Abstract
Aims
We aimed to search for associations between cognitive test results with mortality and rehospitalization in a Swedish prospective heart failure (HF) patient cohort.
Methods and results
Two hundred and eighty‐one patients hospitalized for HF (mean age, 74 years; 32% women) were assessed using cognitive tests: Montreal Cognitive Assessment (MoCA), A Quick Test of Cognitive speed, Trail Making Test A, and Symbol Digit Modalities Test. The mean follow‐up time censored at rehospitalization or death was 13 months (interquartile range, 14) and 28 months (interquartile range, 29), respectively. Relations between cognitive test results, mortality, and rehospitalization risk were analysed using multivariable Cox regression model adjusted for age, sex, body mass index, systolic blood pressure, atrial fibrillation, diabetes, smoking, educational level, New York Heart Association class, and prior cardiovascular disease. A total of 80 patients (29%) had signs of cognitive impairment (MoCA score < 23 points). In the fully adjusted Cox regression model using standardized values per 1 SD change of each cognitive test, lower score on MoCA [hazard ratio (HR), 0.75; confidence interval (CI), 0.60–0.95; P = 0.016] and Symbol Digit Modalities Test (HR, 0.66; CI, 0.48–0.90; P = 0.008) yielded significant associations with increased mortality. Rehospitalization risk (n = 173; 62%) was significantly associated with lower MoCA score (HR, 0.84; CI, 0.71–0.99; P = 0.033).
Conclusions
Two included cognitive tests were associated with mortality in hospitalized HF patients, independently of traditional risk factors. In addition, worse cognitive test scores on MoCA heralded increased risk of rehospitalization.
Keywords: Cognitive dysfunction, Heart failure, Mortality, Rehospitalization
Introduction
Heart failure (HF) is a clinical syndrome characterized by shortness of breath, oedema, and fatigue due to the heart's inability to meet the body's need of oxygenated blood. 1 This inability can be caused by structural or functional defects. Despite the fact that treatment for HF has improved over recent decades, the mortality is still high with an annual mortality rate of 20% 30%, depending on the severity of HF. 2 Every year, HF causes more deaths compared with the most malignant tumours and is one of the most frequent reasons for hospitalization of older adults. 3 It has been increasingly recognized that patients who are hospitalized for HF show signs of cognitive disabilities in executive functions, memory, speech, and mental processing speed. 4 , 5 The reported prevalence of cognitive impairment in HF varies from 30% to 80%, depending on HF severity, study design, and cognitive test assessment. 6 Considering these observations, the term ‘cardiogenic dementia’ was introduced in the 1970s with an aim to describe the relationship between HF and cognitive impairment. 7 Among patients with HF and concomitant cognitive decline, a twofold increase in 30‐day mortality and rehospitalization has been observed, 8 as well as an almost fivefold increase in 1‐year mortality. 9 Poor medical adherence and reduced capacity to recognize early symptoms of HF have been proposed as reasons for early rehospitalization. 10 With an aging population and better survival rate among patients with acute coronary syndrome, the prevalence of patients suffering from HF will keep rising, and therefore, it is of increasing importance to detect patients with HF with signs of cognitive impairment in the clinical setting. Identifying these patients at an early stage and optimizing their medical treatment may not only prevent the progression of HF, but may also delay the development of clinically important cognitive deficits. Therefore, we aimed to search for associations between results of four cognitive tests with post‐discharge mortality and rehospitalization risk in a Swedish prospective HF patient cohort.
Methods
Study population
The HeARt and Brain Failure inVESTigation study (HARVEST) is an ongoing, prospective study undertaken in patients hospitalized for the diagnosis of HF (ICD‐10: I50‐) in the city of Malmö, Sweden. 11 The inclusion criterion for the HARVEST study is admission to the department of internal medicine or cardiology for treatment of newly diagnosed or exacerbated chronic HF. The only exclusion criterion is the inability to deliver an informed consent. In cases of severe cognitive impairment, the relatives are instead being informed and asked for permission on patient's behalf. Between March 2014 and February 2020, a total of 466 consecutive patients hospitalized for HF were included and underwent clinical examination. Cognitive testing in the HARVEST study was first initiated on January 2015, and of the 466 patients included after initiation of the study, 281 participants had complete data set (Figure 1 ). For those participants (n = 185) with missing data on cognitive tests, 155 participants did not participate in the cognitive testing and 30 participants had missing data in one or two tests. Of patients with no data for cognitive tests, 94 of them were included after the initiation of cognitive testing in the HARVEST study. The study was approved by the Ethical Review Board at Lund University, Sweden, and the study complies with the Declaration of Helsinki. A written informed consent was obtained from all participants or relatives as described above.
