COMPANION, and CARE‐HF did not show a significantly worse outcome in diabetic patients. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 Average duration of follow‐up, however, ranged between 12 and 34 months across these studies.
It was therefore our aim to investigate the long‐term effect of DM on survival and left ventricular (LV) reverse remodelling in a real‐world setting over a median follow‐up of 4.8 years.
Methods
Study population
All patients undergoing CRT implantation at the University Heart Center Zurich between November 2000 and January 2015, who provided informed consent, were consecutively enrolled. The study was approved by the local ethics committee (KEK‐ZH‐NR: 2011‐0304). As reported previously, clinical and echocardiographic follow‐up information was gathered by chart review for those patients under follow‐up at our institution and by telephone interview for patients with external follow‐up. 21 This previous publication also provides detailed information of the implantation procedure and post‐operative course.
Endpoints
The endpoints were cardiac survival [defined as the absence of all‐cause mortality, implantation of a ventricular assist device (VAD), or heart transplantation], and LV reverse remodelling as determined by echocardiography at long‐term follow‐up. Two different parameters were utilized to estimate reverse remodelling: improvement of LV ejection fraction (LVEF) by ≥10% or reduction of LV end‐systolic volume index (LVESVI) by ≥15% compared with baseline. Utilization of these particular cut‐off values has been demonstrated to be predictive of survival in this cohort. 21 Long‐term echocardiographic follow‐up occurred at 3.9 [2.3; 5.9] years after CRT implantation.
Statistical analysis
Categorical variables are expressed as frequencies and percentages. Numerical Variables are expressed as mean ± standard deviation or median [inter‐quartile range (IQR)]. Groups were compared using Student's t‐test or Mann–Whitney U‐test for continuous variables, as appropriate, and using contingency χ 2 tests for dichotomous variables. Distribution of continuous variables was assessed using the Kolmogorov–Smirnov test. Survival curves for time‐to‐event analyses were constructed using Kaplan–Meier estimates based on all available data; comparisons of cumulative events were performed using log rank tests. Multivariable analyses were performed utilizing Cox proportional hazard regression analysis for the survival endpoint and logistic regression analysis for the dichotomous LV remodelling endpoints. To correct for confounding variables, we included sex, age, body mass index (BMI), ischaemic cardiomyopathy (ICM) vs. non‐ICM, baseline LVEF, New York Heart Association (NYHA) class III/IV vs. I/II, baseline creatinine, and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) into a model with DM. In addition, we included the initial response to CRT (defined as LVEF improvement ≥ 10% at first follow‐up echocardiography) as a time‐dependent covariate in the Cox regression analysis. This approach was also performed separately for the subgroups of ICM and non‐ICM patients and in diabetic patients only, in order to compare insulin‐dependent DM (IDDM) and non‐insulin‐dependent DM (non‐IDDM). All statistical tests were two‐sided, and statistical significance was accepted for P < 0.05. Analyses were carried out using SPSS software (version 24, IBM).
Results
Study population and baseline characteristics
We investigated 418 patients undergoing CRT implantation between November 2000 and January 2015 at our institution. Among these, DM was present in 105 individuals (25.1%), which was insulin dependent in about a third of cases (n = 38, 36.2% of diabetic patients, Table 1 ). While the prevalence of female patients did not differ significantly between diabetic and non‐diabetic patients, diabetic patients were significantly older and had a higher BMI (Table 1 ). Arterial hypertension (64.8% vs. 49.2%, P = 0.006) and relevant kidney injury as defined by an estimated glomerular filtration rate (Cockroft–Gault) < 60 mL/min were more prevalent in diabetic patients compared with non‐diabetic patients (57.1% vs. 41.2%, P = 0.006). We observed a non‐significant difference in the prevalence of ICM (44.8% vs. 40.9%, P = 0.487). Average baseline LVEF was found to be slightly but significantly lower in diabetic compared with non‐diabetic patients (25.0 ± 7.3 vs. 27.1 ± 8.5%, P = 0.023), while there was no statistically significant difference in the remaining echocardiographic parameters, including LV volumes or right ventricular systolic function (Table 1 ). Importantly, significant differences neither in QRS width nor in baseline NT‐proBNP levels were observed. The majority of patients was in sinus rhythm as determined by 12‐lead electrocardiogram (ECG) at the time of CRT implantation in both groups (69.5% vs. 70.5%, P = 0.957). Atrial fibrillation was diagnosed on the pre‐implantation ECG in 11% of patients. Further information on echocardiographic and electrocardiographic parameters, as well as medical therapy, is listed in Table 1 . CRT with defibrillator (CRT‐D) were implanted in 87.2% and 88.6% of non‐diabetic and diabetic patients, respectively (P = 0.717).
Table 1.
