Abstract
Aims
Our purpose was to investigate the association between the B‐type natriuretic peptide (BNP) level at discharge, the occurrence of worsening renal function (WRF), and long‐term outcomes in patients with heart failure (HF).
Methods and results
We enrolled hospitalized acute HF patients. We divided patients into four groups on the basis of BNP <250 pg/mL (BNP−) or BNP ≥250 pg/mL (BNP+) at discharge and the occurrence of WRF during admission: BNP−/WRF−, BNP−/WRF+, BNP+/WRF−, and BNP+/WRF+. We evaluated the association between BNP at discharge, WRF, and cardiovascular/all‐cause mortality/hospitalization due to HF. Clinical follow‐up was completed in 301 patients. At discharge, percentages of the patients with clinical signs of HF were low and similar among four groups. The median follow‐up period was 1206 days (interquartile range, 733–1825 days). The composite endpoint of cardiovascular mortality and HF hospitalization was significantly different between the four groups [12.9% (BNP−/WRF−), 22.7% (BNP−/WRF+), 35.8% (BNP+/WRF−), and 55.4% (BNP+/WRF+), P < 0.0001]. All‐cause mortality was also different etween the four groups (15.1%, 38.6%, 28.7%, and 39.3%, respectively, P = 0.003). In the multivariate Cox proportional hazards model, the combination of BNP ≥250 pg/mL and WRF showed the highest hazard ratio (HR) for composite endpoint (HR, 5.201; 95% confidence interval, 2.582–11.11; P < 0.0001), and BNP−/WRF+ was associated with increased all‐cause mortality (HR, 2.286; 95% confidence interval, 1.089–4.875; P = 0.03). Patients in BNP+/WRF+ had a higher cardiovascular mortality (28.6%), and those in BNP−/WRF+ had a high non‐cardiovascular mortality (29.5%).
Conclusions
Heart failure patients with BNP ≥250 pg/mL at discharge and in‐hospital occurrence of WRF had the highest risk for the composite endpoint (cardiovascular mortality and HF hospitalization) among groups.
Keywords: Heart failure, B‐type natriuretic peptide, Worsening renal function, Mortality
Introduction
Heart failure (HF) is closely linked with renal impairment in the acute and chronic phases. 1 , 2 Renal dysfunction is a predictor of poor outcome in patients with HF. 1 During hospitalization, worsening renal function (WRF) occurs in approximately one‐third of hospitalized patients with HF. 3 However, whether WRF is an independent predictor of poor prognosis in HF patients is still controversial. 1 , 4 , 5 , 6 Some studies showed that the combination of residual congestion and WRF in patients with HF was associated with high short‐term mortality and high rates of HF hospitalization. 1 , 7 , 8 Regarding long‐term mortality, Lawson et al. showed that WRF was associated with poor prognosis in patients with stable, chronic HF. 9
B‐type natriuretic peptide (BNP) has been used as an important biomarker in HF treatment as mentioned in the congestion grading score. Signs and symptoms of HF alone have limitations in the accuracy as measurement of congestion. Reduction in BNP observed in patients with congestion before discharge may reflect haemodynamic improvement. 10 Nishii et al. showed that increase in BNP without clinical signs of congestion was associated with increased HF rehospitalization. 11 Moreover, BNP is a more reliable predictor of prognosis in patients with HF than improvement of clinical signs of HF. 12 Our purpose was to investigate the association between the occurrence of in‐hospital WRF, the BNP concentration at discharge, and long‐term outcomes in patients with HF.
Methods
Patients
The medical records of a total of 421 patients with acute de novo/decompensated HF who were admitted to our hospital between March 2010 and July 2016 were obtained and screened. They were reviewed by experienced cardiologists; patient history, systolic and diastolic blood pressure, heart rate, echocardiography reports, medication, and laboratory data on admission, during hospitalization, and at discharge were collected. In particular, plasma BNP was measured on admission and within 3 days before discharge. Plasma BNP was measured using a chemiluminescent immunoassay kit (ARCHITECT i2000SR by Abbott Laboratories, Chicago, IL, USA). Other laboratory data during hospitalization were obtained every day or at a few days interval. Patients who were 20 years and older and were diagnosed with HF, based on the criteria of the Framingham study, were eligible. 13 In brief, the Framingham criteria require two major criteria, or one major and two minor criteria to diagnose HF. The major criteria are orthopnoea or paroxysmal nocturnal dyspnoea, neck vein distention, rales, cardiomegaly, acute pulmonary oedema, S3 gallop, increased venous pressure, prolongation of circulation time, and hepatojugular reflux. The minor criteria are ankle oedema, night cough, dyspnoea on exertion, hepatomegaly, tachycardia, and weight loss. We excluded those patients with pulmonary embolism, acute coronary syndrome, bradycardia that required pacemaker implantation, or those on haemodialysis. Patients who had plasma BNP level <100 pg/mL on admission and patients who died during index HF hospitalization were also excluded. To evaluate the relationship between plasma BNP level at discharge, WRF, and outcomes after discharge, we analysed only the patients whose plasma BNP levels at discharge were obtained. We divided 311 patients into four groups by plasma BNP level <250 pg/mL or BNP ≥250 pg/mL at discharge and the occurrence of WRF during admission. We used BNP level <250 pg/mL at discharge for BNP well managed according to previous studies. 14 The four groups were (i) BNP level <250 pg/mL and no occurrence of WRF (BNP−/WRF−, n = 94); (ii) BNP level <250 pg/mL and occurrence of WRF (BNP−/WRF+, n = 45); (iii) BNP level ≥250 pg/mL and no occurrence of WRF (BNP+/WRF−, n = 115); and (iv) BNP level ≥250 pg/mL and occurrence of WRF (BNP+/WRF+, n = 57).
Outcome measures
According to previous reports, WRF was defined as a relative increase in serum creatinine of at least 25% or an absolute increase in serum creatinine ≥0.3 mg/dL from admisson. 8 Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2. The estimated glomerular filtration rate was calculated using CKD Epidemiology Collaboration (CKD‐EPI) equations modified by the Japanese coefficient. 15
The primary outcome was the composite endpoint of cardiovascular mortality and HF hospitalization. We also assessed all‐cause, cardiovascular, and non‐cardiovascular mortality. We defined cardiovascular mortality as deaths attributed to HF, arrhythmia, or myocardial infarction. Follow‐up was performed by clinical visits or telephone calls to the patients, their physicians, or their relatives.
The dose of loop diuretics was expressed as furosemide equivalent for some patients who did not receive furosemide. The formula for conversion from other loop diuretics to furosemide equivalents was as follows: azosemide 30 mg = furosemide 20 mg. 16
The study was compiled in accordance with the Declaration of Helsinki, and the study protocols were approved by the Institutional Review Board. The Institutional Review Board waived the requirement for written informed consent because this study was a retrospective observational study.