FIGURE 1.
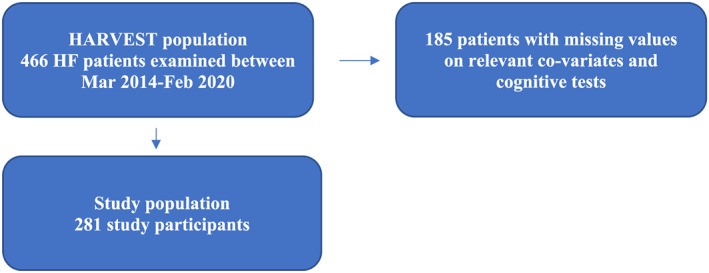
Flowchart of the study population. HARVEST, HeARt and Brain Failure inVESTigation study; HF, heart failure.
Co‐variates
Upon the hospitalization and subsequent admission to the clinical wards, study participants were examined with anthropometric measurements, and blood samples were drawn after overnight fast. Body mass index (BMI) was calculated as kilogrammes per square metre, and data regarding the study participants' medication were collected. Prevalent diabetes was defined as either self‐reported diagnosis of type 2 diabetes, or use of antidiabetic medication, or fasting plasma glucose > 7 mmol/L. Smoking status was self‐reported as yes or no, where never smokers were regarded as non‐smokers, and previous and present‐day smokers were defined as smokers. Trained nurses measured blood pressure using a validated automated blood pressure monitor Boso Medicus (Bosch + Sohn GmbH u. Co. KG, Jungingen, Germany). The upper arm cuff of appropriate size was placed on the right side, and the arm was supported at the heart level. Hypertension was defined as either systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg. Atrial fibrillation (AF) was defined as presence of AF on an electrocardiogram at the time of hospitalization or history of AF according to patient's medical documentation. Self‐reported education level was categorized as 9, 9–12, or >12 study years. Prior cardiovascular disease was defined as prior stroke or prior myocardial infarction.
Echocardiography
Conventional transthoracic echocardiograms were obtained using a Philips IE33 (Philips, Andover, MA, USA) with a 1–5 MHz transducer (S5‐1), or with a GE Vingmed Vivid 7 Ultrasound (GE, Vingmed Ultrasound, Horten, Norway) with a 1–4 MHz transducer (M3S). All studies were performed by experienced sonographers. Cine loops were obtained from standard views (parasternal long axis, apical 4‐chamber and 2‐chamber). Measurements were done offline using Xcelera 4.1.1 (Philips Medical Systems, Netherlands) according to the recommendations of the American Society of Echocardiography.
Laboratory assays
Analyses of high‐density lipoprotein and plasma glucose were carried out at the Department of Clinical Chemistry, Skåne University Hospital in Malmö, participating in a national standardization and quality control system. Low‐density lipoprotein was calculated according to the Friedewald equation.
Assessment of cognitive function
Within 3 days from hospital admission, patients underwent four cognitive tests assessing global cognition [Montreal Cognitive Assessment (MoCA)], 12 cognitive speed [A Quick Test of Cognitive Speed (AQT)], 13 visual attention and task switching [Trailmaking A (TMT A)], 14 and information processing [the digit symbol coding test, Symbol Digit Modalities Test (SDMT)]. 15 The tests were administered by trained nurses.
The Montreal Cognitive Assessment is a one‐page global cognitive test where the cognitive performance is ranked from 0 to 30 points (30 is the highest possible score). 12 The test evaluates eight cognitive domains including visuospatial, executive, short‐term and long‐term memory recall, attention, language, abstraction, delayed recall, and orientation. In the current study, a score below 23 points was regarded as cognitive impairment. 12 , 16 In MoCA, the memory recall assignment contains two learning trials of five nouns where the subject is instructed to repeat the words directly (short‐term recall) and after 5 min (long‐term recall). The visuospatial cognitive ability is assessed using a cube‐drawing and clock‐drawing task. The executive function is partly assessed using a task where the subject is instructed to draw lines between circles numbered 1–5 and circles with letters A–E in an ascending pattern (i.e. 1‐A‐2‐B‐3‐C, etc.). The executive function is also examined by a phonemic fluency task and a two‐item verbal abstraction task. To assess the orientation ability, the subject is instructed to recall the current date, month, year, place, and city. Language is evaluated by a three‐item confrontation naming task where the subject is assigned to name three portrayed animals. The language ability is also assessed in a task of repetition of two syntactically complex sentences. Finally, attention is assessed by three tasks: repetition of a list of digits forward and backwards, a serial subtraction task, and a target detection task.