Demographics and baseline parameters
| All patients | Non‐diabetic patients | All diabetic patients | P‐value | |
|---|---|---|---|---|
| Demographics | ||||
| Number of patients | 418 | 313 (74.9%) | 105 (25.1%) | |
| Female sex | 94 (22.5%) | 69 (22.0%) | 25 (23.8%) | 0.708 |
| Age (years) | 64.6 ± 11.6 | 63.6 ± 12.3 | 67.3 ± 8.8 | 0.005 |
| BMI (kg/m2) | 27.1 ± 5.2 | 26.7 ± 5.1 | 28.2 ± 5.4 | 0.009 |
| Cardiovascular risk factors, co‐morbidities | ||||
| Leading cardiomyopathy | ||||
| Ischaemic | 175 (41.9%) | 128 (40.9%) | 47 (44.8%) | 0.487 |
| Non‐ischaemic | 243 (58.1%) | 185 (59.1%) | 58 (55.2%) | — |
| NYHA class | 29/108/250/27 | 24/88/181/17 | 5/20/69/10 | 0.097 |
| Arterial hypertension | 222 (53.1%) | 154 (49.2%) | 68 (64.8%) | 0.006 |
| Hypercholesterinaemia | 188 (45.0%) | 132 (42.9%) | 56 (53.3%) | 0.063 |
| Peripheral artery disease | 39 (9.3%) | 27 (8.6%) | 12 (11.4%) | 0.393 |
| History of stroke | 35 (8.4%) | 27 (8.6%) | 8 (7.6%) | 0.766 |
| COPD | 37 (8.9%) | 24 (7.7%) | 13 (12.4%) | 0.141 |
| Current smoker | 93 (22.5%) | 72 (23.1%) | 21 (20.6%) | 0.601 |
| Echocardiographic parameters | ||||
| LVEF (%) | 26.6 ± 8.3 | 27.1 ± 8.5 | 25.0 ± 7.3 | 0.023 |
| LVFS (%) | 16.7 ± 8.1 | 17.1 ± 8.4 | 15.2 ± 6.9 | 0.053 |
| LVESVI (mL/m2) | 82.3 ± 34.6 | 81.4 ± 35.7 | 83.8 ± 30.9 | 0.887 |
| LVEDVI (mL/m2) | 109.7 ± 38.3 | 110.5 ± 39.6 | 107.2 ± 34.4 | 0.498 |
| RV FAC (%) | 38.5 ± 13.8 | 39.1 ± 14.1 | 36.7 ± 13 | 0.294 |
| Electrocardiographic parameters | ||||
| Heart rate | 73.0 ± 14.5 | 73.1 ± 14.7 | 72.8 ± 14.1 | 0.864 |
| QRS width (ms) | 156.9 ± 35.1 | 158.1 ± 34.8 | 153.1 ± 36.2 | 0.215 |
| Rhythm on pre‐implantation ECG | ||||
| Sinus rhythm | 293 (70.3%) | 220 (70.5%) | 73 (69.5%) | 0.957 |
| Atrial fibrillation | 46 (11.0%) | 35 (11.2%) | 11 (10.5%) | ‐ |
| Paced rhythm | 78 (18.7%) | 57 (18.2%) | 21 (20.0%) | ‐ |
| Laboratory parameters | ||||
| Creatinine (μmol/L) | 124.9 ± 65.6 | 121.5 ± 63.6 | 135.5 ± 70.6 | 0.066 |
| eGFR (mL/min) Cockroft–Gault | 68.1 ± 33.3 | 70.0 ± 34.7 | 62.4 ± 28.2 | 0.051 |
| eGFR < 60 mL/min | 180 (45.1%) | 124 (41.2%) | 56 (57.1%) | 0.006 |
| NT‐proBNP (ng/L) | 4490.0 ± 7846.9 | 4620.7 ± 8365.9 | 4087.1 ± 6003.3 | 0.598 |
| Baseline medication | ||||
| Aspirin | 193 (46.3%) | 131 (41.9%) | 62 (59.6%) | 0.002 |
| ACE inhibitor/AR blocker | 379 (90.7%) | 286 (91.4%) | 93 (89.4%) | 0.549 |
| Beta‐blocker | 335 (80.3%) | 248 (79.2%) | 87 (83.7%) | 0.326 |
| Spironolactone | 216 (51.9%) | 152 (48.7%) | 64 (61.5%) | 0.023 |
| Loop diuretics | 313 (76.7%) | 223 (72.9%) | 90 (88.2%) | 0.001 |
| Thiazide diuretics | 73 (18.0%) | 55 (18.2%) | 18 (17.8%) | 0.215 |
| Oral anticoagulation | 200 (48.0%) | 160 (51.1%) | 40 (38.5%) | 0.025 |
| Diabetes management | ||||
| HbA1c—NGSP in % | — | — | 7.0 [6.4;8.0] | — |
| <7% | — | — | 34 (47.9%) | — |
| ≥7.0% | — | — | 37 (52.1%) | — |
| Oral antidiabetic therapy | — | — | 59 (57.8%) | — |
| Insulin | — | — | 38 (36.2%) | — |
ACE, angiotensin converting enzyme; AR, angiotensin receptor; BMI, body mass index; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; LVEDVI, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end‐systolic volume index; LVFS, left ventricular fractional shortening; NGSP, National Glycohemoglobin Standardization Program; NYHA, New York Heart Association; RV FAC, right ventricular fractional areal change.
Bold indicates statistically significant findings.
Within the diabetic population, the comparison of IDDM to non‐IDDM patients did not reveal any significant differences in any of the demographic or baseline clinical parameters (Table S1 ). Approximately half of all diabetic patients had an HbA1c level below 7.0%, with a median HbA1c level of 7.0% [IQR: 6.4%; 8.0%] in the overall diabetic population. Comparison of HbA1c levels in IDDM and non‐IDDM subgroups, demonstrated slightly but statistically significantly higher HbA1c levels in insulin‐dependent compared with non‐insulin‐dependent diabetics (7.2% [6.6%; 8.7%] vs. 6.8% [6.3%; 7.6]).
Long‐term clinical follow‐up—diabetic vs. non‐diabetic patients
Patients were followed up for the composite endpoint of all‐cause mortality, heart transplantation, or VAD implantation for a median time of 4.8 [2.8; 7.4] years. This composite endpoint was reached in 58 of 105 (55.2%) diabetic patients and in 130 of 313 (41.5%) non‐diabetic patients (HR 1.50 [1.10; 2.04], P = 0.011) (Figure 1A and Table 2 ). Kaplan–Meier estimates for freedom from the composite endpoint at 5 years were 50% vs. 63% (DM vs. non‐DM, P = 0.011). Among others, further, factors associated with worse outcome for the composite endpoint in univariable analysis were age (HR 1.02 [1.01; 1.04] per year), reduction of LVEF (HR 1.02 [1.01; 1.04] per % LVEF reduction), NYHA class III/IV vs. I/II (HR 1.96 [1.37; 2.80]), ICM (HR 1.64 [1.23; 2.18]), baseline creatinine (HR 1.06[1.05; 1.08] per 10 μmol/L increase), and NT‐proBNP (HR 1.03 [1.02–1.04] per 1000 ng/L increase). Importantly, initial CRT response defined by LVEF improvement ≥ 10% at first follow‐up echocardiography (7.0 [3.9; 9.7] months after implantation) was associated with a significant hazard ratio (HR 0.46 [0.33–0.64]). After multivariable correction for these and further clinically relevant variables (Table 3 ), including initial CRT response as a time‐dependent covariate, adjusted HR for diabetic over non‐diabetic patients remained statistically significant with a hazard ratio of 1.48 [1.02; 2.16] for the composite endpoint (Tables 2 and 3 ).
Figure 1.