Statistical analysis
Data were analysed using JMP 14 (SAS Institute, Inc., Cary, NC, USA). Continuous variables were reported as mean ± standard deviation or median ± interquartile range that represents the 25th to 75th percentiles of the distribution of data. Comparisons between the four groups were performed using ANOVA or the Kruskal–Wallis test for continuous variables, as appropriate. If the difference was significant, a Tukey–Kramer test was used to detect which group contributed to this difference. Categorical variables were compared between the four groups using the χ test or Fisher's exact test, as appropriate. Comparisons between the time of admission and discharge were performed by paired t‐test or Wilcoxon signed‐rank test. We then compared bivariate survival curves for all‐cause, cardiovascular, and non‐cardiovascular mortality and composite endpoint free survival using Kaplan–Meier estimates and tested statistical significance using the log‐rank test. Univariate and multivariate Cox proportional hazards models were used to evaluate the estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between variables and primary/secondary endpoint. Variables with P value <0.10 in univariate analysis and those that had been demonstrated to be associated with primary/secondary endpoint in HF patients by previous literature data were included in multivariate Cox proportional hazards models. Therefore, the variables entered into multivariate analysis of the composite endpoint of cardiovascular mortality and HF hospitalization, including age, sex, New York Heart Association functional class, history of admission for HF, ischaemic heart disease, CKD, left ventricular ejection fraction (LVEF), triple therapy, groups of BNP and WRF, and creatinine at discharge. Triple therapy was defined as the combination usage of beta‐blockers, angiotensin‐converting enzyme inhibitor (ACEI)/angiotensin receptor blockers (ARBs), and aldosterone antagonists. The multivariate analysis of all‐cause mortality included the same variables of multivariate analysis for the composite endpoint of cardiovascular mortality and HF hospitalization. In non‐cardiovascular mortality, we performed multivariate Cox proportional hazards analysis adjusted for age and sex because of low event rate. A two‐sided P value <0.05 was considered significant.
Results
Patient characteristics on admission are shown in Table 1 . WRF developed in 102 patients (32.8%). Patients in BNP−/WRF− were significantly younger than those in the other groups (P = 0.001). Systolic blood pressure was significantly different among the groups (P = 0.03). Left ventricular (LV) diastolic diameter was smaller in patients in BNP−/WRF+ than in those in BNP+/WRF− (P = 0.03). LVEF was higher in patients in BNP−/WRF+ than in those in BNP+/WRF− (P = 0.006) and in BNP+/WRF+ (P = 0.03). At discharge (Table 2 ), beta‐blockers were less frequently used in BNP−/WRF+ and ACEI/ARBs were less frequently used in BNP+/WRF+ (P = 0.03 and P = 0.005, respectively). Cardiothoracic ratio and body weight significantly decreased from the time of admission to discharge (P < 0.0001 for all four groups). The clinical signs of congestion were rarely observed at discharge in the four groups.
Table 1.
Patient characteristics on admission
| Parameter | BNP−/WRF− | BNP−/WRF+ | BNP+/WRF− | BNP+/WRF+ | P value |
|---|---|---|---|---|---|
| n = 94 | n = 45 | n = 115 | n = 57 | ||
| Age (years) | 67.5 ± 14.7 | 73.3 ± 15.3 | 73.4 ± 14.1 * | 74.5 ± 16.3 * | 0.001 |
| Male, n (%) | 62 (66.0) | 22 (48.9) | 77 (67.0) | 32 (56.1) | 0.12 |
| Heart rate (b.p.m.) | 97.6 ± 28.7 | 96.5 ± 23.9 | 97.4 ± 27.0 | 98.6 ± 24.2 | 0.98 |
| Systolic blood pressure (mmHg) | 140.5 ± 34.9 | 149.7 ± 33.1 | 135.9 ± 27.9 | 148.9 ± 36.3 | 0.03 |
| Diastolic blood pressure (mmHg) | 84.1 ± 24.2 | 88.0 ± 23.3 | 84.6 ± 20.7 | 90.1 ± 24.7 | 0.37 |
| NYHA functional class | 3.4 ± 0.6 | 3.5 ± 0.7 | 3.3 ± 0.7 | 3.5 ± 0.6 | 0.28 |
| Body weight on admission (kg) | 63.6 ± 17.0 | 60.1 ± 21.0 | 60.2 ± 14.5 | 59.3 ± 16.1 | 0.41 |
| Coarse crackles on admission, n (%) | 50 (53.2) | 30 (66.7) | 74 (64.3) | 42 (73.7) | 0.03 |
| Peripheral oedema on admission, n (%) | 56 (59.6) | 33 (73.3) | 87 (75.7) | 36 (63.2) | 0.08 |
| CTR on admission (%) | 60.8 ± 6.0 | 59.9 ± 6.0 | 61.7 ± 5.7 | 63.9 ± 8.0 * , † | 0.006 |
| History of admission for heart failure, n (%) | 14 (14.9) | 4 (8.9) | 20 (17.4) | 20 (35.1) | 0.005 |
| HF‐preserved EF, n (%) | 23 (24.5) | 15 (33.7) | 22 (22.6) | 9 (15.8) | 0.29 |
| Ischaemic heart disease, n (%) | 26 (27.7) | 11 (24.4) | 35 (30.4) | 19 (33.3) | 0.76 |
| Atrial fibrillation, n (%) | 33 (35.1) | 14 (31.1) | 51 (44.4) | 25 (43.9) | 0.3 |
