Abstract
Despite mounting evidence of the positive relationship between diabetes mellitus (DM) and heart failure (HF), the entire context of the magnitude of risk for HF in relation to DM remains insufficiently understood. The principal reason is because new‐onset HF (HF occurring in participants without a history of HF) and recurrent HF (HF re‐occurring in patients with a history of HF) are not discriminated. This meta‐analysis aims to comprehensively and separately assess the risk of new‐onset and recurrent HF depending on the presence or absence of DM. We systematically searched cohort studies that examined the relationship between DM and new‐onset or recurrent HF using EMBASE and MEDLINE (from 1 Jan 1950 to 28 Jul 2019). The risk ratio (RR) for HF in individuals with DM compared with those without DM was pooled with a random‐effects model. Seventy‐four and 38 eligible studies presented data on RRs for new‐onset and recurrent HF, respectively. For new‐onset HF, the pooled RR [95% confidence interval (CI)] of 69 studies that examined HF as a whole [i.e. combining HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF)] was 2.14 (1.96–2.34). The large between‐study heterogeneity (I 2 = 99.7%, P < 0.001) was significantly explained by mean age [pooled RR (95% CI) 2.60 (2.38–2.84) for mean age < 60 years vs. pooled RR (95% CI) 1.95 (1.79–2.13) for mean age ≥ 60 years] (P < 0.001). Pooled RRs (95% CI) of seven and eight studies, respectively, that separately examined HFpEF and HFrEF risk were 2.22 (2.02–2.43) for HFpEF and 2.73 (2.71–2.75) for HFrEF. The risk magnitudes between HFpEF and HFrEF were not significantly different in studies that examined both HFpEF and HFrEF risks (P = 0.86). For recurrent HF, pooled RR (95% CI) of the 38 studies was 1.39 (1.33–1.45). The large between‐study heterogeneity (I 2 = 80.1%, P < 0.001) was significantly explained by the proportion of men [pooled RR (95% CI) 1.53 (1.40–1.68) for < 65% men vs. 1.32 (1.25–1.39) for ≥65% men (P = 0.01)] or the large pooled RR for studies of only participants with HFpEF [pooled RR (95% CI), 1.73 (1.32–2.26) (P = 0.002)]. Results indicate that DM is a significant risk factor for both new‐onset and recurrent HF. It is suggested that the risk magnitude is large for new‐onset HF especially in young populations and for recurrent HF especially in women or individuals with HFpEF. DM is associated with future HFpEF and HFrEF to the same extent.
Keywords: Diabetes mellitus, New‐onset heart failure, Recurrent heart failure, Cohort study, Meta‐analysis
Introduction
Heart failure (HF) is a major clinical and public health problem with high prevalence, 1 incurring extraordinary health care expenditures 2 and negatively influencing activities of daily living. 3 Many epidemiological studies have indicated that diabetes mellitus (DM) increases the risk of HF. For example, a recent large cohort study showed a higher risk of hospitalization for HF among patients with than without type 2 DM even if their cardiovascular risk factors were within target ranges. 4
Because recent trials suggested that HF is preventable by specific pharmacological treatment (sodium glucose co‐transporter‐2 inhibitor) 5 and intensified multifactorial interventions, 6 HF has received appropriate attention 7 as one of the most common cardiovascular complications of DM. 8 Estimating the magnitude of HF risk among persons with DM is essential for assessing the importance of HF as a diabetes‐related complication and deciding whether prevention of HF should be given priority among diabetes‐related complications. However, the entire context of the magnitude of risk for HF in relation to DM remains insufficiently understood. Particularly, new‐onset HF (HF occurring without a history of HF) and recurrent HF (HF re‐occurring with a history of HF) are not discriminated. The issues regarding risk of new‐onset and recurrent HF should be discussed separately considering differences in patients' characteristics, therapy goals, and treatments to achieve goals specific to those at high risk for HF but without symptoms of HF compared with those with prior symptoms of HF. 9 In addition, although we should emphasize that it is impossible to compare new‐onset and recurrent HF when the criteria differ between the two conditions, the risk imparted by DM is hypothesized to be quite different between new‐onset and recurrent HF considering the burden of hospitalization after an HF diagnosis even though the cause of such hospitalizations is not necessarily due to HF. 10 Based on this hypothesis, results of many previous cohort studies that combined new‐onset and recurrent HF as the HF outcome would lead to inaccurate conclusions because these studies failed to consider an interaction effect of DM status and a past history of HF even if risk indicators were adjusted for a history of HF.
Previous meta‐analyses of cohort studies that examined the risk of new‐onset HF in relation to DM 2 , 11 included studies on an unselected community population but not on a population selected according to specific characteristics and conditions (e.g. hypertension and renal diseases) that clinicians usually see in a real‐world clinical setting. A recent meta‐analysis that estimated the risk of new‐onset HF failed to exclude studies in which participants with and without a history of HF were combined. 12 Another meta‐analysis of cohort studies limited to patients with a history of HF indicated that DM adversely affected all‐cause death and hospitalization. 13 However, the causes of death or reasons for hospitalization were not specified. This meta‐analysis aims to comprehensively assess the risk of new‐onset and recurrent HF depending on the presence or absence DM.
Methods
We followed the Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) guidelines for conducting meta‐analyses of observational studies. 14 The protocol for this meta‐analysis was registered in advance with the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42019117390).
Search strategy
We used MEDLINE and EMBASE (from 1 Jan 1950 to 28 Jul 2019) as electronic databases for systematic literature searches. Keywords are presented in Appendix 1. Inclusion criteria were (i) cohort study; (ii) DM status of all participants was ascertained before the follow‐up period; (iii) at least 6 months of follow‐up; (iv) exposure is having DM at baseline; (v) referent is not having DM at baseline; (vi) outcome is new‐onset or recurrent HF (see Study outcome); and (vii) the risk indicators [i.e. hazards ratio (HR) or odds ratio (OR)] for HF in relation to DM were described or the risk ratio (RR) could be calculated. Studies that classified new‐onset HF into HF with preserved ejection fraction (EF) (HFpEF) and HF with reduced EF (HFrEF) were also considered. Remarks related to (i) to (v) are in Appendix 2.
We examined the reference lists of publications that met our inclusion criteria to identify additional studies that might be suitable for our purpose. We considered articles published in any language. When there were unclear issues within a study, we contacted the authors for clarification before deciding whether the study met these inclusion criteria. If two or more articles existed for one cohort study, priority for choosing one of these articles was given as follows: (i) direct presentation of data on the HR or the OR and its corresponding 95% confidence interval (CI), (ii) long‐term follow‐up study, and (iii) inclusion of a large number of participants.
Study outcome
As previously mentioned, we considered only studies that separated new‐onset from recurrent HF as the study outcome. We defined new‐onset HF as HF occurring in participants without a history of HF. When the outcome was incident new‐onset HF, included studies had to exclude participants with a history of HF or with current HF. If it was unclear whether such participants were actually excluded, we did not exclude the study if there was no evidence that participants who had history of HF or currently had HF were obviously included. Conversely, even if a study author stated that participants having HF at baseline were excluded, we excluded that study wherein participants were obviously included who had HF of class ≥ II in the New York Heart Association (NYHA) classification or a history of HF of class ≥ II in the Killip classification. We defined recurrent HF as that which re‐occurred in patients with a history of HF although a widely accepted definition does not exist. Thus, when an outcome is recurrent HF, we included only studies that clarified that all participants had already been diagnosed as having HF regardless of the NYHA or Killip classification status.
The endpoints for new‐onset HF were hospitalization due to HF or a doctor's diagnosis of HF and for recurrent HF were hospitalization due to previously diagnosed HF or worsening of existing HF. The HF had to be an independent outcome. Studies that combined endpoints from HF and those from other causes (e.g. all‐cause hospitalizations and cardiovascular events) were excluded. In addition, the endpoints had to include both fatal and non‐fatal events. Studies that included only HF mortality as the endpoint were excluded.
Data extraction
Two authors (S. K. and H. So.) independently extracted the data. Discrepancies were solved by a third author (K. K.). In addition to the risk indicator and its corresponding 95% CI, we extracted the following data: first author, year, study design, cohort name or affiliation, specificity of study population such as underlying diseases, mean age, percentage of men, number of participants and cases, follow‐up duration, percentage of lost to follow‐up, risk indicator, methods for ascertaining DM and HF, endpoint corresponding to the study outcome, and confounding factors. When the study outcome was recurrent HF, we added data on the characteristic of the EF (i.e. reduced/preserved/non‐specified).
If the risk indicator was expressed as HR or OR and its corresponding 95% CI was not directly provided, we calculated the RR and standard error (SE) of the natural logarithm (log) of RR using the formula: where ‘1’ and ‘0’ are having DM and not having DM at baseline, respectively, and ‘C’ and ‘N’ are the number of cases and total number of participants, respectively. These risk indicators were standardized into RR. The HR was considered to be the same as the RR. The OR was transformed into the RR using the formula 15 : where P0 is the incident rate of study endpoints in the referent group. Other remarks with regard to Data Extraction are shown in Appendix 3.
To assess study quality, we adapted the Newcastle‐Ottawa Scale (NOS) for this meta‐analysis. The NOS consists of the following three broad perspectives: selection of study groups (Selection), comparability of groups (Comparability), and ascertainment of the outcome of interest (Outcome). With regard to Comparability, we selected age and coronary heart disease (CHD) as the most important confounders 16 because HF 17 and DM 18 are typical age‐related diseases. Compared with individuals without DM, those with DM have a higher prevalence of CHD, 19 and CHD presents the largest attributable risk for HF among potential risk factors. 1 As to outcome, we used the median of the follow‐up duration in the included studies as a cut‐off value for a sufficient follow‐up duration. Remarks on the criteria for NOS are provided in Appendix 4.
Data synthesis
We separately produced a dataset for estimating the risk of new‐onset and recurrent HF in relation to DM. The RR in each study was pooled with a random‐effects model if between‐study heterogeneity for the magnitude of risk assessed by I 2 was statistically significant. 20 Otherwise, a fixed‐effects model was chosen. The analysis was stratified by each of the pre‐specified study characteristics [i.e. follow‐up duration, mean age, proportion of men, characteristics of risk adjustment, endpoints, and pre‐existing diseases (for new‐onset and recurrent HF) and characteristic of baseline EF status (for recurrent HF)]. With regard to mean age and proportion of men (%), cut‐off values were determined in 5 year and 5% increments, which were close to the median in included studies so that the number of data belonging to the upper and lower values of the cut‐off were as similar as possible. In general, the cut‐off value was close to the median value of the included studies. Based on the stratified analyses, meta‐regression analyses were added to explore the origin of heterogeneity. If a characteristic significantly explained the heterogeneity, that characteristic could be suggested to significantly affect the risk magnitude. Meta‐regression was also performed to compare the risk magnitude between HFpEF and HFrEF with adjustment for each included study.
Publication bias was assessed by two formal tests, Begg's rank correlation test 21 and Egger's regression asymmetry test. 22 If publication bias was statistically detected, we adjusted the pooled RR for publication bias using the trim‐and‐fill method. 23 This method includes (i) the assumption that the funnel plot is symmetrical if there is no publication bias, (ii) detection of hypothetically unpublished data causing the funnel plot to be asymmetrical, and (iii) recalculation of the pooled RR after filling these data as if they had actually existed. Two‐sided P < 0.05 was considered statistically significant. All analyses were based on statistical software STATA version 14 (STATA Corp., College Station, TX, USA).
Results
Literature Searches
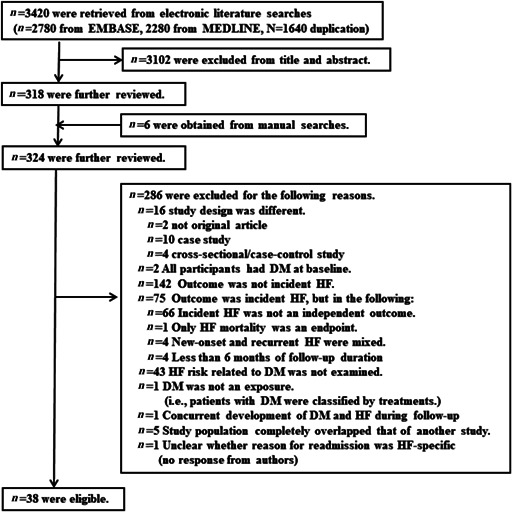
Appendices 5 and 6 are flow charts describing the procedures for selecting studies that examined new‐onset HF and recurrent HF, respectively. Among studies kept for further review after excluding studies at the title and abstract level, it was impossible to judge whether three of these studies were eligible. In one of these, it was unclear whether the reason for re‐hospitalization was HF 24 ; in another, the 95% CI of the RR to calculate its corresponding standard error (SE) was not presented 25 ; and in the third, the RR could not be calculated because of incorrect data on DM status (i.e. DM/impaired glucose tolerance/normal glucose tolerance). 26 We contacted the authors of these studies to clarify these points but received no response. Thus, we did not include those studies in our analysis. Finally, there were 74 studies 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 and 38 studies 85 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 in which we could estimate RRs for new‐onset and recurrent HF, respectively, in relation to DM. One study 85 examined both new‐onset and recurrent HF risk.
Study characteristics
Characteristics of 74 eligible studies of the risk for incident new‐onset HF are shown in Table 1. Ten studies 39 , 46 , 48 , 66 , 68 , 70 , 74 , 76 , 77 , 95 involved studies that were originally trials but were subsequently treated as cohort studies. Most included studies did not differentiate type 1 and type 2 DM. Exceptionally, 10 studies 32 , 38 , 50 , 51 , 52 , 56 , 59 , 75 , 88 , 90 limited DM patients to those with type 2 DM. One differentiated type 1 from type 2 DM. 94 Ranges (median) of mean age and follow‐up duration in the participants of included studies were from 24 to 84 years (62 years) and from 0.8 to 38 years (5.6 years), respectively. Median of proportion of men was 49%. As to the endpoint, 44 studies 27 , 28 , 29 , 30 , 31 , 34 , 36 , 39 , 40 , 41 , 43 , 45 , 46 , 47 , 49 , 50 , 51 , 52 , 54 , 55 , 58 , 59 , 60 , 61 , 64 , 65 , 67 , 72 , 73 , 75 , 76 , 78 , 80 , 82 , 84 , 85 , 86 , 88 , 89 , 92 , 93 , 95 , 96 , 100 used a diagnosis of HF regardless of whether the incident HF resulted in hospitalization. Appendix 7 shows study confounders that were considered when the relationship between DM and new‐onset HF was examined. Most of the included studies (51 studies 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 39 , 40 , 41 , 44 , 45 , 46 , 47 , 50 , 52 , 53 , 58 , 60 , 61 , 62 , 66 , 67 , 68 , 69 , 72 , 73 , 74 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 91 , 92 , 93 , 94 , 95 , 97 , 99 , 100 ) adjusted the RR for new‐onset HF at least for age and CHD. Appendix 8 shows the results of study quality assessments according to the NOS. Mean score [standard deviation (SD)] was 5.4 (1.3) (full marks = 8).
Table 1.