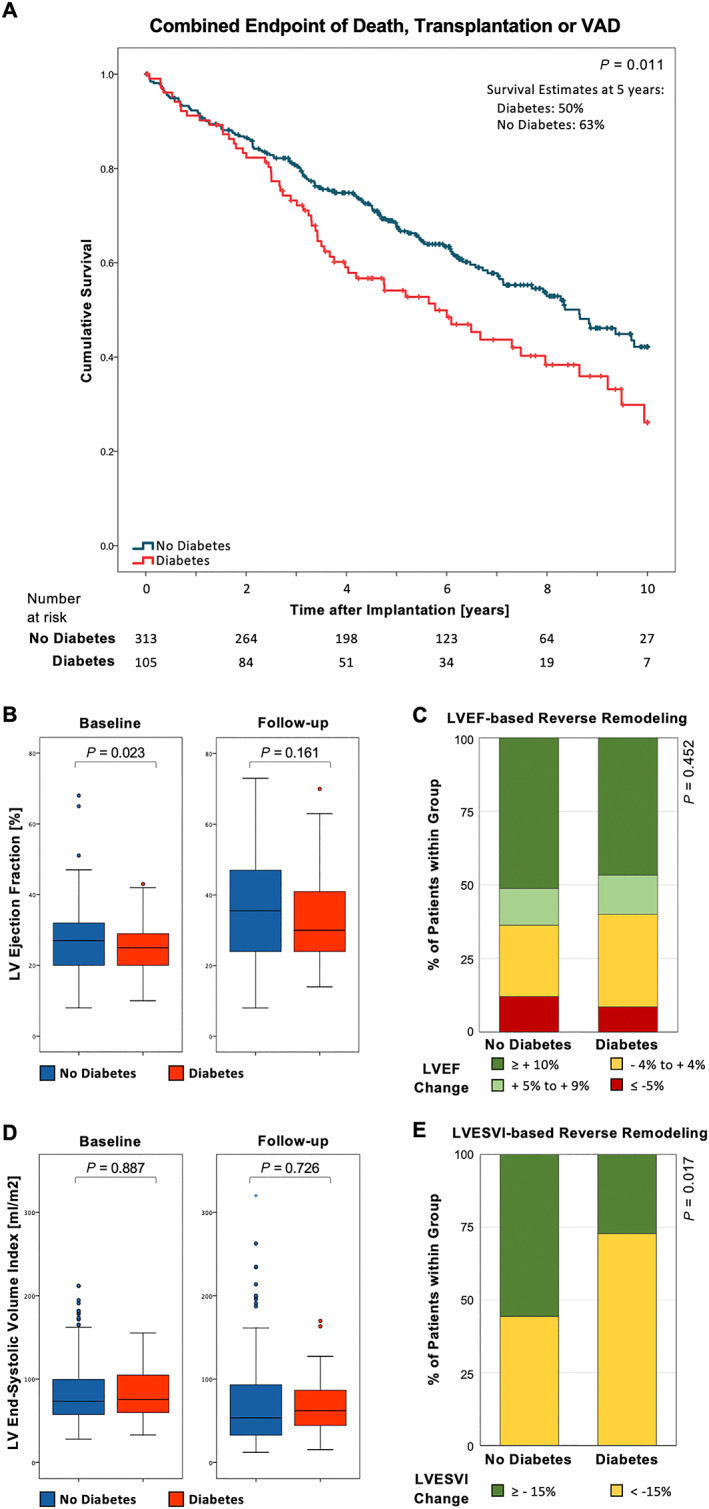
Outcome data stratified for diabetes mellitus (DM) vs. no DM: (A) Kaplan–Meier survival curves for the composite endpoint of all‐cause death, heart transplantation, or ventricular assist device (VAD) implantation. (B) Box plots showing inter‐quartile ranges for left ventricular ejection fraction (LVEF) pre‐CRT and post‐CRT implantation, stratified for DM vs. no DM. Whiskers indicate minima and maxima. (C, D) Stacked bar graph showing categorized left ventricular remodelling in the form of absolute LVEF improvement and relative LVESVI improvement, respectively.
Table 2.
Number of diabetic and non‐diabetic patients reaching endpoints
| All patients | Non‐diabetic patients | Diabetic patients | Statistical comparison | |||
|---|---|---|---|---|---|---|
| aHR | 95% CI | P‐value | ||||
| Composite endpoint of death, transplantation, or VAD | 45.0% (n = 188) | 41.5% (n = 130) | 55.2% (n = 58) | 1.48 | [1.02; 2.16] | 0.041 |
| LV reverse remodelling | ||||||
| By ≥10% LVEF improvement | 47.4% (n = 198) | 48.9% (n = 153) | 44.9% (n = 45) | 0.60 | [0.32; 1.14] | 0.118 |
| By ≥15% LVESVI reduction | 51.5% (n = 123) | 55.7% (n = 102) | 37.5% (n = 21) | 0.45 | [0.21; 0.97] | 0.043 |
The endpoint was defined as a composite of all‐cause mortality, heart transplantation, or ventricular assist device (VAD) implantation. Left ventricular (LV) reverse remodelling was defined as an improvement in LV ejection fraction (LVEF) ≥ 10% or reduction of LV end‐systolic volume index (LVESVI) by ≥15% compared with baseline.
aHR, adjusted hazard ratio; CI, confidence interval.
Table 3.
Univariable and multivariable analyses for reaching the endpoint in the study population
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | aHR | 95% CI | P‐value | |
| Diabetes | 1.50 | [1.10; 2.04] | 0.011 | 1.48 | [1.02; 2.16] | 0.041 |
| Male sex | 1.46 | [1.00; 2.14] | 0.051 | 1.61 | [0.97; 2.69] | 0.070 |
| Age | 1.02 | [1.01; 1.04] | 0.005 | 1.00 | [0.98; 1.02] | 0.829 |
| BMI | 0.98 | [0.95; 1.00] | 0.076 | 0.95 | [0.91; 0.98] | 0.003 |
| LVEF (%) | 0.98 | [0.96; 1.00] | 0.012 | 0.97 | [0.95; 0.99] | 0.008 |
| NYHA ≥ III | 1.96 | [1.37; 2.80] | <0.001 | 2.14 | [1.38; 3.30] | 0.001 |
| Ischaemic cardiomyopathy | 1.64 | [1.23; 2.18] | <0.001 | 1.62 | [1.14; 2.31] | 0.008 |
| Creatinine (10 μmol/L) | 1.06 | [1.05; 1.08] | <0.001 | 1.04 | [1.01; 1.08] | 0.012 |
| NT‐proBNP (1000 ng/L) | 1.03 | [1.02; 1.04] | <0.001 | 1.01 | [0.99, 1.04] | 0.260 |
| Initial CRT response | 0.46 | [0.33; 0.64] | <0.001 | 0.60 | [0.40; 0.89] | 0.011 |
aHR, adjusted hazard ratio; CI, confidence interval; BMI, body mass index; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association class.
Endpoint was defined as a composite of all‐cause mortality, heart transplantation, or ventricular assist device implantation. Initial CRT response was defined as an improvement in left ventricular ejection fraction ≥ 10% compared with baseline at initial follow‐up echocardiography (7.0 [3.9; 9.7]) months after implantation. All variables were included in the multivariate analysis, including CRT response as a time‐dependent covariate.
Bold indicates statistically significant findings.
