Abstract
Aims
The diagnostic and treatment of patients with heart failure with preserved ejection fraction (HFpEF) are both hampered by an incomplete understanding of the pathophysiology of the disease. Novel imaging tools to adequately identify these patients from individuals with a normal cardiac function and respectively patients with HF with reduced EF are warranted. Computing multilayer myocardial strain with feature tracking is a fast and accurate method to assess cardiac deformation. Our purpose was to assess the HFpEF diagnostic ability of multilayer strain parameters and compare their sensitivity and specificity with other established parameters.
Methods and results
We included 20 patients with a diagnosis of HFpEF and, respectively, 20 matched controls. We assessed using feature‐tracking cardiac magnetic resonance longitudinal and circumferential myocardial strain at three distinct layers of the myocardium: subendocardial (Endo‐), mid‐myocardial (Myo‐), and subepicardial (Epi‐). Comparatively, we additionally assessed various others clinical, imaging, and biochemical parameters with a putative role in HFpEF diagnostic: left ventricular end‐diastolic volume (LVEDV), left ventricular mass (LVM), interventricular septum (IVS) wall thickness and free wall thickness, left atrial volume and strain, septal and lateral mitral annular early diastolic velocity (e`), E/e´ ratio, and plasma levels of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP). Global longitudinal strain (GLS) is significantly impaired at Endo (−20.8 ± 4.0 vs. −23.2 ± 3.4, P = 0.046), Myo‐ (−18.0 ± 3.0 vs. −21.0 ± 2.5, P = 0.002), and Epi‐ (−12.2 ± 2.0 vs. −16.2 ± 2.5, P < 0.001) levels. Compared with any other imaging parameter, an Epi‐GLS lower than 13% shows the highest ability to detect patients with HFpEF [area under the curve (AUC) = 0.90 (0.81–1), P < 0.001] and in tandem with NT‐proBNP can diagnose with maximal sensibility (93%) and specificity (100%), patients with HFpEF from normal, composed variable [AUC = 0.98 (0.95–1), P < 0.001]. In a logistic regression model, a composite predictive variable taking into account both GLS Epi and NT‐proBNP values in each individual subject reached a sensitivity of 89% and a specificity of 100% with an AUC of 0.98 (0.95–1), P < 0.001, to detect HFpEF.
Conclusions
Epi‐GLS is a promising new imaging parameter to be considered in the clinical assessment of HFpEF patients. Given its excellent specificity, in tandem with a highly sensitive parameter such as NT‐proBNP, Epi‐GLS holds the potential to greatly improve the current diagnostic algorithms.
Keywords: Multilayer myocardial strain, Heart failure with preserved ejection fraction, NT‐proBNP, Cardiac magnetic resonance, Feature tracking
Background
Approximately half of the patients diagnosed with heart failure (HF) maintain a normal ejection fraction (HFpEF) despite increased left ventricular (LV) filling pressure and lusitropic stiffness. 1 In contrast with HF with reduced EF (HFrEF), in HFpEF, elevated inflammation levels determining microvascular disease, reconfiguration of structural proteins such as titin and interstitial collagen deposition, are the main pathophysiological effectors. 2 Such heterogeneity in pathophysiology is putatively responsible for the difficulty of finding a unifying and accessible imaging marker of disease severity. EF is ipso facto within the normal range, and global longitudinal strain (GLS), a powerful and robust prognostic factor in HFrEF, 3 is only inconstantly decreased in HFpEF patients. 4 Echocardiography‐derived indexes of severity of diastolic dysfunction such as E/e` correlate only moderately with invasively measured LV filling pressure, 5 and, even so, up to one‐third of patients diagnosed with HFpEF have a normal diastolic function. 4
Aims
We hypothesized that benefiting from a superior spatial resolution and complete 3D coverage of the myocardial volume provided by the cine sequences, cardiac magnetic resonance (CMR) feature tracking (FT) can assess longitudinal and circumferential myocardial strain at multiple layer level and potentially augment the accuracy to detect deformation abnormalities in patients with HFpEF.