Characteristics of studies that examined the risk of new‐onset heart failure in relation to diabetes mellitus
| Study source | Design a | Cohort name/affiliation | Population | % men | Age | n | Cases | Dur years b | % LOF | Risk | Methods c | Endpoint | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | HF | ||||||||||||
| Chen (2019) 87 | C | MAX | General patients | 7 | 40.0 | 2.4 × 105 | 1318 | 1.8 | ? | RR | R | R | Hosp |
| Fogarassy (2019) 86 | C | Hungarian NCR | Breast cancer | 1 | 58.1 | 8068 | N/A | 5.9 | 0 | OR | R | R | Dx |
| Magnusssen (2019) 89 | C | BiomarCaRE | General | 48 | 49.5 | 78 657 | 5170 | 12.7 | ? | HR | S | S/R | Dx |
| Winell (2019) 88 | C | Finnish NHDR and CDR | General | — | — | 3.0 × 106 | 3.0 × 105 | 17 | 0 | RR | R | R | Dx |
| Chen (2018) 32 | C | NHI in Taiwan | General | 53 | 60.4 | 68 582 | 8420 | 7.9 | 0 | HR | R | R | Hosp |
| Eggimann (2018) 33 | C | BEAT‐AF | AF | 30 | 68 | 951 | 60 | 3.9 | ? | HR | S | M | Hosp |
| Gong (2018) 34 | C | SCREEN‐HF | Patients at high risk of HF | 55 | 69 | 3847 | 162 | 5.6 | 22 | HR | S | M | Dx |
| Lamblin (2018) 91 | C | CORONOR | CHD d | 78 | 66.0 | 3785 | 211 | 5 | 2 | HR | M | M | Hosp |
| LaMonte (2018) 42 | C | WHI | Post‐menopausal | 0 | 63 | 1.4 × 105 | 2516 | 8.0 | ? | RR | S | S | Hosp |
| Larsson (2018) 94 | C | The 2 cohorts in Sweden | General | 53 | 59.9 | 71 236 | 4246 | 17 | ? | HR | R | R | Hosp |
| McAllister (2018) 90 | C | Scottish DM Register | General | 47 | 53.2 | 3.2 × 106 | 1.2 × 105 | 10 | 0 | RR | R | R | Hosp |
| Rosengren (2018) 35 | C | NDR, Sweden | General | 55 | 62 | 1.6 × 106 | 6.9 × 104 | 5.6 | 1–5 | HR | R | R | Hosp |
| Wandell (2018) 36 | C | PHC in Stockholm | AF | 55 | 74 | 9424 | 2259 | 5.4 | 0 | HR | R | R | Dx |
| Wellings (2018) 37 | C | MIDAS | CHD d | 35 | 63 | 1.1 × 105 | — | 5.0 | 0 | HR | R | R | Hosp |
| Agarwal (2017) 27 | C | HCUP | General patients | 42 | 50.2 | 1.7 × 107 | 2.0 × 105 | 5.0 | 0 | HR | R | R | Hosp |
| Ballotari (2017) 38 | C | REDR | General | 49 | 50 | 3.6 × 105 | 2321 | 3.0 | 0 | RR | R | R | Hosp |
| Chatterjee (2017) 39 | T | WHS | AF | 0 | 69 | 1495 | 187 | 20.6 | 0 | HR | S | S | Dx |
| He (2017) 41 | C | CRIC | RD d | 55 | 58 | 3557 | 432 | 6.3 | ? | HR | S/M | S | Dx |
| Jacobs (2017) 97 | C | HOMAGE | General/high risk patients | 49 | 74.5 | 10 236 | 470 | 3.5 | 0 | HR | ? | R/M | Hosp |
| Kim (2017) 40 | C | Explorys Platform | General patients | 46 | — | 4.5 × 107 | 9.9 × 104 | 10.0 | 0 | OR | M | M | Dx |
| Pandey (2017) 43 | C | ORBIT‐AF | AF | 56 | 74 | 6545 | 236 | 2.0 | 4 | RR | R | M | Dx |
| Policardo (2017) 44 | C | Tuscany Regional Health Care System | General | — | — | — | 2.6 × 104 | 5.0 | 0 | HR | R | R | Hosp |
| Zhang (2017) 31 | C | Montefiore Medical Center | Diastolic dysfunction | 37 | 68 | 7878 | 833 | 5.5 | ? | HR | R | R | Dx |
| Eaton (2016) 28 | C | WHI | Post‐menopausal | 0 | 64 | 42 170 | 1952 | 13.2 | ? | HR | S | S | Hosp |
| Goldhar (2016) 45 | C | Ontario Cancer Registry | Breast cancer | 0 | 52 | 19 074 | — | 5.9 | ? | HR | R | R | Dx |
| Ho (2016) 29 | C | FHS/ PREVEND/ CHS | General | 46 | 60.1 | 22 142 | 1745 | 12.2 | 0 | HR | R | R | Dx |
| Sahle (2016) 46 | T | ANBP‐2 | HT | 59 | 84 | 6083 | 373 | 10.8 | ? | HR | M | M | Dx |
| Silverman (2016) 30 | C | MESA | General e | 53 | 62 | 6742 | 257 | 11.2 | ? | HR | M | M | Dx |
| Chahal (2015) 47 | C | MESA | General e | 47 | 62 | 6814 | 176 | 7.1 | ? | HR | S | M | Dx |
| Donneyong (2015) 48 | T | CaD trial | Post‐menopausal | 0 | 63 | 35 983 | 744 | 7.1 | 0 | RR | ? | S | Hosp |
| Qin (2015) 49 | C | UHCMC | Breast cancer | 0 | 53 | 1153 | 120 | 7.6 | 29 | RR | R | R | Dx |
| Shah (2015) 50 | C | CALIBER | General e | 49 | 47 | 1.9 × 106 | 1.4 × 104 | 5.5 | ? | HR | R | R | Dx |
| Miao (2014) 96 | C | MIMIC II | ICU patients | — | 58.4 | 3048 | 555 | 1 | 0 | HR | M | R | Dx |
| Wong (2014) 51 | C | UPMC | suspected HD | 59 | 55 | 1176 | 46 | 1.3 | ? | RR | M | M | Dx |
| Brouwers (2013) 52 | C | PREVEND | RD d | 50 | 50 | 8569 | 374 | 11.5 | ? | HR | M | M | Dx |
| Ho (2013) 53 | C | The 2nd FHS | General | 46 | 60.0 | 12 631 f | 512 | 7.7 | 0 | HR | M | M | Hosp |
| Hung (2013) 100 | C | NHMD | CHD d | 70 | 63.7 | 15 464 | 1024 | 1 | 13 | OR | R | R | Dx |
| Potpara (2013) 54 | C | Belgrade Atrial Fibrillation Study | AH | 63 | 52 | 842 | 83 | 11.2 | 30 | HR | ? | M | Dx |
| Qureshi (2013) 55 | C | Henry Ford Health System | LT | 52 | 53 | 970 | 98 | 5.3 | 0 | RR | M | M | Dx |
| Agarwal (2012) 56 | C | ARIC | General | 45 | 54 | 13 555 | 1487 | 15.5 | ? | HR | S/M | M | Hosp |
| Nakajima (2012) 57 | C | J‐ACCESS | RD d | 64 | 66 | 2395 | 64 | 3.0 | ? | RR | M | M | Hosp |
| Sato (2012) 98 | C | Okayama RCH | CHD d | 73 | 68.8 | 197 | 23 | 1 | 0 | RR | M | S/M | Hosp |
| Shafazand (2011) 99 | C | Swedish NHDR | CHD d | 64 | 68.9 | 1.8 × 105 | 43 034 | 3 | 0 | HR | R | R | Hosp |
| Roy (2011) 58 | C | CHS | General | 42 | 73 | 5464 | 1134 | 13.0 | ? | HR | M | S | Dx |
| de Simone (2010) 59 | C | SHS phase I | General | 64 | 56 | 2740 | 291 | 11.9 | ? | RR | M | M | Dx |
| Goyal (2010) 60 | C | One Million Person‐Year Follow‐up Study | General | 47 | 38 | 3.6 × 105 | 4001 | 2.9 | ? | HR | R | R | Dx |
| Smith (2010) 61 | C | MDCS | General | 41 | 58 | 5135 | 112 | 13.8 | 1 | HR | S/M | R | Dx |
| van Melle (2010) 62 | C | Heart and Soul Study | CHD d | 82 | 67 | 839 | 77 | 4.1 | 0 | HR | S | S | Hosp |
| Bibbins‐Domingo (2009) 63 | C | CARDIA | General | 44 | 24 | 2637 | 26 | 20.0 | 28 | HR | M | M | Hosp |
| Kenchaiah (2009) 64 | C | PHS | Physicians | 100 | 53 | 21 094 | 1109 | 20.5 | ? | RR | S | S | Dx |
| Leung (2009) 65 | C | Saskatchewan Health beneficiaries | General | 51 | 63 | 5.6 × 105 | 2293 | 1.1 | ? | RR | R | R | Dx |
| Lewis (2009) 66 | T | PEACE | CAD | 82 | 64 | 8211 | 268 | 4.8 | 1 | HR | R | R | Hosp |
| Ruigomez (2009) 67 | C | GPRD in 1996, UK | General | 47 | 64 | 9057 | 386 | 3.6 | 0 | HR | R | M | Dx |
| Nafaji (2008) 92 | C | Perth MONICA Register | CHD d | 15 | 54.5 | 3109 | 406 | 14.4 | 0 | HR | M | R | Dx |
| Aksnes (2007) 68 | T | VALUE | HT | 58 | 66 | 15 245 | 754 | 4.2 | ? | HR | R | M | Hosp |
| Fukuda (2007) 69 | C | Cardiovascular Institute Hospital | AF | 77 | 64 | 248 | 16 | 4.1 | ? | HR | R | M | Hosp |
| Held (2007) 70 | T | ONTARGGET/TRANSCEND | CHD d | 70 | 67 | 30 798 | 668 | 2.4 | 2 | RR | M | M | Hosp |
| Ito (2007) 71 | C | Nagoya City Higashi Municipal Hospital | RD d | 64 | 57 | 100 | 6 | 4.7 | ? | HR | M | M | Hosp |
| Ingelsson (2005) 72 | C | ULSAM | General | 100 | 50 | 2321 | 259 | 28.8 | ? | HR | M | R | Dx |
| Lentine (2005) 73 | C | USRDS | RD d | ‐ | 47 | 27 011 | ‐ | 3.0 | ? | HR | R | R | Dx |
| Bibbins‐Domingo (2004) 74 | T | HERS | CHD d | 0 | 67 | 2391 | 237 | 6.3 | ? | HR | S | M | Hosp |
| Nichols (2004) 75 | C | KPNW | General | 48 | 63 | 17 076 | 1693 | 4.7 | ? | HR | R | R | Dx |
| Wylie (2004) 76 | T | OPUS‐TIMI 16 | CHD d | — | 60.5 | 4681 | 254 | 0.8 | ? | OR | ? | M | Dx |
| Lewis (2003) 77 | T | CARE | CHD d | 87 | 58 | 3860 | 243 | 5.0 | ? | HR | ? | M | Hosp |
| Rigatto (2002) 78 | C | University of Manitoba | RD d | 62 | 38 | 638 | 63 | 8.9 | ? | RR | M | M | Dx |
| Williams (2002) 93 | C | YHAP | General | 42 | 74.3 | 2176 | N/A | 14 | 13 | HR | S | M | Dx |
| Abramson (2001) 95 | T | SHEP | HT | 57 | 71.6 | 4538 | 156 | 4.5 | ? | HR | S | M | Dx |
| He (2001) 79 | C | HHANES‐I | General | 41 | 50 | 13 643 | 1382 | 19.0 | 4 | HR | S | R | Hosp |
| Johansson (2001) 80 | C | GPRD in 2000, UK | General | 52 | 72 | 5000 | 938 | 1.0 | 0 | RR | R | M | Dx |
| Wilhelmsen (2001) 81 | C | MPPS | General | 100 | 52 | 7495 | 937 | 27.0 | 12 | OR | S | R | Hosp |
| Aronow (1999) 82 | C | Hebrew Hospital | General | 32 | 81 | 2893 | 794 | 3.6 | ? | HR | M | M | Dx |
| Chen (1999) 83 | C | New Haven Cohort | General | 41 | 74 | 1749 | 173 | 7.9 | 13 | HR | S | M | Hosp |
| Kannel (1999) 84 | C | FHS | General | 42 | 63 | 15 267 f | 486 | 38.0 | 0 | OR | M | M | Dx |
| Harnett (1995) 85 | C | Royal Victoria Hospital, Montreal | RD d | 65 | 48 | 299 | 76 | 3.4 | 2 | RR | M | M | Dx |
Abbreviations: —, no data; ?, unclear; AF, atrial fibrillation; C, cohort; CHD, coronary heart disease; CKD, chronic kidney disease; Dur, duration of follow‐up; Dx, diagnosed as HF; HD, heart diseases; HDL‐C, high‐density lipoprotein cholesterol; HL, hyperlipidaemia; Hosp, hospitalization due to HF; HR, hazards ratio; HT, hypertension; ICU, intensive care unit; LOF, lost to follow‐up; M, medical records; N/S, not specified; OR, odds ratio; R, registry; RD, renal diseases, RR, calculated risk ratio (not HR); S, self‐report; T, trial; TLV, administration of tolvaptan.
Abbreviations of cohort names: ANBP‐2, Second Australian National Blood Pressure Study; ARIC, Atherosclerosis Risk in Communities study; BEAT‐AF, Basel Atrial Fibrillation Cohort Study; BiomarCaRE, Biomarker for Cardiovascular; CaD, Vitamin D plus calcium; CALIBER, Carbohydrates, Lipids and Biomarkers of Traditional and Emerging Cardiometabolic Risk Factors; CARDIA, Coronary Artery Risk Development in Young Adults Study; CARE, Cholesterol And Recurrent Events; CDR, Causes of Death Register; CHS, Cardiovascular Health Survey; CORONOR, suivi d'une cohorte de patients COROnariens stables en région NORd‐pas‐de‐Calais; CRIC, Chronic Renal Insufficiency Cohort; FHS, Framingham Health Study; GPRD, General Practice Research Database; HCUP, Healthcare Cost and Utilization Project; Health ABC, Health ABC, Health, Aging, and Body Composition Study; HERS, Heart and Estrogen/progestin Replacement Study; HOMAGE, Heart ‘omics’ in AGEing study; J‐ACCESS, Japanese‐Assessment of Cardiac Event and Survival Study; KPNW, Kaiser Permanente Northwest region; MAX, Medicaid Analytic eXtract; MDCS, Malmö Diet and Cancer Study; MESA, Multi‐Ethnic Study of Atherosclerosis; MIMIC II, Multi‐parameter Intelligent Monitoring in Intensive Care; MONICA, MONItoring trends and determinants in CArdiovascular disease; MPPS, Multifactor Primary Prevention Study; MIDAS, Myocardial Infarction Data Acquisition System; NCR, National Cancer Registry; NDR, National Diabetes Register; NHDR, National Hospital Discharge Register (HDR); NHI, National Health Insurance; NHMD, National Hospital Morbidity Database; NHANES I, First National Health and Nutrition Examination Survey; ONTARGET, Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial; OPUS‐TIMI, Oral glycoprotein IIb/IIIa inhibition with orbofiban in patients with unstable coronary syndromes; ORBIT‐AF, Outcomes Registry for Better Informed Treatment of Atrial Fibrillation; PEACE, Prevention of Events with Angiotensin‐Converting Enzyme inhibition study; PHC, primary health care centres; PHS, Physicians' Health Study; PREVEND, Prevention of Renal and Vascular Endstage Disease; RCH, Red Cross Hospital; REDR, Reggio Emilia Diabetes Register; SCREEN‐HF, Screening Evaluation of the Evolution of New Heart Failure; SHS, Strong Heart Study; SHEP, Systolic Hypertension in the Elderly Program Risk.
Assessment in Europe; TRANSCEND, Telmisartan Randomised Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease; UHCMC, University Hospital Case Medical Center; ULSAM, Uppsala Longitudinal Study of Adult Men cohort; UPMC, University of Pittsburgh Medical Center; USRDS, US Renal Data System; VALUE, Valsartan Antihypertensive Long‐term Use Evaluation study; WHI, Women's Health Initiative; WHS, Women's Health Study; YHAP, Yale Health and Aging Project.
Meaning that the study was originally designed as a trial but was then treated as a cohort study.
Mean or median follow‐up duration is indicated.
Methods for confirmation of DM and HF.
In RD, albuminuria, dialysis, CKD, and receiving kidney transplantation are combined as RD. In CHD, angina, myocardial infarction, and cardiovascular diseases are combined as CHD.
Participants with history of coronary heart diseases were excluded at baseline.
Person‐examination based.
Table 2 shows characteristics of the 38 eligible studies that examined the risk for recurrent HF. In comparing those 38 studies with the 74 studies that examined risk for new‐onset HF, the study population was relatively old (median, 67 years; range, from 54 to 79 years), follow‐up duration was relatively short (median, 2.0 years; range, from 0.8 to 7.0 years), and the proportion of men was higher (median, 68%) in the 38 studies. Fourteen studies 102 , 103 , 105 , 108 , 111 , 120 , 122 , 123 , 124 , 125 , 126 , 128 , 132 , 133 were originally designed as trials. All but two included studies 120 , 123 used hospitalization due to HF as the study endpoint. Only three studies 115 , 116 , 136 limited the DM patients to type 2 DM. Half of the included studies [19 studies 85 , 102 , 104 , 106 , 107 , 108 , 110 , 112 , 113 , 115 , 118 , 120 , 122 , 124 , 126 , 127 , 132 , 133 , 134 adjusted the RR at least for age and CHD (Appendix 9)]. Assessment of study quality resulted in a mean score (SD) of 5.5 (1.2) (Appendix 10).
Table 2.