In AQT, the perception and overall cognitive speed are assessed. The test includes three parts with 40 visual stimuli where the first two parts measure the time to name the colour of 40 squares and the shape of 40 geometric figures, respectively. In the third test part, the subject is instructed to combine colours with the geometric 40 figures as fast as possible (red, black, yellow, or blue, combined with circles, squares, rectangles, or triangles). The AQT score in part 3 is regarded as the number of seconds it takes to complete the task. In the current study, we only assessed the third part. Normal time limit is expected to be below 70 s based on data from 300 normally aging adults (ages 15–95). 17
The Trail Making Test consists of two parts (A and B), which together assess executive functions, visual search, scanning, speed of processing, and mental flexibility. 14 In part A (TMT A), the subject is instructed to draw lines between circles numbered 1–25 in an ascending order. In part B (TMT B), the subject is instructed to draw lines between circles numbered 1–14 and circles with letters A–L in an ascending pattern (i.e. 1‐A‐2‐B‐3‐C, etc.). The scores in both parts are regarded as the number of seconds it takes to complete the task. Due to a high amount of missing data in part B (n = 33), the current study only assesses part A. Normative data for the mean value of TMT A in the age category of 70–74 years in regard to educational level (0–12 years and >12 years) have previously been shown as 42 [standard deviation (SD) 15.5] and 40 s (SD 14.5), respectively. 14
The Symbol Digit Modalities Test is used in cognitive screening to evaluate attention, visual scanning, motor speed, and associative learning. 18 Subjects are required to repeatedly pair nine specific symbols to a specific number from 1–9. The obtained scores ranged from 0–110 and are regarded as the correct number of associations within 90 s. 19
Endpoints
Mortality was defined as death by any cause (total mortality) and was retrieved from the National Board of Health and Welfare's Cause of Death Register. Data regarding rehospitalization due to cardiac causes were retrieved from electronic medical charts accessible through the patient's unique national statistical number in the regional hospital database (Melior, Siemens Health Services, Solna, Sweden). All subjects were followed from study inclusion until 31 December 2018.
Statistics
The variables are presented as means (SD). Cox regression model was applied to estimate hazard ratios (HRs) with 95% confidence interval (CI) for each cognitive test per 1 SD increase. Continuous standardized values of each cognitive test were entered as independent variables in separate models. Model 1 was adjusted for age and sex. On top of age and sex, Model 2 was adjusted for BMI, systolic blood pressure, New York Heart Association (NYHA) class at admission, diabetes, educational level, prevalent AF, smoking, and prior cardiovascular disease as independent variables. The time variable was calculated as the follow‐up time between the date of screening and date of first rehospitalization or death until the 31st of February 2020. Group differences in continuous variables between study participants and individuals with missing data were compared using one‐way ANOVA test, whereas categorical variables were compared using Pearson's χ2 test. Group differences in continuous variables between study participants with MoCA scores above and below 23 (and 26) were compared using one‐way ANOVA test, whereas categorical variables were compared using Pearson's χ2 test. All analyses were performed using SPSS Windows version 25.0, and a P value of <0.05 was considered statistically significant.
Results
Patient characteristics
The study population had a mean age of 74 years, consisted predominantly of men (68%), 36% had diabetes, and a high proportion of patients (89%) was considered as NYHA classes III–IV (Table 1 ). During the follow‐up period, a total of 78 (28%) patients died. The most frequent cause of death was HF (n = 26) followed by cardiac arrest (n = 6), cancer (n = 3), and stroke (n = 1), and the rest of the recorded deaths (n = 42) were due to different causes and were defined as ‘other’ in the database. A total of 173 (62%) patients were rehospitalized, with the most common reason of rehospitalization being HF (n = 75) and arrhythmia (n = 14). The rest of the recorded rehospitalizations (n = 84) were due to a diversity of diagnoses and were defined as ‘other’ in the database. One hundred and twenty‐four (44%) individuals had MoCA scores between 18 and 25, and 18 patients (6%) had MoCA scores between 10 and 17. HF patients with MoCA score below 23 were older, were more likely to be women, and had worse result on SDMT, TMT A, and AQT, as compared with patients with MoCA above 23 (Supporting Information, Table S1 ). HF patients with MoCA score below 26 were also more likely to be women, older, and have worse result on SDMT, TMT A, and AQT, as compared with patients with MoCA above 26 (Supporting Information, Table S2 ).
TABLE 1.