Methods
To establish this, we included 20 patients with a diagnosis of HFpEF and, respectively, 20 age‐matched and gender‐matched controls. Diagnosis of HF should have been older than 30 days, the patients were required to be in a stable state with no changes in their HF medication and no HF hospitalization within the previous 7 days, HFpEF was defined in agreement with recent ESC guidelines, 6 as presence of signs and symptoms of HF and LVEF ≥50% at the time of study inclusion plus the existence of one of the following echocardiographic criteria: left atrial volume index > 34 mL/m2, E/e´ > 13 (medial or lateral mitral anulus), LV hypertrophy: septal wall thickness or posterior wall thickness ≥ 13 mm. Exclusion criteria were as follows: atrial fibrillation, symptomatic significant coronary artery disease, co‐existence of any inherited cardiomyopathy or amyloidosis, myocarditis, pulmonary disease, and anaemia. Demographic and baseline characteristics of the study population are presented in Table 1 . All subjects underwent clinical, comprehensive CMR and echocardiographic examinations and a 6 min walking test. All CMR images were acquired using a 1.5 T (Achieva, Philips Healthcare, Best, The Netherlands) MRI scanner with a five‐channel cardiac surface coil in a supine position. Cine images were acquired using electrocardiogram‐gated bSSFP sequence with multiple breath holds at end‐expiration in three LV long‐axis [two‐chamber (2Ch), three‐chamber (3Ch), and four‐chamber (4Ch)] planes. The ventricular two‐chamber and four‐chamber planes were used to plan a stack of short‐axis slices covering the entire LV. The following imaging parameters were used: repetition time (TR) = 3.3 ms, echo time (TE) = 1.6 ms, flip angle = 60°, voxel size = 1.8 × 1.7 × 8.0 mm3 and 50 phases per cardiac cycle in accordance with standards of procedure established in our unit and described previously. 7 Using commercially available software (Medis Suite, version 3.1 and QStrain RE version 2.0, Leiden, The Netherlands) we derived the values of myocardial strain at three distinct layers of the myocardium: subendocardial (Endo‐), mid‐myocardial (Myo‐), and subepicardial (Epi‐) for GLS and circumferential (GCS) strain as previously reported (Figure 1 A ). 8 To compare the diagnostic accuracy, we additionally assessed various others clinical, imaging, and biochemical parameters with a putative role in HFpEF diagnostic: LV end‐diastolic volume (LVEDV), LV mass (LVM), interventricular septum (IVS) wall thickness and free wall thickness, LA volume and strain, septal and lateral mitral annular early diastolic velocity (e`), E/e´ ratio, and plasma levels of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP). 9 All the statistical analyses were performed with IBM SPSS v26 with the exception of comparative receiver operating characteristic analysis, which was performed with Medcalc v19.1.5, using the DeLong formula.
TABLE 1.
Demographics, Baseline Characteristics
| Control (N = 20) | HFpEF (N = 20) | P value | |
|---|---|---|---|
| Demographics | |||
| Male | 12/20 (60%) | 12/20 (60%) | 0.75 |
| Age, y | 68.2 ± 8.1 | 73.3 ± 8.2 | 0.62 |
| LVEF, % | 62.5 ± 5.1 | 61.7 ± 7.2 | 0.84 |
| LVM index (g/m2) | 43.5 ± 13.7 | 47.2 ± 8.5 | 0.21 |
| LVEDV index (mL/m2) | 78 ± 12 | 70 ± 14 | 0.77 |
| IVS thickness (mm) | 9.2 ± 1.7 | 11.4 ± 2.0 | 0.03§ |
| LA area index (cm2/m2) | 20.4 ± 4.1 | 23.7 ± 5.1 | 0.046 |
| LA volume index (mL/m2) | 37.3 ± 7.8 | 44.1 ± 13.9 | 0.068 |
| LA strain (%) | 31.3 ± 6.3 | 21.7 ± 8.2 | <0.001§ |