Comparison of LVEF in diabetic and non‐diabetic patients at their last follow‐up echocardiography after a median of 3.9 [2.3; 5.9] years after CRT implantation did not show a significant difference, neither in absolute numbers nor with respect to the relative change compared with the pre‐implantation LVEF (Figure 1B ): average LVEF after CRT implantation was 33.6% ± 12.8% vs. 36.0% ± 14.4% (P = 0.058), and average change in LVEF was +8.4% ± 14.0% vs. +9.1% ± 14.3% in diabetic and non‐diabetic patients, respectively. The adjusted HR for reaching an LVEF improvement ≥ 10% was 0.60 [0.32; 1.14] in diabetic vs. non‐diabetic patients (Table 2 and Figure 1C ). Similarly, improvements in end‐systolic volumes on long‐term follow‐up were not significantly different between diabetic and non‐diabetic patients (average change: −13.5 ± 31.3 vs. −12.9 ± 40.6 mL/m2 in diabetic vs. non‐diabetic patients, Figure 1D ). However, when utilizing a cut‐off at ≥15% LVESVI reduction, diabetics demonstrated a significantly reduced prevalence of LV reverse remodelling than did non‐diabetics (adjusted HR 0.45 [0.21; 0.97], Table 2 and Figure 1F ).
Diabetes mellitus in ischaemic vs. non‐ischaemic cardiomyopathy
Because diabetes is a strong risk factor for ICM, we assessed the effect of diabetes in CRT patients stratified for ICM vs. non‐ICM. Comparison of diabetic and non‐diabetic patients within the subgroup of patients with ICM did not reveal a significant difference in the composite endpoint (Figure 2A , Table 4 ). In patients with non‐ICM, diabetic patients did worse than did non‐diabetic patients on unadjusted analysis (HR 1.65 [1.06; 2.58], P = 0.027, Figure 2D , Table 4 ). Diabetes was not associated with alterations in LV reverse remodelling as assessed by changes in LVEF or LVESVI in either subgroup (Figure 2B–F ).
Figure 2.
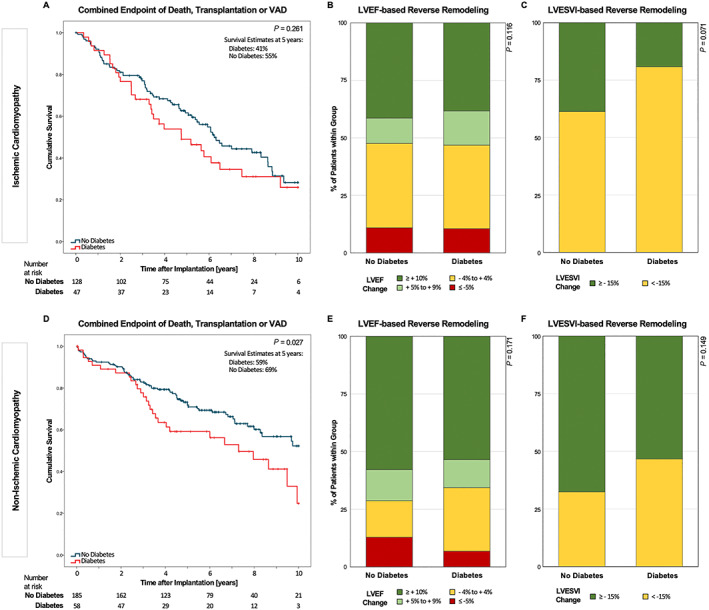
Outcome data stratified for diabetes mellitus (DM) vs. no DM in the subgroups of ischaemic and non‐ischaemic cardiomyopathy. (A) Kaplan–Meier survival curves in the subgroup of ischaemic cardiomyopathy for the composite endpoint of all‐cause mortality, heart transplantation, or ventricular assist device (VAD) implantation. (B, C) Stacked bar graph showing categorized left ventricular remodelling in the form of absolute left ventricular ejection fraction (LVEF) and relative LV end‐systolic volume index (LVESVI) improvement for the subgroup of ischaemic cardiomyopathy, respectively. (D–F) Same as (A–C) for the subgroup of non‐ischaemic cardiomyopathy.
Table 4.
Univariable and multivariable analyses for reaching the endpoint in the study population—stratified by type of underlying cardiomyopathy
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | aHR | 95% CI | P‐value | |
| A. Ischaemic cardiomyopathy | ||||||
| Diabetes | 1.28 | [0.83; 1.97] | 0.261 | 1.55 | [0.92; 2.62] | 0.101 |
| Male sex | 0.70 | [0.40; 1.22] | 0.211 | 0.63 | [0.30; 1.33] | 0.229 |
| Age | 1.02 | [0.99; 1.22] | 0.179 | 1.01 | [0.98; 1.04] | 0.763 |
| BMI | 0.99 | [0.96; 1.03] | 0.803 | 0.99 | [0.94; 1.05] | 0.802 |
| LVEF (%) | 0.97 | [0.94; 0.99] | 0.013 | 0.97 | [0.93; 1.00] | 0.041 |
| NYHA ≥ III | 2.15 | [1.26; 3.68] | 0.005 | 2.12 | [1.13; 3.97] | 0.020 |
| Creatinine (10 μmol/L) | 1.07 | [1.04; 1.09] | <0.001 | 1.03 | [0.97; 1.08] | 0.345 |
| NT‐proBNP (1000 ng/L) | 1.07 | [1.04; 1.10] | <0.001 | 1.05 | [0.99; 1.12] | 0.090 |
| Initial CRT response | 0.64 | [0.41; 1.01] | 0.053 | 0.70 | [0.39; 1.26] | 0.232 |
| B. Non‐ischaemic cardiomyopathy | ||||||
| Diabetes | 1.65 | [1.06; 2.58] | 0.027 | 1.47 | [0.83; 2.61] | 0.451 |
| Male sex | 1.90 | [1.12; 3.21] | 0.017 | 3.12 | [1.53; 6.38] | 0.002 |
| Age | 1.02 | [1.00; 1.04] | 0.076 | 0.99 | [0.97; 1.01] | 0.442 |
| BMI | 0.95 | [0.91; 0.99] | 0.016 | 0.89 | [0.84; 0.94] | <0.001 |
| LVEF (%) | 0.98 | [0.96; 1.01] | 0.214 | 0.97 | [0.94; 1.01] | 0.124 |
| NYHA ≥ III | 1.69 | [1.04; 2.73] | 0.033 | 2.44 | [1.30; 4.60] | 0.006 |
| Creatinine (10 μmol/L) | 1.06 | [1.04; 1.08] | <0.001 | 1.05 | [0.99; 1.09] | 0.084 |
| NT‐proBNP (1000 ng/L) | 1.03 | [1.02; 1.05] | <0.001 | 1.02 | [0.99; 1.05] | 0.259 |
| Initial CRT response | 0.37 | [0.23; 0.60] | <0.001 | 0.45 | [0.25; 0.81] | 0.008 |
aHR, adjusted hazard ratio; BMI, body mass index; CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association class.