Characteristics of studies that examined risk of recurrent heart failure in relation to diabetes mellitus
| Study source | Cohort name/affiliation | Design a | Population | EF | %men | Age | n | Cases | Dur b | LOF | Risk | Methods c | Endpoint | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | HF | |||||||||||||
| Kim (2019) 134 | KorHF | C | N/S | N/S | 50 | 67 | 3162 | 863 | 1.5 | ? | HR | R | ? | Hosp |
| Chen (2018) 101 | Sun Yat‐sen University | C | N/S | N/S | 66 | 64.9 | 587 | 384 | 7.0 | ? | RR | M | M | Hosp |
| Cooper (2018) 102 | HA‐ACTION | T | N/S | ↓ | 77 | 59 | 6214 | — | 1.0 | ? | HR | M | R | Hosp |
| Iorio (2018) 137 | Cardionet® in Trieste | C | N/S | N/S | 57 | 77 | 2314 | 510 | 2.6 | ? | HR | M | R | Hosp |
| Kristensen (2018) 103 | ATMOSPHERE | T | N/S | ↓ | 78 | 63 | 7016 | 1324 | 2.7 | 1 | RR | M | M | Hosp |
| Retwinski (2018) 136 | ESC‐HF‐LT | C | N/S | N/S | 70 | 65.3 | 1080 | 377 | 1 | 0 | RR | M | S/M | Hosp |
| Rorth (2018) 104 | DNPR | C | N/S | N/S | 71 | 54 | 2.6 × 104 | 11 234 | 2.1 | 0 | HR | R | R | Hosp |
| Sandesara (2018) 105 | TOPCAT | T | N/S | → | 49 | 69 | 3385 | 437 | 3.4 | ? | RR | S | R | Hosp |
| Takimura (2018) 135 | Tokyo General Hospital | C | TLV | N/S | 58 | 79 | 1191 | 285 | 1 | 0 | HR | M | M | Hosp |
| Dauriz (2017) 106 | ESC‐HF‐LT | C | N/S | N/S | 72 | 72 | 9428 | 1030 | 1.0 | 32 | HR | S | R | Hosp |
| Farre (2017) 107 | Local Health Department in Catsalut | C | N/S | N/S | 45 | 77 | 8.8 × 104 | 7725 | 1.0 | 0 | OR | R | R | Hosp |
| Kristensen (2017) 108 | I‐PRESERVE | T | N/S | → | 40 | 72 | 4128 | 661 | 3.8 | ? | HR | R | R | Hosp |
| Mohamedali (2017) 109 | ACMC | C | LVAD | ↓ | 78 | 60 | 288 | 57 | 3.1 | 0 | RR | M | ? | Hosp |
| Echouffo‐Tcheugui (2016) 110 | NCDR‐ICD | C | CRT | ↓ | 67 | 75 | 1.8 × 104 | 4380 | 3.0 | ? | RR | M | R | Hosp |
| Kristensen (2016) 111 | PARADIGM‐HF | T | N/S | ↓ | 78 | 64 | 8274 | 1179 | 2.0 | ? | RR | M | R | Hosp |
| Ruigomez (2016) 112 | TWIN | C | N/S | N/S | 52 | 75 | 3516 | 633 | 4.5 | 0 | HR | R | R | Hosp |
| Kaneko (2015) 113 | Shinken | C | N/S | N/S | 70 | 69 | 282 | 55 | 2.5 | ? | HR | M | R | Hosp |
| Takeda (2015) 114 | CUMC | C | LVAD | ↓ | 83 | 57 | 293 | 33 | 2.0 | 3 | HR | M | M | Hosp |
| Carrasco‐Sanchez (2014) 115 | RICA | C | N/S | N/S | 45 | 78 | 1082 | 383 | 1.0 | 0 | HR | S/M | R | Hosp |
| Cubbon (2014) 116 | MCRC | C | N/S | ↓ | 74 | 67 | 628 | 44 | 1.0 | 0 | OR | M | R | Hosp |
| Paoletti (2014) 117 | Four Italian Centre | C | CRT | ↓ | 75 | 70 | 559 | 143 | 2.5 | 0 | HR | M | M | Hosp |
| Sakata (2014) 118 | CHART‐2 | C | Stage C/D | N/S | 68 | 69 | 4736 | — | 3.1 | ? | HR | M | M | Hosp |
| Larina (2013) 119 | RSMU | C | N/S | N/S | 66 | 68 | 248 | 87 | 6.5 | ? | RR | M | M | Hosp |
| Sarma (2013) 120 | EVEREST | T | N/S | ↓ | 74 | 66 | 4131 | 1495 | 0.8 | ? | HR | S | M | Worse |
| Verbrugge (2012) 121 | Ziekenuis OostLimberg | C | CRT | ↓ | 68 | 71 | 172 | 47 | 1.5 | 0 | HR | M | M | Hosp |
| Deedwania (2011) 122 | EPHESUS | T | AMI | ↓ | 69 | 66 | 2238 | 314 | 1.3 | ? | HR | R | R | Hosp |
| Martin (2011) 123 | MADIT‐CRT | T | CRT | ↓ | 75 | 64.6 | 1817 | 329 | 2.0 | ? | RR | ? | M | Worse |
| Aguilar (2010) 124 | DIG | T | N/S | → | 59 | 67 | 987 | 221 | 3.1 | ? | HR | S | ? | Hosp |
| Sze (2010) 125 | MRDIT II | T | ICD/CRT | ↓ | 84 | — d | 1218 | 253 | 1.7 | 1 | HR | ? | M | Hosp |
| MacDonald (2008a) 126 | CHARM | T | N/S | N/S | 68 | 66 | 7599 | 51135 e | 3.1 f | 0 | HR | ? | M | Hosp |
| Macdonald (2008b) 127 | SMR | C | N/S | N/S | 47 | 74 | 1.2 × 105 | 7.0 × 104 | 5.0 | 0 | HR | R | R | Hosp |
| Ghali (2007) 128 | COMPANION | T | NYHA III/IV | ↓ | 68 | 66 | 1519 | 283 | 1.3 | 10 | RR | S | M | Hosp |
| Ruiz‐Ruiz (2007) 129 | HCU Lozano Blesa | C | N/S | N/S | 53 | 73 | 111 | 54 | 1.8 | 0 | OR | M | M | Hosp |
| Formiga (2006) 130 | Hospital Universitari de Bellvitge | C | N/S | N/S | 43 | 79 | 88 | 32 | 0.8 | 0 | RR | M | M | Hosp |
| Garcia (2005) 131 | Hospital Universitari Germans Trias i Pujol | C | N/S | N/S | 27 | 65.3 | 362 | 70 | 1.0 | ? | RR | S | R | Hosp |
| Domanski (2003) 132 | BEST | T | N/S | N/S | 78 | 60 | 2708 | 1045 | 2.0 | 0 | HR | ? | ? | Hosp |
| Shindler (1996) 133 | SOLVD | T | N/S | ↓ | 80 | 61 | 2569 | 80 | 3.4 | ? | HR | S | S | Hosp |
| Harnett (1995) 85 | Royal Victoria Hospital, Montreal | C | dialysis | N/S | 60 | 59 | 133 | 75 | 3.0 | 0 | HR | M | M | Hosp |
Abbreviations: —, no data; ?, unclear; C, cohort; CRT, cardiac resynchronization therapy; Dur, duration of follow‐up; EF, ejection fraction; Hosp, hospitalization due to HF; HR, hazards ratio; ICD, implantable cardioverter–defibrillator; LOF, lost to follow‐up; LVAD, left ventricular assist device placement; M, medical records; N/S, not specified; NYHA, New York Heart Association class; OR, odds ratio; R, registry; RR, calculated risk ratio (not HR); S, self‐report; T, trial; worse, worsening of HF.
Cohort name abbreviations: ACMC, Advocate Christ Medical Center; ATMOSPHERE, Aliskiren Trial of Minimizing OutcomeS for Patients with Heart Failure; BEST, Beta‐blocker Evaluation of Survival Trial; CHARM, Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity programme; CHART‐2, Chronic Heart Failure Analysis and Registry in the Tohoku District‐2; COMPANION, Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure; CUMC, Columbia Presbyterian Medical Center; DIG, Digitalis Investigation Group ancillary study; DNPR, Danish National Patients Registry; EPHESUS, Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study; ESC‐HF‐LT, European Society of Cardiology Heart Failure Long‐Term Registry; EVEREST, Efficacy of Vasopressin Antagonism in Heart Failure Outcome study with Tolvaptan trials; HCU, Hospital Clínico Universitario; HF‐ACTION, Heart Failure and A Controlled Trial Investigating Outcomes of Exercise Training; I‐PRESERVE, Irbesartan in Heart Failure With Preserved Ejection Fraction; KorHF, Korean Heart Failure registry; MADIT‐CRT, Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy; MCRC, Multidisciplinary Cardiovascular Research Centre; NCRD‐ICD, National Cardiovascular Data Registry's Implantable Cardioverter‐Defibrillator Registry; PARADIGM‐HF, Prospective comparison of ARNI with ACE‐I to Determine Impact on Global Mortality and Morbidity in Heart Failure trial; RICA, Registro de Insuficiencia Cardíaca registry; RSMU, Russian State Medical University; SMR, Scottish Morbidity Record database; SOLVD, Studies of Left Ventricular Dysfunction trials; TOPCAT, Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial; TWIN, The Health Improvement Network.
Meaning that the study was originally designed as a trial but then was treated as a cohort study.
Mean or median follow‐up duration is indicated.
Methods for confirmation of DM and HF.
53.2% of patients were 65 years or older.
Estimated value.
Datum was based on follow‐up for all‐cause mortality.
Overall analysis of new‐onset heart failure risk in relation to diabetes mellitus
Among the 74 studies of the risk for incident new‐onset HF, in four, 28 , 29 , 30 , 31 the outcome was separated into HFpEF and HFrEF, and the risk of HF was not examined as a whole. One study 27 only included systolic HF as an endpoint (i.e. diastolic HF was excluded.) The remaining 69 studies estimated DM‐related new‐onset HF risk as a whole (i.e. regardless of EF status). Figure 1 is a forest plot of the RR for new‐onset HF in participants with DM compared with those without DM. Of the 69 included studies, 13 studies 32 , 35 , 36 , 38 , 44 , 50 , 60 , 65 , 80 , 84 , 88 , 89 , 90 presented data on RR by gender; of these, seven studies 32 , 35 , 50 , 87 , 88 , 89 , 90 also examined the risk for HF by age. The RR was above 1 in all included studies. The pooled RR (95% CI) was 2.14 (1.96–2.34). Publication bias was statistically detected not by Egger's test (P = 0.45) but by Begg's test (P = 0.02). However, adjusting the pooled RR for publication did not change the result.
Figure 1.
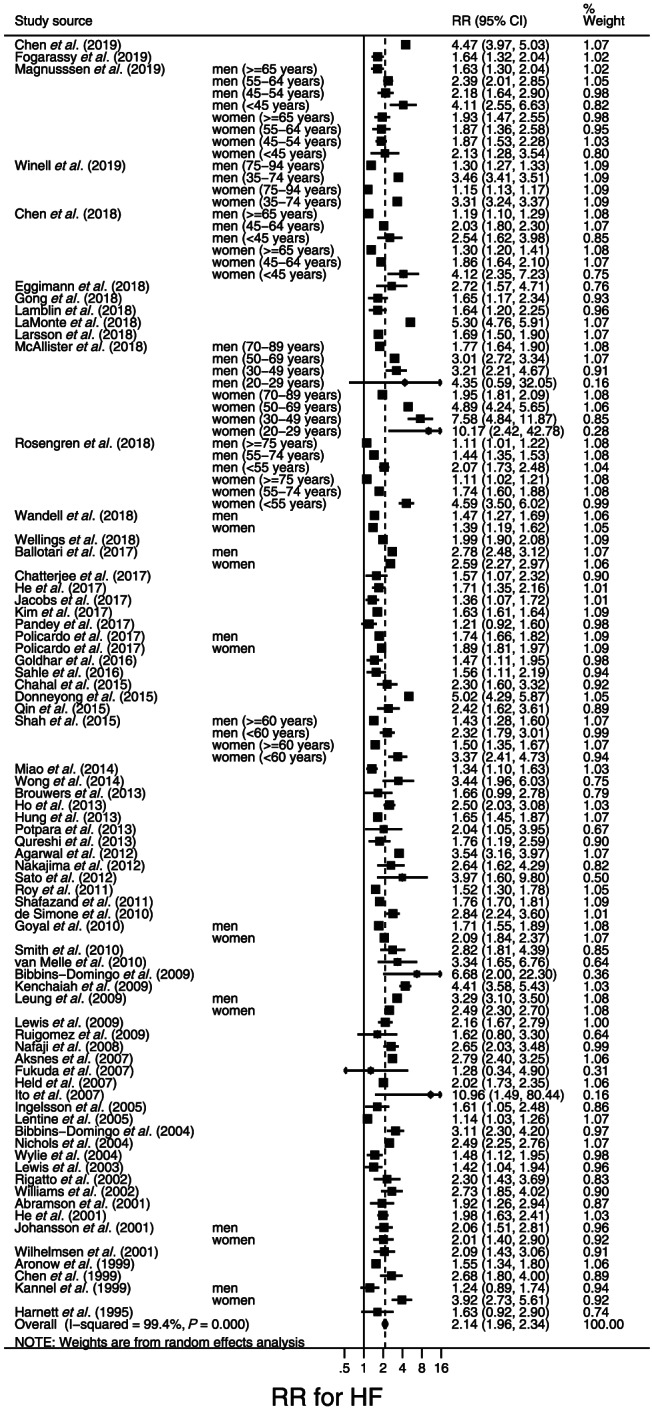
Forest plot of the risk ratios (RRs) for new‐onset heart failure (HF) in participants with diabetes mellitus compared with those without diabetes mellitus. The RRs in each study are indicated by squares. The area of squares is proportional to the study weight (i.e. inverse of square of standard error of the RR). The pooled RR is indicated by a diamond.
Figure 2A is a forest plot of seven studies 28 , 29 , 30 , 31 , 34 , 42 , 55 that examined HFpEF and eight studies 28 , 29 , 30 , 31 , 34 , 42 , 55 , 56 that examined HFrEF risk in relation to DM. The RR (95% CI) was 2.22 (2.02–2.43) for HFpEF and 2.73 (2.71–2.75) for HFrEF. After one study with an extremely large study weight was excluded, 56 the pooled RR (95% CI) was 2.22 (1.98–2.49) (Figure 2B). In seven studies that classified HF into HFpEF and HFrEF and examined both of these risks, there was not a significant difference in the risk magnitude between HFpEF and HFrEF according to the meta‐regression analysis (P = 0.86).
Figure 2.
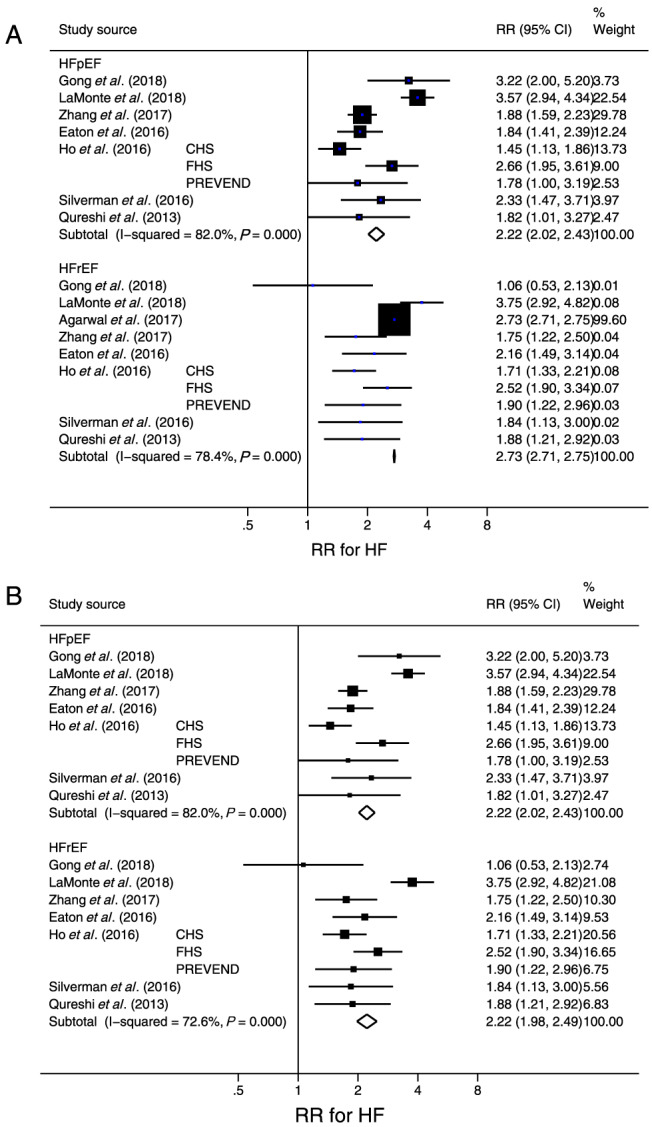
(A) Forest plot of the risk ratio (RR) for new‐onset heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF) in participants with diabetes mellitus compared with those without diabetes mellitus. (B) The forest plot after excluding one study (Agarwal et al.) with an extremely large study weight (i.e. inverse of square of standard error of the RR). The RR in each study is indicated by a square. The area of squares is proportional to the study weight. The pooled RR is indicated by a diamond. Abbreviations: CHS, Cardiovascular Health Survey; FHS, Framingham Health Study; Prevention of Renal and Vascular End‐Stage Disease.
Overall analysis of recurrent heart failure risk in relation to diabetes mellitus
Figure 3 is a forest plot of the RR for recurrent HF in HF patients with DM compared with those without DM. Of the 38 included studies, three studies 124 , 127 , 134 examined risk by gender, and two studies 126 , 137 classified the HF patients by the EF at baseline. The RR was above 1 in all but one study. 103 Statistically significant publication bias was detected not by Begg's test (P = 0.12) but by Egger's test (P = 0.03). Results of the trim‐and‐fill method 23 of adjusting for publication bias suggested seven hypothetically unpublished studies that caused inflation of RR. After these hypothetical studies were included, the RR was slightly deflated to 1.33 (95% CI, 1.27–1.40).
Figure 3.
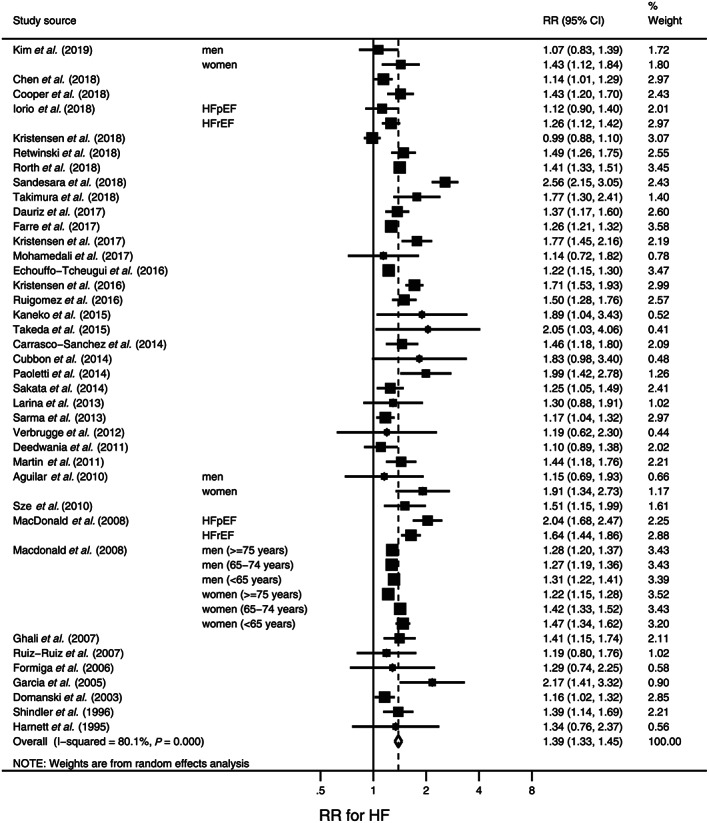
Forest plot of the risk ratios (RRs) for recurrent heart failure (HF) in HF patients with diabetes mellitus compared with those without diabetes mellitus. The RR in each study is indicated by a square. The area of squares is proportional to the study weight (i.e. inverse of square of standard error of the RR). The pooled RR is indicated by a diamond. Abbreviations: pEF, preserved ejection fraction; rEF, reduced ejection fraction.