| Any LGE | 0 (0%) | 9 (45%) | <0.001§ |
| Transmural LGE | 4 (20%) | ||
| Coronary artery disease | 0 (0%) | 11 (55%) | <0.001§ |
| Peripheric artery disease | 0 (0%) | 6 (30%) | 0.008§ |
| Hypertension | 7 (35%) | 14 (70%) | 0.027§ |
| Diabetes | 2 (10%) | 5 (25%) | 0.21 |
| Hypercholesterolaemia | 5 (25%) | 13 (65%) | 0.011§ |
| COPD | 0 (0%) | 1 (5%) | 0.31 |
| Smokers | 6 (30%) | 8 (40%) | 0.51 |
| 6 min walking test (m) | 523 ± 119 | 352 ± 124 | <0.001§ |
| NYHA Class II | 0 (0%) | 11 (55%) | <0.001§ |
| Class III | 0 (0%) | 9 (45%) | <0.001§ |
| Quality of Life Score | 5.2 ± 5.5 | 26.3 ± 21.8 | <0.001§ |
| Borg Score | 7.4 ± 1.7 | 12.2 ± 2.4 | <0.001§ |
| Laboratory values | |||
| Haemoglobin (g/dL) | 13.9 ± 1.1 | 12.8 ± 1.2 | 0.86 |
| Haematocrit | 0.40 ± 0.03 | 0.38 ± 0.03 | 0.94 |
| Creatinine (mg/dL) | 0.87 ± 0.20 | 0.92 ± 0.18 | 0.88 |
| GFR (mL/min) | 81 ± 10 | 71 ± 16 | 0.34 |
| NT‐proBNP (ng/L) | 89 ± 61 | 502 ± 497 | <0.001§ |
| Troponin T (ng/L) | 7 ± 3 | 16 ± 12 | <0.001§ |
| CRP (mg/dL) | 1.3 ± 1.4 | 2.9 ± 2.7 | 0.08 |
| WBC (/nL) | 6.1 ± 1.6 | 7.2 ± 2.4 | 0.19 |
| Medication | |||
| ACE inhibitors | 3 (12%) | 4 (20%) | 0.68 |
| Angiotensin receptor blocker | 5 (25%) | 11 (55%) | 0.05 |
| Calcium antagonist | 4 (20%) | 3 (12%) | 0.68 |
| Mineralocorticoid receptor antagonist | 0 (0%) | 2 (10%) | 0.15 |
| Angiotensin receptor‐neprilysin inhibitor | 0 (0%) | 0 (0%) | n/a |
| Beta‐blocker | 6 (30%) | 10 (50%) | 0.20 |
| Statin | 4 (20%) | 8 (40%) | 0.17 |
| Thiazide diuretic | 5 (25%) | 4 (20%) | 0.71 |
| Loop diuretic | 0 (0%) | 3 (15%) | 0.07 |
Abbreviations: ACE, angiotensin‐converting‐enzyme; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; GFR, glomerular filtration rate; IVS, interventricular septum; LA, left atrium; LGE, late gadolinium enhancement; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVM, left ventricular mass; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; WBC, white blood cell count; Quality of Life Score, Minnesota Living with Heart Failure Questionnaire. Discrete values given as absolute number and percentage of respective HF group. Continuous values given as mean and standard deviation.
FIGURE 1.
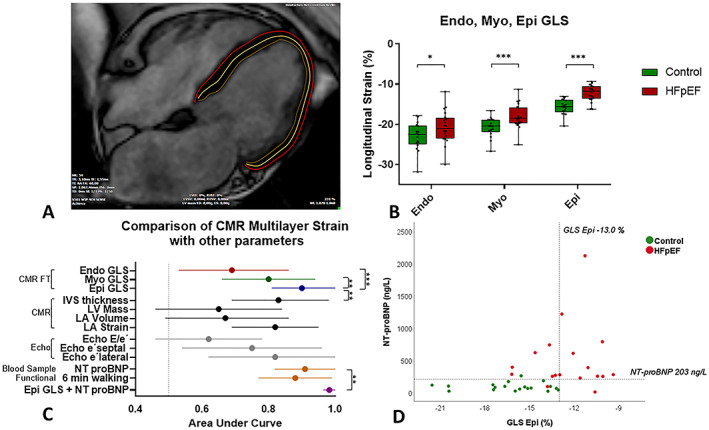
(A) CMR feature tracking multilayer segmentation principle exemplified in a long‐axis four‐chamber end‐diastolic view in a patient with HFpEF. (B) Sub‐endocardial (Endo‐), mid‐myocardial (Myo‐), and sub‐epicardial (Epi‐) global longitudinal strain (GLS) in control and HFpEF patients. (C) Forest Plot of area under curve values: comparison between Endo‐, Myo‐, and Epi‐GLS to detect patients with HFpEF and other parameters with a significant ability to discriminate between HFpEF and control. (D) Scatter Plot with Epi‐GLS and NT‐proBNP values in the two groups, dotted lines represent the cut‐off values (GLS Epi = −13%, NT‐proBNP = 220 ng/L) *P < 0.05, ***P < 0.001, for all the comparisons a P value <0.05 was considered statistically significant. Abbreviations: CMR, cardiac magnetic resonance; Endo, Myo, and Epi, multilayer myocardial strain; FT, feature tracking; HFpEF, heart failure with preserved ejection fraction; LA, left atrium; LVM, left ventricular mass; LVEDV, left ventricular end‐diastolic volume; e´ septal, lateral, peak early diastolic velocity at the septal and lateral mitral annular sites; E/e´, ratio between early trans‐mitral flow velocity and average peak early diastolic velocity; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide.