Endpoint was a composite of all‐cause death, heart transplantation, or ventricular assist device implantation. Initial CRT response was defined as an improvement in left ventricular ejection fraction ≥ 10% compared with baseline at initial follow‐up echocardiography (7.0 [3.9; 9.7] months after implantation. All variables were included in the multivariate analysis, including CRT response as a time‐dependent covariate.
Bold indicates statistically significant findings.
Effect of insulin dependency on outcomes in diabetic cardiac resynchronization therapy patients
To further dissect the role of diabetes in CRT patients, we compared the outcome in IDDM vs. non‐IDDM diabetic CRT patients. We found a trend towards a reduced freedom from the composite endpoint in IDDM vs. non‐IDDM patients (adjusted HR 1.61 [0.80; 3.26], P = 0.185, Table 5 , Figure 3A ). Interestingly, survival curves began to separate only 3 years after CRT implantation. A trend towards an improved LV reverse remodelling in patients with vs. without IDDM did not reach statistical significance (Figure 3B and C ).
Table 5.
Univariable and multivariable analyses for reaching the endpoint in diabetic patients
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | aHR | 95% CI | P‐value | |
| IDDM vs. non‐IDDM | 1.55 | [0.92; 2.61] | 0.102 | 1.61 | [0.80; 3.26] | 0.185 |
| Male sex | 1.27 | [0.66; 2.46] | 0.478 | 2.36 | [0.63; 8.84] | 0.204 |
| Age | 1.00 | [0.98; 1.00] | 0.795 | 1.06 | [1.01; 1.12] | 0.016 |
| BMI | 1.03 | [0.98; 1.08] | 0.208 | 1.08 | [0.99; 1.17] | 0.071 |
| LVEF (%) | 0.93 | [0.89; 0.96] | <0.001 | 0.86 | [0.80; 0.92] | <0.001 |
| NYHA ≥ III | 1.76 | [0.86; 3.58] | 0.122 | 6.45 | [1.97; 21.06] | 0.002 |
| Ischaemic cardiomyopathy | 1.34 | [0.80; 2.24] | 0.272 | 1.73 | [0.85; 3.54] | 0.133 |
| Creatinine (10 μmol/L) | 1.06 | [1.04; 1.09] | <0.001 | 0.99 | [0.88; 1.11] | 0.838 |
| NT‐proBNP (1000 ng/L) | 1.05 | [1.02; 1.08] | 0.002 | 1.09 | [1.00; 1.19] | 0.057 |
| Initial CRT response | 0.90 | [0.54; 1.61] | 0.795 | 1.22 | [0.56; 2.65] | 0.626 |
aHR, adjusted hazard; HR, hazard ratio.
Endpoint was a composite of all‐cause death, heart transplantation, or ventricular assist device implantation. Initial CRT response was defined as an improvement in left ventricular ejection fraction ≥ 10% compared with baseline at initial follow‐up echocardiography (7.0 [3.9; 9.7] months after implantation. All variables were included in the multivariate analysis, including CRT response as a time‐dependent covariate.
Bold indicates statistically significant findings.
Figure 3.
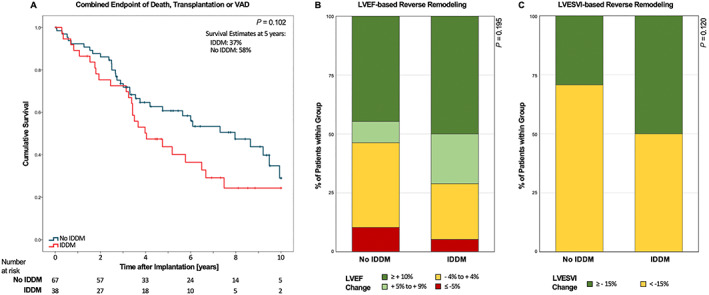
Outcome data in the subgroup of diabetic patients, comparing insulin‐dependent vs. non‐insulin‐dependent DM. (A) Kaplan–Meier survival curves for combined endpoint as indicated. (B, C) Stacked bar graph showing categorized left ventricular remodelling in the form of absolute left ventricular ejection fraction (LVEF) improvement and relative LV end‐systolic volume index (LVESVI) improvement, respectively.
Discussion
Over a median follow‐up of 4.8 years after CRT implantation, diabetic patients had an increased risk to reach the composite endpoint of all‐cause mortality, heart transplantation, or VAD implantation in this single‐centre real‐world cohort. This effect was also evident in the subgroup of patients with non‐ICM in univariable analysis, but not in patients with ICM; this differential effect may indicate that the increased mortality risk associated with DM is mediated by pathophysiological mechanisms distinct from increased atherosclerosis and macrovascular disease. Yet the worse prognosis of patients with ICM compared with non‐ICM may also outweigh the additional risk conferred by DM among patients with ICM. The increased mortality risk associated with DM was not accompanied by a strong signal for impaired LV reverse remodelling, which has been considered a main mechanism underlying improved survival after CRT. 22 , 23 While we did not observe an association between diabetes and LV reverse remodelling defined by an improvement in LVEF ≥ 10% compared with baseline, diabetes was associated with an impaired CRT response if defined as a reduction of LVESVI by ≥15%. This difference may be due to LVESVI being the more sensitive parameter compared with LVEF. However, this result needs to be interpreted with great care owing to larger number of missing data for this variable (data completeness 414/418 for LVEF and 263/418 for LVESVI).
The discrepancy of survival and LV remodelling may hint towards a role for other mechanisms underlying improved survival upon CRT than mere mechanical and hemodynamic changes. 24 , 25 , 26 Besides a predisposition to ischaemic injury, that is, coronary artery disease, further DM‐associated mechanisms have been described to result in myocardial damage (reviewed by Bugger and Abel 27 ). These mechanisms include altered myocardial metabolism and mitochondrial dysfunction, altered cell homeostatic processes regulating apoptosis, and autophagy along with changes in gene regulation. In combination, these molecular alterations result in impaired cardiac contractility and compliance as well as electroanatomical function, all of which are prerequisites for the beneficial effects of CRT. 9 , 12 , 28 , 29 Determining the causes of our findings in this diabetic subpopulation, including the prevalence of atrial and ventricular arrhythmic events, which have been demonstrated to be associated with heart failure outcome, will be subject for further research. 30 Consequently, worse prognosis with worse glycaemic control in DM should be presumed, and there was an according trend with increased mortality risk in patients with IDDM vs. non‐IDDM without statistical significance, which may be related to insufficient statistical power.