Sensitivity analysis of new‐onset heart failure risk in relation to diabetes mellitus
There was large between‐study heterogeneity (I 2 = 99.7%, P < 0.001) (Figure 1). Table 3 shows the results of sensitivity analyses wherein the 69 studies shown in Figure 1 were stratified according to key study characteristics (Table 1). Although a weaker association was observed in limiting the analysis to studies that adjusted the RR for new‐onset HF for age and CHD compared with those without those adjustments, the pooled RR was significant regardless of the adjustment [RR (95% CI), 1.78 (1.70–1.87) vs. 2.71 (2.26–3.25)]. In studies of a population with a mean age < 60 years, the RR was larger for new‐onset HF [pooled RR (95% CI), 2.60 (2.38–2.84)] than in studies with a population having a mean age of ≥60 years [pooled RR (95% CI), 1.95 (1.79–2.13)]. Meta‐regression analysis indicated that the difference in mean age of the study population significantly explained the between‐study heterogeneity in the RR (P < 0.001).
Table 3.
Stratified analysis of risk ratio for new‐onset heart failure in relation to diabetes mellitus using pre‐specified study characteristics
| Variable | n | RR (95% CI) | P value for RR | I 2 (%) | P value for I 2 | Meta‐regression |
|---|---|---|---|---|---|---|
| Total | 106 | 2.14 (1.96–2.34) | <0.001 | 99.7 | <0.001 | |
| Follow‐up period | ||||||
| ≥6 years | 56 | 2.40 (2.14–2.68) | <0.001 | 97.0 | <0.001 | |
| <6 years | 50 | 1.94 (1.69–2.23) | <0.001 | 99.6 | <0.001 | 0.01 |
| Study design | ||||||
| Trial a | 10 | 2.15 (1.62–2.86) | <0.001 | 93.0 | <0.001 | |
| Non‐trial | 96 | 2.14 (1.95–2.35) | <0.001 | 99.5 | <0.001 | 0.92 |
| Mean age a | ||||||
| ≥60 years | 64 | 1.95 (1.79–2.13) | <0.001 | 98.2 | <0.001 | |
| <60 years | 52 | 2.60 (2.38–2.84) | <0.001 | 96.5 | <0.001 | 0.001 |
| % men c | ||||||
| ≥50% | 53 | 2.03 (1.76–2.35) | <0.001 | 99.3 | <0.001 | |
| <50% | 51 | 2.33 (1.99–2.72) | <0.001 | 99.4 | <0.001 | 0.11 |
| Risk adjustment | ||||||
| Both age and CHD | 64 | 1.78 (1.70–1.87) | <0.001 | 91.7 | <0.001 | |
| Failure in adjustment for age and/or CHD | 42 | 2.71 (2.26–3.25) | <0.001 | 99.7 | <0.001 | <0.001 |
| Endpoint | ||||||
| Only hospitalization due to HF | 49 | 2.34 (2.11–2.60) | <0.001 | 97.5 | <0.001 | |
| Including non‐hospitalizations for HF d | 57 | 1.96 (1.73–2.23) | <0.001 | 99.6 | <0.001 | 0.02 |
| Underlying diseases | ||||||
| Non‐hospital‐based study e | 67 | 2.30 (2.02–2.62) | <0.001 | 99.6 | <0.001 | — f |
| RD | 7 | 1.99 (1.36–2.93) | <0.001 | 80.8 | <0.001 | 0.23 |
| AF | 7 | 1.45 (1.32–1.59) | <0.001 | 26.8 | 0.22 | 0.045 |
| CHD | 12 | 1.94 (1.77–2.12) | <0.001 | 79.9 | <0.001 | 0.41 |
| breast cancer | 3 | 1.69 (1.44–1.97) | <0.001 | 50.8 | 0.13 | 0.32 |
| HT | 3 | 2.08 (1.40–3.11) | <0.001 | 81.7 | 0.004 | 0.70 |
| Others g | 7 | 1.99 (1.32–3.00) | 0.001 | 98.0 | <0.001 | 0.40 |
Abbreviations: AF; atrial fibrillation; CHD, coronary heart disease; HT, hypertension; RD, renal disease.
Cohort study that was originally designated as a trial.
Total number of data was different from the other stratified analyses because in this stratified analysis, priority for data extraction was given to data based on subgroup analysis according to age instead of gender if a study provided data on subgroup analysis based on both age and gender. In the other stratified analyses, priority for data extraction was given to data based on the subgroup analysis based on gender.
Incident HF that did not lead to hospitalization.
Including community‐based study or specific populations such as post‐menopausal, nurses, and physicians.
Multivariate regression analysis was performed.
Including non‐specified diseases (i.e. hospital‐based study), preclinical cardiac dysfunction, after liver transplantation, patients at high risk of vascular diseases, and suspected heart diseases.
Stratified analyses of recurrent heart failure risk in relation to diabetes mellitus
Similar to new‐onset HF risk, there was large between‐study heterogeneity (I 2 = 80.1%, P < 0.001) (Figure 3). Results of sensitivity analyses of recurrent HF risk in which the 38 included studies were stratified according to key study characteristics (Table 2) are presented in Table 4. A relatively large association was observed when analysing only studies with proportions of men < 65% [pooled RR (95% CI), 1.53 (1.40–1.68)] compared with studies having ≥65% men [pooled RR (95% CI), 1.32 (1.25–1.39)]. The effect of the proportion of men on between‐study heterogeneity in the RR for recurrent HF was statistically significant (P = 0.01). Studies limiting participants to those having HF with HFpEF showed a larger RR [pooled RR (95% CI), 1.73 (1.32–2.26)] than did studies of only those having HF with HFrEF [pooled RR (95% CI), 1.37 (1.24–1.50)] or when the EF was not specified among HF patients [pooled RR, 1.33 (1.28–1.38)]. Limiting patients to those with HFpEF significantly explained study heterogeneity in the RR for recurrent HF (P = 0.002). Analysis of only studies that adjusted the RR for age and CHD showed that the RR for recurrent HF remained significant [pooled RR, 1.36 (1.30–1.41)].
Table 4.
Stratified analysis of risk ratio for recurrent heart failure in relation to diabetes mellitus using pre‐specified study characteristics
| Variable | n | RR (95% CI) | P value for RR | I 2 (%) | P value for I 2 | Meta‐regression |
|---|---|---|---|---|---|---|
| Total | 47 | 1.39 (1.33–1.45) | <0.001 | 80.1 | <0.001 | |
| Follow‐up period | ||||||
| ≥2 years | 30 | 1.41 (1.32–1.49) | <0.001 | 85.6 | <0.001 | |
| <2 years | 17 | 1.34 (1.26–1.43) | <0.001 | 64.5 | 0.04 | 0.65 |
| Study design | ||||||
| Trial a | 14 | 1.47 (1.28–1.70) | <0.001 | 91.0 | <0.001 | |
| Non‐trial | 33 | 1.33 (1.28–1.38) | <0.001 | 56.9 | <0.001 | 0.23 |
| Mean age b | ||||||
| ≥65 years | 33 | 1.41 (1.34–1.49) | <0.001 | 79.6 | <0.001 | |
| <65 years | 14 | 1.34 (1.23–1.47) | <0.001 | 82.0 | <0.001 | 0.41 |
| Men | ||||||
| ≥65% | 30 | 1.32 (1.25–1.39) | <0.001 | 72.0 | <0.001 | |
| <65% | 17 | 1.53 (1.40–1.68) | <0.001 | 87.0 | <0.001 | 0.01 |
| Risk adjustment | ||||||
| Both age and CHD | 27 | 1.36 (1.30–1.41) | <0.001 | 73.2 | <0.001 | |
| Failure in adjustment for age and/or CHD | 20 | 1.46 (1.28–1.67) | <0.001 | 85.4 | <0.001 | 0.36 |
| Endpoint | ||||||
| Only hospitalization due to HF | 2 | 1.24 (1.12–1.37) | <0.001 | 67.5 | 0.08 | |
| Including non‐hospitalizations for HF c | 45 | 1.40 (1.33–1.46) | <0.001 | 80.5 | <0.001 | 0.52 |
| Special characteristics | ||||||
| Non‐specified | 36 | 1.33 (1.30–1.35) | <0.001 | 83.2 | <0.001 | g |
| After CRT and/or LVAD implantation | 8 d | 1.41 (1.24–1.61) | <0.001 | 51.9 | 0.04 | 0.88 |
| After AMI e | 2 d | 1.25 (1.05–1.48) | 0.01 | 67.5 | 0.08 | 0.26 |
| Others f | 2 | 1.66 (1.26–2.18) | <0.001 | 0.0 | 0.40 | 0.45 |
| EF status | ||||||
| Non‐specified | 24 | 1.33 (1.28–1.38) | <0.001 | 59.6 | <0.001 | — g |
| Reduced EF | 17 | 1.37 (1.24–1.50) | <0.001 | 80.4 | <0.001 | 0.82 |
| Preserved EF | 6 | 1.72 (1.32–2.26) | <0.001 | 86.7 | <0.001 | 0.02 |
Abbreviations: AMI, acute myocardial infarction; CHD, coronary heart disease; CRT, cardiac resynchronization therapy; EF, ejection fraction; LVAD, left ventricular assist device.
Cohort study that was originally designated as a trial.
In one study, 124 data based on the subgroup analysis according to age instead of gender were used.
Worsening of HF that did not lead to hospitalization.
Because one study 125 was included in the two categories indicated as #, total number of data (n = 47) in this stratified analysis was different from that in the overall analysis.
Number of data and RRs are not consistent with those in the text because a sub‐cohort study wherein the cohort was limited to patients having underlying diseases indicated as was excluded from this stratified analysis if the original cohort study existed.
Including patients on dialysis (1 study) and who were administered tolvaptan (1 study).
Multivariate regression analysis was performed.
Discussion
This meta‐analysis is the first to separately assess the risk of new‐onset and recurrent HF in individuals with DM. Current results confirm that DM is a significant risk factor for both new‐onset and recurrent HF. The explanation for these results is that impaired insulin signalling is associated with early changes in the heart such as cardiac stiffness, hypertrophy, and fibrosis. 138
Given that diastolic dysfunction is the first hallmark of diabetic cardiomyopathy, 139 the risk magnitude for HF in individuals with DM would be larger for HFpEF than for HFrEF. That is because among those with HF, the proportion of HFpEF was greater than that of HFrEF in individuals with than without DM. However, the current meta‐analysis revealed no difference in the magnitude of risk between HFpEF and HFrEF. One plausible explanation is that it is difficult to detect the HF in the early stage that is classified as HFpEF, which specifically occurs in patients with DM. 140
The stratified analysis by the study population's mean age suggested that the risk magnitude of new‐onset HF in relation to DM was especially large in relatively young study populations (i.e. in the current meta‐analysis, ≤60 years). Thus, individuals with DM had a high risk of incident HF even if relatively young. A possible explanation is that the relative contribution of DM to HF is larger in the young than in the elderly, as the younger population has not yet experienced the health burdens of aging or age‐associated conditions such as CHD, which might overwhelm the contribution of DM to HF. However, a further plausible explanation should be sought.
According to the results of the meta‐regression analysis wherein the baseline EF status was an explanatory variable, it is suggested that the impact of DM on the risk of recurrent HF is relatively large in HF patients with HFpEF. It is possible that individuals with DM had an especially poor prognosis as compared with those without DM in terms of recurrent HF when the EF is preserved. This possibility is supported by the RELAX (PhosphodiesteRasE‐5 Inhibition to Improve CLinical Status and EXercise Capacity) study reporting that impaired exercise capacity, increased left ventricular hypertrophy, high prevalence of co‐morbidities, and increased biomarkers of fibrosis, oxidative stress, inflammation, and vasoconstrictions in HFpEF patients with DM could contribute to adverse outcomes. 141 Differences in these cardiovascular phenotypes between patients with and without DM were notable among HF cases, in particular HFpEF, indicating that HFpEF is a heterogeneous syndrome. 142
Results of the stratified analysis according to the proportion of men (65%) suggested that the impact of DM on the risk of recurrent HF was stronger in women than in men. This could be explained by deficiencies in managing HF rather than susceptibility of women with DM to recurrent HF. The Euro Heart Survey on Heart Failure indicated that, compared with men, women were less often treated with drugs proven to reduce mortality such as angiotensin‐converting enzyme inhibitors, beta‐blockers, and spironolactone. 143 In addition, women were less likely to undergo assessment of left ventricular function. 143 Another explanation is that, in comparison with men, women have less potential to benefit from management of HF rather than to suffer from deficiencies in management, because women have a higher proportion of HFpEF, 144 for which no effective treatment with a high grade of evidence has been identified. 145
Several limitations should be addressed. First, the follow‐up period varied among studies, which could affect study results. Second, a meta‐analysis of observational studies generally elicits a low grade of evidence. Furthermore, according to the method for assessing the quality of evidence, 146 our findings of large between‐study heterogeneity and statistically significant publication bias might have further downgraded the quality of evidence. However, regarding the suspected publication bias, the RR that was deflated by the adjustment for publication bias was modest. It is unlikely that we need to change the general conclusions. Third, in most studies, type 2 DM was not differentiated from type 1 DM, although most patients with DM have type 2 and many features of cardiac phenotypes are shared by type 1 and type 2 DM. 147 In addition, we could not perform sensitivity analyses based on characteristics of patients with DM at baseline such as duration of DM, haemoglobin A1c, and hypoglycaemic medications including insulin use as most studies lacked these data. These characteristics could substantially affect the results. Fourth, hospitalization has a narrower range of endpoints involved in HF outcomes than a doctor's diagnosis or self‐report of HF that did or did not lead to hospitalization due to HF. The characteristics of endpoints could modify the impact of DM on the risk of HF given that the HF cases with DM were more likely to have experienced hospitalization than those without DM. 141 Lastly, as is inherent to the nature of study‐level meta‐analyses, degrees of confounder adjustments across the included studies varied, which hampers a comprehensive assessment of the impact of a risk factor (i.e. DM in this meta‐analysis) on the outcome (i.e. new‐onset and recurrent HF in this meta‐analysis).
Conclusions
The present results indicate that DM is a significant risk factor for both new‐onset and recurrent HF. It is suggested that the risk magnitude is large for new‐onset HF especially in young populations and for recurrent HF especially in women or those with HFpEF. These findings help to specify the populations that should be the focus of preventive strategies for DM‐related HF. It is also indicated that DM is associated with future HFpEF and HFrEF to the same extent, which could possibly be explained by a current finding that HF in the early stage in patients with DM is difficult to detect.
Conflict of interest
None declared.
Author Contributions
All authors conceived and designed the research; S.K., K.F., C.H., T.S., and M.I. acquired the data; S.K., K.K., and H.So. analysed the data; S.K. drafted the manuscript; and S.K., T.Y., K.K., K.W., H.Sh., T.I., and H.So. interpreted the results and made critical revision of the manuscript for important intellectual content. All authors approved the submission of the final manuscript.
Funding
The study was funded by a Grant‐in‐Aid for Scientific Research from the Japan Society for the Promotion of Science (ID: 19K12840). The sponsor had no influence over the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Acknowledgements
All authors thank Ms. Haga and Ms. Chino in the Niigata University for their excellent secretarial work.
Appendix 1. Study keywords used for electronic literature searches
S1 (retrospective OR retrospectively OR longitudinal OR prospective OR prospectively OR cohort OR followup OR follow‐up OR "follow up" OR period OR observation OR observational OR concurrent) AND (study OR studies)
S2 "odds ratio" OR "OR" OR "RR" OR "relative risk[*1]" OR "hazard ratio[*1]" OR ((incident OR incidence) AND rate[*1]) OR person‐years OR "person years" OR "risk ratio[*1]"
S3 ti((failure OR insufficiency OR decompensation OR incompetence) AND (heart OR cardiac OR myocardial) OR "congestive failure" OR (diabetic AND (heart OR myocardial OR cardiomyopath[*3])))
S4 (MJEMB ("heart failure") OR MJEMB ("congestive heart failure")) OR (MJMESH ("Heart Failure")) OR (MJMESH ("Diabetes Complications") AND MESH ("Heart Failure"))
S5 S4 OR S3
S6 (glycemia OR hyperglucemia OR hyperglycaemia OR hyperglycemia OR glycaemia OR glucose OR diabet[*2]) AND ti (predict[*3] OR risk OR (associated AND factors) OR incident OR incidence OR determinant[*1] OR profile[*1])
S7 ab (glycemia OR hyperglucemia OR hyperglycaemia OR hyperglycemia OR glycaemia OR glucose OR diabet[*2])
S8 (S6 OR S7) AND S5
S9 NOT (RTYPE("Conference Abstract" OR "Conference Paper" OR "Letter" OR "Editorial" OR "Case Reports" OR "Note" OR "Short Survey" OR "Comment" OR “Review”))
S10 S1 AND S2 AND S8 AND S9
- MESH
thesaurus terms of MEDLINE
- EMB
thesaurus terms of EMBASE
- MJ
major thesaurus terms
- RTYPE
publication type
‘ti’ and ‘ab’ indicate that the descriptor terms in parentheses exist in the title and abstract, respectively.