| Hypertension, n (%) | 63 (67.0) | 31 (68.9) | 75 (65.2) | 46 (80.1) | 0.18 |
| Hyperlipidaemia, n (%) | 42 (44.7) | 22 (48.9) | 48 (41.7) | 25 (43.9) | 0.88 |
| Diabetes mellitus, n (%) | 35 (37.2) | 15 (33.3) | 33 (28.7) | 19 (33.3) | 0.63 |
| Chronic kidney disease, n (%) | 36 (38.3) | 19 (42.2) | 62 (53.9) | 32 (56.1) | 0.06 |
| LV diastolic diameter (mm) | 55.0 ± 8.9 | 52.4 ± 9.9 | 56.7 ± 9.4 † | 56.8 ± 8.1 | 0.03 |
| LV systolic diameter (mm) | 43.3 ± 11.2 | 39.2 ± 12.0 | 45.6 ± 11.4 † | 45.8 ± 9.5 † | 0.007 |
| LVEF (%) | 42.6 ± 16.7 | 48.8 ± 14.9 | 39.8 ± 15.7 † | 40.2 ± 13.3 † | 0.009 |
| Laboratory data | |||||
| Albumin (g/dL) | 3.7 ± 0.5 | 3.7 ± 0.6 | 3.6 ± 0.4 | 3.4 ± 0.5 * , † | 0.006 |
| Blood urea nitrogen (mg/dL) | 21.1 ± 12.8 | 18.0 ± 6.4 | 26.4 ± 14.8 * , † | 24.5 ± 13.6 | 0.0009 |
| Creatinine (mg/dL) | 1.06 ± 0.59 | 0.92 ± 0.36 | 1.19 ± 0.56 | 1.30 ± 0.89 † | 0.009 |
| eGFR (mL/min/1.73 m2) | 60.5 ± 19.0 | 61.0 ± 18.0 | 52.3 ± 18.9 * | 50.8 ± 22.8 * , † | 0.001 |
| Sodium (mEq/L) | 139.3 ± 4.2 | 140.4 ± 2.5 | 139.9 ± 4.0 | 138.5 ± 4.2 | 0.06 |
| Chloride (mEq/L) | 104.7 ± 4.6 | 105.7 ± 4.0 | 105.9 ± 4.7 | 105.0 ± 4.7 | 0.22 |
| Potassium (mEq/L) | 4.2 ± 0.5 | 4.1 ± 0.5 | 4.3 ± 0.7 | 4.2 ± 0.5 | 0.17 |
| Haemoglobin (g/dL) | 12.9 ± 2.5 | 12.1 ± 2.8 | 12.4 ± 2.4 | 11.8 ± 2.5 | 0.08 |
| Haematocrit (g/dL) | 38.4 ± 6.7 | 36.7 ± 7.8 | 37.3 ± 6.7 | 35.6 ± 6.8 | 0.09 |
| BNP (pg/mL) | 620.4 ± 454.5 | 420.8 ± 334.8 | 1102.4 ± 936.5 * , † | 1017.4 ± 552.8 * , † | <0.0001 |
| In‐hospital treatment | |||||
| Inotropes, n (%) | 12 (12.8) | 4 (8.9) | 10 (8.7) | 12 (21.1) | 0.14 |
| Intravenous furosemide, n (%) | 58 (61.7) | 32 (71.1) | 76 (66.1) | 46 (80.7) | 0.08 |
| Dose of IV furosemide (mg/day) | 20.9 ± 13.3 | 21.7 ± 13.1 | 19.8 ± 8.3 | 21.0 ± 10.7 | 0.84 |
| Carperitide, n (%) | 51 (54.3) | 29 (64.4) | 76 (66.1) | 40 (70.2) | 0.19 |
| Tolvaptan, n (%) | 13 (13.8) | 10 (22.2) | 23 (20.0) | 19 (33.3) | 0.05 |
| Vasodilator, n (%) | 57 (60.6) | 32 (71.1) | 81 (70.4) | 41 (71.9) | 0.36 |
BNP, B‐type natriuretic peptide; CTR, cardiothoracic ratio; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HF, heart failure; IV, intravenous; LV, left ventricular; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; WRF, worsening renal function.
P < 0.05 compared with BNP−/WRF− group.
P < 0.05 compared with BNP−/WRF+ group.
P < 0.05 compared with BNP+/WRF− group.
Table 2.
Patient characteristics at discharge
| Parameter | BNP−/WRF− | BNP−/WRF+ | BNP+/WRF− | BNP+/WRF+ | P value |
|---|---|---|---|---|---|
| n = 94 | n = 45 | n = 115 | n = 57 | ||
| Body weight at discharge (kg) | 59.7 ± 15.7 | 54.5 ± 17.2 | 55.0 ± 12.6 | 55.7 ± 13.9 | 0.09 |
| Coarse crackles at discharge, n (%) | 2 (2.1) | 1 (2.2) | 2 (1.7) | 0 (0.0) | 0.62 |
| Peripheral oedema at discharge, n (%) | 5 (5.3) | 2 (4.4) | 10 (8.7) | 5 (8.8) | 0.63 |
| CTR at discharge (%) | 53.6 ± 5.9 | 54.5 ± 6.3 | 56.2 ± 6.3 * | 58.3 ± 7.5 * ,† | 0.0001 |
| Length of hospital stay (days) | 16.1 ± 8.2 | 20.4 ± 18.5 | 16.6 ± 10.0 | 22.8 ± 13.9 * , ‡ | 0.002 |
| Laboratory data at discharge | |||||
| Albumin (g/dL) | 3.7 ± 0.4 | 3.6 ± 0.4 | 3.5 ± 0.5 * | 3.4 ± 0.4 * , † | <0.0001 |
| Blood urea nitrogen (mg/dL) | 20.0 ± 9.3 | 23.4 ± 9.0 | 22.8 ± 10.9 | 26.8 ± 14.5 * | 0.004 |
| Creatinine (mg/dL) | 0.98 ± 0.50 | 1.07 ± 0.44 | 1.08 ± 0.44 | 1.43 ± 1.01 * , † , ‡ | 0.0001 |
| eGFR (mL/min/1.73 m2) | 63.7 ± 17.8 | 53.4 ± 20.0 * | 55.7 ± 19.2 * | 46.6 ± 22.1 * , ‡ | <0.0001 |
| Sodium (mEq/L) | 137.9 ± 3.4 | 138.6 ± 2.3 | 139.3 ± 3.0 * | 137.8 ± 4.5 ‡ | 0.01 |
| Chloride (mEq/L) | 103.1 ± 3.8 | 104.5 ± 3.3 | 104.4 ± 4.0 | 103.6 ± 4.8 | 0.07 |
| Potassium (mEq/L) | 4.5 ± 0.4 | 4.5 ± 0.5 | 4.4 ± 0.4 | 4.5 ± 0.6 | 0.3 |
| Haemoglobin (g/dL) | 13.0 ± 2.4 | 11.9 ± 2.4 | 12.6 ± 2.3 | 11.5 ± 2.5 * , ‡ | 0.0009 |
| Haematocrit (%) | 38.7 ± 6.7 | 35.8 ± 6.6 | 37.8 ± 6.3 | 34.4 ± 6.9 * , ‡ | 0.0006 |
| BNP (pg/mL) | 143.7 ± 62.5 | 123.7 ± 62.6 | 667.3 ± 445.8 * , † | 734.8 ± 533.4 * , † | <0.0001 |
| Medication at discharge | |||||
| Beta‐blockers, n (%) | 70 (74.5) | 24 (53.3) | 88 (76.5) | 39 (68.4) | 0.03 |
| ACEI/ARBs, n (%) | 74 (78.7) | 32 (71.1) | 78 (67.8) | 29 (50.9) | 0.005 |
| Loop diuretics, n (%) | 68 (72.3) | 30 (66.7) | 93 (80.9) | 46 (80.7) | 0.18 |
| Aldosterone antagonists, n (%) | 31 (33.0) | 20 (44.4) | 40 (34.8) | 26 (45.6) | 0.3 |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin 2 receptor blocker; BNP, B‐type natriuretic peptide; CTR, cardiothoracic ratio; eGFR, estimated glomerular filtration rate; WRF, worsening renal function.
P < 0.05 compared with BNP−/WRF− group.
P < 0.05 compared with BNP−/WRF+ group.
P < 0.05 compared with BNP+/WRF− group.