Our data add to previous cohort studies, further shifting the balance of existing evidence towards a less favourable long‐term outcome of CRT in diabetic compared with non‐diabetic patients. 11 , 12 , 13 , 14 This appears intuitive considering the role of DM as a strong cardiovascular risk factor and independent predictor of mortality in heart failure in general. However, post hoc analyses of the major randomized controlled CRT trials, including COMPANION, CARE‐HF, and MADIT‐CRT, have not identified any interaction between diabetes and their primary outcome. 16 , 17 , 18 , 19 , 20 More importantly, compared with the majority of previous studies, we provide a markedly longer follow‐up. 11 , 12 , 13 , 14 , 16 , 19 , 20 , 31 This appears to be of particular relevance because survival curves of diabetic and non‐diabetic patients begin to separate no earlier than 2 years after CRT implantation. Therefore, follow‐up time of earlier studies may have been too short to identify an effect. Of note, whereas our data indicate an inferior outcome of CRT associated with DM, the subgroup of diabetic patients in Echo‐CRT, which investigated the effect of CRT in heart failure patients with narrow QRS complex, derived less harm from CRT compared with non‐diabetic patients. 32 This may signal a reduced therapeutic effect of CRT in diabetic patients. Importantly, none of the randomized controlled CRT trials was specifically designed and therefore potentially not powered to assess a differential role of DM in CRT. Our cohort represents a cohort of consecutively enrolled patients in a real‐world setting. While the baseline characteristics of our study are very similar to those of the major clinical trials, patient enrolment was not limited by specific inclusion or exclusion criteria of a study protocol.
Our data furthermore corroborate previous findings, identifying ICM as an independent predictor for all‐cause mortality in heart failure patients with CRT. 33 , 34 , 35 Yet data on the role of DM in patients with ICM compared with non‐ICM are scarce. Intriguingly, the detrimental effect of DM on long‐term outcome after CRT appeared to weigh heavier in patients with non‐ICM compared with those with ICM. Failure to reach significance after adjusting for other risk factors may be attributed to a lack of statistical power in this subgroup of our cohort. However, our findings may also indicate that the additional risk seen with DM in non‐ICM disappears in ICM owing to the overall higher risk of mortality in this patient population. A similar signal was seen in a substudy of Echo‐CRT, where diabetics with non‐ICM derived the least harm from CRT. 32
The limited available data on the role of insulin therapy among diabetic CRT patients are controversial at best. While two post hoc analyses of MADIT‐CRT observed that CRT‐mediated risk reduction was confined to insulin‐dependent diabetics, 18 , 20 insulin use was found to confer additional risk in two well‐conducted cohort studies. 13 , 36 We did not identify a clear signal differentiating the role of IDDM vs. non‐IDDM in our cohort. A trend towards a reduced mortality risk in patients with non‐IDDM compared with patients with IDDM did not reach significance.
Limitations
The present study is an observational, single‐centre, retrospective cohort study. Endpoints were defined post hoc but correspond to those used in large prospective outcome trials. The number of patients in this cohort is limited, as this is a single‐centre study. Consecutive enrolment until January 2015 leads to a certain variance of follow‐up times. Importantly, however, follow‐up is very complete, with median follow‐up of 4.8 years, longer than in most previous studies. Nevertheless, we cannot exclude a type I error while comparing the characteristics of patients with and without diabetes. Moreover, inclusion at a single centre may introduce a selection and/or referral bias. Of note, baseline characteristics of our cohort are very similar to those in most large trials, suggesting that, even if present, selection and referral bias were likely negligible. The baseline characteristics of the diabetic group are different from those of the non‐diabetic group. Diabetic patients are older and had more frequently arterial hypertension, chronic coronary artery disease, and a lower LVEF. Propensity score matching in this study would have been difficult as (i) the relevant variables to calculate the propensity score for the development of diabetes as exposure variable are not available in our (and most other published) study(s) and (ii) matching might substantially reduce the sample size, even if these variables were available. Therefore, we chose a non‐matched cohort approach, adjusting the analysis for the known and available confounders. Importantly, creatinine, along with age and BMI, were accounted for in the multivariable models.
Recent advances in antidiabetic medical therapy with GLP‐1 agonists and SGLT2 inhibitors, which may affect outcome of CRT in diabetic patients, are not considered in this manuscript because the vast majority of CRT implantations was performed prior to marketing of these drugs. 37 Quadripolar coronary sinus leads were utilized in the majority of patients as soon as marketed. Importantly, multipoint pacing was not routinely applied in our patients. Therefore, the effect of multipoint pacing cannot be assessed in this cohort. The effect of quadripolar compared with bipolar leads was not the focus of the current study. 38 While focusing on survival as a hard endpoint, follow‐up of patients outside our centre did not allow us to collect unambiguous data on hospitalizations for worsening heart failure in this cohort, another widely used endpoint. The information provided on heart rhythm at baseline represents the rhythm during pre‐implantation 12‐lead ECG and does not allow differentiation between permanent, persistent, and paroxysmal atrial fibrillation. Finally, as in every retrospective registry, residual confounding cannot be excluded.
Conclusions
Diabetes confers an increased mortality risk in heart failure patients after CRT implantation. This risk appears to weigh heavier in patients with non‐ICM and is not reflected by an impaired reverse remodelling. Intriguingly, survival curves of diabetic and non‐diabetic patients begin to separate no earlier than 2 years after CRT implantation, which is why the follow‐up of earlier studies, including the sub‐studies of the major randomized trials, may have been too short to detect this signal.
Conflict of interest
Dr. Steffel has received consultant and/or speaker fees from Abbott, Amgen, Astra‐Zeneca, Bayer, Berlin‐Chemie/Menarini, Biosense Webster, Biotronik, Boehringer‐Ingelheim, Boston Scientific, Bristol‐Myers Squibb, Daiichi Sankyo, Medscape, Medtronic, Merck/MSD, Novartis, Pfizer, Sanofi‐Aventis, and WebMD. He reports ownership of CorXL. Dr. Steffel has received grant support through his institution from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, Daiichi Sankyo, and Medtronic. Dr. Saguner received educational grants from Abbott, Biotronik, Boston Scientific, and Medtronic. Dr. Flammer received consultant and/or speaker fees from Abbot, Alnylam, Bayer Healthcare, Bristol‐Myers Squibb, Fresenius, Imedos, Mepha, Novartis, Orion Pharma, Pfizer, Roche, and Vifo; and grant support through his institution was from Bayer Healthcare and Novartis. Dr. Breitenstein received educational grants from Biotronik, Biosense Webster, and Actelion; and speaker fees from Abbott, Medtronic, Biotronik, Bayer Healthcare, Bristol‐Myers Squibb, and Pfizer. Dr. Hofer has received speaker fees and/or educational funds from Abbott, Biosense Webster, Biotronik, Medtronik, and Novartis. Dr. Inderbitzin has received educational grants from St. Jude Medical and Medtronic. Dr. Winnik has received consultant and/or speaker fees from Boston Scientific, Abbott, and Boehringer‐Ingelheim. Dr. Winnik has received grant support from Novartis, Bayer, Daichi Sankyo, and Fehling Instruments through his institution. Dr. Trenson has received speaker fees from Boehringer‐Ingelheim and Novartis. He holds a scholarship from the Fund van de Werf.