Results
Global longitudinal strain is significantly impaired at Endo‐ (−20.8 ± 4.0 vs. −23.2 ± 3.4, P = 0.046), Myo‐ (−18.0 ± 3.0 vs. −21.0 ± 2.5, P = 0.002), and Epi‐ (−12.2 ± 2.0 vs. −16.2 ± 2.5, P < 0.001) levels (Figure 1 B ). In contrast, in keeping with previously published meta‐analysis, 5 GCS was similar at any level of myocardium in HFpEF patients (Table S1 .). In a comparative receiver operating characteristic analysis, GLS Epi has the best ability to detect between HFpEF with an area under the curve (AUC) of 0.90 (0.81–1), P < 0.001, compared with 0.80 (0.66–0.94), P = 0.002, for GLS Myo and 0.69 (0.53–0.86), P = 0.046, for GLS Endo (Figure 1C). Additionally, this performance is higher than of any other parameter included in our analysis, excepting only NT‐proBNP that is borderline better with an AUC of 0.91(0.79–1), P < 0.001 (Figure 1 C ). 9 In particular, a threshold value of Epi‐GLS < −13.0% demonstrated an excellent diagnostic specificity (100%) for HFpEF. According to our data, a complementary threshold value for NT‐proBNP > 203 ng/mL was able to detect all but two HFpEF patients, showing very good sensitivity (Figure 1 D ). All numerical data are included in Supporting Information Table S1 . In a logistic regression model, a composite predictive variable taking into account both GLS Epi and NT‐proBNP values in each individual subject reached a sensitivity of 89% and a specificity of 100% with an AUC of 0.98 (0.95–1), P < 0.001, to detect HFpEF (Figures 1 C and S1 .)
Discussion
In line with previous studies, our findings confirmed a decrease of longitudinal strain in patients with HFpEF. 10 Additionally, we showed that, measured selectively at the level of the subepicardial layer, GLS has an increased potential to diagnose HFpEF and discriminate early phases of contractile impairment.
These results may be surprising. It has been previously proposed that a more pronounced vulnerability to ischaemia of subendocardial small‐calibre vasculature irrigating predominantly longitudinally distributed fibres found at this level is responsible for a significant decrease in long‐axis contraction. However, so far, there are no clear clinical evidences to substantiate this assumption and such a model is theoretically flawed. 11 In contrast, several possible explanations for the increased sensitivity of FT Epi‐GLS could be proposed:
Pericardium is a rigid membrane that contains the movements of the heart towards exterior, and thus, the confounding effect of shear strain is absent at this level. 12
Also due to pericardial containment, subepicardial deformation is in a tighter connection with a long axis descend of the mitral valve plane showed also to be an additional index of severity in HFpEF. 13
Left ventricular hypertrophy, observed in up to one‐half of patients with HFpEF, 14 leads to a decrease EDV and thus a false positive increase in strain assessment.