Characteristics of study participants at baseline
| Baseline characteristic | N = 281 |
|---|---|
| Age [years (SD)] | 74 (12) |
| Sex [female, n (%)] | 91 (32) |
| NYHA classes III–IV [n (%)] | 249 (89) |
| Prevalent or history of smoking [n (%)] | 253 (90) |
| ACEi [n (%)] | 154 (55) |
| Beta‐blockers [n (%)] | 252 (90) |
| ARBs [n (%)] | 84 (30) |
| BMI [kg/m2 (SD)] | 28 (6) |
| SBP [mmHg (SD)] | 140 (28) |
| DBP [mmHg (SD)] | 80 (16) |
| Education level | |
| Elementary school, 9 years [n (%)] | 139 (50) |
| Upper secondary school, 9–12 years [n (%)] | 76 (27) |
| College education, >12 years [n (%)] | 61 (22) |
| Diabetes [n (%)] | 102 (36) |
| AF [n (%)] | 149 (53) |
| Prior stroke [n (%)] | 30 (11) |
| Prior myocardial infarction [n (%)] | 100 (36) |
| Nt‐proBNP [pmol/L (min–max)] | 5655 (60–35 000) |
| LVEF (%) | 39 (16) |
| MoCA [points (SD)] | 25 (4) |
| Mild cognitive impairment; MoCA 18–25 [n (%)] | 124 (44) |
| Moderate cognitive impairment; MoCA 10–17 [n (%)] | 18 (6) |
| Severe cognitive impairment; MoCA below 10 [n (%)] | 0 (0) |
| SDMT (points) | 26 (11) |
| TMT A [seconds (SD)] | 62 (34) |
| AQT [seconds (SD)] | 85 (26) |
ACEi, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; AQT, A Quick Test of Cognitive Speed; ARBs, angiotensin receptor blockers; BMI, body mass index; DBP, diastolic blood pressure; LVEF, left ventricular ejection fraction; MoCA, Montreal Cognitive Assessment; NT‐proBNP, N terminal pro brain natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SDMT, Symbol Digit Modalities Test; TMT A, Trail Making Test A.
Association between cognitive test results, mortality, and rehospitalization risk
In the Cox regression analysis adjusted for age and sex, MoCA, SDMT, and AQT scores as continuous variables yielded significant associations with increased risk of death. In the fully adjusted multivariate analysis, lower MoCA (HR, 0.75; CI, 0.60–0.95; P = 0.016) and SDMT scores (HR, 21 0.66; CI, 0.48–0.90; P = 0.008) remained significantly associated with increased risk of death (Table 2 ). Rehospitalization risk was significantly associated with lower MoCA score (HR, 0.84; CI, 0.71–0.99; P = 0.033) in the multivariate analysis (Table 3 ).
TABLE 2.
Cox regression analysis displaying the association between cognitive test results and risk of mortality
| Cognitive tests | HR (CI) | P value |
|---|---|---|
| MoCA | ||
| Model 1 | 0.73 (0.59–0.91) | 0.005 |
| Model 2 | 0.75 (0.60–0.95) | 0.016 |
| SDMT | ||
| Model 1 | 0.64 (0.47–0.86) | 0.003 |
| Model 2 | 0.66 (0.48–0.90) | 0.008 |
| TMT A | ||
| Model 1 | 1.21 (0.99–1.48) | 0.058 |
| Model 2 | 1.20 (0.99–1.47) | 0.068 |
| AQT | ||
| Model 1 | 1.24 (1.01–1.54) | 0.044 |
| Model 2 | 1.20 (0.97–1.49) | 0.092 |
Model 1 is adjusted for sex and age. Model 2 is adjusted for age, sex, body mass index, systolic blood pressure, New York Heart Association class at admission, diabetes, educational level, prevalent atrial fibrillation, smoking, and prior cardiovascular disease.
AQT, A Quick Test of Cognitive Speed; CI, confidence interval; HR, hazard ratio; MoCA, Montreal Cognitive Assessment; SDMT, Symbol Digit Modalities Test; TMT A, Trail Making Test A.
TABLE 3.
Cox regression analysis displaying the association between cognitive test results and risk of rehospitalization
| Cognitive tests | Continuing values | |
|---|---|---|
| HR (CI) | P value | |
| MoCA | ||
| Model 1 | 0.81 (0.69–0.94) | 0.007 |
| Model 2 | 0.84 (0.71–0.99) | 0.033 |
| SDMT | ||
| Model 1 | 0.83 (0.68–0.99) | 0.047 |
| Model 2 | 0.89 (0.73–1.09) | 0.254 |
| TMT A | ||
| Model 1 | 1.13 (0.97–1.32) | 0.115 |
| Model 2 | 1.09 (0.93–1.28) | 0.304 |
| AQT | ||
| Model 1 | 1.08 (0.92–1.26) | 0.246 |
| Model 2 | 1.01 (0.87–1.19) | 0.866 |
Model 1 is adjusted for sex and age. Model 2 is adjusted for age, sex, body mass index, systolic blood pressure, New York Heart Association class at admission, diabetes, educational level, prevalent atrial fibrillation, smoking, and prior cardiovascular disease.