Asterisk (*) and its subsequent number in each bracket indicate allowing inflections within the number of characters.
Appendix 2. Study inclusion criteria
-
1
Cohort study
We also considered a study that was originally designed as a trial such as a randomized controlled trial but then was treated as a cohort study.
-
2
Diabetes status [i.e. whether a participant had diabetes mellitus (DM) or not] of all participants was ascertained before the follow‐up period.
Even if the type of design was a cohort study, a study that concurrently examined whether the participants developed DM and whether they developed HF was excluded.
-
3
At least 6 months of follow‐up
The interest of this study is the chronic effect of DM. Therefore, studies whose outcome was incident early‐onset HF (e.g. HF occurring during hospitalization due to coronary heart disease) were excluded.
-
4
Exposure is having DM at baseline.
Every participant in the risk group had to have DM at baseline. For example, studies were excluded in which the risk group, that is, participants with impaired fasting glucose/impaired glucose tolerance (IFG/IGT), were combined with those with DM.
-
5
Referent is not having DM at baseline.
Studies had to include only participants who did not have DM at baseline. For example, studies were excluded that only included individuals with normal glucose tolerance (i.e. excluded those with IFG/IGT).
Appendix 3. Remarks on data extraction in this meta‐analysis
If two or more risk indicators with different degrees of adjustments for confounding factors were provided within one study, we extracted the most fully adjusted risk indicators in the individual study. If both overall and subgroup analyses (e.g. age, gender, and age and gender) were performed in an individual study, the most finely stratified data (i.e. age and gender in the above example) were extracted. If the risk indicators were provided for each subgroup into which the participants were classified by either gender or age, priority for the overall analysis was given to the data based on the subgroup analysis by gender. In this case, data based on the subgroup analysis by age, instead of those by gender, were used in subsequent stratified analyses.
When a study included participants with type 2 diabetes mellitus (DM) but excluded those with type 1 DM, we simply pooled the data on the risk for HF in individuals with type 2 DM with the data on the risk for HF in studies which type 1 and type 2 DM were combined because type 2 DM accounts for almost all individuals with DM. One study 94 provided data on the risk for HF in individuals with type 1 DM and type 2 DM separately but did not provide data on the risk for HF wherein type 1 and type 2 DM were combined. In this case, we chose the data on type 2 DM.
Appendix 4. Study quality assessment using the Newcastle‐Ottawa Quality Assessment Scale adapted for this meta‐analysis
< For studies that examined the risk of new‐onset heart failure (HF) in relation to diabetes mellitus (DM) >
S: Selection
-
S1. Representative of the cohort
Non‐selected study population except for age and gender* #1
Specific characteristics (e.g. post‐menopausal, specific occupation)
Specific underlying diseases (e.g. coronary heart diseases (CHD), renal diseases, atrial fibrillation)
Study design was originally a trial.
Non‐selected patients
-
S2. Relationship between the analysis sample and the full cohort?
Equal*
Analysis sample was a random sample of the full cohort
The non‐exposed cohort (i.e. individuals without DM) was selected for the exposed cohort (i.e. individuals with DM) (e.g. propensity‐matched cohort)
-
S3. Confirmation of exposure (i.e. whether participants had DM at baseline)
Medical records* #2
Registry* (e.g. accessing study‐specific database, using the code of the International Statistical Classification of Diseases)
Self‐report/questionnaire
Unclear
-
S4. Did the study confirm that the outcome (i.e. incident heart failure) was not present at the beginning of the study? #3
Yes*
Unclear
C: Comparability
-
C1. Did the study control for the most important factors (i.e. age and CHD)?
Yes*
No
O: Outcome
-
O1. Ascertainment of outcome
Medical records (i.e. doctor's diagnosis)
Registry* (e.g. accessing study‐specific database, investigators' reviews using the code of the International Statistical Classification of Diseases)
Self‐report/questionnaire
Unclear
-
O2. Duration of follow‐up
≥6 years *
< 6 years
-
O3. Adequacy of follow‐up of cohorts
Complete follow‐up (i.e. lost to follow‐up rate was zero)*
Not complete follow‐up, but appropriate reasons for the lost to follow‐up were described*
Neither complete follow‐up nor description of appropriate reasons for the lost to follow‐up
Follow‐up rate was unclear
< For studies that examined the risk of recurrent HF in relation to DM >
S: Selection
-
Representative of the cohort
Typical patients with HF*
Typical patients with HF, but limited to patients within specific range of ejection fraction*
Specific characteristics (e.g. receiving cardiac resynchronization therapy or left ventricular assist device placement)
Specific underlying diseases (e.g. CHD)
Study design was originally a trial
-
2
Relationship between the analysis sample and the full cohort?
Equal*
Not equal
-
3
Confirmation of exposure (i.e. whether patients had DM at baseline)
Medical records* #3
Registry* (e.g. accessing study‐specific database, using the code of the International Statistical Classification of Diseases)
Self‐report/questionnaire
Unclear
-
4
Did the study confirm that the outcome (i.e. recurrent episode of HF) was not present at the beginning of the study? #4
C: Comparability
-
Did study control for the most important factors (i.e. age and CHD)?
Yes
No
O: Outcome
-
Ascertainment of outcome
Medical records (i.e. doctor's diagnosis)
Registry* (e.g. accessing study‐specific database, investigators' reviews using the code of the International Statistical Classification of Diseases)
Self‐report/questionnaire
Unclear
-
2
Duration of follow‐up
a) ≥2.1 years*
b) ≤2 years
-
3
Adequacy of follow‐up of cohorts
Complete follow‐up (i.e. lost to follow‐up rate was zero)*
Not complete follow‐up, but appropriate reasons for the lost to follow‐up were described*
Neither complete follow‐up nor description of appropriate reasons for the lost to follow‐up
Follow‐up rate was unclear
NOS scale consists of 7 criteria that are classified into the following 3 broad perspectives: S (Selection), C (Comparability), and O (Outcome). One star (*) corresponds to one point. Full score is 8.
#1 Including population that excluded participants with CHD at baseline.
#2 Including direct measurement of blood glucose levels by the study.
#3 Including direct measurement of blood glucose levels by the study.
#4 In meta‐analysis of risk of recurrent HF, this criterion is not applicable. All studies were given one point.
Appendix 5. Flow chart describing procedures for selection of studies that examined the risk of incident new‐onset heart failure (HF) in relation to diabetes mellitus (DM)
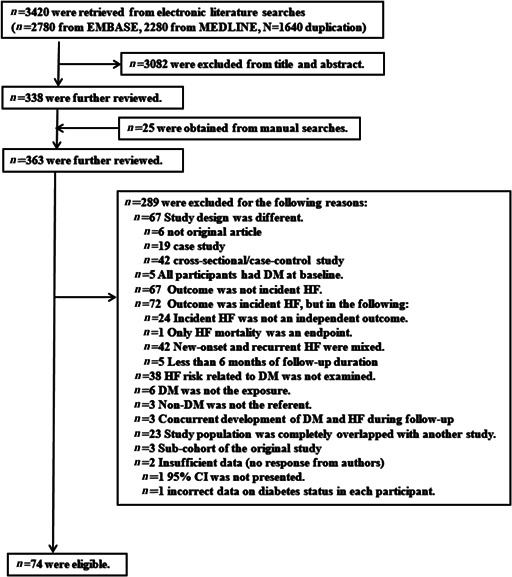
Abbreviation: CI, confidence interval
Appendix 6. Flow chart describing procedures for selection of studies that examined risk of recurrent heart failure (HF) in relation to diabetes mellitus (DM)
Appendix 7. Study confounders considered when the relationship between diabetes mellitus and new‐onset heart failure was examined
| Study source | Confounders |
|---|---|
| Chen (2019) 87 | None |
| Fogarassy (2019) 86 | Age, HT, CHD, stroke, cancer stage, chemotherapies, antihypertensive agents |
| Magnusssen (2019) 89 | Age, gender, smoking, BMI, HT, antihypertensive medication, TC |
| Winell (2019) 88 | (Age), (gender) |
| Chen (2018) 32 | Age, gender, region, CHD, coronary revascularization, medication |
| Eggimann (2018) 33 | Age, BMI, valve surgery, arrhythmia intervention, QTc, BNP |
| Gong (2018) 34 | Age, smoking, BMI, MI, OSA, NT‐proBNP, Hb, calcium channel blocker |
| McAllister (2018) 90 | (Age), (gender) |
| Lamblin (2018) 91 | Age, BMI, HT, multi‐vessel CAD, angina, AF, (CHD) |
| LaMonte (2018) 42 | (Gender) |
| Larsson (2018) 94 | Age, gender, BMI, education, (CHD), FH of MI, smoking, PA, HT, HL, alcohol, DASH diet score |
| Rosengren (2018) 35 | Age, (gender), income, education, marital status, duration of DM, stroke, CHD, AF, renal dialysis or transplantation |
| Wandell (2018) 36 | Age, (gender), obesity, socio‐demography, HT, valvular disease, cardiomyopathy, COPD, OSA |
| Wellings (2018) 37 | Age, gender, race, insurance, HT, (CHD), liver disease, CKD, dyslipidaemia |
| Agarwal (2017) 27 | Age, gender, race, HT, CAD, AF, income, ventricular premature complexes |
| Ballotari (2017) 38 | None |
| Chatterjee (2017) 39 | Age, (gender), race, assignment, smoking, PA, alcohol, BMI, SBP, HL, history of MI, CKD, (AF), medication |
| He (2017) 41 | Age, gender, education, WC, SBP, cystatin C, urine albumin, CVD |
| Jacobs (2017) 97 | Age, gender, BMI, smoking, CAD, HT, SBP, HR, Cre, antihypertensive agents |
| Kim (2017) 40 | Age, gender, smoking, obesity, HT, DM, dyslipidaemia, CHD |
| Pandey (2017) 43 | None |
| Policardo (2017) 44 | Age, (gender), Charlson's index, CVD |
| RD, malignancy, Hb, Na, K, BUN, Cre, baseline EF medication | |
| Zhang (2017) 31 | Age, gender, socioeconomic status, race/ethnicity, HT, MI, PVD, cerebrovascular accident, pulmonary disease, RD, malignancy, Hb, Na, K, BUN, Cre, baseline EF medication |
| Eaton (2016) 28 | Age, education, income, smoking, HT, AF, CHD, chronic lung disease, PA, medication, alcohol, other morbidities, anaemia |
| Goldhar (2016) 45 | Age, (gender), income, rural status, HT, previous MI, chemotherapy regimens, cancer stage |
| Ho (2016) 29 | Age, gender, smoking, alcohol, BMI, HT, MI, LVH, LBBB (left bundle branch block) |
| Sahle (2016) 46 | Age, gender, smoking, BMI, BP, CVD, eGFR, HDL |
| Silverman (2016) 30 | Men: age, gender, race, HrR, HT, BMI, TC, HDL, eGFR, IL‐6, coronary artery calcium score, MI during follow‐up, proBNP, Troponin T, LV mass index; women: age, gender, race, HrR, HT, smoking, HDL, eGFR, IL‐6, coronary artery calcium score, MI during follow‐up, proBNP, troponin T, LV mass index |
| Chahal (2015) 47 | Age, gender, smoking, BMI, SBP, HrR, Cre, LVH, (CVD) |
| Donneyong (2015) 48 | None |
| Qin (2015) 49 | None |
| Shah (2015) 50 | Age, gender, smoking, deprivation, BMI, SBP, HDL, TC, statin, (CHD), antihypertensive drugs |
| Miao (2014) 96 | Age, obesity, arrhythmias, PVD, pulmonary disease, pulmonary vascular disease, HT, hypothyroidism, CKD, LD, AIDS, weight loss, electrolyte disorders |
| Wong (2014) 51 | None |
| Brouwers (2013) 52 | Age, gender, obesity, HT, MI, smoking, AF, HL, Cre, cystatine C, UA, CRP, NT‐proBNP, hs‐TnT |
| Ho (2013) 53 | Age, gender, HT, BMI, HrR, MI, CHD, smoking, valvular disease, HDL, AF, LVH, LBBB |
| Hung (2013) 100 | (CHD), age, gender |
| Potpara (2013) 54 | Age, gender, medication |
| Qureshi (2013) 55 | None |
| Agarwal (2012) 56 | Age, gender, race |
| Nakajima (2012) 57 | None |
| Sato (2012) 98 | (CHD), smoking, HT, MVD |
| Shafazand (2011) 99 | (CHD), age, gender, stroke, AF, valvular disease |
| Roy (2011) 58 | Multiple (65 characteristics) |
| de Simone (2010) 59 | None |
| Goyal (2010) 60 | Age, (gender), CHD, AF, valvular diseases |
| Smith (2010) 61 | Age, gender, BMI, HT, MI, (AF), smoking, MR‐proANP, NT‐proBNP, MR‐proADM, CRP, cystatine C, copeptin |
| van Melle (2010) 62 | Age, gender, race, smoking, BMI, PA, LDL, SBP, MI during follow‐up, LVEF, wall motion abnormality, diastolic dysfunction, CRP, medication |
| Bibbins‐Domingo (2009) 63 | (CHD) |
| Kenchaiah (2009) 64 | None |
| Leung (2009) 65 | Age, gender |
| Lewis (2009) 66 | Age, BMI, MI, bypass surgery, HT, angina, GFR, LVEF, medication |
| Ruigomez (2009) 67 | Age, gender, AF, alcohol, smoking, BMI, HT, hyperlipidaemia, venous thromboembolism, CHD, cardiac diseases, COPD |
| Nafaji (2008) 92 | Age, gender, smoking, HT, ECG, CARP, streptokinase or rTPA |
| Aksnes (2007) 68 | Age, LVH, CHD, DM during follow‐up |
| Fukuda (2007) 69 | Age, gender, HT, structural heart disease, persistent AF, %FS, LAD, LVH |
| Held (2007) 70 | None |
| Ito (2007) 71 | Anaemia (Hb < 10 g/dL) |
| Ingelsson (2005) 72 | (Age), (gender), MI, HT, LVH, smoking, BMI |
| Lentine (2005) 73 | Age, gender, smoking, employment, BMI, cause of ESRD, anaemia, MI, arrhythmia, peripheral artery disease, donors' characteristics, graft function, complications during follow‐up |
| Bibbins‐Domingo (2004) 74 | Age, (gender), smoking, SBP, BMI, ECG, CAD grafting, no. of ischaemic origin, Cre |
| Nichols (2004) 75 | None |
| Wylie (2004) 76 | Age, CHD, BNP, ECG, HrR |
| Lewis (2003) 77 | Age, PA, HT, previous MI, LVEF |
| Rigatto (2002) 78 | Age, SBP, Hb, albumin, cadaveric donor, (CHD) |
| Williams (2002) 93 | Age, gender, HT, MI, PP, depression, functional limitations |
| Abramson (2001) 95 | Age, gender, race, smoking, MI, angina, SBP, DBP, TC, HDL, ECG, trial group, ADL |
| He (2001) 79 | Age, gender, race, CHD |
| Johansson (2001) 80 | Age, smoking, BMI, hyperlipidaemia, prior dyspnea irrelevant to HT, prior co‐morbidity (inc. CHD) |
| Wilhelmsen (2001) 81 | Age, (gender), smoking, alcohol, coffee, BMI, HT, (CHD) |
| Aronow (1999) 82 | Age, gender, race, HT, CHD |
| Chen (1999) 83 | Age, gender, PP, BMI, MI during follow‐up |
| Kannel (1999) 84 | Age, (gender), SBP, LVH, heart rate, (CHD), valve disease |
| Harnett (1995) 85 | Age, DBP, CHD, systolic dysfunction, Hb, albumin, LV mass |
The confounder in parentheses indicates that the risk measure was adjusted for this confounder although the adjustment was not stated.
Abbreviations: ADL, activities of daily living; AF, atrial fibrillation; BMI, body mass index; AIDS, acquired immunodeficiency syndrome; BNP, brain natriuretic peptide; BP, blood pressure; CARP, coronary artery revascularization procedure; CHD, coronary heart disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; Cre, creatinine; CRP, C‐reactive protein; CVA, cerebrovascular accidents; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; EF, ejection fraction; eGFR, estimated glomerular filtration rate; ESRD, end stage renal disease; FS, fractional shortening; GFR, estimated glomerular filtration rate; Hb, haemoglobin; HDL‐C, high‐density lipoprotein cholesterol; HL, hyperlipidaemia; HrR, heart rate; hs‐TnT, high‐sensitivity troponin T; HT, hypertension; IL, interleukin; LAD, left atrial diameter; LBBB, left bundle branch block; LD, liver diseases; LDL‐C, low‐density lipoprotein cholesterol; LT, liver transplantation; LV mass, left ventricular mass; LVH, left ventricular hypertrophy; MI, myocardial infarction; MR‐proANP, mid‐regional pro‐atrial natriuretic peptide; MR pro‐ADM, Mid‐regional pro‐adrenomedullin; MVD, multi‐vessel disease; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; OSA, obstructive sleep apnoea; PA, physical activity; PP, pulse pressure; PVD, peripheral vascular disease; RD, renal diseases; rTPA, recombinant tissue plasminogen activator; SBP systolic blood pressure; TC, total cholesterol; UAE, urine albumin excretion; WC, waist circumference.