Feature tracking relies on adequate contour identification and tracking over the cardiac cycle phases, subepicardial longitudinal benefits from a high contrast difference between T1 values of myocardium, pericardium, and surrounding extracardiac space. 15
In contrast with GLS, and confirming previous studies, GCS is not different at any level of the myocardium between HFpEF and controls. 8 NT‐proBNP has been recently proposed by the most recent guidelines as a major diagnostic criteria for HFpEF. 9
Our findings confirmed that NT‐proBNP is generically a good discriminator of HFpEF from control subjects. However, NT‐proBNP exponentially increase in the restrictive phase of diastolic dysfunction; thus, it might be particularly inefficient in identifying HFpEF patients without or with an earlier stage of diastolic dysfunction. 9 Additionally, NT‐proBNP is not efficient to separate HFpEF from HFrEF. 16 In contrast, we showed previously an excellent specificity for Endo‐GCS, which is normal in HFpEF patients but significantly decreased in HFrEF, to separate HFpEF from HFrEF 8 with various degrees of severity. With this current study, we brought new evidence that Epi‐GLS is specifically decreased in HFpEF patients compared with control and thus, in tandem with Endo‐GCS, constitutes an excellent diagnostic tool to optimally identify patients with HFpEF from both healthy individuals and patients with HFrEF.
Heart failure with preserved ejection fraction is particularly difficult to treat; important therapeutic tools such as β blockers, angiotensin‐converting enzyme inhibitors or angiotensin‐receptor antagonists, and mineralocorticoid‐receptor antagonist, efficient in improving morbidity and mortality in HFrEF patients, all failed to show any benefits in HFpEF. 17 In these conditions, prompt diagnostic and progression monitoring are key factors to direct a salvaging adjuvant therapy such as decreasing an excessive preload or symptoms control. Our study suggests that recent advances in image processing such as multilayer CMR FT potentially increase the diagnostic accuracy in patients suspected of having HFpEF.
Conflicts of Interest
Sebastian Kelle is supported by a grant from Philips Healthcare and received lecture honoraria from Medis. Sebastian Kelle and Burkert Pieske received funding from the DZHK (German Centre for Cardiovascular Research) and by the BMBF (German Ministry of Education and Research). Burkert Pieske reports having received consultancy and lecture honoraria from Bayer Daiichi Sankyo, MSD, Novartis, sanofi‐aventis, Stealth Peptides, and Vifor Pharma and editor honoraria from the Journal of the American College of Cardiology. Radu Tanacli and the other co‐authors report no conflict of interest.
Supporting information
Table S1. ROC Analysis: Multilayer Myocardial Strain and other parameters to detect patients with HFpEF. GLS – global longitudinal strain, GCS – global circumferential strain, LVM left ventricular mass, LVEDV – left ventricular end‐diastolic volume, LA – left atrium, e´ septal, lateral – peak early diastolic velocity at the septal and lateral mitral annular sites, E/e´ – ration between early trans‐mitral flow velocity and average peak early diastolic velocity, NT‐proBNP ‐ N‐terminal prohormone of brain natriuretic peptide. § P < 0.05 (for all the comparisons a P value < 0.05 was considered statistically significant)
Figure S1. ROC Analysis to detect patients with heart failure with preserved ejection fraction (HFpEF) from control: GLS Epi – subepicardial global longitudinal strain, NT‐proBNP ‐ N‐terminal prohormone of brain natriuretic peptide, Combined – logistic regression composite predictor to detect HFpEF. AUCs were respectively: 0.90 (0.81–1), P < 0.001 for GLS Epi, 0.91(0.79–1), P < 0.001 for NT‐proBNP, 0.98 (0.95–1), P < 0.001 for Combined. For all the comparisons a P value < 0.05 was considered statistically significant.
Tanacli, R. , Hashemi, D. , Neye, M. , Motzkus, L. A. , Blum, M. , Tahirovic, E. , Dordevic, A. , Kraft, R. , Zamani, S. M. , Pieske, B. , Düngen, H.‐D. , and Kelle, S. (2020) Multilayer myocardial strain improves the diagnosis of heart failure with preserved ejection fraction. ESC Heart Failure, 7: 3240–3245. 10.1002/ehf2.12826.