AQT, A Quick Test of Cognitive Speed; CI, confidence interval; HR, hazard ratio; MoCA, Montreal Cognitive Assessment; SDMT, Symbol Digit Modalities Test; TMT A, Trail Making Test A.
Risk of mortality and rehospitalization according to Montreal Cognitive Assessment cut‐off score
Using MoCA as a categorical variable with cut‐off values, MoCA < 23 was significantly associated with mortality (HR, 2.17; CI, 1.34–3.50; P = 0.002) and with rehospitalization (HR, 1.62; CI, 1.14–2.29; P = 0.007). MoCA cut‐off score below 26 was significantly associated with rehospitalization but not with mortality (Supporting Information, Table S3 , Figure S1, Figure S2).
Harrell's C‐statistics for evaluation of overall adequacy of risk prediction
Harrell's C‐statistics for evaluation of overall adequacy of risk prediction procedures in the Cox regression model including traditional risk factors (age, sex, BMI, systolic blood pressure, NYHA class at admission, diabetes, educational level, prevalent AF, smoking, and prior cardiovascular disease) for mortality yielded a C‐index of 0.683. An addition of any one of the four tests to that model resulted in a gain in C‐statistics that ranged from −0.1 to 2.2 percentage units, with the highest add‐on value for MOCA < 23 points (Supporting Information, Table S4 ). As for analyses of rehospitalization, the Cox regression model including age, sex, BMI, systolic blood pressure, NYHA class at admission, diabetes, educational level, prevalent AF, smoking, and prior cardiovascular disease yielded a C‐index of 0.602. An addition of any one of the four tests to that model resulted in a gain in C‐statistics that ranged from 0.0 to 1.0 percentage units, with the highest add‐on value for the MOCA test as a continuous variable, together with MOCA < 23 points.
Discussion
In this study, we have demonstrated that two cognitive tests assessing the global cognitive function, cognitive speed, attention, and task switching are associated with post‐discharge mortality in patients hospitalized due to HF, independently of traditional risk factors. Moreover, we have observed that lower performance on MoCA heralds increased risk of rehospitalization in HF patients.
Our findings confirm data from previous studies suggesting cognitive function as a risk marker of mortality and rehospitalization in HF patients. 20 , 21 In the current study, the prevalence of cognitive impairment according to MoCA score below 23 among HF patients was 29%. In prior reports, cognitive impairment has been detected in 25% to 75%. 21 This wide range of prevalence might be attributed to difference in study populations including age, sample size, and HF severity. Additionally, different cognitive tests have been applied to assess the cognitive function, and different criteria in diagnosing HF have been practiced. 6 Further, the cognitive decline observed in HF patients might also be a result of other HF co‐morbidities such as sleep apnoea, anaemia, vitamin deficiency, renal failure, and depression. 22 Plasma level of N terminal pro brain natriuretic peptide did not reveal any correlation and/or association with MoCA Score, despite previously published results in population‐based studies. 23 , 24
Patients included in the current study were divided into two groups according to the results on the global cognitive test MoCA. HF patients with prevalent cognitive impairment defined as MoCA score below 23 were older, more likely to be women, and had lower educational level. Interestingly, the two groups were comparable in terms of HF parameters such as NYHA class and ejection fraction, as well as co‐morbidities such as prevalence of diabetes, AF, and smoking.