Appendix 8. Results of assessing quality of studies that examined the risk of new‐onset heart failure in relation to diabetes mellitus based on the adapted Newcastle‐Ottawa Scale (NOS). The criterion corresponding to each combination of a capital letter and a number is indicated in Appendix 4
| Study source | S1 | S2 | S3 | S4 | C1 | O1 | O2 | O3 | Score |
|---|---|---|---|---|---|---|---|---|---|
| Chen (2019) 87 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 3 |
| Fogarassy (2019) 86 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 6 |
| Magnusssen (2019) 89 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 5 |
| Winell (2019) 88 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Chen (2018) 32 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Eggimann (2018) 33 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 4 |
| Gong (2018) 34 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 5 |
| McAllister (2018) 90 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Lamblin (2018) 91 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| LaMonte (2018) 42 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 3 |
| Larsson (2018) 94 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Rosengren (2018) 35 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 6 |
| Wandell (2018) 36 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Wellings (2018) 37 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 5 |
| Agarwal (2017) 27 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 5 |
| Ballotari (2017) 38 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 5 |
| Chatterjee (2017) 39 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 5 |
| He (2017) 41 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 6 |
| Jacobs (2017) 97 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 5 |
| Kim (2017) 40 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Pandey (2017) 43 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 5 |
| Policardo (2017) 44 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 6 |
| Zhang (2017) 31 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| Eaton (2016) 28 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 5 |
| Goldhar (2016) 45 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| Ho (2016) 29 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Sahle (2016) 46 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Silverman (2016) 30 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 6 |
| Chahal (2015) 47 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 5 |
| Donneyong (2015) 48 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 4 |
| Qin (2015) 49 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 5 |
| Shah (2015) 50 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 5 |
| Miao (2014) 96 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 5 |
| Wong (2014) 51 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 3 |
| Brouwers (2013) 52 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Ho (2013) 53 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Hung (2013) 100 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| Potpara (2013) 54 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5 |
| Qureshi (2013) 55 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 4 |
| Agarwal (2012) 56 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Nakajima (2012) 57 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 4 |
| Sato (2012) 98 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 4 |
| Shafazand (2011) 99 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 6 |
| Roy (2011) 58 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 6 |
| de Simone (2010) 59 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Goyal (2010) 60 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
| Smith (2010) 61 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| van Melle (2010) 62 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 4 |
| Bibbins‐Domingo (2009) 63 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Kenchaiah (2009) 64 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| Leung (2009) 65 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 4 |
| Lewis (2009) 66 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| Ruigomez (2009) 67 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Nafaji (2008) 92 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Aksnes (2007) 68 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 4 |
| Fukuda (2007) 69 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| Held (2007) 70 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 3 |
| Ito (2007) 71 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 4 |
| Ingelsson (2005) 72 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Lentine (2005) 73 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| Bibbins‐Domingo (2004) 74 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 5 |
| Nichols (2004) 75 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 4 |
| Wylie (2004) 76 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 4 |
| Lewis (2003) 77 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 4 |
| Rigatto (2002) 78 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Williams (2002) 93 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Abramson (2001) 95 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 4 |
| He (2001) 79 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Johansson (2001) 80 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 6 |
| Wilhelmsen (2001) 81 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 6 |
| Aronow (1999) 82 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
| Chen (1999) 83 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 6 |
| Kannel (1999) 84 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Harnett (1995) 85 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 6 |
Appendix 9. Study confounders considered when the relationship between diabetes mellitus and recurrent heart failure was examined
| Study source | Confounders |
|---|---|
| Kim (2019) 134 | Age, (gender), BMI, SBP, HR, HT, CHD, Hb, Na, Cre, NT‐proBNP, LVEF, medications |
| Chen (2018) 101 | None |
| Cooper (2018) 102 | Age, gender, race, BMI, SBP, HrR, NYHA, CHD, AF, PVD, COPD, CKD, ACE‐I, ARB, diuretics |
| Iorio (2018) 137 | Age, gender |
| Kristensen (2018) 103 | None |
| Retwinski (2018) 136 | None |
| Rorth (2018) 104 | Age, gender, education, IHD, AF, CKD, COPD, HT, stroke, cancer, medications |
| Sandesara (2018) 105 | None |
| Takimura (2018) 135 | Age, duration after previous HF, Hb, UA, LVEF, LAVI |
| Dauriz (2017) 106 | Age, gender, smoking, BMI, SBP, eGFR, LVEF, IHD, HT, statin, stroke, COPD, Hb |
| Farre (2017) 107 | Age, gender, recent HF, anaemia, valvular disease, IHD, CKD, dialysis, AF, cardiac conduction disorders, cancer, stroke, dementia, cirrhosis number of hospitalization, visits to emergency department |
| Kristensen (2017) 108 | Age, gender, recent HF, LVEF, HrR, eGFR, NT‐proBNP, neutrophils, COPD, MI, ischaemic origin |
| Mohamedali (2017) 109 | None |
| Echouffo‐Tcheugui (2016) 110 | Age, gender, race, LVEF, NYHA, AF, ischaemic cardiomyopathy, ECG (LBBB, wide QRS), cardiac conduction disorders, HF duration, Cre, history of syncope, FH of sudden death, CHD, ventricular tachycardia medications |
| Kristensen (2016) 111 | None |
| Ruigomez (2016) 112 | Age, gender, smoking, alcohol, BMI, residence, IHD, stroke, HT, AF, hyperlipidaemia, COPD, asthma, RD, visiting hospital in the previous year |
| Kaneko (2015) 113 | Age, IHD, DBP, HrR, diuretics |
| Takeda (2015) 114 | None |
| Carrasco‐Sanchez (2014) 115 | Age, NYHA, GFR, Na, BMI, anaemia, PVD, beta‐blocker, ACE‐Is, ARBs |
| Cubbon (2014) 116 | Pulmonary congestion, previous HF, diuretics |
| Paoletti (2014) 117 | None |
| Sakata (2014) 118 | Age, gender, smoking, BMI, SBP, HT, dyslipidaemia, LVEF, HrR, Hb, Cre, BNP, medications |
| Larina (2013) 119 | None |
| Sarma (2013) 120 | Age, gender, smoking, BMI, SBP, EF, Na, BUN, QRS duration, BNP/NT‐proBNP, AF, HT, CKD, stroke, medications |
| Verbrugge (2012) 121 | Obesity, HT, COPD, CKD, NYHA, right ventricular function, ischaemic aetiology of HF |
| Deedwania (2011) 122 | Propensity‐matched for age, gender, smoking, BMI, SBP, DBP, HrR, others (multiple) (previous diseases, laboratory data, medications) |
| Martin (2011) 123 | None |
| Aguilar (2010) 124 | Age, gender, obesity, ischaemic origin, NYHA |
| Sze (2010) 125 | Gender, NYHA, AF, wide QRS, HrR, one of renal function indicators, beta‐blocker, diuretics |
| MacDonald (2008) 126 | 32 covariates (including age, gender, smoking, SBP, DBP, NYHA, LVEF, HrR, IHD, stroke, AT, pacemaker, various medications) |
| Macdonald (2008) 127 | Age, (gender), co‐morbidities (including CHD) |
| Ghali (2007) 128 | None |
| Ruiz‐Ruiz (2007) 129 | None |
| Formiga (2006) 130 | Age, gender, SBP, PIP |
| Garcia (2005) 131 | None |
| Domanski (2003) 132 | Age, gender, BMI, race, Cre, SBP, aetiology of HF, cholesterol, diuretics, vasodilators |
| Shindler (1996) 133 | Age, gender, race, EF, aetiology of left ventricular dysfunction, NYHA |
| Harnett (1995) 85 | Age, IHD, EF, Hb, albumin, DBP, LV mass |
The confounder in parentheses indicates that the risk measure was adjusted for this confounder although the adjustment was not stated.
Abbreviations: ACE‐Is, angiotensin‐converting enzyme inhibitors; AF, atrial fibrillation; AMI, acute myocardial infarction; ARBs, angiotensin receptor blockers; BMI, body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CHD, coronary heart disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; Cre, creatinine; DBP, diastolic blood pressure; EF, ejection fraction; eGFR, estimated glomerular filtration rate; FH, family history; Hb, haemoglobin; HL, hyperlipidaemia; HrR, heart rate; hs‐TnT, high‐sensitivity troponin T; HT, hypertension; ICD, implantable cardioverter–defibrillator; LAVI, left atrial volume index; LBBB, left bundle branch block; LVAD, left ventricular assist device placement, LV mass, left ventricular mass; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association classification; PIP, procollagen type 1; PVD, peripheral vascular disease; RD, renal diseases; SBP systolic blood pressure; TC, total cholesterol; UA, uric acid; VT, ventricular tachycardia.
Appendix 10. Results of assessing quality of studies that examined the risk of recurrent heart failure in relation to diabetes mellitus based on the adapted Newcastle‐Ottawa Scale (NOS). The criterion corresponding to each combination of a capital letter and a number is indicated in Appendix 4
| Study source | S1 | S2 | S3 | S4 | C1 | O1 | O2 | O3 | Score |
|---|---|---|---|---|---|---|---|---|---|
| Kim (2019) 134 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 5 |
| Chen (2018) 101 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Cooper (2018) 102 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| Iorio (2018) 137 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Kristensen (2018) 103 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Retwinski (2018) 136 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 6 |
| Rorth (2018) 104 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Sandesara (2018) 105 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 4 |
| Takimura (2018) 135 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 5 |
| Dauriz (2017) 106 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 5 |
| Farre (2017) 107 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Kristensen (2017) 108 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Mohamedali (2017) 109 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 5 |
| Echouffo‐Tcheugui (2016) 110 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Kristensen (2016) 111 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 5 |
| Ruigomez (2016) 112 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Kaneko (2015) 113 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Takeda (2015) 114 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Carrasco‐Sanchez (2014) 115 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Cubbon (2014) 116 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 6 |
| Paoletti (2014) 117 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Sakata (2014) 118 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Larina (2013) 119 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Sarma (2013) 120 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 4 |
| Verbrugge (2012) 121 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 5 |
| Deedwania (2011) 122 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 4 |
| Martin (2011) 123 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 3 |
| Aguilar (2010) 124 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 4 |
| Sze (2010) 125 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 4 |
| MacDonald (2008) 126 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 6 |
| Macdonald (2008) 127 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Ghali (2007) 128 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 4 |
| Ruiz‐Ruiz (2007) 129 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 6 |
| Formiga (2006) 130 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 6 |
| Garcia (2005) 131 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 4 |
| Domanski (2003) 132 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 5 |
| Shindler (1996) 133 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 4 |
| Harnett (1995) 85 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 6 |
Kodama, S. , Fujihara, K. , Horikawa, C. , Sato, T. , Iwanaga, M. , Yamada, T. , Kato, K. , Watanabe, K. , Shimano, H. , Izumi, T. , and Sone, H. (2020) Diabetes mellitus and risk of new‐onset and recurrent heart failure: a systematic review and meta‐analysis. ESC Heart Failure, 7: 2146–2174. 10.1002/ehf2.12782.
References
- 1. Roger VL. Epidemiology of heart failure. Circ Res 2013; 113: 646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang H, Negishi K, Otahal P, Marwick TH. Clinical prediction of incident heart failure risk: a systematic review and meta‐analysis. Open Heart 2015; 2: e000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolinsky FD, Smith DM, Stump TE, Overhage JM, Lubitz RM. The sequelae of hospitalization for congestive heart failure among older adults. J Am Geriatr Soc 1997; 45: 558–563. [DOI] [PubMed] [Google Scholar]
- 4. Rawshani A, Rawshani A, Franzen S, Sattar N, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, McGuire DK, Rosengren A, Gudbjornsdottir S. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2018; 379: 633–644. [DOI] [PubMed] [Google Scholar]
- 5. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, Group CPC . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 6. Oellgaard J, Gaede P, Rossing P, Rorth R, Kober L, Parving HH, Pedersen O. Reduced risk of heart failure with intensified multifactorial intervention in individuals with type 2 diabetes and microalbuminuria: 21 years of follow‐up in the randomised Steno‐2 study. Diabetologia 2018; 61: 1724–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sattar N, Preiss D. Research digest: heart failure in diabetes comes into focus. Lancet Diabetes Endocrinol 2018; 6: 603. [DOI] [PubMed] [Google Scholar]
- 8. Kasznicki J, Drzewoski J. Heart failure in the diabetic population—pathophysiology, diagnosis and management. Arch Med Sci 2014; 10: 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128: 1810–1852. [DOI] [PubMed] [Google Scholar]
- 10. Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long K, Shah ND, Roger VL. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol 2009; 54: 1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ohkuma T, Komorita Y, Peters SAE, Woodward M. Diabetes as a risk factor for heart failure in women and men: a systematic review and meta‐analysis of 47 cohorts including 12 million individuals. Diabetologia 2019; 62: 1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aune D, Schlesinger S, Neuenschwander M, Feng T, Janszky I, Norat T, Riboli E. Diabetes mellitus, blood glucose and the risk of heart failure: a systematic review and meta‐analysis of prospective studies. Nutr Metab Cardiovasc Dis 2018; 28: 1081–1091. [DOI] [PubMed] [Google Scholar]
- 13. Dauriz M, Mantovani A, Bonapace S, Verlato G, Zoppini G, Bonora E, Targher G. Prognostic Impact of Diabetes on Long‐term Survival Outcomes in Patients With Heart Failure: A Meta‐analysis. Diabetes Care 2017; 40: 1597–1605. [DOI] [PubMed] [Google Scholar]
- 14. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 15. Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta‐analysis. JAMA 2007; 298: 2654–2664. [DOI] [PubMed] [Google Scholar]
- 16. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 17. Shioi T, Inuzuka Y. Aging as a substrate of heart failure. J Cardiol 2012; 60: 423–428. [DOI] [PubMed] [Google Scholar]
- 18. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 19. Nesto RW. Prevalence of and risk factors for coronary heart disease in diabetes mellitus. In: Gersh BJ, Nathan DM, editors. UpToDate 2018.