German Clinical Trials Register (ID: DRKS00015615)
References
- 1. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med 2017; 376: 897. [DOI] [PubMed] [Google Scholar]
- 2. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 3. Sengelov M, Jorgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz‐Hansen T, Nochioka K, Biering‐Sorensen T. Global longitudinal strain is a superior predictor of all‐cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging 2015; 8: 1351–1359. [DOI] [PubMed] [Google Scholar]
- 4. Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation 2015; 132: 402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharifov OF, Schiros CG, Aban I, Denney TS, Gupta H. Diagnostic accuracy of tissue Doppler index E/e' for evaluating left ventricular filling pressure and diastolic dysfunction/heart failure with preserved ejection fraction: a systematic review and meta‐analysis. J Am Heart Assoc 2016; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Group ESCSD . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 7. Lapinskas T, Schnackenburg B, Kouwenhoven M, Gebker R, Berger A, Zaliunas R, Pieske B, Kelle S. Fatty metaplasia quantification and impact on regional myocardial function as assessed by advanced cardiac MR imaging. MAGMA 2018; 31: 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanacli R, Hashemi D, Lapinskas T, Edelmann F, Gebker R, Pedrizzetti G, Schuster A, Nagel E, Pieske B, Dungen HD, Kelle S. Range variability in CMR feature tracking multilayer strain across different stages of heart failure. Sci Rep 2019; 9: 16478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske‐Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA‐PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019; 40: 3297–3317. [DOI] [PubMed] [Google Scholar]
- 10. Morris DA, Ma XX, Belyavskiy E, Aravind Kumar R, Kropf M, Kraft R, Frydas A, Osmanoglou E, Marquez E, Donal E, Edelmann F, Tschope C, Pieske B, Pieske‐Kraigher E. Left ventricular longitudinal systolic function analysed by 2D speckle‐tracking echocardiography in heart failure with preserved ejection fraction: a meta‐analysis. Open Heart 2017; 4: e000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rademakers F, Nagel E. Is global longitudinal strain a superior parameter for predicting outcome after myocardial infarction? JACC Cardiovasc Imaging 2018; 11: 1458–1460. [DOI] [PubMed] [Google Scholar]
- 12. Lee JM, Boughner DR. Mechanical properties of human pericardium. Differences in viscoelastic response when compared with canine pericardium. Circ Res 1985; 57: 475–481. [DOI] [PubMed] [Google Scholar]
- 13. Wenzelburger FW, Tan YT, Choudhary FJ, Lee ES, Leyva F, Sanderson JE. Mitral annular plane systolic excursion on exercise: a simple diagnostic tool for heart failure with preserved ejection fraction. Eur J Heart Fail 2011; 13: 953–960. [DOI] [PubMed] [Google Scholar]
- 14. Shah AM, Shah SJ, Anand IS, Sweitzer NK, O'Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD, Investigators T. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail 2014; 7: 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schuster A, Hor KN, Kowallick JT, Beerbaum P, Kutty S. Cardiovascular magnetic resonance myocardial feature tracking: concepts and clinical applications. Circ Cardiovasc Imaging 2016; 9: e004077. [DOI] [PubMed] [Google Scholar]
- 16. Salah K, Stienen S, Pinto YM, Eurlings LW, Metra M, Bayes‐Genis A, Verdiani V, Tijssen JGP, Kok WE. Prognosis and NT‐proBNP in heart failure patients with preserved versus reduced ejection fraction. Heart 2019; 105: 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernandez‐Ruiz I. The search for an effective HFpEF treatment continues. Nat Rev Cardiol 2019; 16: 647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. ROC Analysis: Multilayer Myocardial Strain and other parameters to detect patients with HFpEF. GLS – global longitudinal strain, GCS – global circumferential strain, LVM left ventricular mass, LVEDV – left ventricular end‐diastolic volume, LA – left atrium, e´ septal, lateral – peak early diastolic velocity at the septal and lateral mitral annular sites, E/e´ – ration between early trans‐mitral flow velocity and average peak early diastolic velocity, NT‐proBNP ‐ N‐terminal prohormone of brain natriuretic peptide. § P < 0.05 (for all the comparisons a P value < 0.05 was considered statistically significant)
Figure S1. ROC Analysis to detect patients with heart failure with preserved ejection fraction (HFpEF) from control: GLS Epi – subepicardial global longitudinal strain, NT‐proBNP ‐ N‐terminal prohormone of brain natriuretic peptide, Combined – logistic regression composite predictor to detect HFpEF. AUCs were respectively: 0.90 (0.81–1), P < 0.001 for GLS Epi, 0.91(0.79–1), P < 0.001 for NT‐proBNP, 0.98 (0.95–1), P < 0.001 for Combined. For all the comparisons a P value < 0.05 was considered statistically significant.