In a Swedish study examining 860 randomly selected elderly individuals from a population‐based cohort, MoCA cut‐offs for cognitive impairment ranged from <25 to <21 for the lowest educated and <26 to <24 for the highest educated, depending on age group. 25 In a newly conducted meta‐analysis including nine studies, a MoCA cut‐off score of 23, rather than the initially recommended score of 26, lowered the false‐positive rate and showed overall better diagnostic accuracy. 16 In comparison with the traditionally used Mini Mental State Examination, growing evidence demonstrates that MoCA yields a higher sensitivity in identifying multiple cognitive deficits in HF. 26 The higher sensitivity of MoCA in detecting cognitive impairment in HF has been suggested as an effect of additional measurement of the executive ability, which is commonly affected in HF patients due to higher burden of cardiovascular disease. 12 However, the use of MoCA has also been criticized due to low specificity in detecting mild cognitive impairment in populations with low base rate of cognitive decline. As the prevalence of cognitive decline among HF patients is comparatively high, this should not create a bias. In an HF population using the same cut‐off score of cognitive decline as in the current study, MoCA yielded a sensitivity of approximately 49% and failed to rule out 30% individuals with normal cognitive ability. 26 In addition to MoCA score, the current study also demonstrated that lower score on SDMT and longer time duration of TMT A indicated increased risk of post‐discharge mortality. As these tests assess cognitive abilities such as attention, visual scanning, and motor speed, there is limited additional value to perform them together in a clinical setting. To find individuals with HF that might be aided of enhanced caretaking in order to manage the treatment, one of these tests performed together with a multidomain test such as MoCA might be sufficient and clinically useful.
In HF, the most affected cognitive abilities are attention, executive function, psychomotor speed, and working memory, while the visuospatial ability and language skills are less affected. 4 Several theories have been proposed to explain the underlying pathophysiology of cognitive impairment and dementia in HF patients including increased risk for stroke and chronically reduced cerebral blood flow. 21 In addition to HF patients, better cardiac function, like peak exercise stroke volume, was associated with higher MoCA scores at baseline and after 6 months in individuals with preserved ejection fraction. 27 The detection of cognitive impairment among elderly patients in general hospital settings is low. 28 The underestimation of prevalent cognitive decline has also been observed in primary care. 29 As cognitive impairment has been linked to increased risk of mortality but also conversion to dementia, the need for cognitive screening has been suggested. 30 A diagnostic approach to HF‐induced cognitive impairment has been proposed to include screening for impaired memory and executive functions, anatomic brain changes (white matter hyperintensities; medial temporal atrophy; frontal lobe and hippocampal atrophy), and elevated levels of interleukin‐6, tumor necrosis factor‐α, cortisol, and epinephrine. 22 The same authors also propose that MoCA should be conducted in all HF patients, either by the referring physician or by a member of the HF team during the patient's first clinic visit and yearly thereafter, in order to recognize possible subtle cognitive changes that might otherwise be overlooked. In the European Society of Cardiology guidelines for the treatment of HF, it is recommended that support from a multidisciplinary HF team in collaboration with specialist dementia support teams, alongside medication compliance aids, tailored self‐care advice, and involvement of family and caregivers, may improve adherence to complex HF medication and self‐care regimens. 1 This approach is expected to improve patient's outcome.
Limitations
Cognitive tests were performed in older patients who were under treatment for acute HF. In the acute setting of HF, some patients may experience delirium, which is a reversible condition of confusion. 1 However, individuals with the clinical picture of delirium were most likely preliminarily excluded. Cognitive tests were performed within 3 days from admission to the hospital. Therefore, it is possible that the cognitive function might have changed during this time. Cognitive testing in the HARVEST study was first initiated on January 2015, and of the 466 patients included after initiation of cognitive testing, 185 patients had missing values on relevant co‐variates and/or cognitive tests. Because individuals with reduced cognitive function are usually less willing to participate in studies, a health selection bias might have occurred. In order to explore whether individuals with missing data differ from those with complete data sets, we have compared the two groups. Group differences in continuous variables between study participants and individuals with missing data were compared using one‐way ANOVA test, whereas categorical variables were compared using Pearson's χ2 test. The results are presented in Supporting Information, Table S5 . Finally, patients were predominantly of European ancestry, and therefore, the results of this study may not be generalizable to other racial/ethnic groups.
Conflict of interest
None declared.
Funding
M.M. was supported by grants from the Medical Faculty of Lund University, Skåne University Hospital, the Crafoord Foundation, the Ernhold Lundstroms Research Foundation, the Region Skåne, the Hulda and Conrad Mossfelt Foundation, the Southwest Skånes Diabetes Foundation, the Kockska Foundation, the Research Funds of Region Skåne, the Swedish Heart and Lung Foundation, and the Wallenberg Center for Molecular Medicine, Lund University.
Supporting information
Figure S1. Kaplan–Meier curves for the risk of mortality among 281 participants in HARVEST stratified according to cut‐off score of at or below 23 on MoCA.
Figure S2. Kaplan–Meier curves for the risk of rehospitalization among 281 participants in HARVEST stratified according to cut‐off score at or below 23 on MoCA.
Table S1. Characteristics of study participants (n = 281) at baseline stratified according to MoCA cut off score at or below 23.