The clinical follow‐up was completed for 301 patients (96.8%). During a median follow‐up period of 1206 days (interquartile range, 733–1825 days), one (1.1%), four (9.1%), 17 (15.7%), and 16 (28.6%) patients died from cardiovascular events in BNP−/WRF−, BNP−/WRF+, BNP+/WRF−, and BNP+/WRF+, respectively (Table 3 ). Patients in BNP+/WRF+ had a higher cardiovascular mortality (28.6%), and those in BNP−/WRF+ had a high non‐cardiovascular mortality (29.5%). Kaplan–Meier curves for all‐cause, cardiovascular, and non‐cardiovascular mortality and composite endpoint are shown in Figure 1 . All‐cause mortality was significantly different between the four groups (P = 0.003). The composite endpoint of cardiovascular mortality and HF hospitalization was also significantly different between the four groups (P < 0.0001).
Table 3.
Causes of death and composite endpoint among four groups
| Causes of death | BNP−/WRF− | BNP−/WRF+ | BNP+/WRF− | BNP+/WRF+ |
|---|---|---|---|---|
| Death, n (%) | 14 (15.1) | 17 (38.6) | 31 (28.7) | 22 (39.3) |
| Cardiovascular, n (%) | 1 (1.1) | 4 (9.1) | 17 (15.7) | 16 (28.6) |
| Non‐cardiovascular, n (%) | 13 (14.0) | 13 (29.5) | 14 (13.0) | 6 (10.7) |
| Cancer, n (%) | 3 (3.2) | 5 (11.4) | 2 (1.9) | 1 (1.8) |
| Infection, n (%) | 2 (2.2) | 2 (4.5) | 2 (1.9) | 2 (3.6) |
| Bleeding, n (%) | 1 (1.1) | 0 (0.0) | 3 (2.8) | 0 (0.0) |
| Composite endpoint, n (%) | 12 (12.9) | 10 (22.7) | 39 (35.8) | 31 (55.4) |
BNP, B‐type natriuretic peptide; WRF, worsening renal function.
Figure 1.
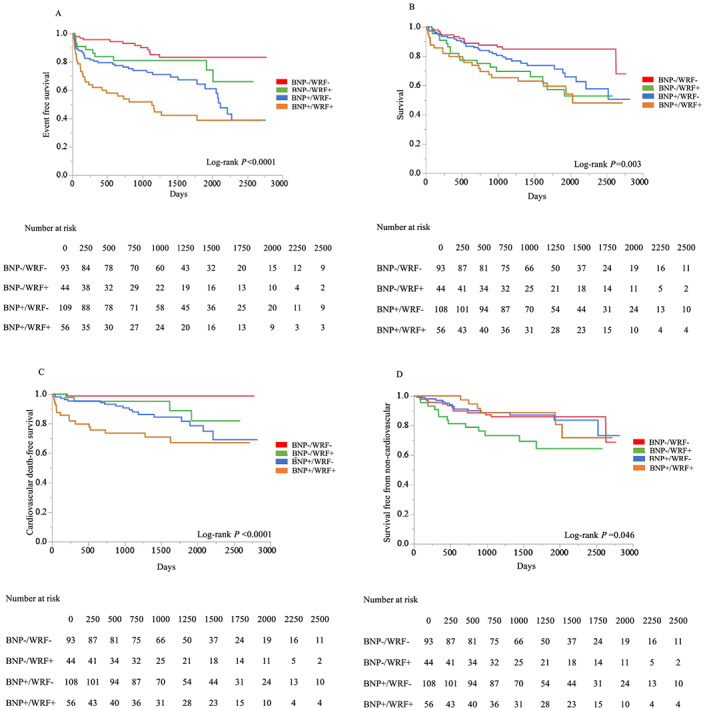
Kaplan–Meier analysis of freedom from the composite endpoint of cardiovascular mortality and heart failure hospitalization (A), overall survival (B), cardiovascular death free survival (C), and survival free from non‐cardiovascular death (D). BNP, B‐type natriuretic peptide; WRF, worsening renal function.
The results of univariate and multivariate Cox proportional hazards models of composite endpoint and all‐cause mortality are shown in Tables 4 and 5 . As shown in Table 4 , history of admission for HF, WRF alone, BNP ≥250 pg/mL alone, and the combination of BNP ≥250 pg/mL and WRF were associated with increased risk of the composite endpoint. The combination of BNP ≥250 pg/mL and WRF showed the highest HR in the multivariate Cox proportional hazards model (HR, 5.201; 95% CI, 2.582–11.11; P < 0.0001). In contrast, patients who received triple therapy (the combination of beta‐blockers, ARB/ACEIs, and aldosterone antagonists) had lower risk of the composite endpoint (HR, 0.342; 95% CI, 0.195–0.577; P < 0.0001).
Table 4.
Univariate and multivariate Cox proportional hazards model analysis of composite endpoint of cardiovascular mortality and heart failure hospitalization
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |||
| Age | 1.027 | 1.011 | 1.044 | 0.0006 | ||||
| Male | 1.053 | 0.693 | 1.624 | 0.81 | ||||
| Heart rate | 0.998 | 0.991 | 1.006 | 0.67 | ||||
| Systolic blood pressure | 0.994 | 0.988 | 1.001 | 0.09 | ||||
| Diastolic blood pressure | 0.987 | 0.977 | 0.997 | 0.006 | ||||
| NYHA functional class | 1.36 | 0.969 | 1.941 | 0.08 | ||||
| History of admission for heart failure | 3.996 | 2.611 | 6.049 | <0.0001 | 3.819 | 2.384 | 6.072 | <0.0001 |
| HF‐preserved EF | 1.101 | 0.659 | 1.764 | 0.7 | ||||
| Ischaemic heart disease | 1.806 | 1.181 | 2.729 | 0.007 | ||||
| Atrial fibrillation | 1.641 | 1.089 | 2.476 | 0.02 | ||||
| Hypertension | 1.219 | 0.784 | 1.953 | 0.39 | ||||
| Hyperlipidaemia | 1.172 | 0.777 | 1.766 | 0.45 | ||||
| Diabetes mellitus | 1.365 | 0.888 | 2.068 | 0.15 | ||||
| CKD | 2.406 | 1.577 | 3.74 | <0.0001 | ||||
| LV diastolic diameter | 0.989 | 0.966 | 1.013 | 0.37 | ||||
| LV systolic diameter | 0.989 | 0.97 | 1.008 | 0.25 | ||||
| LVEF | 1.008 | 0.995 | 1.021 | 0.24 | ||||
| BNP−/WRF+ (vs. BNP−/WRF−) | 1.885 | 0.795 | 4.373 | 0.15 | 2.555 | 1.048 | 6.127 | 0.04 |
| BNP+/WRF− (vs. BNP−/WRF−) | 3.043 | 1.643 | 6.071 | 0.0003 | 3.641 | 1.887 | 7.549 | <0.0001 |
| BNP+/WRF+ (vs. BNP−/WRF−) | 5.617 | 2.958 | 11.39 | <0.0001 | 5.201 | 2.582 | 11.11 | <0.0001 |
| Laboratory data on admission | ||||||||
| Albumin | 0.745 | 0.498 | 1.123 | 0.16 | ||||
| Blood urea nitrogen | 1.019 | 1.009 | 1.028 | 0.0005 | ||||
| Creatinine | 1.524 | 1.203 | 1.863 | 0.001 | ||||
| Sodium | 0.955 | 0.914 | 1.005 | 0.07 | ||||
| Chloride | 0.967 | 0.926 | 1.034 | 0.16 | ||||
| Potassium | 1.204 | 0.839 | 1.671 | 0.3 | ||||
| Haemoglobin | 0.851 | 0.785 | 0.924 | <0.0001 | ||||
| Haematocrit | 0.946 | 0.919 | 0.974 | 0.0002 | ||||
| BNP | 0.999 | 0.999 | 1 | 0.87 | ||||
| Laboratory data at discharge | ||||||||
| Albumin | 0.493 | 0.315 | 0.779 | 0.003 | ||||
| Blood urea nitrogen | 1.035 | 1.02 | 1.049 | <0.0001 | ||||
| Creatinine | 1.651 | 1.3 | 2.022 | 0.0002 | ||||
| Sodium | 0.996 | 0.941 | 1.059 | 0.9 | ||||
| Chloride | 0.976 | 0.93 | 1.028 | 0.35 | ||||