- 20. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 21. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 22. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clin Research Ed) 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000; 56: 455–463. [DOI] [PubMed] [Google Scholar]
- 24. Galofre N, San Vicente L, Gonzalez JA, Planas F, Vila J, Grau J. Predictive factors for readmission in heart failure patients. Med Clin 2005; 124: 285–290. [DOI] [PubMed] [Google Scholar]
- 25. Wolinsky FD, Overhage JM, Stump TE, Lubitz RM, Smith DM. The risk of hospitalization for congestive heart failure among older adults. Med Care 1997; 35: 1031–1043. [DOI] [PubMed] [Google Scholar]
- 26. Alexander M, Grumbach K, Selby J, Brown AF, Washington E. Hospitalization for congestive heart failure. Explaining racial differences. JAMA 1995; 274: 1037–1042. [PubMed] [Google Scholar]
- 27. Agarwal V, Vittinghoff E, Whitman IR, Dewland TA, Dukes JW, Marcus GM. Relation between ventricular premature complexes and incident heart failure. Am J Cardiol 2017; 119: 1238–1242. [DOI] [PubMed] [Google Scholar]
- 28. Eaton CB, Pettinger M, Rossouw J, Martin LW, Foraker R, Quddus A, Liu S, Wampler NS, Hank Wu WC, Manson JE, Margolis K, Johnson KC, Allison M, Corbie‐Smith G, Rosamond W, Breathett K, Klein L. Risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Circ Heart Fail 2016; 9: e002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, Bartz TM, Santhanakrishnan R, Lee DS, Chan C, Liu K, Blaha MJ, Hillege HL, van der Harst P, van Gilst WH, Kop WJ, Gansevoort RT, Vasan RS, Gardin JM, Levy D, Gottdiener JS, de Boer RA, Larson MG. Predicting heart failure with preserved and reduced ejection fraction: The International Collaboration on Heart Failure Subtypes. Circ Heart Fail 2016; 9: e003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Silverman MG, Patel B, Blankstein R, Lima JA, Blumenthal RS, Nasir K, Blaha MJ. Impact of race, ethnicity, and multimodality biomarkers on the incidence of new‐onset heart failure with preserved ejection fraction (from the Multi‐Ethnic Study of Atherosclerosis). Am J Cardiol 2016; 117: 1474–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang L, Liebelt JJ, Madan N, Shan J, Taub CC. Comparison of predictors of heart failure with preserved versus reduced ejection fraction in a multiracial cohort of preclinical left ventricular diastolic dysfunction. Am J Cardiol 2017; 119: 1815–1820. [DOI] [PubMed] [Google Scholar]
- 32. Chen HF, Ho CA, Li CY. Risk of heart failure in a population with type 2 diabetes versus a population without diabetes with and without coronary heart disease. Diabetes Obes Metab 2018; 21: 112–119. [DOI] [PubMed] [Google Scholar]
- 33. Eggimann L, Blum S, Aeschbacher S, Reusser A, Ammann P, Erne P, Moschovitis G, Di Valentino M, Shah D, Schlapfer J, Mondet N, Kuhne M, Sticherling C, Osswald S, Conen D. Risk factors for heart failure hospitalizations among patients with atrial fibrillation. PLoS ONE 2018; 13: e0191736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gong FF, Jelinek MV, Castro JM, Coller JM, McGrady M, Boffa U, Shiel L, Liew D, Wolfe R, Stewart S, Owen AJ, Krum H, Reid CM, Prior DL, Campbell DJ. Risk factors for incident heart failure with preserved or reduced ejection fraction, and valvular heart failure, in a community‐based cohort. Open Heart 2018; 5: e000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosengren A, Edqvist J, Rawshani A, Sattar N, Franzen S, Adiels M, Svensson AM, Lind M, Gudbjornsdottir S. Excess risk of hospitalisation for heart failure among people with type 2 diabetes. Diabetologia 2018; 61: 2300–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wandell P, Carlsson AC, Holzmann MJ, Arnlov J, Sundquist J, Sundquist K. The association between relevant co‐morbidities and prevalent as well as incident heart failure in patients with atrial fibrillation. J Cardiol 2018; 72: 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wellings J, Kostis JB, Sargsyan D, Cabrera J, Kostis WJ, Myocardial Infarction Data Acquisition System Study G. Risk factors and trends in incidence of heart failure following acute myocardial infarction. Am J Cardiol 2018;122:1‐5. [DOI] [PubMed] [Google Scholar]
- 38. Ballotari P, Venturelli F, Greci M, Giorgi Rossi P, Manicardi V. Sex differences in the effect of type 2 diabetes on major cardiovascular diseases: results from a population‐based study in Italy. Int J Endocrinol 2017; 2017: 6039356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chatterjee NA, Chae CU, Kim E, Moorthy MV, Conen D, Sandhu RK, Cook NR, Lee IM, Albert CM. Modifiable risk factors for incident heart failure in atrial fibrillation. JACC Heart Fail 2017; 5: 552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim CH, Al‐Kindi SG, Jandali B, Askari AD, Zacharias M, Oliveira GH. Incidence and risk of heart failure in systemic lupus erythematosus. Heart 2017; 103: 227–233. [DOI] [PubMed] [Google Scholar]
- 41. He J, Shlipak M, Anderson A, Roy JA, Feldman HI, Kallem RR, Kanthety R, Kusek JW, Ojo A, Rahman M, Ricardo AC, Soliman EZ, Wolf M, Zhang X, Raj D, Hamm L, Investigators C. Risk factors for heart failure in patients with chronic kidney disease: the CRIC (Chronic Renal Insufficiency Cohort) Study. J Am Heart Assoc 2017; 17: 6(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. LaMonte MJ, Manson JE, Chomistek AK, Larson JC, Lewis CE, Bea JW, Johnson KC, Li W, Klein L, LaCroix AZ, Stefanick ML, Wactawski‐Wende J, Eaton CB. Physical activity and incidence of heart failure in postmenopausal women. JACC Heart Fail 2018; 6: 983–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pandey A, Kim S, Moore C, Thomas L, Gersh B, Allen LA, Kowey PR, Mahaffey KW, Hylek E, Peterson ED, Piccini JP, Fonarow GC, Investigators O‐A. Patients. Predictors and prognostic implications of incident heart failure in patients with prevalent atrial fibrillation. JACC Heart Fail 2017; 5: 44–52. [DOI] [PubMed] [Google Scholar]
- 44. Policardo L, Seghieri G, Francesconi P, Anichini R, Franconi F, Del Prato S. Gender difference in diabetes related excess risk of cardiovascular events: when does the ‘risk window’ open? J Diabetes Complications 2017; 31: 74–79. [DOI] [PubMed] [Google Scholar]
- 45. Goldhar HA, Yan AT, Ko DT, Earle CC, Tomlinson GA, Trudeau ME, Krahn MD, Krzyzanowska MK, Pal RS, Brezden‐Masley C, Gavura S, Lien K, Chan KK. The temporal risk of heart failure associated with adjuvant trastuzumab in breast cancer patients: a population study. J Natl Cancer Inst 2016; 108: djv301. [DOI] [PubMed] [Google Scholar]
- 46. Sahle BW, Owen AJ, Krum H, Reid CM, Second Australian National Blood Pressure Study Management C. Incidence of heart failure in 6083 elderly hypertensive patients: the Second Australian National Blood Pressure Study (ANBP2). Eur J Heart Fail 2016;18:38‐45. [DOI] [PubMed] [Google Scholar]
- 47. Chahal H, Bluemke DA, Wu CO, McClelland R, Liu K, Shea SJ, Burke G, Balfour P, Herrington D, Shi P, Post W, Olson J, Watson KE, Folsom AR, Lima JA. Heart failure risk prediction in the multi‐ethnic study of atherosclerosis. Heart 2015; 101: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Donneyong MM, Hornung CA, Taylor KC, Baumgartner RN, Myers JA, Eaton CB, Gorodeski EZ, Klein L, Martin LW, Shikany JM, Song Y, Li W, Manson JE. Risk of heart failure among postmenopausal women: a secondary analysis of the randomized trial of vitamin D plus calcium of the women's health initiative. Circ Heart Fail 2015; 8: 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qin A, Thompson CL, Silverman P. Predictors of late‐onset heart failure in breast cancer patients treated with doxorubicin. J Canc Survivorship: Res Pract 2015; 9: 252–259. [DOI] [PubMed] [Google Scholar]
- 50. Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades‐Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A, Hemingway H. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol 2015; 3: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wong TC, Piehler KM, Kang IA, Kadakkal A, Kellman P, Schwartzman DS, Mulukutla SR, Simon MA, Shroff SG, Kuller LH, Schelbert EB. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J 2014; 35: 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, van Veldhuisen DJ, van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community‐based cohort: 11‐year follow‐up of PREVEND. Eur Heart J 2013; 34: 1424–1431. [DOI] [PubMed] [Google Scholar]
- 53. Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG, Levy D. Predictors of new‐onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail 2013; 6: 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Potpara TS, Polovina MM, Licina MM, Marinkovic JM, Lip GY. Predictors and prognostic implications of incident heart failure following the first diagnosis of atrial fibrillation in patients with structurally normal hearts: the Belgrade Atrial Fibrillation Study. Eur J Heart Fail 2013; 15: 415–424. [DOI] [PubMed] [Google Scholar]
- 55. Qureshi W, Mittal C, Ahmad U, Alirhayim Z, Hassan S, Qureshi S, Khalid F. Clinical predictors of post‐liver transplant new‐onset heart failure. Liver Transplant 2013; 19: 701–710. [DOI] [PubMed] [Google Scholar]
- 56. Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, Folsom AR, He M, Hoogeveen RC, Ni H, Quibrera PM, Rosamond WD, Russell SD, Shahar E, Heiss G. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail 2012; 5: 422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nakajima K, Matsuo S, Okuyama C, Hatta T, Tsukamoto K, Nishimura S, Yamashina A, Kusuoka H, Nishimura T. Cardiac event risk in Japanese subjects estimated using gated myocardial perfusion imaging, in conjunction with diabetes mellitus and chronic kidney disease. Circ J 2012; 76: 168–175. [DOI] [PubMed] [Google Scholar]
- 58. Roy B, Pawar PP, Desai RV, Fonarow GC, Mujib M, Zhang Y, Feller MA, Ovalle F, Aban IB, Love TE, Iskandrian AE, Deedwania P, Ahmed A. A propensity‐matched study of the association of diabetes mellitus with incident heart failure and mortality among community‐dwelling older adults. Am J Cardiol. [Research Support, N.I.H., Extramural Research Support, Non‐U.S. Gov't] 2011; 108: 1747–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. de Simone G, Devereux RB, Chinali M, Lee ET, Galloway JM, Barac A, Panza JA, Howard BV. Diabetes and incident heart failure in hypertensive and normotensive participants of the Strong Heart Study. J Hypertens 2010; 28: 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Goyal A, Norton CR, Thomas TN, Davis RL, Butler J, Ashok V, Zhao L, Vaccarino V, Wilson PW. Predictors of incident heart failure in a large insured population: a one million person‐year follow‐up study. Circ Heart Fail 2010; 3: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Smith JG, Newton‐Cheh C, Almgren P, Struck J, Morgenthaler NG, Bergmann A, Platonov PG, Hedblad B, Engstrom G, Wang TJ, Melander O. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol 2010; 56: 1712–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van Melle JP, Bot M, de Jonge P, de Boer RA, van Veldhuisen DJ, Whooley MA. Diabetes, glycemic control, and new‐onset heart failure in patients with stable coronary artery disease: data from the heart and soul study. Diabetes Care 2010; 33: 2084–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bibbins‐Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD, Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med [Research Support, N.I.H., Extramural Research Support, Non‐U.S. Gov't] 2009; 360: 1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kenchaiah S, Sesso HD, Gaziano JM. Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation 2009; 119: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Leung AA, Eurich DT, Lamb DA, Majumdar SR, Johnson JA, Blackburn DF, McAlister FA. Risk of heart failure in patients with recent‐onset type 2 diabetes: population‐based cohort study. J Card Fail 2009; 15: 152–157. [DOI] [PubMed] [Google Scholar]
- 66. Lewis EF, Solomon SD, Jablonski KA, Rice MM, Clemenza F, Hsia J, Maggioni AP, Zabalgoitia M, Huynh T, Cuddy TE, Gersh BJ, Rouleau J, Braunwald E, Pfeffer MA, Investigators P. Predictors of heart failure in patients with stable coronary artery disease: a PEACE study. Circ Heart Fail 2009; 2: 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ruigomez A, Johansson S, Wallander MA, Edvardsson N, Garcia Rodriguez LA. Risk of cardiovascular and cerebrovascular events after atrial fibrillation diagnosis. Int J Cardiol 2009; 136: 186–192. [DOI] [PubMed] [Google Scholar]
- 68. Aksnes TA, Kjeldsen SE, Rostrup M, Omvik P, Hua TA, Julius S. Impact of new‐onset diabetes mellitus on cardiac outcomes in the Valsartan Antihypertensive Long‐term Use Evaluation (VALUE) trial population. Hypertension 2007; 50: 467–473. [DOI] [PubMed] [Google Scholar]
- 69. Fukuda T, Yamashita T, Sagara K, Kato T, Sawada H, Aizawa T. Development of congestive heart failure in Japanese patients with atrial fibrillation. Circ J 2007; 71: 308–312. [DOI] [PubMed] [Google Scholar]
- 70. Held C, Gerstein HC, Yusuf S, Zhao F, Hilbrich L, Anderson C, Sleight P, Teo K, Investigators OT. Glucose levels predict hospitalization for congestive heart failure in patients at high cardiovascular risk. Circulation 2007; 115: 1371–1375. [DOI] [PubMed] [Google Scholar]
- 71. Ito S, Murai S, Sugiura M, Yoshida T, Fukutomi T. Predictors of congestive heart failure in patients on maintenance hemodialysis. Circ J 2007; 71: 1424–1429. [DOI] [PubMed] [Google Scholar]
- 72. Ingelsson E, Arnlov J, Sundstrom J, Zethelius B, Vessby B, Lind L. Novel metabolic risk factors for heart failure. J Am Coll Cardiol 2005; 46: 2054–2060. [DOI] [PubMed] [Google Scholar]
- 73. Lentine KL, Schnitzler MA, Abbott KC, Li L, Burroughs TE, Irish W, Brennan DC. De novo congestive heart failure after kidney transplantation: a common condition with poor prognostic implications. Am J Kidney Dis 2005; 46: 720–733. [DOI] [PubMed] [Google Scholar]
- 74. Bibbins‐Domingo K, Lin F, Vittinghoff E, Barrett‐Connor E, Hulley SB, Grady D, Shlipak MG. Predictors of heart failure among women with coronary disease. Circulation 2004; 110: 1424–1430. [DOI] [PubMed] [Google Scholar]
- 75. Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 2004; 27: 1879–1884. [DOI] [PubMed] [Google Scholar]
- 76. Wylie JV, Murphy SA, Morrow DA, de Lemos JA, Antman EM, Cannon CP. Validated risk score predicts the development of congestive heart failure after presentation with unstable angina or non‐ST‐elevation myocardial infarction: results from OPUS‐TIMI 16 and TACTICS‐TIMI 18. Am Heart J 2004; 148: 173–180. [DOI] [PubMed] [Google Scholar]
- 77. Lewis EF, Moye LA, Rouleau JL, Sacks FM, Arnold JM, Warnica JW, Flaker GC, Braunwald E, Pfeffer MA, Study C. Predictors of late development of heart failure in stable survivors of myocardial infarction: the CARE study. J Am Coll Cardiol 2003; 42: 1446–1453. [DOI] [PubMed] [Google Scholar]
- 78. Rigatto C, Parfrey P, Foley R, Negrijn C, Tribula C, Jeffery J. Congestive heart failure in renal transplant recipients: risk factors, outcomes, and relationship with ischemic heart disease. J Am Soc Nephrol: JASN 2002; 13: 1084–1090. [DOI] [PubMed] [Google Scholar]
- 79. He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow‐up study. Arch Intern Med 2001; 161: 996–1002. [DOI] [PubMed] [Google Scholar]
- 80. Johansson S, Wallander MA, Ruigomez A, Garcia Rodriguez LA. Incidence of newly diagnosed heart failure in UK general practice. Eur J Heart Fail 2001; 3: 225–231. [DOI] [PubMed] [Google Scholar]
- 81. Wilhelmsen L, Rosengren A, Eriksson H, Lappas G. Heart failure in the general population of men—morbidity, risk factors and prognosis. J Intern Med [Research Support, Non‐U.S. Gov't] 2001; 249: 253–261. [DOI] [PubMed] [Google Scholar]
- 82. Aronow WS, Ahn C, Kronzon I. Comparison of incidences of congestive heart failure in older African‐Americans, Hispanics, and whites. Am J Cardiol 1999; 84: 611–612 A619. [DOI] [PubMed] [Google Scholar]
- 83. Chen YT, Vaccarino V, Williams CS, Butler J, Berkman LF, Krumholz HM. Risk factors for heart failure in the elderly: a prospective community‐based study. Am J Med 1999; 106: 605–612. [DOI] [PubMed] [Google Scholar]
- 84. Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med 1999; 159: 1197–1204. [DOI] [PubMed] [Google Scholar]
- 85. Harnett JD, Foley RN, Kent GM, Barre PE, Murray D, Parfrey PS. Congestive heart failure in dialysis patients: prevalence, incidence, prognosis and risk factors. Kidney Int 1995; 47: 884–890. [DOI] [PubMed] [Google Scholar]
- 86. Fogarassy G, Vathy‐Fogarassy A, Kenessey I, Kasler M, Forster T. Risk prediction model for long‐term heart failure incidence after epirubicin chemotherapy for breast cancer—a real‐world data‐based, nationwide classification analysis. Int J Cardiol 2019; 285: 47–52. [DOI] [PubMed] [Google Scholar]
- 87. Chen SK, Barbhaiya M, Fischer MA, Guan H, Yoshida K, Feldman CH, Costenbader KH, Everett BM. Heart failure risk in systemic lupus erythematosus compared to diabetes mellitus and general medicaid patients. Semin Arthritis Rheum 2019; 49: 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Winell K, Pietila A, Salomaa V. Incidence and prognosis of heart failure in persons with type 2 diabetes compared with individuals without diabetes—a nation‐wide study from Finland in 1996–2012. Ann Med 2019; 51: 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Magnussen C, Niiranen TJ, Ojeda FM, Gianfagna F, Blankenberg S, Vartiainen E, Sans S, Pasterkamp G, Hughes M, Costanzo S, Donati MB, Jousilahti P, Linneberg A, Palosaari T, de Gaetano G, Bobak M, den Ruijter HM, Jorgensen T, Soderberg S, Kuulasmaa K, Zeller T, Iacoviello L, Salomaa V, Schnabel RB, BiomarCa REC . Sex‐specific epidemiology of heart failure risk and mortality in Europe: results from the BiomarCaRE Consortium. JACC Heart Fail 2019; 7: 204–213. [DOI] [PubMed] [Google Scholar]