Table S2. Characteristics of study participants (n = 281) at baseline stratified according to MoCA cut off score at or below 26.
Table S3. Associations between death and cognitive tests across cut‐off scores on MoCA
Table S4. Harrell's C‐statistics for evaluation of overall adequacy of risk prediction for mortality and rehospitalization
Table S5. Characteristics of study participants (n = 281) and individuals with missing data (n = 185) at baseline.
Acknowledgements
We thank the research nurses Hjördis Jernhed and Dina Chatziapostolou for valuable contributions, and we thank all the staff at the echocardiographic laboratory at Skåne University Hospital Malmö. The Knut and Alice Wallenberg Foundation is acknowledged for generous support.
Holm, H. , Bachus, E. , Jujic, A. , Nilsson, E. D. , Wadström, B. , Molvin, J. , Minthon, L. , Fedorowski, A. , Nägga, K. , and Magnusson, M. (2020) Cognitive test results are associated with mortality and rehospitalization in heart failure: Swedish prospective cohort study. ESC Heart Failure, 7: 2948–2955. 10.1002/ehf2.12909.
Shared last authorship Martin Magnusson.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl Ed). 2016 2016; 69: 1167. [DOI] [PubMed] [Google Scholar]
- 2. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011; 8: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More ‘malignant’ than cancer? Five‐year survival following a first admission for heart failure. Eur J Heart Fail 2001; 3: 315–322. [DOI] [PubMed] [Google Scholar]
- 4. Leto L, Feola M. Cognitive impairment in heart failure patients. J Geriatr Cardiol 2014; 11: 316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vogels RL, Scheltens P, Schroeder‐Tanka JM, Weinstein HC. Cognitive impairment in heart failure: a systematic review of the literature. Eur J Heart Fail 2007; 9: 440–449. [DOI] [PubMed] [Google Scholar]
- 6. Cannon JA, McMurray JJ, Quinn TJ. ‘Hearts and minds’: association, causation and implication of cognitive impairment in heart failure. Alzheimers Res Ther 2015; 7: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cardiogenic dementia. Lancet 1977; 1: 27–28. [PubMed] [Google Scholar]
- 8. Huynh QL, Negishi K, Blizzard L, Saito M, De Pasquale CG, Hare JL, Leung D, Stanton T, Sanderson K, Venn AJ, Marwick TH. Mild cognitive impairment predicts death and readmission within 30 days of discharge for heart failure. Int J Cardiol 2016; 221: 212–217. [DOI] [PubMed] [Google Scholar]
- 9. Zuccala G, Pedone C, Cesari M, Onder G, Pahor M, Marzetti E, Monaco MR, Cocchi A, Carbonin P, Bernabei R. The effects of cognitive impairment on mortality among hospitalized patients with heart failure. Am J Med 2003; 115: 97–103. [DOI] [PubMed] [Google Scholar]
- 10. Bauer LC, Johnson JK, Pozehl BJ. Cognition in heart failure: an overview of the concepts and their measures. J Am Acad Nurse Pract 2011; 23: 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christensson A, Grubb A, Molvin J, Holm H, Gransbo K, Tasevska‐Dinevska G, Bachus E, Jujic A, Magnusson M. The shrunken pore syndrome is associated with declined right ventricular systolic function in a heart failure population—the HARVEST study. Scand J Clin Lab Invest 2016; 76: 568–574. [DOI] [PubMed] [Google Scholar]
- 12. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 13. Wiig EH, Nielsen NP, Jacobson JM. A Quick Test of Cognitive Speed: patterns of age groups 15 to 95 years. Percept Mot Skills 2007; 104: 1067–1075. [DOI] [PubMed] [Google Scholar]
- 14. Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 2004; 19: 203–214. [DOI] [PubMed] [Google Scholar]
- 15. Sheridan LK, Fitzgerald HE, Adams KM, Nigg JT, Martel MM, Puttler LI, Wong MM, Zucker RA. Normative symbol digit modalities test performance in a community‐based sample. Arch Clin Neuropsychol 2006; 21: 23–28. [DOI] [PubMed] [Google Scholar]
- 16. Carson N, Leach L, Murphy KJ. A re‐examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry 2018; 33: 379–388. [DOI] [PubMed] [Google Scholar]