| Potassium | 0.755 | 0.479 | 1.2 | 0.23 | ||||
| Haemoglobin | 0.81 | 0.736 | 0.888 | <0.0001 | ||||
| Haematocrit | 0.924 | 0.893 | 0.956 | <0.0001 | ||||
| BNP | 1.001 | 1 | 1.001 | 0.004 | ||||
| In‐hospital treatment | ||||||||
| Inotropes | 1.523 | 0.842 | 2.571 | 0.16 | ||||
| Intravenous furosemide | 1.067 | 0.69 | 1.692 | 0.78 | ||||
| Dose of intravenous furosemide (mg/day) | 1.008 | 0.989 | 1.025 | 0.39 | ||||
| Vasodilator | 0.925 | 0.603 | 1.45 | 0.73 | ||||
| Medication at discharge | ||||||||
| Beta‐blockers | 1.01 | 0.643 | 1.641 | 0.97 | ||||
| ACEI/ARBs | 0.33 | 0.218 | 0.497 | <0.0001 | ||||
| Loop diuretics | 1.349 | 0.826 | 2.329 | 0.24 | ||||
| Aldosterone antagonists | 2.436 | 1.616 | 3.689 | <0.0001 | ||||
| Triple therapy | 0.342 | 0.205 | 0.547 | <0.0001 | 0.342 | 0.195 | 0.577 | <0.0001 |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin 2 receptor blocker; BNP, B‐type natriuretic peptide; CI, confidence interval; CKD, chronic kidney disease; EF, ejection fraction; HF, heart failure; LV, left ventricular; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; WRF, worsening renal function.
Multivariate Cox proportional hazards model included age, sex, NYHA functional class, history of admission for heart failure, ischaemic heart disease, CKD, LVEF, triple therapy, groups of BNP and WRF, and creatinine at discharge.
Triple therapy was defined as the combination usage of beta‐blockers, ACEI/ARBs, and aldosterone antagonists.
Table 5.
Univariate and multivariate Cox proportional hazards model analysis of all‐cause mortality
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |||
| Age | 1.083 | 1.059 | 1.108 | <0.0001 | 1.086 | 1.057 | 1.119 | <0.0001 |
| Male | 0.805 | 0.524 | 1.248 | 0.33 | ||||
| Heart rate | 0.99 | 0.981 | 0.998 | 0.01 | ||||
| Systolic blood pressure | 0.997 | 0.99 | 1.003 | 0.34 | ||||
| Diastolic blood pressure | 0.989 | 0.979 | 0.999 | 0.03 | ||||
| NYHA functional class | 1.47 | 1.027 | 2.146 | 0.04 | ||||
| History of admission for heart failure | 2.572 | 1.612 | 4.013 | 0.0001 | 3.262 | 1.911 | 5.500 | <0.0001 |
| HF‐preserved EF | 1.614 | 0.98 | 2.58 | 0.06 | ||||
| Ischaemic heart disease | 0.862 | 0.519 | 1.378 | 0.55 | ||||
| Atrial fibrillation | 1.359 | 0.881 | 2.089 | 0.16 | ||||
| Hypertension | 1.137 | 0.718 | 1.861 | 0.59 | ||||
| Hyperlipidaemia | 1.343 | 0.875 | 2.068 | 0.18 | ||||
| Diabetes mellitus | 0.929 | 0.579 | 1.454 | 0.75 | ||||
| CKD | 1.812 | 1.175 | 2.834 | 0.007 | ||||
| LV diastolic diameter | 0.962 | 0.938 | 0.987 | 0.003 | ||||
| LV systolic diameter | 0.969 | 0.949 | 0.989 | 0.002 | ||||
| LVEF | 1.022 | 1.008 | 1.037 | 0.002 | ||||
| BNP−/WRF+ (vs. BNP−/WRF−) | 2.78 | 1.369 | 5.738 | 0.005 | 2.286 | 1.089 | 4.875 | 0.03 |
| BNP+/WRF− (vs. BNP−/WRF−) | 1.928 | 1.046 | 3.738 | 0.04 | 1.221 | 0.639 | 2.438 | 0.55 |
| BNP+/WRF+ (vs. BNP− /WRF−) | 3.137 | 1.621 | 6.279 | 0.0007 | 1.829 | 0.862 | 3.947 | 0.12 |
| Laboratory data on admission | ||||||||
| Albumin | 0.581 | 0.382 | 0.889 | 0.01 | ||||
| Blood urea nitrogen | 1.015 | 1.003 | 1.025 | 0.02 | ||||
| Creatinine | 1.263 | 0.96 | 1.583 | 0.09 | ||||
| Sodium | 1 | 0.948 | 1.061 | 0.99 | ||||
| Chloride | 0.965 | 0.921 | 1.013 | 0.15 | ||||
| Potassium | 0.95 | 0.636 | 1.383 | 0.8 | ||||
| Haemoglobin | 0.826 | 0.757 | 0.9 | <0.0001 | ||||
| Haematocrit | 0.936 | 0.907 | 0.965 | <0.0001 | ||||
| BNP | 1 | 0.999 | 1 | 0.81 | ||||
| Laboratory data at discharge | ||||||||
| Albumin | 0.395 | 0.248 | 0.634 | 0.0001 | ||||
| Blood urea nitrogen | 1.036 | 1.02 | 1.05 | <0.0001 | ||||
| Creatinine | 1.238 | 0.95 | 1.524 | 0.11 | ||||
| Sodium | 0.97 | 0.917 | 1.031 | 0.32 | ||||
| Chloride | 0.953 | 0.908 | 1.004 | 0.07 | ||||
| Potassium | 0.632 | 0.397 | 1.019 | 0.06 | ||||
| Haemoglobin | 0.747 | 0.672 | 0.826 | <0.0001 | ||||
| Haematocrit | 0.897 | 0.863 | 0.93 | <0.0001 | ||||
| BNP | 1 | 0.999 | 1.001 | 0.07 | ||||
| In‐hospital treatment | ||||||||
| Inotropes | 1.363 | 0.703 | 2.417 | 0.34 | ||||
| Intravenous furosemide | 1.375 | 0.851 | 2.314 | 0.2 | ||||
| Dose of intravenous furosemide (mg/day) | 0.989 | 0.963 | 1.011 | 0.34 | ||||
| Vasodilator | 0.96 | 0.61 | 1.551 | 0.86 | ||||
| Medication at discharge | ||||||||
| Beta‐blockers | 0.427 | 0.277 | 0.662 | 0.0002 | ||||
| ACEI/ARBs | 0.47 | 0.306 | 0.727 | 0.0008 | ||||
| Loop diuretics | 1.175 | 0.714 | 2.038 | 0.54 | ||||
| Aldosterone antagonists | 1.923 | 1.249 | 2.962 | 0.003 | ||||
| Triple therapy | 0.378 | 0.223 | 0.614 | <0.0001 | 0.463 | 0.256 | 0.800 | 0.005 |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin 2 receptor blocker; BNP, B‐type natriuretic peptide; CI, confidence interval; CKD, chronic kidney disease; EF, ejection fraction; HF, heart failure; LV, left ventricular; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; WRF, worsening renal function.