Supporting information
Table S1. Demographics and baseline parameters stratified by insulin‐dependency.
Kahr, P. C. , Trenson, S. , Schindler, M. , Kuster, J. , Kaufmann, P. , Tonko, J. , Hofer, D. , Inderbitzin, D. T. , Breitenstein, A. , Saguner, A. M. , Flammer, A. J. , Ruschitzka, F. , Steffel, J. , and Winnik, S. (2020) Differential effect of cardiac resynchronization therapy in patients with diabetes mellitus: a long‐term retrospective cohort study. ESC Heart Failure, 7: 2773–2783. 10.1002/ehf2.12876.
References
- 1. Cleland JG, Abraham WT, Linde C, Gold MR, Young JB, Claude Daubert J, Sherfesee L, Wells GA, Tang ASL. An individual patient meta‐analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J 2013; 34: 3547–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 3. Woods B, Hawkins N, Mealing S, Sutton A, Abraham WT, Beshai JF, Klein H, Sculpher M, Plummer CJ, Cowie MR. Individual patient data network meta‐analysis of mortality effects of implantable cardiac devices. Heart 2015; 101: 1800–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmidt S, Hurlimann D, Starck CT, Hindricks G, Luscher TF, Ruschitzka F, Steffel J. Treatment with higher dosages of heart failure medication is associated with improved outcome following cardiac resynchronization therapy. Eur Heart J 2014; 35: 1051–1060. [DOI] [PubMed] [Google Scholar]
- 5. Steffel J, Rempel H, Breitenstein A, Schmidt S, Namdar M, Krasniqi N, Holzmeister J, Lüscher TF, Ruschitzka F, Hürlimann D. Comprehensive cardiac resynchronization therapy optimization in the real world. Cardiol J 2014; 21: 316–324. [DOI] [PubMed] [Google Scholar]
- 6. Martens P, Verbrugge FH, Nijst P, Bertrand PB, Dupont M, Tang WH, Mullens W. Feasibility and association of neurohumoral blocker up‐titration after cardiac resynchronization therapy. J Card Fail 2017; 23: 597–605. [DOI] [PubMed] [Google Scholar]
- 7. McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol 2014; 2: 843–851. [DOI] [PubMed] [Google Scholar]
- 8. Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 2004; 27: 1879–1884. [DOI] [PubMed] [Google Scholar]
- 9. Voors AA, van der Horst IC. Diabetes: a driver for heart failure. Heart 2011; 97: 774–780. [DOI] [PubMed] [Google Scholar]
- 10. Sardu C, Marfella R, Santulli G. Impact of diabetes mellitus on the clinical response to cardiac resynchronization therapy in elderly people. J Cardiovasc Transl Res 2014; 7: 362–368. [DOI] [PubMed] [Google Scholar]
- 11. Echouffo‐Tcheugui JB, Masoudi FA, Bao H, Spatz ES, Fonarow GC. Diabetes mellitus and outcomes of cardiac resynchronization with implantable cardioverter‐defibrillator therapy in older patients with heart failure. Circ Arrhythm Electrophysiol 2016; 9, e004132. [DOI] [PubMed] [Google Scholar]
- 12. Hoke U, Thijssen J, van Bommel RJ, van Erven L, van der Velde ET, Holman ER, Schalij MJ, Bax JJ, Delgado V, Marsan NA. Influence of diabetes on left ventricular systolic and diastolic function and on long‐term outcome after cardiac resynchronization therapy. Diabetes Care 2013; 36: 985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shah RV, Altman RK, Park MY, Zilinski J, Leyton‐Mange J, Orencole M, Picard MH, Barrett CD, Heist EK, Upadhyay G, Das R, Singh JP, Das S. Usefulness of hemoglobin A(1c) to predict outcome after cardiac resynchronization therapy in patients with diabetes mellitus and heart failure. Am J Cardiol 2012; 110: 683–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaya E, Senges J, Hochadel M, Eckardt L, Andresen D, Ince H, Spitzer SG, Kleemann T, Maier SSK, Jung W, Stellbrink C, Rassaf T, Wakili R. Impact of diabetes on clinical outcome of patients with heart failure undergoing ICD and CRT procedures: results from the German Device Registry. ESC Heart Fail 2020; 7: 984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun H, Guan Y, Wang L, Zhao Y, Lv H, Bi X, Wang H, Zhang X, Liu L, Wei M, Song H, Su G. Influence of diabetes on cardiac resynchronization therapy in heart failure patients: a meta‐analysis. BMC Cardiovasc Disord 2015; 15: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ghali JK, Boehmer J, Feldman AM, Saxon LA, Demarco T, Carson P, Yong P, Galle EG, Leigh J, Ecklund FL, Bristow MR. Influence of diabetes on cardiac resynchronization therapy with or without defibrillator in patients with advanced heart failure. J Card Fail 2007; 13: 769–773. [DOI] [PubMed] [Google Scholar]
- 17. Hoppe UC, Freemantle N, Cleland JG, Marijianowski M, Erdmann E. Effect of cardiac resynchronization on morbidity and mortality of diabetic patients with severe heart failure. Diabetes Care 2007; 30: 722–724. [DOI] [PubMed] [Google Scholar]
- 18. Kutyifa V, Naqvi SY, Brown M, McNitt S, Goldenberg I, Klein H, Moss AJ. Comparison of long‐term survival benefits with cardiac resynchronization therapy in patients with mild heart failure with versus without diabetes mellitus (from the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy [MADIT‐CRT]). Am J Cardiol 2018; 121: 1567–1574. [DOI] [PubMed] [Google Scholar]
- 19. Martin DT, McNitt S, Nesto RW, Rutter MK, Moss AJ. Cardiac resynchronization therapy reduces the risk of cardiac events in patients with diabetes enrolled in the multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy (MADIT‐CRT). Circ Heart Fail 2011; 4: 332–338. [DOI] [PubMed] [Google Scholar]
- 20. Szepietowska B, Kutyifa V, Ruwald MH, Solomon SD, Ruwald AC, McNitt S, Polonsky B, Thomas S, Moss AJ, Zareba W. Effect of cardiac resynchronization therapy in patients with insulin‐treated diabetes mellitus. Am J Cardiol 2015; 116: 393–399. [DOI] [PubMed] [Google Scholar]