- 90. McAllister DA, Read SH, Kerssens J, Livingstone S, McGurnaghan S, Jhund P, Petrie J, Sattar N, Fischbacher C, Kristensen SL, McMurray J, Colhoun HM, Wild SH. Incidence of hospitalization for heart failure and case‐fatality among 3.25 million people with and without diabetes mellitus. Circulation 2018; 138: 2774–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lamblin N, Meurice T, Tricot O, de Groote P, Lemesle G, Bauters C. First hospitalization for heart failure in outpatients with stable coronary artery disease: determinants, role of incident myocardial infarction, and prognosis. J Card Fail 2018; 24: 815–822. [DOI] [PubMed] [Google Scholar]
- 92. Najafi F, Dobson AJ, Hobbs M, Jamrozik K. Late‐onset heart failure after myocardial infarction: trends in incidence and survival. Eur J Heart Fail 2008; 10: 765–771. [DOI] [PubMed] [Google Scholar]
- 93. Williams SA, Kasl SV, Heiat A, Abramson JL, Krumholz HM, Vaccarino V. Depression and risk of heart failure among the elderly: a prospective community‐based study. Psychosom Med 2002; 64: 6–12. [DOI] [PubMed] [Google Scholar]
- 94. Larsson SC, Wallin A, Hakansson N, Stackelberg O, Back M, Wolk A. Type 1 and type 2 diabetes mellitus and incidence of seven cardiovascular diseases. Int J Cardiol 2018; 262: 66–70. [DOI] [PubMed] [Google Scholar]
- 95. Abramson J, Berger A, Krumholz HM, Vaccarino V. Depression and risk of heart failure among older persons with isolated systolic hypertension. Arch Intern Med 2001; 161: 1725–1730. [DOI] [PubMed] [Google Scholar]
- 96. Miao F, Cai Y, Zhang Y. Risk prediction for heart failure incidence within 1‐year using clinical and laboratory factors. 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC 2014; 26‐30 August 2014; United States: IEEE; 2014. p. 1790‐1793. [DOI] [PubMed]
- 97. Jacobs L, Efremov L, Ferreira JP, Thijs L, Yang WY, Zhang ZY, Latini R, Masson S, Agabiti N, Sever P, Delles C, Sattar N, Butler J, Cleland JGF, Kuznetsova T, Staessen JA, Zannad F, Heart OiAi . Risk for incident heart failure: a subject‐level meta‐analysis from the Heart “OMics” in AGEing (HOMAGE) Study. J Am Heart Assoc 2017; 6: e005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sato T, Ono T, Morimoto Y, Kawai H, Fuke S, Ikeda T, Saito H. Five‐year clinical outcomes after implantation of sirolimus‐eluting stents in patients with and without diabetes mellitus. Cardiovasc Interv Therapeut 2012; 27: 189–195. [DOI] [PubMed] [Google Scholar]
- 99. Shafazand M, Rosengren A, Lappas G, Swedberg K, Schaufelberger M. Decreasing trends in the incidence of heart failure after acute myocardial infarction from 1993–2004: a study of 175,216 patients with a first acute myocardial infarction in Sweden. Eur J Heart Fail 2011; 13: 135–141. [DOI] [PubMed] [Google Scholar]
- 100. Hung J, Teng TH, Finn J, Knuiman M, Briffa T, Stewart S, Sanfilippo FM, Ridout S, Hobbs M. Trends from 1996 to 2007 in incidence and mortality outcomes of heart failure after acute myocardial infarction: a population‐based study of 20,812 patients with first acute myocardial infarction in Western Australia. J Am Heart Assoc 2013; 2: e000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chen YY, Chen Y, Liang SM, Su ZZ, Shu XR, Zhang HF, Wan SH, Wang JF, Xie SL. Prognostic impact of fasting plasma glucose on mortality and re‐hospitalization in patients with acute heart failure. Chin Med J (Engl) 2018; 131: 2032–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cooper LB, Yap J, Tay WT, Teng TK, MacDonald M, Anand IS, Sharma A, O'Connor CM, Kraus WE, Mentz RJ, Lam CS, Hf A, Investigators A‐H. Multi‐ethnic comparisons of diabetes in heart failure with reduced ejection fraction: insights from the HF‐ACTION trial and the ASIAN‐HF registry. Eur J Heart Fail 2018; 20: 1281–1289. [DOI] [PubMed] [Google Scholar]
- 103. Kristensen SL, Mogensen UM, Tarnesby G, Gimpelewicz CR, Ali MA, Shao Q, Chiang Y, Jhund PS, Abraham WT, Dickstein K, McMurray JJV, Kober L. Aliskiren alone or in combination with enalapril vs. enalapril among patients with chronic heart failure with and without diabetes: a subgroup analysis from the ATMOSPHERE trial. Eur J Heart Fail 2018; 20: 136–147. [DOI] [PubMed] [Google Scholar]
- 104. Rorth R, Fosbol EL, Mogensen UM, Kragholm K, Nume AK, Gislason GH, Jhund PS, Petrie MC, McMurray JJV, Torp‐Pedersen C, Kober L, Kristensen SL. Employment status at time of first hospitalization for heart failure is associated with a higher risk of death and rehospitalization for heart failure. Eur J Heart Fail 2018; 20: 240–247. [DOI] [PubMed] [Google Scholar]
- 105. Sandesara PB, O'Neal WT, Kelli HM, Samman‐Tahhan A, Hammadah M, Quyyumi AA, Sperling LS. The prognostic significance of diabetes and microvascular complications in patients with heart failure with preserved ejection fraction. Diabetes Care 2018; 41: 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dauriz M, Targher G, Laroche C, Temporelli PL, Ferrari R, Anker S, Coats A, Filippatos G, Crespo‐Leiro M, Mebazaa A, Piepoli MF, Maggioni AP, Tavazzi L, Registry E‐HHFL‐T . Association between diabetes and 1‐year adverse clinical outcomes in a multinational cohort of ambulatory patients with chronic heart failure: results from the ESC‐HFA Heart Failure Long‐Term Registry. Diabetes Care 2017; 40: 671–678. [DOI] [PubMed] [Google Scholar]
- 107. Farre N, Vela E, Cleries M, Bustins M, Cainzos‐Achirica M, Enjuanes C, Moliner P, Ruiz S, Verdu‐Rotellar JM, Comin‐Colet J. Real world heart failure epidemiology and outcome: a population‐based analysis of 88,195 patients. PLoS ONE 2017; 12: e0172745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kristensen SL, Mogensen UM, Jhund PS, Petrie MC, Preiss D, Win S, Kober L, McKelvie RS, Zile MR, Anand IS, Komajda M, Gottdiener JS, Carson PE, McMurray JJ. Clinical and echocardiographic characteristics and cardiovascular outcomes according to diabetes status in patients with heart failure and preserved ejection fraction: a report from the I‐Preserve Trial (Irbesartan in Heart Failure With Preserved Ejection Fraction). Circulation 2017; 135: 724–735. [DOI] [PubMed] [Google Scholar]
- 109. Mohamedali B, Yost G, Bhat G. Is diabetes mellitus a risk factor for poor outcomes after left ventricular assist device placement? Tex Heart Inst J 2017; 44: 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Echouffo‐Tcheugui JB, Masoudi FA, Bao H, Spatz ES, Fonarow GC. Diabetes mellitus and outcomes of cardiac resynchronization with implantable cardioverter‐defibrillator therapy in older patients with heart failure. Circ Arrhythm Electrophysiol 2016; 9: e004132. [DOI] [PubMed] [Google Scholar]
- 111. Kristensen SL, Preiss D, Jhund PS, Squire I, Cardoso JS, Merkely B, Martinez F, Starling RC, Desai AS, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, McMurray JJ, Packer M, Investigators P‐H, Committees . Risk related to pre‐diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI With ACEI to determine impact on global mortality and morbidity in heart failure trial. Circ Heart Fail 2016; 9: e002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ruigomez A, Michel A, Martin‐Perez M, Garcia Rodriguez LA. Heart failure hospitalization: an important prognostic factor for heart failure re‐admission and mortality. Int J Cardiol 2016; 220: 855–861. [DOI] [PubMed] [Google Scholar]
- 113. Kaneko H, Suzuki S, Goto M, Arita T, Yuzawa Y, Yagi N, Murata N, Kato Y, Kano H, Matsuno S, Otsuka T, Uejima T, Oikawa Y, Sagara K, Nagashima K, Kirigaya H, Sawada H, Aizawa T, Yajima J, Yamashita T. Incidence and predictors of rehospitalization of acute heart failure patients. Int Heart J 2015; 56: 219–225. [DOI] [PubMed] [Google Scholar]
- 114. Takeda K, Takayama H, Colombo PC, Yuzefpolskaya M, Fukuhara S, Han J, Kurlansky P, Mancini DM, Naka Y. Incidence and clinical significance of late right heart failure during continuous‐flow left ventricular assist device support. J Heart Lung Transplant 2015; 34: 1024–1032. [DOI] [PubMed] [Google Scholar]
- 115. Carrasco‐Sanchez FJ, Gomez‐Huelgas R, Formiga F, Conde‐Martel A, Trullas JC, Bettencourt P, Arevalo‐Lorido JC, Perez‐Barquero MM, Investigators R. Association between type‐2 diabetes mellitus and post‐discharge outcomes in heart failure patients: findings from the RICA registry. Diabetes Res Clin Pract 2014;104:410‐419. [DOI] [PubMed] [Google Scholar]
- 116. Cubbon RM, Woolston A, Adams B, Gale CP, Gilthorpe MS, Baxter PD, Kearney LC, Mercer B, Rajwani A, Batin PD, Kahn M, Sapsford RJ, Witte KK, Kearney MT. Prospective development and validation of a model to predict heart failure hospitalisation. Heart 2014; 100: 923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Paoletti Perini A, Bartolini S, Pieragnoli P, Ricciardi G, Perrotta L, Valleggi A, Vergaro G, Michelotti F, Boggian G, Sassone B, Mascioli G, Emdin M, Padeletti L. CHADS2 and CHA2DS2‐VASc scores to predict morbidity and mortality in heart failure patients candidates to cardiac resynchronization therapy. Europace 2014; 16: 71–80. [DOI] [PubMed] [Google Scholar]
- 118. Sakata Y, Miyata S, Nochioka K, Miura M, Takada T, Tadaki S, Takahashi J, Shimokawa H. Gender differences in clinical characteristics, treatment and long‐term outcome in patients with stage C/D heart failure in Japan. Report from the CHART‐2 study. Circ J 2014; 78: 428–435. [DOI] [PubMed] [Google Scholar]
- 119. Larina VN, Bart BY, Vartanyan EA. Factors effecting the decompensation of chronic heart failure in the elderly. Rational Pharmacother Cardiol 2013; 9: 15–24. [Google Scholar]
- 120. Sarma S, Mentz RJ, Kwasny MJ, Fought AJ, Huffman M, Subacius H, Nodari S, Konstam M, Swedberg K, Maggioni AP, Zannad F, Bonow RO, Gheorghiade M, Investigators E. Association between diabetes mellitus and post‐discharge outcomes in patients hospitalized with heart failure: findings from the EVEREST trial. Eur J Heart Fail 2013;15:194‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Verbrugge FH, Dupont M, Rivero‐Ayerza M, de Vusser P, Van Herendael H, Vercammen J, Jacobs L, Verhaert D, Vandervoort P, Tang WH, Mullens W. Comorbidity significantly affects clinical outcome after cardiac resynchronization therapy regardless of ventricular remodeling. J Card Fail 2012; 18: 845–853. [DOI] [PubMed] [Google Scholar]
- 122. Deedwania PC, Ahmed MI, Feller MA, Aban IB, Love TE, Pitt B, Ahmed A. Impact of diabetes mellitus on outcomes in patients with acute myocardial infarction and systolic heart failure. Eur J Heart Fail 2011; 13: 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Martin DT, McNitt S, Nesto RW, Rutter MK, Moss AJ. Cardiac resynchronization therapy reduces the risk of cardiac events in patients with diabetes enrolled in the multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy (MADIT‐CRT). Circ Heart Fail 2011; 4: 332–338. [DOI] [PubMed] [Google Scholar]
- 124. Aguilar D, Deswal A, Ramasubbu K, Mann DL, Bozkurt B. Comparison of patients with heart failure and preserved left ventricular ejection fraction among those with versus without diabetes mellitus. Am J Cardiol 2010; 105: 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sze E, Moss AJ, McNitt S, Barsheshet A, Andrews ML, Zareba W, Goldenberg I. Multicenter Automatic Defibrillator Implantation Trial III. Risk factors for recurrent heart failure events in the Multicenter Automatic Defibrillator Implantation Trial II (MADIT‐II). J Cardiovasc Electrophysiol 2010; 21: 1217–1223. [DOI] [PubMed] [Google Scholar]
- 126. MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, Solomon SD, Granger CB, Swedberg K, Yusuf S, Pfeffer MA, McMurray JJ, Investigators C. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J 2008; 29: 1377–1385. [DOI] [PubMed] [Google Scholar]
- 127. MacDonald MR, Jhund PS, Petrie MC, Lewsey JD, Hawkins NM, Bhagra S, Munoz N, Varyani F, Redpath A, Chalmers J, MacIntyre K, McMurray JJ. Discordant short‐ and long‐term outcomes associated with diabetes in patients with heart failure: importance of age and sex: a population study of 5.1 million people in Scotland. Circ Heart Fail 2008; 1: 234–241. [DOI] [PubMed] [Google Scholar]
- 128. Ghali JK, Boehmer J, Feldman AM, Saxon LA, Demarco T, Carson P, Yong P, Galle EG, Leigh J, Ecklund FL, Bristow MR. Influence of diabetes on cardiac resynchronization therapy with or without defibrillator in patients with advanced heart failure. J Card Fail 2007; 13: 769–773. [DOI] [PubMed] [Google Scholar]
- 129. Ruiz‐Ruiz FJ, Ruiz‐Laiglesia FJ, Samperiz‐Legarre P, Lasierra‐Diaz P, Flamarique‐Pascual A, Morales‐Rull JL, Perez‐Calvo JI. Propeptide of procollagen type I (PIP) and outcomes in decompensated heart failure. Eur J Intern Med 2007; 18: 129–134. [DOI] [PubMed] [Google Scholar]
- 130. Formiga F, Chivite D, Sole A, Manito N, Ramon JM, Pujol R. Functional outcomes of elderly patients after the first hospital admission for decompensated heart failure (HF). A prospective study. Arch Gerontol Geriatr 2006; 43: 175–185. [DOI] [PubMed] [Google Scholar]
- 131. Garcia C, Lupon J, Urrutia A, Gonzalez B, Herreros J, Altimir S, Coll R, Prats M, Rey‐Joly C, Valle V. Prognostic significance of diabetes in a heart failure population: one year mortality and heart failure related hospital admission. Med Clin 2005; 125: 161–165. [DOI] [PubMed] [Google Scholar]
- 132. Domanski M, Krause‐Steinrauf H, Deedwania P, Follmann D, Ghali JK, Gilbert E, Haffner S, Katz R, Lindenfeld J, Lowes BD, Martin W, McGrew F, Bristow MR, Investigators B. The effect of diabetes on outcomes of patients with advanced heart failure in the BEST trial. J Am Coll Cardiol 2003; 42: 914–922. [DOI] [PubMed] [Google Scholar]
- 133. Shindler DM, Kostis JB, Yusuf S, Quinones MA, Pitt B, Stewart D, Pinkett T, Ghali JK, Wilson AC. Diabetes mellitus, a predictor of morbidity and mortality in the Studies of Left Ventricular Dysfunction (SOLVD) Trials and Registry. Am J Cardiol 1996; 77: 1017–1020. [DOI] [PubMed] [Google Scholar]
- 134. Kim HL, Kim MA, Park KT, Choi DJ, Han S, Jeon ES, Cho MC, Kim JJ, Yoo BS, Shin MS, Kang SM, Chae SC, Ryu KH, Kor HFR. Gender difference in the impact of coexisting diabetes mellitus on long‐term clinical outcome in people with heart failure: a report from the Korean Heart Failure Registry. Diabet Med 2019; 36: 1312–1318. [DOI] [PubMed] [Google Scholar]
- 135. Takimura H, Hada T, Kawano M, Yabe T, Takimura Y, Nishio S, Nakano M, Tsukahara R, Muramatsu T. A novel validated method for predicting the risk of re‐hospitalization for worsening heart failure and the effectiveness of the diuretic upgrading therapy with tolvaptan. PLoS ONE 2018; 13: e0207481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Retwinski A, Kosmalski M, Crespo‐Leiro M, Maggioni A, Opolski G, Ponikowski P, Polonski L, Jankowska E, Drzewoski J, Drozdz J. The influence of metformin and the presence of type 2 diabetes mellitus on mortality and hospitalisation in patients with heart failure. Kardiol Pol 2018; 76: 1336–1343. [DOI] [PubMed] [Google Scholar]
- 137. Iorio A, Senni M, Barbati G, Greene SJ, Poli S, Zambon E, Di Nora C, Cioffi G, Tarantini L, Gavazzi A, Sinagra G, Di Lenarda A. Prevalence and prognostic impact of non‐cardiac co‐morbidities in heart failure outpatients with preserved and reduced ejection fraction: a community‐based study. Eur J Heart Fail 2018; 20: 1257–1266. [DOI] [PubMed] [Google Scholar]
- 138. Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res 2018; 122: 624–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Gilca GE, Stefanescu G, Badulescu O, Tanase DM, Bararu I, Ciocoiu M. Diabetic cardiomyopathy: current approach and potential diagnostic and therapeutic targets. J Diabetes Res 2017; 2017: 1310265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Leon LE, Rani S, Fernandez M, Larico M, Calligaris SD. Subclinical detection of diabetic cardiomyopathy with microRNAs: challenges and perspectives. J Diabetes Res 2016; 2016: 6143129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lindman BR, Davila‐Roman VG, Mann DL, McNulty S, Semigran MJ, Lewis GD, de las Fuentes L, Joseph SM, Vader J, Hernandez AF, Redfield MM. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol 2014; 64: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015; 131: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lenzen MJ, Rosengren A, Scholte op Reimer WJ, Follath F, Boersma E, Simoons ML, Cleland JG, Komajda M. Management of patients with heart failure in clinical practice: differences between men and women. Heart 2008; 94: e10. [DOI] [PubMed] [Google Scholar]
- 144. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 145. Andersen MJ, Borlaug BA. Heart failure with preserved ejection fraction: current understandings and challenges. Curr Cardiol Rep 2014; 16: 501. [DOI] [PubMed] [Google Scholar]
- 146. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck‐Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64: 401–406. [DOI] [PubMed] [Google Scholar]
- 147. Bugger H, Abel ED. Rodent models of diabetic cardiomyopathy. Dis Model Mech 2009; 2: 454–466. [DOI] [PubMed] [Google Scholar]