Multivariate Cox proportional hazards model included age, sex, NYHA functional class, history of admission for heart failure, ischaemic heart disease, CKD, LVEF, triple therapy, groups of BNP and WRF, and creatinine at discharge.
Triple therapy was defined as the combination usage of beta‐blockers, ACEI/ARBs, and aldosterone antagonists.
Age, history of admission for HF, and WRF alone were associated with increased all‐cause mortality in the multivariate Cox proportional hazards model (HR, 1.086; 95% CI, 1.057–1.119; P < 0.0001; HR, 3.262; 95% CI, 1.911–5.500; P < 0.0001; HR, 2.286; 95% CI, 1.089–4.875; P = 0.03, respectively) (Table 5 ). The triple therapy was associated with lower all‐cause mortality.
Patients in BNP−/WRF+ had higher HRs for non‐cardiovascular mortality than those in BNP+/WRF− and in BNP+/WRF+ in Cox proportional hazards models adjusted for age and sex (HR, 2.974; 95% CI, 1.364–6.449; P = 0.007; HR, 4.013; 95% CI, 1.522–11.88; P = 0.005, respectively).
Discussion
Our findings were as follows: (i) patients with acute de novo/decompensated HF with a combination of BNP ≥250 pg/mL and in‐hospital WRF (BNP+/WRF+) had the highest cardiovascular mortality and HF hospitalization in the four groups, (ii) in‐hospital WRF alone (BNP−/WRF+) or BNP ≥250 pg/mL alone (BNP+/WRF−) was associated with increased cardiovascular mortality and HF hospitalization in the multivariate Cox proportional hazards model, and (iii) in‐hospital WRF alone was associated with increased all‐cause mortality in Cox proportional hazards model. In addition, in‐hospital WRF alone was associated with increased non‐cardiovascular mortality.
In our multivariate analysis, the combination of BNP ≥250 pg/mL and in‐hospital WRF, and history of admission for HF were the predictors of the composite endpoint. It has been reported that the combination of persistent congestion evaluated by clinical signs and WRF was associated with increased short‐/long‐term cardiovascular mortality. 7 , 17 Our long‐term results are consistent with previous studies. In the present study, some differences in patient characteristics between the four groups were observed. LVEF was lower and LV diastolic diameter was larger in patients in BNP+/WRF− and in BNP+/WRF+ than in those of other groups. Therefore, elevated BNP at discharge might be linked with severe LV dysfunction. Although inotropes were not associated with increased risk of the composite endpoint of cardiovascular mortality and HF hospitalization in the univariate analysis, the frequency of usage of inotropes was numerically higher in patients with the combination of BNP ≥250 pg/mL and WRF (Table 1 ). The frequency of intravenous diuretics administration also tended to be higher in patients with the combination of BNP ≥250 pg/mL and in‐hospital WRF. Therefore, patients with the combination of BNP ≥250 pg/mL and in‐hospital WRF more frequently received intensive pharmacological intervention.
Some studies showed that BNP at discharge was associated with outcome in patients with HF. 14 , 18 Our findings also suggested that patients in BNP+/WRF− and in BNP+/WRF+ had increased risk of composite endpoint of cardiovascular morality and HF hospitalization. Congestion causes HF signs and symptoms and has an important role in multi‐organ dysfunction in patients with HF. 19 In previous studies of congestion in patients with HF and WRF, congestion was assessed by clinical signs and symptoms at discharge and clinical importance of these findings were evaluated. 7 , 17 However, in our study, most of patients had no clinical signs of congestion at discharge. Although clinical signs are important in daily practice to assess patients with HF, they have limitations in terms of the accuracy. 20 Moreover, clinically unrecognized congestion is frequently present in patients without clinical signs and symptoms of volume overload. This subclinical congestion is associated with increased cardiac filling pressures and worse patient outcomes. 21 Because BNP release is triggered by increased volume load and myocardial wall stretch, 22 BNP has been established as a surrogate marker of congestion. Recently, some studies suggested that reduction of BNP or N‐terminal‐proBNP was one of the markers of decongestion in HF. 12 , 23 BNP at discharge is also one of the predictors of prognosis in patients with HF. 14 In addition, Hamatani et al. suggested that the value of BNP at discharge was a more reliable predictor of long‐term mortality than change of BNP (the ratio of discharge to admission BNP). 24 In the present study, the receiver operating characteristic curve analysis revealed a cut‐off value of BNP 254 pg/mL for composite endpoint.
In the present study, cardiovascular mortality was 12.6% and all‐cause mortality was 27.9% during a median follow‐up period of 1206 days. According to sub‐analysis of acute decompensated HF syndromes (ATTEND) registry, all‐cause mortality was 24.4% and non‐cardiovascular mortality was 7.6% during median 513 day follow‐up period. 25 We observed that non‐cardiac deaths accounted for 54.8% of the total deaths. This is clearly higher than the previous study. It could be due to differences in the observation period.