- 17. Kvitting AS, Wimo A, Johansson MM, Marcusson J. A Quick Test of Cognitive Speed (AQT): usefulness in dementia evaluations in primary care. Scand J Prim Health Care 2013; 31: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jaeger J. Digit symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol 2018; 38: 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosano C, Perera S, Inzitari M, Newman AB, Longstreth WT, Studenski S. Digit symbol substitution test and future clinical and subclinical disorders of cognition, mobility and mood in older adults. Age Ageing 2016; 45: 688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dodson JA, Truong TT, Towle VR, Kerins G, Chaudhry SI. Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. Am J Med 2013; 126: 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Doehner W, Ural D, Haeusler KG, Celutkiene J, Bestetti R, Cavusoglu Y, Peña‐Duque MA, Glavas D, Iacoviello M, Laufs U, Alvear RM. Heart and brain interaction in patients with heart failure: overview and proposal for a taxonomy. A position paper from the Study Group on Heart and Brain Interaction of the Heart Failure Association. Eur J Heart Fail 2018; 20: 199–215. [DOI] [PubMed] [Google Scholar]
- 22. Havakuk O, King KS, Grazette L, Yoon AJ, Fong M, Bregman N, Elkayam U, Kloner RA. Heart failure‐induced brain injury. J Am Coll Cardiol 2017; 69: 1609–1616. [DOI] [PubMed] [Google Scholar]
- 23. Mirza SS, de Bruijn RF, Koudstaal PJ, van den Meiracker AH, Franco OH, Hofman A, Tiemeier H, Ikram MA. The N‐terminal pro b‐type natriuretic peptide, and risk of dementia and cognitive decline: a 10‐year follow‐up study in the general population. J Neurol Neurosurg Psychiatry 2016; 87: 356–362. [DOI] [PubMed] [Google Scholar]
- 24. Veugen MGJ, Henry RMA, Brunner‐La Rocca HP, Dagnelie PC, Schram MT, van Agtmaal MJM, van der Kallen CJ, Sep SJ, van Boxtel MP, Bekers O, Meex SJ. Cross‐sectional associations between cardiac biomarkers, cognitive performance, and structural brain changes are modified by age. Arterioscler Thromb Vasc Biol 2018; 38: 1948–1958. [DOI] [PubMed] [Google Scholar]
- 25. Borland E, Nagga K, Nilsson PM, Minthon L, Nilsson ED, Palmqvist S. The Montreal Cognitive Assessment: normative data from a large Swedish population‐based cohort. J Alzheimers Dis 2017; 59: 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hawkins MA, Gathright EC, Gunstad J, Dolansky MA, Redle JD, Josephson R, Moore SM, Hughes JW. The MoCA and MMSE as screeners for cognitive impairment in a heart failure population: a study with comprehensive neuropsychological testing. Heart Lung 2014; 43: 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sugie M, Harada K, Takahashi T, Nara M, Kawai H, Fujiwara Y, Ishikawa J, Tanaka J, Koyama T, Kim H, Sengoku R, Fujimoto H, Obuchi S, Kyo S, Ito H. Peak exercise stroke volume effects on cognitive impairment in community‐dwelling people with preserved ejection fraction. ESC Heart Fail 2018; 5: 876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Douzenis A, Michopoulos I, Gournellis R, Christodoulou C, Kalkavoura C, Michalopoulou PG, Fineti K, Patapis P, Protopapas K, Lykouras L. Cognitive decline and dementia in elderly medical inpatients remain underestimated and underdiagnosed in a recently established university general hospital in Greece. Arch Gerontol Geriatr 2010; 50: 147–150. [DOI] [PubMed] [Google Scholar]
- 29. Lopponen M, Raiha I, Isoaho R, Vahlberg T, Kivela SL. Diagnosing cognitive impairment and dementia in primary health care—a more active approach is needed. Age Ageing 2003; 32: 606–612. [DOI] [PubMed] [Google Scholar]
- 30. Hawkins LA, Kilian S, Firek A, Kashner TM, Firek CJ, Silvet H. Cognitive impairment and medication adherence in outpatients with heart failure. Heart Lung 2012; 41: 572–582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Kaplan–Meier curves for the risk of mortality among 281 participants in HARVEST stratified according to cut‐off score of at or below 23 on MoCA.
Figure S2. Kaplan–Meier curves for the risk of rehospitalization among 281 participants in HARVEST stratified according to cut‐off score at or below 23 on MoCA.
Table S1. Characteristics of study participants (n = 281) at baseline stratified according to MoCA cut off score at or below 23.
Table S2. Characteristics of study participants (n = 281) at baseline stratified according to MoCA cut off score at or below 26.
Table S3. Associations between death and cognitive tests across cut‐off scores on MoCA
Table S4. Harrell's C‐statistics for evaluation of overall adequacy of risk prediction for mortality and rehospitalization
Table S5. Characteristics of study participants (n = 281) and individuals with missing data (n = 185) at baseline.