- 21. Winnik S, Elsener C, Seifert B, Starck C, Straub A, Saguner AM. “Real world” experience in cardiac resynchronization therapy at a Swiss Tertiary Care Center. Swiss Med Wkly 2017; 147: w14425. [DOI] [PubMed] [Google Scholar]
- 22. Linde C, Gold MR, Abraham WT, St John Sutton M, Ghio S, Cerkvenik J, Daubert C, REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) Study Group . Long‐term impact of cardiac resynchronization therapy in mild heart failure: 5‐year results from the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) study. Eur Heart J 2013; 34: 2592–2599. [DOI] [PubMed] [Google Scholar]
- 23. Tan ESJ, Lim J, Chan SP, Seow JT, Singh D, Yeo WT, Lim TW, Kojodjojo P, Seow S‐C. Effect of diabetes mellitus on cardiac resynchronization therapy and to prognosis in heart failure (from the Prospective Evaluation of Asian With Cardiac Resynchronization Therapy for Heart Failure Study). Am J Cardiol 2019; 124: 899–906. [DOI] [PubMed] [Google Scholar]
- 24. Hamdan MH. The benefits of CRT: mechanical remodeling and beyond! Pacing Clin Electrophysiol 2007; 30: 587–590. [DOI] [PubMed] [Google Scholar]
- 25. Henrikson CA, Spragg DD, Cheng A, Capps M, Devaughn K, Marine JE, Calkins H, Tomaselli GF, Berger RD. Evidence for electrical remodeling of the native conduction system with cardiac resynchronization therapy. Pacing Clin Electrophysiol 2007; 30: 591–595. [DOI] [PubMed] [Google Scholar]
- 26. Kamireddy S, Agarwal SK, Adelstein E, Jain S, Saba S. Correlation of electrical and mechanical reverse remodeling after cardiac resynchronization therapy. Ann Noninvasive Electrocardiol 2009; 14: 153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014; 57: 660–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre‐clinical diastolic dysfunction a population‐based study. J Am Coll Cardiol 2010; 55: 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sardu C, Santamaria M, Funaro S, Sacra C, Barbieri M, Paolisso P, Marfella R, Paolisso G, Rizzo MR. Cardiac electrophysiological alterations and clinical response in cardiac resynchronization therapy with a defibrillator treated patients affected by metabolic syndrome. Medicine 2017; 96: e6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sardu C, Santamaria M, Rizzo MR, Barbieri M, di Marino M, Paolisso G, Santulli G, Marfella R. Telemonitoring in heart failure patients treated by cardiac resynchronisation therapy with defibrillator (CRT‐D): the TELECART Study. Int J Clin Pract 2016; 70: 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fantoni C, Regoli F, Ghanem A, Raffa S, Klersy C, Sorgente A, Faletra F, Baravelli M, Inglese L, Salerno‐Uriarte JA, Klein HU, Moccetti T, Auricchio A. Long‐term outcome in diabetic heart failure patients treated with cardiac resynchronization therapy. Eur J Heart Fail 2008; 10: 298–307. [DOI] [PubMed] [Google Scholar]
- 32. Nagele MP, Steffel J, Robertson M, Singh JP, Flammer AJ, Bax JJ, Borer JS, Dickstein K, Ford I, Gorcsan J, Gras D, Krum H, Sogaard P, Holzmeister J, Abraham WT, Brugada J, Ruschitzka F. Effect of cardiac resynchronization therapy in patients with diabetes randomized in EchoCRT. Eur J Heart Fail 2017; 19: 80–87. [DOI] [PubMed] [Google Scholar]
- 33. Barsheshet A, Goldenberg I, Moss AJ, Eldar M, Huang DT, McNitt S, Klein HU, Hall WJ, Brown MW, Goldberger JJ, Goldstein RE, Schuger C, Zareba W, Daubert JP. Response to preventive cardiac resynchronization therapy in patients with ischaemic and nonischaemic cardiomyopathy in MADIT‐CRT. Eur Heart J 2011; 32: 1622–1630. [DOI] [PubMed] [Google Scholar]
- 34. Wasmer K, Kobe J, Andresen D, Zahn R, Spitzer SG, Jehle J, Brachmann J, Stellbrink C, Martens E, Hochadel M, Senges J, Klein H, Eckardt L. Comparing outcome of patients with coronary artery disease and dilated cardiomyopathy in ICD and CRT recipients: data from the German DEVICE‐registry. Clin Res Cardiol 2013; 102: 513–521. [DOI] [PubMed] [Google Scholar]
- 35. Zhang Q, Fung JW, Chan JY, Yip G, Lam YY, Liang YJ, Yu C‐M. Difference in long‐term clinical outcome after cardiac resynchronisation therapy between ischaemic and non‐ischaemic aetiologies of heart failure. Heart 2009; 95: 113–118. [DOI] [PubMed] [Google Scholar]
- 36. Mangiavacchi M, Gasparini M, Genovese S, Pini D, Klersy C, Bragato R, Andreuzzi B, Municinò A, Regoli F, Galimberti P, Ceriotti C, Gronda E. Insulin‐treated type 2 diabetes is associated with a decreased survival in heart failure patients after cardiac resynchronization therapy. Pacing Clin Electrophysiol 2008; 31: 1425–1432. [DOI] [PubMed] [Google Scholar]
- 37. Sardu C, Paolisso P, Sacra C, Santamaria M, de Lucia C, Ruocco A, Mauro C, Paolisso G, Rizzo MR, Barbieri M, Marfella R. Cardiac resynchronization therapy with a defibrillator (CRTd) in failing heart patients with type 2 diabetes mellitus and treated by glucagon‐like peptide 1 receptor agonists (GLP‐1 RA) therapy vs. conventional hypoglycemic drugs: arrhythmic burden, hospitalizations for heart failure, and CRTd responders rate. Cardiovasc Diabetol 2018; 17: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sardu C, Barbieri M, Santamaria M, Giordano V, Sacra C, Paolisso P, Spirito A, Marfella R, Paolisso G, Rizzo MR. Multipolar pacing by cardiac resynchronization therapy with a defibrillators treatment in type 2 diabetes mellitus failing heart patients: impact on responders rate, and clinical outcomes. Cardiovasc Diabetol 2017; 16: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographics and baseline parameters stratified by insulin‐dependency.