Some studies demonstrated that renal insufficiency was an independent predictor of non‐cardiovascular mortality in elderly individuals and in hospitalized patients with HF. 25 , 26 According to the previous study, infections and malignancies were the main causes of non‐cardiovascular death in patients with renal impairment. 26 Wang et al. suggested that renal dysfunction was correlated with infection‐related mortality. 27 A large cohort study showed that renal dysfunction was associated with increased cancer‐related mortality. 28 In patients with critical illness, even transient WRF impaired the renal functional reserve and was associated with poor prognosis. 29 We observed that patients with WRF alone had a higher HR for non‐cardiovascular mortality in Cox proportional hazards models adjusted for age and sex. Although the present study did not include complete data on co‐morbidities, we observed that cancer‐related mortality was higher in BNP−/WRF+ (11.4%) than in other groups (3.2%, 1.9%, and 1.8% in BNP−/WRF−, in BNP+/WRF−, and in BNP+/WRF+, respectively). Patients in BNP−/WRF+ had a higher LVEF and a smaller LV diastolic diameter, which suggested that patients in BNP−/WRF+ might overlap with HF patients with preserved ejection fraction (EF). Dunlay et al. demonstrated that the contribution of non‐cardiac death to all‐cause mortality was greater in HF patients with preserved EF than with reduced EF. 30 Taken together, impairment of renal function reserve expressed by WRF may have an important role in not only cardiovascular mortality or HF readmission but also non‐cardiovascular mortality in patients with HF. Further prospective research to evaluate the association between biomarkers of congestion such as BNP, WRF, and cardiovascular/non‐cardiovascular mortality in patients with HF is needed.
Limitations
This study was a single‐centred retrospective study. Therefore, potential selection biases could not be completely ruled out. We did not obtain the data of changes in prescriptions in outpatient clinics after discharge. In addition, we were unable to obtain the data of undiagnosed co‐morbidities. Therefore, unknown confounders might have influenced the results of analyses even in the multivariate Cox hazards models. Patients with secondary HF might be included because of our inclusion criteria that lack echocardiographic parameters. We added BNP on the Framingham criteria to reduce inclusion of non‐HF patients such as previous reports. 31 , 32 The definition of WRF (a relative increase in serum creatinine of at least 25% or an absolute increase in serum creatinine ≥0.3 mg/dL) might have overestimated the kidney dysfunction compared with the other criteria. Previous reports suggested that timing of in‐hospital WRF, haemoconcentration during decongestive therapy, and blood urea nitrogen increase were associated with prognosis in patients with HF. In the present study, phenotypes of WRF (i.e. transient or permanent) were not well defined because we were not able to assess these factors. 33 , 34 Cardio‐renal syndrome may have affected the clinical course of the patients with CKD; however, we were not able to evaluate the effects of the syndrome. In‐hospital treatment of patients with HF was determined by each treating physician. These findings may not be generally applicable to clinical settings in other regions.
Conclusions
Heart failure patients with in‐hospital occurrence of WRF and BNP ≥250 pg/mL at discharge had the highest risk for the composite endpoint (cardiovascular mortality and HF hospitalization) among groups.
Conflict of interest
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Funding
None.
Okabe, T. , Yakushiji, T. , Kido, T. , Kimura, T. , Asukai, Y. , Shimazu, S. , Saito, J. , Oyama, Y. , Igawa, W. , Ono, M. , Ebara, S. , Yamashita, K. , Yamamoto, M. H. , Amemiya, K. , Isomura, N. , and Ochiai, M. (2020) Poor prognosis of heart failure patients with in‐hospital worsening renal function and elevated BNP at discharge. ESC Heart Failure, 7: 2912–2921. 10.1002/ehf2.12901.
References
- 1. Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta‐analysis. Eur Heart J 2014; 35: 455–469. [DOI] [PubMed] [Google Scholar]
- 2. Rushton CA, Satchithananda DK, Jones PW, Kadam UT. Non‐cardiovascular comorbidity, severity and prognosis in non‐selected heart failure populations: a systematic review and meta‐analysis. Int J Cardiol 2015; 196: 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beldhuis IE, Streng KW, Ter Maaten JM, Voors AA, van der Meer P, Rossignol P, McMurray JJ, Damman K. Renin‐angiotensin system inhibition, worsening renal function, and outcome in heart failure patients with reduced and preserved ejection fraction: a meta‐analysis of published study data. Circ Heart Fail. 2017; 10 :pii: e003588. [DOI] [PubMed] [Google Scholar]
- 4. Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 2010; 122: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okabe T, Yakushiji T, Kido T, Oyama Y, Igawa W, Ono M, Ebara S, Yamashita K, Yamamoto MH, Saito S, Amemiya K, Isomura N, Araki H, Ochiai M. Relationship between worsening renal function and long‐term cardiovascular mortality in heart failure patients. Int J Cardiol 2017; 230: 47–52. [DOI] [PubMed] [Google Scholar]
- 6. Breidthardt T, Weidmann ZM, Twerenbold R, Gantenbein C, Stallone F, Rentsch K, Rubini Gimenez M, Kozhuharov N, Sabti Z, Breitenbücher D, Wildi K, Puelacher C, Honegger U, Wagener M, Schumacher C, Hillinger P, Osswald S, Mueller C. Impact of haemoconcentration during acute heart failure therapy on mortality and its relationship with worsening renal function. Eur J Heart Fail 2017; 19: 226–236. [DOI] [PubMed] [Google Scholar]
- 7. Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, Piovanelli B, Carubelli V, Bugatti S, Lombardi C, Cotter G, Dei Cas L. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail 2012; 5: 54–62. [DOI] [PubMed] [Google Scholar]
- 8. Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, Danesi R, Fontanella B, Lombardi C, Milani P, Verzura G, Cotter G, Dittrich H, Massie BM, Dei CL. Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail 2008; 10: 188–195. [DOI] [PubMed] [Google Scholar]
- 9. Lawson CA, Testani JM, Mamas M, Damman K, Jones PW, Teece L, Kadam UT. Chronic kidney disease, worsening renal function and outcomes in a heart failure community setting: a UK national study. Int J Cardiol 2018; 267: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G, European Society of Cardiology , European Society of Intensive Care Medicine . Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail 2010; 12: 423–433. [DOI] [PubMed] [Google Scholar]
- 11. Nishii M, Inomata T, Takehana H, Naruke T, Yanagisawa T, Moriguchi M, Takeda S, Izumi T. Prognostic utility of B‐type natriuretic peptide assessment in stable low‐risk outpatients with nonischemic cardiomyopathy after decompensated heart failure. J Am Coll Cardiol 2008; 51: 2329–2335. [DOI] [PubMed] [Google Scholar]
- 12. Kagiyama N, Kitai T, Hayashida A, Yamaguchi T, Okumura T, Kida K, Mizuno A, Oishi S, Inuzuka Y, Akiyama E, Suzuki S, Yamamoto M, Shimizu A, Urakami Y, Toki M, Aritaka S, Matsumoto K, Nagano N, Yamamoto K, Matsue Y. Prognostic value of BNP reduction during hospitalization in patients with acute heart failure. J Card Fail 2019; 25: 712–721. [DOI] [PubMed] [Google Scholar]
- 13. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 14. McQuade CN, Mizus M, Wald JW, Goldberg L, Jessup M, Umscheid CA. Brain‐type natriuretic peptide and amino‐terminal pro‐brain‐type natriuretic peptide discharge thresholds for acute decompensated heart failure: a systematic review. Ann Intern Med 2017; 166: 180–190. [DOI] [PubMed] [Google Scholar]
- 15. Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Modification of the CKD epidemiology collaboration (CKD‐EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis 2010; 56: 32–38. [DOI] [PubMed] [Google Scholar]
- 16. Okabe T, Yakushiji T, Kido T, Oyama Y, Igawa W, Ono M, Ebara S, Yamashita K, Yamamoto MH, Saito S, Amemiya K, Isomura N, Ochiai M. The association between high‐dose loop diuretic use at discharge and cardiovascular mortality in patients with heart failure. ESC Heart Fail 2018; 5: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wattad M, Darawsha W, Solomonica A, Hijazi M, Kaplan M, Makhoul BF, Abassi ZA, Azzam ZS, Aronson D. Interaction between worsening renal function and persistent congestion in acute decompensated heart failure. Am J Cardiol 2015; 115: 932–937. [DOI] [PubMed] [Google Scholar]
- 18. Logeart D, Thabut G, Jourdain P, Chavelas C, Beyne P, Beauvais F, Bouvier E, Solal AC. Predischarge B‐type natriuretic peptide assay for identifying patients at high risk of re‐admission after decompensated heart failure. J Am Coll Cardiol 2004; 43: 635–641. [DOI] [PubMed] [Google Scholar]
- 19. Biegus J, Zymliński R, Sokolski M, Siwołowski P, Gajewski P, Nawrocka‐Millward S, Poniewierka E, Jankowska EA, Banasiak W, Ponikowski P. Impaired hepato‐renal function defined by the MELD XI score as prognosticator in acute heart failure. Eur J Heart Fail 2016; 18: 1518–1521. [DOI] [PubMed] [Google Scholar]
- 20. Madhok V, Falk G, Rogers A, Struthers AD, Sullivan FM, Fahey T. The accuracy of symptoms, signs and diagnostic tests in the diagnosis of left ventricular dysfunction in primary care: a diagnostic accuracy systematic review. BMC Fam Pract 2008; 9: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Androne AS, Hryniewicz K, Hudaihed A, Mancini D, Lamanca J, Katz SD. Relation of unrecognized hypervolemia in chronic heart failure to clinical status, hemodynamics, and patient outcomes. Am J Cardiol 2004; 93: 1254–1259. [DOI] [PubMed] [Google Scholar]
- 22. Iwanaga Y, Nishi I, Furuichi S, Noguchi T, Sase K, Kihara Y, Goto Y, Nonogi H. B‐type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol 2006; 47: 742–748. [DOI] [PubMed] [Google Scholar]
- 23. Stienen S, Salah K, Moons AH, Bakx AL, van Pol P, Kortz RAM, Ferreira JP, Marques I, Schroeder‐Tanka JM, Keijer JT, Bayés‐Genis A, Tijssen JGP, Pinto YM, Kok WE. NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide)‐guided therapy in acute decompensated heart failure: PRIMA II randomized controlled trial (can NT‐proBNP‐guided therapy during hospital admission for acute decompensated heart failure reduce mortality and readmissions?). Circulation 2018; 137: 1671–1683. [DOI] [PubMed] [Google Scholar]
- 24. Hamatani Y, Nagai T, Shiraishi Y, Kohsaka S, Nakai M, Nishimura K, Kohno T, Nagatomo Y, Asaumi Y, Goda A, Mizuno A, Yasuda S, Ogawa H, Yoshikawa T, Anzai T, Investigators for the WET‐NaDEF Collaboration Project . Long‐term prognostic significance of plasma B‐type natriuretic peptide level in patients with acute heart failure with reduced, mid‐range, and preserved ejection fractions. Am J Cardiol 2018; 121: 731–738. [DOI] [PubMed] [Google Scholar]
- 25. Wakabayashi K, Ikeda N, Kajimoto K, Minami Y, Keida T, Asai K, Munakata R, Murai K, Sakata Y, Suzuki H, Takano T, Sato N, ATTEND Investigators . Trends and predictors of non‐cardiovascular death in patients hospitalized for acute heart failure. Int J Cardiol 2018; 250: 164–170. [DOI] [PubMed] [Google Scholar]
- 26. Fried LF, Katz R, Sarnak MJ, Shlipak MG, Chaves PH, Jenny NS, Stehman‐Breen C, Gillen D, Bleyer AJ, Hirsch C, Siscovick D, Newman AB. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol 2005; 16: 3728–3335. [DOI] [PubMed] [Google Scholar]
- 27. Wang HE, Gamboa C, Warnock DG, Muntner P. Chronic kidney disease and risk of death from infection. Am J Nephrol 2011; 34: 330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weng PH, Hung KY, Huang HL, Chen JH, Sung PK, Huang KC. Cancer‐specific mortality in chronic kidney disease: longitudinal follow‐up of a large cohort. Clin J Am Soc Nephrol 2011; 6: 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ronco C, Rosner MH. Acute kidney injury and residual renal function. Crit Care 2012; 16: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017; 14: 591–602. [DOI] [PubMed] [Google Scholar]
- 31. Sato N, Kajimoto K, Keida T, Mizuno M, Minami Y, Yumino D, Asai K, Murai K, Muanakata R, Aokage T, Sakata Y, Mizuno K, Takano T, ATTEND Investigators . Clinical features and outcome in hospitalized heart failure in Japan (from the ATTEND Registry). Circ J 2013; 77: 944–951. [DOI] [PubMed] [Google Scholar]
- 32. Matsue Y, Damman K, Voors AA, Kagiyama N, Yamaguchi T, Kuroda S, Okumura T, Kida K, Mizuno A, Oishi S, Inuzuka Y, Akiyama E, Matsukawa R, Kato K, Suzuki S, Naruke T, Yoshioka K, Miyoshi T, Baba Y, Yamamoto M, Murai K, Mizutani K, Yoshida K, Kitai T. Time‐to‐furosemide treatment and mortality in patients hospitalized with acute heart failure. J Am Coll Cardiol 2017; 69: 3042–3051. [DOI] [PubMed] [Google Scholar]
- 33. Krishnamoorthy A, Greiner MA, Sharma PP, DeVore AD, Johnson KW, Fonarow GC, Curtis LH, Hernandez AF. Transient and persistent worsening renal function during hospitalization for acute heart failure. Am Heart J 2014; 168: 891–900. [DOI] [PubMed] [Google Scholar]
- 34. Ruocco G, Palazzuoli A, Ter Maaten JM. The role of the kidney in acute and chronic heart failure. Heart Fail Rev 2020; 25: 107–118. [DOI] [PubMed] [Google Scholar]


